Abstract
Background and Objectives
Pulmonary arterial hypertension (PAH) is a life threatening disease characterized by progressive pulmonary arterial occlusion which may ultimately result in death. Currently, the available treatments are diverse, but no therapy alone can reverse the disease process although they may have some clinical benefits. This study was designed to investigate single and combination therapy of simvastatin and sildenafil, which have different mechanisms of action, in monocrotaline (MCT)-induced PAH.
Meterials and Methods
Rats were randomized to receive saline (control, n=8) or MCT treatment (n=32). MCT treated rats were randomized to vehicle, simvastatin (2 mg/kg/day), sildenafil (25 mg/kg/day) and a combination simvastatin and sildenafil (n=8, respectively). Three weeks later, hemodynamic study and histologic changes of pulmonary arterioles were measured. Proliferating cell nuclear antigen (PCNA) as well as Western blot for endothelial nitric oxide synthase (eNOS) were performed.
Results
Systolic right ventricular pressure was significantly decreased in monotherapy groups (simvastatin and sildenafil) and the combination group compared to MCT group (p<0.05). Right ventricular hypertrophy and medial wall thickness of pulmonary arterioles were significantly attenuated with sole and combination therapy (p<0.05). However, combination therapy did not confer additive benefits over monotherapy. Altered PCNA or eNOS in lung tissue was normalized by either monotherapy or combination therapy.
Conclusion
The results suggest that either simvastatin or sildenafil has the therapeutic potential in MCT-induced PAH, although combination therapy of these two drugs has failed to show greater benefits in the study.
Keywords: Pulmonary circulation, Pulmonary hypertension, Simvastatin, Sildenafil
Introduction
Pulmonary arterial hypertension (PAH) is characterized by abnormal proliferation of vascular endothelial and smooth muscle cells and causes occlusion of pulmonary arterioles which eventually results in right heart failure and death.1-3) Although there is no cure for PAH, newer medical therapies have been shown to improve a variety of clinical end-points, including survival rate, exercise tolerance, hemodynamics and quality of life measures.4)
Basically, there are three classes of medications that produce benefits in PAH: prostanoids, endothelin receptor antagonists, and phosphodiesterase-5 (PDE-5) inhibitors.5) Although they have clinical benefits, these currently available therapies can not reverse the disease process itself.5) Therefore, new therapies based on the understanding of the PAH pathophysiology, especially directed at suppressing inappropriate cellular proliferation in pulmonary arteries, are warranted. The addition a drug with different mechanisms may be reasonable in the event of suboptimal response to monotherapy.2),4)
Simvastatin is a well known drug that confers cardiovascular benefits that exceed its effects in lowering serum cholesterol.6) Recently, it has been demonstrated that simvastatin can reverse established severe PAH and improve survival, and attenuate vascular remodeling in the animal models of pulmonary hypertension.7-10)
One of the PDE-5 inhibitors, sildenafil, is a common choice for decreasing pulmonary arterial pressure and vascular remodeling in animal model of PAH.11),12) In addition, sildenafil was found to improve pulmonary hemodynamics and exercise capacity in patients with PAH.13-16)
In this study, we evaluated the effect of simvastatin or sildenafil treatment in a rat model of monocrotaline (MCT)-induced PAH. In addition, we investigated whether combination of simvastatin and sildenafil would be more effective than the respective monotherapy.
Materials and Methods
Experimental designs
Forty (n=40) male Sprague-Dawley rats (8 weeks old, 180-200 g) were randomly allocated into five groups (each group, n=8): control group was injected with distillated water, MCT group was just injected with MCT (60 mg/kg, subcutaneously, Sigma Chemical Co, St. Louis, MO, USA), simvastatin group was treated with simvastatin (2 mg/kg/day, Hanmi Pharmacy, Korea) in MCT, sildenafil group was treated with sildenafil (25 mg/kg/day, Pfizer Australia Limited) in MCT, and the combination group was treated with simvastatin and sildenafil. Each group was kept in the same room and subjected to the same light/dark cycle. After 21 days, hemodynamic measurement was conducted and tissue samples were obtained for morphometric analysis and Western blot. The experimental protocol was reviewed and approved by the Animal Care and Use Committee of Dongguk University. Animal care and use was in accordance with the guidelines of the National Institutes of Health (Bethesda, MD).
Measurement of systolic right ventricular pressure
The animals were anaesthetized by intraperitonial injection of ketamine (100 mg/kg). For measurement of systolic right ventricular pressure (RVP), the tip of a polyethylene catheter (PE-50, Becton Dickinson, Franklin Lakes, NJ, USA) was inserted into the right ventricle (RV) via the internal jugular vein and a fluid-filled catheter was connected to a pressure transducer (Grass polygraph, Grass instrument Co, Quincy, MA, USA).
Right ventricular hypertrophy and lung morphology
Rats were euthanized by pentothal overdose and then the RV free wall was dissected from the left ventricle plus septum (LV plus S) and weighed separately on the analytic scale. RV hypertrophy (RVH) was assessed by RV-to-LV plus S weight ratio. For the analysis of vascular remodeling, the left lung was paraffin-embedded after fixation with transcardiac infusion of 4% paraformaldehyde. Serial coronal sections in 5 µm thick were obtained at the lower zone of the lung.
Following deparaffinization, sections were stained with hematoxylin-eosin (H&E). The medial wall thickness (MWT) of the pulmonary arteriole was measured at the size of 50-100 µm. The MWT ratio, an index of medial wall hypertrophy, was determined as the average of 10 to 15 fields per slice and calculated as below: MWT=(external diameter-internal diameter)/external diameter.
Immunohistochemical stain of proliferating cell nuclear antigen
Sections were stained with the DAKO kit (DAKO, CH). Briefly, endogenous peroxidase activity was blocked with hydrogen peroxide. After washing in phosphate buffer saline (PBS), the sections were incubated with rat anti-proliferating cell nuclear antigen (PCNA) (1 : 1,000, Sigma, St. Louis, MO, USA). After washing in PBS, the sections were then incubated with biotylated universal anti-mouse, -goat and -rabbit immunoglobulins.
After washing, the sections were incubated with streptavidin conjugated to horseradish peroxidase and reacted with a solution containing diaminobenzidine (DAB) and hydrogen peroxide. The sections were then counter-stained with Mayer's hematoxylin to visualize cell nuclei.
Terminal deoxynucleotidyl transferase dUTP nick end labeling staining
Transferase dUTP nick end labeling (TUNEL) staining (ApopTag kit, Intergen) was employed for the detection of deoxyribonucleic acid fragmentation and apoptotic bodies in lung tissue. Briefly, after deparaffinizing the sections, digesting protein using proteinase K and quenching endogenous peroxidase activity with H2O2 in PBS, slides were placed in equilibration buffer and then in working terminal deoxynucleotidyl transferase (TdT) enzyme, followed by stop/wash buffer. After two drops of anti-digoxigenin-peroxidase were applied to the slides, peroxidase was detected with DAB and examined under microscope (Olympus Bx20). Negative controls were performed using distilled water or PBS for TdT enzyme in preparation for working TdT.
Western blotting of endothelial nitric oxide synthase
Lung tissue was removed and then snap-frozen at -70℃ for western blot analysis. Tissue samples were homogenized in ten volumes of homogenizing buffer. Aliquots were stored at -70℃. Samples of homogenate were run on 7.5% polyacrylamide mini gels (Bio-Rad Mini Protean). After electrophoresis, the protein was transferred to nitrocellulose paper in a Bio-Rad transblot system. Nitrocellulose sheets were washed and incubated with rabbit anti-endothelial nitric oxide synthase (eNOS) (Sigma) overnight at 4℃. The labeling was visualized with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) with an enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Little Chalfont, UK). Immunoblot signal developed by ECL system was quantified using Scion Image software (version 1.59).
Statistical analysis
All data were presented as mean±standard deviation. Data were analyzed by one-way analysis of variance (ANOVA), followed by Tukey's honestly significant different multiple-comparison test. Multiple-comparison tests were only applied when a significant difference was determined by ANOVA (p<0.05). Statistical hypotheses were considered significant if p<0.05.
Results
Changes in right ventricular pressure
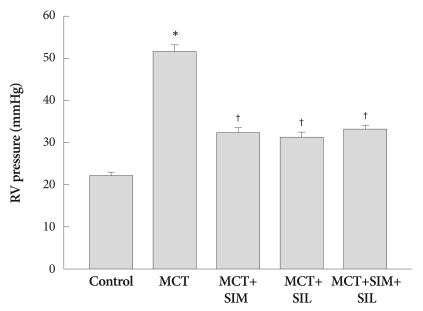
RVP was significantly increased in MCT-induced PAH group compared to that of the controls (51.5±1.5 vs. 22.2±0.7 mmHg, p<0.05), which was markedly suppressed in both single (simvastatin or sildenafil) (32.4±1.2.1, 31.4±1.0 mmHg, p<0.05) and combined treatment groups (33.4±0.7 mmHg, p<0.05). However, combination therapy was not more effective than single therapy with simvastatin or sildenafil (Fig. 1).
Fig. 1.
Right systolic ventricle pressure. Simvastatin (SIM), sildenafil (SIL) and combination therapy (SIM+SIL) prevents the development of pulmonary arterial hypertension in monocrotaline (MCT) treated rats. However, combination therapy does not show additive effects over monotherapy. Values are expressed as mean±SD. *p<0.05 vs. control, †p<0.05 vs. MCT. RV: right ventricle.
Changes in right ventricular hypertrophy
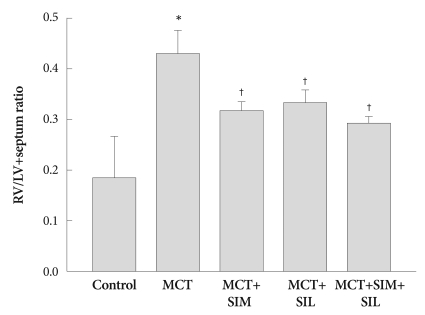
In the MCT group, RVH was developed and significantly increased in RV (LV plus septum ratio) compared to that of the controls (0.45±0.03 vs. 0.26±0.02, p<0.05), which was markedly decreased in the simvastatin, sildenafil and combination treatment groups (0.32±0.02, 0.34±0.02 and 0.29±0.01, p<0.05). Combination therapy tended to diminish RVH more effectively than monotherapy, even though it was not statistically significant (Fig. 2).
Fig. 2.
Ratio of the right ventricle (RV) to left ventricle (LV) plus septum weight. Monocrotaline (MCT)-induced RV hypertrophy was attenuated after simvastatin (SIM), sildenafil (SIL) and combination therapy (SIM+SIL). However, combination therapy did not show additional additive effects over monotherapy. Values are expressed as mean±SD. *p<0.05 vs. control, †p<0.05 vs. MCT.
Changes in pulmonary artery wall thickness
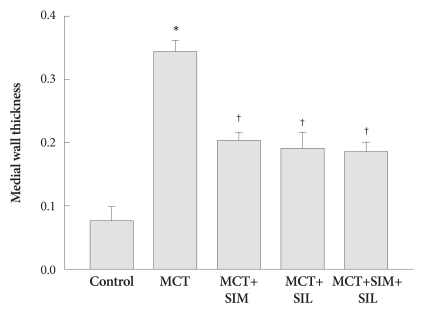
MCT treatment increased MWT (ratio) of pulmonary arteriole compared to that of control (0.34±0.02 vs. 0.08±0.01, p<0.05), which was significantly suppressed in the simvastatin, sildenafil and the combination treatment groups (0.2±0.01, 0.19±0.03 and 0.18±0.01, p<0.05) (Fig. 3). However, combination therapy was not superior to single-agent treatment and did not completely reverse pulmonary arterial wall thickness to normal.
Fig. 3.
Medial wall thickness of pulmonary arterioles. Thickened medial wall in monocrotaline (MCT) treatment group was significantly reduced in simvastatin (SIM), sildenafil (SIL) and combination therapy (SIM+SIL). However, combination therapy did not show additional additive effects over monotherapy. Values are expressed as mean±SD. *p<0.05 vs. control, †p<0.05 vs. MCT.
Changes in cellular proliferation and apoptosis in vascular smooth muscle cells
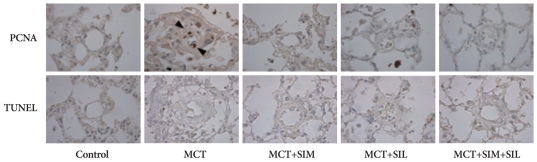
MCT-induced PAH had prominent PCNA positive cells in the medial wall of pulmonary arterioles. Simvastatin, sildenafil and combination therapy showed significant reduction in PCNA-positive cells in MCT-induced PAH (Fig. 4). However, TUNEL-positive cells for detecting apoptosis was rarely seen in both MCT-induced PAH and drug treated groups (Fig. 4).
Fig. 4.
Immunohistochemical stains of proliferating cell nuclear antigen (PCNA) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). Monocrotaline (MCT)-induced pulmonary hypertension showed prominent smooth muscle cell proliferation of the pulmonary artery. Simvastatin (SIM), sildenafil (SIL) and combination therapy (SIM+SIL) reduced cellular proliferation. TUNEL positive cells were not evident in all groups. Arrowheads, PCNA-positive cells.
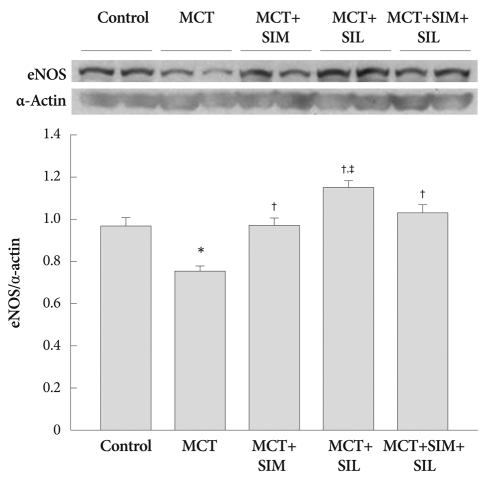
Western blotting of endothelial nitric oxide synthase
As can be seen in Fig. 5, eNOS expression in the lung was significantly depressed after 3 weeks of MCT treatment (p<0.05). The expression of eNOS was significantly up-regulated in the treated groups, compared to that of the MCT group (p<0.05). In particular, eNOS expression in sildenafil-treated group was much higher than that of the combination therapy group (p<0.05) (Fig. 5).
Fig. 5.
Western blots for eNOS in the lung. Expression of lung eNOS was significantly diminished in the monocrotaline (MCT)-induced pulmonary hypertension. However, eNOS expression was normalized to the control level by simvastatin (SIM) and sildenafil (SIL) treatment. However, combination therapy (SIM+SIL) did not show additional additive effects over monotherapy. Values are expressed as mean±SD. *p<0.05 vs. control, †p<0.05 vs. MCT, ‡p<0.05 vs. SIM and combination. eNOS: endothelial nitric oxide synthase.
Discussion
Our data show that simvastatin and sildenafil have therapeutic effects in MCT-induced PAH in rats, even though simvastatin and sildenafil combination therapy has not shown greater clinical benefits than monotherapy with either agent.
Simvastatin has potent antiproliferative and antiapoptotic effects on vascular smooth muscle cells, enhances production of eNOS and exerts anti-inflammatory effects in the cardiovascular system.17-19) In animal studies, simvastatin could reverse or attenuate pulmonary hypertension.9),20),21) A clinical study with small groups of patients demonstrated improvements in 6 min-walk performance and hemodynamics in patients receiving simvastatin daily.22) Our studies have demonstrated reduction of RVP, reduced cellular proliferation and enhanced eNOS expression in simvastatin treated group, which are consistent with the results of previous studies.
Sildenafil is a selective PDE-5 inhibitor, which exerts relaxation of vascular smooth muscle cells by inhibiting cyclic guanosine monophosphate (cGMP) metabolism. Sildenafil has been shown to have beneficial effects in animal and clinical studies.11),12),16) However, although this treatment may slow disease progression, it does not cure the condition. Therefore, combination of two or more drugs with different mechanisms are being widely used.23),24) However, the effect combining simvastatin and sildenafil in PAH is not certain.
In this study, we did not determine the additional benefits of combination therapy over monotherapy with either simvastatin or sildenafil. Consistent with our results, Satoh and Satoh25) reported that the combination of simvastatin (2 mg/kg/day) and sildenafil (5 mg/kg/day) did not show additive effects on MCT-induced PAH. It may be due to the low dosage of sildenafil administered. However, Liu et al.26) reported that low dose sildenafil (1.75 mg/kg/day) can also prevent and reverse pulmonary hypertension effectively. Therefore, further studies are necessary to determine the effective dosage of sildenafil in PAH in animal models.
In addition, two other studies showed beneficial effects of combination therapy.27),28) Recent studies demonstrated that combining simvastatin (20 mg/kg/day) and sildenafil (75 mg/kg/day) in hypoxic animal PAH models had greater therapeutic effects than using either treatment alone and that combination therapy augmented the levels of lung cGMP twice more than monotherapy did, even though the dosage of the two drugs is high enough to reach hepatotoxicity.27) Moreover, in MCT-induced PAH model, the combination of low dose of simvastatin (7 mg/kg/day) and sildenafil (20 mg/kg/day) had greater therapeutic effects than monotherapy in which the dosages of two drugs were similar with that of our study.28) In this study, each drug increased nitric oxide and cGMP significantly. The combination therapy did not show additive benefits, even though there were great improvements in hemodynamic and histologic changes. Consistent with previous studies, our study also showed that eNOS expression was higher in monotherapy with sildenafil than combination therapy. Therefore, further studies, using multiple animal models, different dosage of drugs and different length of follow up are needed in order to assess the effectiveness of combination therapy.
It is well known that MCT-induced PAH is developed by abnormal cellular proliferation of vascular endothelial and smooth muscle cells.1) Thus, many drugs are focused on reducing cellular proliferation via apoptosis. In this study, PCNA-positive staining cells in MCT rats were prominent but rarely seen in simvastatin, sildenafil and their combination therapies. It indicates that simvastatin, sildenafil and their combination therapies effectively reduce cellular proliferations. Several studies showed that apoptosis is one of the most important mechanisms in attenuating PAH.9),10) However, we did not find significant TUNEL-positive cells in MCT-induced PAH and mono- and combined therapy groups with simvastatin and sildenafil. These results may be due to intensive proliferation of smooth muscle cells that developed in the early phase of MCT injection.29) In addition, the apoptotic process was more prominent in the early phase of the therapeutic trial.10) From these results, the timing of treatment appears to be very important in the therapy of PAH.
One of the beneficial effects of simvastatin and sildenafil is increased eNOS expression.11),12) This study also showed that the treatment with simvastatin or sildenafil significantly increased the expression of eNOS, compared to MCT-induced PAH rat. Among them, sildenafil induced the most up-regulation in eNOS expression.
In summary, monotherapy with either simvastatin or sildenafil and combination therapy with both agents attenuated MCT-induced PAH. However, combination therapy with simvastatin and sildenafil was not more effective than monotherapy with either agent. Further studies with different design, including PAH models, drug dosages and duration of treatments, are required to confirm the beneficial effects of the combination therapy.
Acknowledgments
This work was financially supported by a grant from The Korean Society of Circulation in 2007 and a research fund from the Dongguk University.
References
- 1.Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Martin KB, Klinger JR, Rounds SI. Pulmonary arterial hypertension: new insights and new hope. Respirology. 2006;11:6–17. doi: 10.1111/j.1440-1843.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee WS, Kim KH, Jeong DH, et al. Clinical characteristics and prognostic factors of patients with severe pulmonary hypertension. Korean Circ J. 2007;37:265–270. [Google Scholar]
- 4.Lee SH, Rubin LJ. Current treatment strategies for pulmonary arterial hypertension. J Intern Med. 2005;258:199–215. doi: 10.1111/j.1365-2796.2005.01542.x. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Sitbon O, Simmoneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 6.Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84:413–428. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Hu H, Sung A, Zhao G, et al. Simvastatin enhances bone morphogenetic protein receptor type II expression. Biochem Biophys Res Commun. 2006;339:59–64. doi: 10.1016/j.bbrc.2005.10.187. [DOI] [PubMed] [Google Scholar]
- 8.Girgis RE, Li D, Zhan X, et al. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Heart Circ Physiol. 2003;285:H938–H945. doi: 10.1152/ajpheart.01097.2002. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura T, Vaszar LT, Faul JL, et al. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation. 2003;108:1640–1645. doi: 10.1161/01.CIR.0000087592.47401.37. [DOI] [PubMed] [Google Scholar]
- 10.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, et al. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;291:L668–L676. doi: 10.1152/ajplung.00491.2005. [DOI] [PubMed] [Google Scholar]
- 11.Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107:3230–3235. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- 12.Schermuly RT, Kreisselmeier KP, Ghofrani HA, et al. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004;169:39–45. doi: 10.1164/rccm.200302-282OC. [DOI] [PubMed] [Google Scholar]
- 13.Kothari SS, Duggal B. Chronic oral sildenafil therapy in severe pulmonary artery hypertension. Indian Heart J. 2002;54:404–409. [PubMed] [Google Scholar]
- 14.Sastry BK, Narasimhan C, Reddy NK, Raju BS. Clinical efficacy of sildenafil in primary pulmonary hypertension: a randomized, placebo-controlled, double-blind, crossover study. J Am Coll Cardiol. 2004;43:1149–1153. doi: 10.1016/j.jacc.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins MR, Paul GA, Strange JW, et al. Sildenafil versus endothelin receptor antagonist for pulmonary hypertension (SERAPH) study. Am J Respir Crit Care Med. 2005;171:1292–1297. doi: 10.1164/rccm.200410-1411OC. [DOI] [PubMed] [Google Scholar]
- 16.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 17.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 18.Laufs U, Fata VL, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272:31725–31729. doi: 10.1074/jbc.272.50.31725. [DOI] [PubMed] [Google Scholar]
- 19.Son JW, Koh KK, You SM, et al. Effects of simvastatin alone or combined with ramipril on nitric oxide bioactivity and inflammation markers in hypercholesterolemic patients. Korean Circ J. 2003;33:1053–1059. [Google Scholar]
- 20.Girgis RE, Mozammel S, Champion HC, et al. Regression of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1105–L1110. doi: 10.1152/ajplung.00411.2006. [DOI] [PubMed] [Google Scholar]
- 21.Kim MY, Kim YK, Jung YW, Kim WT, Kwon TH, Lee DS. The protective effect of simvastatin on monocrotaline-induced pulmonary hypertension in rats. Korean Circ J. 2008;38:313–319. [Google Scholar]
- 22.Kao PN. Simvastatin treatment of pulmonary hypertension: an observational case series. Chest. 2005;127:1446–1452. doi: 10.1378/chest.127.4.1446. [DOI] [PubMed] [Google Scholar]
- 23.Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. J Eur Respir J. 2005;26:858–863. doi: 10.1183/09031936.05.00075305. [DOI] [PubMed] [Google Scholar]
- 24.Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131:1917–1928. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 25.Satoh M, Satoh A. 3-Hydroxy-3-methylglutaryl (HMG)-COA reductase inhibitors and phosphodiesterase type V inhibitors attenuate right ventricular pressure and remodeling in a rat model of pulmonary hypertension. J Pharm Pharm Sci. 2009;11:118s–130s. doi: 10.18433/j34k5z. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Liu ZY, Guan Q. Oral sildenafil prevents and reverses the development of pulmonary hypertension in monocrotaline-treated rats. Interact Cardiovasc Thorac Surg. 2007;6:608–613. doi: 10.1510/icvts.2006.147033. [DOI] [PubMed] [Google Scholar]
- 27.Zhao L, Sebkhi A, Ali O, et al. Simvastatin and sildenafil combine to attenuate pulmonary hypertension. Eur Respir J. 2009;34:948–957. doi: 10.1183/09031936.00143508. [DOI] [PubMed] [Google Scholar]
- 28.Kuang T, Wang J, Pang B, et al. Combination of sildenafil and simvastatin ameliorates monocrotaline-induced pulmonary hypertension in rats. Pulm Pharmacol Ther. 2010;23:456–464. doi: 10.1016/j.pupt.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMurtry MS, Bonnet S, Michelakis ED, Bonnet S, Haromy A, Archer SL. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamcyin-atorvastatin-simvastatin study. Am J Physiol Lung Cell Mol Physiol. 2007;293:L933–L940. doi: 10.1152/ajplung.00310.2006. [DOI] [PubMed] [Google Scholar]