Abstract
Cardiac amyloidosis describes a clinical disorder caused by infiltration of abnormal insoluble fibrils in the heart, characterized by progressive heart failure and a grave prognosis. Pleural effusion in cardiac amyloidosis may represent a sign of heart failure, but it can also result from pleural infiltration of amyloid, manifested by recurrent large fluid accumulations. Recently, the role of vascular endothelial growth factor (VEGF) has been implicated in the pathogenesis of refractory pleural effusion. We report a case of a 53 year-old female patient with cardiac amyloidosis who presented with recurrent accumulation of large pleural effusions. She was initially treated with high dose loop diuretics, but the pleural effusion persisted, with the daily amount of drainage averaging 1 L/day. Accumulation of pleural fluid did not subside after 3 cycles of melphalan/prednisolone therapy. After the introduction of bevacizumab, an anti-VEGF antibody, the amount of pleural effusion decreased significantly. Efficacy of anti-VEGF therapy for refractory pleural effusions needs to be defined through further studies.
Keywords: Heart disease, Amyloidosis, Pleural effusion, Bevacizumab
Introduction
Amyloidosis is a clinical disorder caused by extracellular deposition of insoluble abnormal fibrils, derived from aggregation of misfolded normally soluble protein.1) Cardiac infiltration of amyloid fibril results in progressive cardiomyopathy with a grave prognosis.2),3) A prominent clinical feature of cardiac amyloidosis is a syndrome of heart failure, characterized by restrictive hemodynamics and progressive deterioration of systolic function. Pleural effusion can develop as a consequence of heart failure, but sometimes it is disproportionate to the degree of heart failure and is resistant to conventional diuretic therapy. The latter complication occurs most often in primary systemic (AL) amyloidosis.4) Refractory pleural effusions are difficult to manage and associated with a poor prognosis.4) Recently, vascular endothelial growth factor (VEGF) has been implicated to play a role in both malignant and nonmalignant pleural effusions through its ability to regulate vascular permeability.5-8) Thus, a therapeutic approach employing anti-VEGF agent might emerge as a potential alternative for managing refractory pleural effusions complicating systemic amyloidosis. Here, we report a case of cardiac amyloidosis in which recurrent pleural effusion could be managed by therapy with bevacizumab, an anti-VEGF antibody.
Case
A 53 year-old female patient was transferred to our cardiology department for the evaluation of hypotension and dyspnea after successful treatment for recombinant tissue-type plasminogen activator for acute right middle cerebral artery infarction. She reported previous episodes of dyspnea and had been diagnosed with cardiac hypertrophy one year previously. But she denied any prior history of coronary heart disease, hypertension, diabetes, or pulmonary diseases. She had not been taking any medication for her cardiac condition.
On transfer, examination revealed blood pressure 90/60 mmHg, heart rate 100/min, body temperature 36.4℃, and respiration rate 22/min. Her face was puffy, and her jugular vein was distended with an estimated pressure of 18 cm H2O. Cardiac sounds were regular, and a gallop sound was not audible. Breath sounds were markedly diminished in the lower half of right lung field. The liver was palpable about 5 cm below the right costal margin. There was marked pitting edema in both lower extremities.
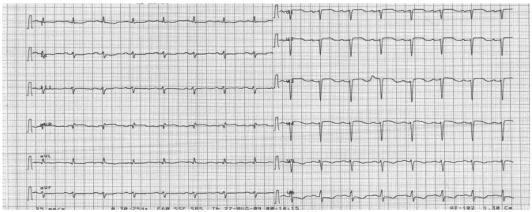
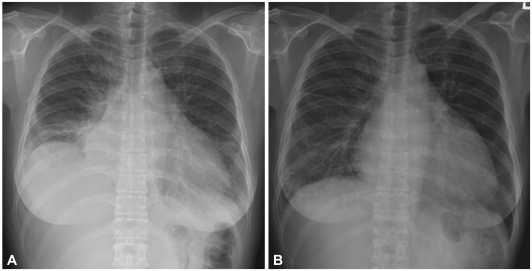
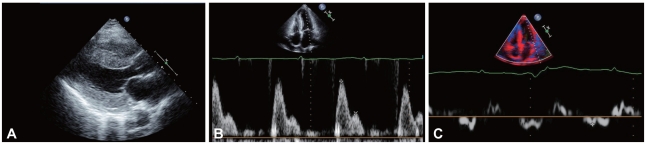
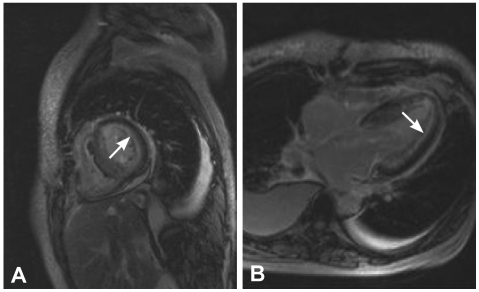
The electrocardiography showed low voltage in the limb leads and a small R wave amplitude across the precordial leads (Fig. 1). The chest film demonstrated moderate cardiomegaly, interstitial congestion and marked pleural effusion in the right lung field (Fig. 2A). Initial laboratory studies revealed normal ranges of renal function test and liver function test, mildly elevated troponin-I (0.52 ng/mL: normal <0.1 ng/mL), and markedly elevated BNP (1,867 pg/mL: normal <100 pg/mL). Serum protein was 6.2 g/dL (normal 6.6-8.4 g/dL), albumin 3.5 g/dL (normal 3.8-5.2 g/dL), and 24 hours urine protein 4.5 g/day (normal 0.03-0.12 g/day). On echocardiography, the septal and free walls of the left ventricle were both moderately thickened (13 mm and 14 mm, respectively), but the left ventricular cavity dimension was not enlarged. Left ventricular wall motion was active, with an estimated ejection fraction in the 65-69% range. Two-dimensional evaluation of the inferior vena cava and Doppler estimation of tricuspid regurgitation revealed elevated right atrial pressure (>20 mmHg) and systolic pulmonary artery pressure (>54 mmHg). Doppler analysis of transmitral inflow showed restrictive pattern with high E/A ratio (2.3) and short deceleration time (119 m/s). Tissue Doppler of mitral annular diastolic velocity (E') was markedly reduced (2.5 cm/s), and estimated left ventricular filling pressure was consequently high (50 mmHg) (Fig. 3). Cardiac magnetic resonance imaging (MRI) revealed a late enhancement of gadolinium over the entire subendocardial circumference with varying degree of extension into the neighboring myocardium (Fig. 4). This particular pattern of late gadolinium enhancement is regarded as a unique and sensitive feature of cardiac amyloidosis.9) Taken together, these electrocardiographic, echocardiographic, and MRI findings strongly suggested a diagnosis of cardiac amyloidosis. The result of renal biopsy confirmed the diagnosis of amyloidosis. Congo-red stain of the specimen revealed apple green birefringence under polarized microscopy (Fig. 5), a finding compatible with amyloid deposit. Serum free-light-chain assay revealed excessive increase in lambda free light chain, resulting in low kappa-to-lambda ratio {free kappa (κ) light chain 18.6 mg/L: reference value 3.3-19.4 mg/L, free lambda (λ) light chain 168.9 mg/L: reference value 5.71-26.3 mg/L, κ/λ ratio 0.11: reference value 0.26-1.65}. Therefore, the patient was diagnosed with primary (AL) cardiac amyloidosis, with manifestations of heart failure, nephrotic syndrome, and massive pleural effusion.
Fig. 1.
Initial standard electrocardiogram showing low voltage in the limb leads (<5 mm) and small R wave amplitude across the precordial leads.
Fig. 2.
A: initial chest roentgenogram showing cardiomegaly, pulmonary congestion and pleural effusion in the right hemithorax, B: chest film taken 1 year after initial admission showing no sign of pleural fluid in the right hemithorax.
Fig. 3.
Results of echocardiographic examination. A: two-dimensional echocardiographic image (parasternal long-axis view) showing normal ventricular dimensions and concentric left ventricular wall thickening. Granular "sparkling" appearance of ventricular wall was not apparent. B: Doppler signals of transmitral inflow showing restrictive pattern with high E/A ratio (2.3) and short deceleration time (119 m/s). C: tissue Doppler echocardiogram of mitral annulus showing markedly reduced diastolic velocity (E') (2.5 cm/s), with elevation of estimated left ventricular filling pressure (50 mmHg).
Fig. 4.
Magnetic resonance images of left ventricular short axial view (A) and 4-chamber long-axis view (B) from post gadolinium delayed enhancement show diffuse subendocardial enhancement in both left (arrows) and right ventricles.
Fig. 5.
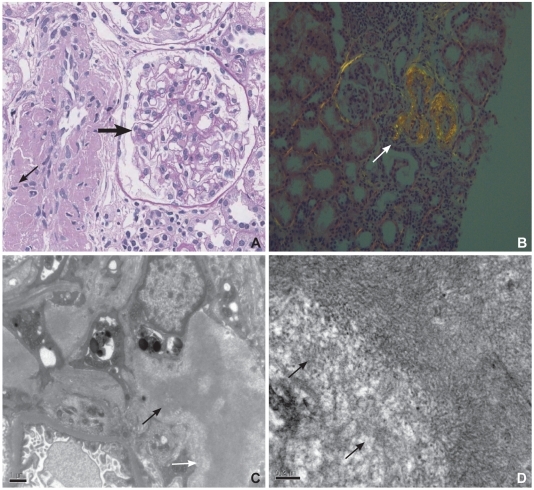
A: microscopic findings of renal tissue. PAS-negative eosinophilic material deposition is observed on the wall (thin arrow) of the interlobular artery and glomerular mesangium (thick arrow) (PAS, ×400). B: view of renal tissue under polarizing microscopy. The interlobular artery shows apple-green birefringence (arrow) on Congo-red staining. C: electron microscopic findings of renal tissue showing tangles of fibrils within mesangial area (arrows) (EM, ×12,500). D: high power view of mesangial tangle showing haphazardly arranged fibrils (arrows) (EM, ×30,000).
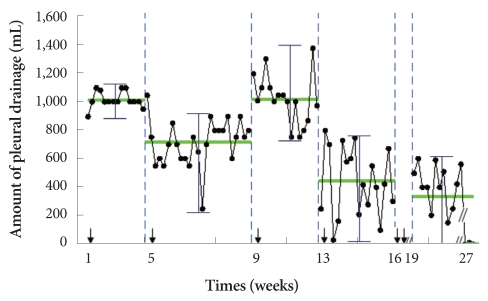
She was initially treated with high dose loop diuretics (intravenous furosemide 120 mg/day) which improved dyspnea symptoms and lower leg edema. However, the accumulation of pleural effusion showed no signs of abatement on her chest film. For the drainage, a pig-tail catheter was inserted into the right hemithorax. Analysis of pleural fluid revealed protein 0.6 g/dL, albumin 0.3 g/dL, LDH 74 IU/L, and 21 nucleated cells (46% polymorphonuclear leukocyte, 27% lymphocyte, 20% monocyte and 7% histiocytes), consistent with transudative effusion. Repeat fluid analysis reported similar results. Pleural biopsies were performed, but pathological examination revealed only fibrosis without signs of amyloid deposit. She was then started on chemotherapy with melphalan (6 mg/m2 for 4 days) and prednisolone (60 mg/m2 for 4 days). Three cycles of chemotherapy elicited the improvement of the kappa-to-lambda ratio (free κ light chain 25.8 mg/L, free λ light chain 51.7 mg/L, κ/λ ratio 0.50), but failed to reduce the accumulation of pleural fluid (Fig. 6). Following discussion of the risk and benefits, bevacizumab, an anti-VEGF antibody, was chosen over chemical pleurodesis for management of her recurrent pleural effusions. Bevacizumab was scheduled to be administered by intravenous infusion, 5 mg/kg/day over 90 minute every 3 weeks. After 1 cycle of bevacizumab therapy, the amount of pleural drainage was markedly decreased to an average of 441 mL/day. Another cycle of bevacizumab therapy was given 3 weeks later. The patient objected to further chemotherapy and so was treated only conservatively thereafter. However, 8 weeks after the completion of the second bevacizumab therapy, the amount of pleural effusion was reduced to nearly zero despite a similar degree of nephrosis on follow-up (Fig. 6). Her general condition was also improved, and she was encouraged to ambulate. With respect to adverse effects of bevacizumab, there were no signs of bleeding or thrombosis, nor aggravation of proteinuria during subsequent follow-up. Chest film taken 1 year after initial admission showed no sign of pleural effusion in the right hemithorax (Fig. 2B).
Fig. 6.
Average drainage of pleural effusion during the chemotherapy. The change of the amount of drained pleural effusion during the chemotherapy. The patient received three cycles of oral melphalan/prednisolone therapy (thin arrow indicates the start of chemotherapy), and 2 cycles of intravenous bevacizumab at the first day of the fourth, and 3 weeks later (thick arrows). The average amount of pleural drainage decreased significantly following the addition of bevacizumab (p<0.01) and no pleural drainage was detectable 8 weeks after the completion of the second bevacizumab therapy.
Discussion
This case represents a challenging case of refractory pleural effusion associated with primary (AL) amyloidosis involving cardiac tissue. In this case, the diagnosis of cardiac amyloidosis was established based upon the characteristic findings on echocardiography and cardiac MRI, together with histology obtained from renal tissue, and the result of serum free-light-chain assay. Recent MRI technique of late gadolinium enhancement generates distinct images for cardiac amyloidosis with a great sensitivity and specificity, so histologic demonstration of amyloid deposit in extracardiac tissue would obviate the need for cardiac biopsy.9)
Pleural effusion in cardiac amyloidosis can occur as a consequence of heart failure, but in some patients with primary (AL) systemic amyloidosis, large pleural effusion develops, which is refractory to diuretic therapy and thoracentesis.4) Presence of large, persistent pleural effusion among those patients is clinically significant, not only because it deteriorates the quality of life but also because it is associated with poor prognosis.4) The prevalence of pleural effusion has not been clearly defined, but in one study 6% of 636 amyloid (AL) patients were inflicted with this complication.10) In contrast, no cases of pleural effusion were reported in 233 patients with secondary (AA) amyloidosis.11)
Mechanisms underlying pleural effusions in AL amyloidosis remain poorly understood. Unlike the transudative nature observed in usual heart failure, the chemistry of pleural effusions in AL amyloidosis varied; about one third to one half of the cases were found to be exudative.4),10) The extraordinarily high rate of exudative effusion suggests that altered hydrostatic forces may not be the principal mechanism driving pleural fluid accumulation. Indeed, in a study comparing 35 AL amyloidosis patients with pleural effusion versus 120 patients without effusion, no significant differences were found in left ventricular indices, pulmonary pressure, or degree of nephrosis.10) Furthermore, both gross and microscopic examination of pleural surface identified the presence of amyloid deposit,10),12) lending strong support to amyloid infiltration being a major factor.
In our case, the pleural effusion was transudative in both of 2 fluid analyses. Nevertheless, the effusion did not respond to high dose loop diuretics, even after signs of pulmonary congestion had cleared. The effusion was also refractory to 3 cycles of melphalan/prednisolone chemotherapy. The effusion recurred rapidly with daily drainage averaging 1 L/day. Refractoriness of transudative effusion to conventional therapy warrants further discussion. The development of transudative effusion indicates that the integrity of pleural membranes is mostly intact.13) Therefore, it is conceivable that impaired pleural fluid absorption is a contributing mechanism. The presence of mesothelial stomata, intercellular openings, and rich networks of submesothelial lymphatics and blood vessels identify parietal pleura as the principal drainage system of the pleural space.14) Blockage of these drainage components by amyloid deposit could lead to the loss of its ability to remove fluid. However, increased fluid secretion is the principal underlying abnormality in the genesis of most effusions, even if obstruction of fluid drainage co-exists.15) One candidate mediator for altered pleural secretion might be VEGF, which enhances permeability of vascular and mesothelial barriers.5),16) VEGF increases capillary and venular leakage by opening the endothelial intracellular junctions and by inducing fenestration development in endothelia.5) Additionally, VEGF increases microvascular permeability by enhancing the activity of vesicular vacuolar organelles, through which macromolecules extravasate.17) Increased VEGF levels have been observed in both the serum and pleural fluid from patients with malignant and non-malignant pleural effusions.6) Although a role of VEGF has been emphasized mostly in exudative effusions,5) it is conceivable that VEGF might also contribute to formation of transudative effusion via the mechanisms cited above.5),17)
Management of large pleural effusion in primary (AL) amyloidosis is a challenging task. Aggressive diuresis or invasive measures like repeated thoracentesis, pleurodesis, and thoracoscopic interventions have only limited efficacy and risk potential adverse effects. Recently, with increasing appreciation of the vital role of VEGF in the formation of pleural effusion, strategic targeting inhibition of VEGF activity is being employed as an alternative measure for managing refractory effusions. In a case report published in 2005, bevacizumab, an anti-VEGF antibody, successfully reduced pleural effusion for the first time in a patient with primary AL cardiac amyloidosis.7) Following this report, 4 cases of successful management of refractory pleural effusions with bevacizumab were described in patients with primary systemic amyloidosis.8) In the present case, recurrence of large pleural effusions was not affected by chemotherapy, but the amount of effusion was remarkably reduced after introduction of bevacizumab therapy. It is unlikely that this benefit was due to the reduction of oncotic pressure, because the degree of nephrosis and proteinuria remained similar throughout the follow-up. Regarding the safety issue, bleeding complications or venous thrombosis were not observed in this patient. Aggravations of proteinuria or heart failure were other concerns, but neither were observed. Thus, bevacizumab therapy improved the pleural effusion in this patient, and appeared clinically beneficial. However, the duration of observations presented in this report was relatively short, and further follow-up will be needed to assess the effect of the therapy. For patients with similar overall clinical presentations, prospective studies are necessary to define the role of bevacizumab. This case, together with previous reports,7),8) suggests the potential of bevacizumab therapy as a novel strategy in patients with systemic amyloidosis and refractory pleural effusions who have few management options.
References
- 1.Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol. 2007;50:2101–2110. doi: 10.1016/j.jacc.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 3.Kim YJ, Choi SO, Kim MK, et al. A case of familial cardiac amyloidosis. Korean Circ J. 2004;34:520–526. [Google Scholar]
- 4.Berk JL. Pleural effusions in systemic amyloidosis. Curr Opin Pulm Med. 2005;11:324–328. doi: 10.1097/01.mcp.0000162378.35928.37. [DOI] [PubMed] [Google Scholar]
- 5.Grove CS, Lee YC. Vascular endothelial growth factor: the key mediator in pleural effusion formation. Curr Opin Pulm Med. 2002;8:294–301. doi: 10.1097/00063198-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Sack U, Hoffmann M, Zhao XJ, et al. Vascular endothelial growth factor in pleural effusions of different origin. Eur Respir J. 2005;25:600–604. doi: 10.1183/09031936.05.00037004. [DOI] [PubMed] [Google Scholar]
- 7.Pichelmayer O, Zielinski C, Raderer M. Response of a nonmalignant pleural effusion to bevacizumab. N Engl J Med. 2005;353:740–741. doi: 10.1056/NEJM200508183530722. [DOI] [PubMed] [Google Scholar]
- 8.Hoyer RJ, Leung N, Witzig TE, Lacy MQ. Treatment of diuretic refractory pleural effusions with bevacizumab in four patients with primary systemic amyloidosis. Am J Hematol. 2007;82:409–413. doi: 10.1002/ajh.20858. [DOI] [PubMed] [Google Scholar]
- 9.Vogelsberg H, Mahrholdt H, Deluigi CC, et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis. J Am Coll Cardiol. 2008;51:1022–1030. doi: 10.1016/j.jacc.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 10.Berk JL, Keane J, Seldin DC, et al. Persistent pleural effusions in primary systemic amyloidosis. Chest. 2003;124:969–977. doi: 10.1378/chest.124.3.969. [DOI] [PubMed] [Google Scholar]
- 11.Smith RR, Hutchins GM, Moore GW, Humphrey RL. Type and distribution of pulmonary parenchymal and vascular amyloid: correlation with cardiac amyloid. Am J Med. 1979;66:96–104. doi: 10.1016/0002-9343(79)90488-1. [DOI] [PubMed] [Google Scholar]
- 12.Bontemps F, Tillie-Leblond I, Coppin MC, et al. Pleural amyloidosis: thoracoscopic aspects. Eur Respir J. 1995;8:1025–1027. [PubMed] [Google Scholar]
- 13.Kinasewitz GT. Transudative effusions. Eur Respir J. 1997;10:714–718. [PubMed] [Google Scholar]
- 14.Albertine KH, Wiener-Kronish JP, Staub NC. The structure of the parietal pleura and its relationship to pleural liquid dynamics in sheep. Anat Rec. 1984;208:401–409. doi: 10.1002/ar.1092080310. [DOI] [PubMed] [Google Scholar]
- 15.Light RW, Hamm H. Malignant pleural effusion: would the real cause please stand up? Eur Respir J. 1997;10:1701–1702. doi: 10.1183/09031936.97.10081701. [DOI] [PubMed] [Google Scholar]
- 16.Mohammed KA, Nasreen N, Hardwick J, Logie CS, Patterson CE, Antony VB. Bacterial induction of pleural mesothelial monolayer barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;281:L119–L125. doi: 10.1152/ajplung.2001.281.1.L119. [DOI] [PubMed] [Google Scholar]
- 17.Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM. Vesiculo-vacuolar barrier organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996;183:1981–1986. doi: 10.1084/jem.183.5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]