Abstract
We investigated the migration of human leukocytes through endothelial cells (EC), and particularly their underlying basement membrane (BM). EC were cultured for 20 days on 3 μm-pore filters or collagen gels to form a distinct BM, and then treated with tumour necrosis factor-α, interleukin-1β or interferon-γ. Neutrophil migration through the cytokine-treated EC and BM was delayed for 20-day compared to 4-day cultures. The BM alone obstructed chemotaxis of neutrophils, and if fresh EC were briefly cultured on stripped BM, there was again a hold-up in migration. In studies with lymphocytes and monocytes, we could detect little hold-up of migration for 20-day versus 4-day cultures, in either the filter- or gel-based models. Direct microscopic observations showed that BM also held-up neutrophil migration under conditions of flow. Treatment of upper and/or lower compartments of filters with antibodies against integrins, showed that neutrophil migration through the endothelial monolayer was dependent on β2-integrins, but not β1- or β3-integrins. Migration from the subendothelial compartment was supported by β1- and β2-integrins for all cultures, but blockade of β3-integrin only inhibited migration effectively for 20-day cultures. Flow cytometry indicated that there was no net increase in expression of β1- or β3-integrins during neutrophil migration, and that their specific subendothelial function was likely dependent on turnover of integrins during migration. These studies show that BM is a distinct barrier to migration of human neutrophils, and that β3-integrins are particularly important in crossing this barrier. The lesser effect of BM on lymphocytes and monocytes supports the concept that crossing the BM is a separate, leukocyte-specific, regulated step in migration.
Abbreviations: EC, endothelial cell; BM, basement membrane
Keywords: Neutrophil, Migration, Integrin, Endothelium, Basement membrane
Introduction
During inflammatory responses, all major classes of leukocytes may be recruited from the blood, although typically neutrophils are the first to arrive in tissue, with monocytes and lymphocytes appearing later. Experimentally, endothelial cells (EC) treated with inflammatory cytokines such as tumour necrosis factor-α (TNF) and interleukin-1β (IL-1) support adhesion and migration of all these leukocytes [1–4]. In flow, leukocytes are first captured by specialised fast-acting endothelial receptors such as selectins or vascular cell adhesion molecule-1 (VCAM-1), and roll on the endothelial surface [5,6]. Chemokines and lipid-derived agents are presented on this surface, and transduce signals through specific receptors. These cause rapid activation of leukocyte integrins which support immobilisation, followed by migration across and through the endothelial monolayer in minutes [7–10]. A proportion of leukocytes are believed to migrate through endothelial cells, but for the majority penetration of the monolayer is via inter-cellular junctions [11]. Intercellular migration appears to be modulated by ligation of endothelial receptors concentrated in that area, including CD31 [12].
While the mechanisms supporting adhesion to the endothelial surface and migration through it have been widely studied, less is known about the kinetics and mechanisms of migration under the endothelial cells and through the basement membrane (BM). In mice it has become clear that transit of neutrophils through the BM is a separately regulated stage in migration in vivo [13,14]. The only study of migration of human leukocytes through BM to date was performed by Huber and Weiss, who demonstrated that over about 20 days, cultured EC laid down a BM with content, morphology and permeability comparable to that seen in vivo [15]. Neutrophils could migrate through the endothelial monolayers and its BM, into an underlying collagen gel, when a chemotactic gradient was applied from below. Interestingly, neutrophils appeared to be delayed above the BM for some minutes before migrating into the gel, although no comparison was made to behaviour observed with short-term cultures without complete BM. Using comparable long-term cultures on solid substrate, we found that time of culture and contact with BM modified the ability of EC themselves to recruit neutrophils, but that nevertheless the BM laid down over 20 days (but not 4 days) modified adhesive and migratory functions of neutrophils [16–18]. Our model did not allow studies of migration through, rather than on, the matrix laid down at different times, and none of the above studies evaluated migration of lymphocytes or monocytes on or through BM.
The involvement of β2-integrins in the initial stages of immobilisation and transendothelial migration has been demonstrated for all the major leukocyte subsets [2,8,9,19–21], but the roles of β1- and β3-integrins in migration are less clear. Recent studies have suggested that integrin ligation is not absolutely required for migration through tissue matrix [22], but this is unlikely to be the case for moving under EC and through BM. Indeed, in mice neutrophils migration through the BM required upregulation of β1-integrins [13,14]. We previously found that pre-treatment of neutrophils with function-blocking antibodies against β1- or β3-integrins had no effect on the ability to cross endothelial monolayers or motility underneath them in short-term cultures [23]. However, neutrophil β1-integrins did influence adhesion to BM from 20-day cultures [17], and leukocyte integrins of each of these families are capable of binding matrix proteins [24]. Thus, evaluation of the roles of different integrins in migration through the subendothelial space requires models where realistic BM is present. Moreover, the leukocytes need to have previously undergone transendothelial migration, because behaviour of leukocytes depends on their prior adhesion/migration history. For instance, in mice, the upregulation of β1-integrin alluded to above, was dependent on ligation of CD31 during the transendothelial migration phase [13]. We and others have shown that human neutrophils also alter their motility and adhesive properties after they migrate through endothelial monolayers [16,23,25].
To clarify the effects of BM on the kinetics of migration of human leukocytes, and to investigate which integrin classes were required for the later stages of migration, we adapted our system for examining recruitment by long-term endothelial cell cultures [16]. EC were cultured on 3 μm pore filters or on collagen gels, so that we could study penetration of BM. Transmigration was studied over prolonged periods in a static system, and filters were incorporated in a novel flow chamber [26], to allow direct observation of migration through the whole construct over shorter periods under flow. We found that the deposited BM represented a significant obstruction to migrating neutrophils, but that this effect was much less marked for lymphocytes or monocytes. In the case of neutrophils, β2-integrins dominated the initial stage of transendothelial migration. While both β1- and β2-integrins influenced subendothelial migration, this was not specific to long-term cultures. However, prolonged endothelial culture revealed a specific role for β3-integrins in migration on and through fully-formed BM.
Materials and methods
Isolation of leukocytes
Venous blood was drawn from healthy volunteers and transferred into tubes containing K2EDTA (1.6 mg/ml final concentration) (Sarstedt, Leicester, UK). Mononuclear cells and neutrophils were isolated using a 2-step density gradient as previously described [27,28] and washed twice in medium 199 (M199; Gibco Invitrogen Compounds, Paisley, UK) supplemented with 0.15% bovine serum albumin (culture-tested BSA; Fraction IV; Sigma-Aldrich, Poole, UK). When desired, lymphocytes were prepared from mononuclear cells by panning on culture plastic to remove contaminating monocytes. Monocytes were isolated as described [29]. Dextran (1 ml 6% w/v MW 500 kDa, in phosphate-buffered saline, PBS; Sigma) was added to 10 ml EDTA-blood, the tube was placed at an angle of 45° from the vertical, and red cells were allowed to sediment for 1 h. The leukocyte-rich supernatant was retrieved and placed on top of 3 ml Nycoprep 1068 medium (Amersham Biosciences, Chalfont St Giles, UK), and centrifuged at 400 g for 15 min. The plasma-rich top was discarded and the middle layer which was rich in monocytes was decanted, washed twice in Ca++/Mg++-free PBS with 0.15% BSA, and resuspended in M199 with BSA. Leukocytes were counted using a Coulter Counter (Coulter Electronics Ltd., Essex, UK) and adjusted to a final desired concentration (0.5 to 2 × 106/ml).
Endothelial cell isolation and culture
Human umbilical vein endothelial cells (HUVEC) were isolated from umbilical cords following maternal consent as previously described [30] and cultured in M199 supplemented with 20% heat-inactivated fetal calf serum (FCS), 2.5 μg/ml amphotericin B, 1 μg/ml hydrocortisone, 10 ng/ml epidermal growth factor (all from Sigma-Aldrich) and 35 μg/ml gentamycin (Gibco Invitrogen Compounds) until confluent. Primary HUVEC were dissociated using trypsin/EDTA (Sigma-Aldrich) and seeded on uncoated low-density 3.0 μm pore polycarbonate Transwell filters (which were placed in matching plates; BD Pharmingen, Oxford, UK) or collagen gels. To form collagen gels, type 1 collagen dissolved in 0.6% acetic acid (2.15 mg/ml; First Link Ltd., West Midlands, UK) was mixed with 10 × M199 (1.66 ml and 0. 34 ml respectively). The pH was neutralised by the addition of 0.18 ml 1 N NaOH, and 1 ml was dispensed into a 6-well plate and allowed to gel at 37 °C. The gel was then equilibrated with HUVEC culture medium for 48 h before seeding with HUVEC. Seeding density was chosen to yield confluent monolayers within 24 h. After culture for 2 to 20 days, HUVEC were stimulated with 0, 1 or 100 U/ml tumour necrosis factor α (TNF) or 50 pg/ml interleukin-1β (IL-1; both from Sigma-Aldrich) for 4 h before assays of neutrophil migration. For studies of mononuclear cells, HUVEC cultured on gels were treated with TNF (100 U/ml) for 4 h for direct comparison to neutrophils; for studies using filters, HUVEC were stimulated with TNF (100 U/ml) plus interferon-γ (IFNγ; 10 ng/ml; Peprotech Inc., London, UK) for 24 h before assay to optimise transmigration [31].
In some experiments, after 4 or 20 days of culture HUVEC were stripped from their underlying substrates as previously described [16], to expose the intact underlying basement membrane on the filter. The EC monolayers were detached by addition of phosphate-buffered saline (PBS) containing 0.5% Triton X-100 and 20 mM NH4OH (all Sigma) pre-warmed to 37 °C. Lysis and detachment of cells was observed microscopically, and when complete, the surface was gently rinsed 5 times with PBS. When required, fresh HUVEC were seeded onto the 4-day or 20-day matrices and cultured for 2 days before treatment with TNF as above. To observe the morphology of deposited matrix, stripped substrates were fixed, processed and examined by scanning electron microscope as described [16].
Migration of leukocytes through endothelial cells and filters under static conditions
Leukocyte adhesion and transmigration was assessed using 24-well format or 6-well format Transwell filters in matching plates. Medium was removed from HUVEC and filters were transferred to wells that had been previously treated with poly(2-hydroxyethyl methacrylate) to prevent cell adhesion to the bottom of the well [32]. M199 + BSA was added to the lower chamber and leukocyte suspension in M199 + BSA was added to the upper chamber (200 μl for 24-well or 1500 μl for 6-well formats; 1 × 106/ml for neutrophils, 2 × 106/ml for mononuclear cells). Leukocytes were allowed to settle, adhere and transmigrate for the desired time period in an incubator maintained at 37 °C, 5% CO2. Migration was stopped at the chosen time by transferring the filter into a clean well. Leukocytes in the original lower chamber were resuspended and removed, the chamber was rinsed with M199 + BSA, and the two samples pooled (fraction 1). Leukocytes in the chamber above the filter were resuspended and removed, the chamber was rinsed with M199 + BSA, and these two samples also pooled (fraction 2). Filters were then fixed and stained with 2% gluteraldehyde containing 1 μg/ml bisbenzamide (fluorescent nuclear stain; Sigma-Aldrich) for 15 min. Filters were cut out and mounted directly onto microscope slides and coverslips were mounted using Vectorshield (Vector Labs, Peterborough, UK).
Leukocytes in fractions 1 and 2 were counted using a Coulter counter. The numbers of leukocytes adherent above (fraction 3) or below (fraction 4) the filter were counted using a fluorescence microscope. Leukocytes in ten fields of a known area were counted. The total number was determined by multiplying the average number per field by the known area of the filter divided by the field area. All numbers were expressed as a percentage of the number of leukocytes added. The percentage of transmigrated cells was calculated by adding fractions 1 and 4. The percentage of adherent cells was calculated by subtracting fraction 2 from 100%.
When evaluating migration of mononuclear cells, the Coulter counter could distinguish between distinct populations of smaller lymphocytes and larger monocytes. When counting the leukocytes adherent below the filter, nuclear morphology was used to estimate the numbers of lymphocytes and monocytes (small round nuclei for lymphocytes, larger kidney-shaped nuclei for monocytes). In this way we obtained estimates of the percentage migration for the two cells types, as well as the more accurate value for the whole population.
When desired, interleukin-8 (IL-8; 10 ng/ml) or stromal-derived factor 1α (SDF1α; 80 ng/ml) (both R&D Systems, Abingdon, UK) was added to the lower wells immediately before addition of leukocytes, to provide an additional chemotactic stimulus. In some of these experiments, stripped filters (i.e., coated with matrix but not EC) were used, and migration of neutrophils through matrix alone was quantified.
Migration of leukocytes through endothelial cells into collagen gels under static conditions
HUVEC on collagen gels were washed with M199 + BSA to remove residual cytokines, and leukocytes (2 ml; 0.5 × 106/ml for neutrophils, 1 × 106/ml for mononuclear cells) were added for 10 min. Non-adherent cells were removed from the HUVEC by gentle washing with medium, and phase-contrast video-microscope recordings were made 0.25 h, 1 h, 3 h and 24 h after the original addition of leukocytes. Five video-fields were recorded. In each field, images were first recorded at the endothelial surface, and then recordings were made as the microscope was focussed gradually down in 100 μm steps. Cells visible with the endothelial monolayer were counted and divided into those which were phase-bright (above EC) and those which were phase-dark (just below EC). Cells within each 100 μm step were counted as they came into focus; these cells were phase-bright. The focal depth of the gels was approximately 300 μm. For a given field of known dimensions, the number of cells counted in the different layers was added and expressed as total adherent cells/mm2. The numbers of migrated cells just below the endothelium, or migrated into the gel were expressed as a proportion of the total adherent cells. The values were averaged for the five fields analysed in each experiment. All manipulations of gels and microscopy were carried out at 37 °C.
Adhesion and migration of neutrophils through endothelial monolayers under flow
Transwell filters (6-well format) coated with HUVEC were cut out and placed on a coverslip, which was incorporated into a bolt-together, parallel-plate, flow chamber as previously described [26]. The flow chamber was placed on the stage of a phase-contrast video-microscope which was enclosed in a chamber at 37 °C, and attached to a perfusion system [8]. HUVEC were perfused with cell-free medium, and then neutrophils were perfused for 4 min, followed by washout with cell-free medium. Flow rate was chosen to exert a wall shear stress of 0.1 Pa (1 dyn/cm2) throughout. Video recordings were made of 5 fields along the centreline of the flow channel following a 2 minute washout, and at chosen intervals afterwards. In between, a single field was recorded for 5 min to allow analysis of migration velocities of neutrophils (see the following discussion). During the later recordings, images were recorded not only of neutrophils interacting with HUVEC on the upper surface of the filter, but also of neutrophils attached to the back of the filter and to the coverslip beneath the filter. This was achieved by focussing the microscope down 10 μm from the top of the filter, to observe the back, and then a further distance (typically ≥ 10 μm) to view the surface of the coverslip.
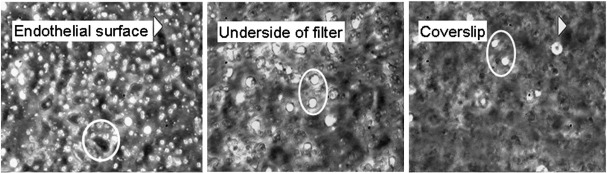
The video recordings of adhesion and migration of the neutrophils were digitised and analysed offline using Image-Pro Plus software (DataCell Ltd., Finchampstead, UK) as described [23]. An example is shown in the Supplemental video. Three classes of adherent neutrophils on HUVEC were counted: (i) rolling adherent cells (which were spherical, and rotated steadily over the surface at a velocity ~ 10 μm/s); (ii) stably-adherent ‘activated’ neutrophils migrating on the top of the endothelial monolayer, which were phase-bright with distorted shape; and (iii) transmigrated neutrophils migrating underneath the monolayer, which were phase-dark. The numbers of neutrophils adherent to the back of the filter and to the coverslip were also counted (see Supplemental Fig. 1). Counts were converted to cells per mm2 using the calibrated microscope field dimensions, and the numbers in all categories added to give the total number of adherent neutrophils. The percentages of adherent neutrophils in each category were then calculated.
Supplementary Fig. 1.
Phase-contrast photomicrographs taken as one focuses down from the endothelial surface, through the filter and onto the coverslip below. Neutrophils on the endothelial surface appear phase-bright with round or distorted outline, while those underneath are phase-dark and spread (circle). Neutrophil on either of the two lower surfaces are phase-bright, some round, some with distorted shape (circled). They do not spread and appear phase-dark like those immediately under the endothelial monolayer.
The velocities of phase-dark neutrophils migrating underneath HUVEC were measured by digitising a sequence of images 1 min apart. In each digitised image, cells were outlined and the position of their centroid determined. Movement was followed for 5 min for ~ 10 cells, and migration velocity (μm/min) was calculated as the average distance moved by the centroid of a cell per minute.
Adhesion of neutrophils to stripped endothelial matrix and purified proteins
HUVEC were cultured in 6-well plates for 4 or 20 days and then the cells were stripped from their underlying substrates as above. The plates were placed on the stage of a phase-contrast video-microscope enclosed at 37 °C. Neutrophils (2 ml) were treated with IL-8 (10 ng/ml), added to the wells and allowed to settle for 5 min. Non-adherent cells were removed by washing the surface twice with M199 + BSA and a series of 5 separate video-fields were recorded. Neutrophils in each field were counted and averaged, and the average number adherent was converted to cells/mm2 using the known field dimensions. For comparison, the same assay was carried out using six-well tissue culture plates coated at 37 °C for 24 h with solutions of the following proteins at 0.2 μg/ml in PBS: laminin purified from human placenta (Chemicon Europe Ltd., Chandlers Ford, UK); vitronectin from human serum (Sigma); fibronectin from human serum (Sigma); collagen type IV from human placenta (Sigma). Residual protein-binding sites were then blocked with PBS containing 1% bovine serum albumin (BSA, culture-tested; Sigma) at 37 °C for 1 h.
Antibody treatment and inhibitory agents
The following function-blocking monoclonal antibodies (mAb) were used (all at a concentration of 10 μg/ml, and mouse IgG1 unless stated): mAb13 against β1-integrin/CD29 (rat IgG2a; Becton Dickinson, Oxford, UK); R6.5E against the β2-integrin/CD18 (a gift from Dr. M. Robinson, Cell Tech, Slough, UK); and SZ21 against the β3-integrin/CD61 (Immunotech, Marseille, France). Control antibodies were mouse IgG1 (Dako, Ely, Cambridgeshire, UK), rat IgG2a (Southern Biotech Associates, Alabama, USA). Neutrophils were incubated with antibodies at 37 °C for 10 min prior to addition to the upper chamber of Transwell filters, perfusion through the flow chamber or addition to coated surfaces. In some experiments, mAb were added to the lower chamber only, or to the lower chamber as well as to neutrophils. Antibodies were present throughout migration through Transwell filters, but not after washout of the flow chambers. In some experiments RGDS peptide (Arg-Gly-Asp-Ser; 0.5 mM; Sigma) was added to neutrophils immediately before analysis.
Surface expression of integrins by neutrophils
To examine changes in integrin surface expression during migration, neutrophils were added to Transwell filters coated with HUVEC and collected from the upper or lower chambers after 2 h. Aliquots from these samples, and an aliquot of the original cell suspension, were incubated on ice for 30 min with one of the following mAb each at 10 μg/ml: PE-conjugated anti-β1-integrin (P5D2; R&D Systems, Abingdon, UK); FITC-conjugated anti-β2-integrin (212701; R&D Systems); unconjugated anti-β3-integrin (SZ21; Immunotech). Neutrophils incubated with SZ21 were centrifuged for 4 min and the pellet resuspended and incubated on ice for 30 min with FITC-conjugated goat anti-mouse IgG1 (Dako). All cells were finally centrifuged for 4 min at 2000 g then resuspended in ice-cold PBS containing 2% BSA. In separate experiments to examine possible internalisation of integrins during migration, neutrophils were labelled with non-blocking unconjugated anti-β1-integrin (419127; R&D) or unconjugated anti-β3-integrin (256809; R&D Systems), and washed twice before addition to Transwell filters coated with HUVEC. Cells were collected from the upper or lower chambers after 2 h. The collected cells, and an aliquot of the originally-labelled cells, were incubated with secondary antibody and washed as above. Samples were analysed using a Coulter XL flow cytometer (Beckman Coulter, High Wycombe, UK) and data expressed as median fluorescence intensity (MFI).
Surface ELISA for stripped substrates
ELISA were carried out using EC grown in 96-well plates (Falcon; Becton Dickinson) for 4 or 20 days and then stripped as above. The following primary mAb were added to surfaces for 1 h at 37 °C: anti-vitronectin (0.8 μg/ml; clone VIT-2; Abcam, Cambridge, UK); anti-laminin (0.1 μg/ml; clone HL-4H3 raised against the P1 pepsin-resistant fragment of laminin from human placenta; Calbiochem, Nottingham, UK); anti-laminin α4-subunit (0.01 μg/ml; clone 3H2, Santa Cruz Biotechnology, Inc., Heidelberg, Germany); and anti-laminin α5-subunit (2 μg/ml clone 3H2, Santa Cruz). Surfaces were washed with PBSA and secondary, horseradish peroxidase-conjugated, goat-anti-mouse antibody (1/2000; Dako) was added for 1 h at 37 °C, and washed out with PBSA. Peroxidase substrate (1,2-phenylenediamine dihydrochloride; Dako) was added for 7 min and the reaction stopped by the addition of 100 μl of 1 M H2SO4. The colour product absorbance was measured at 490 nm using a plate reader, and the signal obtained using a non-specific control antibody (mouse IgG; Dako) was subtracted.
The antibody concentrations were chosen after titration in ELISA on purified vitronectin or laminin from human placenta, coated in plates at 0.2 μg/ml, as were used in the neutrophil adhesion assays described above. It was notable that a much higher concentration of anti-laminin α5-subunit was required to obtain comparable signals to the other antibodies for this mixed laminin type.
Statistical analysis
In all experiments, short-term (2-day or 4-day) and long-term (20-day) cultures of HUVEC were tested on the same day using the same leukocyte isolate. Although the HUVEC themselves were not from the same primary isolates, these comparisons were considered as paired. Effects of multiple treatments or of time were tested using analysis of variance (ANOVA). Comparisons of individual treatments were done by paired t-test.
Results
Effects of BM laid down by HUVEC on porous filters on migration of neutrophils
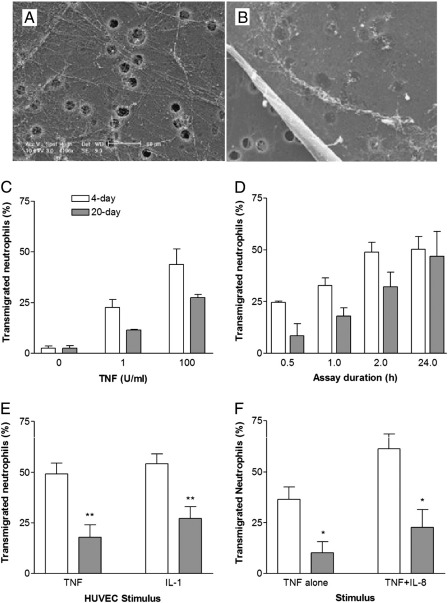
Figs. 1A,B shows scanning electron micrographs of the matrix that had been deposited by HUVEC on 3 μm-pore filters after 4 or 20 days of culture. In agreement with previous observations [16], there were discrete fibrils after 4 days, but a substantial sheet of material which masked the pores after 20 days. In initial experiments, the proportion of neutrophils migrating through HUVEC in 2 h increased with an increasing dose of TNF, but there was a significant reduction in migration through 20-day compared to 4-day cultures (Fig. 1C). The time course of migration was examined for HUVEC treated with 100 U/ml TNF. Migration through 20-day cultures was delayed compared to 4-day cultures during the period up to 2 h but, after 24 h, transmigration had caught up for the 20-day cultures (Fig. 1D). We next tested the stimulus-specificity of the delay by comparing migration through HUVEC treated with TNF or IL-1. With each stimulus, migration through the 20-day cultures was reduced compared to 4-day cultures (Fig. 1E). The above results did not arise because less neutrophils adhered to the longer-term cultures. Gathering data from experiments using 2 h incubations throughout the study, we found that adhesion of neutrophils to day-20 HUVEC treated with 100 U/ml TNF was 95% ± 2% of adhesion to day-4 HUVEC, whereas transmigration through the endothelium and filter was 70 ± 3% of the value for day 4 (mean ± SEM, n = 98).
Fig. 1.
Comparison of transmigration of neutrophils through HUVEC which had been cultured for 4 ( ) or 20 (
) or 20 ( ) days and then treated with TNF or IL-1. A,B. Scanning electron micrographs of matrix deposited by EC on filters after culture for 4 days or 20 days. C. Transmigration after 2 h through HUVEC treated with different doses of TNF. D. Time course of transmigration through HUVEC treated with 100 U/ml TNF. E. Comparison of transmigration through HUVEC treated with TNF (100 U/ml) or IL-1 (50 pg/ml). F. Effect of adding IL-8 to the bottom well on transmigration through HUVEC treated with TNF (100 U/ml). Data are the mean ± SEM from 3 to 7 independent experiments. In C, ANOVA showed a significant effect of TNF dose (p < 0.01) and culture duration (p < 0.01). In D, ANOVA showed a significant effect of culture duration (p < 0.05) and assay time (p < 0.01). In E and F, ANOVA showed significant effects of culture duration (p < 0.01). *p < 0.05, **p < 0.01 compared to 4-day by paired t-test.
) days and then treated with TNF or IL-1. A,B. Scanning electron micrographs of matrix deposited by EC on filters after culture for 4 days or 20 days. C. Transmigration after 2 h through HUVEC treated with different doses of TNF. D. Time course of transmigration through HUVEC treated with 100 U/ml TNF. E. Comparison of transmigration through HUVEC treated with TNF (100 U/ml) or IL-1 (50 pg/ml). F. Effect of adding IL-8 to the bottom well on transmigration through HUVEC treated with TNF (100 U/ml). Data are the mean ± SEM from 3 to 7 independent experiments. In C, ANOVA showed a significant effect of TNF dose (p < 0.01) and culture duration (p < 0.01). In D, ANOVA showed a significant effect of culture duration (p < 0.05) and assay time (p < 0.01). In E and F, ANOVA showed significant effects of culture duration (p < 0.01). *p < 0.05, **p < 0.01 compared to 4-day by paired t-test.
To address the nature of the hold-up further, we added IL-8 to the bottom well of TNF-stimulated HUVEC, to provide an EC-independent chemotactic factor. While IL-8 increased the proportion of neutrophils transmigrating, again there was reduced migration through 20-day cultures compared to 4-day cultures (Fig. 1F). We also stripped EC and tested migration to IL-8 through filters coated with matrix alone. We found that fewer neutrophils underwent chemotaxis through 20-day BM compared to 4-day matrix (21.0 ± 4.7% vs. 50.7 ± 7.1% of cells added; mean ± SEM from 5 paired experiments; p < 0.05 by paired t-test). Thus, the deposited BM acted as a barrier on its own.
To further rule out effects arising from prolonged endothelial culture itself, we cultured fresh HUVEC for 2 days on the matrix that remained after stripping of 4- or 20-day cultures. Control 6- and 22-day cultures of HUVEC that had not been stripped were tested in parallel. Levels of neutrophil transmigration were effectively the same for EC that had been left on their original matrix and for EC that had been seeded onto pre-formed matrix, and transmigration was reduced in either case for 22-day versus 6-day matrices (Fig. 2).
Fig. 2.
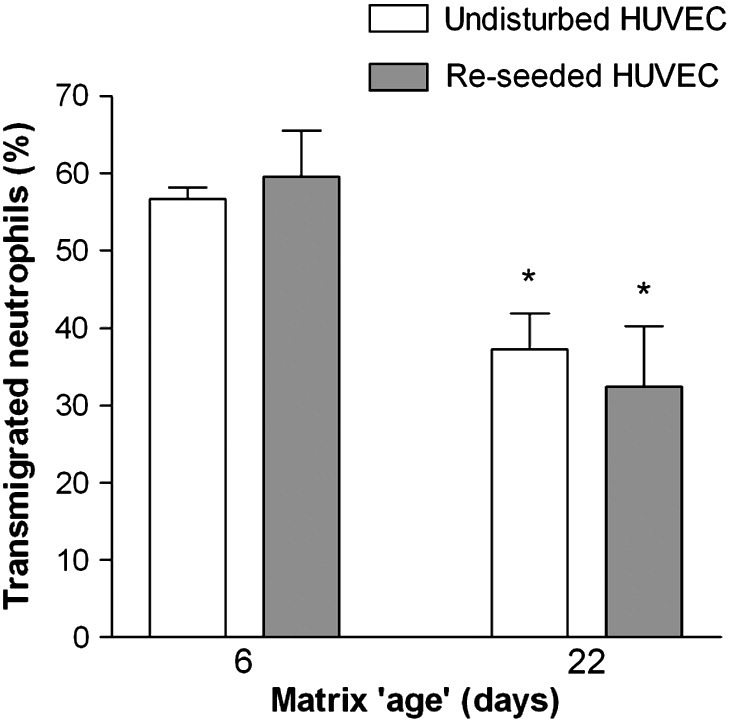
Comparison of transmigration of neutrophils through HUVEC cultured for different durations undisturbed or re-seeded on matrix deposited over different periods. HUVEC were cultured for 4 or 20 days, and then stripped from the underlying matrix. Fresh HUVEC were added to the matrix and cultured for 2 days. Control HUVEC were cultured undisturbed in parallel for 6 or 22 days. The final ‘age’ of matrices was therefore 6 or 22 days. All HUVEC were treated with 100 U/ml TNF for 4 h before neutrophils were added to the upper chamber and allowed to transmigrate for 2 h. Data are the mean ± SEM from three (undisturbed) or five (re-seeded) experiments. ANOVA showed a significant effect of matrix age on transmigration (p < 0.01). *p < 0.05 compared to 6-day matrix by paired t-test.
Effects of BM laid down by HUVEC on porous filters on migration of mononuclear cells
When mononuclear cells were settled on HUVEC that had been treated with TNF + IFN, there were no significant differences between adhesion to short-term or long-term cultures (data not shown). Migration was much less efficient than for neutrophils, and so it was quantified at 2, 4 or 24 h, and not at earlier time points. Judged overall, we did not detect any difference in migration through 4-day or 20-day cultures (Fig. 3A). In these experiments, monocytes represented 15 ± 1% (mean ± SEM from 3 experiments) of the mononuclear cells judged by cell volume measured by a Coulter counter. When we estimated lymphocyte and monocyte migration separately, monocytes migrated more rapidly than lymphocytes, but long-term culture did not hold-up migration in either case (Figs. 3B,C).
Fig. 3.
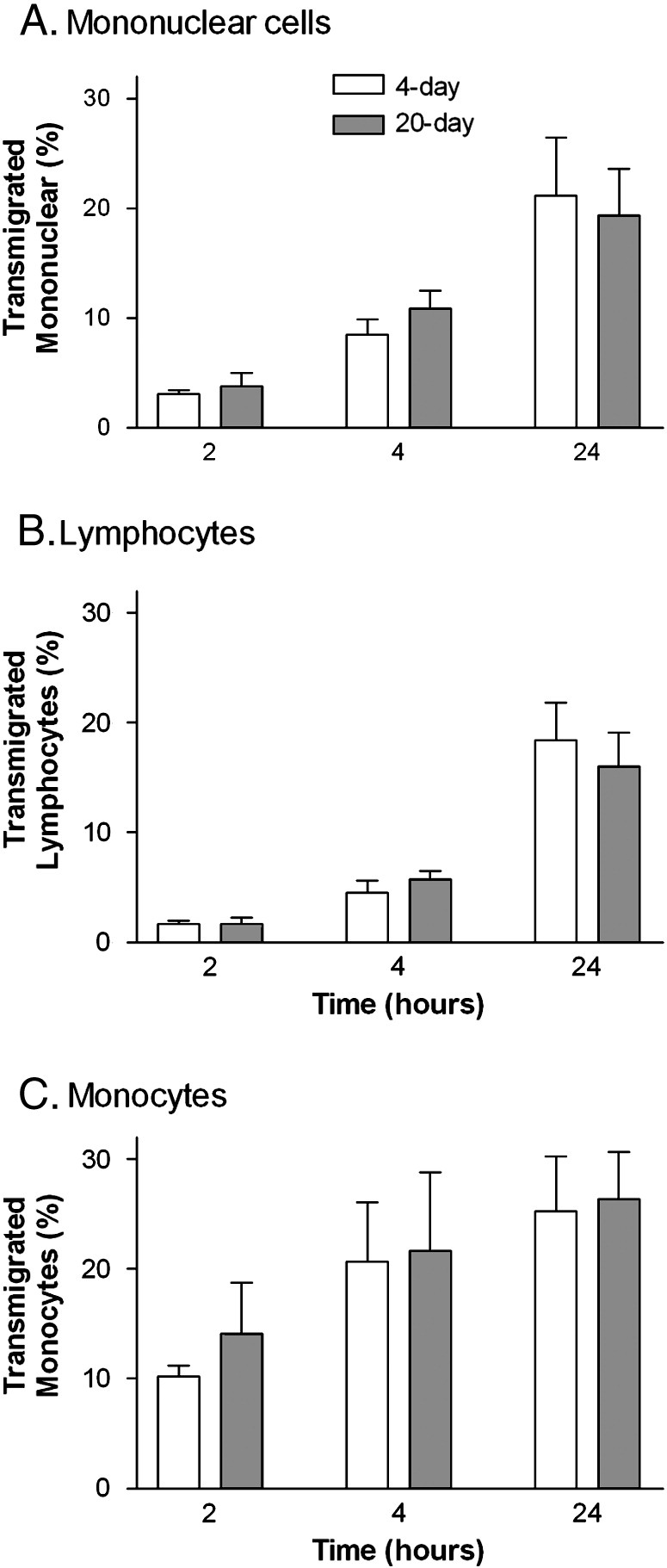
Comparison of transmigration of (A) mononuclear cells, (B), lymphocytes, (C), monocytes, through HUVEC which had been cultured for 4 ( ) or 20 days (
) or 20 days ( ) and then treated with TNF + IFNγ. Data are the mean ± SEM from 3 independent experiments in which migration was analysed after 2, 4 and 24 h.
) and then treated with TNF + IFNγ. Data are the mean ± SEM from 3 independent experiments in which migration was analysed after 2, 4 and 24 h.
In previous studies, we have pointed out that lymphocytes can migrate across endothelial monolayers in minutes, but take many hours to cross endothelial/filter constructs [33]. Given the very low levels of lymphocyte transmigration detected here in the first hours, it is possible that any delay induced by the BM would be undetectable because the delay in crossing the filter itself was much greater and independent of culture duration. We attempted to reduce this delay by adding SDF1α to the bottom well. The level of transmigration through 2-day cultures after 2 h was increased (e.g., 18.1 ± 2.7% of mononuclear cells migrated) but there was still no reduction detected for 20-day cultures (17.4 ± 9.1% of mononuclear cells transmigrated; mean ± SEM; n = 3).
Effects of BM laid down by HUVEC on collagen gels on migration of leukocytes
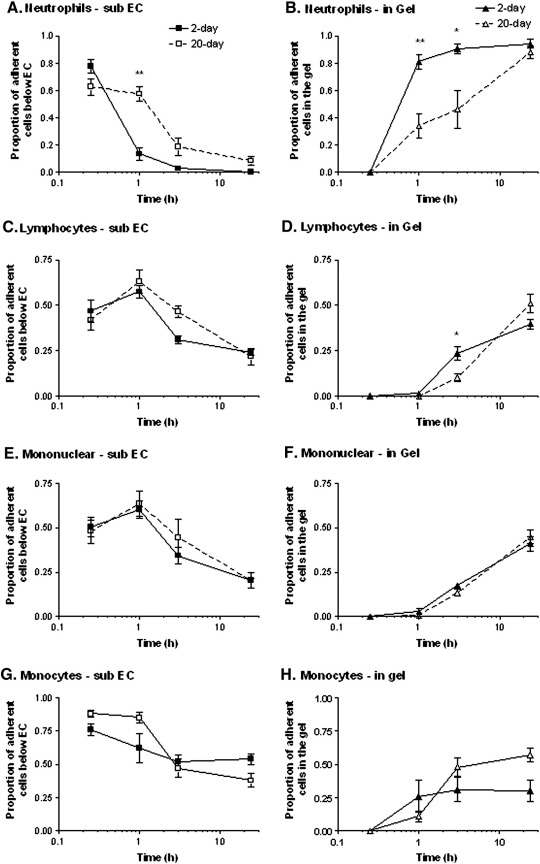
To allow more direct observations of migration through BM, and to avoid potential effects of an obstructive structure beneath, EC were grown on collagen gels for different periods. The various leukocytes were allowed to settle on this surface and microscopic observations were made at chosen times. A similar number of neutrophils adhered to short-term (2-day) or long-term (20-day) cultures of EC on collagen gels, after treatment with TNF (743 ± 67 vs. 622 ± 92 cells/mm2 respectively; mean ± SEM from 4 experiments). In either case, a large proportion of neutrophils migrated through TNF-treated endothelial monolayers within 15 min, and were phase-dark and spread just below the monolayer at that time (Fig. 4A). For 2-day cultures, by 1 h the great majority of migrated neutrophils had entered the gel (Fig. 4B). In comparison, for 20-day cultures, more cells remained immediately below the endothelial monolayer, and less in the gel, at the 1 and 3 h time points (Figs. 4A,B). By 24 h essentially all migrated neutrophils were in the gel in all cases. Few neutrophils remained above the endothelial monolayer.
Fig. 4.
Comparison of transmigration of different leukocytes through HUVEC and into collagen gels after culture for 2 or 20 days and treatment with TNF for 4 h. The proportions of adherent leukocytes were counted below the endothelial monolayer or within the gel, for neutrophils (A,B), lymphocytes (C,D), mononuclear cells (E,F) or monocytes (G,H). Data are the mean ± SEM from 3 or more independent experiments with each type of cell. ANOVA showed a significant effect of culture duration on migration in A (p < 0.05), B (p < 0.01) and D (p < 0.01). *p < 0.05, **p < 0.01 for comparison between 4-day and 20-day cultures by paired t-test.
For isolated lymphocytes, total adhesion was again similar for 2-day or 20-day cultures after treatment with TNF (308 ± 27 vs. 288 ± 49 cells/mm2 respectively; mean ± SEM from 4 experiments). Migration through both cultures was evident within 15 min, but even at 1 h nearly all migrated cells were still just below the monolayer (Figs. 4C,D). After 3 h, a small proportion of lymphocytes had migrated into the gel, and this proportion was slightly but significantly less for 20-day cultures than 2-day cultures. By 24 h, a greater proportion had migrated into the gel, and migration for the 20-day cultures had caught up with the 2-day cultures. In contrast to neutrophils, significant proportions of lymphocytes remained adherent above the monolayer or just below it throughout the 24 h period. We have previously reported the difference in the migratory capability of the two cell types for short-term cultures [33]. Separately, we also tested mixed mononuclear cells in the gel-based model. In this case, the kinetics of leukocyte migration were very similar for the 2-day or 20-day cultures, and at 3 h the lag in entry into the gel for the 20-day cultures was not evident (Figs. 4E,F). The data suggest there was even less delay for monocytes than lymphocytes. To test this independently, we isolated monocytes from blood and tested their migration through TNF-treated HUVEC. Monocytes efficiently crossed the endothelial monolayer (Fig. 4G). They entered the collagen more efficiently than lymphocytes initially, but over 24 h the proportions entering were similar for the two cell types (Fig. 4H). There was no significant delay in monocyte entry for 20-day versus 4-day cultures. Results with the monocytes tended to be more variable than other populations studied, and overall, there were no statistically significant differences in behaviour for the 4-day or 20-day cultures.
Delay of neutrophil migration by the BM directly observed under flow
Since delay of migration was only marked for neutrophils, we made direct observations of flowing neutrophils as they adhered and migrated first through HUVEC and then through the filters. The numbers of neutrophils captured by TNF-treated HUVEC were similar for 4-day or 20-day cultures (1243 ± 189 vs. 1188 ± 176 cells/mm2/106 perfused respectively; mean ± SEM from 7 experiments). The percentages rolling, stationary or transmigrating through the endothelial monolayer were also similar, with slightly more efficient transmigration through the endothelial monolayer for the 20-day cultures (Fig. 5A). However, we noted that while neutrophils soon appeared below the filter for 4-day cultures, it took much longer for this to happen for the 20-day cultures (Fig. 5B). We also noted that there was a tendency for neutrophils to migrate more slowly under the older endothelial monolayer; velocity = 10.5 ± 0.4 μm/min vs. 8.3 ± 0.3 μm/min for 4-day and 20-day cultures respectively (mean ± SEM of means from 3 experiments; p < 0.01 by paired t-test). This slower migration is consistent with our previous report using EC grown on culture plastic and analysed under static conditions [16]. Thus, the formed BM modified neutrophil migration and acted as a barrier to progress under conditions of flow as well as in static assays.
Fig. 5.
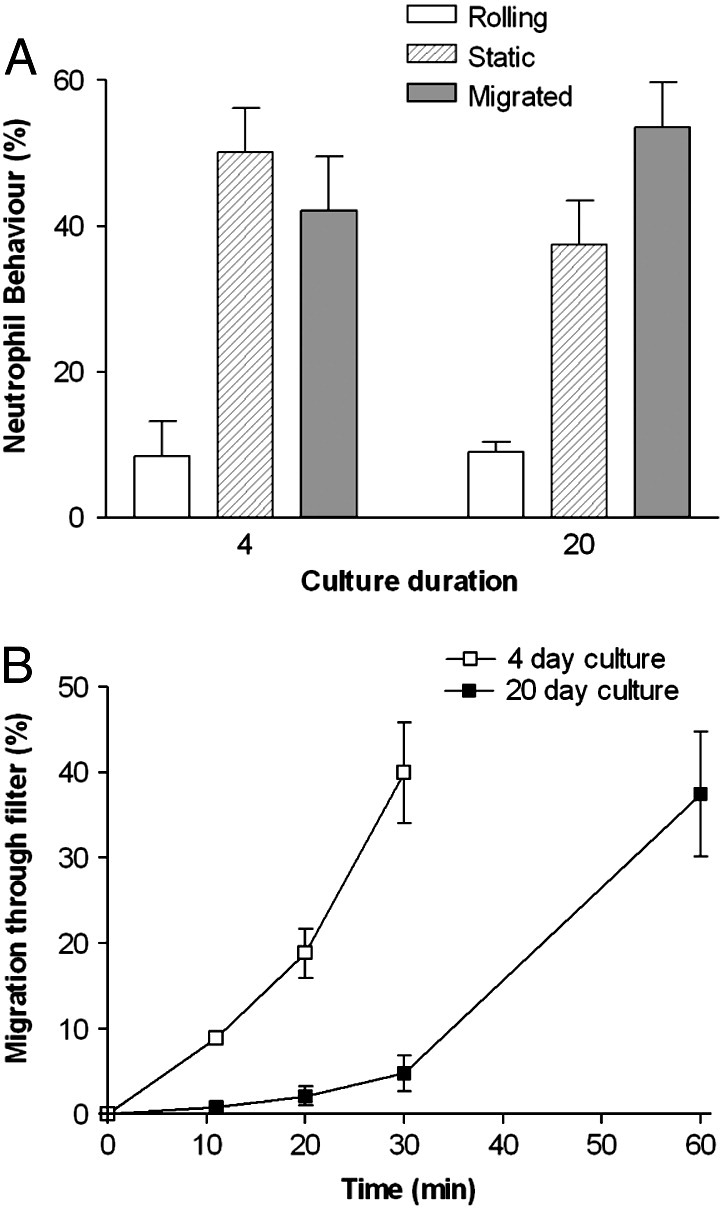
Comparison of behaviour of neutrophils adhering from flow to TNF-treated HUVEC cultured for 4 or 20 days. A. Percentage of adherent neutrophils rolling, stationary adherent or transmigrated through the monolayer after 11 min. B. Percentage of adherent neutrophils that transmigrated through the filter over time. HUVEC were cultured for 4 or 20 days, treated with 100 U/ml TNF for 4 h and transferred to a flow chamber before perfusion of neutrophils for 4 min, followed by washout. Time zero was the start of neutrophil perfusion. Data are the mean ± SEM from 4 experiments. In B, ANOVA showed a significant effect of culture duration on migration (p < 0.01).
Roles of neutrophil integrins during migration through endothelial cells and BM
We investigated whether requirement for specific integrin subsets might explain the hold-up of neutrophils migrating through BM. The effects of function-blocking antibodies against integrins were tested for neutrophils allowed to migrate for 2 h through HUVEC that had been cultured on filters and treated with 100 U/ml TNF. Blockade of β2-integrins in the upper chamber reduced neutrophil adhesion by about 30% but caused a much greater reduction in transmigration after 2 h (Figs. 6A,B). Inhibition of migration at the initial transendothelial stage has previously been reported after β2-integrin blockade. Thus, to test whether β2-integrins were required for migration after this stage, antibody was added to the lower chamber only. Adhesion was no longer reduced, indicating that antibody did not access the cells in the upper chamber; there was, however, some inhibition of transmigration (Figs. 6A,B). These effects were similar for 4- and 20-day cultures. Analysis of surface expression of integrins showed that after transmigration, neutrophils had increased expression of CD18 compared to cells incubated in tubes at 37 °C, although a similar increase was also evident in neutrophils that had been retrieved from the upper chamber (Fig. 6C). Again, changes in expression were similar for neutrophils that had been in contact with 4- or 20-day cultures. Thus, β2-integrins appeared essential for initial migration through endothelial monolayers, and also played a role in migration in the subendothelial compartment regardless of the presence of BM.
Fig. 6.
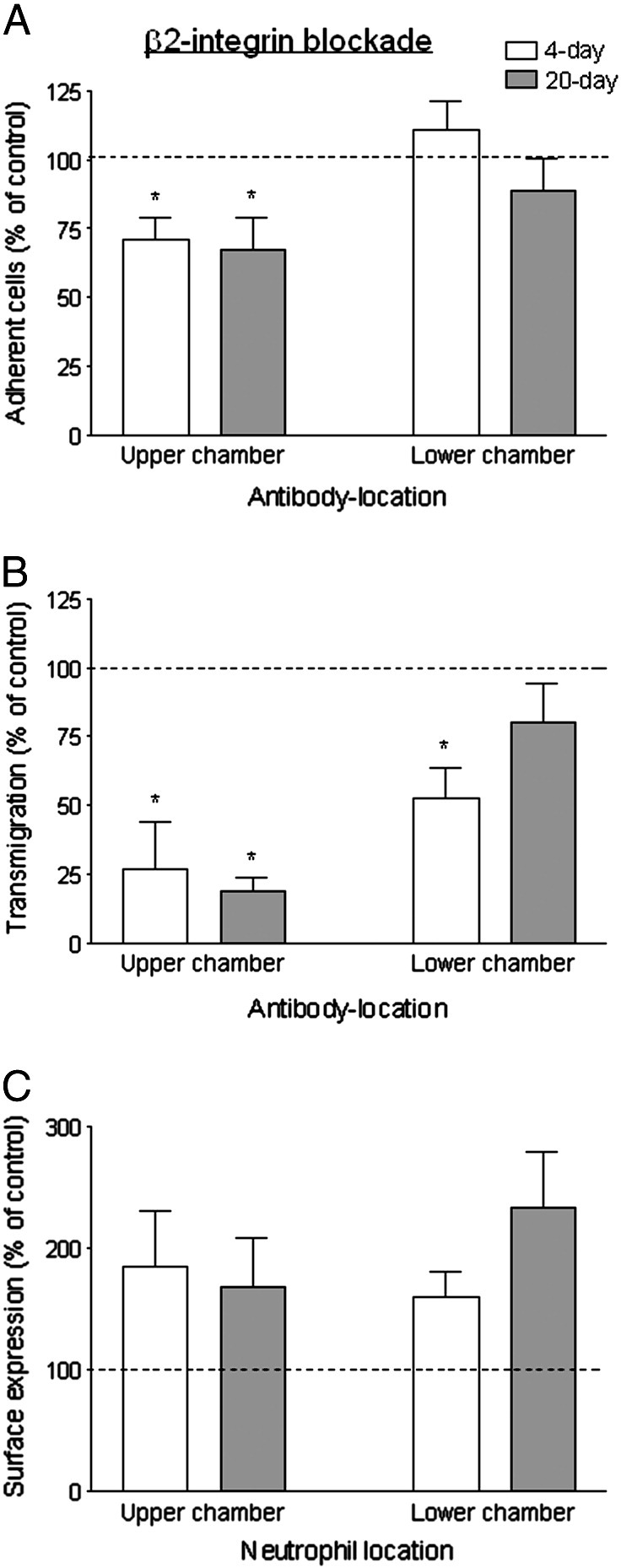
Function and expression of β2-integrins during transmigration of neutrophils through TNF-treated HUVEC cultured for 4 or 20 days. Effects of adding antibody against β2-integrin to the upper chamber of Transwell filters (with neutrophils) or the lower chamber only, on: (A) adhesion to HUVEC; (B) transmigration; (C) surface expression of β2-integrin on neutrophils retrieved from upper or lower chambers (i.e., non-adherent or transmigrated neutrophils respectively). Results for 4-day ( ) and 20-day (
) and 20-day ( ) cultures are shown, expressed relative to untreated control. In A, data are the mean ± SEM from 5 experiments. ANOVA showed a significant effect of treatment of the upper chamber; *p < 0.05 compared to control by paired t-test. In B, data are the mean ± SEM from 5 experiments. ANOVA showed a significant effect of treatment of either type; *p < 0.05 compared to control by paired t-test. In C, data are the mean ± SEM from 3 experiments. ANOVA showed a significant effect of treatment of either type, but there was no significant effect of treatment for the individual days.
) cultures are shown, expressed relative to untreated control. In A, data are the mean ± SEM from 5 experiments. ANOVA showed a significant effect of treatment of the upper chamber; *p < 0.05 compared to control by paired t-test. In B, data are the mean ± SEM from 5 experiments. ANOVA showed a significant effect of treatment of either type; *p < 0.05 compared to control by paired t-test. In C, data are the mean ± SEM from 3 experiments. ANOVA showed a significant effect of treatment of either type, but there was no significant effect of treatment for the individual days.
Blockade of β1-integrins in the upper chamber had no effect on neutrophil adhesion or migration indicating that constitutively-expressed integrin did not play a role in any stage of migration (Figs. 7A,B). To test whether newly-expressed integrin might be effective, we added antibody to the lower chamber as well. In this case, there was a reduction in transmigration for 4-day or 20-day cultures (Fig. 7B) but not adhesion (Fig. 7A). This suggests that β1-integrins were involved in subendothelial migration, whether or not the BM was fully formed, but that pre-existing integrins on the neutrophil surface were not responsible for this function. However, when we analysed surface expression of β1-integrins after transmigration, it was not significantly increased compared to separately-incubated controls or cells washed off the HUVEC monolayer (Fig. 7C). Since there was no net increase in β1-integrin expression, we investigated whether there was any turnover of integrins during migration. Neutrophils were pre-labelled with unconjugated primary antibody before the migration assay, and fluorescently-conjugated secondary antibody was added to cells collected from the different compartments. The level of surface expression detected was not significantly reduced for cells washed off the HUVEC (MFI = 88 ± 5% of controls) but was slightly reduced for transmigrated cells collected from the lower compartment (MFI = 79 ± 5% of controls; p < 0.05 by paired t-test) (mean ± SEM from 4 experiments). This indicates that some-pre-existing β1-integrin was internalised during transmigration, and that there was de novo surface expression at the same time, maintaining overall surface expression approximately constant.
Fig. 7.
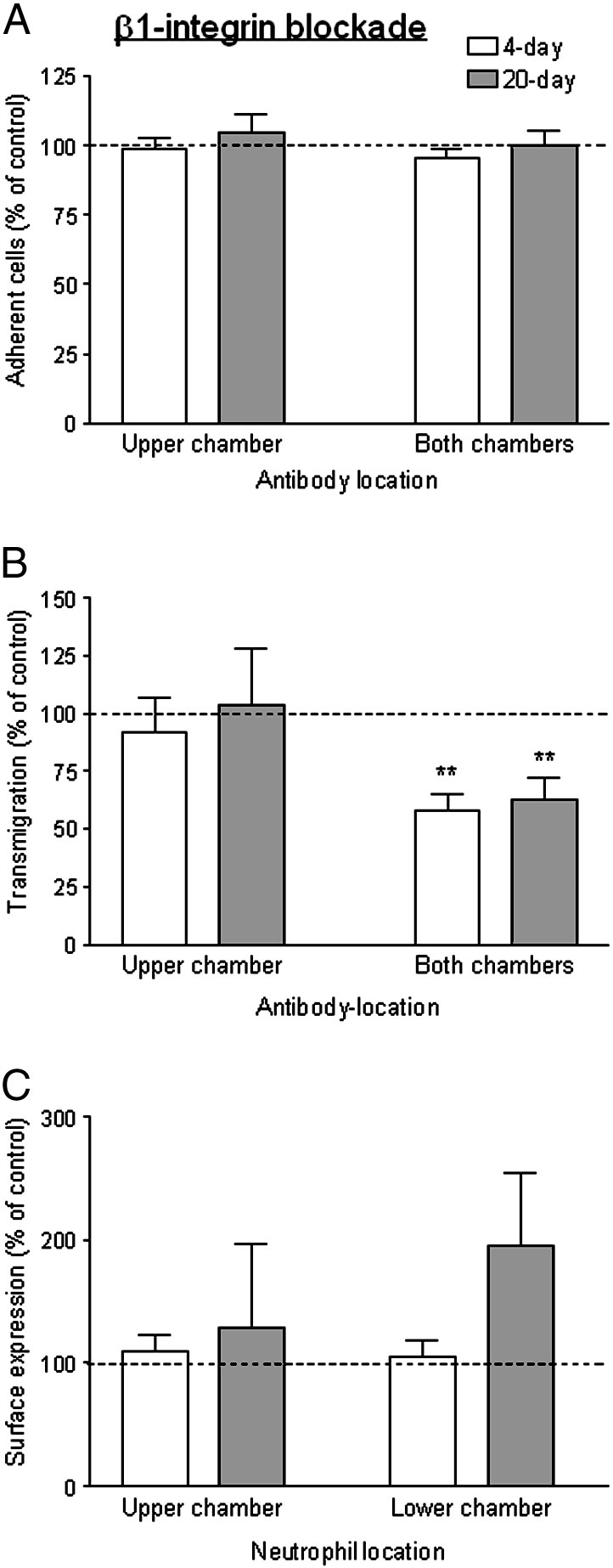
Function and expression of β1-integrins during transmigration of neutrophils through TNF-treated HUVEC cultured for 4 or 20 days. Effects of adding antibody against β1-integrin to the upper chamber of Transwell filters (with neutrophils) or the upper and lower chambers, on: (A) adhesion to HUVEC; (B) transmigration; (C) surface expression of β1-integrin on neutrophils retrieved from upper or lower chambers (i.e., non-adherent or transmigrated neutrophils respectively). Results for 4-day ( ) and 20-day (
) and 20-day ( ) cultures are shown, expressed relative to untreated control. In A, data are the mean ± SEM from 5 experiments. In B, data are the mean ± SEM from 5 experiments. ANOVA showed a significant effect of treatment of both chambers; **p < 0.01 compared to control by paired t-test. In C, data are the mean ± SEM from 3 experiments.
) cultures are shown, expressed relative to untreated control. In A, data are the mean ± SEM from 5 experiments. In B, data are the mean ± SEM from 5 experiments. ANOVA showed a significant effect of treatment of both chambers; **p < 0.01 compared to control by paired t-test. In C, data are the mean ± SEM from 3 experiments.
The roles of integrins in migration noted above were similar for 4- or 20-day cultures. Blockade of β3-integrins in the upper chamber tended to reduce neutrophil migration slightly for 4- or 20-day cultures, although this was not statistically significant (Fig. 8B). Interestingly, when β3-integrins were blocked in both chambers, neutrophil transmigration was significantly decreased specifically through the 20 day cultures, while migration through the 4 day cultures remained slightly but not significantly reduced. None of the treatments affected the number of neutrophils adhering (Fig. 8A). Examination of surface expression of β3-integrins revealed a pattern similar to β1-integrins. There was no net increase in expression during transmigration (Fig. 8C). When neutrophils were pre-labelled with unconjugated primary antibody, the level of expression detected was not reduced for cells washed off the HUVEC (MFI = 102 ± 4% of controls). Transmigrated cells collected from the lower compartment tended to have lost some surface expression (MFI = 83 ± 8% of controls) but this effect did not reach statistical significance (p = 0.08 by paired t-test) (mean ± SEM from 7 experiments). These results indicate that β3-integrins only had a marked role in subendothelial migration when the BM was fully formed, and this was not attributable to integrins initially present on the neutrophils.
Fig. 8.
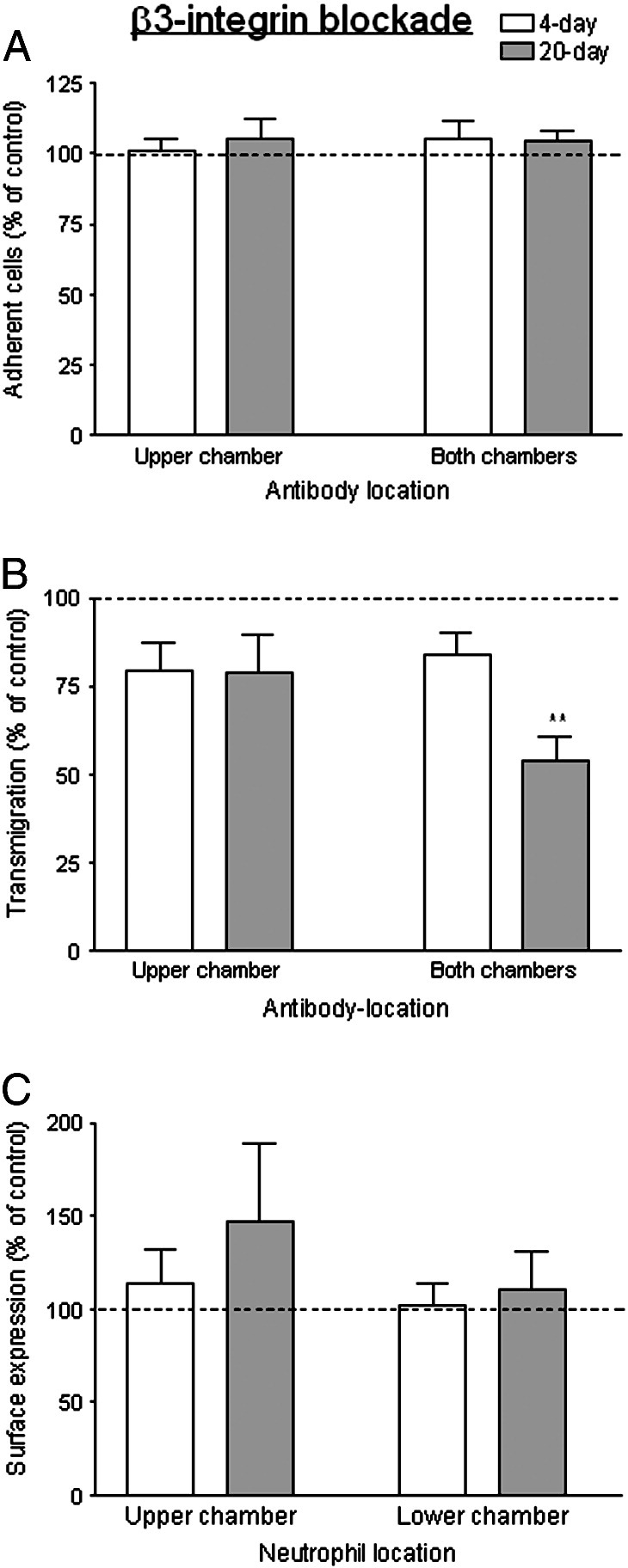
Function and expression of β3-integrins during transmigration of neutrophils through TNF-treated HUVEC cultured for 4 or 20 days. Effects of adding antibody against β3-integrin to the upper chamber of Transwell filters (with neutrophils) or the upper and lower chamber only, on: (A) adhesion to HUVEC; (B) transmigration; (C) surface expression of β3-integrin on neutrophils retrieved from upper or lower chambers (i.e., non-adherent or transmigrated neutrophils respectively). Results are shown for 4-day ( ) and 20-day (
) and 20-day ( ) cultures, expressed relative to untreated control. In A, data are the mean ± SEM from 6 experiments. In B, data are the mean ± SEM from 6 experiments. ANOVA showed a significant effect of treatment of the both chambers; *p < 0.05 compared to control by paired t-test. In C, data are the mean ± SEM from 4 experiments.
) cultures, expressed relative to untreated control. In A, data are the mean ± SEM from 6 experiments. In B, data are the mean ± SEM from 6 experiments. ANOVA showed a significant effect of treatment of the both chambers; *p < 0.05 compared to control by paired t-test. In C, data are the mean ± SEM from 4 experiments.
We also tested effects of combined blockade of different integrins. When antibody against β1-integrins was combined with antibody against β2-integrins (with the latter added only to the lower chamber, to avoid its effects on the initial stages of migration), transmigration was reduced slightly more than for either antibody alone, and the effect was similar for 4-day or 20-day cultures (Fig. 9). When antibody against β3-integrin was added as well, then there was a further reduction in transmigration, but now the effect was greater for 20-day cultures than 4-day cultures (Fig. 9). Thus, again, β3-integrins had a greater role in migration when BM was present.
Fig. 9.
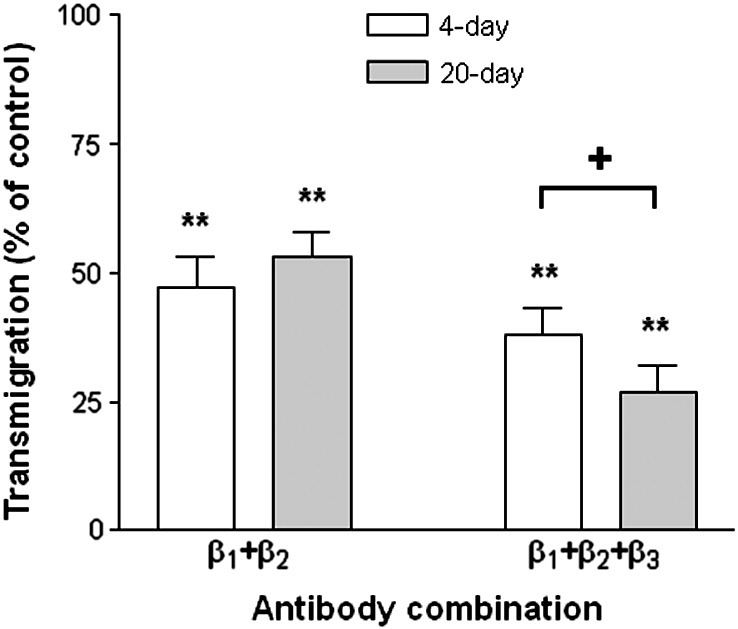
Effects of combinations of antibodies against integrins on transmigration of neutrophils through TNF-treated HUVEC cultured for 4 or 20 days. Antibodies against β1- and β3-integrins were added to the upper and lower chambers, and antibody against β2-integrin was added to the lower chambers only. Results are shown for 4-day ( ) and 20-day (
) and 20-day ( ) cultures, expressed relative to untreated control. Data are the mean ± SEM from 4 experiments. **p < 0.01 compared to control by paired t-test. +p < 0.05 for comparison between 4-day and 20-day cultures by paired t-test.
) cultures, expressed relative to untreated control. Data are the mean ± SEM from 4 experiments. **p < 0.01 compared to control by paired t-test. +p < 0.05 for comparison between 4-day and 20-day cultures by paired t-test.
Although various of the antibody treatments noted above, which had no effect on migration, effectively acted as controls for those that did, we also tested non-specific isotype-matched antibodies in the assays. These antibodies had no significant effects on adhesion or migration for 4-day or 20-day cultures (data not shown).
Effects of integrin blockade on migration directly observed under flow
We made direct microscopic observations of antibody-treated neutrophils migrating in a flow-based assay to check at which stage of migration effects were observed. In earlier studies, we found that antibodies against β2-integrins largely abolished stable adhesion and migration of flowing neutrophils [8,34]. Here, pre-treatment with the antibodies against β1- or β3-integrins (which were present during the 4-minute perfusion of neutrophils but not washout) had no detectable effect on levels of adhesion or of transendothelial migration for 4-day or 20-day cultures (data not shown). This agrees with our previous observations with short-term cultures [23]. However, while neither antibody modified migration velocity of neutrophils in the subendothelial space for 4-day cultures, the antibody against β3-integrins markedly reduced the velocity of migration of the neutrophils under the 20-day cultures (Fig. 10). The cells were observed not only to move more slowly, but to take up more smoothly-rounded and less elongated shapes. Antibody against β1-integrins had a lesser effect (Fig. 10) with no obvious morphological change noted.
Fig. 10.
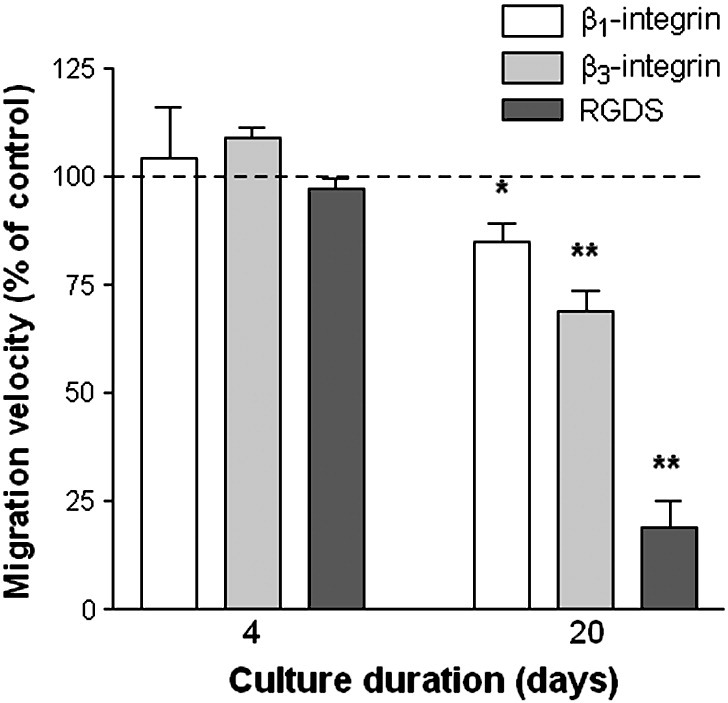
Effects of inhibiting integrin function on migration velocity of neutrophils after migration underneath HUVEC cultured for 4 or 20 days. HUVEC were cultured for 4 or 20 days, treated with 100 U/ml TNF for 4 h and transferred to a flow chamber before perfusion of neutrophils for 4 min, followed by washout. Migration velocity was measured between 11–16 min of washout. Antibody against β1-integrin ( ) or β3-integrin (
) or β3-integrin ( ) was added to neutrophils before perfusion, but was absent from washout medium. RGDS peptide (
) was added to neutrophils before perfusion, but was absent from washout medium. RGDS peptide ( ) was added to neutrophils before perfusion and was also present in washout medium. Data are the mean ± SEM of the mean velocities relative to untreated controls from 3 experiments. *p < 0.05, **p < 0.01 compared to untreated cells by paired t-test.
) was added to neutrophils before perfusion and was also present in washout medium. Data are the mean ± SEM of the mean velocities relative to untreated controls from 3 experiments. *p < 0.05, **p < 0.01 compared to untreated cells by paired t-test.
In these experiments, an antibody may have reached the subendothelial compartment because filters were more vigorously handled when being cut out and loaded in the flow chamber, allowing more leakage than for intact filters. An antibody might also washout gradually after perfusion of the cells. We thus tested the effects of the small RGDS peptide which we could add to large volumes of washout media. For 20-day cultures, the peptide had an even greater effect on slowing migration than the antibody against β3-integrins (Fig. 10), with many cells appearing spread but with smooth outlines lacking pseudopodia. In contrast, there was no discernible effect on behaviour under 4-day cultures (Fig. 10). The peptide had no effects on levels of adhesion from flow or on efficiency of transendothelial migration for either duration of culture (data not shown).
Changes in basement membrane constituents over time and adhesion of neutrophils to them
To evaluate whether the role of β3-integrin might arise from changes in constituents in BM, we measured levels of specific proteins by ELISA. Previously, we showed that concentration of laminin, collagen type IV and fibronectin in a subendothelial matrix increased markedly during culture [16]. Here we analysed the β3-integrin ligand vitronectin and the specific laminin α4- and α5-subunits. Vitronectin was barely detectable above the background in a stripped matrix from 4-day cultures, and showed a small but significant increase in day-20 BM (Fig. 11A). There was a marked, significant increase in laminin between day-4 and day-20; this applied for antibodies recognising the P1 fragment (as used in our previous studies), as well as the α4- and α5-subunits. The signal from the α5-subunit was much lower than from the α4-subunit at both times, especially considering that a much higher antibody concentration was used to detect the former (see Materials and methods).
Fig. 11.
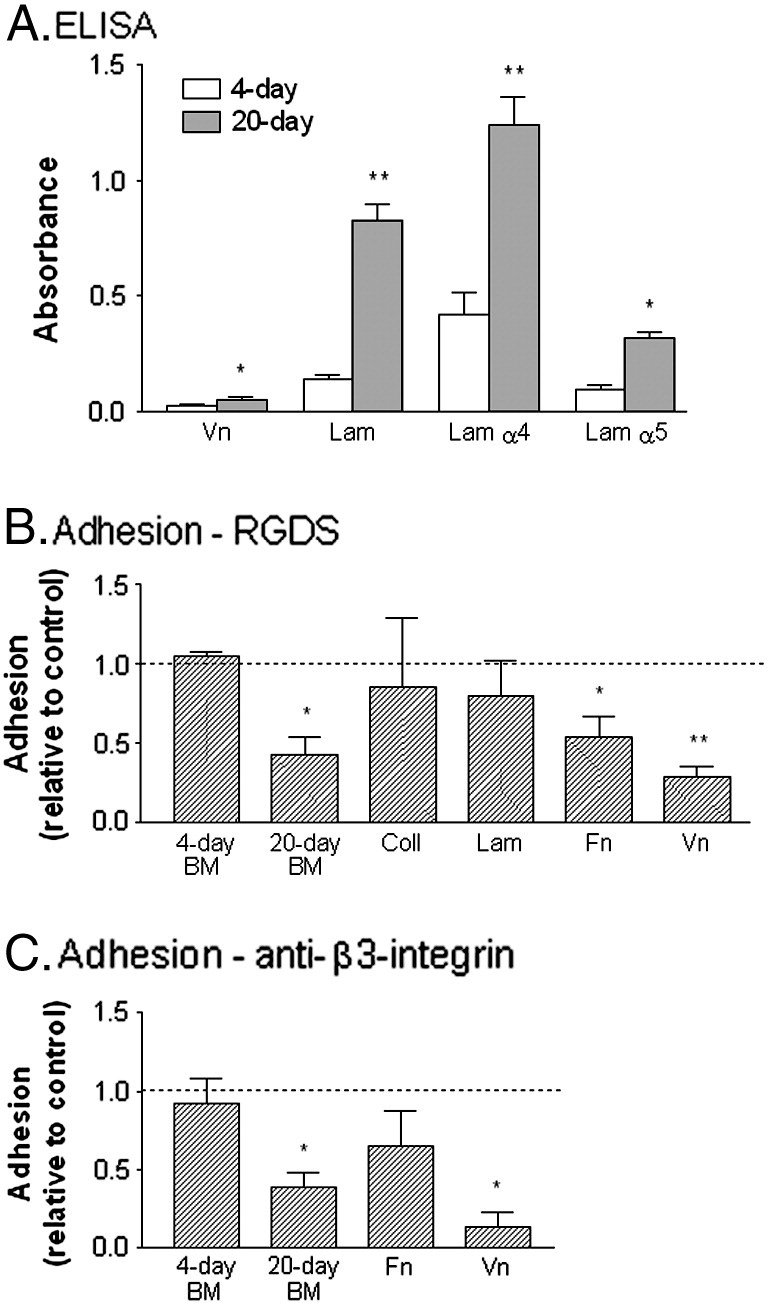
Protein content of matrix from HUVEC cultured for 4 or 20 days, and effects of integrin-inhibition on adhesion of neutrophils to matrix or purified proteins. A. shows absorbance values from ELISA for vitronectin (Vn), laminin (Lam), α4-chain of laminin (Lam α4), α5-chain of laminin (Lam α5) on matrices deposited by HUVEC after culture for 4 days ( ) or 20 days (
) or 20 days ( ). B. shows effect of RGDS peptide on adhesion of neutrophils to 4-day or 20-day BM, collagen type IV (Coll), laminin (Lam), fibronectin (Fn) or vitronectin (Vn). C. shows effect of antibody against β3-integrin on adhesion to 4-day or 20-day BM, fibronectin (Fn) or vitronectin (Vn). In B and C, adhesion is expressed relative to untreated control. Data are the mean ± SEM from 3 or more experiments. *p < 0.05, **p < 0.01 compared to 4-day in A, or untreated control in B and C, by paired t-test.
). B. shows effect of RGDS peptide on adhesion of neutrophils to 4-day or 20-day BM, collagen type IV (Coll), laminin (Lam), fibronectin (Fn) or vitronectin (Vn). C. shows effect of antibody against β3-integrin on adhesion to 4-day or 20-day BM, fibronectin (Fn) or vitronectin (Vn). In B and C, adhesion is expressed relative to untreated control. Data are the mean ± SEM from 3 or more experiments. *p < 0.05, **p < 0.01 compared to 4-day in A, or untreated control in B and C, by paired t-test.
To test the substrate-specificity of β3-integrins further, we analysed adhesion of neutrophils treated with IL-8 to the stripped matrix from 4-day or 20-day cultures and purified proteins. Neither RGDS peptide nor antibody against β3-integrins reduced adhesion to the 4-day matrix significantly; however both reduced adhesion to the 20-day BM (Figs. 11B,C). For purified proteins, RGDS peptide did not inhibit adhesion significantly to type IV collagen or laminin from human placenta, but did inhibit adhesion to purified fibronectin, and most effectively to vitronectin (Fig. 11B). Antibody against β3-integrin tended to reduce binding to fibronectin but this effect was not statistically significant; binding to vitronectin was strongly inhibited (Fig. 11C).
Discussion
We developed models in which human endothelial cells were cultured on filters or gels for prolonged periods to deposit a basement membrane. This enabled us to analyse leukocyte migration through and under the EC, and then through the basement membrane. By comparing kinetics of migration for short-term and long-term cultures, we were able to show that the basement membrane formed a distinct barrier to migration of human neutrophils, delaying their progress away from the subendothelial space. The hold-up in migration imposed by basement membrane was evident in static or flow assays, and was not specific to a particular inducer of migration. Indeed, the stripped basement membrane was itself a barrier to chemotaxis. The barrier function was not so evident for migrating lymphocytes or monocytes, although we could detect a slight delay in lymphocyte migration into collagen gels for long-term cultures. The studies not only revealed a distinct step in leukocyte migration for human cells, but also delivered tractable models for studying the roles of integrins in subendothelial migration of human neutrophils which had not been clearly delineated previously. We found that β2-integrins (but not β1- or β3-integrins) were essential in the first stage of transendothelial migration, and also contributed to subendothelial migration. β1-integrins also contributed to migration in the subendothelial space. However, only β3-integrins played a role specific to migration through basement membrane, and for example, loss of motility under endothelium was observed when their action was inhibited for 20-day but not 4-day cultures. This suggests that a specific interaction between BM and β3-integrins is important in the penetration of this barrier by neutrophils.
Transmigration of lymphocytes or monocytes was much slower than neutrophils, judged by the transit through the filters or into the gels. However, we and others have shown that migration through the endothelial monolayer itself occurs in minutes for lymphocytes, monocytes and neutrophils [33,35,36]. Clearly the filter itself is a barrier to leukocyte migration, which is crossed much more slowly for the mononuclear cells. In the case of collagen gels, for short-term cultures neutrophils quickly dispersed into them. For lymphocytes, mixed mononuclear cells or monocytes, even unobstructed entry into the gel was slower. The tendencies for lymphocytes and monocytes to migrate back and forth across endothelial monolayers, and the fact that many lymphocytes remain associated with the endothelial monolayer rather than migrating away, have been described previously [33,36]. There thus seems to be a fundamental lack of signal(s) to induce efficient subendothelial migration of mononuclear cells in existing in vitro models. Therefore a caveat remains regarding whether BM would be a more distinct barrier to their migration in vivo when more effective signals for onward migration are present. Nevertheless, recent observations of migration of neutrophils and monocytes into the wall of post-capillary venules in mice do suggest that they may use a different mechanism to cross the BM [37]. Based on our work, the BM itself seems less of a specific obstacle for mononuclear cells than neutrophils.
We previously analysed the subsets among peripheral blood lymphocytes (PBL) that migrated in the trans-filter model using immunostaining and flow cytometry [33,38]. We found that the migrated cells contained the same proportions of CD4 and CD8 T-cells as the original PBL, and that in each case, there was enrichment of the memory phenotype (CD45RA-negative) in the migrated population. Using collagen gels in the current study, we found that the proportions of CD4:CD8 cells was again similar for cells retrieved from the gel as for the original PBL (data not shown). Again, the proportion that were CD45RA-negative was enriched in CD4 or CD8 cells in the gel, so that about 80% of migrated CD4 or CD8 cells had this memory phenotype. Selectivity for memory T-cells in transendothelial migration has been reported previously for filter or gel models [39–42]. These results suggest that slow migration away from the endothelial monolayer, and the very modest hold-up due to basement membrane applies to both the main T-cell memory subsets. Whether the same phenomena apply to non-T-cell lymphocytes, or naive T-cells homing through high-endothelial venules, cannot be addressed through the current study. This might usefully be analysed by selecting such cells from PBL and studying them in appropriate culture models.
Long-term culture of EC alters their phenotype, as well as the BM [16–18]. The cells are more sensitive to low dose TNF (judged by the increased efficiency of neutrophil migration through the monolayer itself) and develop a glycocalyx including hyaluronic acid that supports this enhanced recruitment. However, in the present study, attribution of the hold-up in neutrophil transmigration to the BM itself is supported by several findings: neutrophils migrated more slowly through stripped 20-day vs. 4-day BM alone; direct observations under flow showed a delay between appearing under the endothelial monolayer and under the BM/filter construct for 20-day vs. 4-day cultures; when the same EC were seeded on 4-day or 20-day matrices for 2 further days, neutrophil hold-up was seen for 20-day vs. 4-day matrices; while it is possible that the re-seeded EC were themselves modified by the different matrices, slowing of neutrophils underneath re-seeded cells which were seen for 20-day vs. 4-day [16], was reproduced on purified BM alone [17]; EC cultured for 20 days had altered surface properties, but not expression (at mRNA or protein levels) of adhesion molecules or chemokines likely to influence migration [18]; and changes over 20 days in the EC layer itself were generally pro-migratory rather than inhibitory, although only distinct at a low rather than high dose of TNF [16]. Thus, overall, we conclude that it is safe to attribute the hold-up in neutrophil migration to the basement membrane rather than to any linked changes in endothelial cell phenotype.
The adhesive mechanism utilised by leukocytes after they have crossed EC remain incompletely understood. A number of studies have shown the importance of the β2-integrin (CD18) family in neutrophils in the earlier stages of adhesion and transmigration through the endothelial monolayer in static or flow-based models in vitro [8,19,21,43], and in mice or rats in vivo [44,45]. In our own work, their blockade inhibited immobilisation, so that rolling continued and migration was essentially abolished [8,34]. Here, blockade of β2-integrins in the upper compartment of filters greatly reduced transmigration as expected, and we also confirmed previous reports of quantitative upregulation of CD11b and/or CD18 during migration [25,46]. However, the current studies described for the first time a role in migration in the subendothelial space, which we detected by adding antibody only ‘behind’ the coated filters. That the antibody remained in the subendothelial compartment was strongly suggested by the lack of any change in adhesion to the endothelium in those assays, and the much less efficient inhibition of migration compared to addition to the upper compartment.
The role of β1-integrins in human neutrophil migration through endothelium has been less clear. Intravital observations in mice showed a requirement for upregulation of α6β1-integrin for neutrophils to cross the basement membrane after migrating through the endothelial cells [13]. The ability of β1-integrins to take part in the binding of human neutrophils to matrix proteins has been demonstrated in various studies including our own [17,47–50], but most studies did not use EC-deposited matrix, and the neutrophils themselves had not previously migrated through endothelial cells. An earlier study found marked upregulation of expression of β1-integrin, and an increase in their adhesive function, for neutrophils that had undergone chemotaxis through endothelial cells [51]. Recent studies showed that the transendothelial migration process increased the motility of neutrophils and modified their ability to use β1-integrins and αvβ3-integrin to bind peptide substrates, but without evident changes in surface expression [25]. Here we found that pre-treatment with antibodies against β1-integrins had no effect on transmigration across short- or long-term endothelial monolayers in static or flow conditions, but that addition of antibody to the subendothelial compartment did inhibit egress from either type of culture. Total surface expression did not change during migration, but we did obtain evidence that there was turnover of β1-integrins, as transmigrated cells showed loss of surface integrins labelled before the assay. Thus, effects of antibody may have been mediated through blockade of newly-exposed integrin, which played a role in subendothelial migration, with or without the presence of fully-formed BM.
To date there has been even less information on usage of β3-integrins in neutrophil migration through endothelium. Intravital studies in rats found that antibodies added to the vascular compartment inhibited migration across endothelium when the stimulus was formyl peptide, but not when it was IL-1 [52]. It was not clear where the hold-up was. With human cells, β3-integrins were found to regulate migration on purified vitronectin [53], and their blockade inhibited chemotaxis to formyl peptide through protein-coated filters [54]. Our studies clearly indicate that β3-integrins are involved in migration in the subendothelial space and through BM, and that this is only evident for prolonged cultures. This explains why we did not previously find any effects of blockade of this integrin in transmigration studies where subendothelial migration velocity was also analysed [23]. The ability of antibody and RGDS to reduce migration velocity under EC or adhesion to stripped subendothelial matrix, and marked effects on transmigration through endothelial-filter constructs, were only observed for 20-day cultures. In the filter transmigration model, effects were only specific when antibody was added to the back of the filter. Antibody blockade reduced subendothelial velocity for prolonged cultures in the flow model, suggesting that in this system (which requires more vigorous handling of the coated surface) antibody may have leaked into subendothelial space more than in intact filters. RGDS was effective in either system and this small peptide presumably gained access to the sub-endothelium relatively easily. That it acted through β3-integrins (for which it is not strictly specific) in these experiments is evidenced strongly by its lack of effect in 4-day cultures, either viewed directly or in trans-filter assays, where β1- or β2-integrin blockade should have had effects.
We did not detect net upregulation of β3-integrin expression during transmigration, and there was only a slight loss of pre-existing, surface-expressed integrin after transmigration. The fact that addition of antibody to the subendothelial space, and the use of peptide inhibitor, were particularly effective, nevertheless suggest that there is turnover of this integrin during migration. Cycling of integrins is widely considered to regulate cell migration [55], but its role in controlling neutrophil recruitment has not been specifically demonstrated. A problem with studying turnover of both β3-integrin and β1-integrins in these models was that their levels of expression were relatively low (e.g., compared to β2-integrins). This agrees with reports by others, who also found that β3-integrins could take on a role in migration of transmigrated neutrophils, even though difficult to detect by flow cytometry [25].
It is likely that the development of the role of β3-integrins in adhesion to and migration through fully-formed BM arose because of changes in its protein constituents. We previously showed increased levels of laminin, fibronectin and collagen IV in day 20 versus day 4 matrix [16]. In neutrophils, β3-integrins have been shown previously to regulate migration on vitronectin and fibronectin [53,56]. Here, RGDS peptide and antibody against β3-integrin inhibited adhesion to both purified proteins, although more efficiently for vitronectin. In endothelial matrices, we could barely detect vitronectin after 4 days by ELISA, and there was a slight increase in signal after 20 days. We also analysed changes in the main endothelial laminin isoforms, laminin 411 and laminin 511, using ELISA specific for the different α4- and α5-chains. Laminin 411 has been shown to support neutrophil migration via β1- and β2-integrins [e.g., 57,58], but laminin 511 can support interaction with other cell types at least through RGD-dependent or β3-integrin interactions [59,60]. We found that the α4-chain was easily detectable and increased with culture duration. The α5-chain was not detectable in the 4-day matrix using the same antibody concentration as for the α4-chain, but could be detected if a much higher concentration was used. In that case, we saw a several-fold increase after 20-day culture compared to 4-day. Thus two potential β3-integrin ligands, vitronectin and laminin 511, were present only at levels near the detection limit of the ELISA after 4 days and increased significantly by 20 days. It is possible that only by the later time had they reached critical levels to support integrin-binding that had functional effects. Further definition of the specific roles of these and other matrix proteins would require use of antibodies specific for their integrin-binding sites, or methods for manipulating their expression over prolonged periods in endothelial cells.
The above raises questions as to the signals which caused changes in integrin function during transmigration. Ligation of CD31 has been shown to modify adhesive functions of β1-integrins and β2-integrins [61,62], and increases neutrophil motility under EC in our hands [23]. In mice, ligation of CD31 was required for upregulation of expression of α6β1-integrin and thus CD31 indirectly affected the ability of neutrophils to cross BM [13]. However, previously we found no effect of antibody blockade of CD31 on migration from the subendothelial space through filters under flow [26]. Similar antibody treatment in the current model showed no effect on migration through filters coated with 4-day or 20-day cultures (data not shown). Nor could we detect any upregulation of the α6-integrin subunit when transmigrated neutrophils were analysed by flow cytometry (data not shown). Contact with the BM itself seems to modify neutrophil behaviour. In the flow model, neutrophils underneath EC migrated more slowly for long-term than short-term cultures. This agrees with previous observations [16,17],where we also found isolated BM to slow neutrophil migration compared to substrate from short-term cultures or deposited purified BM proteins such as laminin, collagen or fibronectin. An interesting possibility is that the role of β3-integrin was itself regulatory rather than directly adhesive. We found that β1- andβ2-integrins were required to adhere to stripped basement membrane [17] and both clearly influenced subendothelial migration in the current study. Blockade of β3-integrin in the present study had a partial effect on adhesion to stripped BM at 20 days but not 4 days, although it is likely that adequate ligands for the other integrins were present at the later time. In a previous study, we found that β3-integrins regulated the direction of neutrophil migration in flow, although not required for adhesion to the substrate, which was supported by β2-integrins [56]. Thus interaction between β3-integrins and component(s) in the mature BM may regulate the adhesive and migratory functions supported through the other integrins.
Overall, our studies support the concept that migration from the subendothelial space, through basement membrane is a distinct step in the recruitment of leukocytes, which is differently regulated for different leukocyte subsets. They also reveal contributions from β1-, β2- and β3-integrins to migration during this step, but perhaps most interestingly, that β3-integrins are specifically engaged in migration on, and through, basement membrane. The models described here will be useful for further analysis of adhesive mechanisms utilised by human leukocytes in the later stages of recruitment. It has recently been suggested that leukocyte integrins are not needed for migration through tissue matrix [22], although the above indicates that this is not the case for the subendothelial stage. Neither use of purified proteins, or of collagen or similar gels alone, are adequate for modelling this behaviour, because the subsequent migratory behaviour of neutrophils changes during transendothelial migration [16,23,25], and the present studies show that collagen gel alone does not provide the same migration barrier as proteins deposited by EC over time. Integrated models where leukocytes can be followed through the various steps in sequence are required to obtain a full picture of the regulatory mechanism operating at each stage.
The following are the supplementary materials related to this article.
Supplementary video 1
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by a Project Grants from the British Heart Foundation (PG/05/022/18506 and PG/08/128/26525). Umbilical cords were collected with the assistance of the Birmingham Women's Health Care NHS Trust.
References
- 1.Smith C.W., Kishimoto T.K., Abbassi O., Hughes B., Rothlein R., McIntire L.V., Butcher E., Anderson D.C., Abbass O. Chemotactic factors regulate lectin adhesion molecule 1 (LECAM-1)-dependent neutrophil adhesion to cytokine-stimulated endothelial cells in vitro. J. Clin. Invest. 1991;87:609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones D.A., McIntire L.V., Smith C.W., Picker L.J. A two-step adhesion cascade for T cell/endothelial cell interactions under flow conditions. J. Clin. Invest. 1994;94:2443–2450. doi: 10.1172/JCI117612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luscinskas F.W., Ding H., Tan P., Cumming D., Tedder T.F., Gerritsen M.E. L- and P-selectins, but not CD49d (VLA-4) integrins, mediate monocyte initial attachment to TNF-alpha-activated vascular endothelium under flow in vitro. J. Immunol. 1996;157:326–335. [PubMed] [Google Scholar]
- 4.Sheikh S., Rainger G.E., Gale Z., Luu N.-T., Rahman M., Nash G.B. Differing mechanisms of leukocyte recruitment and sensitivity to conditioning by shear stress for endothelial cells treated with tumour necrosis factor-α or interleukin-1. Br. J. Pharmacol. 2005;145:1052–1061. doi: 10.1038/sj.bjp.0706281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Springer T.A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Ann. Rev. Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 6.Cines D.B., Pollak E.S., Buck C.A., Loscalzo J., Zimmerman G.A., McEver R.P., Pober J.S., Wick T.M., Konkle B.A., Schwartz B.S., Barnathan E.S., McCrae K.R., Hug B.A., Schmidt A.M., Stern D.M. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 7.Rainger G.E., Fisher A.C., Nash G.B. Neutrophil rolling is rapidly transformed to stationary adhesion by IL-8 or PAF presented on endothelial surfaces. Am. J. Physiol. 1997;272:H114–H122. doi: 10.1152/ajpheart.1997.272.1.H114. [DOI] [PubMed] [Google Scholar]
- 8.Luu N.T., Rainger G.E., Nash G.B. Differential ability of exogenous chemotactic agents to disrupt transendothelial migration of flowing neutrophils. J. Immunol. 2000;164:5961–5969. doi: 10.4049/jimmunol.164.11.5961. [DOI] [PubMed] [Google Scholar]
- 9.Shulman Z., Shinder V., Klein E., Grabovsky V., Yeger O., Geron E., Montresor A., Bolomini-Vittori M., Feigelson S.W., Kirchhausen T., Laudanna C., Shakhar G., Alon R. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30:384–396. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tull S.P., Yates C.M., Maskrey B.H., O'Donnell V.B., Madden J., Grimble R.F., Calder P.C., Nash G.B., Rainger G.E. Omega-3 fatty acids and inflammation: novel interactions reveal a new step in neutrophil recruitment. PLoS Biol. 2009;7:e1000177. doi: 10.1371/journal.pbio.1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L., Froio R.M., Sciuto T.E., Dvorak A.M., Alon R., Luscinskas F.W. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller W.A. Leukocyte–endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 13.Dangerfield J., Larbi K.Y., Huang M.T., Dewar A., Nourshargh S. PECAM-1 (CD31) homophilic interaction up-regulates alpha6beta1 on transmigrated neutrophils in vivo and plays a functional role in the ability of alpha6 integrins to mediate leukocyte migration through the perivascular basement membrane. J. Exp. Med. 2002;196:1201–1211. doi: 10.1084/jem.20020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S., Voisin M.B., Larbi K.Y., Dangerfield J., Scheiermann C., Tran M., Maxwell P.H., Sorokin L., Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J. Exp. Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber A.R., Weiss S.J. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J. Clin. Invest. 1989;83:1122–1136. doi: 10.1172/JCI113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler L.M., Rainger G.E., Rahman M., Nash G.B. Prolonged culture of endothelial cells and deposition of basement membrane modify the recruitment of neutrophils. Exp. Cell Res. 2005;310:22–32. doi: 10.1016/j.yexcr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Butler L.M., Khan S., Rainger G.E., Nash G.B. Effects of endothelial basement membrane on neutrophil adhesion and migration. Cell. Immunol. 2008;251:56–61. doi: 10.1016/j.cellimm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Butler L.M., Rainger G.E., Nash G.B. A role for the endothelial glycosaminoglycan hyaluronan in neutrophil recruitment by endothelial cells cultured for prolonged periods. Exp. Cell Res. 2009;315:3433–3441. doi: 10.1016/j.yexcr.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furie M.B., Tancinco M.C., Smith C.W. Monoclonal antibodies to leukocyte integrins CD11a/CD18 and CD11b/CD18 or intercellular adhesion molecule-1 inhibit chemoattractant-stimulated neutrophil transendothelial migration in vitro. Blood. 1991;78:2089–2097. [PubMed] [Google Scholar]
- 20.Meerschaert J., Furie M.B. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J. Immunol. 1995;154:4099–4112. [PubMed] [Google Scholar]
- 21.Issekutz A.C., Rowter D., Springer T.A. Role of ICAM-1 and ICAM-2 and alternate CD11/CD18 ligands in neutrophil transendothelial migration. J. Leukoc. Biol. 1999;65:117–126. doi: 10.1002/jlb.65.1.117. [DOI] [PubMed] [Google Scholar]
- 22.Lammermann T., Bader B.L., Monkley S.J., Worbs T., Wedlich-Soldner R., Hirsch K., Keller M., Forster R., Critchley D.R., Fassler R., Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 23.Luu N.T., Rainger G.E., Buckley C.D., Nash G.B. CD31 regulates direction and rate of neutrophil migration over and under endothelial cells. J. Vasc. Res. 2003;40:467–479. doi: 10.1159/000074296. [DOI] [PubMed] [Google Scholar]
- 24.Lindbom L., Werr J. Integrin-dependent neutrophil migration in extravascular tissue. Semin. Immunol. 2002;14:115–121. doi: 10.1006/smim.2001.0348. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez A.L., El Bjeirami W., West J.L., McIntire L.V., Smith C.W. Transendothelial migration enhances integrin-dependent human neutrophil chemokinesis. J. Leukoc. Biol. 2007;81:686–695. doi: 10.1189/jlb.0906553. [DOI] [PubMed] [Google Scholar]
- 26.Chakravorty S., McGettrick H.M., Butler L.M., Buckley C.D., Rainger G.E., Nash G.B. An in vitro model for analysing neutrophil migration into and away from the sub-endothelial space: roles of flow and CD31. Biorheology. 2006;43:71–82. [PubMed] [Google Scholar]
- 27.Rainger G.E., Fisher A., Shearman C., Nash G.B. Adhesion of flowing neutrophils to cultured endothelial cells after hypoxia and reoxygenation in vitro. Am. J. Physiol. 1995;269:1398–1406. doi: 10.1152/ajpheart.1995.269.4.H1398. [DOI] [PubMed] [Google Scholar]
- 28.Rainger G.E., Stone P., Morland C.M., Nash G.B. A novel system for investigating the ability of smooth muscle cells and fibroblasts to regulate adhesion of flowing leukocytes to endothelial cells. J. Immunol. Meth. 2001;255:73–82. doi: 10.1016/s0022-1759(01)00427-6. [DOI] [PubMed] [Google Scholar]
- 29.Luu N.T., Madden J., Calder P.C., Grimble R.F., Shearman C.P., Chan T., Tull S.P., Dastur N., Rainger G.E., Nash G.B. Comparison of the pro-inflammatory potential of monocytes from healthy adults and those with peripheral arterial disease using an in vitro culture model. Atherosclerosis. 2007;193:259–268. doi: 10.1016/j.atherosclerosis.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 30.Cooke B.M., Usami S., Perry I., Nash G.B. A simplified method for culture of endothelial cells and analysis of adhesion of blood cells under conditions of flow. Microvasc. Res. 1993;45:33–45. doi: 10.1006/mvre.1993.1004. [DOI] [PubMed] [Google Scholar]
- 31.McGettrick H.M., Smith E., Filer A., Kissane S., Salmon M., Buckley C.D., Rainger G.E., Nash G.B. Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells. Eur. J. Immunol. 2009;39:113–125. doi: 10.1002/eji.200838232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 33.McGettrick H.M., Hunter K., Moss P.A., Buckley C.D., Rainger G.E., Nash G.B. Direct observations of the kinetics of migrating T-cells suggest active retention by endothelial cells with continual bidirectional migration. J. Leukoc. Biol. 2009;85:98–107. doi: 10.1189/jlb.0508301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahra P., Rainger G.E., Wautier J.L., Luu N.-T., Nash G.B. Each step during transendothelial migration of flowing neutrophils is regulated by the stimulatory concentration of tumour necrosis factor-alpha. Cell Adhes. Commun. 1998;6:491–501. doi: 10.3109/15419069809010797. [DOI] [PubMed] [Google Scholar]
- 35.Luu N.T., Rainger G.E., Nash G.B. Kinetics of the different steps during neutrophil migration through cultured endothelial monolayers treated with tumour necrosis factor-alpha. J. Vasc. Res. 1999;36:477–485. doi: 10.1159/000025690. [DOI] [PubMed] [Google Scholar]
- 36.Bradfield P.F., Scheiermann C., Nourshargh S., Ody C., Luscinskas F.W., Rainger G.E., Nash G.B., Miljkovic-Licina M., Aurrand-Lions M., Imhof B.A. JAM-C regulates unidirectional monocyte transendothelial migration in inflammation. Blood. 2007;110:2545–2555. doi: 10.1182/blood-2007-03-078733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voisin M.B., Woodfin A., Nourshargh S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arterioscler. Thromb. Vasc. Biol. 2009;29:1193–1199. doi: 10.1161/ATVBAHA.109.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGettrick H.M., Buckley C.D., Filer A., Ed R.G., Nash G.B. Stromal cells differentially regulate neutrophil and lymphocyte recruitment through the endothelium. Immunology. 2010;131:357–370. doi: 10.1111/j.1365-2567.2010.03307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pietschmann P., Cush J.J., Lipsky P.E., Oppenheimer-Marks N. Identification of subsets of human T cells capable of enhanced transendothelial migration. J. Immunol. 1992;149:1170–1178. [PubMed] [Google Scholar]
- 40.Bird I.N., Spragg J.H., Ager A., Matthews N. Studies of lymphocyte transendothelial migration: analysis of migrated cell phenotypes with regard to CD31 (PECAM-1), CD45RA and CD45RO. Immunology. 1993;80:553–560. [PMC free article] [PubMed] [Google Scholar]
- 41.Brezinschek R.I., Lipsky P.E., Galea P., Vita R., Oppenheimer-Marks N. Phenotypic characterization of CD4+ T cells that exhibit a transendothelial migratory capacity. J. Immunol. 1995;154:3062–3077. [PubMed] [Google Scholar]
- 42.Oppenheimer-Marks N., Lipsky P.E. Migration of naive and memory T cells. Immunol. Today. 1997;18:456–457. doi: 10.1016/s0167-5699(97)82723-5. [DOI] [PubMed] [Google Scholar]
- 43.Smith C.W., Marlin S.D., Rothlein R., Toman C., Anderson D.C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J. Clin. Invest. 1989;83:2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider T., Issekutz T.B., Issekutz A.C. The role of alpha4 (CD49d) and beta2 (CD18) integrins in eosinophil and neutrophil migration to allergic lung inflammation in the Brown Norway rat. Am. J. Respir. Cell Mol. Biol. 1999;20:448–457. doi: 10.1165/ajrcmb.20.3.3207. [DOI] [PubMed] [Google Scholar]
- 45.Ding Z.M., Babensee J.E., Simon S.I., Lu H., Perrard J.L., Bullard D.C., Dai X.Y., Bromley S.K., Dustin M.L., Entman M.L., Smith C.W., Ballantyne C.M. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 46.Freyer D.R., Morganroth M.L., Todd R.F., III Surface Mo1 (CD11b/CD18) glycoprotein is up-modulated by neutrophils recruited to sites of inflammation in vivo. Inflammation. 1989;13:495–505. doi: 10.1007/BF00916757. [DOI] [PubMed] [Google Scholar]
- 47.Bohnsack J.F. CD11/CD18-independent neutrophil adherence to laminin is mediated by the integrin VLA-6. Blood. 1992;79:1545–1552. [PubMed] [Google Scholar]
- 48.Werr J., Xie X., Hedqvist P., Ruoslahti E., Lindbom L. β1 integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J. Exp. Med. 1998;187:2091–2096. doi: 10.1084/jem.187.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Werr J., Johansson J., Eriksson E.E., Hedqvist P., Ruoslahti E., Lindbom L. Integrin alpha(2)beta(1) (VLA-2) is a principal receptor used by neutrophils for locomotion in extravascular tissue. Blood. 2000;95:1804–1809. [PubMed] [Google Scholar]
- 50.Sixt M., Hallmann R., Wendler O., Scharffetter-Kochanek K., Sorokin L.M. Cell adhesion and migration properties of beta 2-integrin negative polymorphonuclear granulocytes on defined extracellular matrix molecules. Relevance for leukocyte extravasation. J. Biol. Chem. 2001;276:18878–18887. doi: 10.1074/jbc.M010898200. [DOI] [PubMed] [Google Scholar]
- 51.Kubes P., Niu X.F., Smith C.W., Kehrli M.E., Jr., Reinhardt P.H., Woodman R.C. A novel beta 1-dependent adhesion pathway on neutrophils: a mechanism invoked by dihydrocytochalasin B or endothelial transmigration. Faseb J. 1995;9:1103–1111. [PubMed] [Google Scholar]
- 52.Thompson R.D., Wakelin M.W., Larbi K.Y., Dewar A., Asimakopoulos G., Horton M.A., Nakada M.T., Nourshargh S. Divergent effects of platelet-endothelial cell adhesion molecule-1 and beta 3 integrin blockade on leukocyte transmigration in vivo. J. Immunol. 2000;165:426–434. doi: 10.4049/jimmunol.165.1.426. [DOI] [PubMed] [Google Scholar]
- 53.Hendey B., Lawson M., Marcantonio E.E., Maxfield F.R. Intracellular calcium and calcineurin regulate neutrophil motility on vitronectin through a receptor identified by antibodies to integrins alphav and beta3. Blood. 1996;87:2038–2048. [PubMed] [Google Scholar]
- 54.Bruyninckx W.J., Comerford K.M., Lawrence D.W., Colgan S.P. Phosphoinositide 3-kinase modulation of beta(3)-integrin represents an endogenous “braking” mechanism during neutrophil transmatrix migration. Blood. 2001;97:3251–3258. doi: 10.1182/blood.v97.10.3251. [DOI] [PubMed] [Google Scholar]
- 55.Caswell P., Norman J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 2008;18:257–263. doi: 10.1016/j.tcb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Rainger G.E., Buckley C.D., Simmons D.L., Nash G.B. Neutrophils sense flow-generated stress and direct their migration through alphaVbeta3-integrin. Am. J. Physiol. 1999;276:858–864. doi: 10.1152/ajpheart.1999.276.3.H858. [DOI] [PubMed] [Google Scholar]
- 57.Sixt M., Engelhardt B., Pausch F., Hallmann R., Wendler O., Sorokin L.M. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 2001;153:933–946. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wondimu Z., Geberhiwot T., Ingerpuu S., Juronen E., Xie X., Lindbom L., Doi M., Kortesmaa J., Thyboll J., Tryggvason K., Fadeel B., Patarroyo M. An endothelial laminin isoform, laminin 8 (alpha4beta1gamma1), is secreted by blood neutrophils, promotes neutrophil migration and extravasation, and protects neutrophils from apoptosis. Blood. 2004;104:1859–1866. doi: 10.1182/blood-2004-01-0396. [DOI] [PubMed] [Google Scholar]
- 59.Genersch E., Ferletta M., Virtanen I., Haller H., Ekblom P. Integrin alphavbeta3 binding to human alpha5-laminins facilitates FGF-2- and VEGF-induced proliferation of human ECV304 carcinoma cells. Eur. J. Cell Biol. 2003;82:105–117. doi: 10.1078/0171-9335-00297. [DOI] [PubMed] [Google Scholar]
- 60.Atkinson J.J., Adair-Kirk T.L., Kelley D.G., Demello D., Senior R.M. Clara cell adhesion and migration to extracellular matrix. Respir. Res. 2008;9:1. doi: 10.1186/1465-9921-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka Y., Albelda S.M., Horgan K.J., van Seventer G.A., Shimizu Y., Newman W., Hallam J., Newman P.J., Buck C.A., Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of beta 1 integrin-mediated adhesion. J. Exp. Med. 1992;176:245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berman M.E., Muller W.A. Ligation of platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18) J. Immunol. 1995;154:299–307. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary video 1