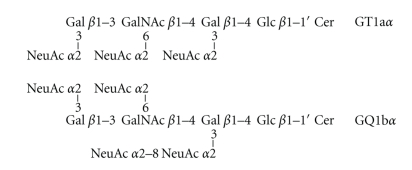Abstract
Conversion of the soluble, nontoxic amyloid β-protein (Aβ) into an aggregated, toxic form rich in β-sheets is a key step in the onset of Alzheimer's disease (AD). It has been suggested that Aβ induces changes in neuronal membrane fluidity as a result of its interactions with membrane components such as cholesterol, phospholipids, and gangliosides. Gangliosides are known to bind Aβ. A complex of GM1 and Aβ, termed “GAβ”, has been identified in AD brains. Abnormal ganglioside metabolism also may occur in AD brains. We have reported an increase of Chol-1α antigens, GQ1bα and GT1aα, in the brain of transgenic mouse AD model. GQ1bα and GT1aα exhibit high affinities to Aβs. The presence of Chol-1α gangliosides represents evidence for genesis of cholinergic neurons in AD brains. We evaluated the effects of GM1 and Aβ1–40 on mouse neuroepithelial cells. Treatment of these cells simultaneously with GM1 and Aβ1–40 caused a significant reduction of cell number, suggesting that Aβ1–40 and GM1 cooperatively exert a cytotoxic effect on neuroepithelial cells. An understanding of the mechanism on the interaction of GM1 and Aβs in AD may contribute to the development of new neuroregenerative therapies for this disorder.
1. Introduction
Alzheimer's disease (AD) is an irreversible, slowly progressive neurodegenerative disease that is the most common form of dementia among people age 65 and older and is characterized by cognitive and behavioral problems. The symptoms are initiated by memory loss and gradually lead to behavior and personality changes with impaired cognitive abilities such as decline of decision making and language disability, and eventually disturbances in recognizing family and friends. These losses are related to the worsening lesion of the connections between certain neurons in the brain. Patients often become anxious or aggressive, or wander away from home. Eventually, patients need total care, and the final outcome is always death. Although there is no cure at present, some therapeutic drugs inhibiting acetylcholinesterase have been used to alleviate the disease symptoms and improve the quality of life for patients with AD [1, 2].
Gangliosides are important constituents of cells; they are especially abundant in neuronal membranes and play a variety of biological functions, including cellular recognition and adhesion as well as signaling [3]. The expression of gangliosides is not only cell type specific and developmentally regulated but also closely related to the differentiation state of the cell [3–6]. Numerous studies have indicated that changes of ganglioside expression patterns and levels during cellular differentiation are closely related to their metabolism, particularly their biosynthesis [3, 4, 6]. Notably, gangliosides may have neuroprotective effects to the cell [7]. Gangliosides do not function as a neurotrophic factor themselves, but they potentiate neurotrophic influences present in the nervous system. In this regard, many scientists have reported the beneficial effects of GM1 treatment in animal models of neurodegeneration and diseases. For example, administration of GM1 protects hippocampal progenitor cells from neuronal injury and reduces hippocampal neurogenesis induced by D-galactose treatment [8]. Saito et al. reported that GM1 and LIGA20 can protect mouse brains from apoptotic neurodegeneration induced by ethanol [9]. In clinical applications or animal studies, many studies have demonstrated the neuroprotective effects of GM1 in diseases such as AD [10], AD model of transgenic mice [11, 12], Parkinson disease [13], stroke [14], and Guillain-Barré syndrome [15].
The pathological hallmarks in the AD brain include senile plaques (SPs) and neurofibrillary tangles. Many scientists believe that the accumulation and aggregation of amyloid β-proteins (Aβs) in SPs in the brain are a central part of the pathogenesis of AD. The conversion of soluble, nontoxic Aβ into aggregated, toxic Aβ rich in β-sheet structures is considered to be the key step in the development of AD. Aβs are able to bind to a variety of biomolecules, including lipids, proteoglycans, and certain proteins [1, 16]. Immunochemical studies revealed that Aβ deposits in AD brain are due to the presence of certain amyloid-associated proteins such as amyloid P component, proteoglycans, and apolipoproteins [17]. The potential significance of the interaction with proteoglycans and Aβs for the pathogenic mechanisms has been reviewed [2].
During aging and neurodegeneration in AD, the physicochemical properties of membranes and lipid metabolism undergo significant alterations [18, 19]. These include multiple changes such as glycosphingolipid (GSL) abnormalities and impairment of neurotrophin signaling, protein trafficking, and protein turnover [20]. These changes can result in imbalances in the proportion of lipids in membranes and/or changed ratios of membrane lipids, which may contribute to the pathogenesis of AD [21–23]. Earlier immunohistochemical studies on the involvement of GSLs using specific monoclonal antibodies revealed that SPs contained GM1 [24], c-series gangliosides [25], and GD1a [26], and these studies implicate that Aβs might be interacting with the above gangliosides. In this paper, we will focus on the role of ganglioside metabolism in the pathogenesis and/or development of AD.
2. Interaction of Amyloid β-Proteins with Gangliosides
A critical question concerning the development of AD is how the soluble, nontoxic Aβ form high in an α-helix-rich structure is converted into an aggregated, toxic form rich in β-sheets in the brain. Terzi et al. first reported that Aβ1–40 undergoes a conformational transition from random coil to aggregated structure rich in β-sheet after addition of lipid vesicles containing negatively charged lipids [27], suggesting that Aβ neurotoxicity may be the result of membrane protein-lipid interactions. Several studies have demonstrated that Aβs bind to gangliosides, especially GM1, resulting in an altered secondary structure of Aβs [28–31]. Aβ binds to membranes containing ganglioside GM1, and upon binding it undergoes a conformational transition from random coil to an ordered structure rich in β-sheet. This interaction appears to be ganglioside-specific because no changes in Aβ1–40 conformation were found in the presence of various phospholipids or sphingomyelin. Aβ binds selectively to gangliosides with a binding affinity ranging from 10−6 to 10−7 M, depending on the type of the sugar moiety present in the ganglioside molecule. On the other hand, the isolated oligosaccharide moieties of gangliosides are ineffective in inducing alterations in the secondary structure of Aβ1–40 [28, 32], suggesting the involvement of the lipid component in their interaction. The sialic acid (NeuAc) moiety of gangliosides interacts with Aβ to induce conformational changes in Aβ [31]. In this regard, we have reported that Aβ1–40 binds to a number of gangliosides with the following order of binding strength: GQ1bα > GT1aα > GQ1b > GT1b > GD3 > GD1a = GD1b > LM1 > GM1 > GM2 = GM3 > GM4 based on surface plasmon resonance studies [33]. Neutral GSLs, including asialo-GM1, generally have a much lower affinity for Aβ1–40 than do gangliosides. The results suggest that the α2,3NeuAc residue on the oligosaccharide core of gangliosides is required for binding. In addition, the α2,6NeuAc residue linked to GalNAc in the α-series of gangliosides contributes significantly to the binding affinity for Aβ. Although several reports documented the GM1-induced alterations in the β-sheet structure of Aβ, Mandel and Pettegrew, on the other hand, reported that GM1 inhibited Aβ from undergoing α-helix to β-sheet conformational changes [34]. This discrepancy clearly needs further clarification. In addition, asialo-GM1 binds specifically with Aβ in a manner that could prevent β-sheet formation. Nakazawa et al. reported that Aβ1–40 strongly perturbed the lipid bilayer structure of liposomes of dimyristoylphosphatidylcholine and GM1 to form a nonlamellar phase (most likely in the micellar phase) [35]. The α-helical peptide conformation is significantly flexible and is approximately equally partitioned between components penetrated into the bilayer and in liquid phase whereas the β-sheet peptide conformation is rigid and is presumably deposited and stacked at the bilayer surface.
The interaction between gangliosides and Aβ appears to be affected by experimental conditions such as pH, ionic strength [30, 31], and metal ions [36, 37]. For example, McLaurin and Chakrabartty have reported that Aβ1–40/Aβ1–42 disrupts acidic lipid membranes, and this disruption is greater at pH 6.0 than at pH 7.0, at which point gangliosides induce Aβ1–40/Aβ1–42 to adopt a novel α/β conformation [30]. A further study indicated that binding of Aβ1–40 to mixed gangliosides or GM1-containing vesicles induced an α-helical structure at pH 7.0 and β-structure at pH 6.0 [31]. Several lines of evidence have indicated that disruption of the homeostatic balance of redox-active biometals such as Cu and Fe can lead to oxidative stress, which plays a key role in the development of AD. Atwood et al. reported that unlike other biometals tested at maximal biological concentrations, Cu2+-induced aggregation of Aβ1–40 occurred as the solution pH was lowered from 7.4 to 6.8 and that the reaction was completely reversible with either chelation or alkalinization [36]. The aggregation-inducing activity of metals is in the following order, Cu2+ > Fe3+ > or = Al3+ > Zn2+ [37].
3. Binding Sites of Amyloid β-Proteins with Gangliosides
The NeuAc residue of the ganglioside head group is important for determining the nature of the conformational change of Aβ [31, 38] or interaction with Aβ [33]. The isolated pentasaccharide head group of GM1 alone, however, does not bind with Aβ, suggesting the need for a polyanionic membrane-like structure [38]. To provide a structural basis for this pathogenic interaction associated with AD, Williamson et al. have demonstrated using NMR on 15N-labelled Aβ1–40 and Aβ1–42 that the interaction with GM1 micelles is localized to the N-terminal region of the peptide, particularly residues His13 to Leu17, which become more helical when GM1 is bound [38]. The key interaction is with His13, which undergoes a GM1-specific conformational change. Zhang et al. reported that the binding site for GM1 was located within residues 52–81 (N terminus) of amyloid precursor protein (APP), resulting in a conformational change of APP [39]. This phenomenon is specific for GM1, but not for GD1a, GT1b, and ceramide, indicating that specific binding depends on the sugar moiety of GM1.
Utsumi et al. reported the association of Aβ1–40 isotopically labeled with GM1 and lyso-GM1 micelles using 920 MHz ultra-high field NMR analyses [40]. The data revealed that: (a) Aβ1–40, upon binding to the gangliosidic micelles, forms discontinuous α-helices at the segments His(14)-Val(24) and Ile(31)-Val(36), and (b) Aβ1–40 lies on hydrophobic/hydrophilic interface of the ganglioside cluster, exhibiting an up-and-down topological mode in which the two α-helices and the C-terminal dipeptide segment are in contact with the hydrophobic interior whereas the remaining regions are exposed to the aqueous environment. These results suggest that the ganglioside clusters serve as a unique platform for binding coupled with conformational transition of Aβ molecules, rendering their spatial rearrangements restricted to promote specific intermolecular interactions. [40]. Further study of NMR analyses of the Aβ interactions with gangliosides using lyso-GM1 micelles as a model system have revealed that the sugar-lipid interface is primarily perturbed upon binding of Aβ to the micelles, underscoring the importance of the inner part of the ganglioside cluster for accommodating Aβ in comparison with the outer carbohydrate branches that provide microbial toxin- and virus-binding sites [41].
4. Accumulation of Specific Ganglioside-Bound Amyloid β-Protein Complex (GAβ) in AD Brain
Choo-Smith et al. have reported that addition of ganglioside-containing vesicles to the Aβ solution dramatically accelerates the rate of fibril formation compared to vesicles without gangliosides [28]. The mechanism of ganglioside-mediated Aβ-fibrillization likely involves an initial step in which the GSL-bound peptide self-associates on the membrane surface, undergoing a conformational transition to a β-sheet structure. This suggests that gangliosides can mediate Aβ assembly to lead to accumulation in the brain, which may be involved in the development of AD. Yanagisawa et al. first reported the presence of membrane-bound Aβ1–42, but not Aβ1–40, which tightly binds to GM1 in the AD brain [42]. This novel Aβ species, named as ganglioside-bound Aβ (GAβ), may act as an endogenous seed for amyloid [42], and exhibited early pathological changes of AD. It was hypothesized that Aβ adopts an altered conformation following interaction with GM1, leading to the generation of GAβ, and then GAβ acts as an endogenous seed for amyloid in AD brain. GAβ has unique characteristics, including an extremely high aggregation potential and an altered pattern of immunoreactivity, which results in seeding for amyloid fibril formation in brain. Thus, GAβ may serve as a seed for toxic amyloid fibril formation. The formation of GAβ serves as one of the critical factors in the development of AD and may provide new insights into the pathophysiology in AD [43]. The occurrence of GAβ in AD brain was further confirmed biochemically by staining with cholera toxin-B subunit (CTXB) that preferentially binds to GM1, and by immunoprecipitation experiments using several anti-Aβ monoclonal antibodies [44]. Recently, the presence of GAβ was confirmed in sections of cerebral cortices of cynomolgus monkeys of different ages, from 4 to 36 years old; especially, GAβ is significantly increased in the brains at ages below 19 years [45]. In this study, the accumulation of GAβ occurred exclusively in the subcellular organelles that are involved in the endocytic pathway. Since Aβ generation and GM1 accumulation likely occur in early endosomes, it suggests that endosomes are intimately involved in the Aβ-associated pathology of AD [45]. In addition, Aβ aggregation in brain is accelerated through an increase in the level of GM1 in neuronal membranes [46, 47]. The effect occurs in a dose-dependent manner; in the presence of lower concentrations of GM1 (approximately 25 μM), Aβ1–40 forms aggregates much more slowly, indicating that an increase in the concentration of GM1 significantly facilitates the aggregation of Aβ. Further studies have indicated that both GM1 and GT1b promote the aggregation and cytotoxicity of Aβ1–40, and these gangliosides, especially GM1, catalyze the formation of neurotoxic fibrils [48]. Moreover, binding of Aβ to GM1 was dependent on cholesterol-induced clustering of GM1 in the host membranes. An increase in the cholesterol concentration in the neuronal membranes accelerates Aβ aggregation through the formation of an endogenous seed [49, 50], consistent with the notion that cholesterol is also a risk factor for AD development. These results further underscore the importance of control of cellular cholesterol and/or ganglioside contents in the pathogenesis of AD [50–52]. Lin et al. reported the role of GM1 and cholesterol on the Aβ-induced cytotoxicity in the plasma membrane [53]. Depletion of GM1 from the plasma membrane would be expected to block the Aβ-induced cytotoxicity. Decreasing the cholesterol level by around 30% could also attenuate the cytotoxicity of Aβ. These findings validate that cholesterol can stabilize the lateral pressure derived from formation of the GM1-Aβ complex on the membrane surface and that both GM1 and cholesterol are essential for Aβ accumulation. Zha et al. reported that GM1 regulated the expression of Aβ in a dose-dependent manner [54]. Exogenously added GM1 increased Aβ levels in mixed rat cortical neurons containing African green monkey epithelial kidney cells (COS7) and human neuroblastoma cells (SH-SY5Y) that were transfected with APP695 cDNA.
Yanagisawa and coinvestigators developed a novel monoclonal antibody, 4396C, raised against GAβ purified from an AD brain [55]. Using this antibody, the presence of GAβ was confirmed in the AD brain, in which GAβ is endogenously generated. The antibody reacted with GM1-bound forms of two Aβ isoforms, Aβ1–40 and Aβ1–42, but not the unbound forms of Aβ1–40 and GM1. Remarkably, using liposomes containing Aβ1–40 and GM1, this antibody completely blocks amyloid fibril formation in a dose-dependent manner, suggesting that it may act to inhibit the initiation of oligomerization-polymerization of Aβ in the brain and serve in the possible development of a novel therapeutic strategy to the GAβ-dependent amyloidogenesis [55, 56]. The age-dependent high-density GM1 clustering at the presynaptic neuritic terminals is a critical step for Aβ deposition in AD. In amyloid-positive synaptosomes prepared from AD brain, GM1 levels are significantly increased when Aβ deposition begins at presynaptic terminals. The antibody against GAβ, 4396C, suppressed Aβ assembly in the synaptosomes [57]. Moreover, peripheral administration of the Fab fragments of 4396C into transgenic mice expressing a mutant amyloid precursor protein gene, following the conjugation of the protein transduction domain of the Tat protein, markedly suppressed Aβ deposition in the brain [58].
Interestingly, the enhanced GAβ-dependent amyloidogenesis under the endocytic dysfunction was suppressed by pretreatment with a sphingomyelinase synthase inhibitor, suggesting that sphingomyelin is also one of the key molecules for GAβ generation, further implying that the interaction of Aβ with membrane lipids is critical in amyloid fibrillization in the brain [59]. In addition, the expression of apolipoprotein E4 may facilitate Aβ assembly in the brain through an increase in the GM1 content in neuronal membranes, which likely induces GAβ generation [43, 47, 60].
In recent years, evidence has been presented that “lipid rafts” are the preferential sites for the formation of the pathological forms of Aβ [61]. GAβ is generated in the membrane raft-like microdomains, comprised of cholesterol, sphingomyelin, and GM1 [62, 63], in which Aβ undergoes a conformational transition from an α-helix-rich structure to a β-sheet-rich structure or oligomerization with the increase in protein density on the membrane. GM1 induced amyloid fibrillization, especially under β-sheet-forming conditions, leading to the generation and seeding of GAβ. Thus, ganglioside binding with Aβ is the initial and common step in the development of a part of human misfolding-type amyloidoses, including AD [64]. The level of GAβ is increased, and its α-helix structure is converted into a β-sheet structure [65]. Thus, the formation of amyloid fibrils or oligomers is likely mediated by gangliosides in lipid rafts [66], and depletion of gangliosides or cholesterol significantly reduces the amount of amyloid deposits [48, 67].
5. Other Gangliosides May Be Involved In the Generation of GAβ
Other gangliosides have been shown to interact with Aβ, which may lead to Aβ accumulation in the brain. The assembly of wild-type and mutant forms of Arctic-, Dutch-, and Flemish-type of Aβs is accelerated in the presence of not only GM1, but also GM3 and GD3 gangliosides. Dutch and Italian-type Aβs require GM3 ganglioside for their assembly [47]. Arctic-type Aβ, in contrast to the wild-type and other variant forms, shows a markedly rapid and higher level of amyloid fibril formation in the presence of sodium dodecyl sulfate or GM1 ganglioside [68]. These results provide evidence that local gangliosides play a crucial role in the region-specific Aβ deposition in the brain [69, 70]. Gangliosides are located mostly on the cell surface and have been demonstrated to modulate neurotrophic activities. The localization of GD1a in dystrophic neurites suggests that such neurites accumulate GD1a as a membranous component. In addition, the accumulation of GD1a in SPs suggests that it may contribute to SP formation [26]. In a study for the interaction of Aβs with GM1 using rat adrenal medulla pheochromocytoma cells (PC12 cells), Wakabayashi et al. used CTXB for detection of GM1 [71]. However, the ganglioside that interacted with Aβs in PC12 cells may not be definitely GM1 because CTXB also strongly reacted also with fucosyl-GM1 and fucosyl-GD1b [72] and PC12 cells express fucosyl gangliosides including fucosyl-GM1 [73, 74] with little or no GM1 [75]. When PC12 cells were cultured in the presence of Aβ1–40 or Aβ1–42, Aβs accumulated in cells expressing fucosyl gangliosides [72]. Thus, the interaction of Aβ with gangliosides to effect amyloid assembly may not be limited to GM1; indeed, other gangliosides should also be involved in “seeding” [1, 72]. Molander-Melin et al. reported that the detergent-resistant membrane fractions from the frontal cortex of AD brains contained a significantly higher concentration of ganglioside GM1 and GM2 [76]. The increased proportions of GM1 and GM2 in lipid rafts at an early AD stage could accelerate the formation of Aβ plaques, which gradually causes membrane raft disruptions and thereby affects cellular functions that are dependent on the presence of such membrane domains.
6. Ganglioside Metabolism in AD Brains and AD Model Mouse Brains
Aβ changes in membrane fluidity could be induced by chemical interactions of the peptide with membrane components such as cholesterol, phospholipids, and gangliosides [77]. Since gangliosides have a strong affinity to Aβs [33], they could participate in conformational changes of Aβs in membrane fluidity. For this reason, ganglioside metabolism has been considered to be closely associated with the pathogenesis of AD [1, 20]. Several earlier studies showed significant changes of ganglioside patterns in AD brain. The concentration of gangliosides decreased in the majority of brain regions, such as the cerebral cortex, hippocampus, and basal telencephalon, especially in the frontal cortex and white matter [78–80]. Kracun et al. reported that the major brain ganglio-N-tetraosyl-series ganglioside species (GT1b, GD1b, GD1a, and GM1) significantly decreased in the frontal and temporal cortices and basal telencephalon of the brains of patients with AD compared with the respective areas in control brain [81, 82]. Brooksbank and McGovern [83] and Crino et al. [84] also reported changes of ganglioside composition in AD brains in which b-series gangliosides, such as GT1b and GD1b, showed a significant decrease, in contrast to a slight increase in GT1a, GD3, GM1, and GM2. These findings suggest that abnormal ganglioside metabolism coincides with the affected cortical areas of neurodegeneration that afflicts AD.
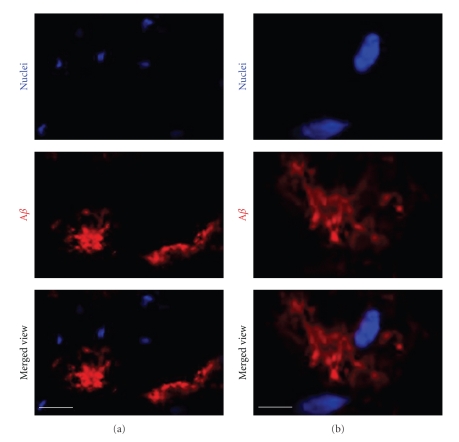
In contrast to these human studies, we found no significant differences in the lipid-bound NeuAc content in the brain slices containing hippocampal/cortical tissue prepared from AD model double transgenic (Tg) mice coexpressing mouse/human chimeric APP with the Swedish mutation and human presenilin-1 with a deletion of exon 9 and age-matched wild-type (WT) mice, even though Aβs were found to be accumulated in the brain (Figure 1) and serum of these Tg AD model mice [85]. In addition, there was no significant difference in the expression levels of major gangliosides (GM1, GD1a, GD1b, and GT1b) in the brains between double Tg and age-matched WT mice. This is consistent with the report by Sawamura et al. [86] who also did not detect notable changes in the major gangliosides in the brain of mutant presenilin-2 Tg mice, despite the remarkable increase in the level of Aβ1–42 and statistically significant lower levels of glycerophospholipids and sphingomyelin. In addition, Bernardo et al. also did not find significant differences in a- or b-series gangliosides between WT and double Tg mice expressing APP with the Swedish mutation and presenilin-1 with a deletion of exon 9 [87]. These studies as well as our recent data indicate no significant changes in the major brain ganglioside metabolism in AD model mice, despite the presence of massive accumulation of Aβ deposits in the brains of these animals. Barrier et al. reported an increase of GM2 and GM3 within the cortices of Tg mice expressing human APP751 with Swedish and London mutations and human presenilin-1 (M1461) [88].
Figure 1.
Immunohistochemical localization of Aβs in the cortex of double transgenic mice coexpressing mouse/human chimeric APP with the Swedish mutation and human presenilin-1 with deletion of exon 9. The coronal brain sections are 12 μm in thickness. Nuclei (blue) and Aβ (red) were stained with Hoechst 33258 and antihuman Aβ antibody, respectively. (a) low-magnification view; (b) high-magnification view. Bar = 20 μm in (a); 5 μm in (b). (Reproduced from [85] with permission).
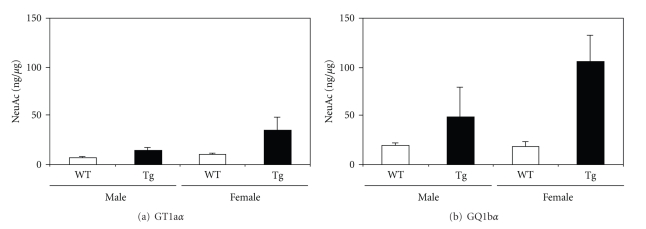
The most consistent and interesting finding of our recent study is the increased expression of cholinergic-specific antigen-1α (Chol-1α) antigens, GT1aα, and GQ1bα (see Scheme 1), especially GQ1bα, in the brain of double Tg mice as compared with those in WT mouse brains (Figure 2). The increase was especially significant in female double Tg mouse brains. No significant differences were found in the expression of GT1aα and GQ1bα between male and female WT mouse brains. These gangliosides are normally minor species in the brain and serve as markers of cholinergic neurons [89, 90]. The expression of Chol-1α antigens in rat brain regions such as the hippocampus is developmentally regulated, and their concentrations increase with aging [91]. Although the functional role of Chol-1α antigens in Tg mice brain has remained obscure, Ando et al. reported that the release of acetylcholine from synaptosomes was inhibited by anti-Chol-1α monoclonal antibody [92]. The memory and learning abilities of rats given anti-Chol-1α antibody were remarkably suppressed. On the contrary, the treatment of Chol-1α antigen induced choline uptake by synaptosomes. As a result of increased choline uptake, acetylcholine synthesis was enhanced by Chol-1α antigens. Chol-1α antigens are specifically expressed in the cholinergic neutrophil and may participate in cognitive functions such as memory and learning. Beneficial effects of Chol-1α antigens were shown to ameliorate decreased functions of synapses from aged brains, suggesting that Chol-1α antigens may play a pivotal role in cholinergic synaptic transmission and participates in cognitive function [93]. Interestingly, Chapman et al. reported the presence of serum antibody in patients with AD that specifically bind to cholinergic neurons [94]. The increasing antibody in the patient's sera may be attributed to the increase of Chol-1α antigens in AD brain. Cholinergic neuronal dysfunction of basal forebrain is observed in patients with AD, and has been linked to decreased neurogenesis in the hippocampus, a region involved in learning and memory [95]. They recently found an increasing number of newborn cells in the dentate gyrus of hippocampus in cholinergic-denervated mice compared to nonlesioned mice, suggesting neurogenesis can occur in Tg mice brain to generate new cells expressing Chol-1α antigens. It would be extremely interesting to enhance neurogenesis in hippocampus of patients and animal models of AD [96–99].
Scheme 1.
Figure 2.
The content of Chol-1α antigens, GT1aα (a) and GQ1bα (b), in AD model mouse brains [85]. GT1aα and GQ1bα extracted from brains of AD model double transgenic mice coexpressing mouse/human chimeric APP with the Swedish mutation and human presenilin-1 with a deletion of exon 9 (Tg) or age-matched wild-type mice (WT) were quantified by densitometric analysis of high-performance thin-layer chromatography immunostaining. n = 3–7. (Reproduced from [85] with permission).
In addition to the above, AD model animals with disrupted ganglioside biosynthesis have been reported to reveal relationships between ganglioside metabolism and aspects of AD. For example, Oikawa et al. crossbred Tg mice expressing human APP having Swedish and London mutations with GM2-synthase knockout mice [100]. The mutant mice expressing GM3 and GD3, but not GM1, GD1a, GD1b, and GT1b, showed a significant increase of Aβ accumulation in vascular tissues and formation of severe dysphonic-form amyloid angiopathy in the brain. In contrast, Bernardo et al. analyzed the AD model of Tg mice expressing APP with the Swedish mutation and presenilin-1 with a deletion of exon 9 that crossbred with mice deficient in GD3-synthase, which catalyzes the synthesis of b-series gangliosides [87]. In the triple mutant mice, b-series gangliosides, including GD3, were completely absent, but GM1 and GD1a were significantly increased. Interestingly, Aβ plaques and associated neuropathology were almost completely eliminated in the triple mutant mice, resulting in cognitive improvement [87]. These observations suggest that b-series gangliosides synthesized by GD3-synthase are one of the major causes of Aβ accumulation and AD. Thus, inhibition of GD3-synthase can be a novel therapeutic target to combat the cognitive deficits, amyloid plaque formation, and neurodegeneration seen in AD.
In this regard, Okada et al. reported that endogenously generated b-series gangliosides may be critical for the repair of damaged neural tissues in vivo [101]. They established a GD3-synthase gene knockout mouse model in which all b-series gangliosides were deleted. However, animals showed no morphological changes in the brains and apparent abnormal behavior. Moreover, no differences in Fas-mediated apoptotic reaction in lymphocytes compared with the wild type were found. The mutant mice, however, exhibited reduced regeneration of axotomized hypoglossal nerves compared with the wild type, suggesting that b-series gangliosides are more important in the repair of damaged nerves rather than in the differentiation of the nervous system.
7. Neurogenesis and Neural Stem Cells in AD Brain
The neurodegenerative process in AD is initially characterized by synaptic damage accompanied by neuronal loss. Neuronal loss leads to cerebral atrophy, which appears to be hallmarks of cognitive impairment in AD [102]. In addition to the alterations in synaptic plasticity and neuronal integrity in mature neuronal circuitries, the neurodegenerative process in AD has recently been shown to be accompanied by alterations in neurogenesis [103, 104]. The hippocampus is one of the regions in the adult brain where neurogenesis occurs throughout life [5]. Many studies have shown that adult neurogenesis is involved in learning and memory. This has led to the hypothesis that impairment in memory during aging and neurodegenerative diseases such as AD involves abnormal neurogenesis [105]. However, neurogenesis in AD and in animal models is not fully studied yet [106]. In AD brains, there is some controversy whether neurogenesis is increased [107] or decreased [107]. Boekhoom et al. reported an apparent increase of neurogenesis markers in AD brains, which may be related to glial and vasculature-associated changes [107]. A number of mouse models of AD displayed reduced neurogenesis [108–110] or enhanced neurogenesis [97]. Several attributes of adult hippocampal neurogenesis suggest that amyloid deposition may influence neurogenesis [111]. Zhang et al. reported that reductions in dentate gyrus neurogenesis in a murine model of amyloid deposition are linked to the deposition of amyloid [112].
The adult mammalian brain contains neural stem cells (NSCs), undifferentiated neural cells characterized by their high proliferative potential and the capacity for self-renewal with retention of multipotency to differentiate into neurons and glial cells, in the subgranular zones of dentate gyrus and the subventricular zone (SVZ) of the lateral ventricles [5]. The possibility that abnormalities in NSCs contribute to the pathogenesis of AD and the cognitive impairments in humans has been suggested [109, 110]. Several papers have described the phenomenon of neurogenesis in hippocampus, and it seems to be enhanced in AD brains. This phenomenon could potentially occur also in the brain of animal models of AD, which points to the possibility of developing strategies for promoting neurogenesis for AD therapy by using NSCs. A number of studies have indicated that Aβs can regulate the proliferation of NSCs and documented the bifunctional roles of Aβs on the cells in a dose-dependent manner. The low concentrations of Aβs have neurogenic effects in some studies [115–117] but cytotoxic effects in other studies [109, 118, 119]. Soluble oligomers of Aβ1–40 and Aβ1–42, but not Aβ40–1, a reversed amino acid sequence, induced neuronal apoptosis [120]. the aggregated form of Aβ1–42 stimulated neurogenesis [117, 121]. In this regard, Aβ1–40 (0.5 μmol/L) significantly reduced proliferation of endothelial progenitor cells by about 65% compared to control whereas Aβ40–1 (0.5 μmol/L), did not affect their proliferation [122]. Gong et al. reported that small, soluble oligomers of Aβ block the reversal long-term potentiation [123]. Controversially, a low micromolar concentration (1 μM) of oligomeric Aβ1–42 increased the proliferation [124] and neurogenesis of adult NSCs [117]. Small peptide, Aβ1–16, had no effect on neuronal proliferation of adult SVZ progenitors [119, 121] Several studies indicated that Aβ25–35 has toxic effects and may induce cell death or apoptosis [109, 110, 118, 125, 126]. In contrast, Li and Zuo reported inhibitory effects of aggregated form of Aβ25–35 (1 mg/mL, 3 μL) on neurogenesis in the SVZ and dentate gyrus after injection into the lateral ventricle of adult mouse [127]. This result indicates that Aβ25–35 could impair neurogenesis in the hippocampus of adult mouse brain.
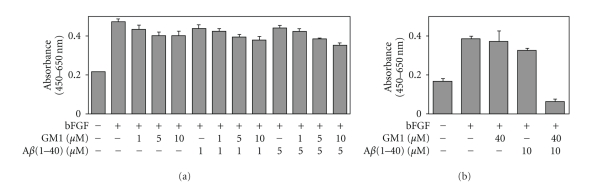
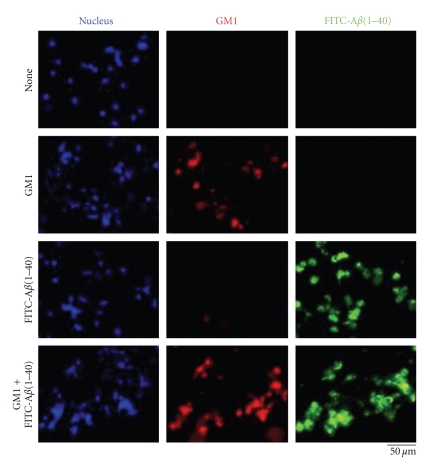
In neuronal cultures prepared from rat hippocampi (embryonic day 18 to 19), it was reported that 25 μM of Aβ25–35 enhanced the metabolism of lipids such as phospholipids (+52%) and gangliosides (+193%), but not cholesterol [128]. In addition, exposure of rat cultured cortical neurons to Aβ25–35 induced a substantial increase of the intracellular GD3 levels [129]. These reports suggest that Aβ can modulate ganglioside metabolism in NSCs. It has been reported that in NSCs, GSLs, including gangliosides, are involved in cellular proliferation via modulation of the Ras-mitogen-activated protein kinase pathway [114]. These findings prompt us to propose that a combination of Aβ and GM1 induces NSC proliferation. Recently, we evaluated the effects of GM1 and Aβ1–40 on mouse neuroepithelial cells (NECs) that are known to be abundant in NSCs [130]. In NECs cultured in the presence of lower concentrations of GM1 (1, 5 or 10 μM) and/or Aβ1–40 (1 or 5 μM), there was no drastic change of the cell number (Figure 3(a)). However, in NECs cultured in the presence of both 40 μM of GM1 and 10 μM of Aβ1–40, a significant reduction of the cell number was detected (Figure 3(b)). These exogenously added GM1 and Aβ1–40 were efficiently incorporated into NECs (Figure 4). In NECs simultaneously treated with GM1 and Aβ1–40, the Ras-mitogen-activated protein kinase pathway important for proliferation was intact, but caspase-3, an executioner for cell death, was activated. Most NECs treated with GM1 and Aβ1–40 were positive for terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling, an indicator of cell death accompanied with DNA fragmentation. These results indicate that Aβ1–40 and GM1 cooperatively exert a cytotoxic effect on NECs, likely via incorporation into NEC membranes where the formation of a complex results in activation of cell death signaling.
Figure 3.
Effects of low (a) and high (b) concentrations of GM1 and Aβ1–40 on NECs. Basic fibroblast growth factor (bFGF; 0 or 5 ng/mL) was added as a mitogen of NECs. The number of NECs cultured in the presence of bFGF for 4 days was estimated by WST-8 assay with (a) GM1 (0, 1, 5 or 10 μM) and Aβ1–40 (0, 1 or 5 μM); with (b) GM1 (0 or 40 μM) and Aβ1–40 (0 or 10 μM). The spectrophotometric absorbance (Abs.) measured at the wavelength of 450 nm (reference, 650 nm) by this assay is highly correlated with the number of living NECs [113]. (Reproduced from [114] with permission).
Figure 4.
Incorporation of exogenously added GM1 and Aβ1–40 into NECs. NECs were cultured in the presence of GM1 (0 or 40 μM) and/or fluorescein isothiocyanate-conjugated Aβ1–40 (FITC-Aβ1–40; 0 or 10 μM) for 2 days, and then stained with biotin-conjugated CTXB, a probe to detect GM1, and rhodamine-conjugated streptavidin. Nuclei were stained with Hoechst 33258. (Reproduced from [114] with permission).
Several reports have indicated that gangliosides added exogenously in the culture medium have bifunctional effects on neural cell proliferation. Gangliosides added exogenously at the concentration of micromolar levels were found to inhibit neuritogenesis in human neuroblastoma cells, SH-SY5Y [131]. However, under physiological conditions, GM1 enhanced nerve growth factor-induced neurite outgrowth, neurite complexity, and neuronal cell survival following nerve growth factor withdrawal using fetal-chick dorsal root ganglia [132] and induced neurite sprouting in culture neurons [133]. GQ1b induced phosphorylation of cell surface proteins in a human neuroblastoma cell line, GOTO [134]. The effects of gangliosides exogenously added remained obscure and seem to vary from one cell line to another and the culture conditions [7]. Therefore, further studies are needed to clarify the relationship between GM1 and Aβ in the proliferation of NECs. In addition, evaluation of the effects of exogenous GM1 on neurogenesis and pathogenesis of AD in pathological conditions, for instance, using AD model mice [135] will be an interesting and fruitful subject for future studies. Many studies showed that NSCs improved neuronal survival in cultured postmortem brain tissue from aged and AD patients [136]. Further studies to understand the roles of GM1 and Aβs on NSCs in AD should contribute to the development of new regenerative therapies of this disease.
8. Conclusions
There is increasing consensus that AD is characterized in the brain by aggregated amyloid deposits in SPs. The aggregation of Aβ plays a pivotal role in the pathogenesis of AD that is intimately linked to neuronal toxicity and inhibition of hippocampal long-term potentiation. At present, there is no cure for AD, although some drugs inhibiting acetylcholinesterase have proved to be current treatment to palliate both cognitive and behavioral symptoms within a limited time. Researchers are looking for new treatments to alter the course of the disease and improve the quality of life for patients with AD and related dementia. Aβ is currently clarified to interact with gangliosides with high affinities. In fact, a complex of GM1 (and possibly other gangliosides) with Aβ, termed GAβ, was found to accumulate in the AD brains. An antibody against GAβ was proved to block amyloid fibril formation, suggesting that it can contribute to the development of a novel therapeutic strategy to AD. In this regard, drugs such as nordihydroguaiaretic acid, rifampicin, and tannic acid are found to be potent inhibitors of the binding of GM1 and Aβ, resulting in inhibition of membrane-mediated formation of Aβ fibrils in vitro. These drugs are useful agents for AD therapy [137]. On the other hand, in AD model mice lacking GD3-synthase, Aβ plaques and associated neuropathology are almost completely eliminated, resulting in cognitive improvement. GD3-synthase and its downstream metabolic products, the b-series gangliosides, can be a novel therapeutic target for repressing neurodegeneration and cognitive deficits that afflict AD patients. Another promising therapeutic strategy for AD is cell replacement therapy using NSCs. Although neurogenesis in AD brains is still controversial, transplantation of NSCs into the damaged brain regions may be beneficial for neural regeneration in AD. For therapeutic use of NSCs in AD, however, it should be essential to fully clarify the effects of Aβs and gangliosides on NSC fate regulation. Future therapies for treating AD will include agents that modulate GSL metabolism, either as primary therapeutics or in combination with other drugs.
The nomenclature for gangliosides is based on the system of Svennerholm [138].
Acknowledgments
This study was supported by USPHS Grants (nos. NS11853, NS26994, and AG027199) to R. K. Yu. The authors are grateful to Dr. Susumu Ando (Tokyo Metropolitan Institute of Gerontology, Japan) for supplying the Chol-1α antigens and the monoclonal antibodies and Dr. Makoto Yanagisawa, Medical College of Georgia, GA for helpful discussions in preparation of this paper.
Abbreviations
- Aβ:
Amyloid β-protein
- AD:
Alzheimer's disease
- APP:
Amyloid precursor protein
- Chol-1α:
Cholinergic-specific antigen-1α
- CTXB:
Cholera toxin B-subunit
- Gaβ:
Ganglioside-bound Aβ or a complex of GM1 and Aβ
- GSL:
Glycosphingolipid
- NEC:
Neuroepithelial cell
- NSC:
Neural stem cell
- SVZ:
Subventricular zone
- NeuAc:
Sialic acid or N-acetyl neuraminic acid
- SP:
Senile plaque
- Tg:
Transgenic
- WT:
Wild type.
References
- 1.Ariga T, McDonald MP, Yu RK. Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease—a review. Journal of Lipid Research. 2008;49(6):1157–1175. doi: 10.1194/jlr.R800007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariga T, Miyatake T, Yu RK. Role of proteoglycans and glycosaminoglycans in the pathogenesis of Alzheimer's disease and related disorders: amyloidogenesis and therapeutic strategies—a review. Journal of Neuroscience Research. 2010;88(11):2303–2315. doi: 10.1002/jnr.22393. [DOI] [PubMed] [Google Scholar]
- 3.Yu RK, Nakatani Y, Yanagisawa M. The role of glycosphingolipid metabolism in the developing brain. Journal of Lipid Research. 2009;50:S440–S445. doi: 10.1194/jlr.R800028-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. Journal of Neurochemistry. 2007;103(6):2327–2341. doi: 10.1111/j.1471-4159.2007.04910.x. [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa M, Yu RK. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 2007;17(7):57R–74R. doi: 10.1093/glycob/cwm018. [DOI] [PubMed] [Google Scholar]
- 6.Yu RK. Development regulation of ganglioside metabolism. Progress in Brain Research. 1994;101:31–44. doi: 10.1016/s0079-6123(08)61938-x. [DOI] [PubMed] [Google Scholar]
- 7.Ledeen RW, Wu G, Lu ZH, Kozireskichuback D, Fang Y. The role of GM1 and other gangliosides in neuronal differentiation: overview and new findings. Annals of the New York Academy of Sciences. 1998;845:161–175. doi: 10.1111/j.1749-6632.1998.tb09669.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Huang Y, Li X, Cui X, Zuo P, Li J. GM1 ganglioside prevented the decline of hippocampal neurogenesis associated with D-galactose. NeuroReport. 2005;16(12):1297–1301. doi: 10.1097/01.wnr.0000174405.24763.bc. [DOI] [PubMed] [Google Scholar]
- 9.Saito M, Mao RF, Wang R, Vadasz C, Saito M. Effects of gangliosides on ethanol-induced neurodegeneration in the developing mouse brain. Alcoholism: Clinical and Experimental Research. 2007;31(4):665–674. doi: 10.1111/j.1530-0277.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 10.Svennerholm L, Bråne G, Karlsson I, Lekman A, Ramström I, Wikkelsö C. Alzheimer disease—effect of continuous intracerebroventricular treatment with GM1 ganglioside and a systematic activation programme. Dementia and Geriatric Cognitive Disorders. 2002;14(3):128–136. doi: 10.1159/000063604. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi K, Kitaguchi N, Nakai S, et al. Novel therapeutic approach for Alzheimer’s disease by removing amyloid β protein from the brain with an extracorporeal removal system. Journal of Artificial Organs. 2010;13(1):31–37. doi: 10.1007/s10047-010-0482-3. [DOI] [PubMed] [Google Scholar]
- 12.Matsuoka Y, Saito M, LaFrancois J, et al. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to β-amyloid. Journal of Neuroscience. 2003;23(1):29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider JS, Roeltgen DP, Mancall EL, Chapas-Crilly J, Rothblat DS, Tatarian GT. Parkinson’s disease improved function with GM1 ganglioside treatment in a randomized placebo-controlled study. Neurology. 1998;50(6):1630–1636. doi: 10.1212/wnl.50.6.1630. [DOI] [PubMed] [Google Scholar]
- 14.Lenzi GL, Grigoletto F, Gent M, et al. Early treatment of stroke with monosialoganglioside GM-1: efficacy and safety results of the early stroke trial. Stroke. 1994;25(8):1552–1558. doi: 10.1161/01.str.25.8.1552. [DOI] [PubMed] [Google Scholar]
- 15.Hughes RA, Cornblath DR. Guillain-Barré syndrome. The Lancet. 2005;366(9497):1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 16.Verdier Y, Penke B. Binding sites of amyloid β-peptide in cell plasma membrane and implications for Alzheimer’s disease. Current Protein and Peptide Science. 2004;5(1):19–31. doi: 10.2174/1389203043486937. [DOI] [PubMed] [Google Scholar]
- 17.Wisniewski T, Ghiso J, Frangione B. Biology of Aβ amyloid in Alzheimer’s disease. Neurobiology of Disease. 1997;4(5):313–328. doi: 10.1006/nbdi.1997.0147. [DOI] [PubMed] [Google Scholar]
- 18.Haughey NJ. Sphingolipids in neurodegeneration. NeuroMolecular Medicine. 2010;12(4):301–305. doi: 10.1007/s12017-010-8135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haughey NJ, Bandaru VVR, Bae M, Mattson MP. Roles for dysfunctional sphingolipid metabolism in Alzheimer’s disease neuropathogenesis. Biochimica et Biophysica Acta. 2010;1801(8):878–886. doi: 10.1016/j.bbalip.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mutoh T, Hirabayashi Y, Mihara T, et al. Role of glycosphingolipids and therapeutic perspectives on Alzheimer’s disease. CNS and Neurological Disorders-Drug Targets. 2006;5(4):375–380. doi: 10.2174/187152706777950710. [DOI] [PubMed] [Google Scholar]
- 21.Gorbenko GP, Kinnunen PKJ. The role of lipid-protein interactions in amyloid-type protein fibril formation. Chemistry and Physics of Lipids. 2006;141(1-2):72–82. doi: 10.1016/j.chemphyslip.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Grimm MOW, Tschäpe JA, Grimm HS, Zinser EG, Hartmann T. Altered membrane fluidity and lipid raft composition in presenilin-deficient cells. Acta Neurologica Scandinavica. 2006;114(185):27–32. doi: 10.1111/j.1600-0404.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 23.Wong PT, Schauerte JA, Wisser KC, et al. Amyloid-β membrane binding and permeabilization are distinct processes influenced separately by membrane charge and fluidity. Journal of Molecular Biology. 2009;386(1):81–96. doi: 10.1016/j.jmb.2008.11.060. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto N, Suzuki Y, Makino Y, Haga C, Kosaka K, Iizuka R. Cell membrane changes in brains manifesting senile plaques: an immunohistochemical study of GM membranous ganglioside. Brain Research. 1990;522(1):152–156. doi: 10.1016/0006-8993(90)91592-5. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H, Hirokawa K, Ando S, Obata K. Immunohistological study on brains of Alzheimer’s disease using antibodies to fetal antigens, C-series gangliosides and microtubule-associated protein 5. Acta Neuropathologica. 1991;81(6):626–631. doi: 10.1007/BF00296372. [DOI] [PubMed] [Google Scholar]
- 26.Nishinaka T, Iwata D, Shimada S, Kosaka K, Suzuki Y. Anti-ganglioside GD1a monoclonal antibody recognizes senile plaques in the brains of patients with Alzheimer-type dementia. Neuroscience Research. 1993;17(2):171–176. doi: 10.1016/0168-0102(93)90093-6. [DOI] [PubMed] [Google Scholar]
- 27.Terzi E, Holzemann G, Seelig J. Self-association of β-amyloid peptide (1–40) in solution and binding to lipid membranes. Journal of Molecular Biology. 1995;252(5):633–642. doi: 10.1006/jmbi.1995.0525. [DOI] [PubMed] [Google Scholar]
- 28.Choo-Smith LP, Garzon-Rodriguez W, Glabe CG, Surewicz WK. Acceleration of amyloid fibril formation by specific binding of Aβ-(1–40) peptide to ganglioside-containing membrane vesicles. Journal of Biological Chemistry. 1997;272(37):22987–22990. doi: 10.1074/jbc.272.37.22987. [DOI] [PubMed] [Google Scholar]
- 29.Matsuzaki K, Horikiri C. Interactions of amyloid β-peptide (1–40) with ganglioside-containing membranes. Biochemistry. 1999;38(13):4137–4142. doi: 10.1021/bi982345o. [DOI] [PubMed] [Google Scholar]
- 30.McLaurin J, Chakrabartty A. Membrane disruption by Alzheimer β-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. Journal of Biological Chemistry. 1996;271(43):26482–26489. doi: 10.1074/jbc.271.43.26482. [DOI] [PubMed] [Google Scholar]
- 31.McLaurin J, Franklin T, Fraser PE, Chakrabartty A. Structural transitions associated with the interaction of Alzheimer β-amyloid peptides with gangliosides. Journal of Biological Chemistry. 1998;273(8):4506–4515. doi: 10.1074/jbc.273.8.4506. [DOI] [PubMed] [Google Scholar]
- 32.Choo-Smith LP, Surewicz WK. The interaction between Alzheimer amyloid β(1–40) peptide and ganglioside G(M1)-containing membranes. FEBS Letters. 1997;402(2-3):95–98. doi: 10.1016/s0014-5793(96)01504-9. [DOI] [PubMed] [Google Scholar]
- 33.Ariga T, Kobayashi K, Hasegawa A, Kiso M, Ishida H, Miyatake T. Characterization of high-affinity binding between gangliosides and amyloid β-protein. Archives of Biochemistry and Biophysics. 2001;388(2):225–230. doi: 10.1006/abbi.2001.2304. [DOI] [PubMed] [Google Scholar]
- 34.Mandal PK, Pettegrew JW. Alzheimer’s disease: NMR studies of asialo (GM1) and Trisialo (GT1b) ganglioside interactions with Aβ(1–40) peptide in a membrane mimic environment. Neurochemical Research. 2004;29(2):447–453. doi: 10.1023/b:nere.0000013750.80925.25. [DOI] [PubMed] [Google Scholar]
- 35.Nakazawa Y, Suzuki Y, Williamson MP, Saitô H, Asakura T. The interaction of amyloid Aβ(1–40) with lipid bilayers and ganglioside as studied by P solid-state NMR. Chemistry and Physics of Lipids. 2009;158(1):54–60. doi: 10.1016/j.chemphyslip.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Atwood CS, Moir RD, Huang X, et al. Dramatic aggregation of alzheimer by Cu(II) is induced by conditions representing physiological acidosis. Journal of Biological Chemistry. 1998;273(21):12817–12826. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- 37.Chen YR, Huang HB, Chyan CL, Shiao MS, Lin TH, Chen YC. The effect of Aβ conformation on the metal affinity and aggregation mechanism studied by circular dichroism spectroscopy. Journal of Biochemistry. 2006;139(4):733–740. doi: 10.1093/jb/mvj083. [DOI] [PubMed] [Google Scholar]
- 38.Williamson MP, Suzuki Y, Bourne NT, Asakura T. Binding of amyloid β-peptide to ganglioside micelles is dependent on histidine-13. Biochemical Journal. 2006;397(3):483–490. doi: 10.1042/BJ20060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Ding J, Tian W, et al. Ganglioside GM1 binding the N-terminus of amyloid precursor protein. Neurobiology of Aging. 2009;30(8):1245–1253. doi: 10.1016/j.neurobiolaging.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Utsumi M, Yamaguchi Y, Sasakawa H, Yamamoto N, Yanagisawa K, Kato K. Up-and-down topological mode of amyloid β-peptide lying on hydrophilic/hydrophobic interface of ganglioside clusters. Glycoconjugate Journal. 2009;26(8):999–1006. doi: 10.1007/s10719-008-9216-7. [DOI] [PubMed] [Google Scholar]
- 41.Yagi-Utsumi M, Kameda T, Yamaguchi Y, Kato K. NMR characterization of the interactions between lyso-GM1 aqueous micelles and amyloid β. FEBS Letters. 2010;584(4):831–836. doi: 10.1016/j.febslet.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Yanagisawa K, Odaka A, Suzuki N, Ihara Y. GM1 ganglioside-bound amyloid β-protein (AB): a possible form of preamyloid in Alzheimer’s disease. Nature Medicine. 1995;1(10):1062–1066. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- 43.Yanagisawa K. Role of gangliosides in Alzheimer’s disease. Biochimica et Biophysica Acta. 2007;1768(8):1943–1951. doi: 10.1016/j.bbamem.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 44.Yanagisawa K, Ihara Y. GM1 ganglioside-bound amyloid β-protein in Alzheimer’s disease brain. Neurobiology of Aging. 1998;19(1):S65–S67. doi: 10.1016/s0197-4580(98)00032-3. [DOI] [PubMed] [Google Scholar]
- 45.Kimura N, Yanagisawa K. Endosomal accumulation of GM1 ganglioside-bound amyloid β-protein in neurons of aged monkey brains. NeuroReport. 2007;18(16):1669–1673. doi: 10.1097/WNR.0b013e3282f0d2ab. [DOI] [PubMed] [Google Scholar]
- 46.Kurganov B, Doh M, Arispe N. Aggregation of liposomes induced by the toxic peptides Alzheimer’s Aβs, human amylin and prion (106–126): facilitation by membrane-bound GM1 ganglioside. Peptides. 2004;25(2):217–232. doi: 10.1016/j.peptides.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto N, Hirabayashi Y, Amari M, et al. Assembly of hereditary amyloid β-protein variants in the presence of favorable gangliosides. FEBS Letters. 2005;579(10):2185–2190. doi: 10.1016/j.febslet.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 48.Okada T, Ikeda K, Wakabayashi M, Ogawa M, Matsuzaki K. Formation of toxic Aβ(1–40) fibrils on GM1 ganglioside-containing membranes mimicking lipid rafts: polymorphisms in Aβ(1–40) fibrils. Journal of Molecular Biology. 2008;382(4):1066–1074. doi: 10.1016/j.jmb.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 49.Yanagisawa K. Cholesterol and Aβ aggregation. Pharmacopsychiatry. 2003;36(2):S127–S129. doi: 10.1055/s-2003-43056. [DOI] [PubMed] [Google Scholar]
- 50.Yanagisawa K, Matsuzaki K. Cholesterol-dependent aggregation of amyloid β-protein. Annals of the New York Academy of Sciences. 2002;977:384–386. doi: 10.1111/j.1749-6632.2002.tb04841.x. [DOI] [PubMed] [Google Scholar]
- 51.Wakabayashi M, Matsuzaki K. Formation of amyloids by Aβ-(1-42) on NGF-differentiated PC12 cells: roles of gangliosides and cholesterol. Journal of Molecular Biology. 2007;371(4):924–933. doi: 10.1016/j.jmb.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Wang SSS, Rymer DL, Good TA. Reduction in cholesterol and sialic acid content protects cells from the toxic effects of β-amyloid peptides. Journal of Biological Chemistry. 2001;276(45):42027–42034. doi: 10.1074/jbc.M102834200. [DOI] [PubMed] [Google Scholar]
- 53.Lin MS, Chen LY, Wang SSS, Chang Y, Chen WY. Examining the levels of ganglioside and cholesterol in cell membrane on attenuation the cytotoxicity of beta-amyloid peptide. Colloids and Surfaces B. 2008;65(2):172–177. doi: 10.1016/j.colsurfb.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Zha Q, Ruan Y, Hartmann T, Beyreuther K, Zhang D. GM1 ganglioside regulates the proteolysis of amyloid precursor protein. Molecular Psychiatry. 2004;9(10):946–952. doi: 10.1038/sj.mp.4001509. [DOI] [PubMed] [Google Scholar]
- 55.Hayashi H, Kimura N, Yamaguchi H, et al. A seed for Alzheimer amyloid in the brain. Journal of Neuroscience. 2004;24(20):4894–4902. doi: 10.1523/JNEUROSCI.0861-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuyama K, Yanagisawa K. Late endocytic dysfunction as a putative cause of amyloid fibril formation in Alzheimer’s disease. Journal of Neurochemistry. 2009;109(5):1250–1260. doi: 10.1111/j.1471-4159.2009.06046.x. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto N, Matsubara T, Sato T, Yanagisawa K. Age-dependent high-density clustering of GM1 ganglioside at presynaptic neuritic terminals promotes amyloid β-protein fibrillogenesis. Biochimica et Biophysica Acta. 2008;1778(12):2717–2726. doi: 10.1016/j.bbamem.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto N, Yokoseki T, Shibata M, Yamaguchi H, Yanagisawa K. Suppression of Aβ deposition in brain by peripheral administration of Fab fragments of anti-seed antibody. Biochemical and Biophysical Research Communications. 2005;335(1):45–47. doi: 10.1016/j.bbrc.2005.06.208. [DOI] [PubMed] [Google Scholar]
- 59.Yuyama K, Yanagisawa K. Sphingomyelin accumulation provides a favorable milieu for GM1 ganglioside-induced assembly of amyloid β-protein. Neuroscience Letters. 2010;481(3):168–172. doi: 10.1016/j.neulet.2010.06.080. [DOI] [PubMed] [Google Scholar]
- 60.Yanagisawa K. GM1 ganglioside and the seeding of amyloid in Alzheimer’s disease: endogenous seed for Alzheimer amyloid. Neuroscientist. 2005;11(3):250–260. doi: 10.1177/1073858405275177. [DOI] [PubMed] [Google Scholar]
- 61.Fantini J, Garmy N, Mahfoud R, Yahi N. Lipid rafts: structure, function and role in HIV, Alzheimer’s and prion diseases. Expert Reviews in Molecular Medicine. 2002;4(27):1–22. doi: 10.1017/S1462399402005392. [DOI] [PubMed] [Google Scholar]
- 62.Kakio A, Nishimoto SI, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Interactions of amyloid β-protein with various gangliosides in raft-like membranes: importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry. 2002;41(23):7385–7390. doi: 10.1021/bi0255874. [DOI] [PubMed] [Google Scholar]
- 63.Mao Y, Shang Z, Imai Y, et al. Surface-induced phase separation of a sphingomyelin/cholesterol/ganglioside GM1-planar bilayer on mica surfaces and microdomain molecular conformation that accelerates Aβ oligomerization. Biochimica et Biophysica Acta. 2010;1798(6):1090–1099. doi: 10.1016/j.bbamem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Matsuzaki K, Kato K, Yanagisawa K. Aβ polymerization through interaction with membrane gangliosides. Biochimica et Biophysica Acta. 2010;1801(8):868–877. doi: 10.1016/j.bbalip.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Matsuzaki K. Interactions between amyloid beta–protein and gangliosides. Tanpakushitsu Kakusan Koso. 2002;47(4):351–356. [PubMed] [Google Scholar]
- 66.Kim SI, Yi JS, Ko YG. Amyloid β oligomerization is induced by brain lipid rafts. Journal of Cellular Biochemistry. 2006;99(3):878–889. doi: 10.1002/jcb.20978. [DOI] [PubMed] [Google Scholar]
- 67.Wakabayashi M, Matsuzaki K. Ganglioside-induced amyloid formation by human islet amyloid polypeptide in lipid rafts. FEBS Letters. 2009;583(17):2854–2858. doi: 10.1016/j.febslet.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto N, Matsubara E, Maeda S, et al. A ganglioside-induced toxic soluble Aβ assembly: its enhanced formation from Aβ bearing the arctic mutation. Journal of Biological Chemistry. 2007;282(4):2646–2655. doi: 10.1074/jbc.M606202200. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto N, Hirabayashi Y, Amari M, et al. Assembly of hereditary amyloid β-protein variants in the presence of favorable gangliosides. FEBS Letters. 2005;579(10):2185–2190. doi: 10.1016/j.febslet.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto N, Van Nostrand WE, Yanagisawa K. Further evidence of local ganglioside-dependent amyloid β-protein assembly in brain. NeuroReport. 2006;17(16):1735–1737. doi: 10.1097/01.wnr.0000239958.53072.14. [DOI] [PubMed] [Google Scholar]
- 71.Wakabayashi M, Okada T, Kozutsumi Y, Matsuzaki K. GM1 ganglioside-mediated accumulation of amyloid β-protein on cell membranes. Biochemical and Biophysical Research Communications. 2005;328(4):1019–1023. doi: 10.1016/j.bbrc.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 72.Yanagisawa M, Ariga T, Yu RK. Fucosyl-GM1 expression and amyloid-β protein accumulation in PC12 cells. Journal of Neuroscience Research. 2006;84(6):1343–1349. doi: 10.1002/jnr.21031. [DOI] [PubMed] [Google Scholar]
- 73.Ariga T, Kobayashi K, Kuroda Y, et al. Characterization of tumor-associated fucogangliosides from PC 12 pheochromocytoma cells. Journal of Biological Chemistry. 1987;262(29):14146–14153. [PubMed] [Google Scholar]
- 74.Yamazaki Y, Horibata Y, Magatsuka Y, Hirabayashi Y, Hashikawa T. Fucoganglioside α-fucosyl(α-galactosyl)-GM1: a novel member of lipid membrane microdomain components involved in PC12 cell neuritogenesis. Biochemical Journal. 2007;407(1):31–40. doi: 10.1042/BJ20070090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ariga T, Macala LJ, Saito M, et al. Lipid composition of PC12 pheochromocytoma cells: characterization of globoside as a major neutral glycolipid. Biochemistry. 1988;27(1):52–58. doi: 10.1021/bi00401a010. [DOI] [PubMed] [Google Scholar]
- 76.Molander-Melin M, Blennow K, Bogdanovic N, Dellheden B, Månsson JE, Fredman P. Structural membrane alterations in Alzheimer brains found to be associated with regional disease development; increased density of gangliosides GM1 and GM2 and loss of cholesterol in detergent-resistant membrane domains. Journal of Neurochemistry. 2005;92(1):171–182. doi: 10.1111/j.1471-4159.2004.02849.x. [DOI] [PubMed] [Google Scholar]
- 77.Eckert GP, Wood WG, Müller WE. Lipid membranes and β-amyloid: a harmful connection. Current Protein and Peptide Science. 2010;11(5):319–325. doi: 10.2174/138920310791330668. [DOI] [PubMed] [Google Scholar]
- 78.Kalanj S, Kracun I, Rosner H, Cosović C. Regional distribution of brain gangliosides in Alzheimer’s disease. Neurologia Croatica. 1991;40(4):269–281. [PubMed] [Google Scholar]
- 79.Kalanj-Bognar S. Ganglioside catabolism is altered in fibroblasts and leukocytes from Alzheimer’s disease patients. Neurobiology of Aging. 2006;27(9):1354–1356. doi: 10.1016/j.neurobiolaging.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 80.Svennerholm L, Gottfries CG. Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II) Journal of Neurochemistry. 1994;62(3):1039–1047. doi: 10.1046/j.1471-4159.1994.62031039.x. [DOI] [PubMed] [Google Scholar]
- 81.Kracun I, Kalanj S, Cosovic C, Talan-Hranilovic J. Brain gangliosides in Alzheimer’s disease. Journal für Hirnforschung. 1990;31(6):789–793. [PubMed] [Google Scholar]
- 82.Kracun I, Kalanj S, Talan-Hranilovic J, Cosovic C. Cortical distribution of gangliosides in Alzheimer’s disease. Neurochemistry International. 1992;20(3):433–438. doi: 10.1016/0197-0186(92)90058-y. [DOI] [PubMed] [Google Scholar]
- 83.Brooksbank BWL, McGovern J. Gangliosides in the brain in adult Down’s syndrome and Alzheimer’s disease. Molecular and Chemical Neuropathology. 1989;11(3):143–156. doi: 10.1007/BF03160048. [DOI] [PubMed] [Google Scholar]
- 84.Crino PB, Ullman MD, Vogt BA, Bird ED, Volicer L. Brain gangliosides in dementia of the Alzheimer type. Archives of Neurology. 1989;46(4):398–401. doi: 10.1001/archneur.1989.00520400054019. [DOI] [PubMed] [Google Scholar]
- 85.Ariga T, Yanagisawa M, Wakade C, et al. Ganglioside metabolism in a transgenic mouse model of alzheimer's disease: expression of Chol-1α antigens in the brain. ASN Neuro. 2010;2(4):233–241. doi: 10.1042/AN20100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sawamura N, Morishima-Kawashima M, Waki H, et al. Mutant presenilin 2 transgenic mice: a large increase in the levels of Aβ342 is presumably associated with the low density membrane domain that contains decreased levels of glycerophospholipids and sphingomyelin. Journal of Biological Chemistry. 2000;275(36):27901–27908. doi: 10.1074/jbc.M004308200. [DOI] [PubMed] [Google Scholar]
- 87.Bernardo A, Harrison FE, McCord M, et al. Elimination of GD3 synthase improves memory and reduces amyloid-β plaque load in transgenic mice. Neurobiology of Aging. 2009;30(11):1777–1791. doi: 10.1016/j.neurobiolaging.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 88.Barrier L, Ingrand S, Damjanac M, Rioux Bilan A, Hugon J, Page G. Genotype-related changes of ganglioside composition in brain regions of transgenic mouse models of Alzheimer’s disease. Neurobiology of Aging. 2007;28(12):1863–1872. doi: 10.1016/j.neurobiolaging.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 89.Ando S, Hirabayashi Y, Kon K, Inagaki F, Tate S, Whittaker VP. A trisialoganglioside containing a sialyl α2-6 N-acetylgalactosamine residue is a cholinergic-specific antigen, Chol-1α. Journal of Biochemistry. 1992;111(3):287–290. doi: 10.1093/oxfordjournals.jbchem.a123751. [DOI] [PubMed] [Google Scholar]
- 90.Hirabayashi Y, Nakao T, Irie F, Whittaker VP, Kon K, Ando S. Structural characterization of a novel cholinergic neuron-specific ganglioside in bovine brain. Journal of Biological Chemistry. 1992;267(18):12973–12978. [PubMed] [Google Scholar]
- 91.Derrington EA, Borroni E. The developmental expression of the cholinergic-specific antigen Chol-1 in the central and peripheral nervous system of the rat. Developmental Brain Research. 1990;52(1-2):131–140. doi: 10.1016/0165-3806(90)90228-q. [DOI] [PubMed] [Google Scholar]
- 92.Ando S, Tanaka Y, Kobayashi S, et al. Synaptic function of cholinergic-specific Chol-1α ganglioside. Neurochemical Research. 2004;29(4):857–867. doi: 10.1023/b:nere.0000018860.75734.a7. [DOI] [PubMed] [Google Scholar]
- 93.Ando S, Tanaka Y, Waki H, Kon K, Iwamoto M, Fukui F. Gangliosides and sialylcholesterol as modulators of synaptic functions. Annals of the New York Academy of Sciences. 1998;845:232–239. doi: 10.1111/j.1749-6632.1998.tb09676.x. [DOI] [PubMed] [Google Scholar]
- 94.Chapman J, Bachar O, Korczyn AD, Wertman E, Michaelson DM. Antibodies to cholinergic neurons in Alzheimer’s disease. Journal of Neurochemistry. 1988;51(2):479–485. doi: 10.1111/j.1471-4159.1988.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 95.Ho NF, Han S, Dawe GS. Effect of voluntary running on adult hippocampal neurogenesis in cholinergic lesioned mice. BMC Neuroscience. 2009;10, article 57 doi: 10.1186/1471-2202-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Greenberg DA, Jin K. Neurodegeneration and neurogenesis: focus on Alzheimer’s disease. Current Alzheimer Research. 2006;3(1):25–28. doi: 10.2174/156720506775697106. [DOI] [PubMed] [Google Scholar]
- 97.Jin K, Galvan V, Xie L, et al. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APP ) mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lopez-Toledano MA, Faghihi MA, Patel NS, Wahlestedt C. Adult neurogenesis: a potential tool for early diagnosis in alzheimer's disease? Journal of Alzheimer's Disease. 2010;20(2):395–408. doi: 10.3233/JAD-2010-1388. [DOI] [PubMed] [Google Scholar]
- 99.López-Toledano MA, Shelanski ML. Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind) Journal of Alzheimer’s Disease. 2007;12(3):229–240. doi: 10.3233/jad-2007-12304. [DOI] [PubMed] [Google Scholar]
- 100.Oikawa N, Yamaguchi H, Ogino K, et al. Gangliosides determine the amyloid pathology of Alzheimer’s disease. NeuroReport. 2009;20(12):1043–1046. doi: 10.1097/WNR.0b013e32832e4b9d. [DOI] [PubMed] [Google Scholar]
- 101.Okada M, Itoh M-I, Haraguchi M, et al. b-series Ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. Journal of Biological Chemistry. 2002;277(3):1633–1636. doi: 10.1074/jbc.C100395200. [DOI] [PubMed] [Google Scholar]
- 102.Gregory GC, Macdonald V, Schofield PR, Kril JJ, Halliday GM. Differences in regional brain atrophy in genetic forms of Alzheimer’s disease. Neurobiology of Aging. 2006;27(3):387–393. doi: 10.1016/j.neurobiolaging.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 103.Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Human Molecular Genetics. 2010;19(1):R12–R20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taupin P. Therapeutic potential of adult neural stem cells. Recent Patents on CNS Drug Discovery. 2006;1(3):299–303. doi: 10.2174/157488906778773670. [DOI] [PubMed] [Google Scholar]
- 105.Shruster A, Melamed E, Offen D. Neurogenesis in the aged and neurodegenerative brain. Apoptosis. 2010;15(11):1415–1421. doi: 10.1007/s10495-010-0491-y. [DOI] [PubMed] [Google Scholar]
- 106.Crews L, Rockenstein E, Masliah E. APP transgenic modeling of Alzheimer’s disease: mechanisms of neurodegeneration and aberrant neurogenesis. Brain Structure and Function. 2010;214(2-3):111–126. doi: 10.1007/s00429-009-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiology of Disease. 2006;24(1):1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 108.Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. Journal of Comparative Neurology. 2006;495(1):70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- 109.Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid β-peptide: implications for the pathogenesis of Alzheimer’s disease. NeuroMolecular Medicine. 2002;1(2):125–135. doi: 10.1385/NMM:1:2:125. [DOI] [PubMed] [Google Scholar]
- 110.Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid β-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. Journal of Neurochemistry. 2002;83(6):1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 111.Ermini FV, Grathwohl S, Radde R, et al. Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. American Journal of Pathology. 2008;172(6):1520–1528. doi: 10.2353/ajpath.2008.060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s disease. Experimental Neurology. 2007;204(1):77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yanagisawa M, Yu RK. O-linked β-N-acetylglucosaminylation in mouse embryonic neural precursor cells. Journal of Neuroscience Research. 2009;87(16):3535–3545. doi: 10.1002/jnr.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yanagisawa M, Nakamura K, Taga T. Glycosphingolipid synthesis inhibitor represses cytokine-induced activation of the Ras-MAPK pathway in embryonic neural precursor cells. Journal of Biochemistry. 2005;138(3):285–291. doi: 10.1093/jb/mvi129. [DOI] [PubMed] [Google Scholar]
- 115.Chen Y, Dong C. Aβ40 promotes neuronal cell fate in neural progenitor cells. Cell Death and Differentiation. 2009;16(3):386–394. doi: 10.1038/cdd.2008.94. [DOI] [PubMed] [Google Scholar]
- 116.Koudinov AR, Berezov TT. Alzheimer’s amyloid-beta (Aβ) is an essential synaptic protein, not neurotoxic junk. Acta Neurobiologiae Experimentalis. 2004;64(1):71–79. doi: 10.55782/ane-2004-1492. [DOI] [PubMed] [Google Scholar]
- 117.López-Toledano MA, Shelanski ML. Neurogenic effect of β-amyloid peptide in the development of neural stem cells. Journal of Neuroscience. 2004;24(23):5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Calafiore M, Battaglia G, Zappalà A, et al. Progenitor cells from the adult mouse brain acquire a neuronal phenotype in response to β-amyloid. Neurobiology of Aging. 2006;27(4):606–613. doi: 10.1016/j.neurobiolaging.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 119.Sotthibundhu A, Li Q-X, Thangnipon W, Coulson EJ. Aβ1-42 stimulates adult SVZ neurogenesis through the p75 neurotrophin receptor. Neurobiology of Aging. 2009;30(12):1975–1985. doi: 10.1016/j.neurobiolaging.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 120.Malaplate-Armand C, Florent-Béchard S, Youssef I, et al. Soluble oligomers of amyloid-β peptide induce neuronal apoptosis by activating a cPLA2-dependent sphingomyelinase-ceramide pathway. Neurobiology of Disease. 2006;23(1):178–189. doi: 10.1016/j.nbd.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 121.Sotthibundhu A, Sykes AM, Fox B, Underwood CK, Thangnipon W, Coulson EJ. β-amyloid1–42 induces neuronal death through the p75 neurotrophin receptor. Journal of Neuroscience. 2008;28(15):3941–3946. doi: 10.1523/JNEUROSCI.0350-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hayashi SI, Sato N, Yamamoto A, et al. Alzheimer disease-associated peptide, amyloid β40, inhibits vascular regeneration with induction of endothelial autophagy. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(11):1909–1915. doi: 10.1161/ATVBAHA.109.188516. [DOI] [PubMed] [Google Scholar]
- 123.Gong Y, Chang L, Viola KL, et al. Alzheimer’s disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(18):10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heo C, Chang K-A, Choi HS, et al. Effects of the monomeric, oligomeric, and fibrillar Aβ42 peptides on the proliferation and differentiation of adult neural stem cells from subventricular zone. Journal of Neurochemistry. 2007;102(2):493–500. doi: 10.1111/j.1471-4159.2007.04499.x. [DOI] [PubMed] [Google Scholar]
- 125.Lee JT, Xu J, Lee JM, et al. Amyloid-β peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. Journal of Cell Biology. 2004;164(1):123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Millet P, Silva Lages C, Haïk S, et al. Amyloid-β peptide triggers Fas-independent apoptosis and differentiation of neural progenitor cells. Neurobiology of Disease. 2005;19(1-2):57–65. doi: 10.1016/j.nbd.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 127.Li X, Zuo P. Effects of Aβ on neurogenesis in the adult mouse subventricular zone and dentate gyrus. Neurological Research. 2005;27(2):218–222. doi: 10.1179/016164105X35585. [DOI] [PubMed] [Google Scholar]
- 128.Cazzaniga E, Bulbarelli A, Cassetti A, et al. β-amyloid (25–35) enhances lipid metabolism and protein ubiquitination in cultured neurons. Journal of Neuroscience Research. 2007;85(10):2253–2261. doi: 10.1002/jnr.21354. [DOI] [PubMed] [Google Scholar]
- 129.Copani A, Melchiorri D, Caricasole A, et al. β-amyloid-induced synthesis of the ganglioside GD3 is a requisite for cell cycle reactivation and apoptosis in neurons. Journal of Neuroscience. 2002;22(10):3963–3968. doi: 10.1523/JNEUROSCI.22-10-03963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yanagisawa M, Ariga T, Yu RK. Cytotoxic effects of GM1 ganglioside and amyloid β-peptide on mouse embryonic neural stem cells. ASN Neuro. 2010;2(1):49–56. doi: 10.1042/AN20090063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hynds DL, Burry RW, Yates AJ. Gangliosides inhibit growth factor-stimulated neurite outgrowth in SH-SY5Y human neuroblastoma cells. Journal of Neuroscience Research. 1997;47(6):617–625. doi: 10.1002/(sici)1097-4547(19970315)47:6<617::aid-jnr7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 132.Leon A, Benvegnu D, Dal Toso R. Dorsal root ganglia and nerve growth factor: a model for understanding the mechanism of GM1 effects on neuronal repair. Journal of Neuroscience Research. 1984;12(2-3):277–287. doi: 10.1002/jnr.490120215. [DOI] [PubMed] [Google Scholar]
- 133.Dreyfus H, Ferret B, Harth S, Gorio A, Freysz L, Massarelli R. Effect of exogenous gangliosides on the morphology and biochemistry of cultured neurons. Advances in Experimental Medicine and Biology. 1984;174:513–524. doi: 10.1007/978-1-4684-1200-0_43. [DOI] [PubMed] [Google Scholar]
- 134.Tsuji S, Yamashita T, Nagai Y. A novel, carbohydrate signal-mediated cell surface protein phosphorylation: ganglioside GQ1b stimulates ecto-protein kinase activity on the cell surface of a human neuroblastoma cell line, GOTO. Journal of Biochemistry. 1988;104(4):498–503. doi: 10.1093/oxfordjournals.jbchem.a122498. [DOI] [PubMed] [Google Scholar]
- 135.Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomolecular Engineering. 2001;17(6):157–165. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 136.Wu L, Sluiter AA, Guo HF, et al. Neural stem cells improve neuronal survival in cultured postmortem brain tissue from aged and Alzheimer patients. Journal of Cellular and Molecular Medicine. 2008;12(5A):1611–1621. doi: 10.1111/j.1582-4934.2007.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Matsuzaki K, Noguch T, Wakabayashi M, et al. Inhibitors of amyloid β-protein aggregation mediated by GM1-containing raft-like membranes. Biochimica et Biophysica Acta. 2007;1768(1):122–130. doi: 10.1016/j.bbamem.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 138.Svennerholm L. The gangliosides. Journal of Lipid Research. 1964;5(4):145–126. [PubMed] [Google Scholar]