Summary
Paget's Disease (PD) is characterized by abnormal osteoclasts (OCL) that secrete high IL-6 levels and induce exuberant bone formation. Because measles virus nucleocapsid gene (MVNP) and the p62P392L mutation are implicated in PD, marrows from 12 PD patients harboring p62P392L and 8 normals were tested for MVNP expression and pagetic OCL formation. 8/12 patients expressed MVNP and formed pagetic OCL in vitro, which were inhibited by antisense-MVNP. 4/12 patients lacked MVNP and formed normal OCL that were hyper-responsive to RANKL but unaffected by antisense-MVNP. Similarly, mice expressing only p62P394L formed normal OCL, while mice expressing MVNP in OCL, with or without p62P394L, developed pagetic OCL and expressed high IL-6 levels dependent on p38MAPK activation. IL-6 deficiency in MVNP mice abrogated pagetic OCL development in vitro. Mice co-expressing MVNP and p62P394L developed dramatic Paget's-like bone lesions. These results suggest that p62P394L and IL-6 induction by MVNP play key roles in PD.
Introduction
Paget's Disease (PD) is characterized by highly localized areas of increased bone resorption coupled to exuberant new bone formation with the primary cellular abnormality in the osteoclasts (OCL) (Roodman and Windle, 2005). OCL in PD are increased in number and size and express a “pagetic phenotype” that distinguishes them from normal OCL. They contain up to 100 nuclei/OCL compared to 3-10 nuclei/OCL in normals. Their precursors are hyper-responsive to RANKL, TNF-α and 1,25(OH)2D3, and form OCL at physiologic concentrations of 1,25(OH)2D3 (10-11M) rather than the pharmacologic 1,25(OH)2D3 concentrations (10-8M) required for normal OCL formation in vitro (Kukita et al., 1990; Kurihara et al., 2004; Menaa et al., 2000). The 1,25(OH)2D3 hyper-responsivity results from elevated levels of a VDR coactivator, TAF12 (formerly TAFII-17) in OCL (Kurihara et al., 2004). Further, OCL in PD secrete high levels of IL-6, which are detectable in marrow plasma and peripheral blood from PD patients (Roodman et al., 1992).
At least 21 mutations in sequestosome 1/p62, a scaffold protein that plays a key role in RANKL signaling in OCL, are linked to PD, with p62P392L the most frequent mutation found (Laurin et al., 2002; Morissette et al., 2006; Ralston, 2002). However, the role of p62P392L in PD is unclear because normal OCL precursors expressing p62P392L are hyper-responsive to RANKL but not to 1,25(OH)2D3, do not express high levels of IL-6 or TAF12 or form bone lesions or OCL characteristic of PD (Hiruma et al., 2008; Kurihara et al., 2007).
Various environmental factors, including measles virus and other paramyxoviruses, have also been implicated in the pathogenesis of PD. We previously found that OCL from ∼70% of PD patients expressed the measles virus nucleocapsid protein (MVNP) gene, and that normal OCL precursors expressing MVNP formed OCL that exhibit the “pagetic phenotype” (Kurihara et al., 2000). Further, 29% of transgenic mice with targeted expression of MVNP to OCL (MVNP mice) developed OCL and bone lesions characteristic of PD (Kurihara et al., 2006).
Therefore, to assess the relative contributions of MVNP and p62P392L in PD, marrows from clinically involved and uninvolved bones of PD patients with p62P392L or normals were tested for MVNP expression, and the effects of antisense-MVNP (AS-MVNP) on the OCL formed determined. To delineate the mechanism(s) responsible for the abnormal OCL activity and bone formation seen with co-expression of MVNP and mutant p62, p62P394L knock-in (p62KI) mice (the mouse equivalent of human p62P392L) were bred to TRAP-MVNP transgenic mice to generate p62KI/MVNP mice. These mice developed increased numbers of pagetic OCL and bone lesions than MVNP mice. Further, the bone lesions in p62KI/MVNP mice were strikingly similar to those seen in Paget's disease. The p62P392L gene increased RANKL sensitivity of OCL precursors while MVNP was responsible for OCL hyper-multinucleation, increased TAF-12 expression, and IL-6 production through enhanced p38MAPK signaling induced by 1,25(OH)2D3. Loss of IL-6 expression in MVNP mice abrogated the formation of pagetic OCL in vitro.
Results
OCL formation in marrow cultures from PD patients and normals
Marrow samples from involved or uninvolved bones of 12 PD patients harboring the p62P392L mutation and 8 age-matched controls were tested for MVNP expression (Table S1). Approximately half of the PD patients had elevated serum alkaline phosphatase (ALP) levels and most had polyostotic PD.
Marrows from 8/12 PD patients expressed MVNP mRNA (Fig. 1A,). In three MVNP+ patients whose involved and uninvolved sites could both be tested, marrow from both sites expressed MVNP. In contrast, MVNP was not detected in normals or in 4/12 PD patients, in either clinically involved or uninvolved bone. The sequence of the MVNP qRT-PCR product from patient #8, who had the highest MVNP expression, completely matched residues 1129-1222 of the Measles virus mRNA for nucleocapsid protein (GenBank accession number X01999). Immunohistochemical analysis confirmed that OCL formed from the marrow of MVNP+ PD patient #8 or MVNP mice expressed MVNP (Fig. 1B). No cross-reactivity to the MVNP antibody was detected in OCL derived from normal human marrow or WT mice.
Fig. 1. MVNP Expression by p62P392L Patient Bone Marrow Cells.

(A) MVNP mRNA expression in p62P392L PD and normal nonadherent bone marrow mononuclear cells (5×105 cells) was determined by qRT-PCR as described in Methods. A ratio of MVNP/actin < 0.3 was considered negative and a ratio of ≥ 0.3 was considered positive for MVNP expression. (B) Detection of MVNP protein in human and murine OCL with an anti-MVNP monoclonal antibody (2D7, Abcam) or control IgG. Cross-reactivity with the anti-MVNP Ab stained nuclei brown in PD patient and MVNP mouse OCL. The arrows show nuclei in OCL that did not react with anti-MVNP.
(See Table S1)
Effects of 1,25(OH)2D3 and RANKL on OCL formation
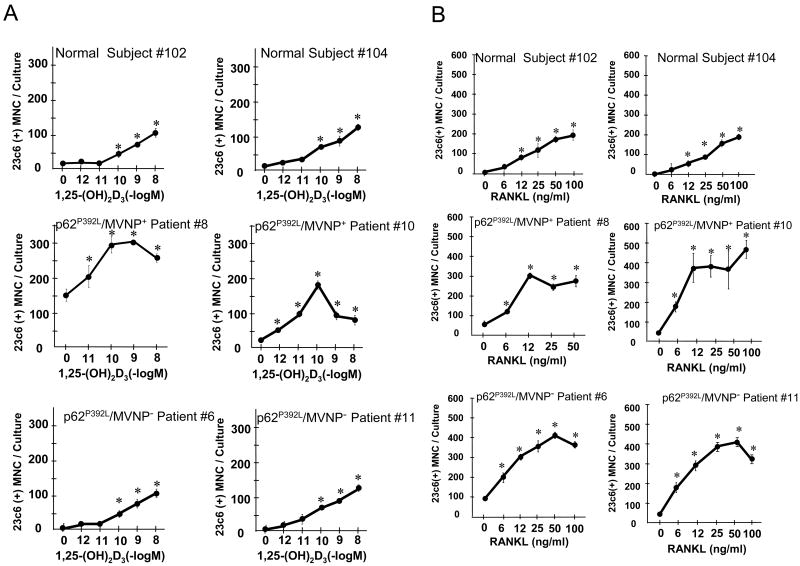
OCL precursors from p62P392L/MVNP+ patients were hyper-responsive to 1,25(OH)2D3 (Fig. 2A, 3C), and formed OCL that were hyper-multinucleated compared to those from normals or p62P392L/MVNP- patients (Fig. 3D, Table S2). However, both p62P392L/MVNP+ and p62P392L/MVNP- OCL precursors were hyper-responsive to RANKL and formed more OCL than normals (Fig. 2B, 3C). Nuclear number/OCL in p62P392L/MVNP- and normal marrow cultures treated with 1,25(OH)2D3 or RANKL did not differ, and were significantly lower than in p62P392L/MVNP+ cultures (Fig. 3D).
Fig. 2. OCL Formation Induced by 1,25-(OH)2D3 and RANKL in Marrow Cultures from Affected Bones of p62P392L Patient and Normals.
Patient and normal OCL formation induced with (A) 1,25-(OH)2D3 or (B) RANKL and M-CSF. Results are expressed as mean ± SD for quadruplicate cultures. *, p<0.01 compared to (A) vehicle or (B) M-CSF alone. Two representative patients or normals are shown. Similar results were seen in 2 independent experiments using different patients and normals.
Fig. 3. AS-MVNP Blocks MVNP and TAF12 Expression and Decreases Osteoclast Formation and Bone Resorption by Osteoclast Precursors from MVNP+ but not MVNP- PD Patients or Normals.
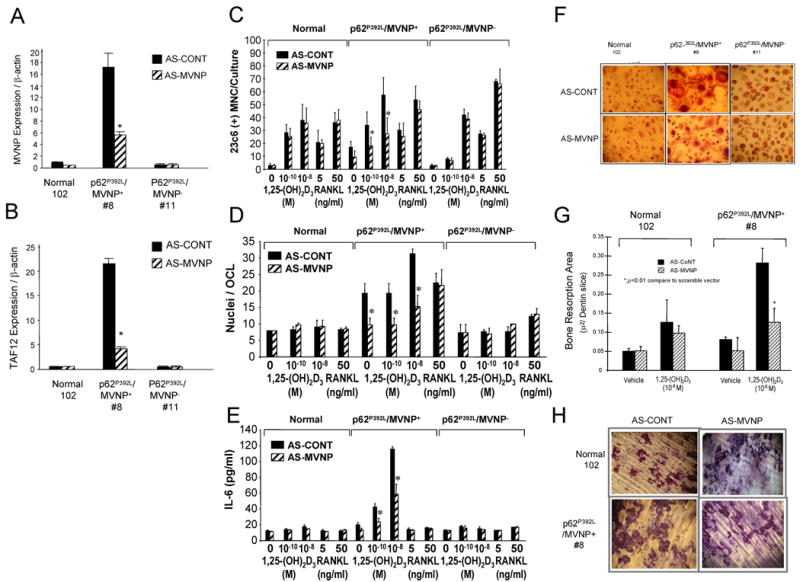
OCL precursors from MVNP+ and MVNP- patients and normals were transduced with AS-MVNP- or AS-CONT and analyzed for (A) MVNP expression; (B) TAF12 mRNA expression; (C) OCL formation per culture, * p<0.01 compared to AS-CONT-transduced CFU-GM treated with the same concentration of 1,25(OH)2D3; (D) Nuclei/OCL, results are the average nuclear number in 50 randomly counted OCL, *, p<0.01 compared to no treatment; (E) IL-6 production; (F) OCL morphology, photomicrograph of OCL in marrow cultures treated with 1,25(OH)2D3 and stained with 23C6 (×100); (G) Bone resorption by OCL derived from involved bones of a MVNP+ patient and normal, *, p<0.05 compared to AS-CONT-transduced cells at the same concentration of 1,25(OH)2D3; (H) Photomicrograph of bone resorption area for (G) (×100). Data in (C), (D) and (E) represent the mean ± SD of aggregate data from 3 MVNP+ patients, 3 MVNP- patients and 4 normals.
(See Table S2)
Effects of an anti-sense MVNP (AS-MVNP) on OCL formation by MVNP+ and MVNP- patients or normals
Transfection of p62P392L/MVNP+ OCL precursors with an AS-MVNP construct decreased expression of MVNP mRNA by nearly 70% (Fig. 3A), and also resulted in an 80% reduction in TAF12 expression (Fig. 3B) as well as significant reductions in OCL formation (Fig. 3C), nuclei/OCL (Fig. 3D) and IL-6 production (Fig. 3E) induced by 1,25(OH)2D3, compared to the scrambled control antisense construct (AS-CONT). Further, the OCL that formed were normal morphologically (Fig. 3F). AS-MVNP also inhibited the increased bone resorption seen in marrow cultures from p62P392L/MVNP+ PD patients when compared to normal OCL (Figs. 3G and 3H). AS-MVNP had no effect on OCL formation, OCL morphology, bone resorption or IL-6 production by p62P392L/MVNP- or normal OCL precursors (Fig. 3).
Characteristics of OCL formation in marrow cultures from mice co-expressing p62P394L and MVNP (p62KI/MVNP)
To determine the mechanism(s) responsible for the effects of p62P392L and MVNP on OCL formation and bone remodeling, knock-in mice expressing the p62P394L mutation (p62KI mice) were bred to transgenic mice expressing MVNP under the control of the tartrate-resistant acid phosphatase (TRAP) promoter (MVNP mice) to generate p62KI/MVNP mice, which were compared to wild type (WT), MVNP and p62KI mice. OCL precursors from MVNP and p62KI/MVNP mice expressed much higher levels of TAF12 than p62KI or WT mice (Fig. 4A), and were hyper-responsive and formed increased numbers of OCL when treated with 1,25(OH)2D3, compared to WT or p62KI mice (Fig 4B). The OCL that formed in marrow cultures from MVNP and p62KI/MVNP mice were also markedly increased in size and number (Fig. 4C), with increased nuclear numbers (Fig. 4D), compared to p62KI or WT mice. OCL precursors from p62KI and p62KI/MVNP mice, and to a lesser extent MVNP mice, were hyper-responsive to RANKL compared to WT mice (Fig. 4B).
Fig. 4. Expression of MVNP, TAF12 and characterization of OCL formation induced by 1,25(OH)2D3 and RANKL in marrow cultures from affected bones of p62KI, MVNP, p62KI/MVNP and WT mice.
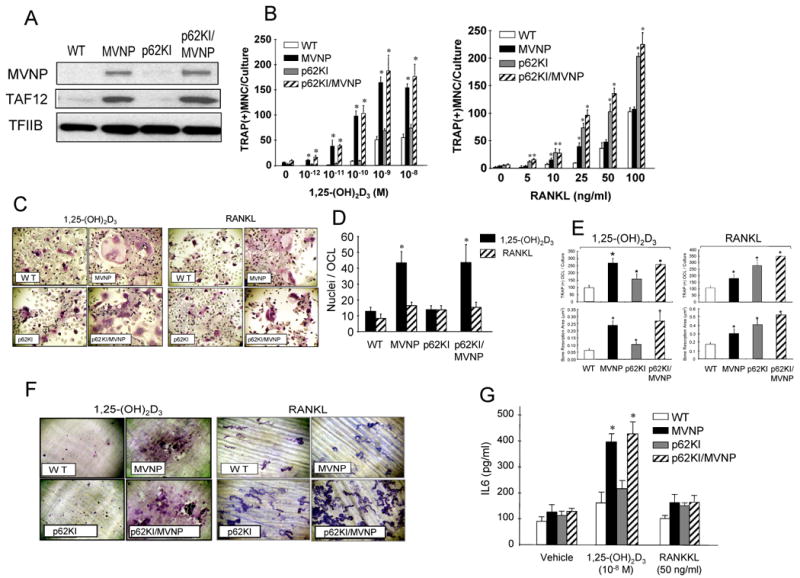
(A) Expression of MVNP and TAF12. CD11b-positive marrow mononuclear cells from MVNP, p62KI, p62KI/MVNP and WT mice were cultured with 10 ng/ml of M-CSF with in 10% FBS and αMEM for 3 days. The nuclear extract (30 μg of protein/lane) were prepared and subjected to immunoblot analysis using antibodies recognizing anti-MVNP (clone 2D7, Abcom) and anti-TAF12 (Protein Tech). Anti-TFIIB (Santa Cruz) is shown as a loading control. (B) OCL formation. *, p<0.01 compared to WT at the same concentration of 1,25(OH)2D3 or RANKL. (C) Morphology and (D) nuclear number/OCL in marrow cultures from MVNP, p62KI, p62KI/MVNP, and WT mice. *, significantly different from 1,25(OH)2D3 or RANKL treatment group, p<0.01. (E) OCL formation by MVNP, p62KI, p62KI/MVNP and WT OCL precursors (top). * p<0.01 compared with WT cultures. Resorption area per dentin slice (bottom). * p<0.05 compared with results from WT cultures. (F) Resorption lacunae formed by OCL on dentin. Original magnification, ×100. (G) IL-6 production in bone marrow cultures from MVNP, p62KI, p62KI/MVNP and WT mice. Results for (B), (D), (E), and (G) are expressed as the mean ± SD for aggregate data from 3 independent experiments
(See Figure S1-A, B)
Similar effects of genotype on bone resorption area in marrow cultures were also seen (Fig. 4E,F). OCL formed in marrow cultures from MVNP or p62KI/MVNP, but not p62KI or WT mice, displayed a marked increase in bone resorption when the cultures were treated with 1,25(OH)2D3 (10-8 M). However, OCL in marrow cultures from mice of all three transgenic or KI genotypes resorbed more dentin than OCL from WT cultures when treated with RANKL (50 ng/ml), reflecting their increased OCL formation.
OCL from both MVNP and p62KI/MVNP mice secreted high levels of IL-6 in response to 1,25(OH)2D3 compared to OCL generated from p62KI or WT mice (Fig. 4G). RANKL modestly increased IL-6 production by OCL from all transgenic mice. These results indicate that expression of MVNP in OCL induces hyper-responsivity to 1,25(OH)2D3, hyper-multinucleation, and increased IL-6 production, while mutant p62 contributes to increased OCL formation and RANKL responsivity in PD patients and transgenic mice. Further, OCL from the p62KI/MVNP mice most accurately recapitulate the phenotypic characteristics of OCL from p62P392L/MVNP+ PD patients.
Effects of MVNP, p62KI and p62KI/MVNP on bone in vivo
Table S3-A depicts the number, age and sex of the animals that were subjected to histological and histomorphometric analysis. In L1-L4 lumbar vertebrae without lesions (see below) from MVNP, p62KI, p62KI/MVNP and WT mice aged 12-14 months, there were no significant differences in the cancellous bone volume, trabecular width, number and separation (Table 1). Cancellous bone structural variables by 2D histomorphometry in L1-L4 were correlated (p<.001) with those by 3D microCT in the 5th lumbar vertebra of 8 mice and in the tibia of 10 mice (data not shown). Although OCL perimeter is increased slightly in MVNP, p62KI and p62KI/MVNP mice compared to WT, these increases are not statistically significant. This likely reflects the small group size and large variation in OCL perimeter, given that we were able to demonstrate a significant increase of OCL in MVNP compared to WT mice with 16-24 mice in each of the groups in the MVNP/IL6-KO experiments (Table S4). Measurement of serum CTX and OCN in transgenic and WT mice showed that both CTX and OCN levels were significantly increased in mice expressing MVNP (Fig. S1-A), while only CTX was increased in p62KI mice.
Table 1. Lumbar vertebral bone structure and remodeling in 12/14 month-old p62KI/MVNP mice measured in vertebrae that did not contain paget-like lesions.
| Genotype | No | BV/TV % | Tb.Wi μm | Tb.N #/mm2 | Tb.Sp μm | Oc.Pm % | Md.Pm % | MAR μm/d | BFR μm2/μm/d |
|---|---|---|---|---|---|---|---|---|---|
| WT | 5 | 15.7 (2.0) |
30.8 (2.4) |
5.1 (0.5) |
167.7 (22.0) |
17.8 (5.6) |
21.7 (3.7) |
1.16 (0.14) |
0.25 (0.07) |
| MVNP | 3 | 13.2 (4.1) |
30.1 (8.3) |
4.4 (0.5) |
201.1 (31.2) |
22.5 (12.8) |
32.1 (10.5) |
1.34 (0.18) |
0.44 (0.19) |
| p62KI Homo | 5 | 17.5 (10.2) |
31.6 (6.1) |
5.3 (2.0) |
182.8 (90.6) |
20.0 (8.9) |
33.1 * (11.3) |
1.10 (0.24) |
0.37 (0.17) |
| p62KI Heter | 12 | 19.6 (7.3) |
34.1 (5.4) |
5.6 (1.7) |
159.2 (65.4) |
25.3 (10.3) |
31.4 * (6.9) |
1.35 (0.24) |
0.44 * (0.16) |
| p62KI Homo/MVNP | 8 | 14.5 (6.3) |
33.4 (6.9) |
4.2 (1.1) |
219.3 (68.3) |
26.0 (8.4) |
29.3 (9.5) |
1.40 * # (0.19) |
0.40 (0.11) |
| p62KI Heter/MVNP | 6 | 14.9 (2.8) |
33.1 (8.8) |
4.6 (0.8) |
189.2 31.3) |
21.9 (13.9) |
21.9 @ (9.1) |
1.17 (0.13) |
0.26 @ (0.12) |
Data are expressed as Mean (SD).
P< 0.05 versus WT;
P< 0.05 versus p62KI Homo;
P< 0.05 versus p62KI Heter.
Mineral apposition rate was modestly increased in the p62KI/MVNP mice, while p62KI mice showed modest increases in mineralized perimeter and bone formation rate, with similar trends seen in MVNP and p62KI/MVNP mice. There were no differences in mineralized perimeter, mineral apposition rate and bone formation rate between MVNP and p62KI/MVNP mice (Table 1).
Despite the lack of significant differences in most histomorphometric parameters among the different genotypes, dramatic bone lesions were found within vertebral bone in 4/10 p62KI/MVNP mice aged 18-26 months and in 1/17 p62KI/MVNP mice aged 12/14 months (Table S3-B). In 2/5 animals with lesions, the lesions were seen in all 4 vertebrae examined. Lesions were not observed in any other group. The morphology of the bone lesions compared with that of normal bone in a WT mouse and a pagetic lesion in a patient is shown in Fig. 5. Morphologically, the lesions in the p62KI/MVNP mice (Fig. 5B,C,F,G,J,K) differed strikingly from normal bone (Fig. 5A,E,I). The lesions were characterized by numerous abnormal OCL, which were large in size, multinuclear, and either in deep resorption cavities or detached from bone. Trabeculae were irregularly thickened and composed primarily of woven bone, and there was extensive marrow fibrosis (Fig. 5C,F,G,J,K). In focal areas, the lesions completely replaced cancellous and cortical bone tissue. There were no detectable differences in the bone lesions between p62KI/MVNP mice that were heterozygous (n=3) or homozygous for p62P394L (n=2). Thus, co-expression of MVNP and p62P394L in mice results in histologic lesions that are strikingly similar to PD (Fig. 5D,H,I).
Figure 5. Histological microphotographs of Paget's-like lesions in vertebrae from a p62KI/MVNP mice compared with the vertebra from WT and the bone lesions from a patient with Paget's disease.
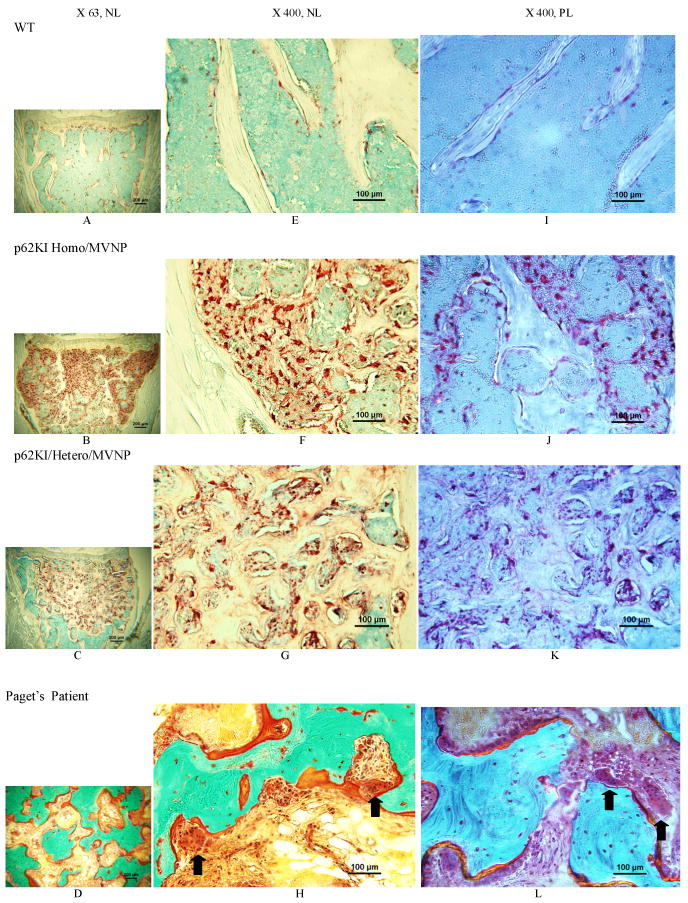
Photos from top to bottom rows were taken from vertebral bone of a WT mouse in the 1st row (A,E,I), a p62 × MVNP homozygous mouse in the 2nd row (B.F.J) and a p62 × MVNP heterozygous mouse in the 3rd row (C,G,K) (TRAP stain); the 4th row (D,H,L) shows bone lesions from a patient with Paget's disease (Goldner's trichrome stain). Photos from left to right columns were taken under bright light with a magnification of 63× (left-A,B,C,D) or 400× (middle-E,F,G,H), or polarizing light with 400× (right- I,J,K,L). The1st row demonstrates normal cancellous and cortical bone (A), osteoclasts (E) and the lamellar pattern of bone (I) in the WT mouse. The 2nd displays diffuse osteolytic lesions within the cancellous bone/marrow compartment and invading cortical bone and growth plate, loss of trabecular microstructure with and irregular thick trabeculae and cortical thinning (B); Extremely numerous, large and multi-nuclear osteoclasts (red) are attached to the bone or in fibrotic marrow (F); and the bone is woven (J) in the p62 × MVNP homozygous mouse. The 3rd row shows a localized lesion within the cancellous/marrow compartment (C); Osteoclasts are numerous, large and multinucleated, both attached or detached from bone, with marrow fibrosis (G); and the bone matrix is comprised of woven bone (K) in the p62 × MVNP heterozygous mouse. The 4th row demonstrates classical paget's lesions: irregular thickened woven bone with “mosaic” pattern, numerous extremely large osteoclasts with many nuclei in deep resorption cavities or detached from bone, plump osteoblasts on osteoid and marrow fibrosis (D,H,L).
(See Table S3-A, B)
MAPKs and NFκB signaling in OCL precursors treated with RANKL or 1,25(OH)2D3
To determine the mechanism(s) responsible for the effects of MVNP and p62P394L on the abnormal OCL formation and activity in p62KI/MVNP mice, signaling pathways previously implicated in OCL formation were analyzed. p38 MAPK activation in response to RANKL was increased in OCL precursors from MVNP, p62KI and p62KI/MVNP compared to WT mice. In contrast, 1,25(OH)2D3 only increased p38 MAPK activation in mice expressing the MVNP gene (Fig. 6A). NFκB, Erk1/2 and JNK responses were similar in MVNP, p62KI, p62KI/MVNP and WT mice in response to 1,25(OH)2D3. However, NFκB signaling was increased in transgenic mice bearing p62P394L in response to RANKL (Fig. S1-B).
Fig. 6. Role of p38MAPK activation in the effects of MVNP on pagetic OCL formation.

(A) Analysis of p38 MAPK activation in MVNP, p62KI, p62KI/MVNP and WT OCL precursors. Lysates from OCL precursors of transgenic or WT mice were induced with RANKL or 1,25(OH)2D3, for the time periods indicated, the lysates prepared and p38MAPK activation determined by Western blot analysis as described in Methods. Similar results were seen in three independent experiments (B) Effects of blocking p38MAPK signaling with a p38 inhibitor (SB203580) in MVNP expressing OCL precursors on IL-6 production, OCL formation and Nuclei/OCL. OCL formation and IL-6 in conditioned media were determined as described in Methods. Results are expressed the mean ± SD for 4 technical replicates. *, significantly different than cells without SB, p<0.01. (C) OCL formation by marrow from MVNP mice lacking IL-6. MVNP mice were interbred with IL-6KO mice and marrow from offspring of the indicated genotypes were tested for pagetic OCL formation. Results are expressed as the mean ± S.D. for 3 independent experiments. *, significantly different from WT cultures, p<0.01. (D) TAF12 expression by MVNP/IL-6KO mice.
(See Table S4)
Since MVNP was responsible for the enhanced 1,25(OH)2D3 responsivity, hyper-multinuclearity, and increased IL-6 production by OCL precursors from p62KI/MVNP mice, and MVNP increased p38MAPK activity to a greater extent than p62P394L, the effects of blocking p38MAPK activity with SB203580 in OCL precursors from MVNP mice were examined. Inhibiting p38MAPK activity blocked the increased OCL formation and IL-6 production by OCL, as well as hyper-multinuclearity of OCL (Fig. 6B). In contrast, blocking NFκB signaling with a NEMO inhibitory peptide in cultures treated with 1,25(OH)2D3 did not affect OCL formation (data not shown).
Role of IL-6 in the effects of MVNP
Because high levels of IL-6 are induced in MVNP but not p62KI OCL, the role of IL-6 in the multiple effects of MVNP on OCL formation was determined by breeding MVNP to IL-6 global knockout (IL-6KO) mice (Kopf et al., 1994). In contrast to MVNP mice, OCL precursors from MVNP/IL-6KO mice were not hyper-responsive to 1,25(OH)2D3 (Fig. 6C), expressed low levels of TAF-12 (Fig. 6D) and formed OCL that contained similar number of nuclei/OCL as WT mice (data not shown). Histomorphometric analysis of WT, MVNP, IL-6KO and MVNP/IL-6KO mice revealed no significant differences in cancellous bone volume, trabecular width, number and separation in the lumbar vertebral bodies (Table S4). MVNP mice showed a significant increase in OCL perimeter and mineral apposition rate and displayed a trend towards increases in mineralized perimeter and bone formation rate, while IL-6KO mice showed a significant decrease in mineralized perimeter and bone formation rate, and a trend towards decrease in mineral apposition rate. MVNP/IL-6KO mice showed a significant increase in OCL perimeter similar to that in MVNP mice, but mineralized perimeter and bone formation rate were reduced in MVNP/IL-6KO mice, similar to IL-6KO mice.
Discussion
Both genetic and environmental etiologies have been proposed for PD. However, their relative contributions to the pathogenesis of PD are unclear. Therefore, we examined 12 PD patients harboring the p62P392L mutation for expression of MVNP, as well as studied mice co-expressing MVNP and p62P394L to address this question. MVNP was expressed in marrow from 8/10 patients for whom specimens from involved sites were available (Table S2), similar to our previous results (Reddy et al., 1995). Most patients (6/8) with elevated ALP levels were MVNP+, while 2/4 patients with normal ALP levels were MVNP+. Interestingly, in 3/3 MVNP+ patients for which specimens from both involved and involved sites were available, the uninvolved specimen also expressed MVNP. Several possibilities could explain this result: 1) MVNP expression in uninvolved bone may represent areas of subclinical PD, where there are very low numbers of pagetic OCL. Clinically detectable PD lesions may only develop when large numbers of pagetic OCL are present. This could require many years to occur, since PD patients rarely develop new lesions during the long course of their disease. 2) MVNP+ patients may have circulating MVNP+ precursors, which are present in peripheral blood admixed with the marrow aspirates. Low numbers of MVNP+ cells have been detected in the blood from PD patients (Reddy et al., 1999).
OCL precursors from all MVNP+ patients formed pagetic OCL in vitro, which were hyper-responsive to 1,25(OH)2D3 and RANKL, formed OCL with increased bone resorbing capacity and had elevated levels of TAF12. In contrast, OCL precursors from MVNP- patients were not hyper-responsive to 1,25(OH)2D3, expressed normal levels of TAF12 and formed normal OCL. OCL precursors from both MVNP- and MVNP+ patients were hyper-responsive to RANKL, most likely reflecting the presence of the p62P392L mutation in all patients, since transfection of p62P392L into normal human OCL precursors or introduction of the p62P394L mutation into mice results in hyper-responsivity of OCL precursors to RANKL. Further, like the MVNP- patients, OCL from marrow cultures of p62KI mice did not display features characteristic of PD (Hiruma et al., 2008; Kurihara et al., 2007). The lack of TAF12 expression and hypersensitivity to 1,25(OH)2D3 in p62P392L/MVNP- patients supports previous reports that normal OCL precursors transfected with p62P392L form normal levels of OCL with normal ratios of nuclei/OCL when treated with 1,25(OH)2D3 (Hiruma et al., 2008; Kurihara et al., 2007). These results suggest that expression of MVNP in OCL precursors is required for formation of OCL expressing a pagetic phenotype in vitro. Consistent with these observations, expression of MVNP but not p62P392L in OCL precursors is sufficient to induce normal OCL precursors to form pagetic OCL and develop pagetic bone lesions in mice (Kurihara et al., 2007; Kurihara et al., 2006).
Interestingly, marrow from MVNP- PD patients, like normal donors, formed OCL that were morphologically normal rather than pagetic, even though the patients have documented PD. The basis for this lack of a PD phenotype in OCL formed in vitro by this subset of patients is unknown. The lack of MVNP expression in some patients suggests that other factors, including viruses, or genes, may be involved in the development of PD in MVNP- patients. For example, we did not screen for Respiratory Syncytial Virus (RSV), which has also been frequently detected by immunohistochemistry in OCL from PD patients (Mills et al., 1984), and could contribute to the development of PD in these patients.
Transfection of OCL precursors from p62P392L/MVNP+ patients with AS-MVNP significantly decreased MVNP expression, resulting in a corresponding decrease in TAF12 mRNA expression, IL-6 production, and hypersensitivity to 1,25(OH)2D3, and generated OCL with a normal morphology. AS-MVNP had no effect on OCL formation from p62P392L/MVNP- patients or normals. Increased expression of TAF12 and IL-6 and 1,25(OH)2D3 hyper-responsivity of OCL precursors are consistent findings in most PD patients with either familial or sporadic PD (Kukita et al., 1990; Mossetti et al., 2005; Neale et al., 2000; Roodman et al., 1992). These results demonstrate that expression of MVNP in OCL of PD patients with p62P392L is responsible for the increased expression of TAF12 and 1,25(OH)2D3 hyper-responsivity of their OCL precursors. The results also suggest that the high IL-6 levels found in marrow and plasma from PD patients (Mossetti et al., 2005; Roodman et al., 1992) result from expression of MVNP or other environmental factors in PD patients, rather than from the p62 mutations linked to PD.
Both MVNP+ and MVNP- PD patients were hyper-responsive to RANKL and formed increased numbers of OCL at lower levels of RANKL than normal OCL precursors. Transfection of MVNP+ or MVNP- OCL precursors with an antisense MVNP had no effect on the increased OCL formation in response to RANKL. These results suggest that p62P392L is responsible for the hyper-responsivity of OCL precursors to RANKL regardless of their MVNP status.
Further, the bone resorbing capacity of OCL formed in marrow cultures from p62P392L/MVNP+ patients was increased, as compared to OCL formed by p62P392L/MVNP- or normal OCL precursors. This most likely reflects that MVNP- OCL were smaller and had fewer nuclei/OCL compared to MVNP+ OCL, since multinucleation enhances the bone resorption capacity of cells (Yagi et al., 2005). These results suggest that MVNP enhances OCL precursor fusion to form hyper-multinucleated OCL with an increased bone resorbing capacity in PD.
In support of our findings in PD patients, OCL formation in bone marrow cultures of p62KI/MVNP mouse OCL precursors was greatly enhanced, the OCL were hyper-multinucleated and the OCL precursors hyper-responsive to 1,25(OH)2D3 as compared to p62KI or WT mice (Fig. 4). Further, the bone resorption capacity was enhanced in p62KI/MVNP cultures. CTX levels were also increased in the serum of MVNP, p62KI and p62KI/MVNP mice (Fig. S1-A). These results support our previous studies that showed that MVNP and p62P394L induce increased levels of OCL formation (Kurihara et al., 2004; Mills et al., 1994). MVNP expression in OCL precursors upregulated TAF12 and made these cells hyper-responsive to 1,25(OH)2D3 as well. OCL precursors from p62KI/MVNP and p62KI mice were more hyper-responsive to RANKL than MVNP mice. IL-6 levels were also increased in marrow cultures of MVNP and p62KI/MVNP mice compared to p62KI mice (Fig. 4G). The basis for the increased IL-6 secretion most likely reflects the increased p38MAPK activation by 1,25(OH)2D3 in MVNP expressing cells (Fig. 6B) (Kurihara et al., 2009).
Our histological finding that the p62KI/MVNP mice developed pagetic-like lesions is consistent with our in vitro OCL formation results. Forty percent of the p62KI/MVNP mice aged 18-26 months showed dramatic bony lesions in their lumbar vertebrae and in 2 of these animals, multiple vertebrae were affected. These lesions, which were more common in the older animals, had many characteristic features of PD, including a dramatically increased number of hyper-multinucleated OCL, irregularly thickened, coarse trabeculae composed primarily of woven bone, large lytic regions in both trabecular and cortical bone, and extensive marrow fibrosis. Further, osteocalcin levels were only elevated in MVNP and p62KI/MVNP mice, consistent with our current and previous histologic results that showed increased bone formation in mice expressing MVNP (Kurihara et al., 2006). Our histomorphometric findings in the p62KI/MVNP mice were also consistent with a pagetic phenotype showing generalized increase in bone turnover, bearing in mind that the histomorphometry was performed on bones without lesions. In this non-lesioned bone MVNP increased osteoclast perimeter by 26% (not statistically significant) in the p62KI/MVNP experiments (Table 1). However, MVNP increased osteoclast perimeter by 23% (statistically significant) in the MVNP/IL-6 KO experiments (Table S4) and by 57% (statistically significant) in our previous study [Table 3, (Kurihara et al., 2006)] compared to WT mice. The reason for the lack of statistical significance in the p62KI/MVNP experiment could be related to the small group sizes for MVNP (n=3) and WT (n=5) mice in Table 1 and the large variation in osteoclast perimeter. This compares with sample sizes of 12-24 in the studies where a significant effect of MVNP on osteoclast perimeter was seen. It is important to note here that the quantitative histomorphometry was performed on bone without lesions and therefore the effects of MVNP on bone remodeling would be expected to be less pronounced. Bone remodeling in non-lesioned bone in patients with Paget's disease is only slightly elevated. It is not clear why we did not find lesions in the MVNP alone animals in the present study, but as this occurs in 29% of MVNP animals, this too may be a sampling issue.
Mineralized perimeter, determined by the use of calcein labeling, was significantly increased in p62KI mice and mineral apposition rate was increased in p62KI/MVNP. The high correlations between structural variables, determined by histomorphometry and microCT at different skeletal sites suggest a generalized expression of the phenotype.
We then examined the mechanisms responsible for MVNP's capacity to induce a pagetic phenotype in MVNP and p62KI/MVNP mice. As shown in Fig. 6, MVNP increased p38MAPK activity in response to 1,25(OH)2D3 to a greater extent than p62P394L. Further, blocking p38MAPK activity decreased OCL numbers, 1,25(OH)2D3 hypersensitivity, formation of hyper-multinucleated OCL, and IL-6 production. Several studies have shown that MVNP can increase IL-6 expression when introduced into cells (Helin et al., 2001; Manchester et al., 1999; Schneider-Schaulies et al., 1993). In addition, IL-6 is a potent inducer of OCL formation (Kurihara et al., 1990), and high levels of IL-6 increase the number of nuclei/OCL in the presence of low levels of 1,25(OH)2D3 (Kurihara et al., 1991). Therefore, to determine if the increased expression of IL-6 by OCL expressing MVNP is responsible for the abnormal OCL activity that we found, we bred MVNP mice to global IL-6 KO mice. The OCL formed from marrow cultures of MVNP/IL-6KO mice cultured in the presence of 1,25(OH)2D3 did not display the characteristics of pagetic OCL. Further, histomorphometric analysis of bones from MVNP/IL-6KO mice revealed that loss of IL-6 also abrogated the increase in mineral apposition rate found in MVNP mice and reduced mineralizing perimeter and bone formation rate. Taken together, these results support that IL-6 is an inducer of the pagetic OCL phenotype and contributes to PD.
The results of our studies suggest that the p62P392L mutation and other genetic mutations linked to PD increase OCL formation and activity. Like our studies with transgenic p62P392L mice (Kurihara et al., 2007), Custer et al recently reported that transgenic mice expressing the mutant VCP gene linked to PD also have increased OCL formation, and develop progressive osteopenia rather than PD (Custer et al., 2010). Genetic mutations linked to PD appear to predispose patients to PD but require co-expression of an environmental factor in OCL precursors for the development of a robust pagetic phenotype. In support of this conclusion, Gennari et al recently reported that there is a strong association between environmental factors and the development of severe PD in patients with a genetic predisposition to PD (Gennari et al., 2010).
Thus, MVNP contributes to the abnormal OCL activity in most but not all the patients with p62P392L mutations we studied, suggesting that there are two types of patients with PD. In one, OCL precursors express MVNP and form OCL in vitro characteristic of pagetic OCL, while in the second, the OCL precursors lack MVNP and form normal-appearing OCL in vitro. These results further suggest that p62P392L enhances the osteoclastogenic capacity of PD patients through enhanced RANKL sensitivity, but that a second stimulus is required for the formation of OCL characteristic of PD (Kurihara et al., 2007). This second stimulus in the MVNP- patients could be another virus or environmental factor analogous to MVNP, or another mutated gene.
Methods
Patients and Age-Matched Healthy Controls
Bone marrow was obtained from involved and uninvolved posterior iliac crests of twelve patients with familial PD carrying the p62P392L mutation and 6 age-matched controls by Dr. Jacques Brown, shipped at room temperature overnight, and placed into culture after receipt. The extent and activity of PD was documented by bone scans, pelvic x-rays and total ALP measurements (Laurin et al., 2002). PD was similarly ruled out in controls. All patients and controls had detectable measles virus IgG antibodies. Prior history of bisphosphonate treatment and clinical status were documented at the time of marrow aspiration. These studies were approved by the University of Pittsburgh and Laval University human studies committees.
Detection of MVNP and TAF12 mRNAs and Sequence Analysis of the MVNP Transcript
MVNP and TAF12 mRNA expression was determined by qRT-PCR with total RNA isolated from primary marrow samples and from AS-MVNP or AS-CONT transduced cells. The gene specific primers were MVNP: 5′- AGT TCC ACA TTA GCA TCT GAA C -3′ (sense) and 5′- TAC TGA TCT TGT CCT CAG TAG -3′ (antisense) (GenBank Accession number M10297X01999), TAF12: 5′ - CTC ATC CAT AAA ACC GGA ACC A - 3′ (sense) and 5′ -TTC AGG GCT AAG ACG ACC TCC - 3′ (antisense) (GenBank Accession number NM 005644), and β-actin: 5′- GTG CGT GAC ATC AAA GAG -3′ (sense) and 5′- GCC ACA GGA TTC CAT ACC -3′ (antisense). The MVNP RT-PCR product from PD patient #8 was cloned into the pCR-TOPO vector (TOPO-TA Cloning Kit, Invitrogen) following the manufacturer's protocol. Three independent clones were sequenced with a M13 forward primer using a CEQ 8000 Automated DNA Sequencer (Beckman-Coulter), and compared with the MVNP sequence in Genbank (Accession number X01999) using Genetyx software (Software Development Co. Ltd., Tokyo, Japan).
Immunohistochemical Detection of MVNP
OCL derived from marrow cultures treated with 10-8M 1,25(OH)2D3 from a normal individual and PD patient #8, and from a WT and an MVNP mouse, were examined for cross-reactivity with a monoclonal antibody against MVNP (clone 2D7) (Abcam, Cambridge, MA) as previously described (Kurihara et al., 2006).
Retroviral Vector Construction and Viral Supernatant Preparation
Recombinant retroviral expression vectors were designed to produce either a previously validated antisense oligonucleotide targeted to 5′ end of the MVNP mRNA (AS-MVNP), or a scrambled antisense control (AS-CONT) (Bell et al., 1997). Briefly, the AS-MVNP oligonucleotide (5′-CTC GGA TAT CCC TAA TCC TGC TCT TGT CCC TGA TAA TAG GAT CTT GAA TCC T -3′) or the AS-CONT oligonucleotide (5′- AGG ATT CAA GAT CCT ATT ATC AGG GAC AAG AGC AGG ATT AGG GAT ATC CGA G-3′) was subcloned into the pLXSN retroviral vector. Recombinant retroviral constructs were transfected into the PT67 amphotropic packaging cell line, and stable clonal cell lines producing recombinant retroviruses at high titer (106 virus particles/mL) were established by selecting for G418 (400 μg/ml) resistance as previously described (Kurihara et al., 2000).
AS-MVNP or AS-CONT Transduction of PD and Normal OCL Precursors
Human marrow mononuclear cells isolated from involved sites of 3 MVNP+, and 3 MVNP- PD patients, and 4 normals were cultured for 96 hours with cytokines and the retroviral supernatant as previously described (Kurihara et al., 2000). The patient marrows were selected for study because they had sufficient cell numbers to perform all experiments. The cells were harvested for short-term CFU-GM clonogenic assays in methylcellulose and transduced CFU-GM resistant to G418 (400 μg/ml) were then used for the studies below.
Long-Term Culture of OCL Precursors Transduced with AS-MVNP or AS-CONT Vectors
AS-MVNP- or AS-CONT-transduced OCL precursor cells (5 × 103 cells/well) from PD patients or normals were cultured for 7 days with 100 pg/ml recombinant human GM-CSF as reported previously (Kurihara et al., 2000). Cells from G418-resistant colonies (5 × 103/culture) were individually collected, and cultured for OCL formation with 1,25(OH)2D3, or RANKL as described previously (Ishizuka et al., 2005). The vitronectin receptor positive multinucleated cells were scored using an inverted microscope. The IL-6 concentration of conditioned media recovered each week and pooled from individual OCL cultures was measured using an ELISA kit for human IL-6 (R&D, Minneapolis, MN).
Osteoclastic Bone Resorption on Dentin Slices
AS-MVNP- or AS-CONT-transduced CFU-GM derived cells (2×106 cells/ml) were dispersed into α-MEM media containing 20% horse serum, and seeded onto mammoth dentin slices (Wako Pure Chemical Industries Ltd. Osaka, Japan) in 96-well multiplates containing 10-10 M 1,25(OH)2D3 or 50 ng/ml RANKL with 10 ng/ml M-CSF to induce OCL formation. After 3 weeks, OCL were removed and pit areas quantified by image analysis as previously described (Ishizuka et al., 2005).
Generation of p62KI/MVNP and MVNP/IL-6KO mice
The Institutional Animal Care and Use Committees at Virginia Commonwealth University, University of Pittsburgh School of Medicine, and the VA Pittsburgh Healthcare System approved all animal studies. Transgenic mice expressing MVNP under the control of the mouse TRAP promoter were previously described (Kurihara et al., 2006), as were knock-in mice carrying a proline-to-leucine substitution at residue 394 of the endogenous mouse SQSTM1/p62 gene (equivalent to human p62P392L) (Hiruma et al., 2008). To establish a colony of mice expressing both the MVNP gene and the p62P394L mutant, MVNP transgenic mice were bred to p62P394L mice. In all subsequent generations, only one of the parents carried the MVNP transgene, and either or both parents were heterozygous or homozygous for the p62 mutation. All data are from mice generated from this p62P394L/MVNP colony (not from the parental p62P394L or MVNP colonies).
Global IL-6KO mice were generated by Kopf et al (Kopf et al., 1994), and obtained from The Jackson Laboratory (stock number 002650). Mice were interbred with MVNP mice as above. All data are from mice generated from this IL-6KO/MVNP colony (not from the parental colonies).
Mouse Bone Marrow Cultures
CD11b(+) cells (2 × 105 cells/well) were prepared as previously described and cultured for 3 days with M-CSF/RANKL or 1,25(OH) 2D3. TRAP-positive cells containing ≥ 3 nuclei were quantitated as described (Kurihara et al., 2006).
Signaling Pathway Activation in OCL Precursor Lysates from p62KI, MVNP, p62KI/MVNP or WT Mice
Cell lysates (50μg) from 1,25(OH)2D3 or RANKL treated OCL precursors of WT or transgenic mice were subjected to electrophoresis on 7.5% SDS-PAGE and the proteins transferred to nitrocellulose membranes and incubated in blocking solution (5% nonfat dry milk in TBS containing 0.1% Tween-20) for 1 h to reduce nonspecific binding. Membranes were then exposed to primary antibodies overnight at 4°C, was hed 3 times, incubated with secondary goat anti-mouse or rabbit IgG HRP-conjugated antibody and the bands detected by chemiluminescence following the manufacturer's directions (Bio-Rad). All blots were densitometrically quantitated, and the results expressed relative to control and normalized to β-actin or TFIID.
Histology processing, evaluation and histomorphometry
Lumbar vertebrae from 60 mice for the p62KI/MVNP experiment and 61 mice for the MVNP/IL-6KO experiment were fixed in 10% buffered formalin at 4°C. The 1st-4th lumbar vertebrae were decalcified in 10% EDTA at 4°C and embedded in paraffin. The 5th lumbar vertebra was embedded without decalcification in methyl methacrylate. Frontal sections were obtained from the decalcified and undecalcified samples. The decalcified sections were stained for TRAP and OCL containing active TRAP were stained red as described by Liu et al (Liu et al., 2003). The undecalcified sections were left unstained for the evaluation of fluorescent labels.
All sections were first evaluated by light microscopy, and then histomorphometric analysis was performed on the cancellous bone/marrow compartment between the cranial and caudal growth plates in the vertebral bodies without lesions using the OsteoMeasureXP™ version 1.01 morphometric program (OsteoMetrics, Inc., Atlanta, GA). Osteoclast perimeter (Oc.Pm) was defined as the length of bone surface covered with TRAP-positive, mono- and multi-nuclear cells. Cancellous bone volume (BV/TV), trabecular width (Tb.Wi), trabecular number (Tb.N), trabecular separation (Tb.Sp), mineralizing perimeter (Md.Pm), mineral apposition rate (MAR) and bone formation (BFR) were quantified. All variables were expressed and calculated according to the recommendations of the ASBMR Nomenclature Committee (Parfitt et al., 1987).
A transiliac crest bone biopsy was taken from a 58-year-old female patient with PD in the ilium. The biopsy sample was embedded without decalcification in methyl methacrylate. The sections were stained with Goldner's Trichrome or left unstained. Histological evaluation was performed under bright field, U-V and polarized light.
Statistical Analysis
Significance was evaluated by a two-sided, unpaired student t-test, or two-way analysis of variance where indicated. p<0.05 was considered significant. The differences in the histomorphometry variables among genotypes were statistically analyzed using ANOVA with Fisher's LSD multiple comparison tests, and the relationship of bone structural variables between 2D histomorphometry and 3D micro-CT was analyzed with linear correlation using the NCSS 2004 program.
Supplementary Material
Acknowledgments
We thank Dr. Fujinami, Ph.D., University of Utah for providing the AS-MVNP construct and Donna Gaspich for preparing the manuscript. We also thank all patients who participated in this study, and Danielle Poulin for her outstanding support in clinical data collection and analysis. We thank Dr. Ramesh Ramakrishnan for assistance with statistical analyses. The research was funded by grant PO1-AR049363 (GDR), R01 AR057308 (GDR), R01 AR057310 (DLG) and RO1 AR053537 (JJW) from NIAMS, NIH/NCRR/CTSA Grant UL1 RR024153 and NIH/NCRR/GCRC Grant M01 RR000056, and a grant from the Paget's Foundation. The materials are the result of work supported with resources and the use of facilities at the VA Pittsburgh Healthcare System, Research and Development, and the VCU Transgenic/Knockout Mouse Core, supported in part by the Massey Cancer Center Support Grant P30 CA016059.
Footnotes
Financial Disclosure: Drs. Noriyoshi Kurihara, Yuko Hiruma, Jolene Windle, David Dempster, Deborah Galson, Kei Yamana, Laëtitia Michou, Côme Rousseau, and Jean Morissette have no conflicts of interest. Dr. Jacques P. Brown reports having served as a consultant to Amgen, Sanofi-Aventis, Warner-Chilcott, Merck, E. Lily, Novartis, Servier. Dr. G. David Roodman reports having served as a consultant to Amgen, Novartis, Celgene and Millennium, and received grant support from Novartis as well.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bell AF, Whitton JL, Fujinami RS. Antisense-mediated resistance to measles virus infection in HeLa cells. J Infect Dis. 1997;176:258–261. doi: 10.1086/517261. [DOI] [PubMed] [Google Scholar]
- Custer SK, Neumann M, Lu H, Wright AC, Taylor JP. Transgenic mice expressing mutant forms VCP/p97 recapitulate the full spectrum of IBMPFD including degeneration in muscle, brain and bone. Hum Mol Genet. 2010;19:1741–1755. doi: 10.1093/hmg/ddq050. [DOI] [PubMed] [Google Scholar]
- Gennari L, Gianfrancesco F, Di Stefano M, Rendina D, Merlotti D, Esposito T, Gallone S, Fusco P, Rainero I, Fenoglio P, et al. SQSTM1 gene analysis and gene-environment interaction in Paget's disease of bone. J Bone Miner Res. 2010;25:1375–1384. doi: 10.1002/jbmr.31. [DOI] [PubMed] [Google Scholar]
- Helin E, Vainionpaa R, Hyypia T, Julkunen I, Matikainen S. Measles virus activates NF-kappa B and STAT transcription factors and production of IFN-alpha/beta and IL-6 in the human lung epithelial cell line A549. Virology. 2001;290:1–10. doi: 10.1006/viro.2001.1174. [DOI] [PubMed] [Google Scholar]
- Hiruma Y, Kurihara N, Subler MA, Zhou H, Boykin CS, Zhang H, Ishizuka S, Dempster DW, Roodman GD, Windle JJ. A SQSTM1/p62 mutation linked to Paget's disease increases the osteoclastogenic potential of the bone microenvironment. Hum Mol Genet. 2008;17:3708–3719. doi: 10.1093/hmg/ddn266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka S, Kurihara N, Reddy SV, Cornish J, Cundy T, Roodman GD. (23S)-25-Dehydro-1{alpha}-hydroxyvitamin D3-26,23-lactone, a vitamin D receptor antagonist that inhibits osteoclast formation and bone resorption in bone marrow cultures from patients with Paget's disease. Endocrinology. 2005;146:2023–2030. doi: 10.1210/en.2004-1140. [DOI] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Kukita A, Chenu C, McManus LM, Mundy GR, Roodman GD. Atypical multinucleated cells form in long-term marrow cultures from patients with Paget's disease. J Clin Invest. 1990;85:1280–1286. doi: 10.1172/JCI114565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara N, Bertolini D, Suda T, Akiyama Y, Roodman GD. IL-6 stimulates osteoclast-like multinucleated cell formation in long term human marrow cultures by inducing IL-1 release. J Immunol. 1990;144:4226–4230. [PubMed] [Google Scholar]
- Kurihara N, Civin C, Roodman GD. Osteotropic factor responsiveness of highly purified populations of early and late precursors for human multinucleated cells expressing the osteoclast phenotype. J Bone Miner Res. 1991;6:257–261. doi: 10.1002/jbmr.5650060307. [DOI] [PubMed] [Google Scholar]
- Kurihara N, Hiruma Y, Zhou H, Subler MA, Dempster DW, Singer FR, Reddy SV, Gruber HE, Windle JJ, Roodman GD. Mutation of the sequestosome 1 (p62) gene increases osteoclastogenesis but does not induce Paget disease. J Clin Invest. 2007;117:133–142. doi: 10.1172/JCI28267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara N, Reddy SV, Araki N, Ishizuka S, Ozono K, Cornish J, Cundy T, Singer FR, Roodman GD. Role of TAFII-17, a VDR binding protein, in the increased osteoclast formation in Paget's Disease. J Bone Miner Res. 2004;19:1154–1164. doi: 10.1359/JBMR.040312. [DOI] [PubMed] [Google Scholar]
- Kurihara N, Reddy SV, Menaa C, Anderson D, Roodman GD. Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J Clin Invest. 2000;105:607–614. doi: 10.1172/JCI8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara N, Windle JJ, Roodman GD. Treatment of Osteoclast (OCL) Precursors Expressing the Measles Virus Nucleocapsid Protein (MVNP) with 1,25-(OH)2D3 Increases Expression of Specific AP-1 Subtypes. 31st Annual American Society Bone and Mineral Meeting; Denver, CO. 2009. Abstract #1033. [Google Scholar]
- Kurihara N, Zhou H, Reddy SV, Garcia Palacios V, Subler MA, Dempster DW, Windle JJ, Roodman GD. Expression of measles virus nucleocapsid protein in osteoclasts induces Paget's disease-like bone lesions in mice. J Bone Miner Res. 2006;21:446–455. doi: 10.1359/JBMR.051108. [DOI] [PubMed] [Google Scholar]
- Laurin N, Brown JP, Morissette J, Raymond V. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet. 2002;70:1582–1588. doi: 10.1086/340731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Yu SF, Li TJ. Multinucleated giant cells in various forms of giant cell containing lesions of the jaws express features of osteoclasts. J Oral Pathol Med. 2003;32:367–375. doi: 10.1034/j.1600-0714.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- Manchester M, Eto DS, Oldstone MB. Characterization of the inflammatory response during acute measles encephalitis in NSE-CD46 transgenic mice. J Neuroimmunol. 1999;96:207–217. doi: 10.1016/s0165-5728(99)00036-3. [DOI] [PubMed] [Google Scholar]
- Menaa C, Reddy SV, Kurihara N, Maeda H, Anderson D, Cundy T, Cornish J, Singer FR, Bruder JM, Roodman GD. Enhanced RANK ligand expression and responsivity of bone marrow cells in Paget's disease of bone. J Clin Invest. 2000;105:1833–1838. doi: 10.1172/JCI9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills BG, Frausto A, Singer FR, Ohsaki Y, Demulder A, Roodman GD. Multinucleated cells formed in vitro from Paget's bone marrow express viral antigens. Bone. 1994;15:443–448. doi: 10.1016/8756-3282(94)90823-0. [DOI] [PubMed] [Google Scholar]
- Mills BG, Singer FR, Weiner LP, Suffin SC, Stabile E, Holst P. Evidence for both respiratory syncytial virus and measles virus antigens in the osteoclasts of patients with Paget's disease of bone. Clin Orthop Relat Res. 1984:303–311. [PubMed] [Google Scholar]
- Morissette J, Laurin N, Brown JP. Sequestosome 1: mutation frequencies, haplotypes, and phenotypes in familial Paget's disease of bone. J Bone Miner Res. 2006;21 2:P38–44. doi: 10.1359/jbmr.06s207. [DOI] [PubMed] [Google Scholar]
- Mossetti G, Rendina D, De Filippo G, Viceconti R, Di Domenico G, Cioffi M, Postiglione L, Nunziata V. Interleukin-6 and osteoprotegerin systems in Paget's disease of bone: relationship to risedronate treatment. Bone. 2005;36:549–554. doi: 10.1016/j.bone.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Neale SD, Smith R, Wass JA, Athanasou NA. Osteoclast differentiation from circulating mononuclear precursors in Paget's disease is hypersensitive to 1,25-dihydroxyvitamin D(3) and RANKL. Bone. 2000;27:409–416. doi: 10.1016/s8756-3282(00)00345-8. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Ralston SH. Pathogenesis of Paget's disease of bone. Clin Rev Bone Miner Metab. 2002;1:109–114. [Google Scholar]
- Reddy SV, Menaa C, Singer FR, Cundy T, Cornish J, Whyte MP, Roodman GD. Measles virus nucleocapsid transcript expression is not restricted to the osteoclast lineage in patients with Paget's disease of bone. Exp Hematol. 1999;27:1528–1532. doi: 10.1016/s0301-472x(99)00097-1. [DOI] [PubMed] [Google Scholar]
- Reddy SV, Singer FR, Roodman GD. Bone marrow mononuclear cells from patients with Paget's disease contain measles virus nucleocapsid messenger ribonucleic acid that has mutations in a specific region of the sequence. J Clin Endocrinol Metab. 1995;80:2108–2111. doi: 10.1210/jcem.80.7.7608263. [DOI] [PubMed] [Google Scholar]
- Roodman GD, Kurihara N, Ohsaki Y, Kukita A, Hosking D, Demulder A, Smith JF, Singer FR. Interleukin 6. A potential autocrine/paracrine factor in Paget's disease of bone. J Clin Invest. 1992;89:46–52. doi: 10.1172/JCI115584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman GD, Windle JJ. Paget disease of bone. J Clin Invest. 2005;115:200–208. doi: 10.1172/JCI24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Schaulies J, Schneider-Schaulies S, Ter Meulen V. Differential induction of cytokines by primary and persistent measles virus infections in human glial cells. Virology. 1993;195:219–228. doi: 10.1006/viro.1993.1363. [DOI] [PubMed] [Google Scholar]
- Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.