SUMMARY
Limited data suggest that low T-helper cell levels may be observed in hepatitis C virus (HCV) monoinfected patients with decompensated liver disease. We sought to determine the distribution and relationship of T-helper cells (CD4) to liver fibrosis in HCV-monoinfected patients before and during pegylated interferon (PegIFN) therapy. CD4 populations were prospectively determined using flow cytometry. All subjects had compensated liver disease. Baseline and subsequent CD4 counts at treatment weeks 12, 24, 36 and 48 and at two time points following treatment discontinuation (weeks 60 and 72) were evaluated. Ishak score was determined by a central pathologist. At baseline, data from 267 subjects were available. Mean age was 50 and 68% were male/Caucasian. HCV viral load was > 800 000 IU/mL in 55%. Nearly half (48%) were Ishak 4–6 with all stages represented. Mean CD4 count was 1004 cells/mm3 ± 400, and 6% had counts <500. There was a trend towards lower CD4 counts among cirrhotic subjects (P = 0.07). A CD4 decrease was noted following PegIFN initiation. Mean CD4 decline was 38.9% and was statistically significant for all fibrosis stages compared with baseline levels, but not between fibrosis levels. CD4 counts <500 cells/mm3 are seen in <10% of HCV-monoinfected subjects. A trend towards lower CD4 counts in subjects with advanced fibrosis was observed. However, at baseline and during/after PegIFN therapy, no significant differences were observed between groups. CD4 counts declined during PegIFN treatment, but returned to baseline after completion. The significance of these findings in terms of disease progression and treatment response requires further evaluation.
Keywords: CD4, fibrosis, HCV, HCV RNA, interferon, stage
INTRODUCTION
Infection with human immunodeficiency virus (HIV) is characterized by depletion of CD3/CD4 + T-helper cell populations leading to immune deficiency and occurrence of opportunistic infections. Low CD4 counts are also associated with increased rates of liver fibrosis in patients co-infected with the hepatitis C virus (HCV) [1,2]. It has been assumed that low CD4 counts are solely attributable to the presence of HIV infection. However, recent reports suggest that low CD4 counts may be observed in patients with HCV infection alone [3,4]. This observation was attributed to the presence of advanced liver disease, with presumed presence of portal hypertension. However, the relationship between CD4 count, and degree of liver fibrosis has not previously been described, nor have the expected range and distribution of CD4 in HCV-monoinfected patients. We hypothesized that liver fibrosis leads to development of portal hypertension which may in turn be associated with a decrease in CD4 count, and that a gradient of CD4 counts should be present when stratified by degree of fibrosis. It is also known that CD4 counts are diminished in HCV/HIV co-infected subjects during administration of interferon. However, the effect of HCV treatment on CD4 counts during interferon-based therapy in HCV-monoinfected subjects has not previously been reported.
METHODS
Patient population
The study cohort was derived from two parent studies designed to determine the efficacy of pegylated interferon (PegIFN) alfa-2a + thymosin alpha-1 in chronic HCV-infected subjects who were previously classified as non-responders to prior interferon-based therapy [5]. The paired studies were virtually identical except that one trial (SciClone 803) permitted enrolment of patients with a baseline fibrosis stage of Metavir 1–3, and the second (SciClone 804) enrolled subjects with more advanced fibrosis (Metavir 3, 4). Enrolled subjects had chronic hepatitis attributable to HCV without other intercurrent liver disease aetiologies. All had compensated liver disease without history of ascites, bleeding varices, or encephalopathy. Both studies captured baseline and subsequent CD4 counts at weeks 12, 24, 36 and 48 during treatment and then at two time points following treatment discontinuation (weeks 60 and 72). CD4 and CD4% were determined in a certified clinical laboratory using standard flow cytometry methodologies in a prospective manner before, during and after drug administration. Because the primary efficacy analysis failed to find any effect of thymosin alpha-1 on treatment outcome, and we failed to find any thymosin effect on CD4 count, data related to CD4, baseline patient characteristics and treatment effect on CD4 were analyzed across both parent studies irrespective of treatment arm.
Although initial study entry was based upon local interpretation of the biopsy using the Metavir (4 stage) scoring system, pretreatment liver biopsies were later evaluated by a single central pathologist (ZDG) and scored using the Ishak (6 stage) scoring system. Data were analyzed using both fibrosis scores (E score) and total Ishak score which includes the inflammatory components of the histological grade.
Statistical analysis
Parametric tests (Student’s T-test, ANOVA, linear regression) and non-parametric tests (Spearman rank correlation) were used to analyze numerical data. A P-value of <0.05 was used to indicate significance with a 2-tailed hypothesis. Statistical analyses were performed using Minitab Software, version 15 (Minitab Incorporated).
RESULTS
CD4 distribution in hepatitis C virus-monoinfected patients
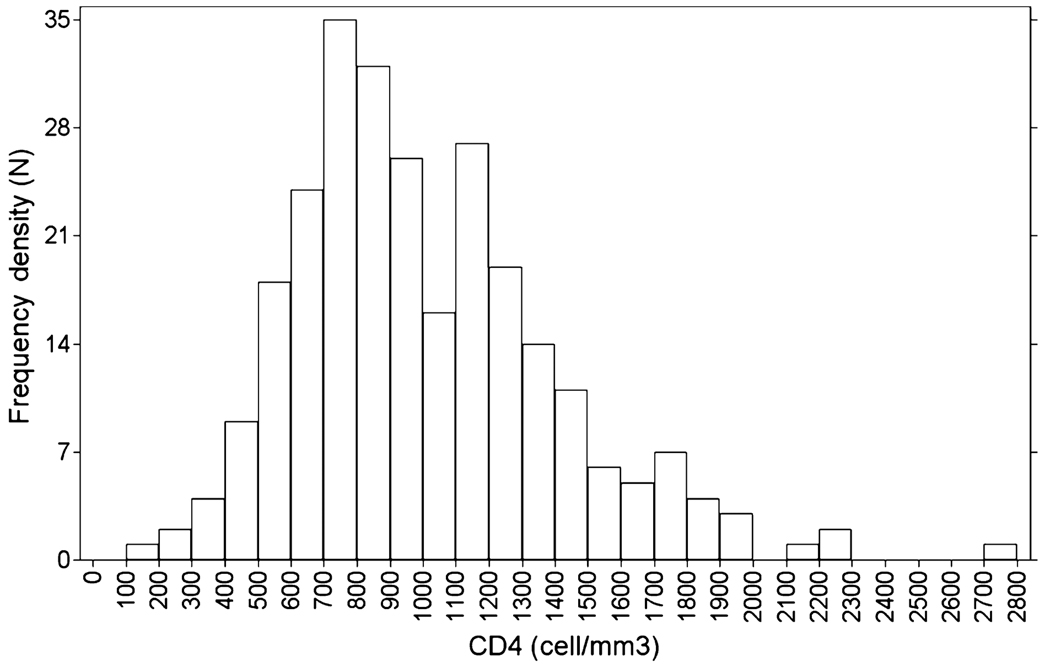
Although >1000 subjects were enrolled in the parent studies, CD4 data were available for only 267 samples because of its inclusion in a protocol amendment after the studies were initiated. Table 1 shows the demographic characteristics of these subjects as well as the CD4 counts. At baseline, 6% had a CD4 count <500 cells/mm3. Levels below this are often associated with mild immunodeficiency and increased risk of immune deficit-related clinical outcomes [6]. Fig. 1 shows the overall distribution of CD4 in HCV-monoinfected subjects.
Table 1.
Demographic characteristics and baseline CD4 T-cell counts of HIV-seronegative patients, stratified by stage of liver fibrosis
| Characteristic | Total Patients |
Fibrosis stage 1 |
Fibrosis stage 2 |
Fibrosis stage 3 |
Fibrosis stage 4 |
Fibrosis stage 5 |
Fibrosis stage 6 |
|---|---|---|---|---|---|---|---|
| Sample size | 267 | 16 | 53 | 70 | 24 | 40 | 48 |
| Age (years) | 50 ± 7 | 48 ± 6 | 49 ± 6 | 51 ± 7 | 49 ± 8 | 52 ± 7 | 50 ± 7 |
| Gender | |||||||
| Male | 181 (68) | 10 (62.5) | 39 (74) | 47 (67) | 17 (71) | 27 (68) | 30 (62.5) |
| Female | 86 (32) | 6 (37.5) | 14 (26) | 23 (33) | 7 (29) | 13 (33) | 18 (37.5) |
| Race | |||||||
| White | 181 (68) | 14 (87.5) | 34 (64) | 45 (64) | 15 (63) | 26 (65) | 36 (75) |
| Black | 53 (20) | 2 (12.5) | 11 (21) | 17 (24) | 6 (25) | 7 (18) | 6 (13) |
| Asian | 3 (1) | 0 | 0 | 0 | 0 | 1 (3) | 2 (4) |
| Hispanic | 25 (9) | 0 | 8 (15) | 6 (9) | 2 (8) | 4 (10) | 4 (8) |
| Other | 4 (1) | 0 | 0 | 2 (3) | 1 (4) | 1 (3) | 0 |
| HCV viral load | |||||||
| High (IU/mL) | 148 (55) | 10 (62.5) | 28 (53) | 41 (59) | 14 (58) | 20 (50) | 27 (56) |
| Low (IU/mL) | 119 (45) | 6 (37.5) | 25 (47) | 29 (41) | 10 (42) | 20 (50) | 21 (44) |
| CD4 T-cell counts | |||||||
| Baseline (cells/mm3) | 1004 ± 400 | 955 ± 329 | 1021 ± 326 | 1017 ± 418 | 1125 ± 363 | 927 ± 347 | 944 ± 479 |
For categorical variables, data represented as number of patients (per cent of patients) in each category; for numerical variables, data represented as mean ± SD.
Fig. 1.
Overall distribution of baseline CD4 counts in HCV monoinfected subjects. n = number of patients.
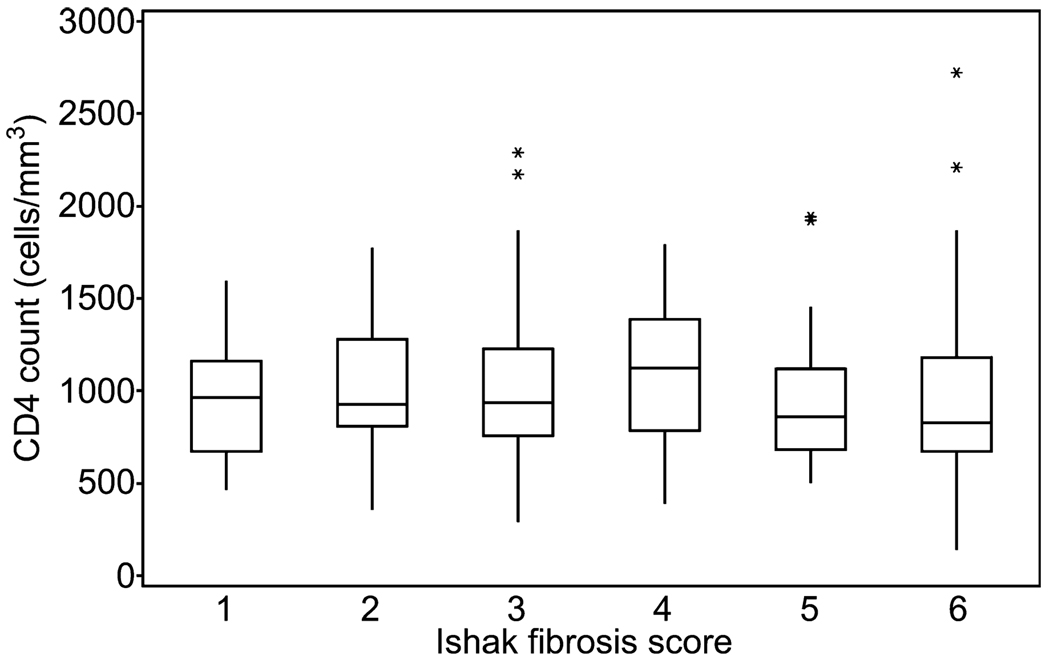
Baseline CD4 counts distributed by Ishak fibrosis stage are shown in Fig. 2. No significant differences between stage and CD4 were noted (P = 0.159). Pooled analysis of Ishak fibrosis stages 1 through 3 compared with stages 4 through 6 determined that no significant association between stage and CD4 count was present (P = 0.487) with this grouping. However, analysis comparing pooled stages 1 through 4 vs stages 5 and 6 (minimal to moderate fibrosis vs cirrhosis) displayed a strong trend for CD4 counts to be associated with Ishak fibrosis score, but failed to reach statistical significance (P = 0.076). No significant association between baseline CD4 counts and age, gender, race, or HCV viral load were noted (P = 0.924, P = 0.355, P = 0.448, P = 0.282). Comparison between CD4 count and total Ishak score determined that there was an overall association between Ishak total score and CD4 count (P = 0.029). However, when the end groups were pooled to ensure that each sample size was >10, there was no significant correlation between total score and CD4 count (P = 0.416). Evaluation of the relationship between the Ishak inflammatory score and CD4 count also yielded no significant correlation (P = 0.271). CD4% was also analyzed. At baseline, this was 48.1 ± 7.5. ANOVA failed to find any statistically meaningful differences when baseline CD4% was compared across fibrosis stages.
Fig. 2.
Baseline CD4 stratified by Ishak fibrosis stage. Asterisk (*) indicates an outlier value.
CD4 count during/after pegylated interferon alfa
Sequential data during PegIFN treatment were available for 122 patients. Data were excluded from this portion of the analysis if there were missing CD4 T-cell counts at any of the four time points when data were collected (n = 130) or the level of liver fibrosis was unknown or not reported (n = 15). The patients did not differ significantly from the primary study set with regard to age, race, gender, fibrosis distribution or HCV viral load. ANOVA failed to find any statistical association between subjects who received PegIFN only and those who received PegIFN + thymosin alpha-1. Therefore, all results were pooled across treatment arm.
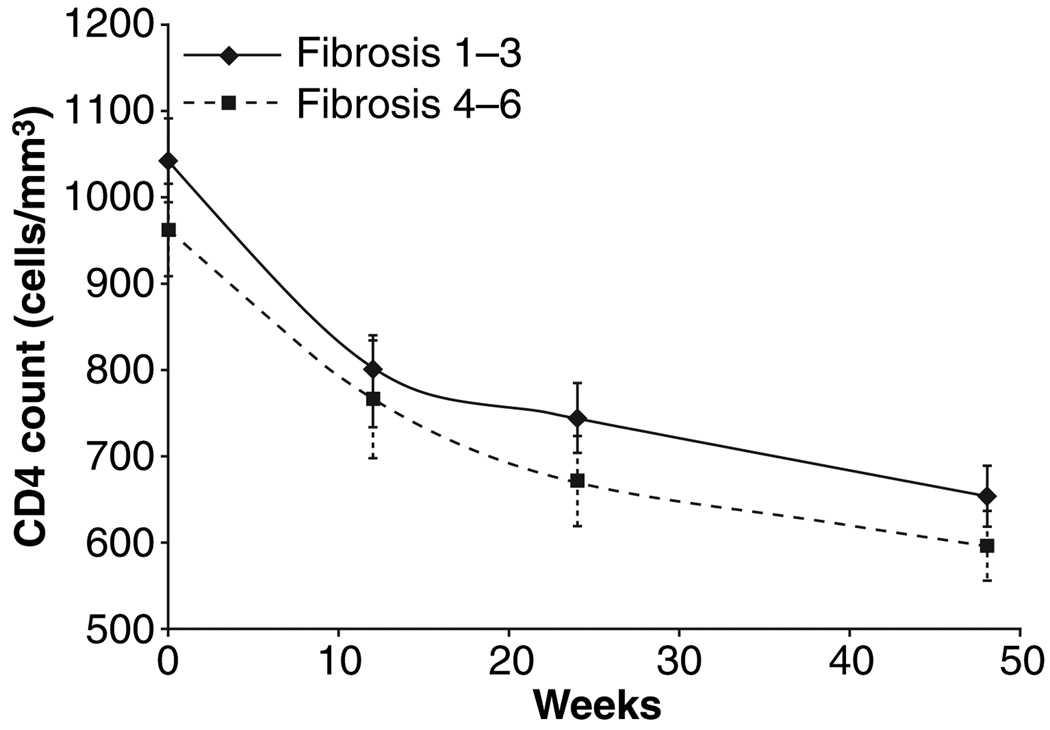
The ranges of baseline CD4 T-cell counts for each level of liver fibrosis 1 through 6 were, respectively, 519–1589, 493–1767, 323–2292, 737–1789, 506–1921, and 542–1863 cells/mm3. Forty-eight weeks after treatment with PegIFN, the CD4 T-cell counts had declined significantly for each stage of liver fibrosis (P = 0.003, P < 0.001, P < 0.001, P < 0.001, P = 0.004, P = 0.002 for each stage 1 through 6, respectively). (Fig. 3) The mean percentage of CD4 decline was 38.9%. However, following mathematic transformation to linear models over time, regression modelling failed to identify any relationship between fibrosis level and CD4 count. Interestingly, there was little variation in CD4% when baseline (48.1%) was compared with weeks 12 (49.6%), 24 (48.3%) and 48 (47.3%) on PegIFN therapy.
Fig. 3.
Mean CD4 counts by fibrosis stage grouping (Ishak 1–3 vs 4–6) over period of treatment with pegylated interferon alfa-2a. Error bar represents standard error of mean. No significant difference between observed outcome at any time point or in group comparison.
Comparison tests were also performed on different groupings by fibrosis level. Using sequential groups of two fibrosis levels, there appeared to be no significant difference between any of the fibrosis level groupings at any of the time points; combined fibrosis levels 1 and 2, levels 3 and 4, and levels 5 and 6 were all determined to be statistically identical at each time point. The same result was obtained when these tests were performed on sequential groupings of three fibrosis levels; combined fibrosis levels 1, 2 and 3 were determined to be statistically identical to levels 4, 5 and 6 at each time point.
Univariate tests were performed on the data categorized by gender as well as the level of liver fibrosis at each time point. For patients with liver fibrosis Stage 1, the mean CD4 T-cell count was different for men and women at weeks 12, 24 and 48 (P = 0.038, P = 0.007, P = 0.045). For all other fibrosis levels, CD4 T-cell counts for men were concluded to be the same as counts for women. There was no significant difference between the levels of liver fibrosis for the male patients; there was no significant pattern to the mean CD4 T-cell counts between the different fibrosis levels across time. Similarly, among women, no mean differences were found by fibrosis levels at each time point. There was no apparent difference between mean CD4 T-cell counts at each time point between high and low HCV viral loads.
DISCUSSION
In patients with HIV, T-helper cell counts are thought to represent the most important surrogate of immune dysfunction and disease progression [7]. Patients with HCV also experience immune dysfunction with progressive liver disease. Anthony et al. reported decreased T-cell responses to HCV core among cirrhotic vs non-cirrhotic patients using ELISPOT assays [8]. Similar results have been described by others [9]. While it is generally assumed that direct infection by HIV represents the dominant source of CD4 depletion among infected patients, it is also recognized that liver fibrosis, associated with development of portal hypertension may also lead to decreased cell populations (lymphocytes, thrombocytes and erythrocytes) via a mechanism of splenic enlargement with increased splenic sequestration of cellular elements [10–12].
Among HIV-infected patients with HCV or HBV, rapid acceleration of fibrotic progression has been described. This process appears to be influenced by the presence of more severe immunodeficiency as determined by low CD4 count. Benhamou first described the association of rapid progression to cirrhosis with CD4 counts <200 cells/mL [1]. While the study population was that of HCV/HIV co-infected patients, HCV-monoinfected patients served as the control groups, and it was assumed that CD4 levels were normal in this comparator cohort. Recently, McGovern et al. provided evidence that HCV-monoinfected patients with advanced decompensated liver disease had lower than population normalized CD4 levels. Clearly, the difference between the two studies is that we examined only patients with compensated liver disease, while the majority of patients who reported had signs and symptoms of end-stage liver disease manifest by identifiable features of portal hypertension in 83% [4].
In terms of PegIFN therapy, there is a consistent and predictable decrease in CD4 count following initiation of therapy. This has previously been described in subjects with HCV/HIV coinfection undergoing therapy with interferon-based regimens [13,14]. These studies reported that CD4% rose during PegIFN treatment. In our dataset, CD4% remained stable over the course of interferon therapy. There is no published literature describing effect of ribavirin on modification of CD4 T-cell subsets including any single agent effect on white blood cells from the product insert. We failed to find any difference between PegIFN effect on T-helper cell populations and degree of hepatic fibrosis, although a trend towards lower CD4 count among cirrhotic subjects (Ishak 5–6) was observed. In terms of treatment outcome, CD4 appears to be an independent predictor of treatment response in HCV/HIV co-infected subjects. Opravil et al. described improved SVR rates among HCV genotype 1 co-infected subjects with higher CD4 counts, who were enrolled in a large, multicenter trial. Those in the highest CD4 quartile demonstrated a 47% SVR rate, while those in the lowest quarter achieved SVR only 33% of the time [15]. Furthermore, specific CD4 T-cell responses were associated with viral clearance following acute infection [16–18]. The effect of baseline CD4 count in HCV monoinfection has not been explored. Although our data are derived from a treatment trial, all subjects were prior treatment non-responders and received the equivalent of PegIFN monotherapy yielding very low SVR rates (<5%) and making analysis of treatment outcome related to CD4 count impossible to analyze. However, the wide range of CD4 counts at baseline and prior to treatment, and the trend towards lower CD4 counts among those with cirrhosis, suggests that CD4 should be evaluated in the context of large clinical trials of HCV-monoinfected patients.
Abbreviations
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- PegIFN
pegylated interferon
- TSS
tropical splenomegaly syndrome
REFERENCES
- 1.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 2.Puoti M, Bonacini M, Spinetti A, et al. Liver fibrosis progression is related to CD4 cell depletion in patients coinfected with hepatitis C virus and human immunodeficiency virus. J Infect Dis. 2001;183:134–137. doi: 10.1086/317644. [DOI] [PubMed] [Google Scholar]
- 3.McGovern BLM, Golan Y, Lawton A, et al. Absolute CD4 T cell subsets are decreased in HIV seronegative patients with cirrhosis. Hepatology. 2003;38:429A. [Google Scholar]
- 4.McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4 + T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431–437. doi: 10.1086/509580. [DOI] [PubMed] [Google Scholar]
- 5.Sherman KE, Gordan SC, Iftikar R, Rodriguez-Torres M, Rustgi V, Di Bisceglie A. Retreatment of treatment experienced HCV patients with pegylated interferon alfa-2A and thymosin alpha-1: pooled analysis of two randomized controlled trials. Hepatology. 2007;46 4 Suppl. 1:1331. [Google Scholar]
- 6.Kaufmann GR, Furrer H, Ledergerber B, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41:361–372. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 7.Benson CA, Kaplan JE, Masur H, Pau A, Holmes KK. Treating opportunistic infections among HIV-infected adults and adolescents. MMWR. 2004;53:1–112. [PubMed] [Google Scholar]
- 8.Anthony D, Post AB, Valdez H, Peterson D, Murphy M, Heeger P. ELISPOT analysis of hepatitis C virus protein-specific IFNg-producing peripheral blood lymphocytes in infected humans with and without cirrhosis. Clin Immunol. 2001;99:232–240. doi: 10.1006/clim.2001.5018. [DOI] [PubMed] [Google Scholar]
- 9.Sreenarasimhaiah J, Jaramillo A, Crippin J, Lisker-Melman M, Chapman WC, Mohanakumar T. Lack of optimal T-cell reactivity against the hepatitis C virus is associated with the development of fibrosis/cirrhosis during chronic hepatitis. Hum Immunol. 2003;64:224–230. doi: 10.1016/s0198-8859(02)00781-4. [DOI] [PubMed] [Google Scholar]
- 10.Holzbach RT, Shipley RA, Clark RE, Chudzik EB. Influence of spleen size and portal pressure on erythrocyte sequestration. J Clin Invest. 1964;43:1125–1135. doi: 10.1172/JCI104997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrella V, Rigotti T, Parachini F, Cantone R. Thrombopenia and cirrhosis. Study of 56 cases. Minerva Med. 1987;78:1523–1525. [PubMed] [Google Scholar]
- 12.Fakunle YM, Oduloju AJ, Greenwood BM. T- and B-lymphocyte subpopulations in the tropical splenomegaly syndrome (TSS) Clin Exp Immunol. 1978;33:239–243. [PMC free article] [PubMed] [Google Scholar]
- 13.Chung R, Andersen J, Volberding P, et al. Peginterferon alpha-2a plus ribavirin versus interferon alpha-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 15.Opravil M, Sasadeusz J, Cooper DA, et al. Effect of baseline CD4 cell count on the efficacy and safety of peginterferon Alfa-2a (40KD) plus ribavirin in patients with HIV/hepatitis C virus coinfection. J Acquir Immune Defic Syndr. 2008;47:36–49. doi: 10.1097/QAI.0b013e31815ac47d. [DOI] [PubMed] [Google Scholar]
- 16.Diepolder HM, Zachoval R, Hoffmann RM, et al. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–1007. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- 17.Thimme R, Bukh J, Spangenberg H, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci. 2002;99:15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urbani S, Amadei B, Fisicaro P, et al. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126–139. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]