Abstract
Impaired mitochondrial function is largely thought to be a core abnormality responsible for disease progression in nonalcoholic fatty liver disease (NAFLD). However, the molecular mechanisms resulting in mitochondrial dysfunction in NAFLD remain poorly understood. This study examined the effects of excessive accumulation of free fatty acids (FFAs) in liver cells on mitochondrial function and the role of the lysosomal-mitochondrial axis on lipotoxicity. Primary mouse hepatocytes, HepG2 and McNtcp.24 cells, were treated with varied concentrations of FFAs with different degrees of saturation for up to 24 hours. Mitochondrial function was monitored by real-time imaging, cytochrome c redistribution, and reactive oxygen species (ROS) production. The temporal relationship of lysosomal and mitochondrial permeabilization was established. Activity of the lysosomal protease cathepsin B was suppressed by genetic and pharmacological approaches. Cathepsin B– knockout mice and wild-type animals were place on a high-carbohydrate diet for 16 weeks, and mitochondrial function and liver damage were assessed. Exposure of liver cells to saturated FFAs resulted in mitochondrial depolarization, cytochrome c release, and increased ROS production. Lysosomal permeabilization and cathepsin B redistribution into the cytoplasm occurred several hours prior to mitochondrial dysfunction. Either pharmacological or genetic inhibition of cathepsin B preserved mitochondrial function. Finally, cathepsin B inactivation protected mitochondria, decreased oxidative stress, and attenuated hepatic injury in vivo.
Conclusion
These data strongly suggest excessive accumulation of saturated FFAs in liver cells directly induce mitochondrial dysfunction and oxidative stress. Our data further suggest this process is dependent on lysosomal disruption and activation of cathepsin B.
Nonalcoholic fatty liver disease (NAFLD) is currently the most common form of chronic liver disease affecting both adults and children in the United States and many other parts of the world.1,2 Non-alcoholic steatohepatitis (NASH) is a stage in the spectrum of NAFLD and is characterized by the accumulation of fat in the liver (steatosis) along with evidence of liver damage, inflammation, and different degrees of scarring or fibrosis.3 NASH is a potentially serious condition, as approximately 25% of patients with NASH may progress to cirrhosis and its feared complications of portal hypertension, liver failure, and hepatocellular carcinoma.4,5 Thus, the clinical implications of NAFLD derive from its common occurrence in both adults and children and its potential to progress to cirrhosis and liver failure.
The pathogenesis of NAFLD/NASH and in particular the mechanisms responsible for liver injury and disease progression remain poorly understood but are of significant biomedical importance, as identification of these processes may help to identify novel therapeutic targets to treat this disease.6 A net retention of lipids, mostly in the form of triglycerides, in hepatocytes is a prerequisite for the development of NAFLD.7 Although enlarged stores of triglycerides in lipid droplets are probably inert and therefore in themselves harmless to cells,8,9 other lipids such as free fatty acids (FFAs) and cholesterol may also overaccumulate.10,11 Increasing evidence suggests that in nonadipose cells, the accumulation of certain FFAs may be cytotoxic.8,12 Mitochondria are involved in many processes essential for liver cell survival, including energy production, redox control, calcium homeostasis, and certain metabolic and biosynthetic pathways. In addition, mitochondria often play an essential role in cell death mechanisms.13 Several lines of evidence suggest that impaired mitochondrial function is a central abnormality responsible for the progression from simple steatosis to steatohepatitis in NAFLD.14,15 Mitochondrial dysfunction is characterized by mitochondrial outer-membrane permeabilization (MOMP). MOMP results in the release of multiple proteins from the mitochondrial intermembrane space (IMS) into the cytosol. This leads to caspase activation in the cytosol,16 disruption of the mitochondrial respiratory chain (MRC), loss of mitochondrial transmembrane potential (ΔΨm), free-radical production, and loss of mitochondrial structural integrity.17 Although the relationships between structural changes, decreased mitochondrial function, and disease progression in NAFLD are becoming clearer, the subcellular and molecular mechanisms resulting in mitochondrial dysfunction in NAFLD remain poorly understood. Thus, the overall objective of the present study was to characterize the fundamental cellular mechanisms that link excess lipid accumulation in liver cells to mitochondrial dysfunction and disease progression in NAFLD. We provide evidence that in both human and murine hepatocytes, saturated FFAs induce mitochondrial dysfunction and increased ROS production. We show that these events are downstream of lysosomal permeabilization and that genetic or pharmacological inhibition of cathepsin B protects against FFA-induced mitochondrial dysfunction and oxidative stress both in vitro and in vivo.
Materials and Methods
Cell Lines and Culture
Hepatocytes were isolated from wild-type C57BL/6 male mice (Jackson Laboratories, Bar Harbor, ME), purified by Percoll gradient centrifugation, cultured as previously described,18,19 and used 4 hours after isolation. A well-differentiated human hepatoblastoma cell line, HepG2 cells, and a well-differentiated rat hepatoma cell line, McNtcp.24 cells, were cultured as previously described.20 Previous studies from our laboratory showed that these cells are sensitive to FFA-induced lysosomal permeabilization in a dose- and time-dependent manner, with kinetics comparable to those observed in primary mouse hepatocytes.21 Cultured cells were treated with various concentrations (0.05–0.5 mM) of long-chain free fatty acids with different degrees of saturation, including palmitate (saturated), oleate (1 double bond), and linoleic (2 double bonds), from Sigma (St. Louis, MO), in media containing 1% bovine serum albumin for as long as 24 hours (0.5, 1, 2, 4, 8, 16, and 24 hours). In selected experiments, cells were incubated with the fatty acid mixture in the presence or absence of the cathepsin B–selective inhibitors CA074 (20 μM, Sigma-Aldrich) or E-64 (10 μM, Roche Diagnostics).
Small Interfering RNA and Transfection
HepG2 cells were grown in 6-well plates and transiently transfected with 30 nmol/L of predesign cathepsin B small interfering RNA (siRNA) (AM16708; Ambion) using 8 mL of the siPORT NeoFXTM Transfection Agent (Ambion) in a total transfection volume of 0.5 mL of OPTI-MEM (Gibco-Invitrogen Corp., Carlsbad, CA). After incubation at 37°C for 5 hours, 1.5 mL of normal growth medium was added. Cells were used for experiments 48 hours after transfection. Inhibition of cathepsin B protein expression was assessed by immunoblot analysis.
LysoTracker Red, Tetramethylrhodamine Methyl Ester Loading and Immunofluorescence
A time course of lysosomal and mitochondrial permeabilization was determined by staining with LysoTracker Red (577/590 nm), an acidophilic fluorescent dye that loads predominantly into lysosomes (Molecular Probes) and tetramethylrhodamine methyl ester (TMRM, 549/573 nm; Molecular Probes), a mitochondrion-selective dye that is released after mitochondrial depolarization in unfixed live cells and visualized under fluorescent microscopy Alternatively, cell were fixed with freshly made 4% para-formaldehyde in PBS for 15 minutes at 4°C. Immunofluorescence detection of cytochrome c was performed using a monoclonal antibody (1:100 dilution, mAb 6H2.B4; BD Biosciences). Cells were then imaged using an inverted laser scanning confocal microscope (model LSM S10; Carl Zeiss, Thornwood, NJ). The percentage of cells displaying positive staining was counted in 30 random fields as previously described by us in detail.22
Cathepsin B Activity Assay
Cathepsin B activity was determined fluorometrically in whole-cell lysates using the InnoZyme cathepsin B activity assay (CBA001; Calbiochem) according to the manufacturer’s instructions. Fluorescence was measured with an excitation wavelength of 360 nm and an emission wavelength of 460 nm. The cathepsin B substrate was coincubated with the cathepsin B inhibitor CA-074 (50 μM) as a negative control. Enzyme activity was defined as fluorescence units per microgram of total protein per 30 minutes.
Measurements of Cellular ROS
The effect of FFA overaccumulation on hepatocyte ROS production was monitored using the nonfluorescent cell-permeant compound 2′,7′-dichlorofluorescin diacetate (DCFH-DA). This technique is based on the principle that on oxidation by ROS, the deesterified form of this compound becomes the fluorescent compound dichlorofluorescin (DCF). FFA-treated cells were loaded with 10 μM DCFH-DA (Molecular Probes) in KRH buffer for 30 minutes at 37°C. The fluorescent compound DCF was monitored at the single-cell level using an inverted fluorescent microscope with excitation and emission wavelengths of 490 and 535 nm, respectively. Digital images were captured using a cooled charged-coupling device camera (KAF-1400; Photometrics, Tucson, AZ), analyzed, and quantitated with fluorescent imaging software (Metafluor Imaging System; Universal Imaging Corp., West Chester, PA). DCF fluorescence is expressed as the percentage of fluorescence at zero minutes.
Immunoblot Analysis
Immunoblot analysis was performed using either whole-cell lysates or cytosolic fractions as previously described.11 Cytosolic extracts were obtained using a selective digitonin permeabilization technique. Briefly, cells were washed once with PBS and then with 1 mL of permeabilization buffer (210 mmol/L D-mannitol, 70 mmol/L sucrose, 10 mmol/L HEPES [pH 7.2], 5 mmol/L sodium succinate, 0.2 mmol/L EGTA, 0.15% BSA, and 10 μg/mL digitonin). Cells were incubated on ice for 5 minutes before the buffer was collected and centrifuged at 13,000g for 10 minutes at 4°C to remove lysosomes and mitochondria. The resulting supernatants were further centrifuged at 100,000g for 1 hour at 4°C. Proteins in the final supernatants (cytosolic extracts) were used for immunoblot analysis. Samples were resolved by 12% SDS-PAGE, transferred to nitro-cellulose membrane, and blotted with appropriate primary antibodies. The membrane was incubated with peroxidase-conjugated secondary antibody (1:10,000 dilution, Biosource International, Camarillo, CA), and the bound antibody was visualized using a chemiluminescent substrate (ECL, Amersham, IL) and Kodak X-OMAT film (Eastman Kodak, Rochester, NY). Primary antibodies were anti– cytochrome c antibody (1:100 dilution, mAb 6H2.B4; BD Biosciences), anti– cathepsin B antibody (1:200 dilution, mAb IM27L; Calbiochem), and goat anti-β-actin (1:2,000)
Animal Studies
These experimental protocols were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic. C57BL/6 cathepsin B– knockout (ctsb−/−) mice and wild-type littermates (ctsb+/+), 20 to 25 g in body weight, for 16 weeks were fed a high-carbohydrate diet ad libitum (consisting of 65% sucrose, 20% casein, 5% corn oil, 4% mineral mixture, 1% vitamin mixture, and 1% orotic acid, Teklad Mills, Madison, WI) starting at 6 to 8 weeks of age (n = 15 in each group). Identical groups of animals (n = 15 in each group) received standard rodent chow to act as controls. Total body weight was measured weekly. Animals in each group were sacrificed at different points on the diet. Hepatic FFA content was measured using the FFA quantification kit from BioVision (kit k612-100) following the manufacturer’s instructions. Mitochondrial integrity and liver injury were assessed by immunoblot analysis of cytosolic proteins; liver histopathology; immunohisto-chemistry for 4-hydroxynonenal (HNE, Cayman Chemicals), a representative lipid peroxide product of oxidative stress; and serum ALT values.
Data Analysis
All data are expressed as the mean ± SD unless otherwise indicated. Differences between groups were compared by analysis of variance followed by a post hoc Bonferroni test to correct for multiple comparisons. Differences were considered to be statistically significant at P < 0.05.
Results
FFAs Induce Dose-Dependent and Saturation-Dependent Mitochondrial Dysfunction
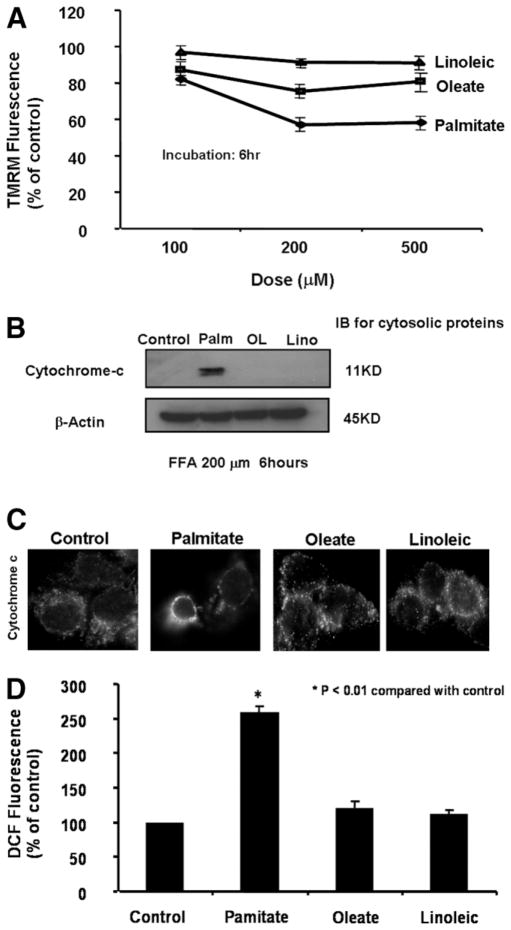
Mitochondrial dysfunction resulting in mitochondrial membrane permeabilization and increased ROS production is thought to represent a central abnormality responsible for progression from simple fatty liver to steatohepatitis.23 However, very little information is currently available regarding the mechanisms leading to mitochondrial dysfunction in NAFLD. Mitochondrial permeabilization manifests at the level of the outer membrane, which allows for the release of cytochrome c, as well as at the level of the inner membrane as a loss of ΔΨm.24 We hypothesized that mitochondrial dysfunction and subsequent mitochondrial ROS production in NAFLD are induced by the accumulation of toxic FFAs. To test this hypothesis, isolated hepatocytes from C57BL/6 mice were initially incubated in the presence or absence of various concentrations of FFAs with different degrees of saturation for up 24 hours. Mitochondrial function was then assessed by real-time imaging of mitochondrial membrane potential, biochemical and morphological assays for cytochrome c release, and single-cell ROS production monitoring. FFA treatment resulted in a significant reduction in TMRM fluorescence that indicated dissipation of ΔΨm that was both dose dependent and dependent on the degree of saturation, with linoleic (polyunsaturated) the least potent and palmitate (saturated) the most potent (Fig. 1A). Similar results were obtained by incubating HepG2 and Mc-Ntcp.24 cells in the same manner (data not shown). Moreover, palmitate uniquely induced cytochrome c release from mitochondria. Release of mitochondria cytochrome c was assessed by immunoblot analysis of the cytosolic fractions (Fig. 1B) and by immunofluorescence using a monoclonal antibody (Fig. 1C). Cytochrome c was absent in the cytosol of control cells as well as in those treated with oleate or linoleic. In contrast, it was present in high abundance in the cytosol from palmitate-treated cells (Fig. 1B). Similarly, cytochrome c immunofluorescence was punctuate in control cells and in cells treated with either oleate or linoleic, consistent with mitochondria localization of this protein. In contrast, palmitate treatment resulted in a diffuse pattern of cytochrome c fluorescence, suggesting redistribution of this protein into the cytosol (Fig. 1C). Finally, palmitate induced mitochondria ROS generation. ROS production was assessed by monitoring DCF fluorescence at the single-cell level (Fig. 1D). Palmitate but not oleate or linoleic resulted in a significant increase in DCF fluorescence compared with the control. Similar results were found with other long-chain-saturated FFAs, such as stearate (data not shown). Thus, collectively these data suggest that long-chain-saturated FFAs induce mitochondrial dysfunction manifested as dissipation of ΔΨm, cytochrome c release, and increased ROS generation.
Fig. 1.
Free fatty acids (FFAs) induced dose- and saturation-dependent mitochondrial dysfunction. Isolated hepatocytes from C57BL/6 mice were incubated in the presence or absence of various concentrations of FFAs with different degrees of saturation for up 24 hours. (A) Mitochondrial permeabilization was determined by staining with tetramethylrhodamine methyl ester (TMRM, 549/573 nm), a mitochondrion-selective dye that is released after mitochondrial depolarization in unfixed live cells and visualized under fluorescent microscopy. (B) Cells were treated with 0.2 mM of various FFAs for 6 hours. Cytosolic fractions were prepared by selective permeabilization with digitonin. Cytosolic proteins were resolved by SDS-PAGE gels, transferred to nitrocellulose, and probed for cytochrome c. β-Actin served as a control for protein loading. (C) Cytochrome c subcellular localization was further investigated by immunofluorescence. Cells were fixed and incubated with anti–cytochrome c antibody (1:100 dilution) for 1 hour at room temperature. (D) Finally, the effect of FFA overaccumulation on hepatocyte ROS production was monitored using the nonfluorescent cell-permeant compound 2′,7′-dichlorofluorescin diacetate (DCFH-DA). This technique is based on the principle that on oxidation by ROS, the deesterified form of this compound becomes the fluorescent compound dichlorofluorescin (DCF). FFA-treated cells were loaded with 10 μM of DCFH-DA (Molecular Probes) in KRH buffer for 30 min at 37°C. The fluorescent compound DCF was monitored at the single-cell level using an inverted fluorescent microscope.
FFAs Induce Mitochondrial Dysfunction Downstream of Lysosomal Permeabilization
We previously demonstrated that incubation of primary mouse hepatocytes as well as HepG2 cells with long-chain FFAs results in rapid lysosomal permeabilization and release of cathepsin B, a lysosomal cysteine protease, into the cytosol.21 We hypothesized that mitochondrial dysfunction and subsequent mitochondrial ROS production in NAFLD are downstream events from lysosomal permeabilization and cathepsin B activation. To test this hypothesis, HepG2 cells were initially incubated in the presence or absence of 0.2 mM FFA for up to 6 hours. The time relationship of lysosomal and mitochondrial permeabilization was determined by staining with the LysoTracker Red (577/590 nm), an acidophilic fluorescent dye that loads predominantly into lysosomes, and tetramethylrhodamine methyl ester (549/573 nm), a mitochondrion-selective dye that is released after mitochondrial depolarization in unfixed live cells, and visualized under fluorescent microscopy (Fig. 2A), as well as by immunoblot analysis for cytosolic cathepsin B and cytochrome c (Fig. 2B). We found a significant reduction in TMRM fluorescence indicating dissipation of ΔΨm occurred several hours after the loss of LysoTracker Red punctuate staining, a pattern consistent with lysosomal permeabilization (Fig. 2A). Similarly, cathepsin B translocation into the cytosol preceded cytochrome c release from mitochondria. Neither cathepsin B nor cytochrome c was identified in the cytosol of control cells. Following treatment with palmitate, cathepsin B was first identified in this fraction at around 1 hour and markedly present at 2 hours, whereas cytochrome c was first weakly identified after 4 hours of incubation and readily detected after 6 hours (Fig. 2B). Taken together, these data suggest that FFAs induce mitochondrial membrane permeabilization downstream of lysosomal breakdown.
Fig. 2.
FFAs induced mitochondrial depolarization and cytochrome c release downstream of lysosomal permeabilization and cathepsin B activation. (A) Cells were incubated with or without 0.2 mM FFA for up to 6 hours. A time course of lysosomal and mitochondrial permeabilization induced by FFAs was determined by staining with LysoTracker Red (LTR) and tetramethylrhodamine methyl ester (TMRM) in unfixed live cells and visualized under fluorescent microscopy. Values represent average fluorescence expressed as percentage of fluorescence at 0 hours. Results are expressed as mean ± SE from 15 to 30 cells per treatment group and 3 independent experiments (*P < 0.05 compared with fluorescence at baseline. (B) Cells were incubated with or without 0.2 mM FFA for up to 6 hours. A time course of cathepsin B and cytochrome c redistribution into the cytoplasm was investigated by immunoblot analysis of cytosolic fractions as detailed in the Materials and Methods section.
Cathepsin B Is Required for FFA-Induced Mitochondrial Dysfunction
We were particularly intrigued by the potential role of cathepsin B in mediating the mitochondrial dysfunction that leads to the progression from simple steatosis to steatohepatitis. This hypothesis stems from the observation that cathepsin B is released in the cytosol in response to FFAs in vitro and that the redistribution of cathepsin B in the cytoplasm is present in human liver tissues from patients with NAFLD.21
To ascertain whether cathepsin B inhibition may protect against FFA-induced mitochondriotoxicity, we suppressed cathepsin B activity using both genetic and pharmacological approaches. We first evaluated cytosolic cytochrome c localization in FFA-treated HepG2 cells in the presence or absence of 20 μM of the selective pharmacological cathepsin B inhibitor CA07 for 6 hours by immunoblotting (Fig. 3). Release of mitochondrial cytochrome c to the cytoplasm was also assessed by confocal microscopy. Increased cytochrome c was readily detectable in the cytosol of FFA-treated cells. This redistribution was almost completely prevented by coincubation with CA074 (Fig. 3A). Consistently, FFA treatment resulted in a diffuse pattern of cytochrome c fluorescence, suggesting release of cytochrome c into the cytosol. In contrast, in cells coincubated with CA074 cytochrome c fluorescence remained punctuate, with a pattern similar to that seen in control cells (Fig. 3B). Next, mitochondrial transmembrane potential was determined using real-time imaging of TMRM fluorescence intensity after incubation with FFAs in the presence or absence of CA074 (Fig. 4A,B). Indeed, FFA treatment resulted in a dramatic reduction in TMRM fluorescence but remained unchanged in cells coincubated with CA074 (Fig. 4A,B). Similar results were obtained by incubating with a second selective cathepsin B inhibitor E-64 (data not shown). To determine whether specific suppression of cathepsin B expression had a similar protective effect on mitochondrial function, we used siRNA to silence cathepsin B expression in HepG2 cells. By this approach, we reduced cathepsin B expression (Fig. 5A) as well as activity (Fig. 5B) to background levels. Mitochondrial function in these cells was assessed as before by monitoring both ΔΨm and cytochrome c release. Cells transfected with a scramble siRNA showed marked cytosolic cytochrome c levels and a diffuse fluorescence pattern consistent with mitochondrial permeabilization after treatment with palmitate (Fig. 6A,B). Cells transfected with cathepsin B siRNA showed no cytosolic cytochrome c and displayed predominantly punctuate cytochrome c fluorescence despite treatment with palmitate, indicating that this maneuver prevented mitochondrial permeabilization (Fig. 6A,B). Collectively, these data strongly suggest that FFAs induce mitochondrial membrane permeabilization and cytochrome c release in a cathepsin B– dependent manner.
Fig. 3.
FFA-induced cytochrome c release was reduced by cathepsin B inhibition. HepG2 cells were treated with 0.2 mM FFA in the presence or absence of a cathepsin B–selective inhibitor (CA074; 20 μM) for 6 hours. Staurosporine (STS; 3 μM) was used as a positive control. (A) Cytosolic fractions were prepared by selective permeabilization with digitonin. Cytosolic proteins were resolved by SDS-PAGE gels, transferred to nitrocellulose, and probed for cytochrome c. β-Actin served as a control for protein loading. (B) Cytochrome c subcellular localization was further investigated by immunofluorescence. Cells were fixed and incubated with anti– cytochrome c antibody (1:100 dilution) for 1 hour at room temperature.
Fig. 4.
FFAs induced loss of mitochondrial membrane potential in a cathepsin B– dependent manner. Cells were incubated with or without FFAs in the presence or absence of a cathepsin B–selective inhibitor (CA074) for 6 hours. Staurosporine (STS; 3 μM) was used as a positive control. (A) Mitochondrial transmembrane potential was then measured by real-time imaging of TMRM fluorescence as already described. (B) Values represent average fluorescence expressed as percentage of fluorescence under the control conditions. Results are expressed as mean ± SE from 15 to 30 cells per treatment group and 3 independent experiments.
Fig. 5.
Cathepsin B siRNA effectively inhibited cathepsin B in HepG2 cells. Cathepsin B expression in HepG2 cells was silenced by siRNA as detailed in the Materials and Methods section. After transfection of HepG2 cells with cathepsin B siRNA, (A) an immunoblot for Bax and β-actin was performed, and (B) cathepsin B activity was measured using a cathepsin B activity assay as detailed in the Materials and Methods section (*P < 0.01 compared with control; Ctsb, cathepsin B; Scr., scramble; siRNA, small interfering RNA.
Fig. 6.

Genetic inhibition of cathepsin B protected mitochondrial function. After 48 hours of transfection with either the cathepsin B or scramble siRNA, cells were treated with palmitate for 6 hours. (A) Cytosolic fractions were prepared by selective permeabilization with digitonin. Cytosolic proteins were resolved by SDS-PAGE gels, transferred to nitrocellulose, and probed for cytochrome c. β-Actin served as a control for protein loading. (B) Cytochrome c subcellular localization was investigated by immunofluorescence. Cells were fixed and incubated with anti– cytochrome c antibody (1:100 dilution) for 1 hour at room temperature.
Inactivation of Cathepsin B Protects Mitochondria and Prevents Liver Injury In Vivo
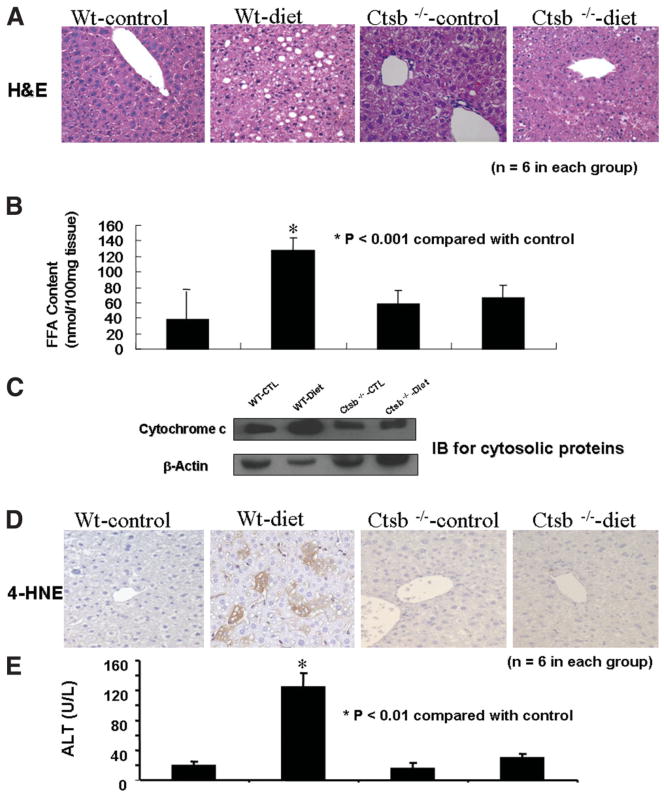
To investigate the in vivo role of cathepsin B in diet-associated fatty liver disease we used ctsb−/− mice. Ctsb−/− mice and their wild-type controls (ctsb+/+) were placed on a high-carbohydrate diet for 16 weeks. On this diet, both ctsb−/− and ctsb+/+ mice developed marked obesity compared with the control mice, which were fed a standard rodent diet. Wild-type mice on the high-carbohydrate diet showed marked steatosis (Fig. 7A) as well as a significant increase in hepatic FFA content (Fig. 7B). These changes were associated with mitochondrial dysfunction, as evidenced by cytochrome c redistribution in the cytosol (Fig. 7C) and increased content of 4-HNE, a marker of oxidative stress (Fig. 7D), as well as liver injury, as shown by elevated serum ALT levels (Fig. 7E) relative to ctsb+/+ on the control diet. In contrast, ctsb−/− mice on the high-carbohydrate diet showed only minimal steatosis, no increase in FFA content and 4-HNE expression, and normal serum ALT values compared with both ctsb−/− and ctsb+/+ mice on the control diet (Fig. 7A-E).
Fig. 7.
Ctsb−/− animals were protected against diet-induced mitochondrial dysfunction and liver injury. Cathepsin B– knockout (ctsb−/−) mice and their wild-type controls (ctsb+/+) were placed on a high-carbohydrate diet for 16 weeks. (A) Representative microphotograph of H&E staining. (B) Hepatic FFA content in the 4 groups of mice. (C) Immunoblots for cytosolic cytochrome c (β-actin served as a control for protein loading). (D) Immunohistochemistry for 4-hydroxynonenal (4-HNE), a representative lipid peroxide product of oxidative stress in liver sections from ctsb−/− and ctsb+/+ animals on a control diet or a high-carbohydrate diet. (E) Serum ALT levels in the 4 groups of mice.
Discussion
The principal findings of this study relate to the mechanisms linking hepatocyte lipid overloading, mitochondrial dysfunction, and liver injury. The results demonstrate that: (1) human and murine hepatocytes exposed to saturated long-chain FFAs showed mitochondrial dysfunction and ROS production downstream of lysosomal permeabilization; (2) inhibition or deletion of cathepsin B, a lysosomal cysteine protease, protected against free fatty acid–induced mitochondrial dysfunction and oxidative stress in vitro; and (3) cathepsin B inactivation attenuated oxidative stress and hepatic injury in a dietary murine model of NAFLD. These results strongly support a central role for the lysosomal-mitochondrial axis in liver damage and disease progression in NAFLD.
Obesity and type 2 diabetes have reached epidemic proportions in most of the Western world. Both conditions are strongly associated with nonalcoholic fatty liver disease.25 The clinicopathological picture of NAFLD resembles that of alcohol-induced liver injury but occurs in persons who consume little or no alcohol.5 Nonalcoholic steatohepatitis (NASH) is a stage in the spectrum of NAFLD and is characterized by the accumulation of fat in the liver (steatosis) along with signs of liver cell damage and inflammation.3 NASH is a potentially serious condition, and about 10% to 25% of patients with NASH may progress to cirrhosis and its significant complications of portal hypertension, liver failure, and hepatocellular carcinoma.4 The clinical implications of NAFLD derive from its common occurrence in both adults and children and its potential to progress to cirrhosis and liver failure.
Several lines of evidence suggest that impaired mitochondrial function is a central abnormality responsible for the progression from simple steatosis to steatohepatitis.26 Studies in experimental models of NAFLD as well as in humans with NAFLD have demonstrated that liver cells have both structural and functional mitochondrial abnormalities.14,15 Structural abnormalities include mitochondrial enlargement and development of crystalline inclusions, whereas functional mitochondrial abnormalities are characterized by enhanced production of reactive oxygen species, accumulation of lipid peroxides, and release of cytochrome c into the cytoplasm. However, the molecular mechanisms responsible for this mitochondrial dysfunction remain poorly understood. A net accumulation of lipids in the liver is a prerequisite for the development of NAFLD.27 Although lipids accumulate mostly in the form of triglycerides, steatosis may results in excess amounts of other lipids such as a variety of FFAs and cholesterol.11,28 Certain FFAs are potentially cytotoxic, and in vitro studies in different cell lines including hepatocytes have implicated increased cellular FFA levels as a trigger of apoptotic cell death.12,29,30 Our current data demonstrates that incubation of human and murine hepatocytes with FFAs results in dose- and saturation-dependent mitochondrial dysfunction. The saturated FFA palmitate at concentrations that mimic the levels of this FFA in the circulation of humans with metabolic syndrome31 induces significant mitochondrial membrane permeabilization and increased ROS production. We have previously reported that cathepsin B, a major lysosomal cysteine protease, is released in the cytosol in response to FFAs in vitro21 and that the redistribution of cathepsin B in the cytoplasm is present in human liver tissues from patients with NAFLD.21 Our current data extend these observations by demonstrating that during FFA treatment of liver cells, lysosome permeabilization and release of cathepsin B into the cytosol is an early event that occurs hours prior to mitochondrial depolarization and cytochrome c redistribution into the cytosol. More importantly, cathepsin B silencing by siRNA or chemical inhibition by 2 selective pharmacological inhibitors significantly prevented FFA-induced mitochondrial dysfunction, proving cathepsin B is essential for FFA-induced mitochondrial dysfunction. Supporting these results are the in vivo studies demonstrating that cathepsin B– knockout mice are protected against diet-induced mitochondrial dysfunction, increased oxidative stress, and liver damage. Taken together, these data strongly suggest a central role for the lysosomal-mitochondrial axis in NAFLD progression.
In summary, the current studies further elucidate the subcellular mechanisms responsible for mitochondrial dysfunction in NAFLD. The results support a model in which FFAs induce lysosomal breakdown, resulting in cathepsin B activation that then triggers mitochondrial dysfunction and increased ROS production. Targeting cathepsin B would appear to be a viable strategy to prevent FFA mitochondrial permeabilization and hepatocyte lipotoxicity.
Acknowledgments
Supported by the National Institutes of Health (grant DK076852) and the AGA Research Scholar Award (to A.E.F.).
Abbreviations
- ctsb
cathepsin B
- FFAs
free fatty acids
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
References
- 1.Wieckowska A, Feldstein AE. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr Opin Pediatr. 2005;17:636–641. doi: 10.1097/01.mop.0000172816.79637.c5. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Brunt EM, Neuschwander-Tetri BA, Oliver D, Wehmeier KR, Bacon BR. Nonalcoholic steatohepatitis: histologic features and clinical correlations with 30 blinded biopsy specimens. Hum Pathol. 2004;35:1070–1082. doi: 10.1016/j.humpath.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 6.Diehl AM. Nonalcoholic steatosis and steatohepatitis IV. Nonalcoholic fatty liver disease abnormalities in macrophage function and cytokines. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1–G5. doi: 10.1152/ajpgi.00384.2001. [DOI] [PubMed] [Google Scholar]
- 7.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–533. viii. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 10.Ginsberg HN. Is the slippery slope from steatosis to steatohepatitis paved with triglyceride or cholesterol? Cell Metab. 2006;4:179–181. doi: 10.1016/j.cmet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 11.McClain CJ, Barve S, Deaciuc I. Good fat/bad fat. Hepatology. 2007;45:1343–1346. doi: 10.1002/hep.21788. [DOI] [PubMed] [Google Scholar]
- 12.Unger RH. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab. 2003;14:398–403. doi: 10.1016/j.tem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Bouchier-Hayes L, Lartigue L, Newmeyer DD. Mitochondria: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2640–2647. doi: 10.1172/JCI26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caldwell SH, Chang CY, Nakamoto RK, Krugner-Higby L. Mitochondria in nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:595–617. x. doi: 10.1016/j.cld.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Fromenty B, Robin MA, Igoudjil A, Mansouri A, Pessayre D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 2004;30:121–138. doi: 10.1016/s1262-3636(07)70098-8. [DOI] [PubMed] [Google Scholar]
- 16.Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 17.Ricci JE, Waterhouse N, Green DR. Mitochondrial functions during cell death, a complex (I–V) dilemma. Cell Death Differ. 2003;10:488–492. doi: 10.1038/sj.cdd.4401225. [DOI] [PubMed] [Google Scholar]
- 18.Feldstein AE, Werneburg NW, Li Z, Bronk SF, Gores GJ. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1339–G1346. doi: 10.1152/ajpgi.00509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 20.Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 21.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 22.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 23.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green DR. Apoptotic pathways: ten minutes to dead. Cell. 2005;121:671–674. doi: 10.1016/j.cell.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 26.Pessayre D, Fromenty B, Mansouri A. Mitochondrial injury in steatohepatitis. Eur J Gastroenterol Hepatol. 2004;16:1095–1105. doi: 10.1097/00042737-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 28.Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, et al. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlstrom S. Studies on fatty acid metabolism in diabetics during exercise. V. Plasma concentration of free fatty acids and glycerol in newly diagnosed, adult diabetics during exercise. Acta Med Scand. 1967;182:363–376. [PubMed] [Google Scholar]