Abstract
OBJECTIVE:
Cardiovascular risk assessment is an accepted practice in adults and correlates with early changes in carotid structure and function. Its clinical use in pediatrics is less common. We sought to determine whether a simple method of clustering cardiovascular risks could detect early atherosclerotic changes in youth. In addition, we compared risk clustering with the accepted Patholobiological Determinants of Atherosclerosis in Youth score to assess its utility for predicting early vascular disease.
PATIENTS AND METHODS:
We collected demographic, anthropometric, laboratory, and vascular measures in a cross-sectional study. The study population (n = 474; mean age: 18 years) was divided into low-risk (0–1) or high-risk (≥2) groups on the basis of the number of cardiovascular risk factors present at evaluation. Group differences and vascular outcomes were compared. General linear models were used to compare clustering cardiovascular risks with the Patholobiological Determinants of Atherosclerosis in Youth score.
RESULTS:
The high-risk group had higher vascular thickness and stiffness compared with the low-risk group (P < .05). Regression models found that clustering cardiovascular risks is associated with abnormal vascular structure and function after adjustment for age, race, and gender. The Patholobiological Determinants of Atherosclerosis in Youth score also is associated with abnormal vascular structure and function but with lower R2 values (P < .05).
CONCLUSIONS:
Cardiovascular risk clustering is a reliable tool for assessing abnormal vascular function. Its simplicity, compared with the Patholobiological Determinants of Atherosclerosis in Youth score, provides an advantageous tool for the practicing clinician to identify those youth who are at higher risk for early cardiovascular disease.
Keywords: adolescence, obesity, carotid artery diseases, risk factors
WHAT'S KNOWN ON THIS SUBJECT:
Cardiovascular risk factors predict the development of premature atherosclerosis. As the number of risk factors increases, so does the extent of these lesions. Assessment of cardiovascular risk factors is an accepted practice in adults but is not used in pediatrics.
WHAT THIS STUDY ADDS:
In this study, the authors discuss how the presence of ≥2 cardiovascular risk factors is associated with vascular changes in adolescents. The findings were compared with the Patholobiological Determinants of Atherosclerosis in Youth risk score to demonstrate that a simple method of clustering is a reliable tool to use in clinical practice.
The presence of cardiovascular risk factors predicts the development of premature atherosclerosis in the aorta and coronary arteries.1,2 As the number of cardiovascular risk factors increase so do the extent of these lesions.1 Furthermore, 1 or more cardiovascular risk factor is present in 85% to 90% of adults with coronary heart disease.3,4
In adults, cardiovascular risk assessment is an accepted practice. The Framingham risk score is a well-established method used to predict a 10-year absolute coronary artery disease risk in people aged 20 years and older.5 In addition, it is used to identify patients who need intensive lifestyle modification. A decrease in the number of risk factors has been associated with a reduction in cardiovascular risk.6,7
Cardiovascular risk factor assessment also has been used in adolescents and young adults. McMahan et al8 developed the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk score to predict the likelihood of advanced atherosclerotic lesions in the coronary arteries and abdominal aorta of subjects aged 15 to 29 years. The PDAY score has been validated using autopsy studies from both young adults and middle-aged subjects.9 In addition, the PDAY risk score has been shown to predict noninvasive changes in carotid intima medial thickness (IMT) in living populations.10 Similar to the Framingham risk score, the PDAY score assigns points to subjects on the basis of the presence and degree of cardiovascular risk factors. To our knowledge, the PDAY score is not routinely used in clinical practice.
We hypothesize that categorizing youth into low- and high-risk groups using cardiovascular risk assessment will be a simple tool for detecting early atherosclerosis. To test this hypothesis, we compared vascular thickness and stiffness in a population of adolescents and young adults classified in low- and high-risk groups. In addition, we compared the PDAY score to our dichotomous assessment of low- and high-risk factors to assess its association with early changes in the vasculature.
PATIENTS AND METHODS
Subjects were recruited as part of a cross-sectional study designed to evaluate the vascular effects of obesity in adolescents and young adults, as previously described.11 Briefly, participants were either recruited from clinics held at the Cincinnati Children's Hospital Medical Center, local physician offices, college campuses, or health fairs. Eligibility criteria included age of 11 to 23 years and no evidence of type 2 diabetes. All subjects with a BMI ≥95th percentile underwent a 2-hour oral glucose tolerance test to exclude the presence of diabetes according to American Diabetes Association criteria.12 Pregnant female subjects were excluded. Participants were divided into 2 groups on the basis of the number of cardiovascular risk factors present at the time of evaluation. Low risk was defined by the presence of 0 to 1 cardiovascular risk factors, whereas high risk was defined as the presence of 2 to 4 risk factors. This classification is based on previous studies using the PDAY score, which have used a similar median score cutoff to stratify their cohort.10 The following were used to define cardiovascular risk factors: (1) a BMI ≥95th percentile, on the basis of Centers for Disease Control and Prevention, National Centers for Health Statistics growth charts13; (2) blood pressure (BP) (systolic or diastolic) ≥95th percentile on the basis of gender, age, and height14; (3) the presence of metabolic derangement, either a fasting glucose 100 mg/dL or more or fasting insulin at the ≥95th percentile (defined as 19.6 mc/L or more derived from healthy control subjects participating in the parent study from which these study data were obtained); and (4) the presence of dyslipidemia (total cholesterol ≥ 210 mg/dL, low-density lipoprotein ≥ 128 mg/dL, triglycerides ≥137 mg/dL, or high-density lipoprotein ≤ 37 mg/dL). Lipid cutoffs were similarly generated from at the ≥95th percentile for healthy control subjects. These risk factors were selected so that a comparison could be made to the PDAY score. Written informed consent was obtained from subjects aged 18 years or older or from a parent or guardian with written assent for subjects younger than 18 years of age, according to the guidelines established by the institutional review board and in accordance with the Declaration of Helsinki.
After a minimum 10-hour overnight fast, participants attended an in-person study visit during which demographic, anthropometric, and laboratory data were collected and vascular studies were performed. Trained personnel obtained 2 measures of height and weight as previously described.15 The average of each was used in the analyses. BMI was calculated as weight in kilograms divided by the square of height in meters. BP was obtained manually with sphygmomanometer (Baum Desktop model with V-Lok cuffs [Copiague, NY]) according to the fourth report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents.14
Fasting plasma lipid profiles were conducted in a standardized laboratory, with low-density lipoprotein cholesterol calculated by using the Friedewald equation. Fasting plasma glucose was measured by using a Hitachi model 704 glucose analyzer (Roche Hitachi, Indianapolis, IN). Plasma insulin was measured by radioimmunoassay with an anti-insulin serum raised in guinea pigs, 125I-labeled insulin (Linco, St Louis, MO), and a double-antibody method to separate bound from free tracer. Hemoglobin A1c was measured in red blood cells by using high-performance liquid chromatography methods.
Carotid Thickness and Stiffness
Carotid ultrasound studies were performed by a single technician. The carotid arteries were evaluated with high-resolution M- and B-mode ultrasonography by using a GE Vivid 7 ultrasound imaging system with a 7.5-MHz linear array transducer (Oceanside, CA). All images were read by a single research-trained vascular technician who was blinded to the group and had more than 3 years' experience reading carotid ultrasound studies and used a Camtronic Medical System (Hartland, WI).
For each subject, the right and left internal carotid segment were examined independently from continuous angles to identify the thickest carotid IMT with the average value used in the analysis. A trace technique was used to measure the maximum carotid thickness from leading edge (lumen-intima) to leading edge (media-adventitia). This technique has been found to be more reproducible than point-to-point measurements with coefficients of variation for repeat trace readings (4.1% to 5.3% vs 5.4% to 7.1%, respectively) (EMU, unpublished data).
To obtain stiffness measurements, M-mode measurements of the common carotid were performed as previously described.11 An optimal 2-dimensional image of the common carotid artery was obtained by placing the M-mode curser 1 cm proximal to beginning of the carotid bulb. The maximal and minimal lumen diameters were read from the M-mode tracing for calculations of Young elastic modulus and Peterson elastic modulus. Young elastic modulus is a measure of arterial wall stiffness controlling for carotid IMT, and the Peterson elastic modulus is a measure of the difference in pressure change required for an increase in vessel diameter.
Vascular Stiffness
Vascular stiffness testing was conducted after 5 minutes of rest in the supine position. Pulse-wave velocity (PWV) was measured with a SphygmoCor SCOR-PVx System (Atcor Medical, Sydney, Australia). The distance from a proximal artery (carotid) to the distal artery recording site (femoral artery) was measured to the nearest 0.1 cm twice, averaged, and entered into the software. A tonometer was used to collect proximal and distal arterial waveforms gated by the R-wave on a simultaneously recorded electrocardiogram. PWV then was calculated as the distance from the carotid-to-distal path length divided by the time delay measured between the feet of the 2 waveforms reported in meters per second.16 Three recordings of PWV were obtained on each subject and were averaged. Repeat measures in our laboratory show a coefficient of variation of less than 5.2% (EMU, unpublished data).
The augmentation index (AIx), which provides information about arterial stiffness and pulse-wave reflections,16 also was collected. The AIx was collected when the SphygmoCor tonometer was placed over the right radial artery. The device analyzes pulse waves using a generalized transfer function validated in a catheterization laboratory to calculate a central aortic pressure wave.17 The AIx was derived from the central pressure waveform by calculating the difference between the main outgoing wave and the reflected wave of the central arterial waveform, expressed as a percentage of the central pulse pressure. Because AIx is affected by heart rate, values were adjusted to a standard heart rate of 75 beats per minute. Reproducibility studies in our laboratory demonstrated a coefficient of variation of less than 7.4% (EMU, unpublished data).
Three measures of brachial artery distensibility (obtained with a DynaPulse Pathway instrument [Pulse Metric, San Diego, CA] as previously described).11 This device derives brachial artery pressure curves from distensibility arterial pressure signals obtained from a standard cuff sphygmomanometer. Repeat measures in our laboratory show a coefficient of variation of less than 9.6% (EMU, unpublished data).
Comparison to PDAY Risk Score
The PDAY risk score is a cardiovascular risk assessment tool used to estimate the probability of advanced atherosclerotic lesions determined at autopsy in the coronary arteries and abdominal aorta in youth and middle-aged subjects.8,9 Points are assigned to unmodifiable risk factors (age and gender) and modifiable risk factors (cholesterol, smoking, BP, BMI, and hyperglycemia) and added together to predict the likelihood of significant lesions.8 This score also has been used to predict early atherosclerosis detected by carotid IMT in adolescents and young adults.10 A large contribution of the score is on the basis of age, with 5 points added to the score for each 5-year age increase over 20 years up to 15 points.8 As a result, in younger populations, a modified PDAY score is used.18 Given the mean age of our study population (18 years), we applied the modified PDAY score.
Statistical Analysis
All analyses were performed with SAS 9.1.3 (SAS, Cary, NC).19
Differences in the low- and high-risk groups were evaluated with a value of P < .05, which indicates significance. Nonnormal values of arterial stiffness and thickness were log transformed. General linear models were constructed to elucidate independent determinates of carotid thickness and stiffness. The full model contained group, age, race, and gender. Height was included in the full model of AIx because height influences the distance of reflection sites to the heart, which in turn affects AIx. BMI, lipids, BP, glucose, and insulin were excluded from the model because they are incorporated into the high- and low-risk clustering score. Similarly, we classified the PDAY score as equal to or less than the median score (PDAY low) and greater than the median score (PDAY high), a method previously used for adolescents and young adults.9 Similar to the above, regression models were constructed to assess how much of the variance in vascular thickness and stiffness could be explained by the PDAY score. The PDAY score was evaluated both continuously and dichotomously. Robustness of all models was assessed using the maximum R2 technique.
RESULTS
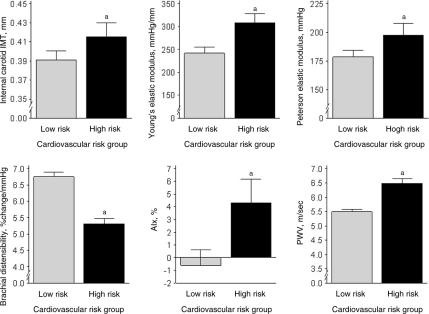
The characteristics of 474 study participants are presented in Table 1. The low-risk (0–1) and high-risk (2–4) groups did not differ according to age, gender distribution, or height. There were more nonwhite subjects in the high-risk group. As dictated by the study design, the low-risk group had a lower BMI and a more favorable cardiovascular risk profile (lower BP, lipids, glucose, and insulin). Carotid thickness and stiffness results according to group are displayed in Table 2 and Fig 1. The high-risk group had significantly thicker and stiffer vessels compared with the low-risk group, as indicated by a higher carotid IMT, Young elastic modulus, Peterson elastic modulus, PWV, AIx, and lower brachial artery distensibility (all P ≤ .05).
TABLE 1.
Characteristics of the Study Population
| Variable | Low Risk (N = 309) | High Risk (N = 165) |
|---|---|---|
| Age, mean ± SD, y | 17.8 ± 3.4 | 18.3 ± 3.4 |
| Female, n (%) | 197 (63.8) | 114 (69.1) |
| Nonwhite, n (%)a | 184 (59.5) | 120 (72.7) |
| Smoker, n | 5 | 6 |
| Height, mean ± SD, cm | 166 ± 11 | 167 ± 10 |
| Weight, mean ± SD, kga | 67 ± 20 | 107 ± 21 |
| BMI, mean ± SD, kg/m2a | 24.2 ± 6.2 | 38.5 ± 7.2 |
| Systolic BP, mean ± SD, mm Hg | 109 ± 10 | 118 ± 11 |
| Diastolic BP, mean ± SD, mm Hga | 61 ± 12 | 66 ± 13 |
| Total cholesterol, mean ± SD, mg/dLa | 163 ± 28 | 174 ± 33 |
| LDL cholesterol, mean ± SD, mg/dLa | 92 ± 23 | 107 ± 30 |
| HDL cholesterol, mean ± SD, mg/dLa | 56 ± 13 | 46 ± 11 |
| Triglycerides, mean ± SD, mg/dLa | 73 ± 32 | 107 ± 66 |
| Fasting insulin, mean ± SD, mIU/mLa | 12 ± 4 | 25 ± 17 |
| Fasting glucose, mean ± SD, mg/dLa | 89 ± 6 | 93 ± 8 |
| Homeostasis model assessment of insulin resistanceb | 2.6 ± 1 | 6.0 ± 4.7 |
| Hemoglobin A1c, mean ± SD, %a | 5.4 ± 0.5 | 5.5 ± 0.4 |
LDL indicates low-density lipoprotein; HDL, high-density lipoprotein.
Significant group differences (P < .05).
The homeostasis model assessment of insulin resistance was calculated as (glucose in mmol/L × fasting insulin)/22.5.
TABLE 2.
Carotid Thickness and Stiffness Measures According to Risk Group
| Variable | Low-Risk Group | High-Risk Group |
|---|---|---|
| Carotid IMT, mean ± SD, mma | 0.39 ± 0.09 | 0.42 ± 0.09 |
| Young elastic modulus, mean ± SD, mm Hg/mma | 242.1 ± 112.8 | 307.8 ± 129.8 |
| Peterson elastic modulus, mean ± SD, mm Hga | 177.7 ± 50.0 | 198.7 ± 62.3 |
| PWV, mean ± SD, m/sa | 5.5 ± 0.8 | 6.5 ± 1.1 |
| AIx, mean ± SD, %a | −0.59 ± 10.8 | 4.32 ± 11.6 |
| Brachial artery distensibility, mean ± SD, % change per mm Hga | 6.8 ± 1.2 | 5.3 ± 1.0 |
Significant group differences (P < .05).
FIGURE 1.
Vascular thickness and stiffness measures according to risk group. a Significant group differences (P < .05).
Regression models using low- versus high-risk clustering score are presented in Table 3. The effect of risk group remained significant after adjusting for demographics. Age and gender were important in explaining the variance in arterial thickness and stiffness. Older age was associated with higher carotid IMT, Young elastic modulus, Peterson elastic modulus, and PWV, and female gender was protective for carotid IMT, Peterson elastic modulus, and brachial artery distensibility. Nonwhite race was associated with adverse levels of carotid IMT, Peterson elastic modulus, and PWV (all estimates P < .05).
TABLE 3.
Independent Determinants of Carotid Structure and Function: Low Versus High Risk
| Variable | Carotid IMT | Young Elastic Modulus | Peterson Elastic Modulus | PWV | AIx | Brachial Artery Distensibility |
|---|---|---|---|---|---|---|
| Intercept, parameter estimate ± SE | −1.13 ± 0.059 | 5.10 ± 0.112 | 4.84 ± 0.79 | 1.28 ± 0.034 | 47.1 ± 8.02 | 1.75 ± 0.030 |
| Age, parameter estimate ± SE | 0.018 ± 0.003 | 0.020 ± 0.006 | 0.023 ± 0.004 | 0.021 ± 0.002 | — | — |
| Gender, female, parameter estimate ± SE | −0.119 ± 0.020 | −0.091 ± 0.026 | — | 0.089 ± 0.018 | ||
| Race, nonwhite, parameter estimate ± SE | 0.086 ± 0.019 | −0.096 ± 0.041 | 0.065 ± 0.026 | 0.078 ± 0.013 | — | — |
| Height, parameter estimate ± SE | — | — | — | — | −0.288 ± 0.048 | — |
| High-risk group, parameter estimate ± SE | 0.048 ± 0.020 | 0.251 ± 0.042 | 0.086 ± 0.026 | 0.144 ± 0.013 | 5.06 ± 1.06 | −0.243 ± 0.018 |
| R2 | 0.17 | 0.11 | 0.13 | 0.42 | 0.11 | 0.32 |
All estimates: P < .05. The low-risk group is the reference to which the high-risk group was compared.
Multivariate models using the PDAY score as a categorical variable are presented in Table 4. Similar to the above models, group was an independent predictor of arterial thickness and stiffness, and age, gender, and race contributed to the models (all P < .05). However, R2 values for each of the carotid thickness and stiffness measures are lower than those explained by clustering of cardiovascular risks into low- versus high-risk groups. When the PDAY score was analyzed as a continuous variable, the resulting models (data not presented) produced a lower R2 value than did the dichotomous assessment of the PDAY score presented in Table 4.
TABLE 4.
Independent Determinants of Carotid Structure and Function: PDAY Risk Score
| Variable | Carotid IMT | Young Elastic Modulus | Peterson Elastic Modulus | PWV | AIx | Brachial Artery Distensibility |
|---|---|---|---|---|---|---|
| Intercept, parameter estimate ± SE | −1.05 ± 0.058 | 4.93 ± 0.126 | 4.96 ± 0.080 | 1.35 ± 0.041 | 49.1 ± 8.1 | 2.82 ± 0.144 |
| Age, parameter estimate ± SE | 0.017 ± 0.003 | 0.018 ± 0.006 | 0.023 ± 0.004 | 0.019 ± 0.002 | — | — |
| Gender, female, parameter estimate ± SE | −0.111 ± 0.020 | 0.086 ± 0.043 | −0.081 ± 0.026 | 0.029 ± 0.014 | — | — |
| Race, nonwhite, parameter estimate ± SE | 0.094 ± 0.019 | — | 0.079 ± 0.026 | 0.099 ± 0.013 | — | 0.050 ± 0.019 |
| Height | — | — | — | — | −0.300 ± 0.049 | — |
| High-risk group, parameter estimate ± SE | 0.045 ± 0.019 | 0.180 ± 0.041 | 0.049 ± 0.025 | 0.093 ± 0.013 | 3.82 | −0.122 ± 0.019 |
| R2 | 0.17 | 0.08 | 0.12 | 0.33 | 0.10 | 0.19 |
All estimates: P < .05. The low-risk group is the reference to which the high-risk group was compared.
Additional analyses were performed to determine whether a single risk factor or a combination of other risk factors increased the R2 values of our models (data not shown). Using BMI alone, R2 values of each of the vascular outcomes were similar to those presented in Table 3. The addition of BP to BMI did not improve these values, nor did substituting homeostasis model assessment of insulin resistance as a risk factor for glucose and insulin. Adding inflammatory markers (interleukin 6 and C-reactive protein) to the models in Table 3 resulted in a decrease in the R2 values.
DISCUSSION
We demonstrated in this study that high-risk youth have increased vascular thickness and stiffness compared with low-risk youth. In addition, clustering cardiovascular risks into low- and high-risk groups is associated with abnormal vascular structure and function after adjustment for age, race, and gender. In our population, both risk-factor clustering and the modified PDAY score were associated with abnormal vascular structure and function, suggesting that clustering of cardiovascular risks is a reliable tool for assessing abnormal vascular function. Its simplicity compared with PDAY risk score assessment likely provides an advantageous tool for the clinician.
Clustering cardiovascular risks is an established method to predict atherosclerosis and coronary heart disease. The Framingham Heart Study first demonstrated the importance of multiple cardiovascular risk factors in the prediction of clinical coronary heart disease in adults.5 Similarly, other large cohort studies, such as the PDAY and Bogalusa Heart Study, also have used cardiovascular risks to predict preclinical atherosclerotic lesions on autopsy examinations in young and middle-aged adults.2,20
With the development of noninvasive cardiovascular-imaging techniques, autopsy studies are no longer the only means to detect preclinical cardiovascular changes in youth. Vascular imaging is an established method to detect early changes in vessel thickness and stiffness that relate to cardiovascular risk factors and an increased risk for coronary artery disease and stroke.21,22 In this study, we demonstrate for all noninvasive measures of the vasculature, that adolescents with 2 or more cardiovascular risk factors had thicker and stiffer vessels compared with their counterparts with 0 to 1 cardiovascular risk factors. The PDAY score also predicted abnormal vessel structure and function, suggesting that these studies are a valid method for assessing target organ damage in high-risk youth.
Previous work has shown that atherosclerosis begins in childhood and progresses to clinical coronary artery disease by the age of 30 years.18,23 Fatty streaks and plaque formation are the result of additive modifiable risk factors such as obesity, dyslipidemia, hypertension, and hyperglycemia.1 In the late 1990s, the Framingham Heart Study was the first to quantify the additive effect of cardiovascular risks to predict clinical coronary events.24 Subsequently, the PDAY score was developed for use in young people to assess the risk for atherosclerotic lesions using data from autopsy studies.8 These scores have been reproduced in different age groups.9,10,25 Although risk scoring is helpful, it can be time consuming. In this study, we demonstrate that a simple, yet practical, technique of risk-factor clustering into low- and high-risk groups also is associated with abnormalities in the vasculature.
We statistically compared low and high clustering risk scoring to the both a dichotomous and continuous PDAY score to determine which assessment was a better predictor of abnormal vasculature. Regression modeling demonstrated that clustering of cardiovascular risks explained more of the variance in carotid thickness and stiffness. For each measure, the low- and high-risk clustering score produced a higher R2 value compared with the PDAY score, except in the internal carotid, where R2 values were similar.
Finally, we assessed whether using other cardiovascular risk factors in our models could increase R2 values of each of the structure and function outcomes. Using BMI as single risk factor, and using a combination of BMI and BP, substituting homeostasis model assessment of insulin resistance for glucose and insulin or adding inflammatory markers did not improve our models. These findings suggest that adiposity is a major risk factor for adverse changes in the vasculature of adolescents. For the purposes of this article, only the model including BMI, BP, hyperglycemia, and lipid measurements was chosen so that it compares with the modified PDAY score. However, it should be noted, as documented in the Fourth Report of Blood Pressure Management in Children and Adolescents,14 adolescents with obesity and elevated BP are at significantly increased risk to develop other components of the metabolic syndrome (high triglycerides, low high-density lipoprotein, and hyperinsulinemia), suggesting that a comprehensive risk-factor profile should be assessed in these individuals.
This study has limitations. First, when developing an easy tool for clinicians, we included risk factors in the PDAY score. Thus, our clustering mechanism is not inclusive of all known cardiovascular risks. Inflammatory markers were omitted because they were not used in the PDAY score and are infrequently measured in pediatric patients. Smoking also was omitted from our clustering score because there were only 11 smokers in our population. Second, we found that in youth with 2 or more cardiovascular risk factors, higher carotid vascular thickness and stiffness compared with those with 0 to 1 risk factor. To date, normal values for this age group have not been established. Therefore, we can not say whether there are no vascular changes in the low-risk group. Finally, our cross-sectional design does not allow us detect when changes occur in the vasculature in relation to the development to risk factors. Similarly, we can not prove that increased cardiovascular risk factors lead to vasculature changes. Longitudinal evaluation is needed to make these conclusions.
CONCLUSIONS
Vascular dysfunction is documented in youth with 2 or more cardiovascular risk factors. These changes are associated with clustering of cardiovascular risk factors. Compared with the PDAY score, clustering provides a simple yet reliable tool that can be used in clinical practice. Therefore, our work suggests that as in adults, assessment of multiple risk factors should be an integral part of care for adolescents to identify those who are at risk for early atherosclerotic disease and in whom risk modification should be used.
ACKNOWLEDGMENT
This study was supported by National Institutes of Health (National Heart, Lung, and Blood Institute) grant R01 HL076269 (Cardiovascular Disease in Adolescents With Type 2 Diabetes).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- PDAY
- Pathobiological Determinants of Atherosclerosis in Youth
- IMT
- intima medial thickness
- BP
- blood pressure
- PWV
- pulse-wave velocity
- AIx
- augmentation index
REFERENCES
- 1. Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults: the Bogalusa Heart Study. N Engl J Med. 1998;338(23):1650–1656 [DOI] [PubMed] [Google Scholar]
- 2. McGill HC, Jr, McMahan CA, Malcom GT, Oalmann MC, Strong JP. Effects of serum lipoproteins and smoking on atherosclerosis in young men and women: the PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol. 1997;17(1):95–106 [DOI] [PubMed] [Google Scholar]
- 3. Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 20 2003;290(7):898–904 [DOI] [PubMed] [Google Scholar]
- 4. Mensah GA, Brown DW, Croft JB, Greenlund KJ. Major coronary risk factors and death from coronary heart disease: baseline and follow-up mortality data from the Second National Health and Nutrition Examination Survey (NHANES II). Am J Prev Med. 2005;29(5 suppl 1):68–74 [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB. Contributions of the Framingham Study to the conquest of coronary artery disease. Am J Cardiol. 15 1988;62(16):1109–1112 [DOI] [PubMed] [Google Scholar]
- 6. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–1389 [PubMed] [Google Scholar]
- 7. Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists' Collaboration. Lancet. 2000;356(9246):1955–1964 [DOI] [PubMed] [Google Scholar]
- 8. McMahan CA, Gidding SS, Fayad ZA, et al. Risk scores predict atherosclerotic lesions in young people. Arch Intern Med. 2005;165(8):883–890 [DOI] [PubMed] [Google Scholar]
- 9. McMahan CA, McGill HC, Gidding SS, et al. PDAY risk score predicts advanced coronary artery atherosclerosis in middle-aged persons as well as youth. Atherosclerosis. 2007;190(2):370–377 [DOI] [PubMed] [Google Scholar]
- 10. McMahan CA, Gidding SS, Viikari JS, et al. Association of Pathobiologic Determinants of Atherosclerosis in Youth risk score and 15-year change in risk score with carotid artery intima-media thickness in young adults (from the Cardiovascular Risk in Young Finns Study). Am J Cardiol. 2007;100(7):1124–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Urbina EM, Kimball TR, McCoy CE, Khoury PR, Daniels SR, Dolan LM. Youth with obesity and obesity-related type 2 diabetes mellitus demonstrate abnormalities in carotid structure and function. Circulation. 2009;119(22):2913–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Diabetes Association Standards of medical care in diabetes: 2010 [published correction appears in Diabetes Care. 2010;33(3):692]. Diabetes Care. 2010;33(suppl 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention Growth charts. Available at: www.cdc.gov/growthcharts Accessed June 1, 2009
- 14. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th report):555–576 [PubMed] [Google Scholar]
- 15. Shah AS, Dolan LM, Kimball TR, et al. Influence of duration of diabetes, glycemic control, and traditional cardiovascular risk factors on early atherosclerotic vascular changes in adolescents and young adults with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94(10):3740–3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605 [DOI] [PubMed] [Google Scholar]
- 17. O'Rourke MF, Gallagher DE. Pulse wave analysis. J Hypertens Suppl. 1996;14(5):S147–S157 [PubMed] [Google Scholar]
- 18. McGill HC, Jr, McMahan CA, Gidding SS. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation. 2008;117(9):1216–1227 [DOI] [PubMed] [Google Scholar]
- 19. SAS Software [computer program]. Release 9.1.3 Cary, NC: SAS Institute; 2007 [Google Scholar]
- 20. Newman WP, 3rd, Freedman DS, Voors AW, et al. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis: the Bogalusa Heart Study. N Engl J Med. 1986;314(3):138–144 [DOI] [PubMed] [Google Scholar]
- 21. Brohall G, Oden A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med. 2006;23(6):609–616 [DOI] [PubMed] [Google Scholar]
- 22. Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: the Muscatine Study. Circulation. 2001;104(23):2815–2819 [DOI] [PubMed] [Google Scholar]
- 23. Strong JP, McGill HC., Jr The natural history of coronary atherosclerosis. Am J Pathol. 1962;40:37–49 [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847 [DOI] [PubMed] [Google Scholar]
- 25. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421 [PubMed] [Google Scholar]