Abstract
CONTEXT:
No formal comparison has been made between the pediatric post–highly active antiretroviral therapy (HAART) outcomes of resource-limited and developed countries.
OBJECTIVE:
To systematically quantify and compare major baseline characteristics and clinical end points after HAART between resource-limited and developed settings.
METHODS:
Published articles and abstracts (International AIDS Society 2009, Conference on Retroviruses and Opportunistic Infections 2010) were examined from inception (first available publication for each search engine) to March 2010. Publications that contained data on post-HAART mortality, weight-for-age z score (WAZ), CD4 count, or viral load (VL) changes in pediatric populations were reviewed. Selected studies met the following criteria: (1) patients were younger than 21 years; (2) HAART was given (≥3 antiretroviral medications); and (3) there were >20 patients. Data were extracted for baseline age, CD4 count, VL, WAZ, and mortality, CD4 and virologic suppression over time. Studies were categorized as having been performed in a resource-limited country (RLC) or developed country (DC) on the basis of the United Nations designation. Mean percentage of deaths per cohort and deaths per 100 child-years, baseline CD4 count, VL, WAZ, and age were calculated for RLCs and DCs and compared by using independent samples t tests.
RESULTS:
Forty RLC and 28 DC publications were selected (N = 17 875 RLCs; N = 1835 DC). Mean percentage of deaths per cohort and mean deaths per 100 child-years after HAART were significantly higher in RLCs than DCs (7.6 vs 1.6, P < .001, and 8.0 vs 0.9, P < .001, respectively). Mean baseline CD4% was 12% in RLCs and 23% in DCs (P = .01). Mean baseline VLs were 5.5 vs 4.7 log10 copies per mL in RLCs versus DCs (P < .001).
CONCLUSIONS:
Baseline CD4% and VL differ markedly between DCs and RLCs, as does mortality after pediatric HAART. Earlier diagnosis and treatment of pediatric HIV in RLCs would be expected to result in better HAART outcomes.
Keywords: HIV/AIDS, pediatric, HAART, mortality, resource-limited
Highly active antiretroviral therapy (HAART) results in marked survival benefits for HIV-infected people.1,2 In contrast to adults, who may defer HAART for several years, nearly half of HIV-1 infected children in Africa will die by the age of 2 if they are not treated.3,4 By 2005 in Africa, where ∼90% of the world's HIV-infected children reside, children represented 13% of the population in need of antiretroviral treatment (ART) and only ∼5% of the population receiving ART.5,6 The number of children receiving ART has since increased; however, children are still less likely than adults to receive therapy.5
Many factors impede the use of HAART in resource-limited settings, particularly in pediatric populations. Lack of infrastructure, health care professionals, and technology to diagnose HIV-1 and monitor treatment have initially delayed treatment of both adults and children.7 The threat of poor adherence and viral resistance continues to be a concern in resource-limited settings.8,9 Children face additional barriers to treatment including dosing, formulations, higher costs for pediatric antiretroviral drugs, and high infant mortality rates.3,10–13
Beginning in 2004, African countries began expanding access to antiretroviral medications as funding became available.14,15 Better descriptions of clinical diagnosis, staging, and management of HIV-infected children facilitated scale-up of treatment.16 A number of publications in which treatment outcomes for pediatric populations in resource-limited settings were described have recently emerged. These studies, including 2 recent reviews by Sutcliffe et al14 and Ciaranello et al,17 reference outcomes from developed-countries (DCs) publications for informal comparison; however, no study has systematically compared outcomes and characteristics of pediatric ART between resource-limited countries (RLCs) and DCs.
The purpose of this study was to review the literature to quantify and compare major clinical end points and baseline characteristics for children receiving HAART in DCs versus RLCs.
METHODS
Search Strategy
A systematic literature search was performed through March 2010 for all studies for which outcomes (mortality, weight-for-age z score [WAZ], CD4%, and viral load [VL]) were reported after initiation of HAART in pediatric patients. The following databases were searched: PubMed, EBSCO, Global Health Host, AIDSLine, and the Cochrane Library. Conference abstracts from the International AIDS Society 2009 and Conference on Retroviruses and Opportunistic Infections 2010 were searched, because these data likely have not had time to be published. Search terms included “pediatric,” “children,” “HIV,” “HAART,” “Africa,” “resource-limited,” “developing country,” “outcomes,” “mortality,” “efficacy,” and “adherence” (or equivalents of these terms [ie, HIV-1, ART, antiretrovirals, ARV, therapy, treatment]). This search strategy was supplemented by searching references in the bibliographies of articles.
Study Selection
Observational cohorts and clinical trial studies were selected for review on the basis of predefined criteria. Full-length articles published in a language other than English were included if they had an abstract in English. Studies were selected on the basis of the following criteria: (1) patients were younger than 21 years and not limited to a narrow age range such as <24 months or >13 years), (2) patients had received HAART (≥3 antiretroviral medications), (3) the sample size was >20 patients, and (4) patients had at least 6 months' follow-up on HAART. Outcome measures included mortality, weight change, CD4 counts and percentages, and VLs. Two authors reviewed the reports and came to agreement on inclusion or exclusion of the publications.
Data Extraction
In addition to the outcome measures, information was extracted on the focus of the study, regimen, previous ART exposure including prevention of mother-to-child transmission, time from presentation to initiation of HAART, disease severity, predictors of mortality, orphan status, hospitalization, follow-up time, percentage of patients lost to follow-up, and intent-to-treat versus as-treated analysis. Articles were separated into 2 categories, RLCs or DCs, according to rankings by the United Nations Statistics Division.18 Articles were then subcategorized according to geographic location. Studies were also grouped on the basis of the cohorts' previous HAART exposure: HAART-naive (previous mono/dual therapy or antiretroviral naive) or HAART-exposed (3-drug regimen including a protease inhibitor or a nonnucleoside reverse transcriptase inhibitor).
Multiple reports were reviewed for the same study, and individual studies were compared for overlap. Overlap was evaluated by reviewing authors, location, date, duration, and specific interventions. When results overlapped, data from the largest cohort, most recent publication, or longest follow-up time were selected. Multiple reports for the same or overlapping cohort were included if they each provided unique outcome data (eg, 1 reported mortality, 1 reported CD4%, or each reported CD4 count at different time points). Unique outcome data were extracted and added so that no overlapping data points were used in calculations. Reports were excluded if data were not used (see Appendix).
Calculations and Statistical Analysis
The mortality percentage was collected directly or calculated from reported results. Deaths per 100 child-years (DPCY) was calculated by using the number of deaths and time of the mortality measurement, unless reported directly in the article. The total number of child-years was estimated as the sum of years contributed by living patients at the time of mortality measurement and one-half of this follow-up time for deceased patients. These estimations are likely to be less accurate with longer follow-up times.
For articles in which mortality rates at multiple follow-up time points were provided, the mortality measurement nearest 12 months' follow-up was used, because it was the most commonly used follow-up time point.
Mean baseline characteristics and mortality rates were calculated for comparison between RLCs and DCs. All mean calculations were weighted on the basis of cohort size. Hereafter, weighted means will be referred to simply as means. The means, ranges, SDs, and confidence intervals of HAART-naive studies were calculated for both mortality percentage and DPCY for each geographic subregion, RLCs, and DCs. In addition, the RLC and DC means, ranges, SDs, and confidence intervals for baseline characteristics including CD4 T-cell percentage, VL (log10 copies per mL), age, and WAZ were calculated for HAART-naive studies and for all studies that included HAART-experienced cohorts. CD4% and the percentage of patients who achieved virologic suppression were graphed over time, and the mean levels 12 months after HAART were calculated. The RLC and DC baseline values and outcomes were compared by using independent-samples t tests.
RESULTS
Study Selection and Characteristics
The initial literature search produced 723 publications: 313 published articles and 410 conference abstracts. Abstracts, methods, and/or results were reviewed, and 199 reports were found to contain some relevant selection criteria. Of these reports, 131 were excluded for reasons listed in Fig 1 and the Appendix, and the remaining 68 were used for analysis (RLCs = 40, total N = 18 882 and 17 875 approximately correcting for overlap; DCs = 28, total N = 3150 and 1835 approximately correcting for overlap). Characteristics of included studies are summarized in Table 1.
FIGURE 1.
Study-selection flowchart. ARV indicates antiretroviral medication.
TABLE 1.
Study Characteristics of Pediatric Cohorts in RLCs and DCs
| Study Authors (Year) | Location | N | Age, Mean (b) or Median (a) | Study Details | Follow-up Time, Total (b) or Median (a), mo |
|---|---|---|---|---|---|
| RLCs | |||||
| Hien et al19 (2009) | Burkina Faso | 52 | 6.8 ya | IAS abstract | 24b |
| Fassinou et al20 (2004) | Cote d'Ivoire | 78 | 7.2 ya | 21% HAART-experienced | 21a |
| Rouet et al21 (2006) | Cote d'Ivoire | 78 | 6.5 ya | PI vs NNRTI comparison | 36a |
| Nyandiko et al22 (2006) | Kenya | 279 | 6.0 ya | Rural, orphan comparison | 34a |
| Song et al23 (2007) | Kenya | 29 | 8.5 yb | Adult comparison | 15b |
| Van Winghem et al24 (2008) | Kenya | 648 | 5.5 ya | Adherence: MSF | 48b |
| Wamalwa et al25 (2007) | Kenya | 67 | 4.4 ya | Early response | 9a |
| Reddi et al26 (2007) | KwaZulu Natal | 151 | 5.7 ya | 16% HAART-experienced | 8a |
| Leyenaar et al27 (2009) | Lesotho | 284 | 2.2 ya | BIPAI center of excellence | 14b |
| Cohen et al28 (2009) | Lesotho | 283 | NR | IAS abstract, rural: MSF | 24b |
| Bong et al29 (2007) | Malawi | 439 | 6.0 ya | FDC | 24b |
| —16 (2006) | Malawi | A: 436 | <15 y | FDC | 6b |
| B: 233 | FDC | 12b | |||
| Weigel et al30 (2010) | Malawi | 497 | 8.0 ya | CROI abstract, growth | 24b |
| Marazzi et al31 (2006) | Mozambique | 297 | 4.4 yb | Integrated public health program | 9a |
| Vaz et al32 (2009) | Mozambique | 1007 | 3.0 ya | IAS abstract, growth | 48b |
| van Griensven et al33 (2008) | Rwanda | 315 | 7.2 ya | Nurse-based care: MSF | 45b |
| Diack MBaye et al34 (2005) | Senegal | 98 | 5.0 ya | Non-specific focus | 36b |
| Barth et al35 (2008) | South Africa | 66 | 8 mo to 11 y | Rural, ART-naive | 12b |
| Eley36 (2006) | South Africa | 409 | 1.9 ya | Severe clinical disease | 12b |
| Jaspan et al37 (2008) | South Africa | 391 | 2.2 ya | PI vs NNRTI comparison | 48b |
| Jooste et al38 (2005) | South Africa | 100 | 1–14 y | Non-specific focus | 6b |
| Smit et al39 (2009) | South Africa | 615 | 1.8 ya | IAS abstract: Cape Town | 46b |
| Blè et al40 (2007) | Tanzania | 59 | 3 mo to 11 y | Orphan study | 12b |
| Kamya et al41 (2007) | Uganda | 250 | 9.2 yb | Genotypic mutations | 14b |
| Bolton-Moore et al42 (2007) | Zambia | 2938 | 6.8 ya | Providers (nonphysicians) | 12a |
| Gupta et al43 (2009) | Zambia | 103 | 8.0 ya | IAS: Triomune FDC | 36b |
| Walker et al44 (2007) | Zambia | 93 | 8.8 ya | Non-specific focus | 24b |
| Janssens et al45 (2007) | Cambodia | 212 | 6.0 ya | Split FDC | 36b |
| Myung et al13 (2007) | Cambodia | 117 | 5.5 ya | DOT | 26b |
| Zhang et al46 (2007) | China | A: 51 | NR | HAART-naive | 13b |
| B: 32 | NR | HAART-experienced | 13b | ||
| Rajasekaran et al47 (2009) | India | 295 | 7.6 yb | Non-specific focus | 10a |
| Kline et al48 (2007) | Romania | 414 | 13.0 yb | 82% drug-experienced | 51a |
| Aurpibul et al49 (2009) | Thailand | 225 | 7.4 ya | IAS: growth | 55b |
| Lapphra et al40 (2008) | Thailand | 139 | 6.0 ya | Siriraj Hospital | 36a |
| Puthanakit et al51 (2007) | Thailand | 192 | 7.6 yb | Chiang Mai Hospitals | 29a |
| Romanelli et al52 (2006) | Brazil | 43 | 2.4 yb | Dual vs triple antiretroviral therapy | 48b |
| Martins et al53 (2009) | Brazil | 196 | NR | IAS: growth | 6b |
| Martins et al54 (2009) | Brazil | 196 | NR | IAS: immunosuppression | 6b |
| George et al12 (2007) | Haiti | 236 | 6.3 ya | Treatment-naive | 20a |
| Severe et al55 (2005) | Haiti | 94 | <13 y | Adult and child study | 12b |
| DCs | |||||
| Ghaffari et al56 (2004) | US | 40 | 7.1 ya | PI: University of Florida, Gainesville | 22b |
| King et al57 (2005) | US | 41 | 6.4 ya | PACTG 403, PI nelfinavir | 11b |
| Krogstad et al58 (1999) | US | 62 | 3 mo to 13 y | PI nelfinavir, age groups | 10a |
| Krogstad et al11 (2002) | US | 192 | 6.2 ya | PACTG 377 | 11b |
| McKinney et al59 (2007) | US | 37 | 10.5 ya | PACTG 1021 | 22b |
| Melvin et al60 (2002) | US | 36 | 6.0 ya | 5 patients overlap PACTG | 29a |
| Patel et al61 (2008) | US | 1236 | NR | PACTG 219 10-y follow-up | 70a |
| Rosenblatt et al62 (2005) | US | 192 | 6.2 ya | PACTG 377 | 11b |
| Soh et al63 (2003) | US | 702 | 6.7 ya | PACTG 219 CD4 response | 48b |
| Spector et al64 (2000) | US | 57 | 8.0 ya | PACTG 382 | 11b |
| Starr et al65 (1999) | US | 57 | 8.0 yb | PACTG 382 | 11b |
| Watson et al66 (1999) | US | 72 | NR | Adherence and efficacy | 9a |
| Wiznia et al67 (2000) | US | 192 | 6.2 ya | PACTG 377 | 11b |
| Yogev et al68 (2002) | US | 245 | 7.4 ya | PACTG 338 subset | 11b |
| Bracher et al69 (2007) | Denmark | 49 | 6.7 ya | Long term follow-up | 72b |
| Teglas et al70 (2001) | France | 33 | 12.5 ya | Efavirenz study | 9b |
| Thuret et al71 (1999) | France | 22 | 6.5 ya | Non-specific focus | 21b |
| Wintergerst et al72 (2008) | Germany | 33 | 8.2 ya | Efavirenz study | 50a |
| Fraaij et al73 (2005) | Netherlands | 31 | 5.1 ya | Prospective PI study | 48b |
| Scherpbier et al74 (2007) | Netherlands | 36 | 6.6 ya | Efavirenz study | 11a |
| van Rossum et al75 (2002) | Netherlands | 32 | 5.4 ya | Non-specific focus | 22b |
| van Rossum et al76 (2000) | Netherlands | 28 | 6.0 ya | Non-specific focus | 6b |
| Verweel et al77 (2002) | Netherlands | 24 | 5.2 ya | HAART effect on growth | 22b |
| Nadal et al78 (2000) | Switzerland | A: 37 | 6.3 ya | Ritonavir | 28a |
| B: 237 | 7.8 ya | Nelfinavir | 28a | ||
| Rudin et al79 (2008) | Switzerland | 133 | 6.3 ya | PI comparison | 66b |
| Judd et al80 (2007) | UK, Ireland | 156 | NR | CHIPS 2003–2006 antiretroviral-naive | >9 |
| Walker et al81 (2004) | UK, Ireland | 265 | 4.2 ya | CHIPS, antiretroviral-naive | 24b |
| PENTA82 (2002) | 8 countries | 103 | 5.3 ya | PENTA | 11b |
IAS indicates International AIDS Society; PI, protease inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; MSF, Medecins Sans Frontiere; BIPAI, Baylor International Pediatric AIDS Initiative; —, No author provided; FDC, fixed-dose combination treatment; CROI, Conference on Retroviruses and Opportunistic Infections; DOT, directly observed therapy; NR, not reported; PACTG, Pediatric AIDS Clinical Trial Group; CHIPS, Collaborative HIV Paediatric Study; PENTA, Paediatric European Network for Treatment of AIDS.
Mean/total.
Median.
Baseline Mean/Median Age
The mean baseline age in RLC studies was 5.4 and 5.7 years in HAART-naive and all studies, respectively (Table 2). In DCs, the mean age of patients in HAART-naive and all studies was 6.5 and 6.7 years, respectively. There was no significant difference in the mean/median age at baseline between RLCs and DCs for HAART-naive cohorts (P = .1) or all studies (P = .2).
TABLE 2.
Pooled Summary Statistics: Comparison of RLCs and DCs
| RLCs | DCs | P | |
|---|---|---|---|
| Mean mortality, % | |||
| HAART-naive | 7.6 (0 to 18.8)a ± <0.1b (8937) | 1.6 (0 to 3.8)a ± <0.1b (1241) | <.001 |
| Ref No. | 12, 13, 16, 19, 22–25, 27, 28, 31, 33, 35, 42, 44, 45, 47, 51, and 52 | 59, 61, 69, 71, 73, 79, and 82 | |
| All studiesc | 7.5 (0 to 18.8)a ± <0.1b (9663) | 1.7 (0 to 3.8)a ± <0.1b (1277) | <.001 |
| Ref No. | Additionally 21, 26, 46, and 48 | Additionally 74 | |
| Mean DPCY | |||
| HAART-naive | 8.0 (0 to 41.5)a ± 4.1b (8937) | 0.9 (0 to 1.1)a ± 1.2b (1241) | <.001 |
| Ref No. | 12, 13, 16, 19, 22–25, 27, 28, 31, 33, 35–42, 44, 45, 47, 51, and 52 | 59, 61, 69, 71, 73, 79, and 82 | |
| All studiesc | 7.7 (0 to 41.5)a ± 3.8b (9663) | 1.0 (0 to 3.1)a ± 1.2b (1277) | <.001 |
| Ref No. | Additionally 21, 26, 46, and 48 | Additionally 74 | |
| Mean baseline CD4% | |||
| HAART-naive | 12 (5 to 20)a ± 0.1b (8437) | 23 (7 to 47)a ± 0.2b (647) | .01 |
| Ref No. | 12, 13, 19, 22, 25, 27, 29, 32, 33, 35–37, 39, 41, 42, 44, 45, 47, and 50–52 | 11, 56, 57, 59, 66, 69, 71, 73, 78, and 82 | |
| All studiesc | 12 (5 to 20)a ± 0.1b (8666) | 23 (7 to 47)a ± 0.1b (719) | .003 |
| Ref No. | Additionally 21 and 26 | Additionally 60 and 74 | |
| Mean baseline VL, log10 copies per mL | |||
| HAART-naive | 5.5 (5.1 to 6.1)a ± 2.7b (1882) | 4.7 (4.4 to 5.2)a ± 2.2b (647) | <.001 |
| Ref No. | 12, 19, 23, 25, 34–37, 41, 46A;, 51, and 52 | 56, 57, 59, 66, 67, 69, 71, 73, 78, and 82 | |
| All studiesc | 5.5 (4.9 to 6.1)a ± 2.7b (1992) | 4.7 (3.6 to 5.2)a ± 2.0b (752) | <.001 |
| Ref No. | Additionally 21 and 46B | Additionally 60, 70, and 74 | |
| Mean baseline age, y | |||
| HAART-naive | 5.4 (1.8 to 10.0)a ± 4.0b (9494) | 6.5 (5.1 to 10.5)a ± 3.4b (575) | .1 |
| Ref No. | 12, 13, 19, 22–25, 27, 29, 31, 34, 36, 37, 39, 41, 42, 44, 45, 46, 47, and 50–52 | 56, 57, 59, 67, 69, 71, 73, 78, and 82 | |
| All studiesc | 5.7 (1.8 to 13.0)a ± 3.8b (10169) | 6.7 (5.1 to 12.5)a ± 2.9b (680) | .2 |
| Ref No. | Additionally 21, 26, 46B, and 48 | Additionally 60, 70, and 74 | |
| Mean baseline WAZ | |||
| HAART-naive | −2.2 (−3.8 to −1.6)a ± 2.1b (5748) | −0.4 (−0.8 to −0.3)a ± 0.1b (180) | <.001 |
| Ref No. | 13, 19, 23, 27, 30, 33, 36, 37, 40, 42, 44, 46, 49, 53, and 55 | 56, 59, 65, 71, and 77 | |
| All studiesc | −2.2 (−3.8 to −1.5)a ± 2.0b (6009) | −0.4 (−0.8 to −0.3)a ± 0.1b (180) | <.001 |
| Ref No. | Additionally 20, 26, and 46B | — |
Baseline WAZ
The mean WAZ for children who were initiated on HAART in RLCs was −2.2 for both HAART-naive and all studies combined (Table 2). In DCs, the mean WAZ for both HAART-naive and all studies was −0.4. There was a large and statistically significant difference between baseline WAZ in RLCs and DCs (P < .001).
Post-HAART Mortality Outcomes
The mortality analysis included 38 cohorts: 30 cohorts from RLCs (N = 9663) and 8 cohorts from DCs (N = 1277). Only outcomes for patients on HAART (≥3 antiretroviral medications) were used in the analysis. Several calendar studies were excluded, because the authors reported mortality rates for birth cohorts without separating outcomes for patients on no ART, mono/dual therapy, or HAART; these studies revealed decreased mortality rates after the introduction of HAART.2,80,83–85 In addition, 11 study reports provided mortality data but were not included in pooled analysis because of overlap with larger or more recent studies.*
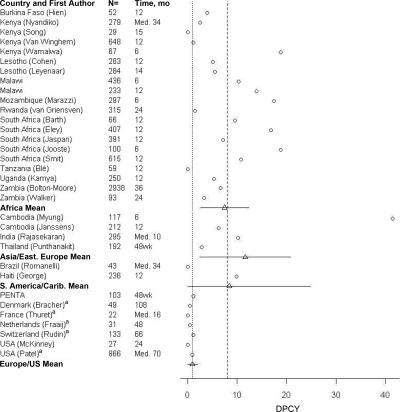
Post-HAART mortality data for HAART-naive studies are shown in Fig 2 and Table 3. Geographic subregions used for RLCs were Africa (20 studies), Asia/Eastern Europe (4 studies), and South America/Caribbean (2 studies). The mean mortality rates in Africa, Asia/Eastern Europe, and South America/Caribbean were 7.4%, 8.8%, and 7.6% per cohort and 7.5, 11.9, and 8.4 DPCY, respectively. In US and European HAART-naive studies, the mean mortality rates were 1.6% per cohort and 0.9 DPCY.
FIGURE 2.
Pediatric DPCY after HAART: HAART-naive cohorts. a Includes mono/dual ART-experienced patients. Vertical line (…), DC mean DPCY for HAART-naive studies; vertical line (—), RLC mean DPCY for HAART-naive studies; horizontal lines, 95% confidence intervals for weighted mean.
TABLE 3.
Pediatric Mortality Rates After HAART in RLCs and DCs
| Country | N | Time | Lost to Follow-up, % | Mortality, % | DPCY |
|---|---|---|---|---|---|
| RLCs | |||||
| Burkina Faso (Hien et al19) | 52 | 12 mo | 2 | 3.8 | 3.9 |
| Kenya (Nyandiko et al22) | 279 | 34 mo (median) | 11 | 6.8 | 2.5 |
| Kenya (Song et al23) | 29 | 15 mo | 3 | 0 | 0 |
| Kenya (Van Winghem et al24) | 648 | 12 mo | NR | 1.1 | 1.1 |
| Kenya (Wamalwa et al25) | 67 | 6 mo | NR | 9.0 | 18.8 |
| Lesotho (Cohen et al28) | 283 | 12 mo | 2 | 5.0 | 5.1 |
| Lesotho (Leyenaar et al27) | 284 | 14 mo | 1 | 6.3 | 5.5 |
| Malawi A16 | 436 | 6 mo | 11 | 5.0 | 10.3 |
| Malawi B16 | 233 | 12 mo | 15 | 13.0 | 13.9 |
| Mozambique (Marazzi et al31) | 297 | 6 mo | NR | 8.4 | 17.5 |
| Rwanda (van Griensven et al33) | 315 | 24 mo | 4 | 2.5 | 1.4a |
| South Africa (Barth et al35) | 66 | 12 mo | 17 | 9.0 | 9.5 |
| South Africa (Eley36) | 407 | 12 mo | NR | 15.4 | 16.8 |
| South Africa (Jaspan et al37) | 391 | 12 mo | 2 | 6.9 | 7.2 |
| South Africa (Jooste et al38) | 100 | 6 mo | 3 | 9.0 | 18.8 |
| South Africa (Smit et al39) | 615 | 12 mo | NR | 10.2 | 10.8 |
| Tanzania (Bl+è et al40) | 59 | 12 mo | 0 | 0 | 0 |
| Uganda (Kamya et al41) | 250 | 12 mo | 1 | 5.2 | 5.3 |
| Zambia (Bolton-Moore et al42) | 2938 | 36 mo | 13 | 8.3 | 6.6a |
| Zambia (Walker et al44) | 93 | 24 mo | NR | 6.5 | 3.3 |
| Africa mean | n/a | n/a | NR | 7.4 ± 0.6b | 7.5 ± 4.9b |
| Cambodia (Myung et al13) | 117 | 6 mo | NR | 18.8 | 41.5 |
| Cambodia (Janssens et al45) | 212 | 12 mo | 2 | 6.0 | 6.2 |
| India (Rajasekaran et al47) | 295 | 10 mo (median) | 2 | 8.1 | 10.2 |
| Thailand (Puthanakit et al51) | 192 | 48 wk | NR | 6.7 | 2.8a |
| Asia/Eastern Europe mean | n/a | n/a | NR | 8.8 ± <0.1b | 11.9 ± 9.2b |
| Brazil (Romanelli et al52) | 43 | 34 mo (median) | 9.3 | 0 | 0 |
| Haiti (George et al12) | 236 | 12 mo | 10 | 9.0 | 9.9 |
| South America/Caribbean mean | n/a | n/a | NR | 7.6 ± 0.1b | 8.4 ± 16.4b |
| DCs | |||||
| PENTA82 | 103 | 48 wk | NR | 1.0 | 1.1 |
| Denmark (Bracher et al69)c | 49 | 108 mo | NR | 2.0 | 0.4a |
| France (Thuret et al71)c | 22 | 16 mo (median) | NR | 0 | 0 |
| Netherlands (Fraaij et al73)c | 31 | 48 mo | 16 | 3.1 | 0.4 |
| Switzerland (Rudin et al79)c | 133 | 66 mo | 1 | 3.8 | 1.1a |
| US (McKinney et al59) | 27 | 24 mo | NR | 0 | 0 |
| US (Patel et al61)c | 866 | 70 mo (median) | NR | 1.4 | 0.9a |
| Mean | n/a | n/a | NR | 1.6 ± 0.2b | 0.9 ± 1.2b |
NR indicates not reported; n/a, not applicable.
Directly reported number of child-years of follow-up.
95% confidence intervals for weighted mean.
Included mono/dual ART-experienced patients.
Comparisons between RLCs and DCs are listed in Table 2. Post-HAART mortality rates for HAART-naive cohorts in RLCs were ∼5 and 9 times greater than in DCs: 7.6% vs 1.6% and 8.0 vs 0.9 for mortality percentage and DPCY, respectively (P = .002 and P < .001, respectively). Mean mortality rates for all studies that included previously mono/dual protease inhibitor– and nonnucleoside reverse transcriptase inhibitor–treated children were 7.5% vs 1.7% mortality percentage and 7.7 vs 1.0 DPCY for RLCs and DCs, respectively.
Baseline and Post-HAART CD4 Levels
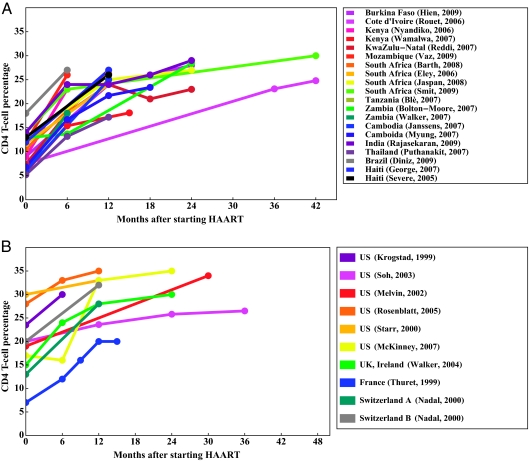
Forty-two study reports provided unique data for either baseline CD4% or CD4% over time: 25 from RLCs and 17 from DCs. Twenty-three RLC and 13 DC studies were pooled for baseline CD4%, restricting to 1 value from overlapping cohorts (Table 2). HAART-naive studies had mean baseline CD4% values of 12% (range: 5%–20%) and 23% (range: 7%–47%) for RLCs and DCs, respectively (P = .01). Twenty reports from RLCs and 9 reports from DCs described changes in CD4% after HAART initiation (Fig 3). In this graphical presentation, overlap exists between the Pediatric AIDS Clinical Trial Group publications (namely, refs 58, 60, 62, 63, and 65). Mean CD4% 12 months after HAART was significantly different between RLC and DC studies: 24% and 27%, respectively (P = .03).
FIGURE 3.
CD4 T-cell percentage change over time. A, RLCs; B, DCs.
Baseline VLs and Post-HAART Virologic Suppression
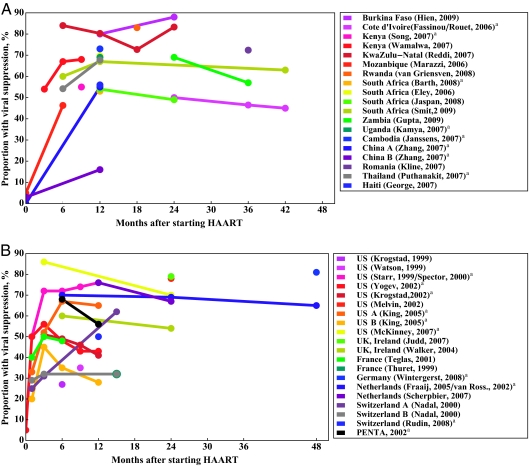
Forty-three study reports provided unique data for either baseline VL or percent virologic suppression: 21 from RLCs and 22 from DCs. Fourteen RLC and 14 DC studies reported baseline VL (Table 2). Baseline VLs in HAART-naive studies were 5.5 log10 copies per mL in RLCs and 4.7 log10 copies per mL in DCs (P < .001). Nineteen RLC and 20 DC reports described the percentage of patients who achieved virologic suppression (Fig 4). Viral suppression was defined as <400 copies per mL. Six study reports only defined viral suppression as <50 copies per mL.26,39,43,51,60,72 Overlap exists between refs 11, 58, 64, 65, and 68; the Paediatric European Network for Treatment of AIDS overlaps with ref 81 but not with ref 80, because data from this reference were extracted for the 2003–2006 birth cohort. Twelve months after HAART, the mean percentage of children who achieved viral suppression was 65% in RLC and 49% in DC studies, and there was no significant difference between the 2 groups (P = .4). Eleven of the DC and 7 of the RLC studies reported using an intention-to-treat approach when evaluating the rate of virologic suppression.†
FIGURE 4.
Percentage of patients who achieved viral suppression over time. A, RLCs; B, DCs. a Intention-to-treat analysis.
Predictors of Mortality
Weighted least-squares regression was used to determine if differences in mortality between RLCs and DCs diminished after controlling for baseline WAZ, CD4%, or VL. Adjusting for baseline CD4 level, the mortality difference between RLCs and DCs persisted (6.7% mortality difference; P = .01), and there was negligible evidence of confounding. There were fewer studies for which VL and WAZ were reported; however, the mortality difference between RLCs and DCs seemed to be confounded by baseline WAZ and VL.
Studies from both RLCs and DCs revealed associations between mortality rate, baseline CD4%, and VL.11,12,13,36,42,61,63,68 Low WAZ was a risk factor for mortality.12,26,27,40,44,77 Several RLC studies revealed that younger age was associated with mortality, whereas DC studies revealed conflicting findings regarding age and mortality.12,22,36,51,73,80 Finally, 2 RLC studies revealed that orphans had a higher mortality rate, although programs with >50% orphans achieved relatively low mortality rates overall.22,23,45
Additional Study Characteristics
Information was also collected surrounding initiation of HAART. RLC studies referenced various World Health Organization (WHO) pediatric ART guidelines that recommend initiation of HAART at WHO stage 3 or 4 or at a CD4% of <15%, <20%, <15%, or <20%, depending on age, or <200 cells per μL. The authors of ref 45 (Cambodia) noted that 37% of evaluated children did not meet initiation criteria, and 10% of eligible children died before initiation. DC studies often did not report specific initiation criteria. Between 61% and 99% of children in RLC studies initiated HAART at WHO stage 3 or 4 disease.27,36 Between 10% and 62% of children in DC studies had Centers for Disease Control and Prevention class C disease.78,82 Only 1 RLC study42 (Zambia) reported median age at diagnosis: 5.5 years. The authors of ref 69 (Denmark) reported median age of diagnosis at 1.5 years; median age of initiation was 6.7 years. Another study80 found that between 2004 and 2006 foreign-born children presented later than UK-born children: 7.6 versus 0.8 years, respectively. Four RLC studies reported time between diagnosis and HAART initiation ranging from a median of 53 days to 26 months.20,33,40,44 RLC studies reported that <10% of subjects received antiretroviral medications in attempt to prevent vertical transmission (<1%–12%); however, it is possible that perinatal nevirapine exposure was not systematically ascertained or was underreported.16,20,25,26,27,33,38,41 Prevention of mother-to-child transmission was reported as being widely available in DCs, but rates of utilization were not specified.11,61,67,80
DISCUSSION
In this study, we determined and compared baseline status and outcomes of children who initiated HAART in RLCs and DCs. As anticipated, mortality rates were dramatically lower with HAART than in studies before HAART and <10% in both settings. Mortality rates were higher in RLCs than in DCs, but the mortality difference observed was less than would have been expected on the basis of general childhood mortality estimates from those regions, which suggests that the added contact with care providers enhances survival beyond baseline, likely by prevention of common infectious diseases. RLC cohorts involved children with significantly lower baseline CD4 counts and WAZ and higher VLs, all of which would be expected to also contribute to increased mortality rates. Efforts to initiate HAART earlier would be expected to identify children before substantial immunosuppression, which should translate into improved survival rates.
Comparisons between observational studies have inherent limitations; nonetheless, these comparisons are important for the evaluation of programs and to guide future treatment. Although authors of recent reviews have described post-HAART outcomes in Africa, none has systematically compared outcomes between regions.14 RLC outcomes have been compared informally to DC outcomes without careful consideration of the cohort selection criteria or treatment regimens. Informal comparisons have been made to DC study reports that provided outcomes according to calendar years, including patients not on ART and those on HAART for years, infant studies, overlapping studies, and cohorts of < 20 patients.2,14,17,80–85,89–91 Publications included in this study were systematically screened on the basis of age, cohort size, and regimens. Outcome data were extracted to avoid analysis of data from overlapping cohorts, and standardized outcomes were compared.
The post-HAART mortality rates for HAART-naive children were fivefold to ninefold greater in RLCs than in DCs. Although not directly comparable, this difference was less than the difference between overall mortality rates for children between the ages of 1 and 5 years in RLCs and DCs (exceptions included Brazil and Thailand).92 Six studies from Zambia, Kenya, Rwanda, and Tanzania found that post-HAART mortality rates fell below 3.5 DPCY despite higher regional child mortality rates (range: 37–56 deaths per 1000 live births between the ages of 1 and 5 years, as estimated by subtracting infant mortality rates from mortality rates of children <5 years old).22–24,33,40,44,92 Although HIV contributes to overall child mortality rates in high HIV-prevalence areas, HAART seems to provide some survival benefits to patients, perhaps by simply bringing children in contact with medical services.93,94
The CD4 and VL data provide important contextual information for interpretation of the mortality results by demonstrating that HAART programs in RLCs have reported efficient and comparable increases in CD4% and declines in VLs as DC programs. Significantly lower CD4 levels observed in RLCs 12 months after HAART are likely a result of markedly lower baseline CD4 levels. In contrast, post-HAART VL-suppression rates were not significantly different between DCs and RLCs after HAART despite significantly higher baseline VL in RLCs.
Strengths of this analysis include the large number of studies evaluated, the study-selection process, and the standardized comparisons of mortality rates, baseline CD4 percentage, and baseline VLs. This comprehensive comparison spanned 11 years of publications, during which drug regimens, guidelines, and patient populations evolved, particularly in DCs. Although later publications from DCs would be expected to use more potent medications, earlier study reports described larger HAART-naive cohorts, which provides better comparison of baseline characteristics and perhaps slightly underestimates the difference between DCs and RLCs.74 A limitation of the study is that outcomes were likely biased toward better outcomes, because children lost to follow-up could include unreported deaths or immunologic and virologic nonresponders. We excluded cohorts that had only included children with a minimum amount of follow-up for the same reason. The studies we included had survivor bias (mean baseline age: >5 years); in RLCs, untreated HIV-infected children have only 50% survival rates below 2 years. As-treated analysis also results in overestimation of virologic suppression, because the proportion of children who achieved virologic suppression has been reported within the denominator of patients with VL data rather than the total number of patients who initiated treatment.
Low baseline CD4, WAZ, and high VL levels were identified by individual studies as strong predictors of mortality in both RLCs and DCs. In multivariate analysis, mortality-rate differences between RLCs and DCs persisted even after adjusting for baseline CD4 count. However, we found that at least a portion of the mortality-rate difference was attributable to differences in WAZ or VL in these different settings. In RLCs, children were older at the time of diagnosis and had more advanced disease, and the majority of deaths occurred in the first 6 months of treatment.‡ Earlier identification of children could improve post-HAART outcomes by identifying children with less advanced disease.95,96 Revised 2010 WHO treatment guidelines recommend treatment for all children younger than 24 months and a new CD4 threshold of 25% or 750 cells per μL for children aged 2 to 5 years. Additional studies are needed to evaluate the effect of these new guidelines on post-HAART outcomes.97
CONCLUSIONS
Pediatric HAART programs in RLCs are successfully achieving a reduction in HIV- related mortality; however, post-HAART mortality rates remain higher than the rates in DCs. Currently, children in RLCs begin HAART at higher baseline VLs and lower baseline CD4 levels. Continuing to improve child health with interventions including nutritional support and prevention and treatment of coinfections may additionally improve survival rates. With increased availability of treatment and earlier treatment, regions with high HIV prevalence should realize marked declines in HIV-related child mortality.
ACKNOWLEDGMENTS
We thank Romel D. Mackelprang, Neil S. Geisler, Patrick Danaher, and Enrique Peacock-Lopez for their contributions to the creation of the figures.
APPENDIX.
Excluded Studies
| Country | Author | Reason for Exclusion |
|---|---|---|
| 1. Cote d'Ivoire | Adje-Toure et al98 | Overlap with Fassinou et al20, subset analysis excluded patients who died |
| 2. Ethiopia | Biadgilign et al99 | Adherence study, cross-sectional |
| 3. Nigeria | Onankpa et al100 | Epidemiology study, no post-HAART outcomes reported |
| 4. South Africa | Cowburn et al101 | Mortality not reported, hospitalization study |
| 5. South Africa 2004 | Eley102 | Overlap with Eley,36 data not used in analysis |
| 6. South Africa | Prendergast et al103 | Limited to infants followed from birth |
| 7. South Africa | van Kooten et al104 | Cohort too small (17 patients) |
| 8. South Africa | Violari et al95 | Age limited to 6–12 wk, early vs delayed antiretroviral medication |
| 9. Togo | Atakouma et al105 | Cross-sectional study |
| 10. Togo | Polisset et al106 | Adherence study, no post-HAART outcomes reported |
| 11. Argentina | Fallo et al107 | Calendar-year comparisons |
| 12. Brazil | Matida et al108 | Calendar surveillance |
| 13. Brazil | Candiani et al109 | No. on HAART not specified |
| 14. Guatemala | Samayoa et al110 | Results combine HAART and non-HAART |
| 15. Jamaica | Evans-Gilbert et al111 | Mortality not reported, hospitalization |
| 16. Cambodia | Madec et al112 | Age limited to >13 y |
| 17. India | Kumarasamy et al113 | Excluded patients with follow-up at <18 mo |
| 18. India | Lodha et al114 | Excluded patients with follow-up at <3 mo |
| 19. India | Natu et al115 | Mortality not reported |
| 20. India | Pensi et al116 | Cohort too small (13 patients) |
| 21. Romania | Ferris et al117 | Overlap with Kline et al,48 focus on disclosure |
| 22. Romania 2004 | Kline et al88 | Overlap with Kline et al,48 data not used in analysis |
| 23. Thailand | Chearskul et al118 | Overlap with Lapphra et al40, data not used in analysis |
| 24. Thailand | Koekkoek et al119 | Cohorts too small (16 patients) |
| 25. Thailand | Plipat et al120 | Cohort too small (19 patients) |
| 26. Multiple | O'Brien et al10 | Overlap with multiple studies |
| 27. Multiple | Arrive et al121 | Overlap with multiple studies |
| 28. Multiple | KIDS ART-LINC122 | Overlap with multiple studies |
| 29. Multiple | Saez-Llorens et al123 | Did not isolate data from RLCs and DCs |
| 30. Africa/Romania | Weidle et al124 | Mortality not reported, dosing study |
| 31. Lat. America | Hazra et al125 | Results combined HAART- and non–HAART-treated patients |
| Europe | ||
| 32. Belgium | Hainaut et al126 | Cohort too small (4 patients), age limited to <2 mo |
| 33. France | Aboulker et al127 | Age limited to <3 mo |
| 34. France | Faye et al128 | Age limited to <1 y |
| 35. Germany | Funk et al90 | Cohort too small (16 patients) |
| 36. Germany 1998 | Wintergerst et al129 | Cohort too small (15 patients) |
| 37. Italy | Canani et al130 | Cohort too small (10 patients) |
| 38. Italy | Chiappini et al131 | Overlap with de Martino et al87, calendar study |
| 39. Italy | de Martino et al87 | Overlap with PENTA,82 data not used in analysis |
| 40. Italy | Vigano et al132 | Cohort too small (11 patients), heavily pretreated |
| 41. Netherlands 1998 | Cohen et al133 | Cohort too small (13 patients) |
| 42. Spain | Larru et al134 | Limited to patients whose conditions failed to respond HAART |
| 43. Spain 2006, 2004 | Resino et al135,85 | Calendar study |
| 44. Spain 2003 | Sanchez et al136 | Overlap with Larru et al134, Kaplan-Meier survival |
| 45. Spain | Guillen Martin et al137 | Epidemiology/immigrant study, no post-HAART outcomes reported |
| 46. Switzerland | Steiner et al138 | Overlap with Nadal et al78/Rudin et al79 excludes patients with <72 wk follow-up, growth study, |
| 47. UK, Ireland | Gibb et al91 | Overlap with PENTA,82 calendar study |
| 48. UK, Ireland | Doerholt et al89 | Age limited to <12 mo |
| 49. 9 countries | Newell et al139 | Overlap with PENTA82/Scherpbier et al74, results combined HAART- and non–HAART-treated patients |
| 50. Europe 2009 | Goetghebuer et al140 | Limited to infants followed from birth |
| 51. US 2001 | Abrams et al141 | Calendar study |
| 52. US 2003 | Benjamin et al142 | PACTG 300 mono/dual treatment, growth study |
| 53. US 2004 | Berrien et al143 | Adherence study, post-HAART outcomes not reported |
| 54. US 2001 | Blazevic et al144 | Cohort too small (11 patients) |
| 55. US 2000 | Borkowsky et al145 | Overlap with PACTG 338 reports, data not used in analysis |
| 56. US 2010 | Brady et al146 | Calendar/birth-cohort study |
| 57. US 2005 | Brogly et al147 | Overlap with PACTG 219C reports, calendar study, <24 y of age |
| 58. US 2004 | Brundage et al148 | Overlap with PACTG 382 reports, data not used in analysis |
| 59. US 2001 | Buchacz et al149 | Overlap with PACTG 219 reports, growth measures not comparable |
| 60. US 2003 | Caudill et al83 | Results combined HAART- and non–HAART-treated patients |
| 61. US 2005 | Chadwick et al150 | Age limited between 4 wk and 24 mo |
| 62. US 2008 | Chadwick et al151 | Age limited to <6 mo |
| 63. US 2001 | Chougnet et al152 | Overlap with Mueller et al168,169, excluded patients with clinical/immune decline |
| 64. US 2002, 2004 | Church et al153,9 | Cohort too small (14 patients), Enfuvirtide study |
| 65. US 1999 | Essajee et al154 | Limited to severely immunocompromised patients |
| 66. US 2004, 2007 | Flynn et al155 | Age limited to 8–22 y |
| 67. US 2007 | Glikman et al157 | Cohort too small (9 patients), adherence study |
| 68. US 2006 | Gona et al158 | Overlap with PACTG 219, calendar comparison, opportunistic-infection study |
| 69. US 2001 | Gortmaker et al2 | Overlap with PACTG 219, calendar study |
| 70. US 2001 | Jankelevich et al159 | Excluded patients with follow up at <96 wk |
| 71. US 2001 | Johnston et al160 | Immune-reconstitution study |
| 72. US 2009 | King et al161 | Age limited to 10–18 y, pharmacokinetic study |
| 73. US 1998 | Kline et al162 | Cohort too small (12 patients) |
| 74. US 2006 | Lee et al163 | Overlap with PACTG 219, quality-of-life study |
| 75. US 2000 | Lindsey et al84 | Meta-analysis included mono/dual/HAART |
| 76. US 1997, 2004 | Luzuriaga et al164,165 | Age limited between 2 wk and 24 mo |
| 77. US 2005 | McConnell et al166 | Calendar study |
| 78. US 1997 | Melvin et al167 | Cohort too small (9 patients) |
| 79. US 1998, 1998 | Mueller et al168,169 | Follow-up included 16 wk of monotherapy and only 12 wk on HAART, overlap with Jankelevich et al159 |
| 80. US 2000 | Nachman et al170 | Overlap with PACTG 338, data not used in analysis |
| 81. US 1999 | Palumbo et al171 | Mono and dual therapy study |
| 82. US 2008 | Patel et al172 | Overlap with PACTG 219, CD4% comparison between patients with and without HAART initiation |
| 83. US 1999 | Pelton et al86 | Results combined HAART- and non–HAART-treated patients |
| 84. US 2005 | Pelton et al173 | Overlap with PACTG 338, data not used in analysis |
| 85. US 2001 | Polis et al174 | Overlap with Mueller et al168,169, monotherapy |
| 86. US 2000 | Reddington et al175 | Overlap with PACTG 219 is unclear, adherence study |
| 87. US 2008 | Robbins et al176 | Limited to patients whose conditions failed to respond to HAART therapy, pharmacokinetic study |
| 88. US 1997 | Rutstein et al177 | Results combined HAART- and non–HAART-treated patients |
| 89. US 2005 | Storm et al178 | PACTG 219, cross-sectional quality-of-life study |
| 90. US 2002 | Van Dyke et al179 | Overlap with PACTG 377, adherence subset, data not used in analysis |
| 91. US 2004 | Viani et al180 | Calendar comparison |
| 92. US 2007 | Wiznia et al181 | Required HAART for 4 mo before study initiation, Enfuvirtide study |
| IAS Abstracts 2009 | ||
| 93. Kenya | Ayaya et al182 | Results combined HAART- and non–HAART-treated patients |
| 94. Kenya | McGrath et al183 | Growth study comparison of children <3 y/>3-y patient outcomes |
| 95. Kenya | Owiso et al184 | Calendar-study comparison |
| 96. Kenya | Wamalwa et al185 | Overlap with published study, data not used in analysis |
| 97. KwaZulu Natal | Ndirangu et al186 | Overlap with Reddi et al,26 growth study |
| 98. Malawi | Braun et al187 | Limited to “infant” cohort, age not specified |
| 99. Malawi | Dow et al188 | Age limited to <6 wk |
| 100. Malawi | Kabue et al189 | Limited to patients failing first line HAART |
| 101. Malawi | Kabue et al190 | Limited to “infant” cohort age not specified |
| 102. Swaziland | Chouraya et al191 | Age limited to <12 mo |
| 103. South Africa | Colvin et al192 | Mortality not reported |
| 104. South Africa | Coovadia et al193 | Post-PMTCT study |
| 105. South Africa | Fatti et al194 | Overlap with Eley,36 data not used in analysis |
| 106. South Africa | Fenner et al195 | Comparison of <5 y/>5-y patient outcomes |
| 107. South Africa | Kaplan et al196 | Overlap unclear, hospital not listed |
| 108. Uganda | Kekitiinwa et al197 | Limited to malnourished children |
| 109. Cambodia | Augustinova et al198 | Age limited to <18 mo |
| 110. Cambodia | Isaakidis et al199 | Cross-sectional survey |
| 111. Cambodia | Sophan et al200 | Limited to patients whose conditions failed to respond to first-line HAART |
| 112. Indian | Pandian et al201 | Overlap with published study, data not used in analysis |
| 113. Thailand | McConnel et al202 | Overlap with Puthanakit et al51 |
| 114. Brazil | Rezende et al203 | Overlap with Romanelli et al52, limited to patients with follow-up at >48 wk |
| 115. Multiple | Carter et al204 | Comparison of children <12 mo/>12 mo |
| 116. Multiple | Hansudewechakul et al205 | Overlap multiple studies |
| 117. Southern Africa | Davies et al206 | IeDEA, virologic failure study, does not report virologic suppression |
| 118. US | Palumbo et al207 | IMPAACT trial, age limited to <36 mo |
| 119. Unspecified | Bognon et al208 | Results combined HAART- and non–HAART-treated patients |
| CROI 2010 | ||
| 120. South Africa | Venkatesh et al209 | Limited to infants followed from birth |
| 121. Uganda | Achan et al210 | Outcomes reported virologic failure |
| 122. Southern Africa | Becquet et al211 | Survival study, not HAART-focused |
| 123. India | Pandian et al212 | Overlap with Rajasekaran et al47 |
| 124. Thailand | Sudjaritruk et al213 | No mortality reported, hospitalization study |
| 125. Spain | Palladino et al214 | Fosamprenavir study, experimental |
| 126. US | Nachman et al215 | Cohort too small (10 patients) |
PENTA indicates Paediatric European Network for Treatment of AIDS; PACTG, Pediatric AIDS Clinical Trial Group; IAS, International AIDS Society; PMTCT, prevention of mother-to-child transmission; IeDEA, International Epidemiologic Databases to Evaluate AIDS; IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Group; CROI, Conference on Retroviruses and Opportunistic Infections.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- HAART
- highly active antiretroviral therapy
- ART
- antiretroviral treatment
- RLC
- resource-limited country
- DC
- developed country
- WAZ
- weight-for-age z score
- VL
- viral load
- DPCY
- deaths per 100 child-years
- WHO
- World Health Organization
REFERENCES
- 1. Hammer S, Squires K, Hughes M, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337(11):725–733 [DOI] [PubMed] [Google Scholar]
- 2. Gortmaker SL, Hughes M, Cervia J, et al. ; Pediatric AIDS Clinical Trials Group Protocol 219 Team Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345(21):1522–1528 [DOI] [PubMed] [Google Scholar]
- 3. Newell M, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364(9441):1236–1243 [DOI] [PubMed] [Google Scholar]
- 4. Spira R, Lepage P, Msellati P, et al. Natural history of human immunodeficiency virus type 1 infection in children: a five-year prospective study in Rwanda. Mother-to-Child HIV-1 Transmission Study Group. Pediatrics. 1999;104(5). Available at: www.pediatrics.org/cgi/content/full/104/5/e56 [DOI] [PubMed] [Google Scholar]
- 5. Joint United Nations Programme on HIV/AIDS 2008 report on the global AIDS epidemic. Available at: www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp Accessed December 23, 2009
- 6. Boerma J, Stanecki K, Newell M, et al. Monitoring the scale-up of antiretroviral therapy programmes: methods to estimate coverage. Bull World Health Organ. 2006;84(2):145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Academy of Pediatrics, Committee on Pediatric AIDS, Section on International Child Health; Havens PL, Gibb DM. Increasing antiretroviral drug access for children with HIV infection. Pediatrics. 2007;119(4):838–845 [DOI] [PubMed] [Google Scholar]
- 8. Gill C, Hamer D, Simon J, Thea D, Sabin L. No room for complacency about adherence to antiretroviral therapy in sub-Saharan Africa. AIDS. 2005;19(12):1243–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Church JA, Hughes M, Chen J, et al. ; Pediatric AIDS Clinical Trials Group P1005 Study Team Long term tolerability and safety of enfuvirtide for human immunodeficiency virus 1-infected children. Pediatr Infect Dis J. 2004;23(8):713–718 [DOI] [PubMed] [Google Scholar]
- 10. O'Brien D, Sauvageot D, Zachariah R, Humblet P. In resource-limited settings good early outcomes can be achieved in children using adult fixed-dose combination antiretroviral therapy. AIDS. 2006;20(15):1955–1960 [DOI] [PubMed] [Google Scholar]
- 11. Krogstad P, Lee S, Johnson G, et al. ; Pediatric AIDS Clinical Trials Group 377 Study Team Nucleoside-analogue reverse-transcriptase inhibitors plus nevirapine, nelfinavir, or ritonavir for pretreated children infected with human immunodeficiency virus type 1. Clin Infect Dis. 2002;34(7):991–1001 [DOI] [PubMed] [Google Scholar]
- 12. George E, Noël F, Bois G, et al. Antiretroviral therapy for HIV-1-infected children in Haiti. J Infect Dis. 2007;195(10):1411–1418 [DOI] [PubMed] [Google Scholar]
- 13. Myung P, Pugatch D, Brady M, et al. Directly observed highly active antiretroviral therapy for HIV-infected children in Cambodia. Am J Public Health. 2007;97(6):974–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sutcliffe C, van Dijk J, Bolton C, Persaud D, Moss W. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8(8):477–489 [DOI] [PubMed] [Google Scholar]
- 15. WHO and UNAIDS release update that focuses on HIV prevention. IAVI Rep. 2005;9(5):20. [PubMed] [Google Scholar]
- 16. Antiretroviral therapy for children in the routine setting in Malawi. Trans R Soc Trop Med Hyg. 2007;101(5):511–516 [DOI] [PubMed] [Google Scholar]
- 17. Ciaranello A, Chang Y, Margulis A, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009;49(12):1915–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. United Nations Statistics Division Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. Available at: http://unstats.un.org/unsd/methods/m49/m49regin.htm Accessed April 30, 2010
- 19. Hien H, Nacro B, Zouré E, et al. Once-a-day paediatric HAART with DDI+3TC+EFV in West Africa: 24 month virological and immunological outcomes [abstract MOPEB031]. ANRS12103/12167 trial. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa Available at: www.ias2009.org/abstract.aspx?elementId=200722693 Accessed December 15, 2010 [Google Scholar]
- 20. Fassinou P, Elenga N, Rouet F, et al. Highly active antiretroviral therapies among HIV-1 infected children in Abidjan, Cote d'Ivoire. AIDS. 2004;18(14):1905–1913 [DOI] [PubMed] [Google Scholar]
- 21. Rouet F, Fassinou P, Inwoley A, et al. ; ANRS 1244/1278 Programme Enfants Yopougon Long-term survival and immuno-virological response of African HIV-1-infected children to highly active antiretroviral therapy regimens. AIDS. 2006;20(18):2315–2319 [DOI] [PubMed] [Google Scholar]
- 22. Nyandiko W, Ayaya S, Nabakwe E, et al. Outcomes of HIV-infected orphaned and non-orphaned children on antiretroviral therapy in western Kenya. J Acquir Immune Defic Syndr. 2006;43(4):418–425 [DOI] [PubMed] [Google Scholar]
- 23. Song R, Jelagat J, Dzombo D, et al. Efficacy of highly active antiretroviral therapy in HIV-1 infected children in Kenya. Pediatrics. 2007;120(4). Available at: www.pediatrics.org/cgi/content/full/120/4/e856 [DOI] [PubMed] [Google Scholar]
- 24. Van Winghem J, Telfer B, Reid T, et al. Implementation of a comprehensive program including psycho-social and treatment literacy activities to improve adherence to HIV care and treatment for a pediatric population in Kenya. BMC Pediatr. 2008;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wamalwa D, Farquhar C, Obimbo E, et al. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. J Acquir Immune Defic Syndr. 2007;45(3):311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reddi A, Leeper S, Grobler A, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr. 2007;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leyenaar J, Novosad P, Ferrer K, et al. Early clinical outcomes in children enrolled in human immunodeficiency virus infection care and treatment in Lesotho. Pediatr Infect Dis J. 2010;29(4):340–345 [DOI] [PubMed] [Google Scholar]
- 28. Cohen R, Lynch S, Bygrave H, et al. Nurse-driven, community-supported HIV/AIDS care and treatment: 2 year antiretroviral treatment outcomes from a primary care level programme in rural Lesotho [abstract MOAD102]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa Available at: www.ias2009.org/abstract.aspx?elementId=200722512 Accessed December 15, 2010 [Google Scholar]
- 29. Bong C, Yu J, Chiang H, et al. Risk factors for early mortality in children on adult fixed-dose combination antiretroviral treatment in a central hospital in Malawi. AIDS. 2007;21(13):1805–1810 [DOI] [PubMed] [Google Scholar]
- 30. Weigel R, Keiser O, Gumulira J, et al. Growth response to ART in HIV-infected children from Lilongwe, Malawi [paper 848]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; San Francisco, CA Available at: www.retroconference.org/2010/Abstracts/37992.htm Accessed December 15, 2010 [Google Scholar]
- 31. Marazzi M, Germano P, Liotta G, Buonomo E, Guidotti G, Palombi L. Pediatric highly active antiretroviral therapy in Mozambique: an integrated model of care [in Italian]. Minerva Pediatr. 2006;58(5):483–490 [PubMed] [Google Scholar]
- 32. Vaz P, Santos P, Augusto O, Macassa E, Blanche S, Andersson S. The impact of antiretroviral treatment on growth of HIV-1 infected children in Maputo, Mozambique [abstract WEPEB197]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa Available at: www.ias2009.org/abstract.aspx?elementId=200722321 Accessed December 15, 2010 [Google Scholar]
- 33. van Griensven J, De Naeyer L, Uwera J, Asiimwe A, Gazille C, Reid T. Success with antiretroviral treatment for children in Kigali, Rwanda: experience with health center/nurse-based care. BMC Pediatr. 2008;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diack MBaye A, Signaté Sy H, Diagne Guèye NR, et al. Epidemiological and clinical aspects of paediatric HIV infections in Albert-Royer Paediatric Hospital (Dakar, Senegal) [in French]. Arch Pediatr. 2005;12(4):404–409 [DOI] [PubMed] [Google Scholar]
- 35. Barth R, van der Meer J, Hoepelman A, et al. Effectiveness of highly active antiretroviral therapy administered by general practitioners in rural South Africa. Eur J Clin Microbiol Infect Dis. 2008;27(10):977–984 [DOI] [PubMed] [Google Scholar]
- 36. Eley B. Addressing the paediatric HIV epidemic: a perspective from the Western Cape region of South Africa. Trans R Soc Trop Med Hyg. 2006;100(1):19–23 [DOI] [PubMed] [Google Scholar]
- 37. Jaspan H, Berrisford A, Boulle A. Two-year outcomes of children on non-nucleoside reverse transcriptase inhibitor and protease inhibitor regimens in a South African pediatric antiretroviral program. Pediatr Infect Dis J. 2008;27(11):993–998 [DOI] [PubMed] [Google Scholar]
- 38. Jooste J, Van Zyl A, Baker A, Crawford W, Jassen A. Antiretroviral treatment in the Northern Cape. S Afr Med J. 2005;95(11):812. [PubMed] [Google Scholar]
- 39. Smit EJ, Rabie H, Prozesky H, Cotton M. Pediatric outcome 42 months after highly active antiretroviral therapy availability in the public sector: the Tygerberg Children's Hospital experience [abstract MOPEB079]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa Available at: www.ias2009.org/abstract.aspx?elementId=200721774 Accessed December 15, 2010 [Google Scholar]
- 40. Blè C, Floridia M, Muhale C, et al. Efficacy of highly active antiretroviral therapy in HIV-infected, institutionalized orphaned children in Tanzania. Acta Paediatr. 2007;96(7):1090–1094 [DOI] [PubMed] [Google Scholar]
- 41. Kamya MR, Mayanja-Kizza H, Kambugu A, et al. ; Academic Alliance for AIDS Care and Prevention in Africa Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46(2):187–193 [DOI] [PubMed] [Google Scholar]
- 42. Bolton-Moore C, Mubiana-Mbewe M, Cantrell R, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298(16):1888–1899 [DOI] [PubMed] [Google Scholar]
- 43. Gupta RK, Ford D, Kabamba D, et al. Two year virological outcomes in HIV-1 infected Zambian children using adult Triomune (fixed dose combination d4T, 3TC and NVP) [abstract MOPEB056]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa Available at: www.ias2009.org/abstract.aspx?elementId=200722143 Accessed December 15, 2010 [Google Scholar]
- 44. Walker AS, Mulenga V, Ford D, et al. ; CHAP Team The impact of daily cotrimoxazole prophylaxis and antiretroviral therapy on mortality and hospital admissions in HIV-infected Zambian children. Clin Infect Dis. 2007;44(10):1361–1367 [DOI] [PubMed] [Google Scholar]
- 45. Janssens B, Raleigh B, Soeung S, et al. Effectiveness of highly active antiretroviral therapy in HIV-positive children: evaluation at 12 months in a routine program in Cambodia. Pediatrics. 2007;120(5). Available at: www.pediatrics.org/cgi/content/full/120/5/e1134 [DOI] [PubMed] [Google Scholar]
- 46. Zhang F, Haberer J, Zhao Y, et al. Chinese pediatric highly active antiretroviral therapy observational cohort: a 1-year analysis of clinical, immunologic, and virologic outcomes. J Acquir Immune Defic Syndr. 2007;46(5):594–598 [DOI] [PubMed] [Google Scholar]
- 47. Rajasekaran S, Jeyaseelan L, Ravichandran N, Gomathi C, Thara F, Chandrasekar C. Efficacy of antiretroviral therapy program in children in India: prognostic factors and survival analysis. J Trop Pediatr. 2009;55(4):225–232 [DOI] [PubMed] [Google Scholar]
- 48. Kline M, Rugina S, Ilie M, et al. Long-term follow-up of 414 HIV-infected Romanian children and adolescents receiving lopinavir/ritonavir-containing highly active antiretroviral therapy. Pediatrics. 2007;119(5). Available at: www.pediatrics.org/cgi/content/full/119/5/e1116 [DOI] [PubMed] [Google Scholar]
- 49. Aurpibul L, Puthanakit T, Taecharoenkul S, Sirisanthana V. Impact of non-nucleoside reverse transcriptase inhibitor (NNRTI)-based antiretroviral therapy (ART) on weight and height of antiretroviral-naive HIV-infected children [abstract CDB103]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa Available at: www.ias2009.org/abstract.aspx?elementId=200721497 Accessed December 15, 2010 [Google Scholar]
- 50. Lapphra K, Vanprapar N, Chearskul S, et al. Efficacy and tolerability of nevirapine- versus efavirenz-containing regimens in HIV-infected Thai children. Int J Infect Dis. 2008;12(6):e33–e38 [DOI] [PubMed] [Google Scholar]
- 51. Puthanakit T, Aurpibul L, Oberdorfer P, et al. Hospitalization and mortality among HIV-infected children after receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;44(4):599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Romanelli R, Pinto J, Melo L, Vasconcelos M, Pereira RM. Effectiveness of dual and triple antiretroviral therapy in the treatment of HIV-infected children [in Portuguese]. J Pediatr (Rio J). 2006;82(4):260–265 [DOI] [PubMed] [Google Scholar]
- 53. Martins Oliveira Diniz L, Marie Martins Maia M, Silveira Camargos L, Custódio Amaral L, Goulart E, Andrade Pinto J. Long-term effects of HAART on weight and height among Brazilian children infected with HIV [abstract CDB105]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa Available at: www.ias2009.org/abstract.aspx?elementId=200722217 Accessed December 15, 2010 [Google Scholar]
- 54. Martins Oliveira Diniz L, Marie Martins Maia M, Silveira Camargos L, Custódio Amaral L, Goulart E, Andrade Pinto J. Long-term effects of HAART on CD4+ cell percentage among Brazilian children infected with HIV [abstract MOPEB087]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa Available at: www.ias2009.org/abstract.aspx?elementId=200722230 Accessed December 15, 2010 [Google Scholar]
- 55. Severe P, Leger P, Charles M, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353(22):2325–2334 [DOI] [PubMed] [Google Scholar]
- 56. Ghaffari G, Passalacqua D, Caicedo J, Goodenow M, Sleasman J. Two-year clinical and immune outcomes in human immunodeficiency virus-infected children who reconstitute CD4 T cells without control of viral replication after combination antiretroviral therapy. Pediatrics. 2004;114(5). Available at: www.pediatrics.org/cgi/content/full/114/5/e604 [DOI] [PubMed] [Google Scholar]
- 57. King J, Nachman S, Yogev R, et al. Efficacy, tolerability and pharmacokinetics of two nelfinavir-based regimens in human immunodeficiency virus-infected children and adolescents: pediatric AIDS clinical trials group protocol 403. Pediatr Infect Dis J. 2005;24(10):880–885 [DOI] [PubMed] [Google Scholar]
- 58. Krogstad P, Wiznia A, Luzuriaga K, et al. Treatment of human immunodeficiency virus 1-infected infants and children with the protease inhibitor nelfinavir mesylate. Clin Infect Dis. 1999;28(5):1109–1118 [DOI] [PubMed] [Google Scholar]
- 59. McKinney RJ, Rodman J, Hu C, et al. ; Pediatric AIDS Clinical Trials Group Protocol P1021 Study Team Long-term safety and efficacy of a once-daily regimen of emtricitabine, didanosine, and efavirenz in HIV-infected, therapy-naive children and adolescents: Pediatric AIDS Clinical Trials Group Protocol P1021. Pediatrics. 2007;120(2). Available at: www.pediatrics.org/cgi/content/full/120/2/e416 [DOI] [PubMed] [Google Scholar]
- 60. Melvin A, Lewis P, Mohan K, Naugler W, Frenkel L. Efficacy and toxicity of antiretroviral therapy using 4 or more agents: application of a strategy for antiretroviral management in human immunodeficiency virus-infected children. Arch Pediatr Adolesc Med. 2002;156(6):568–573 [DOI] [PubMed] [Google Scholar]
- 61. Patel K, Hernan M, Williams P, et al. ; Pediatric AIDS Clinical Trials Group 219/219C Study Team Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis. 2008;46(4):507–515 [DOI] [PubMed] [Google Scholar]
- 62. Rosenblatt H, Stanley K, Song L, et al. Immunological response to highly active antiretroviral therapy in children with clinically stable HIV-1 infection. J Infect Dis. 2005;192(3):445–455 [DOI] [PubMed] [Google Scholar]
- 63. Soh C, Oleske J, Brady M, et al. ; Pediatric AIDS Clinical Trials Group Long-term effects of protease-inhibitor-based combination therapy on CD4 T-cell recovery in HIV-1-infected children and adolescents. Lancet. 2003;362(9401):2045–2051 [DOI] [PubMed] [Google Scholar]
- 64. Spector S, Hsia K, Yong F, et al. Patterns of plasma human immunodeficiency virus type 1 RNA response to highly active antiretroviral therapy in infected children. J Infect Dis. 2000;182(6):1769–1773 [DOI] [PubMed] [Google Scholar]
- 65. Starr S, Fletcher C, Spector S, et al. Combination therapy with efavirenz, nelfinavir, and nucleoside reverse-transcriptase inhibitors in children infected with human immunodeficiency virus type 1. Pediatric AIDS Clinical Trials Group 382 Team. N Engl J Med. 1999;341(25):1874–1881 [DOI] [PubMed] [Google Scholar]
- 66. Watson D, Farley J. Efficacy of and adherence to highly active antiretroviral therapy in children infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 1999;18(8):682–689 [DOI] [PubMed] [Google Scholar]
- 67. Wiznia A, Stanley K, Krogstad P, et al. Combination nucleoside analog reverse transcriptase inhibitor(s) plus nevirapine, nelfinavir, or ritonavir in stable antiretroviral therapy-experienced HIV-infected children: week 24 results of a randomized controlled trial—PACTG 377. Pediatric AIDS Clinical Trials Group 377 Study Team. AIDS Res Hum Retroviruses. 2000;16(12):1113–1121 [DOI] [PubMed] [Google Scholar]
- 68. Yogev R, Lee S, Wiznia A, et al. ; Pediatrics AIDS Clinical Trials Group 338 Study Team Stavudine, nevirapine and ritonavir in stable antiretroviral therapy-experienced children with human immunodeficiency virus infection. Pediatr Infect Dis J. 2002;21(2):119–125 [DOI] [PubMed] [Google Scholar]
- 69. Bracher L, Valerius N, Rosenfeldt V, et al. Long-term effectiveness of highly active antiretroviral therapy (HAART) in perinatally HIV-infected children in Denmark. Scand J Infect Dis. 2007;39(9):799–804 [DOI] [PubMed] [Google Scholar]
- 70. Teglas J, Quartier P, Treluyer J, Burgard M, Gregoire V, Blanche S. Tolerance of efavirenz in children. AIDS. 2001;15(2):241–243 [DOI] [PubMed] [Google Scholar]
- 71. Thuret I, Michel G, Chambost H, et al. Combination antiretroviral therapy including ritonavir in children infected with human immunodeficiency. AIDS. 1999;13(1):81–87 [DOI] [PubMed] [Google Scholar]
- 72. Wintergerst U, Hoffmann F, Jansson A, et al. Antiviral efficacy, tolerability and pharmacokinetics of efavirenz in an unselected cohort of HIV-infected children. J Antimicrob Chemother. 2008;61(6):1336–1339 [DOI] [PubMed] [Google Scholar]
- 73. Fraaij P, Verweel G, van Rossum A, et al. Sustained viral suppression and immune recovery in HIV type 1-infected children after 4 years of highly active antiretroviral therapy. Clin Infect Dis. 2005;40(4):604–608 [DOI] [PubMed] [Google Scholar]
- 74. Scherpbier H, Bekker V, Pajkrt D, Jurriaans S, Lange J, Kuijpers T. Once-daily highly active antiretroviral therapy for HIV-infected children: safety and efficacy of an efavirenz-containing regimen. Pediatrics. 2007;119(3). Available at: www.pediatrics.org/cgi/content/full/119/3/e705 [DOI] [PubMed] [Google Scholar]
- 75. van Rossum A, Geelen S, Hartwig N, et al. Results of 2 years of treatment with protease-inhibitor–containing antiretroviral therapy in Dutch children infected with human immunodeficiency virus type 1. Clin Infect Dis. 2002;34(7):1008–1016 [DOI] [PubMed] [Google Scholar]
- 76. van Rossum A, Niesters H, Geelen S, et al. Clinical and virologic response to combination treatment with indinavir, zidovudine, and lamivudine in children with human immunodeficiency virus-1 infection: a multicenter study in the Netherlands. On behalf of the Dutch Study Group for Children With HIV-1 infections. J Pediatr. 2000;136(6):780–788 [DOI] [PubMed] [Google Scholar]
- 77. Verweel G, van Rossum A, Hartwig N, Wolfs T, Scherpbier H, de Groot R. Treatment with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children is associated with a sustained effect on growth. Pediatrics. 2002;109(2). Available at: www.pediatrics.org/cgi/content/full/109/2/e25 [DOI] [PubMed] [Google Scholar]
- 78. Nadal D, Steiner F, Cheseaux J, et al. Long-term responses to treatment including ritonavir or nelfinavir in HIV-1-infected children [published correction appears in Infection. 2000;28(6):402]. Pediatric AIDS Group of Switzerland. Infection. 2000;28(5):287–296 [DOI] [PubMed] [Google Scholar]
- 79. Rudin C, Burri M, Shen Y, Rode R, Nadal D. Long-term safety and effectiveness of ritonavir, nelfinavir, and lopinavir/ritonavir in antiretroviral-experienced HIV-infected children. Pediatr Infect Dis J. 2008;27(5):431–437 [DOI] [PubMed] [Google Scholar]
- 80. Judd A, Doerholt K, Tookey P, et al. ; Collaborative HIV Paediatric Study (CHIPS); National Study of HIV in Pregnancy and Childhood (NSHPC) Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996–2006: planning for teenage and adult care. Clin Infect Dis. 2007;45(7):918–924 [DOI] [PubMed] [Google Scholar]
- 81. Walker A, Doerholt K, Sharland M, Gibb D. Response to highly active antiretroviral therapy varies with age: the UK and Ireland Collaborative HIV Paediatric Study. AIDS. 2004;18(14):1915–1924 [DOI] [PubMed] [Google Scholar]
- 82. Paediatric European Network for Treatment of AIDS Comparison of dual nucleoside-analogue reverse-transcriptase inhibitor regimens with and without nelfinavir in children with HIV-1 who have not previously been treated: the PENTA 5 randomised trial. Lancet. 2002;359(9308):733–740 [DOI] [PubMed] [Google Scholar]
- 83. Caudill S, Goldman T, Marconi K. Evaluation of pediatric HIV care provided in Ryan White CARE Act Title IV Women, Infants, Children, and Youth Clinics. AIDS Patient Care STDS. 2003;17(2):65–73 [DOI] [PubMed] [Google Scholar]
- 84. Lindsey J, Hughes M, McKinney R, et al. Treatment-mediated changes in human immunodeficiency virus (HIV) type 1 RNA and CD4 cell counts as predictors of weight growth failure, cognitive decline, and survival in HIV-infected children. J Infect Dis. 2000;182(5):1385–1393 [DOI] [PubMed] [Google Scholar]
- 85. Resino S, Resino R, Maria Bellón J, et al. ; Spanish Group of Pediatric HIV Infection Clinical outcomes improve with highly active antiretroviral therapy in vertically HIV type-1-infected children. Clin Infect Dis. 2006;43(2):243–252 [DOI] [PubMed] [Google Scholar]
- 86. Pelton S, Johnson D, Chadwick E, Baldwin Z, Yogev R. A one year experience: T cell responses and viral replication in children with advanced human immunodeficiency virus type 1 disease treated with combination therapy including ritonavir. Pediatr Infect Dis J. 1999;18(7):650–652 [DOI] [PubMed] [Google Scholar]
- 87. de Martino M, Tovo P, Balducci M, et al. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. Italian Register for HIV Infection in Children and the Italian National AIDS Registry. JAMA. 2000;284(2):190–197 [DOI] [PubMed] [Google Scholar]
- 88. Kline M, Matusa R, Copaciu L, Calles N, Kline N, Schwarzwald H. Comprehensive pediatric human immunodeficiency virus care and treatment in Constanta, Romania: implementation of a program of highly active antiretroviral therapy in a resource-poor setting. Pediatr Infect Dis J. 2004;23(8):695–700 [DOI] [PubMed] [Google Scholar]
- 89. Doerholt K, Duong T, Tookey P, et al. ; Collaborative HIV Paediatric Study Outcomes for human immunodeficiency virus-1-infected infants in the United kingdom and Republic of Ireland in the era of effective antiretroviral therapy. Pediatr Infect Dis J. 2006;25(5):420–426 [DOI] [PubMed] [Google Scholar]
- 90. Funk M, Linde R, Wintergerst U, et al. Preliminary experiences with triple therapy including nelfinavir and two reverse transcriptase inhibitors in previously untreated HIV-infected children. AIDS. 1999;13(13):1653–1658 [DOI] [PubMed] [Google Scholar]
- 91. Gibb D, Goodall R, Giacomet V, McGee L, Compagnucci A, Lyall H. Adherence to prescribed antiretroviral therapy in human immunodeficiency virus-infected children in the PENTA 5 trial. Pediatr Infect Dis J. 2003;22(1):56–62 [DOI] [PubMed] [Google Scholar]
- 92. United Nations Children's Fund Information by country and programme. Available at: www.unicef.org/infobycountry/index.html Accessed April 17, 2010).
- 93. Adetunji J. Trends in under-5 mortality rates and the HIV/AIDS epidemic. Bull World Health Organ. 2000;78(10):1200–1206 [PMC free article] [PubMed] [Google Scholar]
- 94. Newell M, Brahmbhatt H, Ghys P. Child mortality and HIV infection in Africa: a review. AIDS. 2004;18(suppl 2):S27–S34 [DOI] [PubMed] [Google Scholar]
- 95. Violari A, Cotton M, Gibb D, et al. ; CHER Study Team Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Eley B. Antiretroviral therapy during infancy: essential intervention for resource-limited settings. Expert Rev Anti Infect Ther. 2008;6(5):585–589 [DOI] [PubMed] [Google Scholar]
- 97. World Health Organization Antiretroviral Therapy for HIV in Infants and Children: Towards Universal Access—Recommendations for a Public Health Approach. 2010 revision Geneva, Switzerland: World Health Organization; 2010. Available at: www.who.int/hiv/pub/paediatric/infants/en/index.html Accessed July 26, 2010 [PubMed] [Google Scholar]
- 98. Adjé-Touré C, Hanson D, Talla-Nzussouo N, Borget M, Kouadio L, Tossou O, et al. Virologic and immunologic response to antiretroviral therapy and predictors of HIV type 1 drug resistance in children receiving treatment in Abidjan, Côte d'Ivoire. AIDS Res Hum Retroviruses. 2008;24(7):911–917 [DOI] [PubMed] [Google Scholar]
- 99. Biadgilign S, Deribew A, Amberbir A, Deribe K. Adherence to highly active antiretroviral therapy and its correlates among HIV infected pediatric patients in Ethiopia. BMC Pediatr. 2008;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Onankpa B, Airede L, Paul I, Dorcas I. Pattern of pediatric HIV/AIDS: a five-year experience in a tertiary hospital. J Natl Med Assoc. 2008;100(7):821–825 [DOI] [PubMed] [Google Scholar]
- 101. Cowburn C, Hatherill M, Eley B, Nuttall J, Hussey G, Reynolds L, et al. Short-term mortality and implementation of antiretroviral treatment for critically ill HIV-infected children in a developing country. Arch Dis Child. 2007;92(3):234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Eley B, Nuttall J, Davies M, Smith L, Cowburn C, Buys H, et al. Initial experience of a public sector antiretroviral treatment programme for HIV-infected children and their infected parents. S Afr Med J. 2004;94(8):643–646 [PubMed] [Google Scholar]
- 103. Prendergast A, Mphatswe W, Tudor-Williams G, Rakgotho M, Pillay V, Thobakgale C, et al. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS. 2008;22(11):1333–1343 [DOI] [PubMed] [Google Scholar]
- 104. van Kooten Niekerk NK, Knies MM, Howard J, Rabie H, Zeier M, van Rensburg A, et al. The first 5 years of the family clinic for HIV at Tygerberg Hospital: family demographics, survival of children and early impact of antiretroviral therapy. J Trop Pediatr. 2006;52(1):3–11 [DOI] [PubMed] [Google Scholar]
- 105. Atakouma D, Tsolenyanu E, Gbadoe A, Gbetoglo V, Lawson-Evi K, Agbere A, et al. [Primary results of antiretroviral treatment among HIV/AIDS infected children in Lomé (Togo)]. Arch Pediatr. 2007;14(10):1178–1182 [DOI] [PubMed] [Google Scholar]
- 106. Polisset J, Ametonou F, Arrive E, Aho A, Perez F. Correlates of Adherence to Antiretroviral Therapy in HIV-Infected Children in Lomé, Togo, West Africa. AIDS Behav. 2009;13(1):23–32 [DOI] [PubMed] [Google Scholar]
- 107. Fallo A, DobrzanskiNisiewicz W, Sordelli N, Cattaneo M, Scott G, López E. Clinical and epidemiologic aspects of human immunodeficiency virus-1-infected children in Buenos Aires, Argentina. Int J Infect Dis. 2002;6(1):9–16 [DOI] [PubMed] [Google Scholar]
- 108. Matida L, Marcopito L, Succi R, Marques H, Della Negra M, Grangeiro A, et al. Improving survival among Brazilian children with perinatally-acquired AIDS. Braz J Infect Dis. 2004;8(6):41–423 [DOI] [PubMed] [Google Scholar]
- 109. Candiani T, Pinto J, Cardoso C, Carvalho I, Dias A, Carneiro M, et al. Impact of highly active antiretroviral therapy (HAART) on the incidence of opportunistic infections, hospitalizations and mortality among children and adolescents living with HIV/AIDS in Belo Horizonte, Minas Gerais State, Brazil. Cad Saude Publica. 2007;23 Suppl 3:S414–423 [DOI] [PubMed] [Google Scholar]
- 110. Samayoa B, Anderson M, Grazioso C, Rivera B, Harrison M, O'Brien W, et al. Experience of a pediatric HIV clinic in Guatemala City. Rev Panam Salud Publica. 2009;25(1):51–55 [DOI] [PubMed] [Google Scholar]
- 111. Evans-Gilbert T, Pierre R, Steel-Duncan J, Rodriguez B, Whorms S, Hambleton I, et al. Antiretroviral drug therapy in HIV-infected Jamaican children. West Indian Med J. 2004;53(5):322–326 [PubMed] [Google Scholar]
- 112. Madec Y, Laureillard D, Pinoges L, Fernandez M, Prak N, Ngeth C, et al. Response to highly active antiretroviral therapy among severely immuno-compromised HIV-infected patients in Cambodia. AIDS. 2007;21(3):351–359 [DOI] [PubMed] [Google Scholar]
- 113. Kumarasamy N, Venkatesh K, Devaleenol B, Poongulali S, Mothi S, Solomon S. Safety, tolerability and effectiveness of generic HAART in HIV-infected children in South India. J Trop Pediatr. 2009;55(3):155–159 [DOI] [PubMed] [Google Scholar]
- 114. Lodha R, Upadhyay A, Kabra SK. Antiretroviral therapy in HIV-1 infected children. Indian Pediatr. 2005;42(8):789–796 [PubMed] [Google Scholar]
- 115. Natu S, Daga S. Antiretroviral therapy in children: Indian experience. Indian Pediatr. 2007;44(5):339–343 [PubMed] [Google Scholar]
- 116. Pensi T. Fixed Dose Combination of Lamivudine, Stavudine and Nevirapine in the Treatment of Pediatric HIV infection: A Preliminary Report. Indian Pediatr. 2007;44(7):519–521 [PubMed] [Google Scholar]
- 117. Ferris M, Burau K, Schweitzer A, Mihale S, Murray N, Preda A, et al. The influence of disclosure of HIV diagnosis on time to disease progression in a cohort of Romanian children and teens. AIDS Care. 2007;19(9):1088–1094 [DOI] [PubMed] [Google Scholar]
- 118. Chearskul P, Chokephaibulkit K, Chearskul S, Phongsamart W, Plipat N, Lapphra K, et al. Effect of antiretroviral therapy in human immunodeficiency virus-infected children. J Med Assoc Thai. 2005;88 Suppl 8:S221–S231 [PubMed] [Google Scholar]
- 119. Koekkoek S, Eggermont L, De Sonneville L, Jupimai T, Wicharuk S, Apateerapong W, et al. Effects of highly active antiretroviral therapy (HAART) on psychomotor performance in children with HIV disease. J Neurol. 2006;253(12):1615–1624 [DOI] [PubMed] [Google Scholar]
- 120. Plipat N, Cressey T, Vanprapar N, Chokephaibulkit K. Efficacy and plasma concentrations of indinavir when boosted with ritonavir in human immunodeficiency virus-infected Thai children. Pediatr Infect Dis J. 2007;26(1):86–88 [DOI] [PubMed] [Google Scholar]
- 121. Arrivé E, Kyabayinze DJ, Marquis B, Tumwesigye N, Kieffer MP, Azondekon A, et al. Cohort profile: the paediatric antiretroviral treatment programmes in lower-income countries (KIDS-ART-LINC) collaboration. Int J Epidemiol. 2008;37(3):474–480 [DOI] [PubMed] [Google Scholar]
- 122. The KIDS-ART-LINC Collaboration Low Risk of Death, but Substantial Program Attrition, in Pediatric HIV Treatment Cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008;49(5):523–531 [DOI] [PubMed] [Google Scholar]
- 123. Saez-Llorens X, Violari A, Ndiweni D, Yogev R, Cashat M, Wiznia A, et al. Long-term safety and efficacy results of once-daily emtricitabine-based highly active antiretroviral therapy regimens in human immunodeficiency virus-infected pediatric subjects. Pediatrics. 2008;121(4):e827–e835 [DOI] [PubMed] [Google Scholar]
- 124. Weidle P, Abrams E, Gvetadze R, Rivadeneira E, Kline M. A simplified weight-based method for pediatric drug dosing for zidovudine and didanosine in resource-limited settings. Pediatr Infect Dis J. 2006;25(1):59–64 [DOI] [PubMed] [Google Scholar]
- 125. Hazra R, Stoszek SK, Freimanis Hance L, Pinto J, Marques H, Peixoto M, et al. Cohort Profile: NICHD International Site Development Initiative (NISDI): a prospective, observational study of HIV-exposed and HIV-infected children at clinical sites in Latin American and Caribbean countries. Int J Epidemiol. 2009;38(5):1207–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hainaut M, Peltier C, Gérard M, Marissens D, Zissis G, Levy J. Effectiveness of antiretroviral therapy initiated before the age of 2 months in infants vertically infected with human immunodeficiency virus type 1. Eur J Pediatr. 2000;159(10):778–782 [DOI] [PubMed] [Google Scholar]
- 127. Aboulker J, Babiker A, Chaix M, Compagnucci A, Darbyshire J, Debré M, et al. Highly active antiretroviral therapy started in infants under 3 months of age: 72-week follow-up for CD4 cell count, viral load and drug resistance outcome. AIDS. 2004;18(2):237–245 [DOI] [PubMed] [Google Scholar]
- 128. Faye A, Bertone C, Teglas J, Chaix M, Douard D, Firtion G, et al. Early multitherapy including a protease inhibitor for human immunodeficiency virus type 1-infected infants. Pediatr Infect Dis J. 2002;21(6):518–525 [DOI] [PubMed] [Google Scholar]
- 129. Wintergerst U, Hoffmann F, Sölder B, Notheis G, Petropoulou T, Eberle J, et al. Comparison of two antiretroviral triple combinations including the protease inhibitor indinavir in children infected with human immunodeficiency virus. Pediatr Infect Dis J. 1998;17(6):495–499 [DOI] [PubMed] [Google Scholar]
- 130. Canani R, Spagnuolo M, Cirillo P, Guarino A. Decreased needs for hospital care and antibiotics in children with advanced HIV-1 disease after protease inhibitor-containing combination therapy. AIDS. 1999;13(8):1005–1006 [DOI] [PubMed] [Google Scholar]
- 131. Chiappini E, Galli L, Tovo P, Gabiano C, Lisi C, Gattinara G, et al. Changing patterns of clinical events in perinatally HIV-1-infected children during the era of HAART. AIDS. 2007;21(12):1607–1615 [DOI] [PubMed] [Google Scholar]
- 132. Viganò A, Schneider L, Giacomet V, Bianchi R, Cicero M, Soster F, et al. Efficacy and tolerability of multiple drug therapy in HIV-infected children. J Infect. 2005;50(5):404–411 [DOI] [PubMed] [Google Scholar]
- 133. Cohen Stuart JW, Slieker WA, Rijkers GT, Noest A, Boucher CA, Suur MH, et al. Early recovery of CD4+ T lymphocytes in children on highly active antiretroviral therapy. Dutch study group for children with HIV infections. AIDS. 1998;12(16):2155–2159 [DOI] [PubMed] [Google Scholar]
- 134. Larrú B, Resino S, Bellón J, de José M, Fortuny C, Navarro M, et al. Long-term response to highly active antiretroviral therapy with lopinavir/ritonavir in pre-treated vertically HIV-infected children. J Antimicrob Chemother. 2007 [DOI] [PubMed] [Google Scholar]
- 135. Resino S, Bellón JM, Ramos JT, Resino R, Gurbindo MD, Mellado MJ, et al. Impact of highly active antiretroviral therapy on CD4+ T cells and viral load of children with AIDS: a population-based study. AIDS Res Hum Retroviruses. 2004;20(9):927–931 [DOI] [PubMed] [Google Scholar]
- 136. Sánchez J, Ramos Amador J, Fernández de Miguel S, González Tomée M, Rojo Conejo P, Ferrnado Vivas P, et al. Impact of highly active antiretroviral therapy on the morbidity and mortality in Spanish human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2003;22(10):863–867 [DOI] [PubMed] [Google Scholar]
- 137. Guillén Martín S, Ramos Amador J, Resino García R, Bellón Cano J. [Epidemiological trends in new diagnoses of HIV-1 infection in children]. An Pediatr (Barc). 2005;63(3):199–202 [DOI] [PubMed] [Google Scholar]
- 138. Steiner F, Kind C, Aebi C, Wyler-Lazarevitch C, Cheseaux J, Rudin C, et al. Growth in human immunodeficiency virus type 1-infected children treated with protease inhibitors. Eur J Pediatr. 2001;160(10):611–616 [DOI] [PubMed] [Google Scholar]
- 139. Newell M, Patel D, Goetghebuer T, Thorne C. CD4 cell response to antiretroviral therapy in children with vertically acquired HIV infection: is it associated with age at initiation? J Infect Dis. 2006;193(7):954–962 [DOI] [PubMed] [Google Scholar]
- 140. Goetghebuer T, Haelterman E, Le Chenadec J, Dollfus C, Gibb D, Judd A, et al. Effect of early antiretroviral therapy on the risk of AIDS/death in HIV-infected infants. AIDS. 2009;23(5):597–604 [DOI] [PubMed] [Google Scholar]
- 141. Abrams E, Weedon J, Bertolli J, Bornschlegel K, Cervia J, Mendez H, et al. Aging cohort of perinatally human immunodeficiency virus-infected children in New York City. New York City Pediatric Surveillance of Disease Consortium. Pediatr Infect Dis J. 2001;20(5):511–517 [DOI] [PubMed] [Google Scholar]
- 142. Benjamin DJ, Miller W, Benjamin D, Ryder R, Weber D, Walter E, et al. A comparison of height and weight velocity as a part of the composite endpoint in pediatric HIV. AIDS. 2003;17(16):2331–2336 [DOI] [PubMed] [Google Scholar]
- 143. Berrien V, Salazar J, Reynolds E, McKay K. Adherence to antiretroviral therapy in HIV-infected pediatric patients improves with home-based intensive nursing intervention. AIDS Patient Care STDS. 2004;18(6):355–363 [DOI] [PubMed] [Google Scholar]
- 144. Blazevic V, Jankelevich S, Steinberg S, Jacobsen F, Yarchoan R, Shearer G. Highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children: analysis of cellular immune responses. Clin Diagn Lab Immunol. 2001;8(5):943–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Borkowsky W, Stanley K, Douglas S, Lee S, Wiznia A, Pelton S, et al. Immunologic response to combination nucleoside analogue plus protease inhibitor therapy in stable antiretroviral therapy-experienced human immunodeficiency virus-infected children. J Infect Dis. 2000;182(1):96–103 [DOI] [PubMed] [Google Scholar]
- 146. Brady M, Oleske J, Williams P, Elgie C, Mofenson L, Dankner W, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53(1):86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Brogly S, Williams P, Seage Gr, Oleske J, Van Dyke R, McIntosh K. Antiretroviral treatment in pediatric HIV infection in the United States: from clinical trials to clinical practice. JAMA. 2005;293(18):2213–2220 [DOI] [PubMed] [Google Scholar]
- 148. Brundage R, Yong F, Fenton T, Spector S, Starr S, Fletcher C. Intrapatient variability of efavirenz concentrations as a predictor of virologic response to antiretroviral therapy. Antimicrob Agents Chemother. 2004;48(3):979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Buchacz K, Cervia J, Lindsey J, Hughes M, Seage Gr, Dankner W, et al. Impact of protease inhibitor-containing combination antiretroviral therapies on height and weight growth in HIV-infected children. Pediatrics. 2001;108(4):E72. [DOI] [PubMed] [Google Scholar]
- 150. Chadwick E, Rodman J, Britto P, Powell C, Palumbo P, Luzuriaga K, et al. Ritonavir-based highly active antiretroviral therapy in human immunodeficiency virus type 1-infected infants younger than 24 months of age. Pediatr Infect Dis J. 2005;24(9):793–800 [DOI] [PubMed] [Google Scholar]
- 151. Chadwick E, Capparelli E, Yogev R, Pinto J, Robbins B, Rodman J, et al. Pharmacokinetics, safety and efficacy of lopinavir/ritonavir in infants less than 6 months of age: 24 week results. AIDS. 2008;22(2):249–255 [DOI] [PubMed] [Google Scholar]
- 152. Chougnet C, Jankelevich S, Fowke K, Liewehr D, Steinberg S, Mueller B, et al. Long-term protease inhibitor-containing therapy results in limited improvement in T cell function but not restoration of interleukin-12 production in pediatric patients with AIDS. J Infect Dis. 2001;184(2):201–205 [DOI] [PubMed] [Google Scholar]
- 153. Church JA, Cunningham C, Hughes M, Palumbo P, Mofenson LM, Delora P, et al. Safety and antiretroviral activity of chronic subcutaneous administration of T-20 in human immunodeficiency virus 1-infected children. Pediatr Infect Dis J. 2002;21(7):653–659 [DOI] [PubMed] [Google Scholar]
- 154. Essajee S, Kim M, Gonzalez C, Rigaud M, Kaul A, Chandwani S, et al. Immunologic and virologic responses to HAART in severely immunocompromised HIV-1-infected children. AIDS. 1999;13(18):2523–2532 [DOI] [PubMed] [Google Scholar]
- 155. Flynn P, Rudy B, Douglas S, Lathey J, Spector S, Martinez J, et al. Virologic and immunologic outcomes after 24 weeks in HIV type 1-infected adolescents receiving highly active antiretroviral therapy. J Infect Dis. 2004;190(2):271–279 [DOI] [PubMed] [Google Scholar]
- 156. Flynn P, Rudy B, Lindsey J, Douglas S, Lathey J, Spector S, et al. Long-term observation of adolescents initiating HAART therapy: three-year follow-up. AIDS Res Hum Retroviruses. 2007;23(10):1208–1214 [DOI] [PubMed] [Google Scholar]
- 157. Glikman D, Walsh L, Valkenburg J, Mangat P, Marcinak J. Hospital-based directly observed therapy for HIV-infected children and adolescents to assess adherence to antiretroviral medications. Pediatrics. 2007;119(5):e1142–e1148 [DOI] [PubMed] [Google Scholar]
- 158. Gona P, Van Dyke R, Williams P, Dankner W, Chernoff M, Nachman S, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296(3):292–300 [DOI] [PubMed] [Google Scholar]
- 159. Jankelevich S, Mueller B, Mackall C, Smith S, Zwerski S, Wood L, et al. Long-term virologic and immunologic responses in human immunodeficiency virus type 1-infected children treated with indinavir, zidovudine, and lamivudine. J Infect Dis. 2001;183(7):1116–1120 [DOI] [PubMed] [Google Scholar]
- 160. Johnston A, Valentine M, Ottinger J, Baydo R, Gryszowka V, Vavro C, et al. Immune reconstitution in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy: a cohort study. Pediatr Infect Dis J. 2001;20(10):941–946 [DOI] [PubMed] [Google Scholar]
- 161. King J, Acosta E, Yogev R, Wiznia A, Kraimer J, Graham B, et al. Steady-state pharmacokinetics of lopinavir/ritonavir in combination with efavirenz in human immunodeficiency virus-infected pediatric patients. Pediatr Infect Dis J. 2009;28(2):159–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kline M, Fletcher C, Harris A, Evans K, Brundage R, Remmel R, et al. A pilot study of combination therapy with indinavir, stavudine (d4T), and didanosine (ddI) in children infected with the human immunodeficiency virus. J Pediatr. 1998;132(3 Pt 1):543–546 [DOI] [PubMed] [Google Scholar]
- 163. Lee G, Gortmaker S, McIntosh K, Hughes M, Oleske J. Quality of life for children and adolescents: impact of HIV infection and antiretroviral treatment. Pediatrics. 2006;117(2):273–283 [DOI] [PubMed] [Google Scholar]
- 164. Luzuriaga K, Bryson Y, Krogstad P, Robinson J, Stechenberg B, Lamson M, et al. Combination treatment with zidovudine, didanosine, and nevirapine in infants with human immunodeficiency virus type 1 infection. N Engl J Med. 1997;336(19):1343–1349 [DOI] [PubMed] [Google Scholar]
- 165. Luzuriaga K, McManus M, Mofenson L, Britto P, Graham B, Sullivan J. A trial of three antiretroviral regimens in HIV-1-infected children. N Engl J Med. 2004;350(24):2471–2480 [DOI] [PubMed] [Google Scholar]
- 166. McConnell MS, Byers RH, Frederick T, Peters VB, Dominguez KL, Sukalac T, et al. Trends in antiretroviral therapy use and survival rates for a large cohort of HIV-infected children and adolescents in the United States, 1989–2001. J Acquir Immune Defic Syndr. 2005;38(4):488–494 [DOI] [PubMed] [Google Scholar]
- 167. Melvin A, Mohan K, Arcuino L, Edelstein R, Frenkel L. Clinical, virologic and immunologic responses of children with advanced human immunodeficiency virus type 1 disease treated with protease inhibitors. Pediatr Infect Dis J. 1997;16(10):968–974 [DOI] [PubMed] [Google Scholar]
- 168. Mueller B, Nelson RJ, Sleasman J, Zuckerman J, Heath-Chiozzi M, Steinberg S, et al. A phase I/II study of the protease inhibitor ritonavir in children with human immunodeficiency virus infection. Pediatrics. 1998;101(3 Pt 1):335–343 [DOI] [PubMed] [Google Scholar]
- 169. Mueller B, Sleasman J, Nelson RJ, Smith S, Deutsch P, Ju W, et al. A phase I/II study of the protease inhibitor indinavir in children with HIV infection. Pediatrics. 1998;102(1 Pt 1):101–109 [DOI] [PubMed] [Google Scholar]
- 170. Nachman S, Stanley K, Yogev R, Pelton S, Wiznia A, Lee S, et al. Nucleoside analogs plus ritonavir in stable antiretroviral therapy-experienced HIV-infected children: a randomized controlled trial. Pediatric AIDS Clinical Trials Group 338 Study Team. JAMA. 2000;283(4):492–498 [DOI] [PubMed] [Google Scholar]
- 171. Palumbo P, Raskino C, Fiscus S, Pahwa S, Schutzbank T, Spector S, et al. Virologic and immunologic response to nucleoside reverse-transcriptase inhibitor therapy among human immunodeficiency virus-infected infants and children. J Infect Dis. 1999;179(3):576–583 [DOI] [PubMed] [Google Scholar]
- 172. Patel K, Hernán M, Williams P, Seeger J, McIntosh K, Dyke R, et al. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis. 2008;46(11):1751–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Pelton S, Stanley K, Yogev R, Fletcher C, McIntosh K, Wiznia A, et al. Switch from ritonavir to indinavir in combination therapy for HIV-1-infected children. Clin Infect Dis. 2005;40(8):1181–1187 [DOI] [PubMed] [Google Scholar]
- 174. Polis M, Sidorov I, Yoder C, Jankelevich S, Metcalf J, Mueller B, et al. Correlation between reduction in plasma HIV-1 RNA concentration 1 week after start of antiretroviral treatment and longer-term efficacy. Lancet. 2001;358(9295):1760–1765 [DOI] [PubMed] [Google Scholar]
- 175. Reddington C, Cohen J, Baldillo A, Toye M, Smith D, Kneut C, et al. Adherence to medication regimens among children with human immunodeficiency virus infection. Pediatr Infect Dis J. 2000;19(12):1148–1153 [DOI] [PubMed] [Google Scholar]
- 176. Robbins B, Capparelli E, Chadwick E, Yogev R, Serchuck L, Worrell C, et al. Pharmacokinetics of High-Dose Lopinavir/Ritonavir with and without Saquinavir or Non-Nucleoside Reverse Transcriptase Inhibitors in HIV Infected Pediatric and Adolescent Patients Previously Treated with Protease Inhibitors - Study PACTG P1038. Antimicrob Agents Chemother. 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Rutstein R, Feingold A, Meislich D, Word B, Rudy B. Protease inhibitor therapy in children with perinatally acquired HIV infection. AIDS. 1997;11(12):F107–F111 [DOI] [PubMed] [Google Scholar]
- 178. Storm D, Boland M, Gortmaker S, He Y, Skurnick J, Howland L, et al. Protease inhibitor combination therapy, severity of illness, and quality of life among children with perinatally acquired HIV-1 infection. Pediatrics. 2005;115(2):e173–e182 [DOI] [PubMed] [Google Scholar]
- 179. Van Dyke R, Lee S, Johnson G, Wiznia A, Mohan K, Stanley K, et al. Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have human immunodeficiency virus infection. Pediatrics. 2002;109(4):e61. [DOI] [PubMed] [Google Scholar]
- 180. Viani R, Araneta M, Deville J, Spector S. Decrease in hospitalization and mortality rates among children with perinatally acquired HIV type 1 infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39(5):725–731 [DOI] [PubMed] [Google Scholar]
- 181. Wiznia A, Church J, Emmanuel P, Eppes S, Rowell L, Evans C, et al. Safety and efficacy of enfuvirtide for 48 weeks as part of an optimized antiretroviral regimen in pediatric human immunodeficiency virus 1-infected patients. Pediatr Infect Dis J. 2007;26(9):799–805 [DOI] [PubMed] [Google Scholar]
- 182. Ayaya S, Nyandiko W, Vreeman R, Wafula S, Rotch J. Progression of HIV Infection in children attending the academic model providing access to healthcare (AMPATH) clinics in Western Kenya [abstract CDB006]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 183. McGrath C, Chung M, Richardson B, et al. Growth in HIV-1-infected children in Kenya following the initiation of HAART [abstract WEPEB191]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 184. Owiso G, Odawo P, Njoroge A, Meresey J, Muriithi C. Partnering with community health workers to improve pediatrics HIV testing and ART adherence [abstract WEPEB279]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 185. Wamalwa D, Obimbo E, Farquhar C, et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya [abstract MOPEB086]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 186. Ndirangu J, Bland R, Newell ML. Weight and weight gain in HIV-infected children receiving antiretroviral therapy in rural KwaZulu-Natal [abstract WEPEB188]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 187. Braun M, Kabue M, Chirwa M, et al. Challenges in pediatric HIV referral: evidence for PMTCT, infant diagnosis, and pediatric HIV clinic integration in Lilongwe, Malawi [abstract WEPED218]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 188. Dow A, Dube Q, Chirambo CM, Moore M, Heyderman R, Van Rie A. Community based early infant testing and treatment: experience from Blantyre, Malawi [abstract CDB098]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 189. Kabue MM, Buck WC, Kazembe PN, Kline MW. Discontinuation of standard first-line antiretroviral therapy in a cohort of 1434 Malawian children [abstract WEPED175]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kabue MM, Braun M, Aetker L, et al. ART initiation and increased survival of infants traced from PMTCT to pediatric HIV care: highlighting the need for program coordination in Lilongwe, Malawi [abstract WEPDD103]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 191. Chouraya C, Mahdi MA, Kieffer MP, Lukhele B. Implementing early antiretroviral treatment for infants in Swaziland [abstract CDB078]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 192. Colvin CJ, Knight L, Van Cutsem G, et al. Paediatric outcomes after five years on ART in Khayelitsha Township, South Africa [abstract CDB109]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 193. Coovadia A, Abrams E, Strehlau R, et al. Randomized clinical trial of switching to nevirapine-based therapy for infected children exposed to nevirapine prophylaxis [abstract MOAB103]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 194. Fatti G, Bock P, Grimwood A, Wampold S, Eley B. Antiretroviral therapy outcomes in rural and urban children attending public health facilities in South Africa [abstract MOPEB077]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 195. Fenner L, Keiser O, Brinkhof M, et al. Mortality, loss to follow-up and transfer-out in paediatric ART programmes in Southern Africa [abstract WEPEB276]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 196. Kaplan R, Bekker LG, Zwane E, Campbell E, Orrell C, Wood R. Long term programmatic outcomes for adults and children at a primary health care antiretroviral clinic in South Africa [abstract MOAD105]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 197. Kekitiinwa A, Maganda A, Tumbu P, Asiimwe Rwego A, Kiboneka E. Mortality rate among malnourished HIV-infected children in Kampala, Uganda: implications for time to initiate HAART [abstract MOPEB046]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 198. Augustinova D, Ban B, Kong C, et al. Health care of children diagnosed HIV positive before 18 months in Chea Chumneas Hospital, Takhmao, Cambodia [abstract CDC026]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 199. Isaakidis P, Raguenaud ME, Te V, et al. High survival and treatment success sustained after up to three years of ART for children in Cambodia [abstract MOAB102]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 200. Sophan S, Vibol U, Chanatheany H, et al. Lopinavir/ritonavir-based second line antiretroviral treatment in children at National Pediatric Hospital, Phnom Penh, Cambodia [abstract CDD020]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 201. Gnana Durai Pandian AG, Kandaswamy R, Nadol P, Chandrasekar S. Clinical and immunological response to highly active antiretroviral therapy (HAART) in paediatric patients - a retrospective cohort study at Government Hospital of Thoracic Medicine, India [abstract CDB102]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 202. McConnell M, Yuktanont P, Siangphoe U, et al. Survival rates following expansion of the national pediatric antiretroviral treatment program, Thailand, 2000–2005 [abstract MOAB101]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 203. Rezende R, Maia M, Diniz L, Camargos L, Amaral L, Pinto J. Impact of HAART in growth parameters of HIV-infected children in Minas Gerais, Brazil [abstract CDB104]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 204. Carter RJ, Katyal M, Toro P, Abrams EJ. Immunologic response and survival of infants initiating antiretroviral treatment (ART) at less than one year of age compared to older children enrolled at MTCT-Plus Initiative sites in 8 African countries and Thailand [abstract MOPEB048]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 205. Hansudewechakul R, Naiwatanakul T, Faikratok W, et al. Clinical outcomes in a community-based pediatric HIV care network in Chiang Rai, Thailand, 2002–2008 [MOPEB084]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 206. Davies MA, Wood R, Van Cutsem G, et al. Virologic failure and second-line antiretroviral therapy (ART) in children in South Africa: the international epidemiologic databases to evaluate AIDS (IeDEA) Southern Africa collaboration [abstract MOAB104]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 207. Palumbo P, Violari A, Lindsey J, et al. Nevirapine (NVP) vs lopinavir-ritonavir (LPV/r)-based antiretroviral therapy (ART) in single dose nevirapine (sdNVP)-exposed HIV-infected infants: preliminary results from the IMPAACT P1060 trial [abstract LBPEB12]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 208. Bognon T, Azondekon A, Homawoo E, et al. Networking between medical centers and ART pediatric site: what are the benefits for children? [abstract WEPDD105]. Presented at: 5th International AIDS Society conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa [Google Scholar]
- 209. Venkatesh K, De Bruyn G, Marinda E, et al. Morbidity and Mortality among Infants Born to HIV-infected Women in South Africa: Implications for Child Health in Resource-limited Settings [paper 841]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19 2010; San Francisco, CA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Achan J, Ruel T, Li P, et al. Incidence of Early Virological Failure and the Evolution of Antiretroviral Drug Resistance Mutations in Ugandan Children [paper 849]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19 2010; San Francisco, CA [Google Scholar]
- 211. Becquet R, et al. Survival of Children HIV-infected Perinatally or through Breastfeeding: A Pooled Analysis of Individual Data from Sub-Saharan Africa [paper 840]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19 2010; San Francisco, CA [Google Scholar]
- 212. Pandian PG, Chandran P, Kandasamy C. Persistence of Stunting after HAART in HIV-infected Children in South India [paper 847]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19 2010; San Francisco, CA [Google Scholar]
- 213. Sudjaritruk T, Aurpibul L, Puthanakit T, Sirisanthana T, Sirisanthan V. Causes of Hospitalization for HIV+ Children: Comparison of the Pre-PCP Prophylaxis, Pre-ART, and ART Era [paper 856]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19 2010; San Francisco, CA [Google Scholar]
- 214. Palladino C, Briz V, Negre-Policarpo S, et al. Long-term (>180 Weeks) Efficacy and Safety of Fosamprenavir in HIV-infected Pediatric Patients in Clinical Practice [paper 876]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19 2010; San Francisco, CA [Google Scholar]
- 215. Nachman S, Samson P, Acosta E, et al. Pharmacokinetic, Safety, and Efficacy Data on Cohort IIA; Youth Aged 6 to 11 Years from IMPAACT P1066: A Phase I/II Study to Evaluate Raltegravir in HIV-1-infected Youth [paper 873]. Presented at: 17th Conference on Retroviruses and Opportunistic Infections; February 16–19 2010; San Francisco, CA [Google Scholar]