Abstract
OBJECTIVE:
Our purpose was to assess early infant-feeding patterns in a cohort of low-income black mothers and to examine associations between maternal perception of infant temperament and complementary feeding (CF) before 4 months.
METHODS:
We used cross-sectional data from the 3-month visit (n = 217) of the Infant Care, Feeding and Risk of Obesity Study to assess relationships between early feeding of solids or juice and 6 dimensions of perceived infant temperament. Descriptive statistics were used to assess infant-feeding patterns, and logistic regression models were fit for each diet-temperament relationship found significant in the bivariate analyses.
RESULTS:
Seventy-seven percent of the infants were fed solid foods at 3 months, 25% were fed juice, and 6% were exclusively breastfed. In multivariable analyses, 2 dimensions of perceived infant temperament were associated with early feeding of solid foods (distress-to-limitations odds ratio [OR]: 1.97 [95% confidence interval (CI): 1.12–3.44]; activity-level OR: 1.75 [95% CI: 1.07–2.85]), whereas 1 dimension, low-intensity pleasure, was associated with early feeding of juice (OR: 0.51 [95% CI: 0.34–0.78]). Maternal characteristics significantly associated with early CF included breastfeeding, obesity, and depressive symptoms.
CONCLUSIONS:
Low-income black mothers may represent a priority population for interventions aimed at improving adherence to optimal infant feeding recommendations. That maternal perceptions of several domains of perceived infant temperament are related to early CF suggests that this is an important factor to include in future observational research and in the design of interventions.
Keywords: infancy, temperament, complementary feeding, breastfeeding, overweight
WHAT'S KNOWN ON THIS SUBJECT:
Several qualitative studies have revealed that caregivers use infant fussing as a cue for beginning complementary feeding (CF). Despite a higher prevalence of early CF among black infants, few studies have quantitatively examined the role of maternal perception of infant fussiness.
WHAT THIS STUDY ADDS:
Results of this study show that in a cohort of low-income, black, first-time mothers, early CF was highly prevalent and that maternal perception of infant temperament, breastfeeding, and maternal obesity and depression were important factors related to early CF.
The prevalence of overweight among US infants and toddlers has increased by ∼60% in the past 30 years.1 The prevalence is higher among non-Hispanic black (black) people (10.3%) than non-Hispanic white (white) people (8.7%) but not Hispanic people (12.5%).2 This disparity in overweight prevalence is concerning in light of research that has linked large infant size and/or rapid postnatal growth with child and adult overweight.3–6
Factors related to such growth patterns include early complementary feeding (CF)7–10 and, conversely, early discontinuation of exclusive breastfeeding (EBF),11–15 both of which are disproportionately high among black infants. The current prevalence of EBF through 3 months is 18.8% for black infants compared with 35.0% and 35.7% among white and Hispanic infants, respectively.16 National data also indicate that black mothers are least likely to delay solid food until 4 months (37.5%, 55.4%, and 59.5% for black, Hispanic, and white mothers, respectively).17
On the basis of studies that have revealed associations between maternal perception of fussy infant temperament and rapid growth or large size in infancy,18–21 it has been hypothesized that a fussy infant temperament may lead parents to use food as a soothing technique.18,19,22 This suggested causal mechanism has support in qualitative research on maternal infant-feeding decisions; several studies have found that mothers use infant fussing or crying to determine when their infant is hungry or when to first begin CF, particularly with solid food.23–28
Yet, few studies have quantitatively examined the relationship between maternal perception of fussy infant temperament and early CF, and in those that have,18,19,29 dietary measurement was suboptimal and/or the sample consisted of predominately middle-class and white subjects. No studies were identified that examined this relationship among low-income black mothers and infants, a population in which overweight and early CF are disproportionately high. The objective of our study was twofold: (1) to assess infant-feeding patterns from 0 to 4 months in a cohort of low-income, black, first-time mothers; and (2) to examine associations between maternal perception of infant temperament and early introduction of CF.
METHODS
Study Design and Participants
Data are from the Infant Care, Feeding and Risk of Obesity Study (Infant Care Study), an observational cohort of mother-infant dyads from 3 to 18 months after delivery. First-time black mothers aged 18 to 35 years were recruited through the North Carolina Supplemental Nutrition Program for Women Infants and Children (WIC) and assessed during in-home visits at infant ages 3, 6, 9, 12, and 18 months. For this study, data were largely from the 3-month visit (n = 217), at which 42 infants were between 2.7 and 2.9 months old, 162 were between 3.0 and 3.9 months old, and 1 was ≥4.0 months old. Exclusion criteria for the Infant Care Study included delivery at ≤35 weeks' gestation or presence of any of the following conditions: Down syndrome, epilepsy, cleft lip/palate, cerebral palsy, failure to thrive, mental retardation, severe food allergies, and any condition that might affect appetite, feeding, or growth. Data were collected from 2003 to 2007. The institutional review board of the University of North Carolina at Chapel Hill approved this study.
Study Measures and Variable Creation
Infant Diet
During each home visit, mothers completed an infant diet history (IDH) and a 24-hour dietary recall (DR). The IDH was similar to that used in other national studies of infant feeding30; mothers were asked how often they fed their infant a list of foods/beverages during the first, second, and third months. Using IDH data, we created 6 categories to describe feeding patterns across the first 3 months: (1) breast milk only; (2) formula only; (3) breast milk and formula; (4) breast milk and solids/juice; (5) formula and solids/juice; and (6) breast milk, formula, and solids/juice. For infants younger than 3 months at the first home visit, IDH data were taken from the second (6-month) home visit, during which mothers recalled foods and beverages fed during the third month.
The 24-hour DR was administered and analyzed via the 2005 version of the Nutrition Data System for Research (NDS-R) (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). This methodology was similar to that used in the Feeding Infants and Toddlers Study31 and has been shown among infants to produce similar patterns of intakes of food groups compared with 3-day weighed food records.32,33 To improve estimates of usual food intakes, the Infant Care Study collected 2 additional 24-hour DRs, which were taken by telephone on random, nonconsecutive days within 2 weeks of the home visit.34 Study personnel were trained by an NDS-R–certified staff member of the University of North Carolina at Chapel Hill Nutrition Epidemiology Core (National Institutes of Health grant DK56350). Because the 24-hour DRs were collected concurrent with the assessment of temperament for all infants, these data were used to create 2 dichotomous, dependent variables: one represented early introduction of solid foods, and the other represented early introduction of juice.
Infant Temperament
Maternal perception of infant temperament was measured by using 6 subscales from the Infant Behavior Questionnaire-Revised (IBQ-R).35 The IBQ-R is a valid and reliable questionnaire that, in its entirety, contains 14 subscales, each of which captures a separate dimension of perceived infant temperament. The choice of subscales to include in the Infant Care Study questionnaire was based on previous research of infant temperament and growth.18,22 Items are organized according to caregiving context (eg, feeding, sleeping) and ask the parent to estimate how often the infant responded in a specific way, from 1 (never) to 7 (always), during the previous week (2 weeks for some items). An example item for each subscale used in our study is as follows: (1) smile and laughter, “How often during the last week did the infant smile or laugh when given a toy?” (2) activity level (AL), “When put into the bath water, how often did the infant splash or kick?” (3) distress to limitations (DTL), “When placed on his/her back, how often did the infant fuss or protest?” (4) low-intensity pleasure (LIP), “When playing quietly with one of his/her favorite toys, how often did the infant show pleasure?” (5) duration of orienting, “How often during the last week did the infant stare at a mobile, crib bumper picture for 5 minutes or longer?” and (6) soothability, “When patting or gently rubbing some part of the infant's body, how often did s/he soothe immediately?” Each IBQ-R dimension has good internal consistency with Cronbach's α coefficients in the range of 0.77 to 0.90 for infants aged 3 to 6 months.34 For the Infant Care Study sample at 3 months, all α coefficients were in the range of “respectable” (0.70–0.80) to “very good,” with the exception of soothability, which was “acceptable” (α = 0.60).36 We included all 6 temperament dimensions, because there is no standard definition of the domains that comprise “fussiness.”37
Covariates
Choice of covariates was based on the literature17,24,38 and conceptual significance. Variables included infant birth weight, weight-for-length z score, gestational age, gender, and history of reflux; maternal age, education, BMI, breastfeeding, depression, and social desirability; and having a grandmother living in the household.
Statistical Methods
Descriptive statistics were used to assess infant-feeding patterns. For all independent variables (temperament dimensions and covariates), bivariate associations with each dependent variable were examined by using t and χ2 statistics. For each temperament-CF association found in the bivariate analyses to be significant, a multivariable logistic regression model that included the temperament dimension of interest and all covariates was fit by using the backward-elimination procedure.39 Interactions between temperament variables and select covariates (breastfeeding, infant gender, and soothability) were assessed. However, no interactions were significant, and we present only results from the main-effects models. Among formula-fed infants, a 2-sided independent-samples t test was used to test whether energy intakes differed between infants fed only formula and those also given solids or juice. Stata 10 (Stata Corp, College Station, TX) was used for all analyses. Statistical significance was set at P < .05.
RESULTS
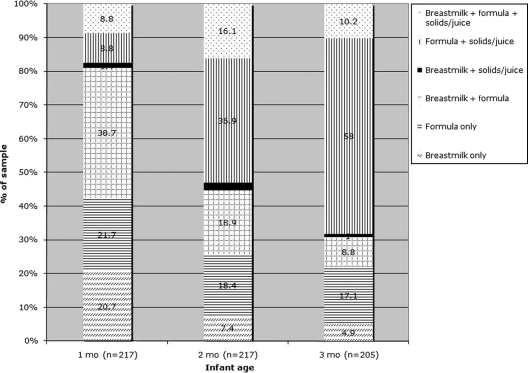
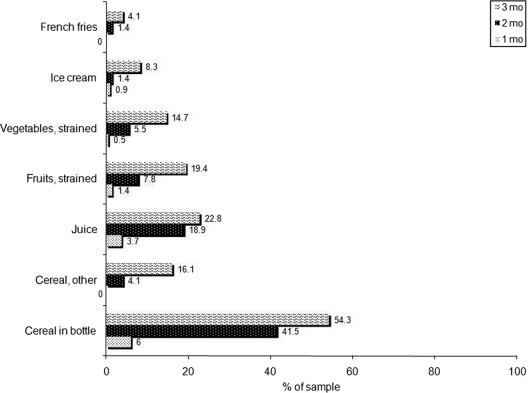
Approximately 70% of the infants were fed some breast milk during the first month; ∼20% were EBF (Fig 1). These proportions declined to ∼25% and ∼5% during the third month, respectively. Feeding of solids/juice was prevalent as early as 1 month. By the second month, the most common feeding pattern was formula and solids/juice. Infant cereal, particularly in a bottle, was the most commonly fed solid food (Fig 2).
FIGURE 1.
Prevalence of feeding patterns according to infant age. All data are from IDHs administered at 3 or 6 months. At the first home visit, 46 mothers, who reported that their infant was not yet old enough, did not complete a 3-month IDH. For these mother-infant pairs, 3-month IDH data from the second (6-month) home visit were examined. Twelve of these mother-infant pairs were lost to follow-up.
FIGURE 2.
Types of solids/juice fed according to infant age. All data are from IDHs administered at 3 or 6 months. At the first home visit, 46 mothers, who reported that their infant was not yet old enough, did not complete a 3-month IDH. For these mother-infant pairs, 3-month IDH data from the second (6-month) home visit were examined. Twelve of these mother-infant pairs were lost to follow-up. Sample sizes: 217 (1 month); 217 (2 months); and 205 (3 months).
Maternal perception of temperament variables significantly associated with early introduction of solids in bivariate analyses were DTL (P < .01) and AL (P < .01) (Table 1). For juice, a significant association was found for LIP (P < .01) (Table 1).
TABLE 1.
Sample Characteristics and Bivariate Associations With Feeding Solid Foods or Juice Before 4 Months (N = 217)
| Sample Characteristic | Total Sample | Solids at <4 moa |
Juice at <4 moa |
||
|---|---|---|---|---|---|
| Yes, 77% (N = 167) | No, 23% (N = 50) | Yes, 25% (n = 54) | No, 75% (N = 163) | ||
| Temperament dimension, mean (SD) | |||||
| Smile and laughter (n = 206) | 4.8 (1.0) | 4.9 (1.0) | 4.7 (1.1) | 4.8 (0.9) | 4.8 (1.0) |
| AL (n = 206) | 4.1 (0.8) | 4.2 (0.8)b | 3.8 (0.9) | 4.2 (0.8) | 4.1 (0.8) |
| DTL (n = 206) | 3.5 (0.7) | 3.5 (0.8)b | 3.2 (0.6) | 3.6 (0.7) | 3.4 (0.8) |
| LIP (n = 206) | 5.0 (0.9) | 4.9 (0.9) | 5.0 (1.1) | 4.6 (0.9)b | 5.1 (0.9) |
| Duration of orienting (n = 206) | 4.0 (1.0) | 4.1 (1.0) | 3.8 (1.0) | 4.1 (0.8) | 4.0 (1.1) |
| Soothability (n = 205) | 5.0 (0.8) | 5.0 (0.8) | 5.1 (0.9) | 4.9 (0.8) | 5.0 (0.9) |
| Infant age, mean (SD), wk | 3.3 (0.3) | 13.1 (1.2)c | 12.6 (1.2) | 13.4 (1.3)c | 12.9 (1.2) |
| Infant birth weight, mean (SD), kg | 3.2 (0.5) | 3.2 (0.5) | 3.2 (0.5) | 3.2 (0.4) | 3.2 (0.5) |
| Gestational age, mean (SD), wk | 41.1 (9.7) | 41.2 (10.1) | 40.7 (8.3) | 40.2 (8.0) | 41.4 (10.2) |
| Infant gender, male, % (n) | 47 (101) | 48 | 40 | 46 | 47 |
| Infant overweight, % (n)d | 14 (31) | 15 | 12 | 15 | 13 |
| Infant weight-for-length z score, mean (SD) | 0.6 (1.0) | 0.6 (1.0) | 0.6 (1.0) | 0.7 (0.9) | 0.5 (1.0) |
| History of infant reflux, % (n) | 19 (41) | 19 | 20 | 20 | 18 |
| Maternal age, mean (SD), y | 22.7 (3.8) | 22.4 (3.5) | 23.6 (4.6) | 23.0 (4.3) | 22.5 (3.6) |
| Maternal education, any college (n = 214), % (n) | 43 (91) | 38b | 59 | 47 | 41 |
| Maternal weight status (BMI), % (n) | |||||
| Underweight (<18.5) | 2 (5) | 2 | 4 | 2 | 3 |
| Normal (18.5–24.9) | 25 (55) | 24 | 30 | 32 | 23 |
| Overweight (25–29.9) | 28 (60) | 26 | 34 | 31 | 26 |
| Obese (≥30) | 45 (97) | 48 | 32 | 35 | 48 |
| Any breastfeeding, % (n)e | 21 (45) | 15c | 40 | 15c | 23 |
| Maternal depression (n = 213), % (n)f | 29 (62) | 32 | 20 | 40 | 26 |
| Maternal social desirability (n = 209), mean (SD)g | 6.9 (2.0) | 6.9 (2.0) | 7.1 (1.9) | 7.0 (1.9) | 6.9 (2.0) |
| Grandmother living in household, % (n) | 46 (100) | 48 | 40 | 41 | 48 |
The χ2 test for categorical variables and t test for continuous variables were used.
A slightly higher proportion of mothers reported feeding solids or juice when assessed by 24-hour DRs than the 3-month IDH. Percent agreement between the 2 dietary methods was 73% for solids and 77% for juice. Although 1 infant was older than 4 months (ie, 4.2 months) at the 3-month visit, all analyses were unchanged by his or her inclusion; therefore, this infant's data were retained in the current analyses.
P < .05.
P < .01.
At >90th percentile on the 2000 Centers for Disease Control and Prevention National Center for Health Statistics growth charts.
Any breastfeeding in 24-hour recalls.
Score ≥ 16 on the Center for Epidemiological Studies Depression Scale.
Marlow-Crowne Social Desirability Scale.
In multivariable analyses, results were similar for the DTL and AL subscales (Table 2). Infants perceived to have a 1-unit higher score on either temperament scale were nearly twice as likely to be fed solid food before 4 months. Maternal obesity produced even stronger odds ratios than the temperament dimensions, whereas any breastfeeding and maternal college education were protective against early solid feeding. Because of the small number of mothers in the breastfeeding and solids/juice category, we could not examine associations according to type of milk-feeding.
TABLE 2.
Results of Multivariate Logistic Regression Examining Factors Associated With Feeding Solid Foods or Juice Before 4 Months
| Adjusted Odds Ratio (95% Confidence Interval) | |
|---|---|
| Model 1: final model for DTL and feeding solid foods (N = 198) | |
| DTL | 1.97 (1.12–3.44)a |
| Soothabilityb | 0.73 (0.44–1.20) |
| Any breastfeedingc | 0.33 (0.15–0.76)d |
| Infant gender | 0.50 (0.22–1.13) |
| Infant age, wk | 1.39 (1.01–1.93)a |
| Maternal obesity, BMI ≥ 30 | 2.34 (1.03–5.30)a |
| Maternal education, any college | 0.36 (0.16–0.82)a |
| Maternal social desirabilityd | 1.08 (0.88–1.34) |
| Model 2: final model for AL and feeding solid foods (N = 198) | |
| AL | 1.75 (1.07–2.85)a |
| Soothabilityb | 0.66 (0.40–1.09) |
| Any breastfeedingc | 0.32 (0.14–0.73)d |
| Infant gender | 0.47 (0.21–1.06) |
| Infant age, wk | 1.33 (0.96–1.84) |
| Maternal obesity, BMI ≥ 30 | 2.55 (1.13–5.75)a |
| Maternal education, any college | 0.40 (0.18–0.91)a |
| Maternal social desirabilitye | 1.05 (0.85–1.29) |
| Model 3: final model for LIP and feeding juice (N = 197) | |
| LIP | 0.51 (0.34–0.78)d |
| Soothabilityb | 1.01 (0.64–1.59) |
| Infant age, wk | 1.70 (1.26–2.28)f |
| Birth weight, kg | 0.49 (0.22–1.07) |
| Maternal obesity, BMI ≥ 30 | 0.48 (0.23–0.99)a |
| Maternal education, any college | 1.88 (0.91–3.89) |
| Maternal depressiong | 2.97 (1.32–6.69)d |
| Maternal social desirabilitye | 1.12 (0.92–1.36) |
One infant was 4.2 months old, but results of the analyses were unchanged with his or her exclusion.
P < .05.
Included to control for maternal perception of infant's ability to be soothed.
Any breastfeeding in 24-hour recalls.
P < .01.
Marlow-Crowne Social Desirability Scale.
P < .001.
Score ≥ 16 on the Center for Epidemiological Studies Depression Scale.
The inverse association between the LIP subscale and early feeding of juice remained significant in the final adjusted model (model 3) (Table 2). A higher score on the LIP subscale represents infants perceived to gain greater satisfaction from low-stimulus activities such as rocking/swaying. Unlike the findings for early solids, obese mothers were less likely to feed juice before 4 months, as were mothers with depressive symptoms.
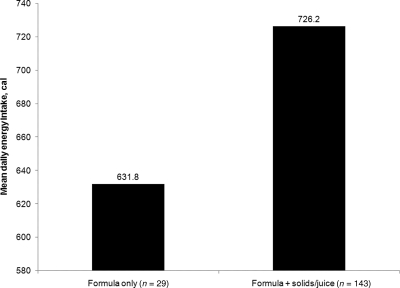
Among formula-fed infants, those fed either solids or juice had an average daily energy intake ∼100 cal greater than infants given only formula (P < .05) (Fig 3).
FIGURE 3.
Mean daily energy intake among formula-fed infants according to feeding-pattern category (P < .05).
DISCUSSION
Several studies have linked fussy infant temperament to rapid growth or large size during infancy18–20 and greater adiposity in childhood.22 Despite the suggested causal mechanism that parents use food to quiet fussy infants, and the ample qualitative evidence that supports this supposition,23–28 few quantitative studies have investigated the relationship between infant temperament and early CF. In this study, we examined, in a cohort of low-income black mother-infant dyads, early infant-feeding patterns and associations between maternal perception of infant temperament and CF. We have documented a high prevalence of nonadherence to optimal infant-feeding recommendations40 and demonstrate that multiple domains of perceived infant temperament plus other maternal characteristics are related to early CF.
The prevalence of EBF at 3 months in this sample (∼5%) was much lower than the national prevalence for all black mothers (18.8%).16 This result is concerning and may be due to the confluence in this sample of several factors independently associated with lower rates of EBF, including low income, WIC participation, and Southeastern residence.17,41 Conversely, the prevalence of infants who were consuming solid foods before 4 months was slightly higher than national estimates for all black infants (76% vs 62.5%, respectively).17 National estimates for early juice consumption according to race have not been published, but the prevalence in this sample (24%) was notably higher than that for all infants at 3 months of age (6.7%).17 These findings are similar to those of Bronner et al,42 who found that black mothers participating in WIC introduced solid foods and juice earlier than recommended, some as early as 7 to 10 days after delivery.
We believe this is the first quantitative study to examine maternal perception of fussy infant temperament and early CF in a sample of low-income black mother-infant pairs. The literature on this topic is small and results have been mixed,18,19,29 likely because of differences in dietary measurement and categorization of the exposure rather than true differences in the underlying phenomenon. Two studies, one in a predominately middle-class US sample18 and the other from northeastern United Kingdom,19 published similar findings that infant feeding, defined as breastfeeding versus bottle-feeding, was not associated with perception of fussy infant temperament. However, categorizing infant feeding as breastfeeding versus bottle-feeding is problematic, because breastfeeding mothers may express milk and feed via a bottle, and both breast- and formula-feeding mothers might place CF (with, eg, infant cereal or juice) in a bottle. Such broad categorization makes these results difficult to interpret.
We are aware of only 1 quantitative study on maternal perception of fussy infant temperament and feeding that had adequate dietary measurement.29 In a large sample of 6-month-old Norwegian infants, Niegel et al29 found an inverse association between full breastfeeding and maternal perception of fussy infant temperament (r = −0.11; P = .0000) as measured by the Infant Characteristics Questionnaire.43 That our study found, in a very different population, a similar direction of association between perception of fussiness and early CF lends support to the notion that feeding is influenced by maternal perceptions of infant characteristics. The evidence is further strengthened by qualitative studies from multiethnic and socioeconomic samples in which mothers were shown to use infant fussing/crying to decide when to initiate solid food and feeding in general.23–28
Although there is no standard definition of the domains that comprise “fussiness,”37 DTL has been the IBQ-R construct most often used in research related to overweight; studies have consistently found positive associations between perception of DTL and greater weight or adiposity in young children.19,21,22 We also document a significant association between DTL and early CF but show that it is important to measure additional domains of temperament, particularly AL and LIP. Mothers may perceive higher levels of infant activity as fussiness, representing an infant who is more difficult to manage, whereas lower LIP scores may capture more subtle forms of fussiness, for which mothers use juice rather than solids to appease. Soothability was unrelated to early CF in our study, which suggests that mothers give CF on the basis of the initial presentation of fussiness and not necessarily the ease with which an infant is calmed by non–feeding-related interventions, such as rocking or patting. The soothability scale of the IBQ-R does not include feeding in its list of ways to soothe an infant.
Similar to national studies on infant-feeding practices,17,39 we document protective effects of breastfeeding and maternal college education on the likelihood of feeding solids before 4 months. How breastfeeding may decrease risk of early introduction of solids is unclear, but at least 2 studies have demonstrated an association between breastfeeding during infancy and lower maternal control over feeding at 12 and 18 months.44,45 The authors suggested that breastfeeding may encourage feeding styles that are more responsive to infant signs of hunger or fullness; it is possible that the process of breastfeeding may also encourage styles that are more responsive to signs of developmental readiness for solids.
Although it has been shown that obese mothers are less likely to initiate and sustain breastfeeding,46,47 we believe this is the first study to demonstrate an association between maternal obesity and early introduction of solid foods. One possible explanation is that obese mothers have larger infants,48–50 whom they perceive to need more than breast milk and/or formula for adequate growth. That maternal obesity was negatively associated with early introduction of juice is also a novel finding. Although controversial,51 several studies have revealed positive associations between juice consumption and childhood overweight.52,53 Given an obese versus a normal-weight mother, or a mother who is simply overweight 3 months after delivery, it is possible that WIC staff place a greater emphasis on the American Academy of Pediatrics' recommendation to delay introduction of juice until 6 months of age.54
Our finding that maternal depressive symptoms are associated with early feeding of juice adds to a growing body of research on suboptimal parenting behaviors related to child health.55 Specific to overweight, mothers with depressive symptoms are less likely to initiate or sustain breastfeeding56–58 and are more likely to put an infant to bed with a bottle57 or allow a toddler to watch television for ≥2 hours/day.59 These maternal behaviors may reflect the need of mothers who feel overwhelmed to find easier ways to care for their infant. Maternal depressive symptoms have also been associated with more forceful, indulgent, and uninvolved feeding styles.60 However, McLearn et al56 used data from a national study of parent-infant dyads and found no association between maternal depressive symptoms and CF before 4 months. Interactions according to maternal age, race, marital status, and education were not tested; thus, discrepant findings may be a result of differences in sample characteristics.
Finally, results of our exploratory analysis among formula-fed infants suggest that early CF may be associated with higher energy intakes. This finding is similar to results from the Avon Longitudinal Study of Parents and Children that, among infants fed formula or both breast milk and formula, earlier introduction of CF was significantly associated with greater caloric intake (670.2, 635.1, and 618.0 cal for infants aged 1–2, 3, and ≥4 months, respectively; Ptrend < .0001), which, in turn, was associated with higher weight and BMI at 1, 2, 3, and 5 years of age.9 Other studies have found a similar relationship between early CF (<4 months) and greater weight gain during infancy.7,8
Potential limitations of our study include the cross-sectional design and, in the views of some people, indirect assessment of infant temperament via maternal report. Although the cross-sectional nature does not allow determination of the direction of association, results of qualitative studies support the hypothesis that infant fussiness both precedes and affects maternal feeding decisions. Regarding maternal report versus direct assessment of infant temperament, questionnaires that use maternal report have the advantage of capturing the extensive knowledge that caregivers have of children's behavior in multiple situations, whereas direct observations in the home or laboratory by an observer are limited by the number of contexts and the frequency with which a child can be rated.61 More important is that, for the purpose of interventions to delay early CF, maternal perception of infant temperament may be the more salient measure. Maternal-report measures are also relatively inexpensive to administer and amenable for use in clinical settings.61
CONCLUSIONS
Our study suggests a high prevalence of early CF among low-income, black, first-time mothers. That several domains of perceived infant temperament are related to early CF and that caloric intakes may be higher among infants who receive versus those who do not receive CF before 4 months demonstrates that maternal perception of infant behavior is an important factor to include in future research. Development of counseling methods to help mothers respond to infant behavior in ways that are supportive of optimal feeding seem warranted. The finding of a protective effect of breastfeeding against early introduction of solid foods in the context of predominant use of formula highlights the need for breastfeeding interventions targeted to this population.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health grants R01 HD42219-02, NIH DK56350, and T32 HD057824-01.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- CF
- complementary feeding
- EBF
- exclusive breastfeeding
- Infant Care Study
- Infant Care, Feeding and Risk of Obesity Study
- WIC
- Supplemental Nutrition Program for Women Infants and Children
- IDH
- infant diet history
- DR
- dietary recall
- IBQ-R
- Infant Behavior Questionnaire-Revised
- AL
- activity level
- DTL
- distress to limitations
- LIP
- low-intensity pleasure
REFERENCES
- 1. Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA. 2002;288(14):1728–1732 [DOI] [PubMed] [Google Scholar]
- 2. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the united states, 1999–2004. JAMA. 2006;295(13):1549–1555 [DOI] [PubMed] [Google Scholar]
- 3. Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life: a systematic review. Obes Rev. 2005;6(2):143–154 [DOI] [PubMed] [Google Scholar]
- 5. Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9(5):474–488 [DOI] [PubMed] [Google Scholar]
- 6. Ong KK, Emmett P, Northstone K, et al. Infancy weight gain predicts childhood body fat and age at menarche in girls. J Clin Endocrinol Metab. 2009;94(5):1527–1532 [DOI] [PubMed] [Google Scholar]
- 7. Kramer MS, Barr RG, Leduc DG, Boisjoly C, McVey-White L, Pless IB. Determinants of weight and adiposity in the first year of life. J Pediatr. 1985;106(1):10–14 [DOI] [PubMed] [Google Scholar]
- 8. Baker JL, Michaelsen KF, Rasmussen KM, Sorensen TI. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr. 2004;80(6):1579–1588 [DOI] [PubMed] [Google Scholar]
- 9. Ong KK, Emmett PM, Noble S, Ness A, Dunger DB; ALSPAC Study Team Dietary energy intake at the age of 4 months predicts postnatal weight gain and childhood body mass index. Pediatrics. 2006;117(3). Available at:www.pediatrics.org/cgi/content/full/117/3/e503 [DOI] [PubMed] [Google Scholar]
- 10. Schack-Nielsen L, Sorensen TI, Mortensen EL, Michaelsen KF. Late introduction of complementary feeding, rather than duration of breastfeeding, may protect against adult overweight. Am J Clin Nutr. 2010;91(3):619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hediger ML, Overpeck MD, Kuczmarski RJ, Ruan WJ. Association between infant breastfeeding and overweight in young children. JAMA. 2001;285(19):2453–2460 [DOI] [PubMed] [Google Scholar]
- 12. Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity: a systematic review. Int J Obes Relat Metab Disord. 2004;28(10):1247–1256 [DOI] [PubMed] [Google Scholar]
- 13. Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162(5):397–403 [DOI] [PubMed] [Google Scholar]
- 14. Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115(5):1367–1377 [DOI] [PubMed] [Google Scholar]
- 15. Singhal A, Lanigan J. Breastfeeding, early growth and later obesity. Obes Rev. 2007;8(suppl 1):51–54 [DOI] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention Breastfeeding among U.S. children born 1999–2007, CDC National Immunization Survey. Available at: www.cdc.gov/breastfeeding/data/NIS_data/index.htm Accessed June 10, 2010
- 17. Grummer-Strawn LM, Scanlon KS, Fein SB. Infant feeding and feeding transitions during the first year of life. Pediatrics. 2008;122(suppl 2):S36–S42 [DOI] [PubMed] [Google Scholar]
- 18. Carey WB. Temperament and increased weight gain in infants. J Dev Behav Pediatr. 1985;6(3):128–131 [PubMed] [Google Scholar]
- 19. Darlington AS, Wright CM. The influence of temperament on weight gain in early infancy. J Dev Behav Pediatr. 2006;27(4):329–335 [DOI] [PubMed] [Google Scholar]
- 20. Niegel S, Ystrom E, Vollrath ME. Is difficult temperament related to overweight and rapid early weight gain in infants? A prospective cohort study. J Dev Behav Pediatr. 2007;28(6):462–466 [DOI] [PubMed] [Google Scholar]
- 21. Slining MM, Adair L, Goldman BD, Borja J, Bentley M. Infant temperament contributes to early infant growth: a prospective cohort of African American infants. Int J Behav Nutr Phys Act. 2009;6:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wells JC, Stanley M, Laidlaw AS, Day JM, Stafford M, Davies PS. Investigation of the relationship between infant temperament and later body composition. Int J Obes Relat Metab Disord. 1997;21(5):400–406 [DOI] [PubMed] [Google Scholar]
- 23. Baughcum AE, Burklow KA, Deeks CM, Powers SW, Whitaker RC. Maternal feeding practices and childhood obesity: a focus group study of low-income mothers. Arch Pediatr Adolesc Med. 1998;152(10):1010–1014 [DOI] [PubMed] [Google Scholar]
- 24. Bentley M, Gavin L, Black MM, Teti L. Infant feeding practices of low-income, African-American, adolescent mothers: an ecological, multigenerational perspective. Soc Sci Med. 1999;49(8):1085–1100 [DOI] [PubMed] [Google Scholar]
- 25. Corbett KS. Explaining infant feeding style of low-income black women. J Pediatr Nurs. 2000;15(2):73–81 [DOI] [PubMed] [Google Scholar]
- 26. Heinig MJ, Follett JR, Ishii KD, Kavanagh-Prochaska K, Cohen R, Panchula J. Barriers to compliance with infant-feeding recommendations among low-income women. J Hum Lact. 2006;22(1):27–38 [DOI] [PubMed] [Google Scholar]
- 27. Hodges EA, Hughes SO, Hopkinson J, Fisher JO. Maternal decisions about the initiation and termination of infant feeding. Appetite. 2008;50(2–3):333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scott JA, Binns CW, Graham KI, Oddy WH. Predictors of early introduction of solid foods in infants: results of a cohort study. BMC Pediatr. 2009;9:60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niegel S, Ystrom E, Hagtvet KA, Vollrath ME. Difficult temperament, breastfeeding, and their mutual prospective effects: the Norwegian mother and child cohort study. J Dev Behav Pediatr. 2008;29(6):458–462 [DOI] [PubMed] [Google Scholar]
- 30. Fein SB, Labiner-Wolfe J, Shealy KR, Li R, Chen J, Grummer-Strawn LM. Infant feeding practices study II: study methods. Pediatrics. 2008;122(suppl 2):S28–S35 [DOI] [PubMed] [Google Scholar]
- 31. Ziegler P, Briefel R, Clusen N, Devaney B. Feeding Infants and Toddlers Study (FITS): development of the FITS survey in comparison to other dietary survey methods. J Am Diet Assoc. 2006;106(1 suppl 1):S12–S27 [DOI] [PubMed] [Google Scholar]
- 32. Lanigan JA, Wells JC, Lawsom MS, Lucas A. Validation of food diary method for assessment of dietary energy and macronutrients in infants and children aged 6–24 months. Eur J Clin Nutr. 2001;55(2):124–129 [DOI] [PubMed] [Google Scholar]
- 33. Fisher JO, Butte NF, Mendoza PM, et al. Overestimation of infant and toddler energy intake by 24-h recall compared with weighed food records. Am J Clin Nutr. 2008;88(2):407–415 [DOI] [PubMed] [Google Scholar]
- 34. Thompson FE, Subar AF. Dietary assessment methodology. In: Coulston AM, Boushey CJ. eds. Nutrition in the Prevention and Treatment of Disease. 2nd ed. San Diego, CA: Elsevier Academic Press; 2008:3–41 [Google Scholar]
- 35. Gartstein MA, Rothbart MK. Studying infant temperament via the revised infant behavior questionnaire. Infant Behav Dev. 2003;26:64–86 [Google Scholar]
- 36. DeVellis RF. Scale Development: Theory and Applications. Thousand Oaks, CA: Sage; 2003 [Google Scholar]
- 37. Rothbart MK. Commentary: differentiated measures of temperament and multiple pathways to childhood disorders. J Clin Child Adolesc Psychol. 2004;33(1):82–87 [DOI] [PubMed] [Google Scholar]
- 38. Hendricks K, Briefel R, Novak T, Ziegler P. Maternal and child characteristics associated with infant and toddler feeding practices. J Am Diet Assoc. 2006;106(1 suppl 1):S135–S148 [DOI] [PubMed] [Google Scholar]
- 39. Kleinbaum DG, Kupper LL, Nizam A, Muller KE. Applied Regression Analysis and Other Multivariable Methods. Belmont, CA: Thomson Brooks/Cole; 2008 [Google Scholar]
- 40. American Academy of Pediatrics, Committee on Nutrition Supplemental foods for infants. In: Kleinman R. ed. Pediatric Nutrition Handbook. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 1998:43–54 [Google Scholar]
- 41. Li R, Darling N, Maurice E, Barker L, Grummer-Strawn LM. Breastfeeding rates in the United States by characteristics of the child, mother, or family: the 2002 national immunization survey. Pediatrics. 2005;115(1). Available at: www.pediatrics.org/cgi/content/full/115/1/e31 [DOI] [PubMed] [Google Scholar]
- 42. Bronner YL, Gross SM, Caulfield L, et al. Early introduction of solid foods among urban African-American participants in WIC. J Am Diet Assoc. 1999;99(4):457–461 [DOI] [PubMed] [Google Scholar]
- 43. Bates JE, Freeland CA, Lounsbury ML. Measurement of infant difficultness. Child Dev. 1979;50(3):794–803 [PubMed] [Google Scholar]
- 44. Fisher JO, Birch LL, Smiciklas-Wright H, Picciano MF. Breast-feeding through the first year predicts maternal control in feeding and subsequent toddler energy intakes. J Am Diet Assoc. 2000;100(6):641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taveras EM, Scanlon KS, Birch L, Rifas-Shiman SL, Rich-Edwards JW, Gillman MW. Association of breastfeeding with maternal control of infant feeding at age 1 year. Pediatrics. 2004;114(5). Available at: www.pediatrics.org/cgi/content/full/114/5/e577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rasmussen KM. Association of maternal obesity before conception with poor lactation performance. Annu Rev Nutr. 2007;27:103–121 [DOI] [PubMed] [Google Scholar]
- 47. Thulier D, Mercer J. Variables associated with breastfeeding duration. J Obstet Gynecol Neonatal Nurs. 2009;38(3):259–268 [DOI] [PubMed] [Google Scholar]
- 48. Nohr EA, Vaeth M, Baker JL, Sorensen TI, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008;87(6):1750–1759 [DOI] [PubMed] [Google Scholar]
- 49. Nohr EA, Vaeth M, Baker JL, Sorensen TI, Olsen J, Rasmussen KM. Pregnancy outcomes related to gestational weight gain in women defined by their body mass index, parity, height, and smoking status. Am J Clin Nutr. 2009;90(5):1288–1294 [DOI] [PubMed] [Google Scholar]
- 50. Margerison Zilko CE, Rehkopf D, Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am J Obstet Gynecol. 2010;202(6):574.e1–574.e8 [DOI] [PubMed] [Google Scholar]
- 51. Nicklas TA, O'Neil CE, Kleinman R. Association between 100% juice consumption and nutrient intake and weight of children aged 2 to 11 years. Arch Pediatr Adolesc Med. 2008;162(6):557–565 [DOI] [PubMed] [Google Scholar]
- 52. Dennison BA, Rockwell HL, Baker SL. Excess fruit juice consumption by preschool-aged children is associated with short stature and obesity [published correction appears in Pediatrics. 1997;100(4):733]. Pediatrics. 1997;99(1):15–22 [PubMed] [Google Scholar]
- 53. Dennison BA, Rockwell HL, Nichols MJ, Jenkins P. Children's growth parameters vary by type of fruit juice consumed. J Am Coll Nutr. 1999;18(4):346–352 [DOI] [PubMed] [Google Scholar]
- 54. American Academy of Pediatrics, Committee on Nutrition The use and misuse of fruit juice in pediatrics. Pediatrics. 2001;107(5):1210–1213 [DOI] [PubMed] [Google Scholar]
- 55. Field T. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav Dev. 2010;33(1):1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McLearn KT, Minkovitz CS, Strobino DM, Marks E, Hou W. Maternal depressive symptoms at 2 to 4 months post partum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279–284 [DOI] [PubMed] [Google Scholar]
- 57. Paulson JF, Dauber S, Leiferman JA. Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics. 2006;118(2):659–668 [DOI] [PubMed] [Google Scholar]
- 58. Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Paediatr. 2007;96(4):590–594 [DOI] [PubMed] [Google Scholar]
- 59. McLearn KT, Minkovitz CS, Strobino DM, Marks E, Hou W. The timing of maternal depressive symptoms and mothers' parenting practices with young children: implications for pediatric practice. Pediatrics. 2006;118(1). Available at: www.pediatrics.org/cgi/content/full/118/1/e174 [DOI] [PubMed] [Google Scholar]
- 60. Hurley KM, Black MM, Papas MA, Caulfield LE. Maternal symptoms of stress, depression, and anxiety are related to nonresponsive feeding styles in a statewide sample of WIC participants. J Nutr. 2008;138(4):799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rothbart M, Bates J. Temperament. In: Damon W, Lerner RM. eds. Handbook of Child Psychology: Volume 3—Social Emotional and Personality Development. Hoboken, NJ: John Wiley and Sons; 2006:99–166 [Google Scholar]