Abstract
An enzyme-linked immunosorbent assay (elisa) for the detection of antigen secreted by viable Taenia solium metacestodes (Ag-elisa) was applied to 43 pre-treatment and 47 follow-up cerebrospinal fluid (CSF) samples from Peruvian patients with neurocysticercosis demonstrated by computed tomography and enzyme-linked immunoelectrotransfer blot assay. The sensitivity of the assay was 86%. Negative pre-treatment results in the Ag-elisa test were restricted to patients with only a single live cyst or only enhancing lesions. Patients with hydrocephalus had higher levels of circulating antigen. There was no difference between antigen levels in CSF taken before and immediately after treatment (day 14). Levels of parasite antigen were significantly positively correlated with the number of live cysts detected by tomography and were also proportional to the number and intensity of antibody reactions recognized by the immunoblot diagnostic test. In contrast, there was a negative correlation with the number of enhancing lesions revealed by tomography, supporting the hypothesis that enhancing lesions correspond to a terminal, moribund stage of the parasite. The use of antigen-detection tests specific for viable metacestodes has immediate utility in the clinical context, not only providing important information on the viability of the parasites but also leading to an improved understanding of the pathogenesis of neurocysticercosis before and after drug treatment.
Keywords: neurocysticercosis, Taenia solium, diagnosis, antigen detection, enzyme-linked immunosorbent assay
Introduction
Human Taenia solium neurocysticercosis is endemic in many developing countries where pig production forms part of the local economy. The disease is currently recognized as the main cause of acquired epilepsy in the world (Commission on Tropical Diseases, 1994). Until recently, serological diagnosis was limited by the poor sensitivity and specificity of the available assays. The enzyme-linked immunoelectrotransfer blot (EITB) assay (Tsang et al., 1989), based on the detection of specific antibody to defined parasite glycoprotein antigens, provided an improved antibody detection assay. However, antibody detection has 2 important limitations. Firstly, it may indicate only exposure to infection and not necessarily the presence of established, viable infection and, secondly, antibody may persist long after the parasite has been eliminated through immune mechanisms and/or drug therapy (Harrison et al., 1989; Garcia et al., 1997a). In the former case, the presence of antibodies to T. solium in a patient with neurological symptoms, living in an endemic zone, may result in false diagnosis of neurocysticercosis and delay the search for other pathological conditions. In the latter, antiparasitic therapy may be unnecessarily indicated since the parasites may not be viable.
Various assays have been developed for the detection of T. solium antigens in serum or cerebrospinal fluid (CSF) since 1985, with variable results (Estrada & Kuhn, 1985; Tellez-Giron et al., 1987; Correa et al., 1989; Estrada et al., 1989; Choromanski et al., 1990; Chen et al., 1991; Wang et al., 1992). Our group has developed a monoclonal antibody based enzyme-linked immunosorbent assay (Ag-elisa) designed to detect the presence of excretory–secretory antigens from viable parasites (Harrison et al., 1989). This assay performed well in comparison to other available tests when tested using CSF from a group of clinically diagnosed neurocysticercosis patients, and exhibited negligible background readings when tested with a panel of CSF of neurological patients from a non-endemic zone (Correa et al., 1989). The present study assessed the ability of the Ag-elisa to detect T. solium antigen in CSF samules from a series of 50 Peruvian patients whose diagnosis of neurocysticercosis was made on the basis of computed tomography (CT) scans and serum antibody detection by EITB, in order to evaluate its potential and clinical application for diagnosis and follow-up.
Materials and Methods
Samples
Archive CSF samples from patients participating in a therapeutic trial for neurocysticercosis (Garcia et al., 1997b) were used in this study. In the original trial, diagnosis of neurocysticercosis was based on the identification of at least one active cysticercotic lesion on a CT scan and a positive EITB assay result with serum. Lesions seen on CT were recorded as follows. (i) Live cysts: hypodense, rounded lesions visible in non-contrasted series. (ii) Enhancing lesions: isodense zones which became hyperdense after the injection of contrast dye. (iii) Calcified lesions: hyperdense images which did not alter in appearance after the injection of contrast dye. ‘Active’ lesions included live cysts with or without contrast enhancement, and enhancing lesions (Garcia et al., 1997b).
Patients were treated with albendazole at 400 mg twice daily for either 7 or 14 d, together with dexamethasone 1·5 mg orally 3 times daily for 5 d, reduced to 0·5 mg 3 times daily on days 6 and 7 and then withdrawn. CSF samples were routinely obtained at baseline or 14 d after treatment, and occasionally at follow-up visits (scheduled every 3 months) if a lumbar puncture was indicated by the attendant neurologist.
Processing
The Aa-elisa was performed with slight modifications from the original report (Harrison et al., 1989) as follows: microplate wells (Immulon® 2, flat bottom, Dynatech Laboratory) were coated with 100 μL (5 μg/mL) of 50% saturated (NH4)2SO4 precipitate of HP10 monoclonal antibody (mab) to T. saginata in carbonate buffer (pH 9·6), and left overnight at 4°C. Unbound antibody was removed by washing the wells with saline containing 0·05% (w/v) Tween®20. Free binding sites were blocked with 1% (w/v) bovine serum albumin (BSA) diluted in phosphate-buffered saline (pH 7·3) containing 0·05% (w/v) Tween® 20 (PBS–Tween) for one hour at room temperature. After washing the microplate wells again, 100 μL of undiluted CSF per well were added and incubated for 30 min at 37°C. After washing. 100 μL per well of biotin-conjugated HP10 mab dilute; 1/2000 in BSA–PBS–Tween were added and incubated for 30 min at 37°C. Plates were washed and streptavidin/peroxidase conjugate (Pierce), diluted 1/10000 in BSA–PBS–Tween, was added (100 μL/well) and the plates were incubated for 30 min at 37°C. The microplates were then washed and 100 μL of 3′,3′,5′,5′-tetramethylbenzidine (TMB liquid substrate system; Sigma) were added to each well. The plates were incubated for 15 min at room temperature. The reaction was stopped by adding 100 μL of 0·2m H2SO4 and read at 450 nm on a Titertek Multiska®. A sample was considered positive if the specific optical density (OD) value was greater than the mean of the values obtained with negative CSF samples plus 3 standard deviations (SD).
EITB assays were performed using 50 μL of CSF, as described by Tsang et al. (1989). Positive identification was based on visualizing T. solium-specific antibodies reacting to any one of 7 purified T. solium glycoprotein antigens (GP-ags: diagnostic bands GP50, GP42-39, GP24, GP18, GP14 and GP13; the number indicates the molecular mass in kDa).
Analysis
Assay sensitivity was calculated as the number of elisa-positive cases/total number of patients. Correlations between number of GP-ags recognized by EITB and OD levels, and between OD levels and number of active lesions, were analysed by using a non-parametric test (Spearman’s rank correlation coefficient). Differences in mean absorbance values between groups were analysed by Student’s t test.
Results
A total of 90 samples was processed, from 43 patients for whom archive pre-treatment CSF samples were available. The 47 post-treatment archive samples were recovered as follows: day 7 (n=2), day 14 (n=37), day 28 (n=2), day 60 (n=2), and days 150, 240, 540 and 720 (one each). Thirty-one patients had 2 samples taken at different times (usually at baseline and day 14 after therapy onset), 7 patients had 3, and one had 4. The patients included 23 males and 20 females, mean age 40·6 years (sd=16·6). The female patients tended to be older (mean age 45·7 years [sd=17·8] versus 36·13 years [sd=14·4], P=0·058). The type and number of cysticercotic lesions in these patients are shown in the Table.
Table.
Pre-treatment circulating antigen levels determined by elisa according to type and number of lesions in 43 Peruvian patients with neurocysticercosis
| Type of lesion | Frequency | Mean | No. of lesions Median |
Range | Optical densitya |
Percentage positive |
|---|---|---|---|---|---|---|
| Active lesions | ||||||
| Live cysts only | 24 | 10·1 | 2·0 | 1–180 | 0·983±0·113 | 87·5 |
| Single cyst | 8 | 1·0 | 1·0 | – | 0·625±0·231 | 62·5 |
| Two or more cysts | 16 | 14·7 | 4·0 | 2–180 | 1·165±0·103 | 100 |
| Enhancing lesions only | 9 | 8·8 | 5·5 | 2–23 | 0·463±0·178 | 67 |
| Cysts+enhancing lesions | 10 | 26·5 | 9·0 | 2–103 | 1·003±0·172 | 100 |
| Hydrocephalus | 9 | 6·3 | 5·0 | 2–19 | 1·366±0·101 | 100 |
Mean±standard error.
All baseline pre-treatment CSF samples reacted with 2 or more GP-ags in the EITB assay (median no. 6 GP-ags; 18 samples reacted with all 7). In the Ag-elisa, the cut-off OD level was 0·102, and the sensitivity was 86·0% with the pre-treatment samples (37/43). The mean absorbance level of positive samples was 1·050 (sd=0.513). There was a positive relationship between baseline antigen levels and number of GP-ags recognized in the EITB assay (correlation coefficient 0·49, P=0·001). This correlation was not found if all 90 samples were analysed (correlation coefficient 0·41, P<0.001).
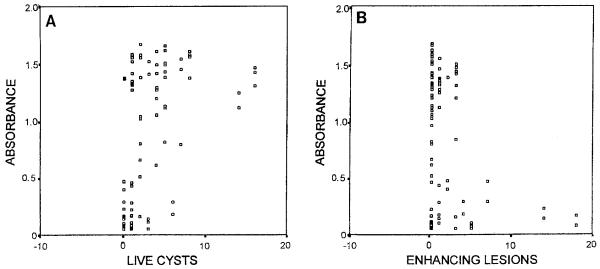
A significant correlation was found between the number of live cysts and absorbance values in the Ag-elisa with the 43 pre-treatment samples (correlation coefficient 0·48, P=0·001) and also with the 47 post-treatment samples (Figure, A). Conversely, there was a negative trend between the number of enhancing lesions and absorbance values, which was significant if all the samples were analysed (correlation coefficient −0·27, P=0·012) (Figure, B). No statistically significant difference was found between antigen levels at baseline and those at day 14 (P=0·88, 37 paired samples). CSF samples from patients with hydrocephalus had significantly higher antigen levels than did those from patients without this condition (mean OD=1·366 [sd=0·302] versus 0·751 [sd=0·567], P<0·001).
Figure.
Scatter plots of absorbance values versus number of live cysts (A) and number of enhancing lesions (B) in 90 cerebrospinal fluid samples from neurocysticercosis patients. Cases with more than 20 cysts or more than 20 enhancing lesions are not represented.
Six of the 43 pre-treatment samples gave negative results in the Ag-elisa: 3 in the group of patients with only enhancing lesions, i.e., with no live cyst (3/9), and 3 in the group of patients with a single live cyst (3/9). All 23 pre-treatment samples from patients with more than one cyst (16 without enhancing lesions and 7 with enhancing lesions) contained detectable levels of the secreted parasite antigen.
Discussion
Human cysticercosis is a major cause of neurological pathology in most developing countries (Medina et al., 1990; Garcia et al., 1993; Commission on Tropical Diseases, 1994) and is an emerging disease in developed countries that receive immigrants from endemic zones (Schantz et al., 1992; Evans et al., 1997b; White, 1997). Its clinical diagnosis is difficult, because of the variable number, size and location of lesions, and the immune reaction of the host (Nash & Neva, 1984; Del Brutto & Sotelo, 1988). Imaging techniques permit the visualization and identification of parasites in most cases (Del Brutto & Sotelo, 1988), but these examinations are expensive and the facility is scarce in endemic zones (Garcia et al., 1994). The application of a reliable and specific antigen-detection test, as presented in this work, would clarify the diagnosis in cases where imaging techniques are either not aiailable or not conclusive. In addition, the elisa permits quantitative monitoring of circulating parasite antigen levels, which are related to the number of viable parasites and hence to the effectiveness of antiparasitic therapy.
The sensitivity of the antigen detection test was 86%, one of the highest values of the variable results reported to date (Estrada & Kuhn, 1985; Tellez-Giron et al., 1987; Correa et al., 1989; Estrada et al., 1989; Choromanski et al., 1990; Chen et al., 1991; Wang et al., 1992), and only the invasive nature of CSF sampling argues against its routine use for diagnosis of neurocysticercosis. The specificity of these assays when applied to human serum samples is currently undergoing detailed investigation (E. Sciutto, L. J. S. Harrison & H. H. Garcia, unpublished work). A potential bias in this study arose from the fact that all patients were seropositive by EITB assay; thus we selected patients with a demonstrated immunological response to T. solium. EITB-negative patients are usually those with a single lesion on CT in whom a definitive diagnosis of cysticercosis is questionable. Thus the results presented here do not apply to this subgroup of patients, where indeed the major diagnostic problem arises. However, a similar criticism may also be levelled at other serological studies where patients were also selected by having unequivocal CT images or histopathological diagnoses.
It should be noted that, with those patients whose antigen detection test was negative, independent CT evaluation indicated that the infection was limited to a single live cyst or to multiple degenerating lesions. Interestingly, in 2 patients with a single cyst infection, follow-up samples 2 weeks after the beginning of therapy became Ag-elisa positive (data not shown), raising the possibility of confirming the diagnosis in this problematic group of patients by examining CSF immediately after treatment. Although the natural evolution of neurocysticercosis is poorly understood, clinical observations suggest that the immune rejection of cysts does not commence until several years after the initial infection (Dixon & Lipscomb, 1961; Evans et al., 1997a). ‘Enhancing’ lesions are in the midst of this degenerative process, and it is still a matter of controversy whether such cysts are, or are not, irreversibly damaged. Our findings support the former hypothesis.
In addition to the value of the viable parasite antigen detection assay in diagnosis, it may also contribute to our understanding of the pathogenesis of human neurocysticercosis—in particular, the process of parasite death and degeneration. For example, although anti-parasitic therapy with either praziquantel or albendazole is effective at killing most central nervous system parasites, there is still controversy with regard to its long-term clinical benefits. Whether recurrence of seizures in a subgroup of treated patients is due to the persistence of lesions could also be determined by prospectively comparing antigen levels according to clinical evolution after therapy.
The use of T. saginata as a model for T. solium has facilitated development of the antigen assay as applied to human cysticercosis and indeed minimizes the risks of cysticercosis infection in laboratory personnel (Parkhouse et al., 1996). In comparison to other clinical tests such as CT scan, this assay is highly economical and reproducible as the target antigen is very stable even under field conditions. The use of this new tool for clinical diagnosis and monitoring of human neurocysticercosis in endemic zones will assist clinical management, perhaps clarify therapeutic controversies, and may also lead to a deeper insight into the pathogenesis of the disease.
Acknowledgements
We are indebted to L. Verastegui, L. Zapata, V. Villaseca and all the personnel of the Laboratory of Cysticercosis of Instituto de Ciencias Neurologicas, Lima, for help in sample management and data collection, and to Drs Dolores Correa and Carlton Evans for helpful discussions.
This study was funded in part by grants no. CT95-0002 from the INCO-DC programme of the European Union, and no. 1-U01 A135984-01 from the National Institutes of Health, USA. We also acknowledge the British Council for support in the form of a Higher Education Link Project between the University of Edinburgh and the Universidad Peruana Cayetano Heredia.
References
- Chen JP, Zhang XY, Tan W, Liu MF, Liu GL, Hu YX. Determination of circulating antigen in cysticercosis patients using McAb-based ELISA. Chung Kuo Chi Sheng Chung Hsueh Yu Chi Sheng Chung Ping Tsa Chih. 1991;9:122–125. In Chinese, with English summary. [PubMed] [Google Scholar]
- Choromanski L, Estrada JJ, Kuhn RE. Detection of antigens of larval Taenia solium in the cerebrospinal fluid of patients with the use of HPLC and ELISA. Journal of Parasitology. 1990;76:69–73. [PubMed] [Google Scholar]
- Commission on Tropical Diseases Relationship between epilepsy and tropical diseases. Epilepsia. 1994;35:89–93. International League Against Epilepsy. [PubMed] [Google Scholar]
- Correa D, Sandoval MA, Harrison LJS, Parkhouse RME, Plancarte A, Meza-Lucas A, Flisser A. Human neurocysticercosis: comparison of enzyme immunoassay capture techniques based on monoclonal and polyclonal antibodies for the detection of parasite products in cerebrospinal fluid. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1989;83:814–816. doi: 10.1016/0035-9203(89)90340-4. [DOI] [PubMed] [Google Scholar]
- Del Brutto OH, Sotelo J. Neurocysticercosis: an update. Reviews of Infectious Diseases. 1988;10:1075–1087. doi: 10.1093/clinids/10.6.1075. [DOI] [PubMed] [Google Scholar]
- Dixon HBF, Lipscomb FM. Cysticercosis: an analysis and follow-up of 450 cases. Medical Research Council, Special Report Series. 1961;299:1–58. [Google Scholar]
- Estrada JJ, Kuhn RE. Immunochemical detection of antigens of larval Taenia solium and anti-larval antibodies in the cerebrospinal fluid of patients with neurocysticercosis. Journal of the Neurological Sciences. 1985;71:39–48. doi: 10.1016/0022-510x(85)90035-8. [DOI] [PubMed] [Google Scholar]
- Estrada JJ, Estrada JA, Kuhn RE. Identification of Taenia solium antigens in cerebrospinal fluid and larval antigens from patients with neurocysticercosis. American Journal of Tropical Medicine and Hygiene. 1989;41:50–55. [PubMed] [Google Scholar]
- Evans CAW, Gonzalez AE, Gilman RH, Verastegui M, Garcia HH, Chavera A, Tsang VCW, Pilcher JB, The Cysticercosis Working Group in Peru Immunotherapy for porcine cysticercosis: imulications for prevention of human diseas. American Journal of Tropical Medicine and Hygiene. 1997a;56:33–37. doi: 10.4269/ajtmh.1997.56.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C, Gilman RH, Garcia HH, Friedland JS. Controversies in the management of cysticercosis. Emerging Infectious Diseases. 1997b;3:403–405. doi: 10.3201/eid0303.970324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia HH, Gilman R, Martinez M, Tsang VCW, Pilcher JB, Herrera G, Diaz F, Porras M, Alvarado M, Orrillo E, Torres P, Miranda E, The Cysticercosis Working Group in Peru Cysticercosis as a major cause of epilepsy in Peru. Lancet. 1993;341:197–200. doi: 10.1016/0140-6736(93)90064-n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García HH, Herrera G, Gilman RH, Tsang VCW, Pilcher JB, Diaz F, Candy EJ, Miranda E, Naranjo J, The Cysticercosis Working Group in Peru Discrepancies between cerebral computed tomography and Western blot in the diagnosis of neurocysticercosis. American Journal of Tropical Medicine and Hygiene. 1994;49:190–195. doi: 10.4269/ajtmh.1994.50.152. [DOI] [PubMed] [Google Scholar]
- García HH, Gilman RH, Catacora M, Verastegui M, Gonzalez AE, Tsang VCW, The Cysticercosis Working Group in Peru Serological evolution of neurocysticercosis patients after antiparasitic therapy. Journal of Infectious Diseases. 1997a;175:486–489. doi: 10.1093/infdis/175.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García HH, Gilman RH, Horton J, Martinez M, Herrera G, Altamirano J, Cuba JM, Rios-Saavedra N, Verastegui M, Boero J, Gonzalez AE, The Cysticercosis Working Group in Peru Albendazole therapy for neurocysticercosis: a prospective double blind trial comparing 7 vs. 14 days of treatment. Neurology. 1997b;48:1421–1427. doi: 10.1212/wnl.48.5.1421. [DOI] [PubMed] [Google Scholar]
- Harrison LJS, Joshua GWP, Wright SH, Parkhouse RME. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunology. 1989;11:351–370. doi: 10.1111/j.1365-3024.1989.tb00673.x. [DOI] [PubMed] [Google Scholar]
- Medina M, Rosas E, Rubio F, Sotelo J. Neurocysticercosis as the main cause of late-onset epilepsy in Mexico. Archives of Internal Medicine. 1990;150:325–327. [PubMed] [Google Scholar]
- Nash TE, Neva FA. Recent advances in the diagnosis and treatment of cerebral cysticercosis. New England Journal of Medicine. 1984;311:1492–1496. doi: 10.1056/NEJM198412063112307. [DOI] [PubMed] [Google Scholar]
- Parkhouse RME, Garate T, Benitez L, Kirkham P, Brookes SM, Wright S, Harrison LJS. Approaches towards a vaccine for human, porcine and bovine cysticercosis. In: solium T, Garcia HH, Martinez SM, editors. Taeniasis/Cisticercosis por. Ed. Universo; Lima: 1996. pp. 65–78. [Google Scholar]
- Schantz PM, Moore AC, Munoz JL, Hartman BJ, Schaefer JA, Aron AM, Persaud D, Sarti E, Wilson M, Flisser A. Neurocysticercosis in an orthodox Jewish community in New York City. New England Journal of Medicine. 1992;327:692–695. doi: 10.1056/NEJM199209033271004. [DOI] [PubMed] [Google Scholar]
- Tellez-Giron E, Ramos MC, Dufour L, Alvarez P, Montante M. Detection of Cysticercus cellulosae antigens in cerebrospinal fluid by dot enzyme-linked immunosorbent assay (Dot-elisa) and standard elisa. American Journal of Tropical Medicine and Hygiene. 1987;37:169–l73. doi: 10.4269/ajtmh.1987.37.169. [DOI] [PubMed] [Google Scholar]
- Tsang V, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) Journal of Infectious Diseases. 1989;159:50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- Wang CY, Zhang HH, Ge LY. A MAb-based elisa for detecting circulating antigen in CSF of patients with neurocysticercosis. Hybridoma. 1992;11:825–827. doi: 10.1089/hyb.1992.11.825. [DOI] [PubMed] [Google Scholar]
- White AC. Neurocysticercosis: a major cause of neurological disease worldwide. Clinical Infectious Diseases. 1997;24:101–113. doi: 10.1093/clinids/24.2.101. [DOI] [PubMed] [Google Scholar]