Abstract
Clinical studies show that men are more likely to develop disorders affecting midbrain dopaminergic pathways, such as drug addiction and Parkinson’s disease (PD). Although a great deal of focus has been given to the role of oestrogen in the maintenance of midbrain dopaminergic pathways, little is known about how testosterone influences these pathways. In the present study, we used stereological analysis of tyrosine hydroxylase-immunoreactive (TH-IR) cell bodies to determine how testosterone influences the dopaminergic cell bodies of the substantia nigra pars compacta (SNpc) and ventral tegmental area (VTA). Rats and mice were castrated at post-natal day (PN) 60, and these midbrain cell populations were counted on PN 90. One month after castration, TH-IR cell number had increased in the SNpc and VTA of rats and mice. Replacement with testosterone or the non-aromatisable analogue dihydrotestosterone (DHT) in castrated animals reduced TH-IR cell number in the SNpc and VTA in rats. In mice, the decrease of TH-IR cell number with testosterone or DHT replacement was observed only in the SNpc. The apparent increase in TH-IR neurone number after castration is not explained by an increase in TH expression because the number of nondopaminergic cells (TH-immunonegative, TH-IN) did not decrease proportionally after castration. TH-IN cell number did not change after castration or hormone replacement in rat or mouse SNpc or VTA. These findings suggest that testosterone may play a suppressive role in midbrain dopaminergic pathways.
Keywords: dopamine, androgens
Clinical studies show that many disorders of dopaminergic dysfunction have a higher incidence in men than women (1–3). For example, men are more likely to develop drug addiction (although women may develop addiction more quickly) (2, 4, 5) and Parkinson’s disease (PD) than women (6, 7). The mechanisms mediating these differences are poorly understood. Although a great deal of focus has been placed on the stimulatory and protective effects of oestrogen in dopaminergic systems (5, 8), considerably less is known about the role of androgens.
Studies from our laboratory and others have reported that adult male rats are less active in the open field than females under baseline conditions and after treatment with psychostimulant drugs (9–11). Castration has been reported to increase locomotor responses to amphetamine and cocaine and also to increase dopamine release (9, 10, 12, 13), although negative effects have also been reported (14, 15). Testosterone replacement results in a decrease of these dopaminergic responses, suggesting that androgens may have a suppressive effect on dopaminergic function (12, 16). The most comprehensive investigation of androgen effects on dopaminergic function has focused on the dopaminergic projections to the cortex. An elegant series of studies by Kritzer and colleagues showed that castration caused an increase in dopaminergic innervation to these areas that is reversed by testosterone. This is accompanied by worsening in working memory and extradimensional set shifting in castrated animals (17–19).
Animal models of neurotoxin-induced damage also provide support for androgen suppression of dopaminergic activity. Males experience more severe behavioural deficits and cell loss than females after treatment with the dopamine neurotoxin 6-hydroxydopamine (6-OHDA) (20). Castration in rats protects striatal dopamine content from 6-OHDA lesion compared to intact males (21, 22) and testosterone replacement in castrated mice blocks protection of striatal dopamine from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (23, 24).
Despite accumulating evidence that androgens can influence forebrain dopamine systems and the demonstration that as many as 50% of tyrosine hydroxylase (TH)-positive neurones in the substantia nigra pars compacta (SNpc) and ventral tegmental area (VTA) express androgen receptors (25–27), there has been little study of whether androgen regulates dopaminergic neurone number as well as innervation density of specific projections. The present study aimed to determine whether androgens affect TH-immunoreactive (TH-IR) cell number in the SNpc and VTA. To answer this question, we used unbiased stereology to estimate dopaminergic neurone number in the SNpc and VTA in sham castrated and castrated adult rats and mice and castrated rats and mice that had received testosterone and dihydrotestosterone (DHT) replacement.
Materials and methods
Animals, surgery, hormone replacement and housing
In Experiment 1, Sprague-Dawley male rats castrated or sham castrated on postnatal day (PN) 60 ± 5 were purchased from Charles River Laboratories (Raleigh, NC, USA). Animals were segregated by surgical condition and housed in plastic cages under a 12 : 12 h light/dark cycle with ad lib access to food and water. In a parallel study, male C57Bl/6J mice sham castrated or castrated at PN 60 ± 5 (Charles River Laboratories) were separated by surgical condition and housed in plastic cages under a 12 : 12 h light/dark cycle with ad libitum access to food and water. Rats and mice were transcardially perfused with 10% neutral buffered formalin (VWR International, West Chester, PA, USA) on PN 90. In a second experiment, castration or sham surgery was performed under aseptic conditions on adult rats (PN 60) when animals were under ketamine and xylazine (80 : 10 mg/kg) anaesthesia. For castration, an incision was made through the skin at the tip of the scrotum. Pressure was placed on the abdomen to push the testis downward. A small incision was made through the connective tissue of the scrotum and the tissue sac containing the epididymis. The cauda epididymis was pulled out, accompanied by the testis, caput epididymis, vas deferens and spermatic blood vessels. The blood vessels and vas deferens were severed, and testis and epididymis were removed. The incision was closed with a wound clip. All animals received acetaminophen (500 mg/kg) before surgery and in the drinking water for 48 h after surgery.
Starting on the day of surgery, animals received daily s.c. injections of vehicle (sesame oil) or testosterone (1 mg/kg) or DHT (0.5 mg/kg). In a separate study involving mice, surgeries were performed by the supplier and animals shipped the next morning. Subcutaneous injections of hormones were started the day after receipt. Mice received daily s.c. injections of vehicle or testosterone (0.1 mg/day) or DHT (0.05 mg/day). Animals were housed until PN 90 for perfusion.
Tissue preparation and immunohistochemistry
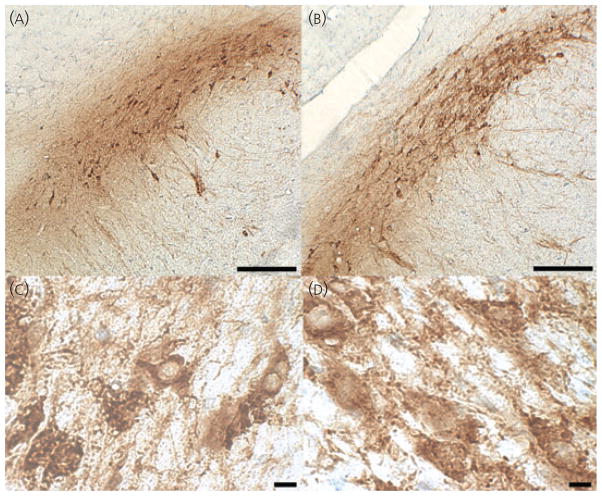
Animals were deeply anaesthetised with sodium pentobarbital and transcardially perfused with 10% neutral buffered formalin. After perfusion, the brains were extracted and post-fixed overnight in 10% formalin. Brains were then equilibrated in a 30% sucrose cryoprotectant solution and stored at 4 °C. Serial coronal sections (30 μm) were cut on a cryostat and thawmounted onto slides. For rats, every third section was collected and, for mice, every second section was collected. Sections were allowed to dry overnight at 37 °C. Heat mediated antigen retrieval was performed to increase immunoreactivity of the tissue for TH (28, 29). Sections were pressure cooked (Deni electric pressure cooker; Keystone Manufacturing Company, Buffalo, NY, USA) at high pressure for 1 min and 30 s in citrate buffer (pH 6.0) (30). This length of time allowed for optimal staining without compromising cell morphology. Sections were rinsed in phosphate-buffered saline and incubated in 0.3% hydrogen peroxide-methanol for 30 min to quench endogenous peroxidase. Sections were rinsed and blocked in 0.5% bovine serum albumin + 0.3% Triton X-100 for 15 min at room temperature. After blocking, sections were incubated in primary antibody diluted in blocking buffer (dilution 1 : 10 000; Immunostar, Inc., Hudson, WI, USA) overnight at 4 °C. The specificity of this antibody has been verified by the supplier, which demonstrated a lack of cross reactivity with all other phenotypic proteins for catecholaminergic neurones (dihydropteridine reductase, dopamine β hydroxylase, phenylethanolamine-N-methyltransferase) and also verified the presence of expression in cells transfected with the protein. Other laboratories have also verified specificity by western blotting (31). The next day, sections were rinsed and incubated in a biotinylated horse anti-mouse secondary antibody (dilution 1 : 1000; Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature. The sections were then rinsed and incubated in avidin-biotin complex for 1 h at room temperature followed by rinsing and staining with diaminobenzidine (DAB) (Vector Laboratories). Sections were rinsed, dehydrated through graded alcohols, mounted and coverslipped. To estimate the number of cells that were TH-immunonegative (TH-IN), sections in a subset of animals (from the hormone replacement experiment) were counterstained with 0.5% cresyl violet after DAB staining and coverslipped. Photomicrographs showing the stained material at low and high magnification in rat and mouse are presented in Fig. 1.
Fig. 1.
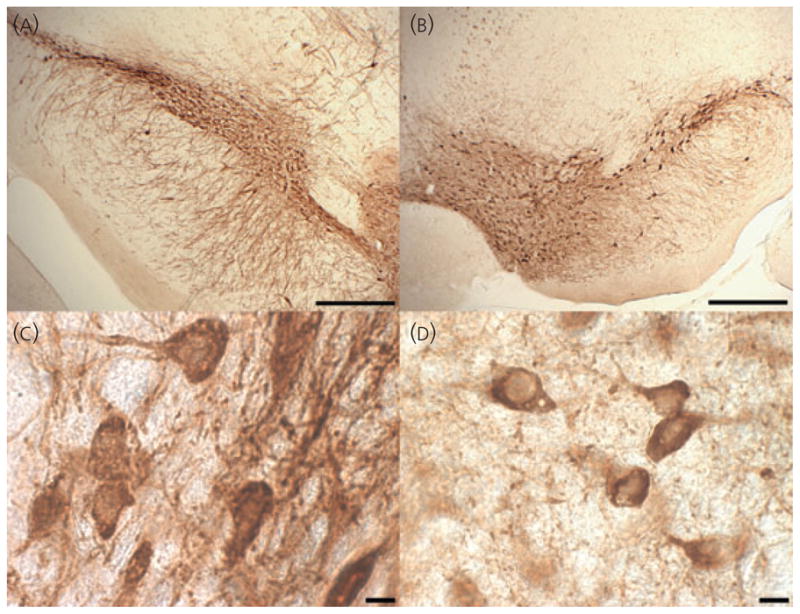
Low and high magnification photomicrographs of tyrosine hydroxylase staining in rat and mouse. (A) Rat substantia nigra pars compacta (SNpc) at × 5. (B) Mouse SNpc and ventral tegmental area at × 5. (C) Rat SNpc at × 100. (D) Mouse SNpc at × 100. Scale bars on top images = 400 μm. Scale bars on bottom images = 10 μm. [For clarity, Fig. 1. is reproduced in the companion paper: Journal of Neuroendocrinology 2010; 22: 226–237).
Unbiased stereology
Unbiased stereological estimation of the total number of TH-IR cell bodies in the SNpc and VTA was performed using the optical fractionator method (32). In rats, every third section was collected through the extent of the midbrain, and every sixth section was analysed for cell counting in rostral–caudal fashion, resulting in a total of six to eight sections sampled for both the right and left sides of the brain. For mice, every second section was collected through the extent of the midbrain and every fourth was analysed for stereology, resulting in six or seven sections per animal. A computerised counting system containing a Nikon Optiphot-2 microscope (Nikon, Tokyo, Japan), a camera (Dage MTI, Michigan City, IN, USA) and motorised stage (Ludl Electronic Products, Hawthorne, NY, USA) was used to estimate the total number of cells. Each region of interest was projected onto a monitor, traced at low (× 4) magnification and a sampling grid was superimposed on the traced region by the StereoInvestigator software (MicroBrightField, Williston, VT, USA). After shrinkage, final thickness of the sections used averaged 12 μm. Therefore, a 40 × 40 μm counting frame with a dissector height of 8 μm was used. Each counting frame was randomly spaced 80 μm apart and guard zones of 2 μm from the top and bottom of the section were used. Individual cell bodies were visualised with a × 100 oil immersion lens (numerical aperture = 1.3). Cells that were stained for TH and at least 10 μm in diameter were counted as TH-IR. Cells that were stained with cresyl violet but not TH and were at least 10 μm in diameter were counted as TH-IN. Sufficient cells were counted to achieve a coefficient of error that was ≤ 0.10. The stereologer was blinded to all surgical and treatment groups for each experiment.
Cell size
To verify that neither surgery nor endocrine treatment influenced cell size, cell diameters were counted in a subset of animals from the replacement study. To estimate cell size, seven to ten representative TH-IR cells from the SNpc and VTA were randomly selected by sampling one section from the same location (rostral–caudal and lateral–medial) for each animal. All measured cells and sections were matched in their rostral–caudal location across animals. A 40 × 40 μm counting frame was used for both rats and mice, with a sampling grid spacing of 200 × 200 μm for rats and 120 × 120 μm for mice. Because cell shape varies from spherical to ovoid, the ‘diameter’ was measured along the longest axis of TH-IR cells contained within the counting frame. The mean value of cell diameters from each animal was used to represent cell size. The mean ± SEM were computed for each experimental group (cell size from each animal constituted n = 1). The mean cell size in the SNpc and VTA were measured for all experimental groups for which the identical section was available (n = 5 per group for rats and n = 3 per group for mice).
Prostate weights
Prostate weights were collected as a measure of successful replacement in comparison with intact controls. After fixation of tissues by transcardial perfusion, the ventral prostate was removed. All connective tissues associated with prostates were removed prior to weighing.
Light microscopy
Low magnification photomicrographs were obtained using a Leitz Diaplan microscope (Leitz Wetzlar GmbH, Wetzlar, Germany) at × 6.4 magnification using a Sony DCX-500 Color Digital Camera (Sony Corp., Tokyo, Japan).
Statistical analysis
All statistical analyses were performed using ANOVA (NCSS, Kaysville, UT, USA) with P < 0.05 considered statistically significant. Cell number was analysed by one-way ANOVA with treatment (sham, castrated, testosterone or DHT replaced) as the between subjects factor. Tissue weights were analysed by one-way ANOVA with treatment (sham, castrated, testosterone or DHT replaced) as the between subjects factor. Cell diameter was analysed by three-way ANOVA repeated measures with area as within and species and treatment as between subjects factors). Post-hoc analysis was performed using the Fisher’s least significant difference test to determine group differences. Post-hoc tests were conducted on all planned comparisons (Sham versus cast, cast versus testosterone, cast versus DHT).
Results
Effect of castration on TH-IR cell number
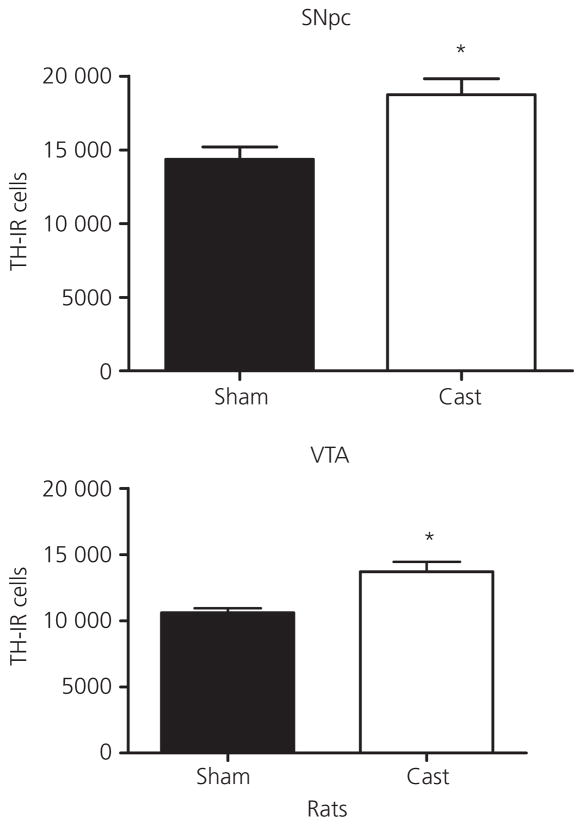
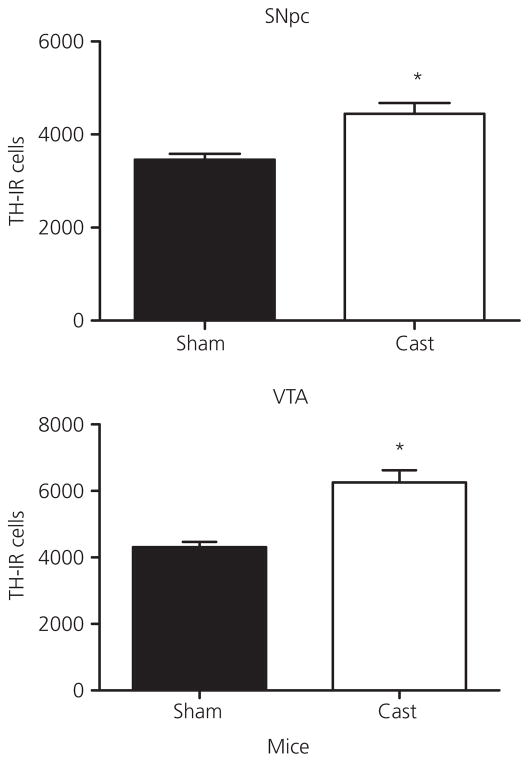
To investigate how the loss of testicular hormones influences TH-IR cell number in the SNpc and VTA, animals were sham castrated or castrated at PN 60 and neurones counted 1 month post-surgery. Figure 2 shows representative high and low magnification images of SNpc from a representative sham and castrated rat section that were stained for TH and counterstained with cresyl violet. Castration at PN 60 resulted in an increase in TH-IR cell number the SNpc and VTA of male rats (Fig. 3) and mice (Fig. 4) at PN 90 relative to the sham surgery controls. In rats, ANOVA revealed a significant effect of castration in the SNpc (F1,10 = 10.0, P < 0.05) and VTA (F1,9 = 11.6, P < 0.05). In mice, ANOVA also revealed a significant effect of castration in the SNpc (F1,10 = 13.8, P < 0.01) and VTA (F1,10 = 23.7, P < 0.01).
Fig. 2.
Low and high magnification of tyrosine hydroxylase staining with counterstaining with cresyl violet in sham and castrated male rat to illustrate the increase in cell body number but not size after castration. (A) Sham substantia nigra pars compacta (SNpc) at × 10. (B) Castrated SNpc at × 10. (C) Sham SNpc at × 100. (D) Castrated SNpc at × 100. Scale bars on top images = 200 μm. Scale bars on bottom images = 10 μm.
Fig. 3.
Total tyrosine hydroxylase-immunoreactive neurone (TH-IR) number in the substantia nigra pars compacta (SNpc) and ventral tegmental area (VTA) of castrated (Cast) compared to sham cast male rats after 1 month of castration during adulthood. Data are represented as the mean ± SEM (n = 4–5 per group). *Statistically significantly different from sham cast, for SNpc: P < 0.02 and for VTA: P < 0.05.
Fig. 4.
Total tyrosine hydroxylase-immunoreactive neurone (TH-IR) number in the substantia nigra pars compacta and ventral tegmental area of castrated (Cast) compared to sham cast male mice after 1 month of castration during adulthood. Data are represented as the mean ± SEM (n = 5 per group). *Statistically significantly different from sham cast, P < 0.01.
Testosterone suppresses TH-IR cell number in SNpc
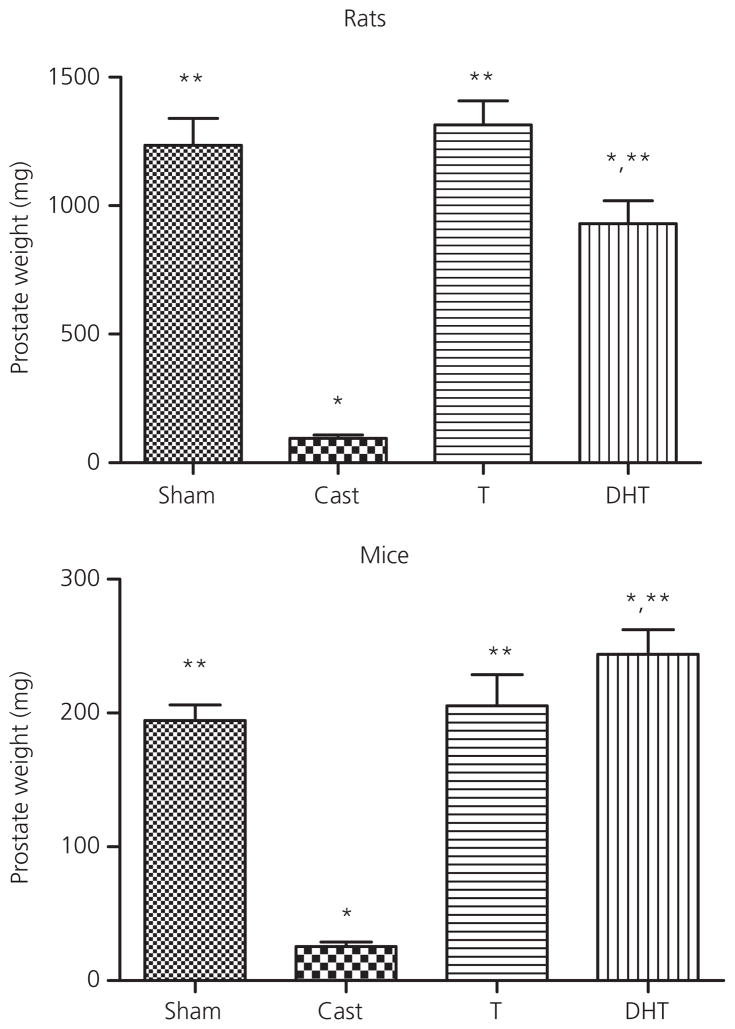
All hormone replacements were confirmed with the collection of ventral prostate weights (Fig. 5). For rats, ANOVA showed a significant effect of treatment (F3,25 = 33.4, P < 0.0001). Post-hoc analysis revealed that castration resulted in a significant reduction in prostate weight, whereas replacement with testosterone restored prostate weights to that of the sham controls. Prostate weights from DHT-treated rats were significantly higher than castrated animals but lower than sham controls. In mice, ANOVA indicated a significant effect of treatment (F3,28 = 38.8, P < 0.0001). Post-hoc tests revealed that castration decreased prostate weight relative to the sham controls and that replacement with testosterone or DHT resulted in prostate weights greater than the castrated animals. DHT replacement also yielded prostate weights that were greater than those of the sham animals.
Fig. 5.
Effect of testosterone (T) and dihydrotestosterone (DHT) on prostate weight in male rats and mice compared to vehicle-treated sham castrated (Sham) and castrated (Cast) animals. Animals were castrated or sham castrated at postnatal day (PN) 60 and treated with vehicle or testosterone or DHT for 1 month post-surgery. Data are represented as the mean ± SEM (n = 6–7 per group for rat and n = 5–8 per group for mice). *Statistically different from sham castrated; **statistically different from castrated (P < 0.0001).
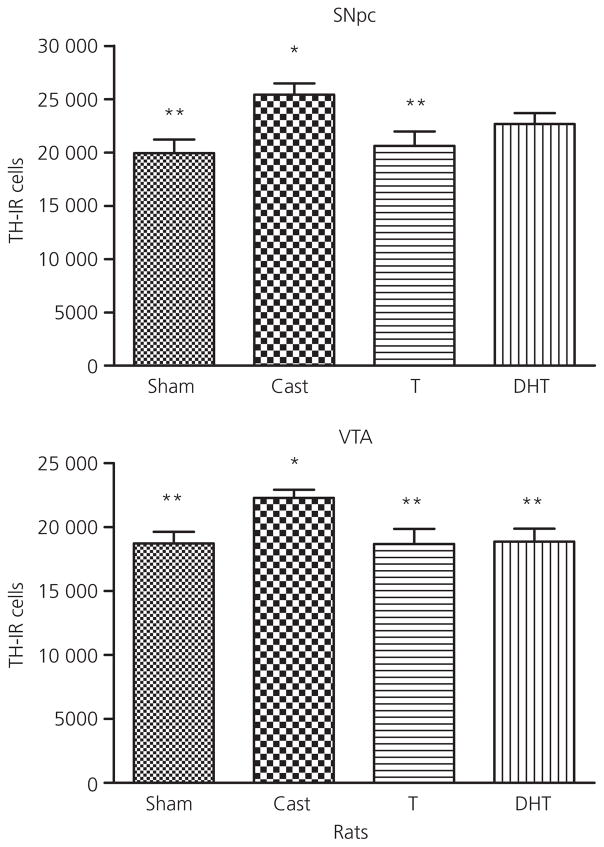
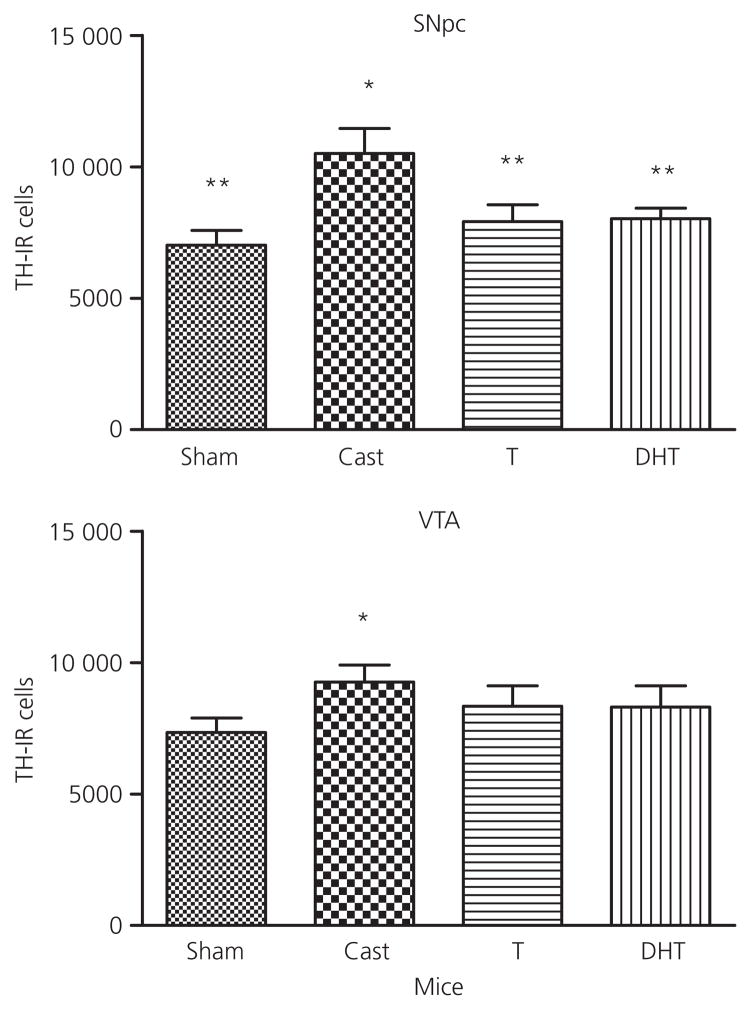
TH-IR cell number was increased after castration relative to sham controls in the rat SNpc and VTA (Fig. 6). The global ANOVA indicated a significant effect of treatment (F3,25 = 4.1, P < 0.02) in the SNpc, whereas the global analysis of VTA was not significant (F3,25 = 2.7, P < 0.08). Post-hoc tests of the planned comparisons revealed that castration resulted in an increase in TH-IR cell number in both regions relative to the sham controls. This increase was reversed by testosterone in both areas. DHT treated-animals were intermediate and were not significantly different from castrated animals in SNpc. In mice, treatment affected TH-IR cell number in the SNpc (F3,28 = 4.6, P < 0.011) (Fig. 7). Castration increased cell number relative to sham, and testosterone and DHT both significantly decreased cell number relative to castration. The global ANOVA for treatment in the VTA was not significant but the planned comparisons showed that castration increased cell number. Neither testosterone nor DHT significantly decreased cell number in the mouse VTA compared to castrated animals.
Fig. 6.
Effect of testosterone (T) and dihydrotestosterone (DHT) on tyrosine hydroxylase-immunoreactive neurone number in the substantia nigra pars compacta (SNpc) and ventral tegmental area (VTA) of male rats compared to vehicle-treated sham castrated (Sham) and castrated animals (Cast). Animals were castrated or sham castrated at postnatal day (PN) 60 and treated with vehicle or testosterone or DHT for 1 month post-surgery. Data are expressed as the mean ± SEM (n = 5–7 per group). *Statistically different from sham castrated; **statistically different from castrated, P < 0.05.
Fig. 7.
Effect of testosterone (T) and dihydrotestosterone (DHT) on tyrosine hydroxylase-immunoreactive neurone number in the substantia nigra pars compacta (SNpc) and ventral tegmental area (VTA) of male mice compared to vehicle-treated sham castrated (Sham) and castrated animals (Cast). Animals were castrated or sham castrated at postnatal day (PN) 60 and treated with vehicle or testosterone or DHT for 1 month post-surgery. Data are expressed as the mean ± SEM (n = 5–8 per group). *Statistically different from sham castrated; **statistically different from castrated, P < 0.05.
Testosterone effects on TH-IN neurone number
The number of TH-IN neurones was estimated in control and hormone-replaced animals to determine whether the increase in TH-IR cell bodies in the SNpc and VTA after castration was a result of increased TH expression. In rat, no differences between treatment groups were observed in TH-IN cell number in the SNpc and VTA (Table 1). TH-IR and total neurone number in the SNpc in castrated animals differed from sham (F3,24 = 4.1, P < 0.02) and (F3,24 = 4.5, P < 0.01), respectively, but TH-IN cells in castrated or testosterone-treated rats did not differ from shams. For total neurone number, both sham and testosterone differed from castrated in a post-hoc test of planned comparisons. In the VTA, the global ANOVA was not significant but TH-IR neurone number in castrated animals differed from sham by post-hoc test of planned comparisons, and both sham-surgery and testosterone-treated animals differed from castrated animals. There were no differences between treatment groups of TH-IN cells observed in the VTA. Similar results were obtained with mice (Table 2). TH-IR and total neurone number in castrated animals differed from sham (F3,27 = 4.6, P < 0.01) and (F3,27 = 5.7, P < 0.004), respectively, for the ANOVA by treatment. Sham, testosterone-treated and DHT-treated differed significantly from castrated in post-hoc tests of planned comparisons. TH-IN cells in castrated, testosterone-treated and DHT-treated mice did not differ from shams. In the VTA, the global ANOVA was not significant but TH-IR and total neurone number in castrated animals differed from sham by post-hoc test of planned contrasts. Although the global ANOVA for TH-IN cell in the VTA was not significant, the post-hoc test of planned contrasts indicated a significant difference between testosterone-treated animals and castrated animals, although sham animals did not differ from castrated animals.
Table 1.
Estimated Total Number of Tyrosine Hydroxylase-Immunoreactive (TH-IR), TH-Immunonegative (-IN) and Total Neurones in the Substantia Nigra Pars Compacta (SNpc) and Ventral Tegmental Area (VTA) of Intact, Castrated and Castrated, Hormone-Replaced Male Rats.
| Treatment | TH-IR cells | TH-IN cells | Total cells |
|---|---|---|---|
| SNpc | |||
| Sham castrated | 19 944 ± 1270** | 3 517 ± 802 | 22 875 ± 1031** |
| Castrated | 25 415 ± 1076* | 2 811 ± 303 | 28 227 ± 1 176* |
| Testosterone | 20 619 ± 1340** | 4 383 ± 997 | 25 002 ± 979** |
| DHT | 22 654 ± 1034 | 2 629 ± 374 | 25 284 ± 883 |
| VTA | |||
| Sham castrated | 18 725 ± 914** | 2 372 ± 653 | 20 702 ± 930 |
| Castrated | 22 281 ± 636* | 1 721 ± 375 | 20 289 ± 3958 |
| Testosterone | 18 761 ± 1191** | 2 124 ± 469 | 20 795 ± 972 |
| DHT | 18 958 ± 939** | 1 676 ± 321 | 20 635 ± 892 |
TH-IR and TH-IN cell number was determined in male hormone replacement groups. Results are expressed as the mean ± SEM, n = 5–7.
P < 0.05 or better relative to sham-castrated.
P < 0.05 or better relative to castrated.
DHT, dihydrotestosterone.
Table 2.
Estimated Total Number of Tyrosine Hydroxylase-Immunoreactive (TH-IR), TH-Immunonegative (TH-IN) and Total Neurones in the Substantia Nigra Pars Compacta (SNpc) and Ventral Tegmental Area (VTA) of Intact, Castrated and Castrated, Hormone-Replaced Male Mice.
| Treatment | TH-IR cells | TH-IN cells | Total cells |
|---|---|---|---|
| SNpc | |||
| Sham castrated | 7 022 ± 564** | 1 089 ± 288 | 8 111 ± 540** |
| Castrated | 10 508 ± 947* | 1 403 ± 191 | 11 911 ± 1015* |
| Testosterone | 7 923 ± 639** | 910 ± 176 | 8 478 ± 623** |
| DHT | 8 030 ± 400** | 1 116 ± 370 | 9 146 ± 511** |
| VTA | |||
| Sham castrated | 7 348 ± 549 | 394 ± 69 | 7 742 ± 505 |
| Castrated | 9 246 ± 641* | 671 ± 107 | 9 917 ± 639* |
| Testosterone | 8 345 ± 780 | 312 ± 61** | 8 657 ± 777 |
| DHT | 8 297 ± 790 | 580 ± 236 | 8 877 ± 737 |
TH-IR and TH-IN cell number was determined in male hormone replacement groups. Results are expressed as the mean ± SEM, n = 5–8.
P < 0.05 relative to sham-castrated.
P < 0.05 relative to castrated.
DHT, dihydrotestosterone.
Androgen does not affect cell size
Castration and/or hormone replacement could theoretically influence cell size so much that TH-IR cells no longer met the size criteria used to positively assign a cell as dopaminergic. To verify that this was not the case, we measured cell diameter in ten cells at the same rostral/caudal and medial/lateral location to obtain an average cell size per animal, and computed means for five rats from each treatment group and for three mice from each treatment group. Preliminary analysis indicated that cell size did not vary with species, and so all the data were analysed by three-way ANOVA (species × treatment × region) with region as a repeated variable. These data are shown in Table 3. ANOVA indicated a significant effect of region (F1,60 = 11.68, P < 0.002), with cells in the SNpc being larger than those in the VTA. Cell size did not vary significantly by species or surgical/endocrine state. All of the cell diameters were above the size threshold for identification as dopaminergic (10 μm).
Table 3.
Estimated Cell Diameter of Tyrosine Hydroxylase-Immunoreactive (TH-IR) Cells in the Substantia Nigra Pars Compacta (SNpc) and Ventral Tegmental Area (VTA) of Rat and Mice that were Castrated and Hormone-Replaced.
| Treatment | Rat |
Mouse |
||
|---|---|---|---|---|
| SNpc | VTA | SNpc | VTA | |
| Sham castrated | 23.2 ± 1.3 | 21.4 ± 0.8 | 22.1 ± 1.0 | 20.8 ± 1.2 |
| Castrated | 23.5 ± 1.9 | 20.2 ± 0.6 | 22.1 ± 1.0 | 21.4 ± 0.8 |
| Testosterone | 24.5 ± 0.8 | 22.2 ± 0.9 | 22.1 ± 1.2 | 21.0 ± 1.7 |
| DHT | 24.2 ± 2.0 | 19.4 ± 0.6 | 23.1 ± 2.1 | 20.0 ± 1.4 |
Data are expressed as the mean ± SEM, n = 5 per group for rat treatment groups, and 3 per group for mouse treatment groups. DHT, dihydrotestosterone.
Discussion
The present study showed that castration in adulthood increases TH-IR cell number in the SNpc and VTA of both rats and mice relative to the intact controls. In these same areas, testosterone replacement reduced TH-IR neurone number to that of sham castrated controls. Findings with DHT were intermediate because TH-IR neurone numbers in DHT-treated animals were equal to testosterone-treated animals but were not statistically different from castrated animals. These findings show that testosterone has a tonic, ongoing role in regulating midbrain TH-IR neurone survival in adult male rodents and suggest that androgen receptors could contribute to this effect on cell number.
These findings contrast with a recent report that castration does not affect dopaminergic neurone number in the SNpc and VTA (33), which employed a different duration of hormone deprivation and a different method for estimating neurone number. However, they are consistent with previous studies showing that castration in rats or mice results in increased dopamine release relative to gonadally intact males (13, 14, 34). Furthermore, these findings are consistent with reports that testosterone either fails to protect from or worsens neurotoxin-induced damage to dopaminergic neurones. In mice, testosterone does not protect against depletion of striatal dopaminergic concentration after MPTP lesion (23). In rats receiving unilateral 6-OHDA lesions, castration was shown to prevent the loss of striatal dopamine (DA) content, whereas testosterone replacement made it worse (21, 22), suggesting that androgens do not play a tropic or protective role in the nigrostriatal pathway. The weight of available evidence indicates that testicular hormones may tonically suppress dopaminergic neurone number, but certainly are not neuroprotective for dopaminergic neurones in the midbrain.
The suppressive effects of testosterone on TH-IR cell number may explain in part why male rats exhibit less psychostimulant-stimulated behaviour and dopamine release than females. We have shown that males exhibit less cocaine-induced locomotion and less electrically stimulated dopamine release (9, 11, 35, 36). Several other studies have also reported that testosterone replacement decreases cocaine and amphetamine-stimulated behaviour relative to castrated animals (12, 16, 37), although these differences are partly the result of differences in amphetamine metabolism (38). One study has shown that testosterone can decrease dopaminergic function: in castrated mice, potassium-stimulated striatal dopamine output was greater than in testosterone-replaced mice (34). However, there are few studies comparing dopaminergic neurone function in intact and castrated adult males, so the impact of cell survival on terminal function remains to be established. Such studies are ongoing in our laboratory.
The effective reduction in dopaminergic cell number by testosterone in both rats and mice suggests that gonadal steroid hormone receptors in part mediate these effects. The androgen receptor (AR) is one candidate to mediate effects of testosterone on dopaminergic neurone number. An increasing weight of evidence supports an important role for AR, especially in organisational effects of testosterone in the brain (39). Studies have shown that a significant subset of TH-IR cells in the SNpc and VTA expresses AR (25–27). AR is particularly common in TH-IR cells projecting to cortical regions in male rats, with estimates that approximately 25% of the dopaminergic neurones projecting to the frontal cortex express AR (26, 27). AR is also expressed in at least 10–12% of neurones in the VTA that project to the nucleus accumbens (18). However, testosterone can also act via aromatisation to oestradiol, and both oestrogen receptor (ER) α and ERβ are expressed in subsets of cells in the SNpc and VTA, although fewer TH-IR cells express ER than AR (18, 26, 40). Results with DHT, the non-aromatisable testosterone derivative, were mildly suggestive that AR contributes to this effect. However, the present study is not definitive.
The effects of oestradiol aromatised from testosterone must be considered, as they mediate many effects of testosterone in the mammalian brain (41). Although a role for aromatase in sexual differentiation of catecholamine neurones is well described in quail (42), there has been very little study of aromatase in rodent midbrain dopamine neurones. In addition, a recent study suggests that DHT is also metabolised to a compound, 5α-androstan-3β, 17β-diol (3β-diol), which has actions via ERβ. Therefore, effects of either testosterone or DHT could reflect actions on an oestrogen receptor (43). Three findings suggest that oestradiol does not mediate the suppressive effects of testosterone or DHT on dopaminergic neurone number observed in the present study. First, a companion study in this issue (44) shows that, in females, oestradiol increases rather than decreases cell number in this midbrain area. Second, a recent study in mice showed that oestradiol similarly increased dopaminergic neurone number in the SNpc of male mice (45). Finally, another study of dopaminergic neurone function reported that aromatase knockout mice had lower dopaminergic neurone number than wild-type (46). Even more intriguing, the latter study reported that these animals had an increase in circulating testosterone. The opposite finding would be expected if testosterone treatment was decreasing neurone number in the present study after aromatisation to oestradiol. In sum, although the findings with DHT were somewhat indeterminate, the weight of evidence suggests that aromatisation of testosterone to oestradiol does not suppress DA neurone number.
The global increase in DA neurone number in both SNpc and VTA after castration was unexpected because AR expression is wide-spread in the VTA but much sparser in the VTA (17, 26). This reported distribution of AR would have predicted that greater effects might be expected in the VTA than in the SNpc. However, global effects of androgen deprivation also occur in the locus coeruleus, where TH-IR cell number is increased in male wild-type mice castrated during adolescence relative to intact animals (47). One challenge to interpreting the latter study is the effects of androgen on TH expression, which was enhanced in noradrenergic neurones in the locus coeruleus.
The finding that the number of TH-IN cells did not change significantly suggests that the increased dopaminergic neurone number observed after castration in the present study does not reflect a gain in TH expression, which would increase the number of TH-IR cells with a corresponding decrease in TH-IN cells. Although, in rats, the number of TH-IN cells fell slightly (by approximately 1000 cells), it could not account for the much larger increase in TH-IR cells (more than 5000 cells). In mice, a similar trend was noted, with a slight decrease in TH-IN cells that was not proportionate to the rise in TH-IR. The lack of effect on TH-IN cells distinguishes the SNpc and VTA from the noradrenergic neurones in the locus coeruleus in which testosterone may influence TH expression through actions on ERβ (43). Androgen effects on TH expression are probably highly dependent on cell context because another study which has evaluated testosterone effects on transcriptional control of TH showed that testosterone increased TH expression in SK-N-BE(2)C or MN9D cells that were transfected with AR (48). If androgen does increase TH expression, then a decrease rather than an increase after castration would be predicted. The present results suggest that testosterone aromatisation to oestradiol would not mediate decreases in TH expression because oestradiol increases TH in those neurones in which it acts, and effects in the SNpc have not been reported (48–51).
One intriguing explanation for the present results could be that castration increased the production of new TH-IR cells, either by enhancing maturation from a precursor or through de novo neurogenesis. It is well established that neurogenesis occurs in the adult hippocampus (52, 53). Studies have shown that hippocampal neurogenesis can be influenced by gonadal hormones (54, 55). However, a low rate of neurogenesis has been shown to occur in the adult rodent midbrain, with very few if any of these newly-formed cells adopting a dopaminergic phenotype (56–58). Furthermore, the cell diameter of TH-IR cells did not change at all after castration, which might have been expected if newly-differentiated cells were contributing to the population. However, the estimates of cell size were underpowered in comparison to estimates of cell number, and it is possible that a modest change in cell size was not detected. In addition, a recent study showing that oestradiol increased dopaminergic cell number in SNpc of adult mice supports the possibility (45). Although dopaminergic stimulation of adult neurogenesis in the subventricular zone has been widely documented, the formation of new DA neurones is controversial at best (59, 60).
Androgen could also decrease TH neurone survival or change phenotypic expression. The data reported in the present study support the former conclusion, although findings with TH-IN cells are somewhat ambiguous. Furthermore, the ability of castration to increase apparent neurone number in just 1 month, in males that have experienced adult levels of androgen for many weeks, argues against this possibility. Given the extremely slow rate of cell replacement in the SNpc that was noted above, the slowing of neurone elimination would not likely result in such an abrupt increase in cell number in a month. An effect on dopaminergic phenotype may be a more likely explanation because phenotypic expression can change quickly. Sex-determining factors could also contribute to the determination of dopaminergic phenotype. Sry, the testis-determining gene, may be important in regulating expression of TH, one phenotypic protein, independent of androgen. The present results suggest that Sry and androgen could function in opposition to maintain TH expression and/or neurone number. Neurones with an inducible dopaminergic phenotype have been described in the striatum after dopamine denervation (61). Evaluation of expression of other phenotypic markers of dopaminergic neurones including Nurr-1, Lmx1b or might be useful to resolve this question.
In summary, the present study shows that testicular hormones contribute to regulation of midbrain DA neurone number, either through regulation of cell survival or through the expression of dopaminergic phenotype. These changes occur uniformly across both the SNpc and VTA rather than being restricted to the small population of DA neurones that express AR or ER. Future studies will be necessary to elucidate the specific gonadal steroid hormone receptor and mechanism by which this occurs.
Acknowledgments
We would like to thank Dr Dona Chikaraishi for assistance with these experiments. This research was supported by grant DA09079.
References
- 1.McGrath JJ. Variations in the incidence of schizophrenia: data versus dogma. Schizophr Bull. 2006;32:195–197. doi: 10.1093/schbul/sbi052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the US population. Drug Alcohol Depend. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- 3.Baldereschi M, Carlo AD, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, Amaducci L, Inzitari D. Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. Neurology. 2000;55:1358–1363. doi: 10.1212/wnl.55.9.1358. [DOI] [PubMed] [Google Scholar]
- 4.Chen K, Kandel D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend. 2002;68:65–85. doi: 10.1016/s0376-8716(02)00086-8. [DOI] [PubMed] [Google Scholar]
- 5.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyons KE, Hubble JP, Troster AI, Pahwa R, Koller WC. Gender differences in Parkinson’s disease. Clin Neuropharmacol. 1998;21:118–121. [PubMed] [Google Scholar]
- 7.Wooten GF, Cabassa J, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry. 2004;75:637–639. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman GE, Merchenthaler I, Zup SL. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine. 2006;29:217–231. doi: 10.1385/ENDO:29:2:217. [DOI] [PubMed] [Google Scholar]
- 9.Walker QD, Cabassa J, Kaplan KA, Li S, Haroon J, Spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychpharmacology. 2001;25:118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 10.Festa D, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast scan cyclic voltammetry. Neuroscience. 2000;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- 12.Beatty WW, Dodge AM, Traylor KL. Stereotyped behavior eleicited by amphetamine in the rat: influences of the testes. Pharmacol Biochem Behav. 1982;16:565–568. doi: 10.1016/0091-3057(82)90416-6. [DOI] [PubMed] [Google Scholar]
- 13.Dluzen DE, Ramirez VD. Effects of orchidectomy on nigro-striatal dopaminergic function: behavioral and physiological evidence. J Neuroendocrinol. 1989;1:285–290. doi: 10.1111/j.1365-2826.1989.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 14.Becker JB, Ramirez VD. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981;204:361–372. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- 15.Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- 16.Savageau MM, Beatty WW. Gonadectomy and sex differences in the behavioral repsonses to amphetamine and apomorphine of rats. Pharmacol Biochem Behav. 1981;14:17–21. doi: 10.1016/0091-3057(81)90097-6. [DOI] [PubMed] [Google Scholar]
- 17.Kritzer MF. Effects of acute and chronic gonadectomy on the catecholamine innervation of the cerebral cortex in adult male rats: insensitivity of axons immunoreactive for dopamine-beta-hydroxylase to gonadal steroids, and differential sensitivity of axons immunoreactive for tyrosine hydroxylase to ovarian and testicular hormones. J Comp Neurol. 2000;427:617–633. [PubMed] [Google Scholar]
- 18.Creutz LM, Kritzer MF. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J Comp Neurol. 2004;476:348–362. doi: 10.1002/cne.20229. [DOI] [PubMed] [Google Scholar]
- 19.Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Tamas A, Lubics A, Szalontay L, Lengvari I, Reglodi D. Age and gender differences in behavioral and morphological outcome after 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Behav Brain Res. 2005;158:221–229. doi: 10.1016/j.bbr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Gillies GE, Murray HE, Dexter D, McArthur S. Sex dimorphisms in the neuroprotective effects of estrogen in an animal Model of Parkinson’s disease. Pharmacol Biochem Behav. 2004;78:513–522. doi: 10.1016/j.pbb.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Murray HE, Pillai AV, McArthur SR, Razni N, Datla DP, Dexter DT, Gillies GE. Dose-and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: differential actions of estrogen in males and females. Neuroscience. 2003;116:213–222. doi: 10.1016/s0306-4522(02)00578-x. [DOI] [PubMed] [Google Scholar]
- 23.Dluzen DE. Effects of testosterone upon MPTP-induced neurotoxicity of the nigrostriatal dopaminergic system. Brain Res. 1996;715:13–118. doi: 10.1016/0006-8993(95)01566-3. [DOI] [PubMed] [Google Scholar]
- 24.Ekue A, Boulanger JF, Morissette M, Di Paolo T. Lack of effect of testosterone and dihydrotestosterone compared to 17b-oestadiol in 1-methyl-4-phenyl-1,2,3,6, tetrahydropyridine-mice. J Neuroendocrinol. 2002;14:731–736. doi: 10.1046/j.1365-2826.2002.00833.x. [DOI] [PubMed] [Google Scholar]
- 25.Kritzer MF. Selective colocalization of immunoreactivity for intracellular gonadal hormone receptors and tyrosine hydroxylase in the ventral tegmental area, substantia nigra, and retrorubral fields in the rat. J Comp Neurol. 1997;379:247–260. doi: 10.1002/(sici)1096-9861(19970310)379:2<247::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 2008;28:9525–9535. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heritage AS, Stumpf WE, Sar M, Grant LD. (3-H)-dihydrotestosterone in catecholamine neurons of rat brain stem: combined localization by autoradiography and formaldehyde-induced fluorescence. J Comp Neurol. 1981;200:289–307. doi: 10.1002/cne.902000208. [DOI] [PubMed] [Google Scholar]
- 28.Norton AJ, Jordan S, Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994;173:371–379. doi: 10.1002/path.1711730413. [DOI] [PubMed] [Google Scholar]
- 29.Tischler AS. Triple immunohistochemical staining for bromodeoxyuridine and catecholamine biosynthetic enzymes using microwave antigen retrieval. J Histochem Cytochem. 1995;43:1–4. doi: 10.1177/43.1.7822757. [DOI] [PubMed] [Google Scholar]
- 30.Shi S, Imam SA, Young L, Cote RJ, Taylor CR. Antigen retrieval immunohistochemistry under the influence of pH using monoclonal antibodies. J Histochem Cytochem. 1995;43:193–201. doi: 10.1177/43.2.7822775. [DOI] [PubMed] [Google Scholar]
- 31.Darmopil S, Muneton-Gomez VC, de Ceballos ML, Bernson M, Moratalla R. Tyrosine hydroxylase cells appearing in the mouse striatum after dopamine denervation are likely to be projection neurones regulated by L-DOPA. Eur J Neurosci. 2008;27:580–592. doi: 10.1111/j.1460-9568.2008.06040.x. [DOI] [PubMed] [Google Scholar]
- 32.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 33.McArthur S, McHale E, Gillies GE. Striatal susceptibility to a dopaminergic neurotoxin is independent of sex hormone effects on cell survival and DAT expression but is exacerbated by central aromatase inhibition. J Neurochem. 2007;100:678–692. doi: 10.1111/j.1471-4159.2006.04226.x. [DOI] [PubMed] [Google Scholar]
- 34.Shemisa K, Kunnathur V, Liu B, Salvaterra TJ, Dluzen DE. Testosterone modulation of striatal dopamine output in orchidectomized mice. Synapse. 2006;60:347–353. doi: 10.1002/syn.20309. [DOI] [PubMed] [Google Scholar]
- 35.Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008;89:314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychpharmacology. 2006;31:1193–1202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- 37.Menniti FS, Baum MJ. Differential effects of estrogen and androgen on locomotor activity induced in castrated male rats by amphetamine, a novel environment, or apomorphine. Brain Res. 1981;216:89–107. doi: 10.1016/0006-8993(81)91280-4. [DOI] [PubMed] [Google Scholar]
- 38.Camp DM, Becker JB, Robinson TE. Sex differences in the effects of gonadectomy on amphetamine-induced rotational behavior in rats. Behav Neural Biol. 1986;46:491–495. doi: 10.1016/s0163-1047(86)90527-3. [DOI] [PubMed] [Google Scholar]
- 39.Zuloaga DG, et al. The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Horm Behav. 2008;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creutz LM, Kritzer MF. Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. J Comp Neurol. 2002;446:288–300. doi: 10.1002/cne.10207. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Segura LM. Aromatase in the brain: not just for reproduction anymore. J Neuroendocrinol. 2008;20:705–712. doi: 10.1111/j.1365-2826.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 42.Balthazart J, Baillien M, Ball GF. Interactions between aromatase (estrogen synthase) and dopamine in the control of male sexual behavior in quail. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:37–55. doi: 10.1016/s1096-4959(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 43.Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson ML, Ho CC, Day AE, Walker QD, Franics R, Kuhn CM. Oestrogen receptors enhance dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010;22:226–237. doi: 10.1111/j.1365-2826.2010.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tripanichkul W, Jaroensuppaperch EO, Finkelstein DI. Estrogen enhances the number of nigral dopaminergic neurons of adult male mice without affecting nigral neuroglial number and morphology. Neurosci Lett. 2008;435:210–214. doi: 10.1016/j.neulet.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 46.Morale MC, L’Episcopo F, Tiroli C, Giaquinta G, Caniglia S, Testa N, Arcieri P, Serra PA, Lupo G, Alberghina M, Harada N, Honda S, Panzica GC, Marchetti B. Loss of aromatase cytochrome P450 function as a risk factor for Parkinson’s disease? Brain Res Rev. 2008;57:431–443. doi: 10.1016/j.brainresrev.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Pendergast JS, Tuesta LM, Bethea JR. Oestrogen receptor β contributes to the transient sex difference in tyrosine hydorxylase expression in the mouse locus coeruleus. J Neuroendocrinol. 2008;20:1155–1164. doi: 10.1111/j.1365-2826.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 48.Jeong H, Kim MS, Kwon J, Kim KS, Seol W. Regulation of the transcriptional activity of the tyrosine hydroxylase gene by androgen receptor. Neurosci Lett. 2006;396:57–61. doi: 10.1016/j.neulet.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 49.Kohama SG, Bethea CL. Steroid regulation of tyrosine hydroxylase messenger ribonucleic acid in dopaminergic subpopulations of monkey hypothalamus. Endocrinology. 1995;136:1790–1800. doi: 10.1210/endo.136.4.7895692. [DOI] [PubMed] [Google Scholar]
- 50.Sabban EL, Maharjan S, Nostramo R, Serova LI. Divergent effects of estradiol on gene expression of catecholamine biosynthetic enzymes. Physiol Behav. 2010;99:163–168. doi: 10.1016/j.physbeh.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Serova LI, Maharjan S, Huang A, Sun D, Kaley G, Sabban EL. Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in brain catecholaminergic regions varies with mode of administration. Brain Res. 2004;2:1–8. doi: 10.1016/j.brainres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to abberant network reorganization in the adult rat hipocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brannvall K, Bogdanovic N, Korhonen L, Lindholm D. 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. Eur J Neurosci. 2005;21:871–878. doi: 10.1111/j.1460-9568.2005.03942.x. [DOI] [PubMed] [Google Scholar]
- 54.Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- 55.Galea LAM. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–343. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Frielingsdorf H, Schwarz K, Brundin P, Mohapel P. No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc Natl Acad Sci USA. 2004;101:10177–10182. doi: 10.1073/pnas.0401229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lie DC, Dziewczapolsk G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisen J, Janson AM. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci USA. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borta A, Hoglinger GU. Dopamine and adult neurogenesis. J Neurochem. 2007;100:587–595. doi: 10.1111/j.1471-4159.2006.04241.x. [DOI] [PubMed] [Google Scholar]
- 60.Dewing P, Chiang CW, Sinchak K, Sim H, Fernagut PO, Kelly S, Chesselet MF, Micevych PE, Albrecht KH, Harley VR, Vilain E. Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol. 2006;16:415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 61.Huot P, Parent A. Dopaminergic neurons intrinsic to the striatum. J Neurochem. 2007;101:1441–1447. doi: 10.1111/j.1471-4159.2006.04430.x. [DOI] [PubMed] [Google Scholar]