Abstract
Background
Alcohol abuse disorders emerge over time with repeated consumption of ethanol, but not all ethanol drinkers develop these disorders. There are pre-existing characteristics that indicate which drinkers are most likely to abuse alcohol. Adolescence, novelty seeking, and high stress reactivity are among the characteristics of the most vulnerable individuals. In addition, an individual’s response to his or her first exposure to the drug influences future consumption. We assessed an array of behavioral and hormonal characteristics in adolescent (28-day-old) male rats before exposure to ethanol, and then determined which rats were most prone to high levels of alcohol drinking.
Methods
The assessments consisted of measures of anxiety (elevated plus maze), response to novelty (open field locomotion, novel object exploration), and circulating corticosterone levels after mild restraint and after the elevated plus maze task. After this test battery, the rats were placed in lickometer cages nightly (5 pm to 9 am) for evaluation of fluid consumption. Rats were first habituated to the cages with water in the lickometer bottles, and then given 10% (v/v) ethanol for 3 nights as the only available fluid. After this forced ethanol exposure, the rats were allowed to choose between 8% ethanol and water for 10 consecutive nights. After 2 nights of abstinence, the rats were again placed in the lickometer cages and given a choice between 8% ethanol and water to assess ethanol consumption in response to alcohol deprivation, a measure of relapse-like behavior.
Results
Ethanol consumption on the third day of forced consumption was significantly correlated with ethanol consumption on days 8 to 10 of the choice phase, which in turn was significantly correlated to relapse-like consumption. Preference for ethanol was also significantly correlated with early consumption. Novel object exploration, open field activity, open arm time in the elevated plus maze, initial water consumption, and circulating corticosterone levels did not significantly predict deprivation-stimulated consumption.
Conclusions
These results suggest that consumption during early exposure to ethanol establishes a pattern leading to development of increased alcohol consumption and preference in adolescent male rats. In addition, they represent an animal model of the well-described observation that humans who consume large quantities of ethanol during early exposure are the most likely to repeat heave drinking behavior. Furthermore, early consumption is distinct from novelty seeking, anxiety, and stress hormone levels which are also thought to contribute to vulnerability to alcoholism.
Keywords: Alcohol, Drinking, Behavior, Anxiety, Postdeprivation Effect
Alcohol abuse disorders Result from the interaction between an individual’s genetic and environmental susceptibility and repeated intake of alcohol over time. No one can become an alcoholic without repeatedly consuming alcohol, but only a small percentage of all drinkers become alcoholics. In the United States, where alcohol is commonly available, only about 8% of the population can be classified as alcohol-abusing or dependent (NIAAA, 2001– 2002). Substantial research efforts are aimed at determining what makes some individuals susceptible to alcoholism, while others can consume as casual drinkers. Personality characteristics such as impulsivity and risk-taking influence initial drug taking (Clark et al., 2005; Cloninger et al., 1988; Kreek et al., 2005;Masse and Tremblay, 1997), while comorbid psychiatric disorders, genetics, and environmental factors such as stressors contribute to regular use and the transition to addiction (Kreek et al., 2005).
Stress and the hypothalamic pituitary adrenal axis response to it may also impact the development of dependence. Alcohol is often used to self-medicate symptoms of stress and anxiety (Kushner et al., 2000). Furthermore, an atypical response to stress may contribute to relapse once a person has become addicted (Brown et al., 1995; Kreek et al., 2005). In fact, stress is one of the most common explanations given for relapse among abstinent alcoholics (Brown et al., 1995). Studies in rodent models have shown that exposure to stressors can initiate relapse-like behaviors in alcohol-drinking rats (Vengeliene et al., 2003).
The quality of a person’s response to his or her early experiences with alcohol significantly influences subsequent drinking behavior. For example, alcoholism is rare among populations that have genetically low levels of aldehyde dehydrogenase activity due to genetic polymorphism that inactivates aldehyde dehydrogenase-2 (Goedde et al., 1982). This enzyme metabolizes acetaldehyde, a metabolite of alcohol that contributes to unpleasant side effects of ethanol consumption (Goedde et al., 1982). Reduced activity of this enzyme leads to acetaldehyde accumulation after alcohol consumption, leading to aversive effects (Goedde et al., 1983). In contrast, individuals who are relatively insensitive to ethanol and require more alcohol to become intoxicated are more likely to become alcohol dependent (Schuckit et al., 2006, 2004; Wilhelmsen et al., 2003). This predisposition is also genetically based (Duranceaux et al., 2006; Hinckers et al., 2006; Schuckit et al., 2005). Thus, genetic factors can either increase or decrease risk of alcoholism.
Age is another key factor influencing development of alcoholism. Younger users are more at risk than older users. According to one study, approximately 38% of people who begin use of any drug before age 15 develop dependence, whereas only 4% of those who initiate after age 25 do so (Robins and Przybeck, 1985). These statistics demonstrate a striking vulnerability in younger users, but also underscore the point that not all users become addicted. In fact, experimenting with alcohol is normal during this age range. The Monitoring the Future Study has revealed that approximately 73% of twelfth graders, 61% of tenth graders, and 40% of eighth graders have tried alcohol at least once. Fifty-six percent of twelfth graders have been drunk at least once. However, regular use of alcohol is less common. Less than 50% of twelfth graders claimed to have used alcohol in the past 30 days and less than 30% had participated in binge drinking in the past 30 days. The current study was undertaken in an effort to develop a model that will be useful to understand biological mechanisms differentiating casual alcohol users from those who escalate intake.
The adolescent rat provides a model of neurobehavioral development that is highly relevant for adolescent humans. Adolescent rats undergo a growth spurt, puberty, and social realignment similar to human teenagers (Spear, 2000b). Rats have proven to be a useful model for ethanol use during adolescence (Spear, 2000a). There is some evidence that adolescent rats consume more ethanol than adult rats (Brunell and Spear, 2005; Doremus et al., 2005; Vetter et al., 2007), but this is not evident in all strains (Bell et al., 2006; Siegmund et al., 2005).
Aversive responses to ethanol are clearly different between adolescent and adult rodents. Adolescent rats are less sensitive to hangover-induced anxiety (Doremus et al., 2003; Varlinskaya and Spear, 2004a) and also to the social inhibitory effects of high ethanol doses (Varlinskaya and Spear, 2004b). Adolescent rats are also less sensitive to the sedative effects of ethanol (Little et al., 1996;Moy et al., 1998; Silveri and Spear, 1998; White et al., 2002). These observations suggest that insensitivity to aversive effects may be one reason that some adolescents drink more often and in larger quantities than adults and are more likely to become alcohol dependent.
The purpose of the present study was to evaluate the range of alcohol consumption levels in an adolescent population, and to examine which individual differences might predict intake and relapse-like behavior. Rather than use a genetically-selected strain, we took advantage of the high degree of interindividual variability inherent in the outbred Sprague-Dawley strain. To model relapse-like behavior, we used the alcohol deprivation effect, which is a long-standing behavioral model of alcohol craving and relapse (Spanagel and Holter, 1999). In this model, rats consume ethanol by choice for an extended period of time, and then are deprived of it. When ethanol is again available after deprivation, rats consume significantly more than they did before deprivation (Sinclair and Senter, 1968). This strategy is thought to model the observation that human alcoholics who attempt to abstain from alcohol consume higher quantities upon relapse than they had previously consumed when drinking regularly (Rodd et al., 2004).
Outbred rats, like humans, exhibit a wide range of individual sensitivity to ethanol. We behaviorally phenotyped adolescent rats before exposure to ethanol and then used multivariate statistical methods to identify factors that predict postdeprivation drinking in adolescent rats. We hypothesized that stress hormone levels, anxiety, novelty seeking behaviors, and initial intake of ethanol would combine to predict relapse-like behavior. We found that early ethanol intake is predictive of relapse-like behavior, but that the other behavioral and hormonal markers we assessed did not predict increased postdeprivation consumption. This system provides an animal model in which we can examine biological mechanisms mediating consumption of ethanol during free-choice and postdeprivation periods.
MATERIALS AND METHODS
Animals
Forty-eight male CD (Sprague–Dawley) rats were purchased from Charles River Laboratories (Raleigh, NC) and delivered at 21 days of age (weighing approximately 100 g at the start of the study). Thirty-six rats were used in the initial characterization and 12 rats were used to confirm the findings. Rats were housed 2 to 3 per cage with food and water available ad libitum. Cages were maintained on a ventilated rack with a 12:12-hour light: dark cycle (lights on at 7 am).
Experimental Design
Screening began on Day 28 (Prescreen Day 1) with collection of blood to assess corticosterone levels during a mild restraint stress (see blood collection procedure). On Day 2, each rat was videotaped in the novel object task (see below). On Day 3, rats were tested in the locomotor box task as another measure of novelty seeking behavior, and then immediately placed on the elevated plus maze (EPM) to measure anxiety-like behavior. This was followed by a second collection of blood to evaluate corticosterone levels in response to exposure to the locomotor box and EPM.
At 35 days of age, rats were placed in lickometer cages equipped with bottles at each end. The computer program Quick-Lick Win (Davis & Henderson, v.1.0.0) detected and recorded the number of licks on each bottle. Rats were placed in cages between 4 and 5 pm each day and removed between 8 and 9 am the following day for 14 consecutive days. Water was always available in the home cage, and liquids were available in the lickometer cages as described below. Bottles were filled and weighed each night, and reweighed in the morning to determine the mass of each liquid consumed. Mass consumed was converted to volume and then g/kg based on the density of the fluids. On the first night, rats were placed in the cage to acclimate to the lickometers with water in both bottles. The following 3 nights (Forced Consumption Period), rats were exposed to a solution of 10% ethanol (v/v) in both bottles. We chose a relatively high concentration during this phase to be sure that the rats would overcome any neophobia for the taste of ethanol, and to acquaint them with its pharmacological effects if they drank even a small amount. For the next 10 nights (Days 1 to 10 of Choice Consumption Period), rats were in a 2-bottle choice paradigm in which they were exposed to a bottle of 8% ethanol (v/v) and a bottle of water. We switched to the lower concentration during this phase in an effort to increase the volume consumed and potentially increase interindividual variability during the choice phase. To avoid placement preference, bottle position was alternated in the lickometer cage each night. After 10 days of choice consumption, rats were deprived of ethanol for 2 nights, and then allowed to choose between 8% ethanol and water to assess alcohol deprivation-stimulated drinking. The light cycle during all measured consumption tests was the same as in the home cages (12:12, lights on at 7 am). Food was available ad libitum in both the home cages and lickometer cages.
Blood Collection From Saphenous Vein
Rats in their home cages were placed on a heating pad set at medium for 5 to 10 minutes to stimulate blood flow. Each rat was restrained in a Decapi-cone (Braintree Scientific, Braintree, MA). The hind leg was held above the joint for shaving which also served as a tourniquet to assist in blood collection. The area around the vein was coated with a thin layer of petroleum jelly. A 25-guage needle was used to puncture the vein and removed to allow blood flow outside the skin. Approximately 300 ul of blood was collected in EDTA-coated Sarstedt Multivette tubes. Following blood collection, direct pressure was applied to the vein for hemostasis (Adapted from Hem et al., 1998). During this procedure, the rat was restrained for a total of 2 to 3 minutes. Blood was placed on ice until centrifugation at 3,000 × g and the serum was frozen at −80°C for later analysis of corticosterone levels using an RIA kit according to kit directions (DPC Los Angeles, CA). Although this procedure may be stressful to the rats, preliminary studies showed that the experience did not affect subsequent behavior in the elevated plus maze (Schramm-Sapyta et al., 2006).
Novel Object Task
An object (aluminum foil ball, sea shell, Lego pyramid, or shiny pink holiday ornament) was placed in the rats’ cages 18 hours before the experiment was performed. One hour before the experiment, rats were moved to the experimental room for habituation (filtered cage lids were removed but wire racks were left in place). For the test, a video camera was used to record 2 cages simultaneously (separated by piece of black Plexiglas). An incandescent lamp provided illumination of 20 to 25 lux inside the cages. Each rat was briefly placed in a holding cage while the home cage was prepared with 1 novel object and the familiar object in view of the camera. The rat was then placed back in his home cage in front of the camera and activity was recorded for 5 minutes. While each rat was tested, his cagemates were left in the holding cage. After each individual’s test, all rats were returned to the home cage with the familiar object and allowed to re-acclimate for at least 15 minutes before the next cagemate was tested.
Video recordings were later scored for the time each rat spent exploring both the novel and familiar objects. Exploring was defined as chewing on, playing with, and sniffing the object. Time when the animal stood on an object as a means to smell something else or fell on it was not counted as object exploration. Most rats spent 0 to 2 seconds exploring the familiar object, and there was significant inter-individual variation (ranging from 3 to 150 seconds) in the time spent exploring the novel object. Preliminary experiments demonstrated that exploration of the novel object was maximal during the first 5 minutes, and that the time spent exploring the novel object was not a function of the particular object chosen.
Locomotor Activity
Rats were placed in locomotor boxes (Hamilton–Kinder, 40 × 40 × 40 cm) in the same room as the EPM for 5 minutes immediately before placement on EPM. Light in the chamber was provided by an incandescent torch lamp (7 lux). The floor of the box was covered with corn cob bedding. Infrared beams surrounding each box detected the animal’s position. Beam breakages were then converted into position and distance by Hamilton-Kinder software. The dependent measure of interest was total distance traveled (inches). Rats that traveled a greater distance were deemed to be more active in response to novelty.
Elevated Plus Maze
The EPM measures anxiety by comparing time spent in the open versus closed arms of the maze, and general activity by measuring transitions between arms (Pellow et al., 1985). The maze is made of sealed wood painted black, and arranged in a “+ ” shape that is elevated 90 cm above the floor. Two of the arms (north and south) are open and are 50 cm long and 10 cm wide. The other arms (east and west) are enclosed with walls that are 36 cm high on the 2 long sides. The areas surrounding the EPM were made visually neutral by hanging white curtains on all 4 sides. The room was dimly lit by an incandescent torch light so that the brightness on the open arms was approximately 7 lux, and in the closed arms was approximately 3.5 lux. Rats were placed in the center of the EPM and their behavior was recorded for 5 minutes using a low-light recording mode. Video recordings were later scored for the number of entries into and time spent in each arm. Percentage of time in the open arms is calculated as:
Measured Ethanol Drinking
Ethanol consumption was measured in standard rat housing cages (45 × 24 × 20 cm) with lick-blocks attached at each end (a front/-back arrangement). A bottle containing approximately 100 ml of water or ethanol, as described, was placed in each lick-block nightly. Lick-blocks were connected to a computer equipped with QuickLick-Win software which recorded licks on each bottle. Nightly lick patterns did not differ between water and ethanol-containing bottles (data not shown). Total amount consumed was measured by weighing the bottles before and after the consumption session and then calculating the difference.
Data Analysis
All statistical analyses were performed using JMP 7 statistical software (SAS Institute, Cary, NC). Distributions of the measures were tested for normality using the Shapiro–Wilk test. Non-normal distributions were transformed appropriately (see Table 2). Data were analyzed using multiple-linear regression and correlation.
Table 2.
Correlations (R values) Among Alcohol Consumption Measures
| Ethanol-only (forced) day 3 |
Choice drinking, days 8 to 10 (log transformed) |
Postdeprivation ethanol drinking (square root transformed) |
|
|---|---|---|---|
| Ethanol-only (forced) day 3 | 0.37*,** | 0.53* | |
| Choice drinking days 8 to 10 (log transformed) | 0.37*,** | 0.59*,** | |
| Preference, choice days 8 to 10 (log transformed) | 0.27** | 0.97*,** | 0.53*,** |
| Preference, postdeprivation (log transformed) | 0.51* (in confirmatory group, R = 0.56, p = 0.06) | 0.54*,** | 0.95*,** |
Significant correlation, p < 0.05, in initial characterization group, n = 36 rats.
Significant correlation, p < 0.05 in confirmatory group, n = 12.
RESULTS
Prescreen Phenotyping Measures
Values obtained during the prescreen phenotyping from the novel object task, circulating corticosterone collections, open field locomotion, and elevated plus maze task are listed in Table 1. All parameters measured in these rats fell within the range of expected values based on the literature and our prior experience.
Table 1.
Values Obtained in Prescreen Test Battery
| Measurement | Mean (n = 36) | SD |
|---|---|---|
| Time exploring novel object (seconds) | 39 | 38 |
| Time exploring familiar object (seconds) | 1.0 | 2.6 |
| Corticosterone, restrained in decapi-cone only (ng/ml) | 185 | 83 |
| Corticosterone post-EPM (ng/ml) | 302 | 95 |
| Locomotor distance, inches | 924 | 155 |
| EPM % open arm time | 24 | 12 |
| EPM open entries | 4.6 | 2.0 |
| In confirmatory group (n = 12): 4.5 | 2.3 |
Consumption During Ethanol-Only Phase
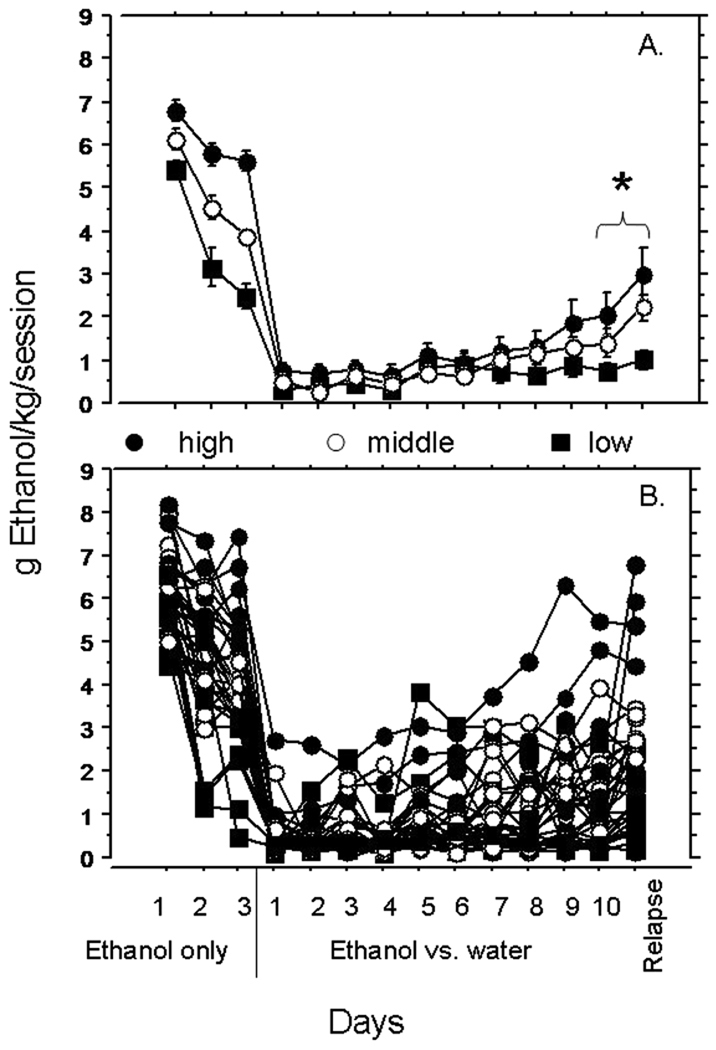
During the 3 days when ethanol was the only available fluid, the rats as a group exhibited a general decline in ethanol consumption [RMANOVA, F(2,70) = 49.48, p < 0.0001; see Fig. 1A]. There was a significant decrease in consumption from days 1 to 2 [F(1,35) = 39.47, p < 0.0001] and from day 2 to day 3 [F(1,35) = 6.81, p = 0.0132]. There was substantial inter-individual variation in consumption which increased across the 3 days (see Fig. 1B).
Fig. 1.
Time course of ethanol consumption. (A) Average consumption (g/kg) across the time course of the experiment. Error bars, standard error, *, significant difference between choice day 10 and relapse day, RMANOVA, p < 0.05. (B) Grams of ethanol consumed per kilogram by individual rats during 10% ethanol-only (forced) days 1 to 3, 8% ethanol versus water (choice) days 1 to 10, and relapse day. Closed circles, rats that were in the highest tertile of consumption on forced day 3, open circles, middle tertile, closed squares, lowest tertile.
Consumption During Ethanol Versus Water Choice Phase
During the choice consumption phase, rats initially avoided the ethanol-containing solution and consumed mostly water. Over the course of 10 days, however, the group exhibited an increase in ethanol consumption [RMANOVA, F(9,315) = 13.005, p < 0.0001; see Fig. 1]. Consumption on day 10 was significantly greater than consumption on day 1 of the choice phase [RMANOVA, F(1,35) = 19.567, p < 0.0001]. There was also an increase in inter-individual variation in consumption (see Fig. 1B). This result suggests that individual patterns of consumption emerged gradually over the 10 days of the 2-choice period. On average, the rats exhibited an increase in consumption following the deprivation period. Ethanol intake (g/kg) was significantly greater on the rebound day than on day 10 of the choice phase [RMANOVA, F(1, 35) = 8.677, p = 0.0057; Fig. 1A].
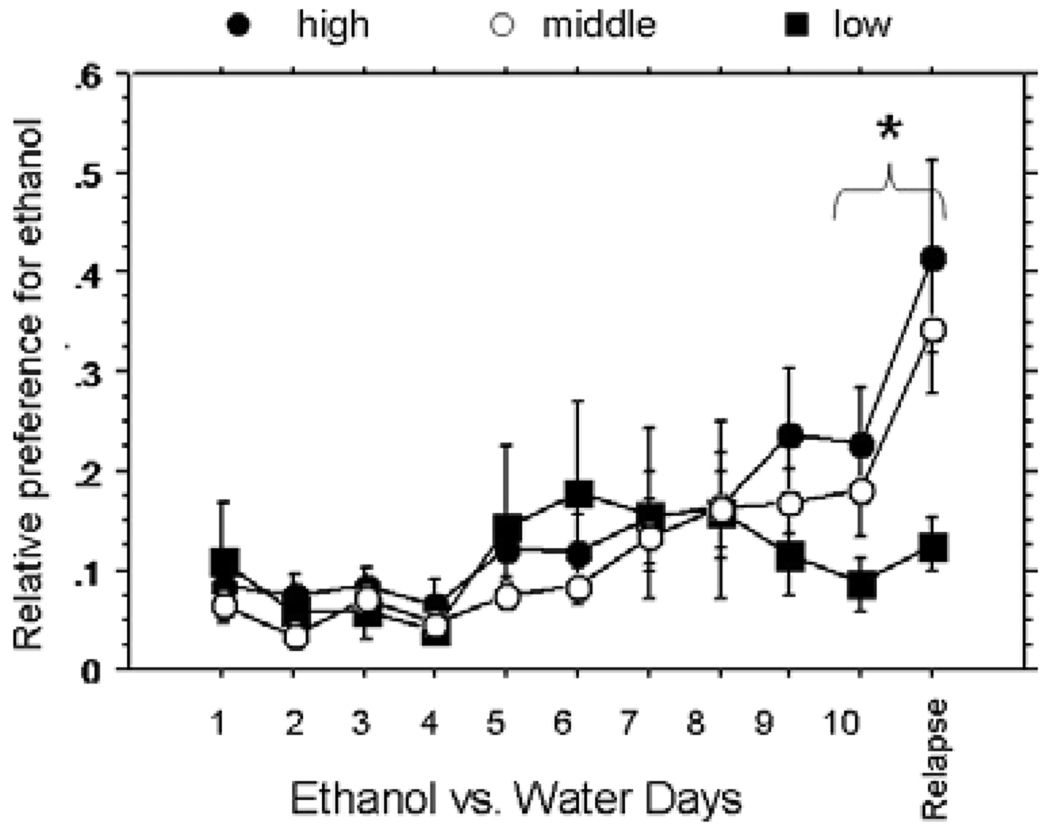
Preference for ethanol (percent of total fluid intake) also increased over the course of 10 days of choice consumption in a pattern similar to g/kg consumption [RMANOVA, F(9,315) = 7.156, p < 0.0001; Fig. 2]. There was a significant increase in preference for ethanol from choice day 1 to choice day 10 [F(1,35) = 7.417, p = 0.0100, RMANOVA], and from choice day 10 to the relapse day [F(1, 35) = 11.201, p=0.002, RMANOVA, Fig. 2]. Alcohol preference was significantly correlated with total alcohol (g/kg) intake on choice days 8 to 10 (R2 = 0.95 p < 0.0001) and on the relapse day (R2 = 0.90, p < 0.0001), but not on choice day 1, when little ethanol was consumed by all of the rats.
Fig. 2.
Time course of ethanol preference. Ethanol fraction of total fluid consumption during 8% ethanol versus water (choice) days 1 to 10, and relapse day. Closed circles, rats that were in the highest tertile of consumption on forced day 3, open circles, middle tertile, closed squares, lowest tertile. Error bars, standard error, *, significant difference between choice day 10 and relapse day, RMANOVA, p < 0.05.
Individual Differences in Consumption
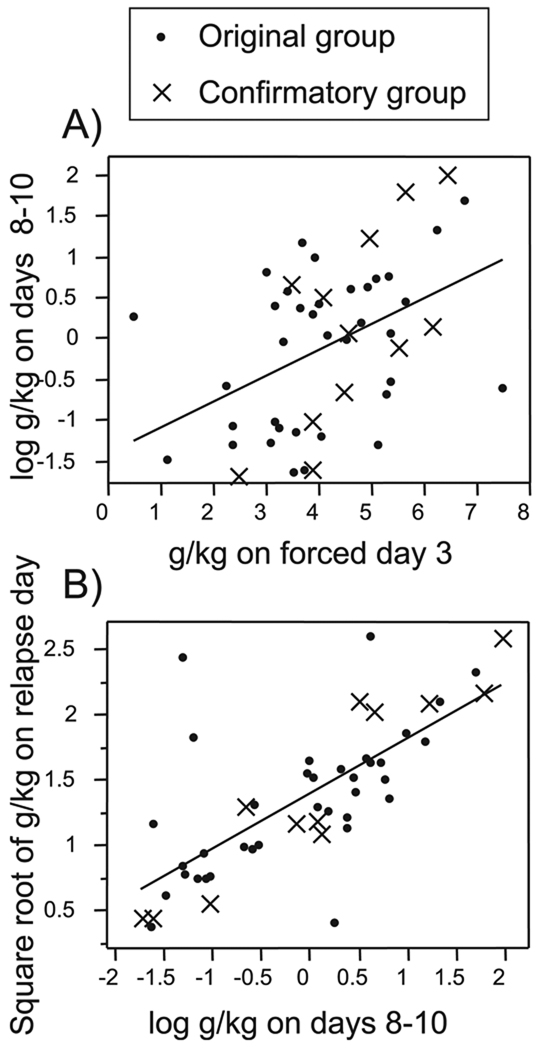
Individual variation in ethanol consumption patterns began to emerge during the forced consumption phase, and was maintained throughout the course of the experiment. As shown in Fig. 1, there is variance in gram EtOH consumed per kilogram during the 3 days of ethanol-only consumption. This variance largely disappears when water is offered as an option (choice days 1 to 2), but re-emerges over 10 days of choice consumption. Individual levels of ethanol consumption which were established by day 3 of the ethanol-only phase reemerged over the 10 days of the choice consumption period. There is a significant positive correlation between ethanol consumption on forced day 3 and ethanol consumption on choice days 8 to 10 (R2 = 0.13, p=0.029; Fig. 3A) as well as a significant correlation between forced day 3 and relapse day (R2 = 0.28, p = 0.0009; Table 2). However, the level of early consumption does not fully account for the variation in consumption in the later days measured. Consumption on forced day 3 accounts for only 13% of the variance (R2) in consumption on days 8 to 10, and 28% of the variance in consumption on the relapse day. Thus, other factors, as yet unidentified, determine the level of postdeprivation consumption.
Fig. 3.
Scatterplot showing the relationship between early and later ethanol consumption. (A) g/kg consumed on forced day 3 is significantly correlated with g/kg consumed on choice days 8 to 10. (B) g/kg consumed on choice days 8 to 10 is significantly correlated with g/kg consumed on relapse day. Closed circles, original group; X’s, confirmatory group; Lines, best fit by least squares regression. See text and Table 2 for statistical details.
By days 8 to 10 of choice consumption, patterns are strongly established. There is a significant correlation between consumption on days 8 to 10 of the choice phase and consumption on the relapse day (p = 0.0002; Fig. 3B). The variance in consumption on days 8 to 10 accounts for 35% (R2 = 0.35) of the variance in relapse consumption.
These correlations are probably not the result of consistent patterns of thirst. There was no correlation between consumption of water during the first night in the lickometer cages and consumption of ethanol on ethanol-only nights, nor was there a significant correlation between water consumption on the first night and ethanol consumption on days 8 to 10 of the choice phase, or postdeprivation ethanol consumption (data not shown).
Patterns of alcohol preference were similarly established early in the experiment and maintained through the postdeprivation consumption test. As shown in Table 2, consumption (g/kg) on Forced day 3 was significantly correlated with postdeprivation preference (R2 = 0.26, p = 0.0015). In addition, preference on days 8 to 10 was significantly correlated to preference on relapse day (R2 = 0.22, p = 0.0039). As mentioned above, preference was highly correlated to consumption within days 8 to 10 and relapse day (i.e., preference on days 8 to 10 was correlated with g/kg consumption on days 8 to 10, etc.). These data suggest that preference, along with consumption pattern, is established early in the experiment.
Ability of Prescreen to Predict Alcoholism-Like Behaviors
We hypothesized that our prescreen measures would be useful for predicting relapse-like behavior. To test these predictions, we assessed correlations between postdeprivation ethanol consumption and each of the prescreen measures individually. In this analysis, only the number of entries into the open arms of the EPM was significantly correlated with postdeprivation ethanol consumption (R2 = 0.17, p = 0.0138). None of the prescreen measures were correlated with drinking on days 8 to 10 of the choice phase or consumption on forced day 3.
We also tested whether the prescreen measures could account for additional variance in relapse drinking when combined with consumption on forced day 3. In a multiple linear regression model, a significant beta coefficient was obtained for open arm entries in the elevated plus maze when combined with consumption on forced day 3. However, the model containing both forced day 3 and open arm entries together (R2 = 0.39; p = 0.0003) did not account for significantly more variance in rebound drinking than forced day 3 alone (R2 = 0.28; p = 0009) (F-ratio, 2 factor model vs. 1 factor model = 1.12; p > 0.05). Similarly, a model containing choice days 8 to 10 and open arm entries did not predict significantly more variance in relapse drinking than a model containing choice days 8 to 10 alone. Overall, our prescreen was not useful for predicting postdeprivation consumption.
Test of Predictive Value
As we performed so many correlational analyses in the original data set, it was possible that we obtained false-positive results. Therefore, to confirm our conclusions we tested an additional 12 rats to see if the same relationships emerged. As shown in Table 2, consumption on forced day 3 was again correlated with consumption on days 8 to 10 of the choice phase (R2 = 0.46, p = 0.0153) and there was a trend toward a correlation between forced day 3 and rebound consumption (R2 = 0.25, p = 0.099). Postdeprivation consumption was also significantly correlated to ethanol consumption on choice days 8 to 10, as in the original model (R2 = 0.89, p < 0.0001).
Our findings relating to alcohol preference were nearly but not exactly replicated in the confirmatory group. Preference on days 8 to 10 was significantly correlated to consumption on forced day 3, which was not observed in the original group (R2 = 0.39, p = 0.029, Table 2). Preference on the relapse day was nearly significantly correlated to consumption on forced day 3 (R2 = 0.31, p = 0.06, Table 2). The relationship between entries into the open arms of the elevated plus maze and relapse consumption was not replicated, suggesting that the weak correlation in the original group may have been a false positive result. All other relationships were replicated in the confirmatory group as indicated in Table 2.
Based on the results from these 2 separate groups of rats, we conclude that relapse-like ethanol consumption and preference in adolescent male rats is partially predicted by early ethanol consummatory behaviors. Individual consumption and preference levels continue to emerge across the choice phase, but are partially established early in the experiment.
DISCUSSION
There are 2 principal findings from these experiments. First, individual patterns of ethanol consumption emerged quickly in adolescent male rats. The rats that consumed the most ethanol postdeprivation were among the heaviest drinkers by their third exposure to alcohol. After an initial decrease when water was presented as an option, some individuals increased ethanol consumption over the 10-day choice period. Thus, the rats that eventually exhibited high relapse consumption were the individuals that had high early consumption levels. Additional patterns of alcohol consumption continued to emerge, as shown by the fact that the heaviest drinkers on the relapse day included additional individuals that increased drinking during the choice phase. This phenomenon was also evident in the preference data. Preference for alcohol during choice and postdeprivation consumption periods was partly determined by early intake, and postdeprivation preference was strongly related to choice consumption. Thus, early drinking behavior seems to predict both future intake and preference.
The second finding is that several behavioral phenotypes which we assessed were not significantly correlated with either initial intake or relapse drinking. Most notably, novelty seeking (as assessed either by novel object exploration or by locomotion in an open field) was not correlated with ethanol consumption at any phase. This was surprising since epidemiological studies have demonstrated that novelty seeking is related to initiation of drug and alcohol experimentation in human adolescents (Cloninger et al., 1988; Conway et al., 2003; Kreek et al., 2005; Masse and Tremblay, 1997), although at least one other study has failed to demonstrate an association between alcohol intake and novelty seeking in adult male Wistar rats (Bienkowski et al., 2001). In contrast, there are several studies which have demonstrated a relationship between novelty-seeking and self-administration of psychostimulants (Abreu-Villaca et al., 2006; Cain et al., 2005; Deminiere et al., 1992; Grimm and See, 1997; Mitchell et al., 2005; Piazza et al., 1990; Pierre and Vezina, 1997; Suto et al., 2001) but see (Sutton et al., 2000), and one study that demonstrated a relationship between novelty seeking and operant self-administration of ethanol (Nadal et al., 2002). The lack of correlation that we and Bienkowski et al. observed may be due to drug- or method-specific effects. It is possible that the underlying (dopaminergic) mechanisms mediating novelty seeking in rodents are more relevant to psychostimulant intake than alcohol intake, or that operant self-administration of any drug is more related to novelty seeking than voluntary drinking.
It was surprising that circulating corticosterone levels were not significantly correlated with ethanol consumption at any phase of the experiment, considering the large body of literature suggesting that stress and stress hormones play a large factor in both ethanol abuse (Funk et al., 2006; Menzaghi et al., 1994; Merlo Pich et al., 1995; Rassnick et al., 1993; Valdez et al., 2002; Zimmermann et al., 2007) and alcohol withdrawal-induced anxiety (Baldwin et al., 1991; Menzaghi et al., 1994). Our findings contrast with a previous report showing that rhesus macaques with high stress-induced circulating cortisol levels as juveniles consumed more alcohol as young adults (Fahlke et al., 2000). It is possible that the transient response to a mild stressor in a rodent, which we measured, is not related to alcohol intake in the same way that intense chronic stress (such as childhood trauma or maternal separation) is related to alcohol intake in humans and primates. A study comparing several alcohol-preferring rat lines demonstrated that ethanol consumption in response to a stressor was strain-specific (Vengeliene et al., 2003). Thus, rats may model genetic but not environmental contributors to the link between stress hormones and alcohol intake.
One important question emerging from these findings relates to the mechanisms that drive early ethanol consumption. None of our prescreen measures were significantly correlated with ethanol consumption on forced day 3. Consumption on day 3 is likely related to multiple factors that were not included in our prescreen. It is likely that animals that find alcohol initially rewarding are most likely to repeat drinking. In addition, animals that are insensitive to the aversive effects of ethanol are also more likely to repeat drinking. In addition, impulsivity may play a role, as has been demonstrated in genetically modified and inbred mice ((Bowers and Wehner, 2001; Logue et al., 1998) but see (McMillen et al., 1998)). It is possible that natural variation in all of these behavioral characteristics in our outbred strain of rats contributed to variation in ethanol intake.
Initial insensitivity to ethanol may have been another significant predictor of alcohol intake. Previous studies have shown that adolescent rats are relatively insensitive to the sedating effects of ethanol (Little et al., 1996; Moy et al., 1998; Silveri and Spear, 1998), but have not examined interindividual differences in the sedative effects. We hypothesize that insensitivity to ethanol may be a predictor of alcohol consumption which is developmentally regulated and also interacts with individual variation. This possibility is under investigation in our laboratory.
This study focused on adolescent male rats. We do not yet know whether similar findings would emerge in female rats, or whether any of our predictors would be relevant in females. There are clear sex differences in alcohol consumption which emerge during puberty (Almeida et al., 1998; Blanchard et al., 1993; Cailhol and Mormede, 2001; Jones and Whitfield, 1995; Lancaster et al., 1996; Piano et al., 2005), as well as clear sex differences in sedative (Cha et al., 2006; Webb et al., 2002) and aversive (Cailhol and Mormede, 2002; Foy and Foy, 2003) effects. Future studies will focus on whether differential predictors are related to intake in males versus females.
In conclusion, our results suggest that early exposure to ethanol may initiate a pattern of heavy drinking and increased vulnerability to relapse in some adolescent rats. By using natural variation in an outbred strain of rats, our model allows examination of multiple etiologies of alcoholism, which complements existing genetic models focusing on a single heritable characteristic. This method could be used in future studies which examine factors that emerge with repeated exposure to ethanol.
REFERENCES
- Abreu-Villaca Y, Queiroz-Gomes Fdo E, Dal Monte AP, Filgueiras CC, Manhaes AC. Individual differences in novelty-seeking behavior but not in anxiety response to a new environment can predict nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res. 2006;167:175–182. doi: 10.1016/j.bbr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. J Clin Invest. 1998;101:2677–2685. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Koros E, Kostowski W. Novelty-seeking behaviour and operant oral ethanol self-administration in Wistar rats. Alcohol Alcohol. 2001;36:525–528. doi: 10.1093/alcalc/36.6.525. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, Glick SD. Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res. 1993;17:968–973. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Wehner JM. Ethanol consumption and behavioral impulsivity are increased in protein kinase Cgamma null mutant mice. J Neurosci. 2001;21:RC180. doi: 10.1523/JNEUROSCI.21-21-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res. 2001;25:594–599. [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Conditioned taste aversion and alcohol drinking: strain and gender differences. J Stud Alcohol. 2002;63:91–99. [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Cha YM, Li Q, Wilson WA, Swartzwelder HS. Sedative and GABAergic effects of ethanol on male and female rats. Alcohol Clin Exp Res. 2006;30:113–118. doi: 10.1111/j.1530-0277.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Clark DB, Cornelius JR, Kirisci L, Tarter RE. Childhood risk categories for adolescent substance involvement: a general liability typology. Drug Alcohol Depend. 2005;77:13–21. doi: 10.1016/j.drugalcdep.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Bohman M. Childhood personality predicts alcohol abuse in young adults. Alcohol Clin Exp Res. 1988;12:494–505. doi: 10.1111/j.1530-0277.1988.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Conway KP, Kane RJ, Ball SA, Poling JC, Rounsaville BJ. Personality, substance of choice, and polysubstance involvement among substance dependent patients. Drug Alcohol Depend. 2003;71:65–75. doi: 10.1016/s0376-8716(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Deminiere JM, Piazza PV, Guegan G, Abrous N, Maccari S, Le Moal M, Simon H. Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res. 1992;586:135–139. doi: 10.1016/0006-8993(92)91383-p. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Duranceaux NC, Schuckit MA, Eng MY, Robinson SK, Carr LG, Wall TL. Associations of variations in alcohol dehydrogenase genes with the level of response to alcohol in non-Asians. Alcohol Clin Exp Res. 2006;30:1470–1478. doi: 10.1111/j.1530-0277.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol Clin Exp Res. 2000;24:644–650. [PubMed] [Google Scholar]
- Foy MR, Foy JG. Reversal of long-delay conditioned taste aversion learning in rats by sex hormone manipulation. Integr Physiol Behav Sci. 2003;38:203–213. doi: 10.1007/BF02688854. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedde HW, Agarwal DP, Harada S. The role of alcohol dehydrogenase and aldehyde dehydrogenase isozymes in alcohol metabolism, alcohol sensitivity, and alcoholism. Isozymes Curr Top Biol Med Res. 1983;8:175–193. [PubMed] [Google Scholar]
- Goedde HW, Meier-Tackmann D, Agarwal DP, Harada S. Physiological role of aldehyde dehydrogenase isozymes. Prog Clin Biol Res. 1982;114:347–362. [PubMed] [Google Scholar]
- Grimm JW, See RE. Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiol Behav. 1997;61:755–761. doi: 10.1016/s0031-9384(96)00532-x. [DOI] [PubMed] [Google Scholar]
- Hem A, Smith AJ, Solberg P. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret and mink. Lab Anim. 1998;32:364–368. doi: 10.1258/002367798780599866. [DOI] [PubMed] [Google Scholar]
- Hinckers AS, Laucht M, Schmidt MH, Mann KF, Schumann G, Schuckit MA, Heinz A. Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biol Psychiatry. 2006;60:282–287. doi: 10.1016/j.biopsych.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Jones BC, Whitfield KE. Sex differences in ethanol-related behaviors in genetically defined murine stocks. Recent Dev Alcohol. 1995;12:223–230. doi: 10.1007/0-306-47138-8_14. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Logue SF, Swartz RJ, Wehner JM. Genetic correlation between performance on an appetitive-signaled nosepoke task and voluntary ethanol consumption. Alcohol Clin Exp Res. 1998;22:1912–1920. [PubMed] [Google Scholar]
- Masse LC, Tremblay RE. Behavior of boys in kindergarten and the onset of substance use during adolescence. Arch Gen Psychiatry. 1997;54:62–68. doi: 10.1001/archpsyc.1997.01830130068014. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Means LW, Matthews JN. Comparison of the alcohol-preferring P rat to the Wistar rat in behavioral tests of impulsivity and anxiety. Physiol Behav. 1998;63:371–375. doi: 10.1016/s0031-9384(97)00442-3. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Rassnick S, Heinrichs S, Baldwin H, Pich EM, Weiss F, Koob GF. The role of corticotropin-releasing factor in the anxiogenic effects of ethanol withdrawal. Ann N Y Acad Sci. 1994;739:176–184. doi: 10.1111/j.1749-6632.1994.tb19819.x. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Cunningham CL, Mark GP. Locomotor activity predicts acquisition of self-administration behavior but not cocaine intake. Behav Neurosci. 2005;119:464–472. doi: 10.1037/0735-7044.119.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Duncan GE, Knapp DJ, Breese GR. Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcohol Clin Exp Res. 1998;22:1485–1492. [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162:333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Piano MR, Carrigan TM, Schwertz DW. Sex differences in ethanol liquid diet consumption in Sprague-Dawley rats. Alcohol. 2005;35:113–118. doi: 10.1016/j.alcohol.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1:339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berl) 1997;129:277–284. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Robins LN, Przybeck TR. Age of onset of drug use as a factor in drug and other disorders. NIDA Res Monogr. 1985;56:178–192. [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG, Brown SA. Testing the level of response to alcohol: social information processing model of alcoholism risk–a 20-year prospective study. Alcohol Clin Exp Res. 2004;28:1881–1889. doi: 10.1097/01.alc.0000148111.43332.a5. [DOI] [PubMed] [Google Scholar]
- Schuckit M, Smith T, Pierson J, Danko G, Beltran IA. Relationships among the level of response to alcohol and the number of alcoholic relatives in predicting alcohol-related outcomes. Alcohol Clin Exp Res. 2006;30:1308–1314. doi: 10.1111/j.1530-0277.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Wilhelmsen K, Smith TL, Feiler HS, Lind P, Lange LA, Kalmijn J. Autosomal linkage analysis for the level of response to alcohol. Alcohol Clin Exp Res. 2005;29:1976–1982. doi: 10.1097/01.alc.0000187598.82921.27. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000a;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000b;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology (Berl) 2001;158:175–180. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Karanian DA, Self DW. Factors that determine a propensity for cocaine-seeking behavior during abstinence in rats. Neuropsychopharmacology. 2000;22:626–641. doi: 10.1016/S0893-133X(99)00160-8. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004a;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Changes in sensitivity to ethanol-induced social facilitation and social inhibition from early to late adolescence. Ann N Y Acad Sci. 2004b;1021:459–461. doi: 10.1196/annals.1308.064. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res. 2003;27:1048–1054. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B, Burnett PW, Walker DW. Sex differences in ethanol-induced hypnosis and hypothermia in young Long-Evans rats. Alcohol Clin Exp Res. 2002;26:695–704. [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen KC, Schuckit M, Smith TL, Lee JV, Segall SK, Feiler HS, Kalmijn J. The search for genes related to a low-level response to alcohol determined by alcohol challenges. Alcohol Clin Exp Res. 2003;27:1041–1047. doi: 10.1097/01.ALC.0000075551.02714.63. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Blomeyer D, Laucht M, Mann KF. How gene-stress-behavior interactions can promote adolescent alcohol use: the roles of predrinking allostatic load and childhood behavior disorders. Pharmacol Biochem Behav. 2007;86:246–262. doi: 10.1016/j.pbb.2006.09.024. [DOI] [PubMed] [Google Scholar]