Dma1 ubiquitinates the SIN scaffold, Sid4, to impede the mitotic localization of Plo1 kinase
Exact functions for yeast homologues of Chfr and Rnf8, members of a small ubiquitin ligase family with important cell cycle control roles, have remained elusive. Identification of the first ubiquitination target for fission yeast Dma1 now offers insight into how it regulates signalling by the septation initiation network.
Keywords: cytokinesis, Dma1, Plo1, septation initiation network, Sid4
Abstract
Proper cell division requires strict coordination between mitotic exit and cytokinesis. In the event of a mitotic error, cytokinesis must be inhibited to ensure equal partitioning of genetic material. In the fission yeast, Schizosaccharomyces pombe, the checkpoint protein and E3 ubiquitin ligase, Dma1, delays cytokinesis by inhibiting the septation initiation network (SIN) when chromosomes are not attached to the mitotic spindle. To elucidate the mechanism by which Dma1 inhibits the SIN, we screened all SIN components as potential Dma1 substrates and found that the SIN scaffold protein, Sid4, is ubiquitinated in vivo in a Dma1-dependent manner. To investigate the role of Sid4 ubiquitination in checkpoint function, a ubiquitination deficient sid4 allele was generated and our data indicate that Sid4 ubiquitination by Dma1 is required to prevent cytokinesis during a mitotic checkpoint arrest. Furthermore, Sid4 ubiquitination delays recruitment of the Polo-like kinase and SIN activator, Plo1, to spindle pole bodies (SPBs), while at the same time prolonging residence of the SIN inhibitor, Byr4, providing a mechanistic link between Dma1 activity and cytokinesis inhibition.
Introduction
At the end of each cell division cycle, chromosomes segregate to opposite sides of the cell and a cytokinetic ring (CR) assembles and constricts between them to physically separate the two new cells. Clearly, it is critical that chromosome segregation occurs before ring constriction and, thus, mitosis and cytokinesis must be coupled to ensure that each new cell inherits the proper genetic complement. In the fission yeast, Schizosaccharomyces pombe, the septation initiation network (SIN) confers proper coordination by triggering contractile ring constriction once mitosis is complete (for review see McCollum and Gould, 2001; Krapp et al, 2004b). Thus, precise activation of the SIN is required for the fidelity of each cell division.
SIN signalling is restricted to the spindle pole bodies (SPBs) and is initiated by the GTPase, Spg1 (Schmidt et al, 1997). Upon conversion to its GTP-bound form during metaphase, its effector kinase Cdc7 (Sohrmann et al, 1998) is recruited, followed by Sid1–Cdc14 (Guertin et al, 2000). In anaphase B, the downstream SIN kinase, Sid2–Mob1, which localizes constitutively to SPBs, concentrates at the CR before constriction and is thought to transduce the signal to constrict (Sparks et al, 1999; Hou et al, 2000; Salimova et al, 2000). To prevent premature septation in interphase, a bipartite GAP complex comprising Cdc16 and Byr4 binds and inhibits Spg1 at SPBs (Song et al, 1996; Furge et al, 1998; Jwa and Song, 1998; Krapp et al, 2008). The GAP complex also regulates asymmetric distribution of SIN activity at the two SPBs throughout mitosis (Cerutti and Simanis, 1999; Li et al, 2000). In metaphase, Byr4–Cdc16 are absent from both SPBs, but are then recruited to the old SPB during anaphase B, thus permitting SIN activity only on the new SPB (Sohrmann et al, 1998; Grallert et al, 2004). Asymmetric distribution of SIN activity is critically important for the SIN to trigger septation precisely and also to silence the SIN after the completion of cytokinesis (Garcia-Cortes and McCollum, 2009).
Two scaffold proteins, Sid4 and Cdc11, provide the spatial cues for assembly of the SIN and its regulators at SPBs (Krapp et al, 2001, 2003, 2004a; Tomlin et al, 2002; Morrell et al, 2004), while the conserved Polo-like kinase, Plo1, temporally regulates SIN signalling (Mulvihill et al, 1999; Tanaka et al, 2001). Among its other activities, Plo1 has a key role in forming the contractile ring, predicting the site of division, driving septum formation (Ohkura et al, 1995; Bahler et al, 1998), and plo1+ overexpression activates the SIN pathway (Ohkura et al, 1995; Mulvihill et al, 1999). To execute these events faithfully, Plo1 localization within the cell is controlled precisely. Plo1 concentrates on the mitotic, but not the interphase SPB (Mulvihill et al, 1999), partly through association with the SIN scaffold, Sid4 (Morrell et al, 2004), suggesting that Plo1 might directly target one or more SIN components to drive septum formation. A pathway homologous to the SIN, called the mitotic exit network (MEN), exists in Saccharomyces cerevisiae (for reviews see Bardin and Amon, 2001; McCollum and Gould, 2001; de Bettignies and Johnston, 2003; Seshan and Amon, 2004). While the Plo1 target at the SIN has not yet been identified in S. pombe, the S. cerevisiae Plo1 homologue, Cdc5, phosphorylates and inhibits the GAP complex, Bub2–Bfa1, allowing MEN activation (Hu et al, 2001; Geymonat et al, 2003).
In addition to the SIN/MEN pathways that function during every cell cycle, multiple checkpoint pathways also control mitotic progression. For instance, in the event that chromosomes are not properly attached to the mitotic spindle during metaphase, the spindle assembly checkpoint (SAC) inhibits the anaphase-promoting complex/cyclosome to prevent anaphase onset and mitotic exit (for reviews see Malmanche et al, 2006; Varetti and Musacchio, 2008; Zich and Hardwick, 2010). In addition to the SAC, studies in yeast have identified a SAC-independent pathway required to inhibit cytokinesis when chromosomes are not properly attached to the mitotic spindle (Alexandru et al, 1999; Beltraminelli et al, 1999; Gardner and Burke, 2000). In S. pombe, one effector of the SAC-independent pathway is the checkpoint protein, Dma1 (Murone and Simanis, 1996; Guertin et al, 2002). Dma1 localizes to SPBs and the division site, and dma1+ overexpression prevents the SIN kinases from assembling at SPBs (Guertin et al, 2002). Furthermore, Dma1 binds the SIN scaffold, Sid4, and delays recruitment of Plo1 to SPBs during a checkpoint response (Guertin et al, 2002). However, the mechanism by which this occurs is unknown.
At its N terminus, Dma1 has a Forkhead-associated (FHA) domain, which is predicted to interact with phosphothreonine residues (Durocher et al, 2000; Durocher and Jackson, 2002; Mahajan et al, 2008). At its C terminus, Dma1 contains a ring-finger (RF) domain, which most likely confers E3 ubiquitin ligase activity to the protein (for reviews see Joazeiro and Weissman, 2000; Deshaies and Joazeiro, 2009). The FHA domain is required for Dma1 localization to SPBs and the cell division site, and both domains are required for proper checkpoint function (Guertin et al, 2002). Dma1 belongs to a small class of proteins that encode both FHA and RF domains, of which there are two in humans, CHFR (Scolnick and Halazonetis, 2000) and RNF8 (Kolas et al, 2007). CHFR (CHeckpoint protein with FHA and RF domains) is a tumour suppressor protein, which has been implicated in a mitotic checkpoint termed the antephase checkpoint (Scolnick and Halazonetis, 2000; Matsusaka and Pines, 2004). RNF8 is also a checkpoint protein, which has a role in the DNA damage response pathway (Huen et al, 2007; Kolas et al, 2007; Wang and Elledge, 2007). Two homologues exist in S. cerevisiae, Dma1 and Dma2, which are functionally redundant and are required for the spindle position checkpoint (Fraschini et al, 2004). While all of these proteins share little sequence similarity outside of their FHA and RF domains, all participate in cell cycle checkpoints (for review see Brooks et al, 2008), which might imply a conserved mode of action.
To elucidate the mechanism of Dma1 inhibition of the SIN, we screened all SIN components as potential Dma1 substrates and found that the SIN scaffold, Sid4, is ubiquitinated in a Dma1-dependent manner and that Sid4 ubiquitination is required to prevent cytokinesis during a mitotic checkpoint arrest. Furthermore, when the spindle checkpoint is activated in the absence of Sid4 ubiquitination, Plo1 prematurely accumulates at SPBs and the GAP component, Byr4, is driven off SPBs earlier compared with wild type cells. Our data indicate that Dma1 ubiquitinates the SIN scaffold protein, Sid4, to antagonize Plo1 localization and access to SIN substrates in order to delay cytokinesis.
Results
The SIN scaffold, Sid4, is ubiquitinated in vivo
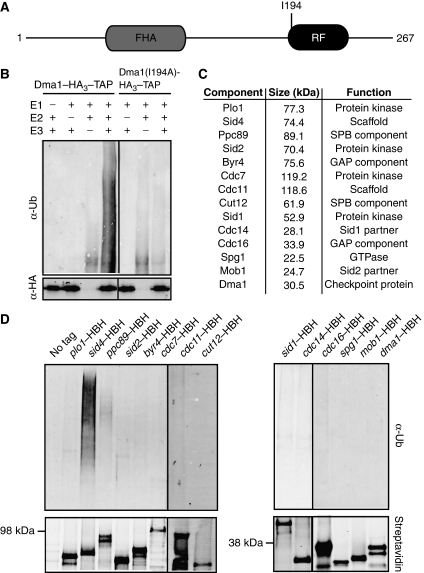
dma1+ encodes a RF domain, which is predicted to have E3 ubiquitin ligase activity (Figure 1A). To determine whether Dma1 is in fact a functional E3 ubiquitin ligase, Dma1 was tagged at its endogenous C terminus with HA3–TAP and purified from S. pombe lysates. When the TAP eluate was incubated with an E1-activating enzyme and the E2-conjugating enzyme, Ubc13–Uev1a, Dma1 catalysed formation of polyubiquitin chains in vitro (Figure 1B, left panel). To be sure that the polyubiquitin chains were formed in a Dma1-specific manner and were not a product of another E3 contaminant present in the TAP eluate, a conserved hydrophobic residue within the RF domain (I194) that is expected to disrupt interaction with its cognate E2 enzyme (Katoh et al, 2003) was mutated to alanine (Figure 1A). When the Dma1(I194A)–HA3–TAP eluate was incubated with the E1 and E2 enzymes, polyubiquitin chains were not formed (Figure 1B, right panel). Taken together, these data indicate that the predicted RF domain of Dma1 confers ubiquitin ligase activity to the protein.
Figure 1.
The SIN scaffold, Sid4, is ubiquitinated in vivo. (A) Schematic diagram of Dma1 protein with relative positions of Dma1 FHA and RF domains and the I194A point mutation indicated. (B) In vitro ubiquitination assay using an E1-activating enzyme, the human E2-conjugating enzyme, Ubc13/Uev1a and either dma1–HA3–TAP or dma1(I194A)–HA3–TAP purified from S. pombe lysates arrested by the nda3-KM311 mutation. (C) List of SIN and SPB proteins screened for in vivo ubiquitination. (D) In vivo ubiquitination assay of proteins listed in C. Each protein was purified from checkpoint-activated cells (nda3-KM311) and visualized by immunoblot using fluorescently labelled streptavidin (bottom panels) and a Ubiquitin antibody (top panels).
Given that the Dma1 RF domain is required to maintain a spindle checkpoint arrest and that dma1+ antagonizes SIN signalling by perturbing Plo1 SPB localization (Guertin et al, 2002), we reasoned that Dma1 performed its checkpoint function by targeting Plo1 or other SIN component(s) for ubiquitination. Therefore, the in vivo ubiquitination status of Plo1 and every SIN component (Figure 1C) was examined in checkpoint-activated cells (Figure 1D). We also tested the ubiquitination status of the SPB component Ppc89, which is required for Sid4 association with the SPB, and Cut12, with which Plo1 also interacts at the SPB (Flory et al, 2002; MacIver et al, 2003) (Figure 1C). Each protein was tagged at its endogenous C terminus with a His6–BIO–His6 (HBH) epitope and purified from denatured lysates using Ni2+–NTA and streptavidin resin (Tagwerker et al, 2006). Proteins were purified from cells in which the spindle checkpoint had been activated using a reversible cold-sensitive mutation in the β-tubulin gene (nda3-KM311) (Hiraoka et al, 1984) and the ubiquitination status was determined by immunoblotting for ubiquitin. To validate that each protein was indeed purified, we also blotted with streptavidin, which recognizes the biotinylated epitope. Through this approach, we found that the SIN scaffold, Sid4, was the only protein tested to be robustly ubiquitinated in vivo (Figure 1D).
Sid4 is ubiquitinated in a Dma1-dependent manner
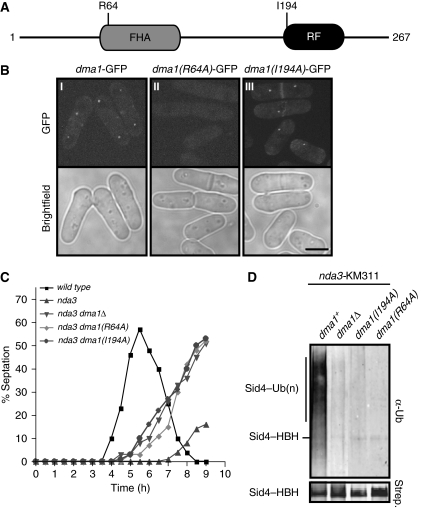
The finding that Sid4 is ubiquitinated in vivo during a checkpoint arrest suggests that it might be a Dma1 substrate. In this regard, it is noteworthy that Dma1 and Sid4 were shown previously to interact with each other by yeast two-hybrid analysis (Guertin et al, 2002). We therefore examined whether Sid4 ubiquitination required Dma1. Mutants were generated in which either the entire coding region of dma1+ was deleted or single mutations within the dma1+ coding region (R64 or I194) were mutated to alanine and integrated at the endogenous dma1+ locus (Figure 2A). Mutating R64 to alanine is predicted to disrupt interaction with phosphothreonine residues (Durocher and Jackson, 2002) and impedes localization of Dma1 to SPBs and the cell division site (Figure 2B, compare panels I and II), while the I194A mutation eliminates Dma1 E3 ligase activity (Figure 1B), but does not disrupt its localization to SPBs or the division site (Figure 2B, compare panels I and III).
Figure 2.
Sid4 ubiquitination requires Dma1 function. (A) Schematic diagram of Dma1 domains and positions of the R64A and I194A mutations. The R64A mutation prevents interaction with phosphothreonine motifs and I196A inactivates ubiquitin ligase activity. (B) Localization of dma1–GFP (panel I), dma1(R64A)–GFP (panel II) and dma1(I194A)–GFP (panel III) in cells growing in log phase. Scale bar, 5 μm. (C) Spindle checkpoint assay. Cells of the indicated strains were synchronized at 32°C in G2 by centrifugal elutriation, shifted to 18°C, and the septation index of each strain determined every 30 min for 9 h. (D) In vivo ubiquitination status of Sid4–HBH in nda3-KM311 dma1Δ or nda3-KM311 dma1 mutants.
To validate that the dma1 mutants compromise Dma1 function, each dma1 mutant was combined with the nda3-KM311 mutation and tested for checkpoint function. Cells were synchronized in G2 by centrifugal elutriation, shifted to the restrictive temperature (18°C) to activate the spindle checkpoint, and septation indices were measured at 30 min intervals for 9 h. While the nda3-KM311 dma1+ strain maintained a checkpoint arrest for ∼7 h, the nda3-KM311 dma1(R64A) and nda3-KM311 dma1(I194A) mutant strains could not maintain an arrest and formed aberrant septa at ∼5 h, which is comparable with nda3-KM311 dma1Δ cells (Figure 2C). Thus, the R64A and I194A mutations compromise Dma1-dependent checkpoint function.
We next examined Sid4 ubiquitination in checkpoint activated (nda3-KM311) dma1Δ, dma1(R64A) and dma1(I194A) mutants. Cells were shifted to 18°C for 5 h to activate the spindle checkpoint and Sid4 ubiquitination was examined. Strikingly, in the absence of Dma1 protein, activity or localization, Sid4 ubiquitination was abolished (Figure 2D). These data indicate that Sid4 is ubiquitinated in a Dma1-dependent manner.
Sid4 ubiquitination is required for Dma1-dependent checkpoint function
To determine if Sid4 ubiquitination is required for the Dma1-dependent checkpoint arrest, a ubiquitination deficient sid4 allele was generated. Ubiquitin transfer often occurs in a sequence-independent manner and can occur on multiple substrate lysines, making site identification challenging (for reviews see Laney and Hochstrasser, 1999; Pickart, 2001). Sid4 contains 49 lysines (Figure 3A, top diagram) and mutating all 49 sites simultaneously would likely disrupt protein function. Thus, four sid4 mutants were made, in which clusters of lysine residues were mutated that, collectively, cover every lysine within Sid4 (Supplementary Figure 1A). As sid4+ is essential for viability, we first tested whether the four mutants could rescue the temperature-sensitive sid4–SA1 mutant at the restrictive temperature (data not shown) and as they all could, each was then integrated at the endogenous sid4+ locus to examine its in vivo ubiquitination status. Surprisingly, all four mutants were still ubiquitinated in vivo (Supplementary Figure 1B). Therefore, in order to create a ubiquitin-deficient sid4 allele, we needed to generate a mutant that would eliminate more lysine residues simultaneously. However, all four mutants generated above were severely cold sensitive (data not shown), indicating that Sid4 function was already compromised and adding more mutations would likely exacerbate these phenotypes.
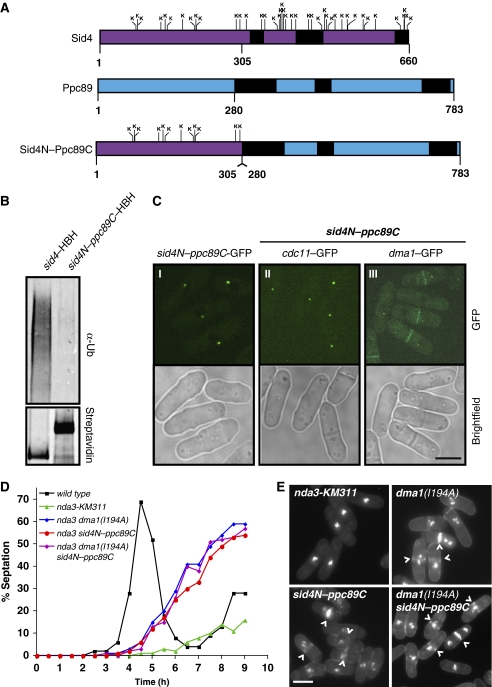
Figure 3.
Sid4 ubiquitination is required to maintain a checkpoint arrest. (A) Schematic diagrams of Sid4 with relative positions of all 49 lysines (top), Ppc89 (middle) and the Sid4N–Ppc89C fusion mutant (bottom). Predicted coiled-coil regions are shown in black. (B) In vivo ubiquitination of Sid4–HBH and Sid4N–Ppc89C–HBH. (C) Localization of Sid4N–Ppc89C–GFP (panel I), Cdc11–GFP (panel II) and Dma1–GFP (panel III) in sid4N–ppc89C–HBH mutant cells. Scale bar, 5 μm. (D) Spindle checkpoint assay. Cells of the indicated strains were blocked at 32°C in S phase with hydroxyurea, released into hydroxyurea-free media at 18°C, and the septation index of each strain was determined every 30 min for 9 h. (E) Cells from each of the strains examined in 3D at the 7 h time point stained with methyl blue, which stains the septa, and DAPI, which stains DNA. (^) indicate septated cells that have bypassed the checkpoint. Scale bar, 5 μm.
Thus, as an alternative means of eliminating relevant Sid4 lysine residues without disrupting protein function, we made use of previous structure and function analyses of Sid4 and the core SPB protein, Ppc89. The N-terminal 300 amino acids of Sid4 are required for direct binding to Plo1 (Morrell et al, 2004), Cdc11 (Tomlin et al, 2002) and Dma1 (Guertin et al, 2002), indicating that this region contains the essential SIN scaffolding activity of Sid4. The C termini of both Sid4 and Ppc89 contain several predicted coiled-coil regions (Figure 3A, top and middle diagram, respectively), which are only required for their SPB localization (Rosenberg et al, 2006). In fact, replacing the Sid4 C terminus with the SPB targeting region of Ppc89 (Figure 3A, bottom diagram) rescues both the temperature-sensitive sid4–SA1 allele at 36°C and the sid4Δ (Rosenberg et al, 2006). Thus, the sid4N–ppc89C fusion mutant, which eliminates ∼76% of Sid4 lysines on the protein, was integrated at the endogenous sid4+ locus and tested for in vivo ubiquitination. While Sid4–HBH was robustly ubiquitinated, ubiquitination of the Sid4N–Ppc89C–HBH mutant was essentially eliminated (Figure 3B). Importantly, sid4N–ppc89C mutant cells were wild type for morphology and were not temperature sensitive. These data indicate that Dma1 targets the Sid4 C terminus for ubiquitination in vivo.
As expected, Sid4N–Ppc89C–GFP localized to SPBs properly (Figure 3C, panel I) and Cdc11–GFP, whose localization depends on Sid4, also localized to SPBs normally (Figure 3C, panel II). Furthermore, Cdc11–GFP intensities at SPBs are not significantly altered in sid4N–ppc89C mutant cells compared with wild-type cells (Supplementary Figure 2A and B). Thus, as predicted by its wild type morphology, the Sid4N–Ppc89C mutant does not disrupt the SIN scaffold complex. To ensure that the loss of Sid4N–Ppc89C ubiquitination was not due to a failure to recruit Dma1, we examined Dma1–GFP localization and found that it was present at SPBs in sid4N–ppc89C mutant cells (Figure 3C, panel III), consistent with the previous observation that Dma1 interacts with the Sid4 N-terminal 300 amino acids (Guertin et al, 2002). Thus, while the Sid4 N terminus binds Dma1, its C terminus is required for ubiquitination. Collectively, these data indicate that the sid4N–ppc89C mutant retains full scaffolding and essential SIN functions of sid4+, but is unable to be ubiquitinated in vivo even in the presence of Dma1.
We then assessed the checkpoint function of the sid4N–ppc89C mutant. Cells were arrested in S phase with hydroxyurea (HU), released synchronously at 18°C to activate the spindle checkpoint, and septation indices were measured at 30 min intervals for 9 h. The nda3-KM311 strain maintained a checkpoint arrest for ∼7 h (Figure 3D and E, top left panel); however, the nda3-KM311 sid4N–ppc89C mutant formed aberrant septa (marked with (^) in Figure 3E, bottom left panel) at ∼5 h, which phenocopied the dma1–RF mutant (nda3-KM311 dma1(I194A)) (Figure 3D and E, top right panel). Importantly, a double dma1(I194A) sid4N–ppc89C mutant septated with similar kinetics as either mutant alone and did not display any other additive effects (Figure 3D and E, bottom right panel), suggesting that these mutants bypass a checkpoint arrest via the same mechanism. Thus, Sid4 ubiquitination is necessary to inhibit cytokinesis during a dma1-dependent checkpoint arrest.
Sid4 ubiquitination antagonizes Plo1 recruitment to SPBs during a checkpoint response
When the spindle checkpoint is activated in the absence of dma1+, Plo1 is recruited to SPBs earlier (Guertin et al, 2002). Because our data suggest that Dma1 ubiquitinates Sid4 when a mitotic checkpoint is activated, we tested if Sid4 ubiquitination was the biochemical signal that perturbs Plo1 recruitment to SPBs by measuring the timing of Plo1 recruitment to SPBs in checkpoint-activated sid4N–ppc89C cells. Endogenously expressed Plo1 fused to a single GFP is difficult to visualize in vivo. Thus, to improve visualization three tandem copies of GFP were fused to the C terminus of Plo1 (Plo1–GFP3) and used in the subsequent experiments.
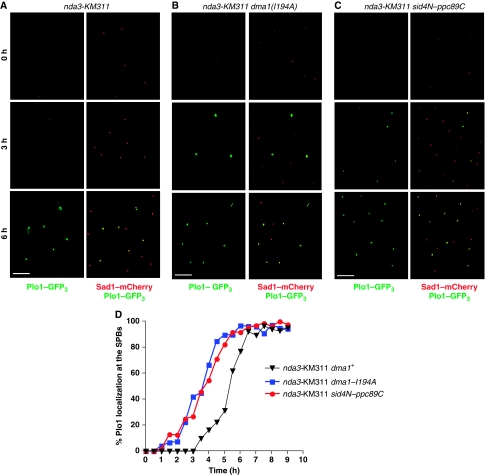
nda3-KM311, nda3-KM311 dma1(I194A) and nda3-KM311 sid4N–ppc89C cells were synchronized in G2 by lactose gradient sedimentation, shifted to 18°C to activate the spindle checkpoint, and Plo1–GFP3 was visualized at 30 min intervals for 9 h. In dma1+ cells, Plo1–GFP3 was not visible on SPBs until ∼4 to 5 h (Figure 4A and D). However, in the dma1(I194A) mutant, Plo1–GFP3 was detected at SPBs ∼2 h earlier compared with dma1+ cells and cells failed to arrest in mitosis (Figure 4B and D), which is similar to the premature recruitment observed previously for dma1Δ cells (Guertin et al, 2002). Similarly, Plo1–GFP3 was recruited to SPBs earlier in sid4N–ppc89C mutant cells (Figure 4C and D). It should be noted that when cells are arrested in prometaphase by the nda3-KM311 mutation, Plo1 localizes to both SPBs; however, because the mitotic spindle does not form and SPBs do not separate in this arrest, Plo1's signal in the later time points is slightly obscured by the fact that it is localizing on two SPBs that are sometimes overlapping in the Z axis. To be sure that we were quantitating SPB-localized Plo1, Plo1–GFP3 was colocalized with the constitutive SPB marker, Sad1–mCherry (Hagan and Yanagida, 1995; Figure 4A–C, right panels). These data suggest that when the spindle checkpoint is activated, Sid4 ubiquitination antagonizes Plo1 recruitment to SPBs and thereby prevents it from reaching its substrates and activating the SIN.
Figure 4.
Sid4 ubiquitination delays Plo1 recruitment to the SPBs when the spindle checkpoint is activated. (A–C) nda3-KM311 (A), nda3-KM311 dma1(I194A) (B) or nda3-KM311 sid4N–ppc89C (C) cells were synchronized at 32°C in G2 by lactose gradient sedimentation, released to 18°C to activate the spindle checkpoint, and Plo1-GFP3 and Sad1-mCherry localization at the SPBs were imaged periodically for 9 h. In each panel, the images on the left show Plo1–GFP3 localization alone and the images on the right show merged images of Plo1–GFP3 colocalized with Sad1–mCherry at each of the times indicated. Scale bar, 10 μm. (D) The kinetics of Plo1 recruitment to SPBs was measured for each of the strains shown by calculating the percentage of cells with Plo1–GFP3 on SPBs at each time point.
Sid4 ubiquitination antagonizes Plo1 recruitment to SPBs during interphase
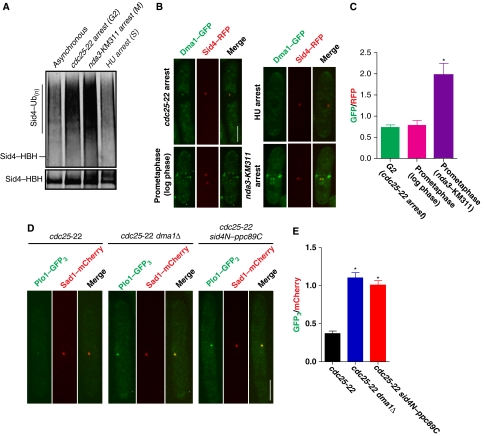
As Dma1 can be detected at SPBs in the absence of checkpoint induction, we examined a potential role for Sid4 ubiquitination during normal cell cycle progression. While Sid4 was most robustly ubiquitinated during a mitotic arrest, as expected, it was also ubiquitinated in G2 cells, but significantly less ubiquitination was detected during S phase (Figure 5A). As Sid4 ubiquitination levels fluctuate throughout the cell cycle, we tested if Dma1 concentration at SPBs was also cell cycle dependent by measuring Dma1–GFP intensities at SPBs in different cell cycle stages and comparing these intensities with the constitutive SPB marker Sid4–RFP (Morrell et al, 2004). Dma1–GFP intensity was detected at low levels in prometaphase cells grown to log phase under permissible conditions (Figure 5B and C) and was significantly increased during a mitotic checkpoint arrest (nda3-KM311 arrest) (Figure 5B and C), suggesting that Dma1 concentrates at SPBs in response to mitotic stress. Dma1-GFP was also detected in cells arrested in G2 (cdc25-22) (Figures 5B and C), although with significantly decreased intensity compared with nda3-KM311-arrested cells, and it was not detected on SPBs in cells arrested in S phase (Figure 5B and C). Thus, the levels of Sid4 ubiquitination correlate with the concentration of SPB-localized Dma1.
Figure 5.
Sid4 ubiquitination prevents Plo1 recruitment to SPBs during interphase. (A) In vivo ubiquitination of Sid4–HBH in asynchronous cells or cells arrested in G2 (cdc25-22), prometaphase (nda3-KM311) or S phase (hydroxyurea; HU). (B) Representative images showing Dma1–GFP and Sid4–RFP localization in a G2 arrest (cdc25-22 arrest), an S-phase arrest (HU arrest), prometaphase cell growing in log phase, and a mitotic arrest when the checkpoint is active (nda3-KM311 arrest). Scale bar, 5 μm. (C) Quantitation of relative Dma1–GFP/Sid4-RFP intensity ratios for each of the cell cycle stages shown in B plotted as arbitrary units. For each cell cycle stage, Dma1–GFP and Sid4–RFP intensities were measured for at least 20 cells and averaged; error bars represent standard error of the mean, *P<0.05. (D) Representative images showing Plo1–GFP3 and Sad1–mCherry localization at SPBs during a cdc25-22 arrest in wild type (left panels), dma1Δ (middle panels) and sid4N–ppc89C (right panels) cells. Scale bar, 5 μm. (E) Quantitation of relative Plo1–GFP3/Sad1–mCherry intensity ratios at SPBs for each of the strains shown in D plotted in arbitrary units. For each strain, Plo1–GFP3 and Sad1–mCherry intensities were measured for at least 20 cells and averaged; error bars represent standard error of the mean, *P<0.05.
Plo1 localization to SPBs is also cell cycle regulated, accumulating at SPBs upon commitment to mitosis (Mulvihill et al, 1999). As Sid4 ubiquitination antagonizes Plo1 localization at SPBs and Sid4 is ubiquitinated in interphase cells, we wondered if the absence of Sid4 ubiquitination would allow Plo1 to concentrate at SPBs in interphase. Thus, dma1+, dma1Δ or sid4N–ppc89C cells were arrested in G2 using the temperature-sensitive cdc25-22 mutation, and Plo1–GFP3 intensities at SPBs were measured relative to Sad1–mCherry. While Plo1–GFP3 was only detected at low levels in dma1+ cells (Figure 5D and E), Plo1–GFP3 intensities at SPBs were significantly increased in dma1Δ cells (Figure 5D and E). A similar increase in Plo1–GFP3 intensities was observed in sid4N–ppc89C cells, in which Sid4 ubiquitination is abolished (Figure 5D and E). These data suggest that Sid4 ubiquitination antagonizes Plo1 localization to SPBs during interphase and during a mitotic checkpoint arrest.
Byr4 is a potential Plo1 target
While the direct SIN target(s) of Plo1 have not yet been identified in S. pombe, the S. cerevisiae Plo1 homologue, Cdc5, is known to phosphorylate and inhibit the Byr4 ortholog and GAP component Bfa1, resulting in MEN activation (Hu et al, 2001; Geymonat et al, 2003). Bfa1 phosphorylation by Cdc5 inhibits its GAP activity in vitro and also ejects it from SPBs (Hu et al, 2001; Geymonat et al, 2003). S. pombe Byr4 is also a phosphoprotein (Song et al, 1996) and is hyperphosphorylated just before septation (Krapp et al, 2008). Thus, the potential of Plo1 SPB recruitment influencing Byr4 phosphorylation status and SPB localization was examined.
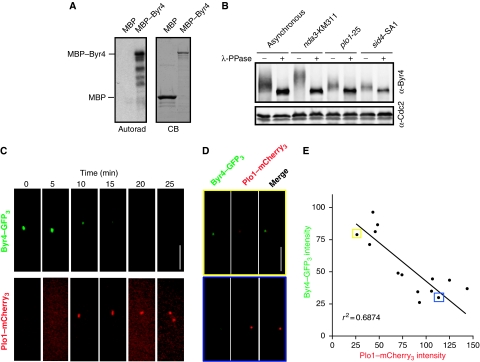
First, Byr4 was tested as a Plo1 substrate in vitro. MBP and MBP–Byr4 were produced in E. coli, and purified on amylose resin. When purified proteins were incubated with Plo1 purified from baculovirus-infected insect cells and 32γ-ATP, we found that Plo1 could directly phosphorylate full-length Byr4 (Figure 6A). Next, Byr4 phosphorylation in vivo was examined. As previously reported (Song et al, 1996), Byr4 was hyperphosphorylated in a mitotic arrest (Figure 6B). However, in a temperature-sensitive plo1 mutant (plo1-25) that had been synchronized and shifted to the restrictive temperature, the extent of Byr4 phosphorylation was drastically reduced (Figure 6B). Significantly, the degree of Byr4 phosphorylation in the plo1-25 mutant was comparable with Byr4 phosphorylation in sid4–SA1 mutant cells at the restrictive temperature (Figure 6B), indicating that Byr4 must be associated with the SPBs to become phosphorylated. Taken together, these data suggest that Plo1 contributes to the majority of Byr4 phosphorylation at the SPB.
Figure 6.
Byr4 is a potential Plo1 target. (A) Left, autoradiograph of recombinant MBP and MBP–Byr4 phosphorylated in vitro by Plo1 kinase. Right, Coomassie blue (CB) gel of purified MBP and MBP–Byr4 proteins. (B) Gel shifts of endogenous Byr4 immunoprecipitated from asynchronous, nda3-KM311, plo1-25 or sid4-SA1 temperature-sensitive cells, which were synchronized in S phase by hydroxyurea and released at the restrictive temperature. Immunoprecipitates were treated with (+) or without (−) λ-phosphatase and detected by immunoblotting using an anti-Byr4 serum. (C) A Byr4–GFP3 Plo1–mCherry3 strain was imaged via time-lapse microscopy and a representative montage is depicted. (D) A Byr4–GFP3 Plo1–mCherry3 strain was grown to log phase and imaged. Top and bottom panels show representative images of cells in which Byr4–GFP3 or Plo1–mCherry3, respectively, localization to the SPB predominates. (E) Byr4–GFP3 and Plo1–mCherry3 fluorescence intensities were measured and plotted against each other. A linear regression analysis was performed to calculate the best-fit line, r2=0.687. The data points boxed in yellow and blue represent the intensity calculations for the top and bottom panels shown in D, respectively.
We next examined the timing of Byr4 and Plo1 localization at SPBs relative to each other via time-lapse microscopy. To visualize Plo1 and Byr4 in the same cells, Byr4 was tagged at its C terminus with three tandem copies of GFP (Byr4–GFP3) and Plo1 was tagged at its C terminus with three tandem copies of mCherry (Plo1–mCherry3). byr4–GFP3 plo1–mCherry3 cells were morphologically wild type, were not temperature sensitive and did not display any observable cell cycle defects, suggesting that Byr4 and Plo1 functions were not significantly compromised. In a representative movie, Byr4–GFP3 was detected until the 10 min time point, when Plo1–mCherry3 was first detected on SPBs, and continued to decrease until it was undetectable at 20 min, just before SPB separation (Figure 6C). We also quantitated the relative intensities of Byr4–GFP3 and Plo1–mCherry3 at SPBs in an asynchronous population of cells (Figure 6D). In the few cells in which both proteins were detected at SPBs, Byr4 and Plo1 intensities showed a strong negative correlation (Figure 6D and E). Collectively, these data suggest that Plo1 phosphorylation of Byr4 at SPBs promotes Byr4 dissociation from SPBs.
Sid4 ubiquitination is required to prolong Byr4 residence on SPBs during a checkpoint arrest
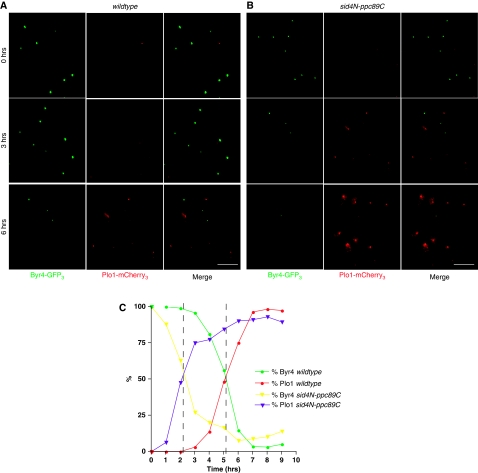
We next examined the kinetics of Byr4 and Plo1 localization during a checkpoint response in nda3-KM311 and nda3-KM311 sid4N–ppc89C mutant cells. Cells were synchronized in G2, shifted to 18°C to activate the checkpoint and Byr4–GFP3 and Plo1–mCherry3 were visualized periodically for 9 h. In nda3-KM311 sid4+ cells, Byr4–GFP3 was maintained on SPBs for ∼3 h (Figure 7A and C). However, in the absence of Sid4 ubiquitination (nda3-KM311 sid4N–ppc89C mutant), Byr4–GFP3 began to disappear from SPBs after just 1 h and was absent from almost 100% of the cells by 5 h (Figure 7B and C). In both strains, the time in which SPB-localized Byr4 was absent in 50% of the cells (∼5 h in sid4+ cells and ∼2 h in the sid4N–ppc89C mutant) corresponds to the same time in which SPB-localized Plo1 was detected in 50% of the cells (Figure 7C, intersections marked by dashed lines). These data suggest that when a mitotic checkpoint is activated, Sid4 ubiquitination antagonizes Plo1 SPB recruitment in order to retain Byr4 on SPBs and inhibit SIN signalling.
Figure 7.
Sid4 ubiquitination is required to prolong Byr4 residence on SPBs when a mitotic checkpoint is activated. (A, B) nda3-KM311 (A) and nda3-KM311 sid4N–ppc89C (B) cells were synchronized in G2 by lactose gradient sedimentation, shifted to 18°C to activate the spindle checkpoint, and Byr4–GFP3 and Plo1–mCherry3 localizations at the SPBs were imaged periodically for 9 h. Representative images of Byr4–GFP3, Plo1–mCherry3 and the merged images are shown for the times indicated. Scale bar, 10 μm. (C) At each time point, the percentage of cells with Byr4–GFP3 and Plo1–mCherry3 were calculated and plotted over time. Dashed lines represent the time in which the plots for Byr4 and Plo1 intersect (∼5 h for nda3-KM311 cells and ∼2 h for nda3-KM311 sid4N–ppc89C cells).
Discussion
Ubiquitin-mediated inhibition of cytokinesis
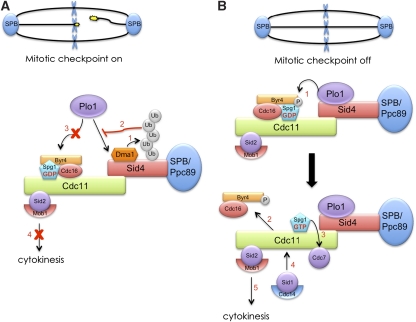
Mitotic exit and cytokinesis must be coupled for proper partitioning of genetic material. In S. pombe, this entrainment is achieved by the SIN. Here, we have presented new evidence regarding how Dma1 influences SIN signalling. Our data indicate that when chromosomes are not attached properly to the mitotic spindle, Dma1 concentrates at SPBs and ubiquitinates the SIN scaffold, Sid4 (Figure 8A, step 1). We propose that Sid4 ubiquitination antagonizes Plo1 localization at SPBs (Figure 8A, step 2) to restrict its ability to activate the SIN and cytokinesis (Figure 8A, steps 3 and 4).
Figure 8.
Model of Dma1 inhibition of the SIN during a mitotic checkpoint. (A) Proposed mechanism of Dma1 inhibition of the SIN when the mitotic checkpoint is active. (B) Mechanism of SIN activation when chromosomes are properly attached to the mitotic spindle and the checkpoint is satisfied.
The SIN pathway consists of several protein kinases, which assemble sequentially at the SPBs. The first kinase on the scene is Plo1, which directly binds the SIN scaffold, Sid4 (Morrell et al, 2004). Once recruited, Plo1 initiates the SIN pathway presumably by phosphorylating one or more SIN components directly; however, its direct target(s) have remained unknown to date. Here, we find that the GAP component, Byr4, is a likely Plo1 target (Figure 8B, step 1). Additionally, the observation that Plo1 and Byr4 localization to SPBs are negatively correlated suggests a model wherein Plo1 ejects Byr4 from SPBs (Figure 8B, step 2), highlighting yet another conserved mechanism between the S. pombe SIN and the S. cerevisiae MEN. Subsequently, expulsion of the GAP complex from SPBs relieves the inhibition on the GTPase, Spg1, which subsequently facilitates recruitment of the SIN kinases, Cdc7 and Sid1–Cdc14 (Figure 8B, steps 3 and 4, respectively), and finally allows Sid2–Mob1 to accumulate at the division site to trigger cytokinesis (Figure 8B, step 5). During a checkpoint response, the delayed Plo1-mediated phosphorylation of Byr4 would detain Byr4 on SPBs and thereby prevent cytokinesis from occurring before chromosome segregation (Figure 8A, steps 3 and 4).
We have also uncovered a potential role for Dma1 during normal cell cycle progression. Here, we find that Dma1's SPB concentration varies according to cell cycle stage and, consistent with our model, its concentration correlates with the degree of Sid4 ubiquitination. It is possible that in addition to its role as a mitotic checkpoint protein, Dma1 cooperates with other SIN inhibitors to minimize SIN activity during interphase. However, these basal levels of Dma1 and Sid4 ubiquitination are likely not sufficient when SIN activity must be kept low for longer periods, such as during a checkpoint arrest. Thus, Dma1 might be ‘activated' during a checkpoint response, at least in part, through additional SPB recruitment. This is supported by the fact that Dma1 intensities increase significantly at SPBs and Sid4 ubiquitination is observed more robustly when a mitotic checkpoint is activated. In order to understand how Dma1 responds to a mitotic checkpoint, it will be pertinent to identify upstream factors that regulate the extent of Dma1 recruitment to SPBs during normal cell cycle progression and in response to a mitotic checkpoint.
Distinct roles of SPB-localized Plo1 kinase in mitosis and cytokinesis
Plo1 accumulates at the mitotic, but not the interphase SPB, through association with Sid4 (Morrell et al, 2004) and at least two other SPB components, Cut12 (Mulvihill et al, 1999) and Pcp1 (Fong et al, 2010). Its recruitment upon commitment to mitosis is dependent on cyclin-dependent kinase activity; however, a hypermorphic Cut12 mutant (stf1-1) can bypass a cdc25-22 arrest by increasing Plo1 recruitment to SPBs and increasing its kinase activity suggesting that the Plo1–Cut12 interaction promotes mitotic entry (Mulvihill et al, 1999). Similarly, Plo1's association with Pcp1 also seems to have a role in promoting mitotic entry as the mitotic defects observed in a temperature-sensitive pcp1 mutant that exhibits reduced Plo1 localization at SPBs can be rescued by a wee1 mutant that causes premature mitotic entry (Fong et al, 2010). Here, we find that the absence of Sid4 ubiquitination also allows Plo1 to accumulate at SPBs in interphase; however, this on its own did not affect normal cell cycle progression and cells did not bypass a cdc25-22 arrest. Given that Cut12 (Bridge et al, 1998) and Pcp1 (Flory et al, 2002) associate with the nuclear side of the SPB, while Sid4 resides on the cytoplasmic face (our unpublished data), suggests that the role of Plo1 in promoting mitotic entry is spatially restricted to the nuclear SPB surface, while its association with Sid4 on the cytoplasmic surface may have a distinct role in regulating cytokinesis.
How does Sid4 ubiquitination antagonize Plo1?
The fact that Sid4 is ubiquitinated might suggest that Sid4 polyubiquitination signals it for degradation by the proteasome, thereby preventing access of Plo1 to core SIN components. However, mimicking a checkpoint response by overexpressing dma1+ does not alter Sid4 protein levels or disrupt its localization at SPBs (Guertin et al, 2002), and fluorescence recovery after photobleaching experiments indicate that Sid4 is stably bound to the SPB (Morrell et al, 2004). Furthermore, the other major SIN scaffold protein, Cdc11, whose localization to the SPBs depends on Sid4, remains localized to SPBs during dma1+ overexpression (Guertin et al, 2002), and its intensity at SPBs is not affected in the sid4N–ppc89C mutant, indicating that Dma1 does not disrupt the Cdc11–Sid4 scaffold complex. An alternative possibility is that ubiquitination physically masks the Plo1 binding site. However, both Plo1 (Morrell et al, 2004) and Dma1 (Guertin et al, 2002) physically interact with the N terminus of Sid4, while Sid4 ubiquitination appears to occur on the C terminus. We do not rule out this possibility, however, due to the lack of information about Sid4's three-dimensional conformation. Sid4 is predicted to contain several intrinsically unstructured regions and it has been proposed that some scaffold proteins are intrinsically unstructured to increase their flexibility and versatility for the proteins that they bind (reviewed in Cortese et al, 2008). Potentially, ubiquitination might induce a structural change within Sid4 that alters the Plo1 binding site, reducing Sid4 affinity for Plo1. To address these outstanding questions, structural studies of Sid4 will be required.
From our studies, it is clear that Sid4 is ubiquitinated in vivo. However, the type of ubiquitin modification formed on Sid4 remains to be characterized. Because Sid4 is not targeted for degradation, it is unlikely that it is polyubiquitinated with K48-linked chains. Our in vitro studies indicate that Dma1 forms polyubiquitin chains with the E2 enzyme complex, Ubc13–Uev1a, which specifically forms K63-linked chains (Hofmann and Pickart, 1999). Also, the Dma1-related proteins, CHFR (Bothos et al, 2003), RNF8 (Plans et al, 2006) and S. cerevisiae Dma1 and Dma2 (Loring et al, 2008), have all been shown to function with Ubc13 in vitro and/or in vivo. K63-linked polyubiquitin chains are not typically associated with proteasome-mediated degradation, but regulate proteins by other mechanisms (for reviews see Ikeda and Dikic, 2008; Pickart and Fushman, 2004). Recent studies indicate that the linear architecture of K63-linked chains can provide a scaffold to recruit proteins with ubiquitin-binding domains in a spatially and temporally regulated manner (Kim et al, 2007; Komander et al, 2009; Sims and Cohen, 2009). Potentially, K63-linked chains could recruit an unidentified factor to Sid4 that antagonizes either Plo1 binding or its kinase activity.
Yet another possibility is that Sid4 is not polyubiquitinated, but multiubiquitinated. Our observation that mutating all endogenous lysines within Sid4 in large clusters has no impact on the extent of Sid4 ubiquitination supports this idea and indicates that Dma1 has loose specificity towards its target lysine(s). We have attempted to examine Sid4 ubiquitination in vitro to address this issue and to validate Sid4 as a direct Dma1 substrate. However, given that Dma1 binds Sid4 through its FHA domain, it is likely that Sid4 must first be phosphorylated on a threonine residue in order to interact with Dma1; establishing the proper in vitro conditions will require phospho-characterization of Sid4. Thus, characterizing the type of ubiquitin modification on Sid4 will be a challenging, yet important future endeavour necessary to understand the detailed mechanism by which it antagonizes Plo1 SPB recruitment.
Conservation of mechanism
Although previously assumed based on its domain architecture, we have shown here for the first time that Dma1 is in fact a bona fide ubiquitin ligase. Four other proteins with similar architecture and activity, the human tumour suppressor protein, CHFR (Scolnick and Halazonetis, 2000), human RNF8 (Tuttle et al, 2007) and S. cerevisiae proteins, Dma1 and Dma2 (Fraschini et al, 2004), have also been implicated in mitotic checkpoints for which the pertinent substrates are unknown. It has been reported that CHFR can directly ubiquitinate the human Polo-like kinase, Plk1, in Xenopus laevis extracts (Kang et al, 2002) and downregulates Plk1 protein levels in human cells (Shtivelman, 2003). These studies suggest that CHFR might directly ubiquitinate Polo-like kinases, targeting them for degradation. However, other reports indicate that a CHFR-dependent checkpoint arrest does not require the function of the proteasome at all, as cells can arrest when treated with proteasomal inhibitors (Matsusaka and Pines, 2004). Here, we find that in S. pombe, Plo1 localization to SPBs is at least in part regulated by ubiquitination of its scaffold rather than ubiquitination of itself. Indeed, we obtained no evidence that Plo1 or Dma1 was ubiquitinated during a mitotic checkpoint response. Whether similar mechanisms operate in other organisms to control Polo-like kinase activity during mitotic checkpoints will be important future studies.
Materials and methods
Yeast methods
Yeast strains (Supplementary Table 1) were grown in yeast extract media supplemented with appropriate amino acids (Moreno et al, 1991). For in vivo ubiquitination assays, strains were grown in 100 ml of 4 × YE media, with the exception of cdc11-linkerHBH and cut12-linkerHBH, which were grown in 2L 4 × YE media. For nda3-KM311 arrests, cultures were shifted to 18°C for 6.5 h before harvesting. For cdc25-22 arrests, cultures were shifted to 36°C for 3.5 h before analysis.
For gene replacements at endogenous loci, mutant open reading frames plus at least 500 bps of 5′- and 3′-flanking nucleotides were subcloned into the pIRT2 plasmid containing the LEU2+ marker. For dma1+ gene replacements, a haploid dma1Δ strain was transformed with either pIRT2–dma1(R64A) or pIRT2–dma1(I194A) and stable integrants were selected by resistance to 5′-FOA. For sid4+ gene replacements, a diploid sid4+/sid4Δ strain was transformed with pIRT2–sid4 mutant constructs and grown on minimal media lacking leucine, adenine and uracil. Transformants were allowed to sporulate and stable haploid integrants were selected based on resistance to 5′-FOA. Mutants were validated by colony PCR with primers outside of the 5′- and 3′-flanking regions.
Cell synchronization methods
For HU block and release experiments, cells were grown to log phase at 32°C before adding HU to a final concentration of 12 mM. After 2 h, a second dose of HU (6 mM final concentration) was added to the cells and after 3.5 h, HU was washed out and cells were released at 18°C.
For synchronization by lactose gradient, cells were grown to log phase at 32°C and sedimented by centrifugation on a 7–30% lactose gradient. Small G2 cells were extracted from the gradient, washed with fresh media and inoculated in media pre-cooled to 18°C.
In vivo ubiquitination assay
Proteins of interest were tagged at their endogenous C termini with a HBH affinity tag with the exception of Cdc11 and Cut12, which were tagged with a linker-HBH affinity tag. Tagged proteins were purified using a modified version of the two-step tandem affinity purification under fully denatured conditions (Tagwerker et al, 2006). Cell pellets were lysed by bead disruption into buffer 1 (8 M urea, 300 mM NaCl, 50 mM NaPO4, 0.5% NP40 and 4 mM Imidazole, pH 8) and incubated with Ni2+-NTA agarose beads (Qiagen) for 3–4 h at room temperature. After incubation, beads were washed 4 × with buffer 3 (8 M urea, 300 mM NaCl, 50 mM NaPO4, 0.5% NP40 and 20 mM Imidazole, pH 6.3) and eluted in buffer 4 (8 M urea, 200 mM NaCl, 50 mM NaPO4, 0.5% NP40 and 2% SDS, 100 mM Tris and 10 mM EDTA, pH 4.3). The pH of the eluate was adjusted to 8 before adding streptavidin ultra-link resin (Pierce) and incubated overnight at room temperature. After the second incubation, streptavidin beads were washed 4 × with buffer 6 (8 M urea, 200 mM NaCl, 2% SDS and 100 mM Tris, pH 8) and 1 × with buffer 7 (8 M urea, 200 mM NaCl and 100 mM Tris, pH 8). Purified proteins were detected on a western blot using a ubiquitin antiserum (Sigma) and fluorescently labelled streptavidin (Licor).
In vitro ubiquitination assay
The E1-activating enzyme and E2 (Ubc13/Uev1a) were purchased from Boston Biochem. Dma1–HA3–TAP and Dma1(I194A)–HA3–TAP were purified from S. pombe lysates using a tandem affinity purification method (Gould et al, 2004). All components were incubated in a reaction buffer containing 50 mM Tris–HCl (pH 7.5), 2.5 mM MgCl2 and 0.5 mM DTT. Reactions were incubated at room temperature for 90 min before adding SDS sample buffer to quench the reaction. To assess Dma1 activity, samples were run on a 3–8% Tris-Acetate gel (Invitrogen) and immunoblotted with anti-Ub (Sigma).
In vitro kinase assays
MBP and MBP–Byr4 fusion proteins were purified on amylose beads (NEB) in column buffer (20 mM Tris (pH 7.0), 150 mM NaCl, 2 mM EDTA and 0.1% NP40) and eluted with maltose. One μg of recombinant protein was used as the substrate in each reaction. Kinase reactions were performed in protein kinase buffer (10 mM Tris pH7.4, 10 mM MgCl2 and 1 mM DTT) at 30°C for 30 min. Reactions were quenched by adding SDS sample buffer, and proteins were separated by SDS–PAGE. Phosphorylated proteins were visualized by autoradiograph and relative protein quantities were assessed by Coomassie blue staining.
S. pombe protein methods
Cell pellets were lysed by bead disruption, and immunoprecipitations were performed in either NP40 buffer for native lysates, or in NP40 buffer containing SDS for denatured lysates as previously described (Gould et al, 1991). For gel shifts, denatured lysates were treated with Lambda phosphatase (New England Biolabs) in 25 mM HEPES-NaOH (pH 7.4), 150 mM NaCl, and 1 mM MnCl2 and incubated at 30°C for 30 min with shaking. Proteins were separated on an 8% Tris-glycine gel and immunoblotted with a Byr4 antiserum (CoCalico).
Microscopy methods
All fluorescence microscopy was performed using a spinning disk confocal microscope (Ultraview LCI; PerkinElmer) with a 100 × NA 1.40 Plan-Apochromat oil immersion objective and either a 488-nm argon (GFP) ion or a 594 nm helium neon (RFP and mCherry) laser. Images were processed using a charge-coupled device camera (Orca-ER; Hamamatsu Photonics) and Metamorph 7.1 software (MDS Analytical Technologies). For imaging cdc25-22-arrested cells, slides, coverslips and immersion oil were pre-heated to 36°C, and cells were imaged on an objective heated to 36°C. For nda3-KM311 strains, cells were fixed in 70% ethanol for 30 min before imaging.
DAPI and methyl blue images were obtained with a personal DeltaVision System equipped with an Olympus IX71 microscope using a 100 × NA 1.40 UPlansApo oil immersion objective. Images were processed using a Cool Snap HQ2 camera and Softworx® software.
Quantitative microscopy was performed using ImageJ software available at: http://rsbweb.nih.gov/ij/. Average GFP fluorescence intensities at SPBs were measured for at least 20 cells with background correction for each. Average RFP or mCherry fluorescence intensities were measured similarly, and final values for each cell are expressed as Green/Red ratios. Measurements for the 20 cells in each group were averaged for statistical analysis.
Supplementary Material
Acknowledgments
We thank Anna Feoktistova, Ping Liang and Liping Ren for their technical assistance and Dr Dannel McCollum for strains used in this study and helpful discussions. We also thank members of the Gould lab, especially Rachel Roberts-Galbraith, Dawn Clifford-Hart, Adam Bohnert, Matthew Broadus and Aurélie Clement for critically reading the manuscript and many helpful discussions. AEJ is supported by the Cellular, Biochemical and Molecular Sciences Training Program, NIH T32 GM08554. This work was supported by the Howard Hughes Medical Institute, of which KLG is an investigator.
Author contributions:AEJ performed all of the experiments; AEJ and KLG designed and interpreted the experiments.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alexandru G, Zachariae W, Schleiffer A, Nasmyth K (1999) Sister chromatid separation and chromosome re-duplication are regulated by different mechanisms in response to spindle damage. EMBO J 18: 2707–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler J, Steever AB, Wheatley S, Wang Y, Pringle JR, Gould KL, McCollum D (1998) Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J Cell Biol 143: 1603–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin AJ, Amon A (2001) Men and sin: what's the difference? Nat Rev Mol Cell Biol 2: 815–826 [DOI] [PubMed] [Google Scholar]
- Beltraminelli N, Murone M, Simanis V (1999) The S. pombe zfs1 gene is required to prevent septation if mitotic progression is inhibited. J Cell Sci 112(Pt 18): 3103–3114 [DOI] [PubMed] [Google Scholar]
- Bothos J, Summers MK, Venere M, Scolnick DM, Halazonetis TD (2003) The Chfr mitotic checkpoint protein functions with Ubc13-Mms2 to form Lys63-linked polyubiquitin chains. Oncogene 22: 7101–7107 [DOI] [PubMed] [Google Scholar]
- Bridge AJ, Morphew M, Bartlett R, Hagan IM (1998) The fission yeast SPB component Cut12 links bipolar spindle formation to mitotic control. Genes Dev 12: 927–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks L III, Heimsath EG Jr, Loring GL, Brenner C (2008) FHA-RING ubiquitin ligases in cell division cycle control. Cell Mol Life Sci 65: 3458–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti L, Simanis V (1999) Asymmetry of the spindle pole bodies and spg1p GAP segregation during mitosis in fission yeast. J Cell Sci 112(Pt 14): 2313–2321 [DOI] [PubMed] [Google Scholar]
- Cortese MS, Uversky VN, Dunker AK (2008) Intrinsic disorder in scaffold proteins: getting more from less. Prog Biophys Mol Biol 98: 85–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bettignies G, Johnston LH (2003) The mitotic exit network. Curr Biol 13: R301. [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Joazeiro CA (2009) RING domain E3 ubiquitin ligases. Annu Rev Biochem 78: 399–434 [DOI] [PubMed] [Google Scholar]
- Durocher D, Jackson SP (2002) The FHA domain. FEBS Lett 513: 58–66 [DOI] [PubMed] [Google Scholar]
- Durocher D, Taylor IA, Sarbassova D, Haire LF, Westcott SL, Jackson SP, Smerdon SJ, Yaffe MB (2000) The molecular basis of FHA domain:phosphopeptide binding specificity and implications for phospho-dependent signaling mechanisms. Mol Cell 6: 1169–1182 [DOI] [PubMed] [Google Scholar]
- Flory MR, Morphew M, Joseph JD, Means AR, Davis TN (2002) Pcp1p, an Spc110p-related calmodulin target at the centrosome of the fission yeast Schizosaccharomyces pombe. Cell Growth Differ 13: 47–58 [PubMed] [Google Scholar]
- Fong CS, Sato M, Toda T (2010) Fission yeast Pcp1 links polo kinase-mediated mitotic entry to gamma-tubulin-dependent spindle formation. EMBO J 29: 120–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschini R, Bilotta D, Lucchini G, Piatti S (2004) Functional characterization of Dma1 and Dma2, the budding yeast homologues of Schizosaccharomyces pombe Dma1 and human Chfr. Mol Biol Cell 15: 3796–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furge KA, Wong K, Armstrong J, Balasubramanian M, Albright CF (1998) Byr4 and Cdc16 form a two-component GTPase-activating protein for the Spg1 GTPase that controls septation in fission yeast. Curr Biol 8: 947–954 [DOI] [PubMed] [Google Scholar]
- Garcia-Cortes JC, McCollum D (2009) Proper timing of cytokinesis is regulated by Schizosaccharomyces pombe Etd1. J Cell Biol 186: 739–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RD, Burke DJ (2000) The spindle checkpoint: two transitions, two pathways. Trends Cell Biol 10: 154–158 [DOI] [PubMed] [Google Scholar]
- Geymonat M, Spanos A, Walker PA, Johnston LH, Sedgwick SG (2003) In vitro regulation of budding yeast Bfa1/Bub2 GAP activity by Cdc5. J Biol Chem 278: 14591–14594 [DOI] [PubMed] [Google Scholar]
- Gould KL, Moreno S, Owen DJ, Sazer S, Nurse P (1991) Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J 10: 3297–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KL, Ren L, Feoktistova AS, Jennings JL, Link AJ (2004) Tandem affinity purification and identification of protein complex components. Methods 33: 239–244 [DOI] [PubMed] [Google Scholar]
- Grallert A, Krapp A, Bagley S, Simanis V, Hagan IM (2004) Recruitment of NIMA kinase shows that maturation of the S. pombe spindle-pole body occurs over consecutive cell cycles and reveals a role for NIMA in modulating SIN activity. Genes Dev 18: 1007–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Chang L, Irshad F, Gould KL, McCollum D (2000) The role of the sid1p kinase and cdc14p in regulating the onset of cytokinesis in fission yeast. EMBO J 19: 1803–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Venkatram S, Gould KL, McCollum D (2002) Dma1 prevents mitotic exit and cytokinesis by inhibiting the septation initiation network (SIN). Dev Cell 3: 779–790 [DOI] [PubMed] [Google Scholar]
- Hagan I, Yanagida M (1995) The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol 129: 1033–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y, Toda T, Yanagida M (1984) The NDA3 gene of fission yeast encodes beta-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell 39(2 Pt 1): 349–358 [DOI] [PubMed] [Google Scholar]
- Hofmann RM, Pickart CM (1999) Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96: 645–653 [DOI] [PubMed] [Google Scholar]
- Hou MC, Salek J, McCollum D (2000) Mob1p interacts with the Sid2p kinase and is required for cytokinesis in fission yeast. Curr Biol 10: 619–622 [DOI] [PubMed] [Google Scholar]
- Hu F, Wang Y, Liu D, Li Y, Qin J, Elledge SJ (2001) Regulation of the Bub2/Bfa1 GAP complex by Cdc5 and cell cycle checkpoints. Cell 107: 655–665 [DOI] [PubMed] [Google Scholar]
- Huen MS, Grant R, Manke I, Minn K, Yu X, Yaffe MB, Chen J (2007) RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131: 901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda F, Dikic I (2008) Atypical ubiquitin chains: new molecular signals. Protein modifications: beyond the usual suspects' review series. EMBO Rep 9: 536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joazeiro CA, Weissman AM (2000) RING finger proteins: mediators of ubiquitin ligase activity. Cell 102: 549–552 [DOI] [PubMed] [Google Scholar]
- Jwa M, Song K (1998) Byr4, a dosage-dependent regulator of cytokinesis in S. pombe, interacts with a possible small GTPase pathway including Spg1 and Cdc16. Mol Cells 8: 240–245 [PubMed] [Google Scholar]
- Kang D, Chen J, Wong J, Fang G (2002) The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. J Cell Biol 156: 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh S, Hong C, Tsunoda Y, Murata K, Takai R, Minami E, Yamazaki T, Katoh E (2003) High precision NMR structure and function of the RING-H2 finger domain of EL5, a rice protein whose expression is increased upon exposure to pathogen-derived oligosaccharides. J Biol Chem 278: 15341–15348 [DOI] [PubMed] [Google Scholar]
- Kim H, Chen J, Yu X (2007) Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science 316: 1202–1205 [DOI] [PubMed] [Google Scholar]
- Kolas NK, Chapman JR, Nakada S, Ylanko J, Chahwan R, Sweeney FD, Panier S, Mendez M, Wildenhain J, Thomson TM, Pelletier L, Jackson SP, Durocher D (2007) Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 318: 1637–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komander D, Reyes-Turcu F, Licchesi JD, Odenwaelder P, Wilkinson KD, Barford D (2009) Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep 10: 466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Cano E, Simanis V (2003) Mitotic hyperphosphorylation of the fission yeast SIN scaffold protein cdc11p is regulated by the protein kinase cdc7p. Curr Biol 13: 168–172 [DOI] [PubMed] [Google Scholar]
- Krapp A, Cano E, Simanis V (2004a) Analysis of the S. pombe signalling scaffold protein Cdc11p reveals an essential role for the N-terminal domain in SIN signalling. FEBS Lett 565: 176–180 [DOI] [PubMed] [Google Scholar]
- Krapp A, Collin P, Cano Del Rosario E, Simanis V (2008) Homoeostasis between the GTPase Spg1p and its GAP in the regulation of cytokinesis in S. pombe. J Cell Sci 121(Pt 5): 601–608 [DOI] [PubMed] [Google Scholar]
- Krapp A, Gulli MP, Simanis V (2004b) SIN and the art of splitting the fission yeast cell. Curr Biol 14: R722–R730 [DOI] [PubMed] [Google Scholar]
- Krapp A, Schmidt S, Cano E, Simanis V (2001) S. pombe cdc11p, together with sid4p, provides an anchor for septation initiation network proteins on the spindle pole body. Curr Biol 11: 1559–1568 [DOI] [PubMed] [Google Scholar]
- Laney JD, Hochstrasser M (1999) Substrate targeting in the ubiquitin system. Cell 97: 427–430 [DOI] [PubMed] [Google Scholar]
- Li C, Furge KA, Cheng QC, Albright CF (2000) Byr4 localizes to spindle-pole bodies in a cell cycle-regulated manner to control Cdc7 localization and septation in fission yeast. J Biol Chem 275: 14381–14387 [DOI] [PubMed] [Google Scholar]
- Loring GL, Christensen KC, Gerber SA, Brenner C (2008) Yeast Chfr homologs retard cell cycle at G1 and G2/M via Ubc4 and Ubc13/Mms2-dependent ubiquitination. Cell Cycle 7: 96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver FH, Tanaka K, Robertson AM, Hagan IM (2003) Physical and functional interactions between polo kinase and the spindle pole component Cut12 regulate mitotic commitment in S. pombe. Genes Dev 17: 1507–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan A, Yuan C, Lee H, Chen ES, Wu PY, Tsai MD (2008) Structure and function of the phosphothreonine-specific FHA domain. Sci Signal 1: re12. [DOI] [PubMed] [Google Scholar]
- Malmanche N, Maia A, Sunkel CE (2006) The spindle assembly checkpoint: preventing chromosome mis-segregation during mitosis and meiosis. FEBS Lett 580: 2888–2895 [DOI] [PubMed] [Google Scholar]
- Matsusaka T, Pines J (2004) Chfr acts with the p38 stress kinases to block entry to mitosis in mammalian cells. J Cell Biol 166: 507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum D, Gould KL (2001) Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol 11: 89–95 [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Morrell JL, Tomlin GC, Rajagopalan S, Venkatram S, Feoktistova AS, Tasto JJ, Mehta S, Jennings JL, Link A, Balasubramanian MK, Gould KL (2004) Sid4p-Cdc11p assembles the septation initiation network and its regulators at the S. pombe SPB. Curr Biol 14: 579–584 [DOI] [PubMed] [Google Scholar]
- Mulvihill DP, Petersen J, Ohkura H, Glover DM, Hagan IM (1999) Plo1 kinase recruitment to the spindle pole body and its role in cell division in Schizosaccharomyces pombe. Mol Biol Cell 10: 2771–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murone M, Simanis V (1996) The fission yeast dma1 gene is a component of the spindle assembly checkpoint, required to prevent septum formation and premature exit from mitosis if spindle function is compromised. EMBO J 15: 6605–6616 [PMC free article] [PubMed] [Google Scholar]
- Ohkura H, Hagan IM, Glover DM (1995) The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev 9: 1059–1073 [DOI] [PubMed] [Google Scholar]
- Pickart CM (2001) Mechanisms underlying ubiquitination. Annu Rev Biochem 70: 503–533 [DOI] [PubMed] [Google Scholar]
- Pickart CM, Fushman D (2004) Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol 8: 610–616 [DOI] [PubMed] [Google Scholar]
- Plans V, Scheper J, Soler M, Loukili N, Okano Y, Thomson TM (2006) The RING finger protein RNF8 recruits UBC13 for lysine 63-based self polyubiquitylation. J Cell Biochem 97: 572–582 [DOI] [PubMed] [Google Scholar]
- Rosenberg JA, Tomlin GC, McDonald WH, Snydsman BE, Muller EG, Yates JR III, Gould KL (2006) Ppc89 links multiple proteins, including the septation initiation network, to the core of the fission yeast spindle-pole body. Mol Biol Cell 17: 3793–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimova E, Sohrmann M, Fournier N, Simanis V (2000) The S. pombe orthologue of the S. cerevisiae mob1 gene is essential and functions in signalling the onset of septum formation. J Cell Sci 113(Pt 10): 1695–1704 [DOI] [PubMed] [Google Scholar]
- Schmidt S, Sohrmann M, Hofmann K, Woollard A, Simanis V (1997) The Spg1p GTPase is an essential, dosage-dependent inducer of septum formation in Schizosaccharomyces pombe. Genes Dev 11: 1519–1534 [DOI] [PubMed] [Google Scholar]
- Scolnick DM, Halazonetis TD (2000) Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature 406: 430–435 [DOI] [PubMed] [Google Scholar]
- Seshan A, Amon A (2004) Linked for life: temporal and spatial coordination of late mitotic events. Curr Opin Cell Biol 16: 41–48 [DOI] [PubMed] [Google Scholar]
- Shtivelman E (2003) Promotion of mitosis by activated protein kinase B after DNA damage involves polo-like kinase 1 and checkpoint protein CHFR. Mol Cancer Res 1: 959–969 [PubMed] [Google Scholar]
- Sims JJ, Cohen RE (2009) Linkage-specific avidity defines the lysine 63-linked polyubiquitin-binding preference of rap80. Mol Cell 33: 775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrmann M, Schmidt S, Hagan I, Simanis V (1998) Asymmetric segregation on spindle poles of the Schizosaccharomyces pombe septum-inducing protein kinase Cdc7p. Genes Dev 12: 84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Mach KE, Chen CY, Reynolds T, Albright CF (1996) A novel suppressor of ras1 in fission yeast, byr4, is a dosage-dependent inhibitor of cytokinesis. J Cell Biol 133: 1307–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks CA, Morphew M, McCollum D (1999) Sid2p, a spindle pole body kinase that regulates the onset of cytokinesis. J Cell Biol 146: 777–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagwerker C, Flick K, Cui M, Guerrero C, Dou Y, Auer B, Baldi P, Huang L, Kaiser P (2006) A tandem affinity tag for two-step purification under fully denaturing conditions: application in ubiquitin profiling and protein complex identification combined with in vivo cross-linking. Mol Cell Proteomics 5: 737–748 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Petersen J, MacIver F, Mulvihill DP, Glover DM, Hagan IM (2001) The role of Plo1 kinase in mitotic commitment and septation in Schizosaccharomyces pombe. EMBO J 20: 1259–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin GC, Morrell JL, Gould KL (2002) The spindle pole body protein Cdc11p links Sid4p to the fission yeast septation initiation network. Mol Biol Cell 13: 1203–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle RL, Bothos J, Summers MK, Luca FC, Halazonetis TD (2007) Defective in mitotic arrest 1/ring finger 8 is a checkpoint protein that antagonizes the human mitotic exit network. Mol Cancer Res 5: 1304–1311 [DOI] [PubMed] [Google Scholar]
- Varetti G, Musacchio A (2008) The spindle assembly checkpoint. Curr Biol 18: R591–R595 [DOI] [PubMed] [Google Scholar]
- Wang B, Elledge SJ (2007) Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc Natl Acad Sci USA 104: 20759–20763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zich J, Hardwick KG (2010) Getting down to the phosphorylated ‘nuts and bolts' of spindle checkpoint signalling. Trends Biochem Sci 35: 18–27 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.