Abstract
Objective
Our goal was to test whether formin homology protein 1 (FHOD1) plays a significant role in the regulation of SMC differentiation, and if so, whether Rho-kinase (ROCK)-dependent phosphorylation in the diaphanous auto-inhibitory domain is an important signaling mechanism that controls FHOD1 activity in SMC.
Methods and Results
FHOD1 is highly expressed in aortic SMCs and in tissues with a significant SMC component. Exogenous expression of constitutively active FHOD1, but not WT, strongly activated SMC-specific gene expression in 10T1/2 cells. Treatment of SMC with the RhoA activator, sphingosine-1-phosphate (S1P), increased FHOD1 phosphorylation at T1141 and this effect was completely prevented by inhibition of ROCK with Y-27632. Phosphomimetic mutations to ROCK target residues enhanced FHOD1 activity suggesting that phosphorylation interferes with FHOD1 auto-inhibition. Importantly, knock-down of FHOD1 in SMC strongly inhibited S1P-dependent increases in SMC differentiation marker gene expression and actin polymerization suggesting that FHOD1 plays a major role in RhoA-dependent signaling in SMC.
Conclusions
Our results indicate that FHOD1 is a critical regulator of SMC phenotype and is regulated by ROCK-dependent phosphorylation. Thus, further studies on the role of FHOD1 during development and the progression of cardiovascular disease will be important.
Introduction
Smooth muscle cell (SMC) differentiation is an important component of vascular development, and defective control of this process in adult animals has been shown to contribute to a variety of cardiovascular pathologies, including atherosclerosis and restenosis (see 1 for review). It is well known that SMC differentiation marker gene expression is regulated by serum response factor (SRF) binding to conserved CArG cis elements within the SMC-specific promoters. The myocardin family of SRF co-factors (myocardin and the Myocardin-Related Transcription Factors, MRTF-A/MKL-1 and MRTF-B/MKL-2) are also critical and have been shown to be required for SMC differentiation marker gene expression in a variety of in vitro and in vivo models 2–5. Thus, identifying the signaling mechanisms by which extrinsic cues regulate SRF/myocardin factor activity will be critical for our understanding of the control of SMC phenotype.
Miralles et al. were the first to demonstrate that MRTF-A activity is controlled by the small GTPase, RhoA and that MRTF-A nuclear localization was enhanced by RhoA-dependent actin polymerization 6. Studies from our lab and others have shown that regulation of MRTF-A and MRTF-B by this mechanism plays an important role in the regulation of SMC phenotype in at least some SMC sub-types 7–9. Of the RhoA effector proteins, Rho-kinase (ROCK) is the most well-studied and has been shown to enhance actin polymerization through LIM-kinase mediated inhibition of cofilin and to stimulate contractility by inhibiting myosin phosphatase. Although the ROCK inhibitor, Y-27632, attenuates SMC-specific transcription 10, 11, it is clear that other RhoA effectors are also involved.
Actin polymerization is also regulated by formin proteins that directly catalyze actin polymerization through two highly conserved formin homology domains (FH1 and FH2) that bind profilin and actin, respectively (see 12, 13 for reviews). Members of the diaphanous-related formin (DRF) sub-family (mDia1, mDia2, mDia3, and FHOD1) exist in an auto-inhibited state maintained by a molecular interaction between the N-terminal diaphanous inhibitory domain (DID) and the C-terminal diaphanous auto-regulatory domain (DAD) (see figure 1A). Importantly, the DRFs interact with specific RhoGTPases (Rho, Rac, Cdc42) through a conserved GTPase-binding domain (GBD) positioned near the DID. Studies of mDia1 auto-inhibition have demonstrated that RhoA binding to the GBD interferes with the DID-DAD interaction thus promoting mDia1-mediated actin nucleation 14, 15. mDia2 is also activated by RhoA, and we have recently shown that both of these DRFs are highly expressed in SMC and are critical for RhoA's effects on SMC-specific gene expression and MRTF nuclear localization 16.
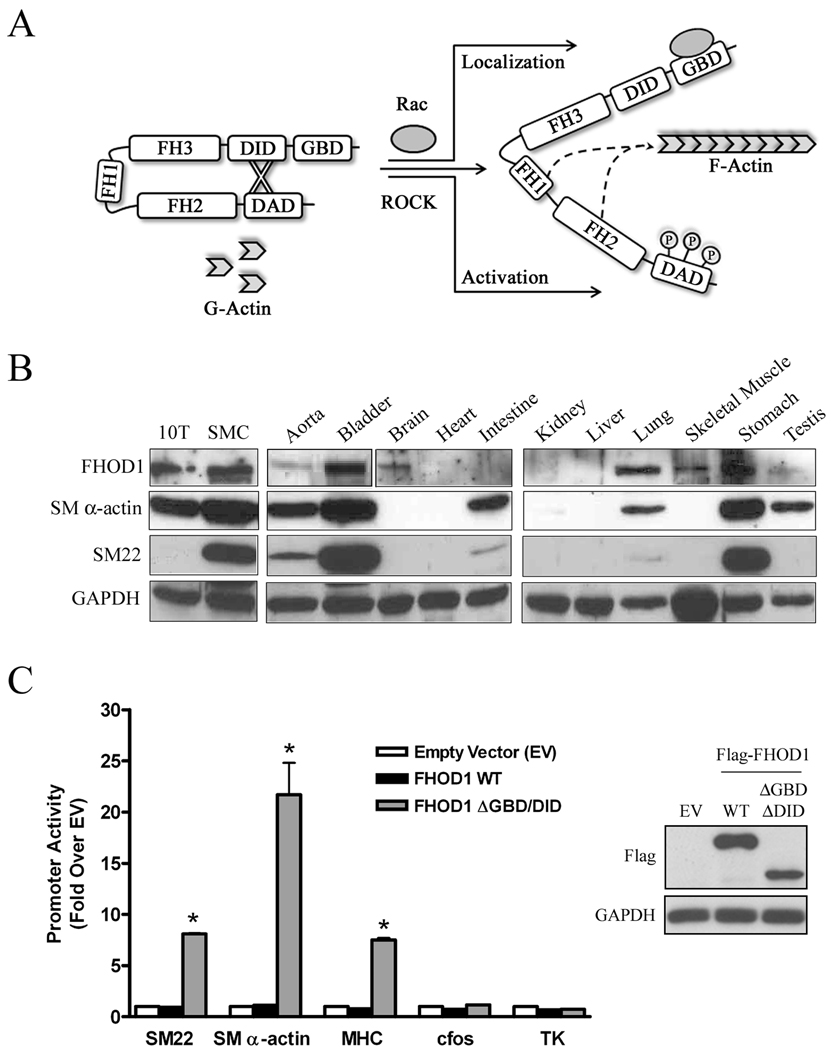
Figure 1. FHOD1 is expressed in SMC-containing tissues and stimulated SMC-specific gene transcription.
A) Molecular regulation of FHOD1. ROCK-dependent phosphorylation of three conserved residues within the DAD domain activates FHOD1 while binding to Rac regulates FHOD1 subcellular localization. B) Western Blot of FHOD1, SM α-actin, SM22, and GAPDH expression in the indicated cell-lines and tissues from adult C57/Bl6 mice. C) Flag-tagged wild-type (WT) or ΔGBD/DID FHOD1 variants were transfected into 10T1/2 cells along with luciferase reporter constructs driven by the indicated promoter. Luciferase activity or was analyzed at 24h and is expressed relative to plus empty expression vector (EV). Protein expression (inset), * p<0.05 versus plus empty vector.
FHOD1 has not been as extensively studied, but based upon several early observations we assumed it was not a major regulator of SMC differentiation marker gene expression. First, FHOD1 binds only to Rac, which unlike RhoA, has only minimal effects on SMC-specific gene expression 10. Second, Rac binding does not activate FHOD1, but serves as a mechanism for localizing FHOD1 to the plasma membrane 17. Finally, although FHOD1 was shown to be expressed in human SMC and endothelial cell lines 18, 19, it was unclear whether FHOD1 was expressed at appreciable levels in SMC in vivo. Importantly, Takeya et al. recently demonstrated that FHOD1 was phosphorylated by ROCK at multiple S/T residues in the DAD domain in endothelial cells and that this phosphorylation interfered with the DID-DAD interaction 19. These studies were of considerable interest because they were the first to identify an activation mechanism for FHOD1, and more importantly, they implicated FHOD1 as an actin nucleating protein downstream of RhoA. Thus, we wanted to test whether FHOD1 played a significant role in the regulation of SMC differentiation, and if so, whether ROCK-dependent phosphorylation was an important signaling mechanism that controlled FHOD1 activity in SMC.
Materials and Methods
Plasmids and Reagents
cDNA encoding human FHOD1 and MRTF-A/B plasmids were a kind gift from Michael Mendelsohn (Tuffs Medical Center, Boston, MA) and Da-Zhi Wang (Havard, Boston, MA), respectively. pCMV-Myc-ROCKΔ3 was a generous gift from Keith Burridge (University of North, Chapel Hill, NC). Full-length and truncation mutations of FHOD1 were generated by PCR and sub-cloned into the expression plasmid pcDNA3.1 (Clonetech). Aspartate substitutions at Ser1131, Ser1137, and Thr1141 were generated using the Quikchange Site-Directed Mutagenesis Kit (Stratagene). The anti-MRTF-A antibody was generated by us and has been previously described 9. The SM22α Ab was a kind gift from Mario Gimona (University of Salzburg, Austria), All other Abs were purchased commercially: FHOD1 and FHOD1 pThr1141 (ECM Biosciences), M2-flag and SM α-actin (Sigma), GAPDH (Santa Cruz), SM-MHC (Abbiotec), α-actin, ERK and pERK (Cell Signaling). Y-27632 and Latrunculin B were purchased from Calbiochem and S1P from Cayman Chemicals.
Cell Culture, Transient Transfections and Reporter Gene Assays
SMCs from mouse thoracic aorta were isolated and cultured as previously described 7. The maintenance and transfection of multipotential 10T1/2 cells were performed as previously described 16. In brief, cells were maintained in 48 well plates in 10% serum and were transfected 24 h after plating at 70–80% confluency using the transfection reagent, TransIT-LT1 (Mirus), as per protocol. Luciferase assays (Promega) were conducted 24 h following transfection. The SM22, SM α-actin, and cfos promoters have been previously described 10, 20.
siRNA Knockdown
FHOD1 was depleted from primary mouse aortic SMCs using siRNA (Dharmacon) with following sequences: Sense 5’- GGAAGAGCGGCAGAAGAUUGAGGTT, Anti-sense 5’- CCUCAAUCUUCUGCCGCUCUUCCTT. Cells were transfected with gene-specific siRNA (100nM) or non-targeted control (NTC) siRNA designed against GFP using DharmaFECT reagent 1. After 72 h of knockdown, cells were serum starved for 24 h and left untreated or treated with S1P (10μM) for an additional 24 h or 12.5 minutes (Erk phosphorylation).
Western Blots
Samples were obtained from adult C57/Bl6 mice or confluent SMCs and 10T1/2 cell and lysed in buffer A (50 mM Tris (pH 7.2), 150 mM NaCl, 2 mM EDTA, 1% NP40, 0.5% Triton X-100) and cleared by centrifugation (4°C for 15 minutes at 14,000 rpm) and protein concentrations were determined by BCA assay (Pierce). Protein lysates were run on SDS polyacrylamide gels, transferred to nitrocellulose, and probed with the indicated antibodies.
Immunoflourescence
10T1/2 cells were plated and transfected in 4-well chamber slides, maintained overnight in media containing 10% serum. Cells were serum-starved for 16 h, fixed in 3.7% paraformaldehyde/PBS for 20 min, and permeabilized in 0.5% Triton X-100/PBS for 3–4 min. The M2 Flag (1:500), SM α-actin (1:2000), or MRTF-A (1:250) were diluted in 20% Goat Serum / 3% BSA in PBS and incubated with the fixed cells for 2 hours. Texas Red or FITC (Jackson ImmunoResearch) conjugated secondary antibodies were used at (1:1000) while AlexaFluor 555 Phalloidin (Molecular Probes) and DAPI (Molecular Probes) were used at 1:100 and 1nM, respectively.
Actin sedimentation assays
SMCs transfected with control or FHOD1 siRNA (see above) were subject to actin sedimentation assays as previously described 21. In brief, cell lysates were centrifuged at 100,000 × g for 1h and the supernatant (G-actin fraction) and pellet (F-actin fraction) were subjected to Western Blot analysis for α-actin.
Results
FHOD1 is expressed in SMC-containing tissues and stimulates SMC-specific gene transcription
To begin to address whether FHOD1 was an important regulator of SMC phenotype, we measured FHOD1 expression in mouse tissues and several cell lines by Western Blot (figure 1B). Although FHOD1 was detected in many tissues, in general, its expression was relatively high in many tissues that exhibited strong SMC differentiation marker gene expression including bladder, lung, stomach, and primary aortic SMCs. To determine whether FHOD1, like mDia1 and mDia2, could stimulate SMC-specific transcription, we expressed flag-tagged FHOD1 variants in multi-potential 10T1/2 cells along with several SMC-specific promoter-luciferase constructs. 10T1/2 cells serve as an excellent model for studying the signaling pathways that regulate SMC phenotype because we and others have shown that SMC differentiation marker gene expression can be dramatically up-regulated by exogenously expressed proteins or by treatment with various agonists such as TGF-β and sphingosine-1-phosphate (S1P) 7, 22. Figure 1C demonstrates that expression of WT FHOD1 had no effect on any of the promoters tested reflecting the auto-inhibited nature of the full-length molecule. However, expression of a constitutively active FHOD1 variant that lacks the N-terminal auto-inhibitory domain (ΔGBD/DID) strongly stimulated SMC-specific promoter activity (by 8–25 fold) even though it was expressed at a slightly lower level than the WT variant. Importantly, constitutively active FHOD1 had no effect on the CArG-containing c-fos promoter or on the CArG-independent minimal thymidine kinase (TK) promoter suggesting that this effect was at least somewhat specific.
FHOD1 activity in SMC is regulated by ROCK
Although constitutively active FHOD1 was sufficient to activate SMC-specific transcription, we were interested in whether FHOD1 activity in our SMC differentiation models was controlled by ROCK-dependent phosphorylation. To test this, we measured SM22 promoter activity in 10T1/2 cells co-expressing FHOD1 and constitutively active variants of RhoA (L63) or ROCK (Δ3). As shown in figure 2A, expression of L63RhoA or ROCKΔ3 by themselves enhanced SM22 promoter activity around 6 fold and 4 fold, respectively while expression of FHOD1 had little effect. However, co-expression of FHOD1 with L63RhoA resulted in a synergistic activation of SM22 promoter activity (to approximately 15 fold) suggesting that these signaling molecules cooperate to regulate SMC-specific expression. Importantly, this increase was significantly attenuated by the ROCK inhibitor, Y-27632, and a similar synergy was observed upon co-expression of ROCKΔ3 with FHOD1. In contrast, co-expression of FHOD1 with constitutively active Rac (L61) did not lead to synergistic activation of SMC differentiation marker gene expression, but did result in FHOD1 localization to the plasma membrane and a relatively small increase in cortical actin polymerization (figure S1).
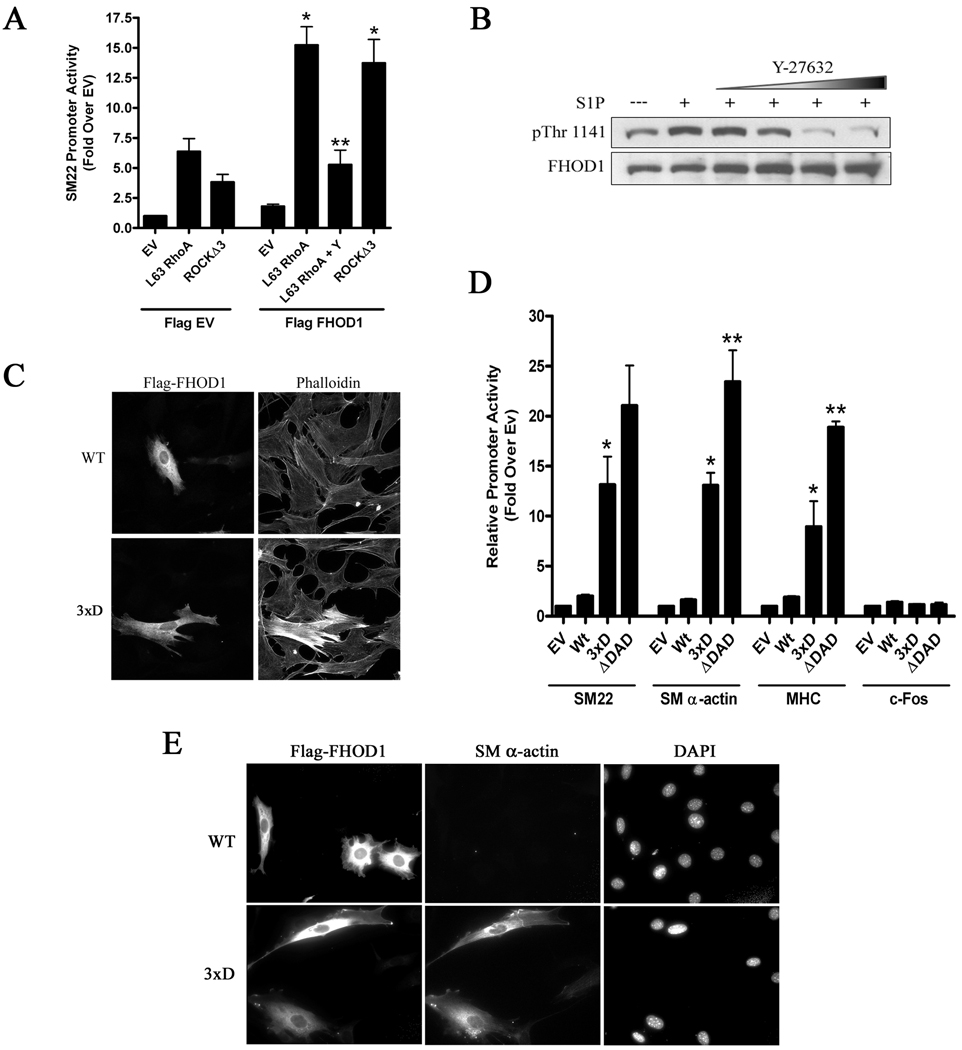
Figure 2. ROCK-dependent phosphorylation of FHOD1 activated SMC-specific gene expression.
A) SM22 luciferase and constitutively active RhoA (L63) or ROCK (Δ3) were transfected into 10T1/2 cells plus/minus flag-FHOD1. In some experiments cells were treated with Y-27632 (Y) for 8h prior to luciferase determination. * p<0.05 versus minus FHOD1, ** p<0.05 versus L63 RhoA + FHOD1. B) Primary mouse SMCs were serum starved for 24h and then pre-treated with increasing amounts of Y-27632 (10, 25, 50, 100μM) for 90 m and the phosphatase inhibitor, calyculin A (50nM), for 5 m. After addition of S1P (10μM), FHOD1 phosphorylation was measured at 7.5 m using a phospho-specific antibody against Thr-1141. C and E) 10T1/2 cells were transfected with WT FHOD1 or an FHOD1 variant containing phosphomimetic mutations to the three ROCK target residues within the auto-inhibitory domain (3xD). Cells were serum starved in 0.5% FBS containing media for 24h, and then stained for actin polymerization (C) or endogenous SM α-actin expression (E). D) The indicated flag-FHOD1 variants and luciferase reporters were transfected into 10T1/2 cells and luciferase activity was measured at 24h. * p<0.05 versus Wt. ** p<0.05 versus 3xD.
It has been shown in endothelial cells that FHOD1 was phosphorylated by ROCK at three highly conserved sites (S1131, S1137, and T1141) just C-terminal to the core DAD domain and that phosphorylation of these residues activated FHOD1 by interfering with the DID-DAD interaction 19. To test whether endogenous FHOD1 was phosphorylated by ROCK in SMC we used a phospho-specific antibody against T1141. As shown in figure 2B, treatment of cells with S1P, a lipid agonist previously shown to activate RhoA in SMC 7, resulted in a significant increase in T1141 phosphorylation. Importantly, S1P-dependent and basal T1141 phosphorylation were inhibited by Y-27632 strongly indicating that ROCK phosphorylates FHOD1 in SMC. To directly test if phosphorylation activates FHOD1 in SMC, we generated an FHOD1 variant that contained phosphomimetic aspartic acid mutations to all three residues shown to be phosphorylated by ROCK (3xD). In comparison to WT, the 3xD variant significantly enhanced FHOD1 activity as measured by actin polymerization (figure 2C), SMC-specific promoter activity (figure 2D), and endogenous SM α-actin expression (figure 2E). Interestingly, the 3xD variant was not quite as active as the constitutively active FHOD1 variant lacking the entire DAD domain (ΔDAD).
FHOD1 activity regulates MRTF nuclear localization
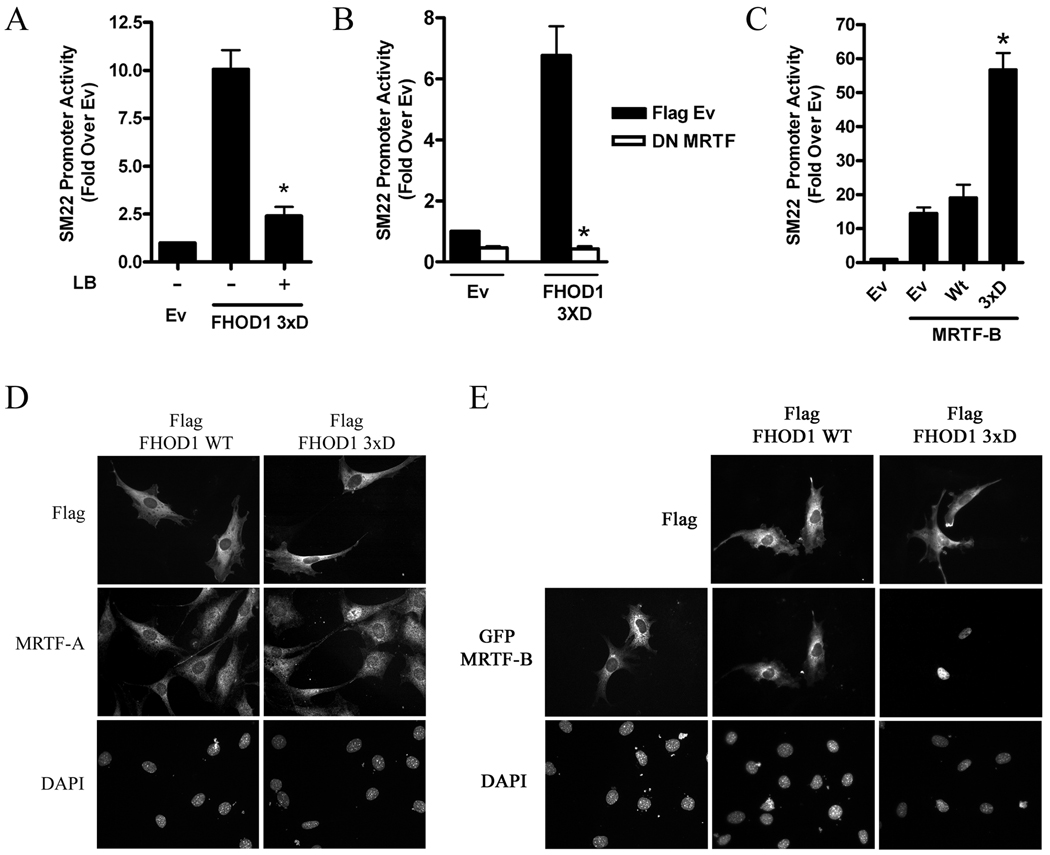
Based upon our previous studies on mDia1 and mDia2, we hypothesized that the effect of FHOD1 on SMC-specific transcription was mediated by an actin polymerization-dependent enhancement of MRTF nuclear localization. In support of this idea, the ability of the FHOD1 3xD variant to stimulate SM22 promoter activity was strongly inhibited by the actin polymerization inhibitor, latrunculin B (figure 3A) or by co-expression with a dominant negative MRTF (figure 3B). Furthermore, the 3xD variant synergistically enhanced transactivation of the SM22 promoter by MRTF-B, an effect not observed with WT FHOD1 (figure 3C). In excellent agreement with these findings, endogenous MRTF-A (figure 3D) and GFP-MRTF-B (figure 3E) localized to the nucleus in 100% of cells expressing the phosphomimetic FHOD1 variant while cells expressing Wt FHOD1 exhibited little MRTF nuclear localization (16 +/− 5% for MRTF-A, p<0.05; and 3 +/− 2 % for GFP-MRTF-B, p<0.05).
Figure 3. FHOD1 regulated MRTF sub-cellular localization and activity.
A) 10T1/2 cells were transfected with the 3xD FHOD1 variant and SM22 luciferase. Some cells were treated with 10μM latrunculin B (LB) for 6h prior to luciferase determination. * p<0.05 versus untreated. B) The 3xD FHOD1 variant was co-transfected with dominant negative MRTF and SM22 luciferase. * p<0.05 versus FHOD1 plus EV. C) SM22 luciferase, MRTF-B, and either WT or 3xD FHOD1 were co-transfected into 10T1/2 cells. * p<0.05 versus Wt FHOD1 plus MRTF-B. D and E) Localization of endogenous MRTF-A (D) or GFP-MRTF-B (E) in serum starved 10T1/2 cells co-expressing Wt or 3xD FHOD1.
FHOD1 is required for S1P-dependent SMC differentiation
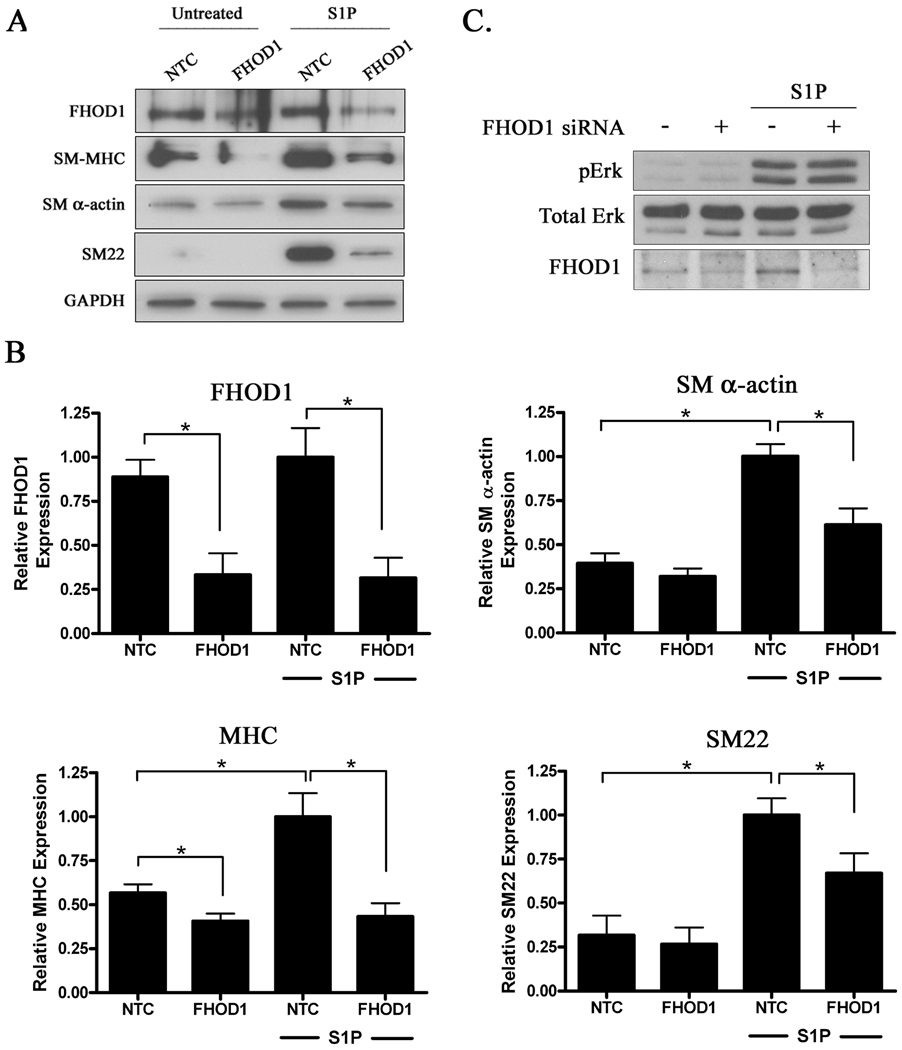
Since we have previously shown that S1P up-regulates SMC differentiation marker gene expression through a RhoA-dependent increase in actin polymerization 7, we wanted to determine whether endogenous FHOD1 was required for this response. We used standard siRNA methods to knock-down FHOD1 expression in SMCs, and as shown in figure 4b, we consistently achieved about a 60–70% reduction in FHOD1 protein when compared to cells transfected with nontargeted control siRNA. Treatment of serum-starved SMC with S1P resulted in a significant increase in endogenous SM MHC, SM22, and SM α-actin expression in control cells, and importantly this increase was significantly attenuated in the FHOD1 knock-down SMC (figure 4a). Knockdown of FHOD1 had no effect on GAPDH expression, tubulin expression (data not shown) or on S1P-dependent activation of ERK (figure 4c), indicating that depletion of FHOD1 did not have significant negative effects on normal cell function or other S1P-dependent signaling pathways.
Figure 4. Knock-down of FHOD1 in SMC inhibited SMC differentiation marker gene expression.
A) Primary aortic SMC were transfected with non-targeted control (NTC) or FHOD1 siRNAs for 72h. Following 24h of complete serum starvation, cells were treated with S1P for an additional 24h. Lysates were subjected to Western Blot analysis using the indicated antibody. B) Quantification of FHOD1 and SM marker gene expression from five independent experiments. Protein expression was normalized to GAPDH expression and is expressed relative to NTC cells treated with S1P set to 1. * p<0.05. C) Control and FHOD1 knockdown SMC were treated with S1P for 12.5 minutes. ERK activity was then measured by Western Blotting using a phospho-specific ERK antibody.
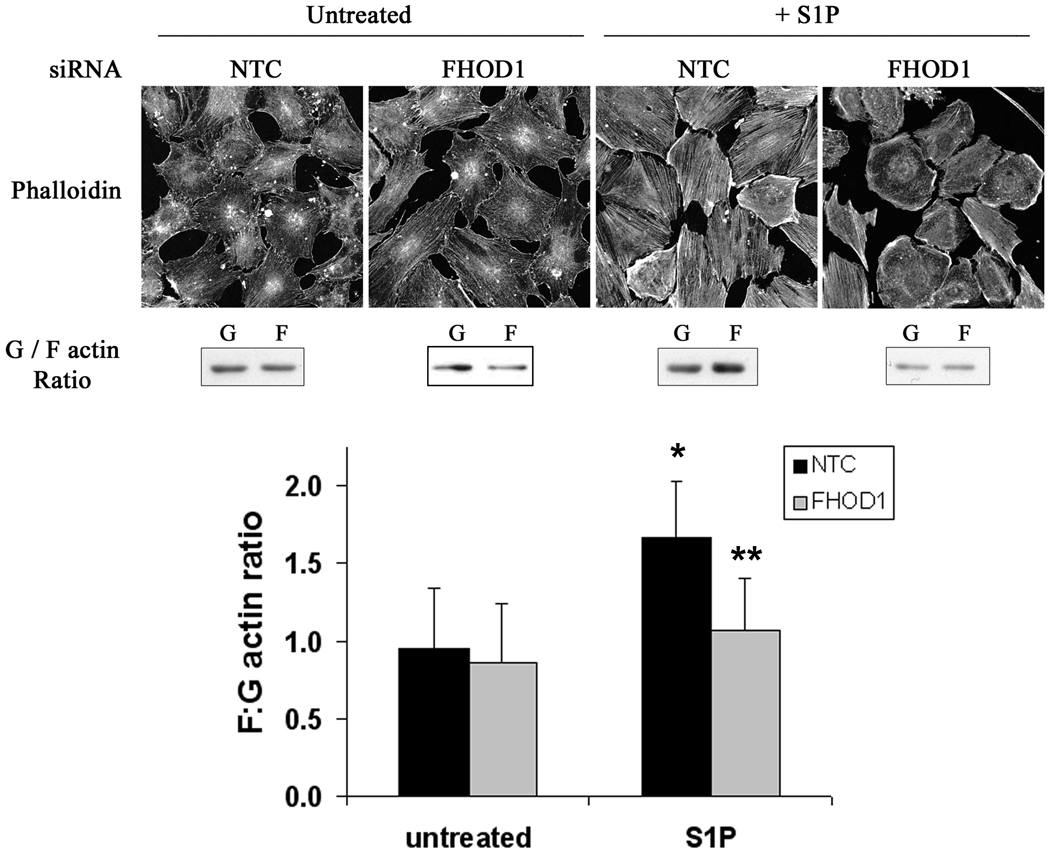
We also measured changes in actin polymerization in this model. As shown in figure 5, treatment of control SMCs with S1P increased stress fiber formation as measured by phalloidin staining and significantly increased the ratio of F-actin to G-actin as measured by actin sedimentation assays. Importantly, knock-down of FHOD1 significantly attenuated these S1P-induced changes. These results are in excellent agreement with the down regulation of SMC differentiation marker gene expression observed in FHOD1 depleted SMC and strongly suggest that FHOD1 plays a critical role in the RhoA-dependent regulation of SMC phenotype.
Figure 5. Knock-down of FHOD1 inhibited S1P-dependent actin polymerization.
SMCs were plated on 10μg/mL fibronectin, depleted of FHOD1 by siRNA for 72h, serum starved for 24h, and treated with S1P for 30 min. Actin polymerization was measured by phalloidin staining (top panels) and actin sedimentation assays (middle panels; see methods for more details). The results of three separate actin sedimentation experiments are quantified in the bottom panel. * p<0.05 versus untreated NTC; ** p<0.05 versus S1P-treated NTC.
Discussion
Extensive evidence indicates that RhoA activity stimulates SMC differentiation marker gene expression by promoting MRTF activity (reviewed in 23). This effect is mediated by increased actin polymerization and we have previously demonstrated that the RhoA effectors, mDia1 and mDia2, were involved in this response. We now demonstrate that an additional member of the mDia family, FHOD1, is also highly expressed in SMC and regulates RhoA-dependent stimulation of actin polymerization and SMC differentiation marker gene expression. Interestingly, FHOD1 activity in SMC is not directly regulated by an interaction with RhoA. Instead, our data indicate that FHOD1 is regulated by ROCK-dependent phosphorylation.
It is clear that disruption of the auto-inhibitory DID-DAD interaction is required for DRF activation. Crystal structure studies have revealed that the highly conserved amphipathic core DAD sequence, MDSLLEAL, binds tightly to a hydrophobic pocket on the DID surface15. In addition, a highly conserved basic domain just C-terminal to the core DAD contributes to the DID-DAD interaction strongly suggesting that electrostatic forces are also important 24. Although GTPase binding is critical for the activation of most DRFs, it is becoming clear that other mechanisms are also involved (see 12 for review). Our results indicate that FHOD1 is phosphorylated by ROCK in SMC and provide further support for the idea that phosphorylation disrupts the DID-DAD interaction to activate FHOD1 19. Interestingly, the 3xD phosphomimetic did not stimulate SMC-specific transcription as strongly as the constitutively active ΔDAD variant, perhaps suggesting that other factors are required for full FHOD1 activation in SMC. Providing additional support for this alternative activation mechanism, we have recently demonstrated that mDia2 activity is also enhanced by ROCK-dependent phosphorylation of two conserved S/T residues just C-terminal to the DAD basic domain (Staus and Mack, unpublished observations). Whether mDia1 or mDia3 are regulated by phosphorylation has not been examined, but this mechanism could provide specificity to DRF activation and additional spatial and/or temporal control over FHOD1 and mDia2 activity.
Because RhoA activates a number of downstream effectors that regulate actin polymerization and cytoskeletal remodeling, it has been somewhat difficult to determine the precise contributions of each to the control of SMC differentiation marker gene expression. The negative effects of Y-27632 on SMC differentiation marker gene expression have been attributed mainly to attenuation of LIM kinase/cofilin signaling. 7, 25–27. However, in light of the current results, decreased FHOD1 and mDia2 activity must also be taken into account. Our demonstration of relatively high FHOD1 expression in SMC when coupled with the results of our gain/loss of function experiments indicate that FHOD1 plays a significant role in regulating SMC phenotype. Interestingly, individual knock-downs of mDia1 or mDia2 also decreased SMC-specific gene expression in this model (data not shown) suggesting that each of these DRFs has a non-redundant function required for full MRTF activation, or alternatively, that these proteins interact functionally or physically to regulate actin polymerization. In support of the latter, we have recently demonstrated that endogenous mDia2 and FHOD1 could be co-immunoprecipitated from SMC lysates (Staus and Mack, unpublished observations). Interestingly, we have not yet observed statistically significant changes in endogenous MRTF-A nuclear localization in FHOD1 knockdown SMC upon S1P-treatment (at least at early time-points), and it is possible that FHOD1 knockdown inhibits SMC-specific gene expression by other mechanisms downstream of MRTF nuclear localization, perhaps at the level of transcription complex formation. Clearly, additional experiments will be required to determine the precise importance of each of the RhoA-dependent DRFs and whether they have specific functions in regulating SMC differentiation.
Activation of FHOD1 by DAD phosphorylation may present a unique mechanism by which different signaling pathways can converge to regulate SMC phenotype. For example, cGMP-dependent protein kinase (PKG) has been shown to promote the contractile SMC phenotype in vitro and in vivo but the mechanism by which this occurs is not well understood 28, 29. Interestingly PKG has also been shown to phosphorylate the FHOD1 DAD at Ser-1131 18, and it is intriguing to postulate that phosphorylation-dependent activation of FHOD1 may explain the effects of PKG on SMC marker gene expression.
In summary, our results indicate that FHOD1 plays a critical role in RhoA-dependent regulation of SMC-specific gene expression and that ROCK-dependent phosphorylation regulates FHOD1 activity in SMC (Figure 6). The importance of FHOD1 in SMC differentiation during vascular development and in the progression of cardiovascular disease will be critical areas for future investigation.
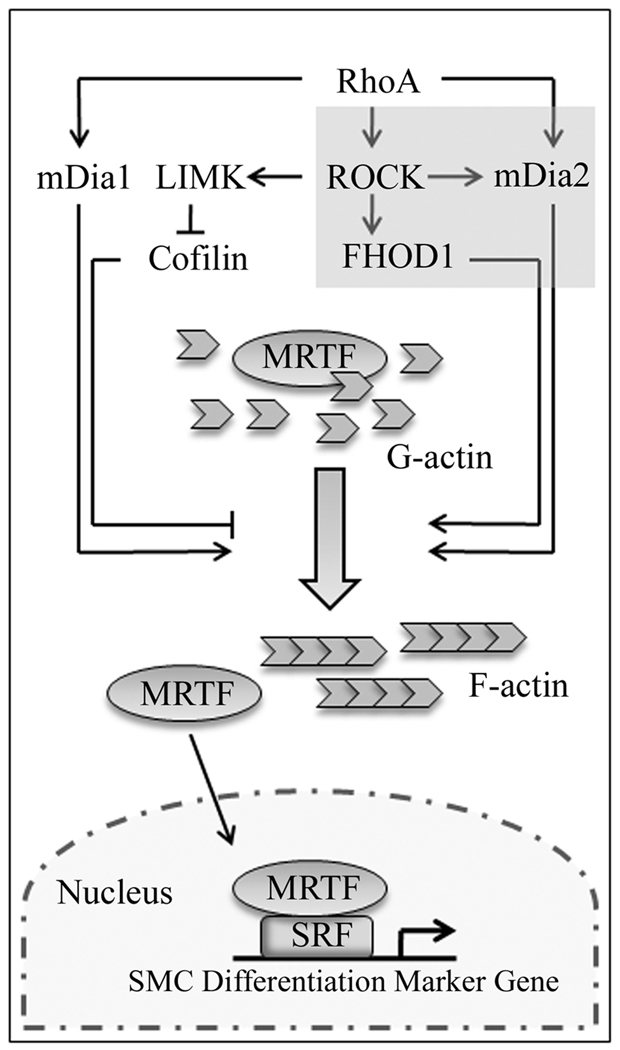
Figure 6. The RhoA-dependent signaling pathways that regulate SMC differentiation.
RhoA activates mDia1, mDia2, and ROCK through direct binding. ROCK activity stimulates actin polymerization through LIM-kinase-mediated inhibition of cofilin. Our recent results (shaded box) suggest that ROCK also enhances the activities of FHOD1 and mDia2 through direct phosphorylation of the DAD auto-regulatory domains of these proteins. Collectively these pathways reduce cellular G-actin pools leading to MRTF nuclear accumulation, and our data suggest that all three DRFs are required for full RhoA-dependent activation of SMC-specific gene expression.
Supplementary Material
Acknowledgements
none
Sources of Funding - This work was supported by NIH grants HL-070953 (CPM) HL102446 (JMT) and American Heart Association Grant 07152340U (DPS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures - none
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Landerholm TE, Dong XR, Lu J, Belaguli NS, Schwartz RJ, Majesky MW. A role for serum response factor in coronary smooth muscle differentiation from proepicardial cells. Development. 1999;126:2053–2062. doi: 10.1242/dev.126.10.2053. [DOI] [PubMed] [Google Scholar]
- 3.Niu Z, Yu W, Zhang SX, Barron M, Belaguli NS, Schneider MD, Parmacek M, Nordheim A, Schwartz RJ. Conditional mutagenesis of the murine serum response factor gene blocks cardiogenesis and the transcription of downstream gene targets. J Biol Chem. 2005;280:32531–32538. doi: 10.1074/jbc.M501372200. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Chang S, Qi X, Richardson JA, Olson EN. Requirement of a myocardin-related transcription factor for development of mammary myoepithelial cells. Mol Cell Biol. 2006;26:5797–5808. doi: 10.1128/MCB.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Zhu X, Chen M, Cheng L, Zhou D, Lu MM, Du K, Epstein JA, Parmacek MS. Myocardin-related transcription factor B is required in cardiac neural crest for smooth muscle differentiation and cardiovascular development. Proc Natl Acad Sci U S A. 2005;102:8916–8921. doi: 10.1073/pnas.0503741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 7.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-Phosphate Stimulates Smooth Muscle Cell Differentiation and Proliferation by Activating Separate Serum Response Factor Co-factors. J Biol Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- 8.Cen B, Selvaraj A, Burgess RC, Hitzler JK, Ma Z, Morris SW, Prywes R. Megakaryoblastic leukemia 1, a potent transcriptional coactivator for serum response factor (SRF), is required for serum induction of SRF target genes. Mol Cell Biol. 2003;23:6597–6608. doi: 10.1128/MCB.23.18.6597-6608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinson JS, Medlin MD, Lockman K, Taylor JM, Mack CP. Smooth muscle cell-specific transcription is regulated by nuclear localization of the myocardin-related transcription factors. Am J Physiol Heart Circ Physiol. 2007;292:H1170–H1180. doi: 10.1152/ajpheart.00864.2006. [DOI] [PubMed] [Google Scholar]
- 10.Mack CP, Somlyo AV, Hautmann M, Somlyo AP, Owens GK. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J Biol Chem. 2001;276:341–347. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- 11.Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV, Owens GK. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a rho kinase/myocardin/SRF-dependent mechanism. Circ Res. 2004;95:406–414. doi: 10.1161/01.RES.0000138582.36921.9e. [DOI] [PubMed] [Google Scholar]
- 12.Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol. 11:62–74. doi: 10.1038/nrm2816. [DOI] [PubMed] [Google Scholar]
- 13.Schonichen A, Geyer M. Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochim Biophys Acta. 1803:152–163. doi: 10.1016/j.bbamcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Higgs HN. Dissecting requirements for auto-inhibition of actin nucleation by the formin, mDia1. J Biol Chem. 2005;280:6986–6992. doi: 10.1074/jbc.M411605200. [DOI] [PubMed] [Google Scholar]
- 15.Nezami AG, Poy F, Eck MJ. Structure of the autoinhibitory switch in formin mDia1. Structure. 2006;14:257–263. doi: 10.1016/j.str.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Staus DP, Blaker AL, Taylor JM, Mack CP. Diaphanous 1 and 2 regulate smooth muscle cell differentiation by activating the myocardin-related transcription factors. Arterioscler Thromb Vasc Biol. 2007;27:478–486. doi: 10.1161/01.ATV.0000255559.77687.c1. [DOI] [PubMed] [Google Scholar]
- 17.Schulte A, Stolp B, Schonichen A, Pylypenko O, Rak A, Fackler OT, Geyer M. The human formin FHOD1 contains a bipartite structure of FH3 and GTPase-binding domains required for activation. Structure. 2008;16:1313–1323. doi: 10.1016/j.str.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, El-Zaru MR, Surks HK, Mendelsohn ME. Formin homology domain protein (FHOD1) is a cyclic GMP-dependent protein kinase I-binding protein and substrate in vascular smooth muscle cells. J Biol Chem. 2004;279:24420–24426. doi: 10.1074/jbc.M313823200. [DOI] [PubMed] [Google Scholar]
- 19.Takeya R, Taniguchi K, Narumiya S, Sumimoto H. The mammalian formin FHOD1 is activated through phosphorylation by ROCK and mediates thrombin-induced stress fibre formation in endothelial cells. Embo J. 2008;27:618–628. doi: 10.1038/emboj.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mack CP, Thompson MM, Lawrenz-Smith S, Owens GK. Smooth muscle alpha-actin CArG elements coordinate formation of a smooth muscle cell-selective, serum response factor-containing activation complex. Circ Res. 2000;86:221–232. doi: 10.1161/01.res.86.2.221. [DOI] [PubMed] [Google Scholar]
- 21.Jahraus A, Egeberg M, Hinner B, Habermann A, Sackman E, Pralle A, Faulstich H, Rybin V, Defacque H, Griffiths G. ATP-dependent membrane assembly of F-actin facilitates membrane fusion. Mol Biol Cell. 2001;12:155–170. doi: 10.1091/mbc.12.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res. 2007;100:633–644. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 24.Wallar BJ, Stropich BN, Schoenherr JA, Holman HA, Kitchen SM, Alberts AS. The basic region of the diaphanous-autoregulatory domain (DAD) is required for autoregulatory interactions with the diaphanous-related formin inhibitory domain. J Biol Chem. 2006;281:4300–4307. doi: 10.1074/jbc.M510277200. [DOI] [PubMed] [Google Scholar]
- 25.Zhao XH, Laschinger C, Arora P, Szaszi K, Kapus A, McCulloch CA. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J Cell Sci. 2007;120:1801–1809. doi: 10.1242/jcs.001586. [DOI] [PubMed] [Google Scholar]
- 26.Harvey KA, Welch Z, Sliva D, Siddiqui RA. Role of Rho kinase in sphingosine 1-phosphate-mediated endothelial and smooth muscle cell migration and differentiation. Mol Cell Biochem. doi: 10.1007/s11010-010-0461-2. [DOI] [PubMed] [Google Scholar]
- 27.Jeon ES, Park WS, Lee MJ, Kim YM, Han J, Kim JH. A Rho kinase/myocardin-related transcription factor-A-dependent mechanism underlies the sphingosylphosphorylcholine-induced differentiation of mesenchymal stem cells into contractile smooth muscle cells. Circ Res. 2008;103:635–642. doi: 10.1161/CIRCRESAHA.108.180885. [DOI] [PubMed] [Google Scholar]
- 28.Anderson PG, Boerth NJ, Liu M, McNamara DB, Cornwell TL, Lincoln TM. Cyclic GMP-dependent protein kinase expression in coronary arterial smooth muscle in response to balloon catheter injury. Arterioscler Thromb Vasc Biol. 2000;20:2192–2197. doi: 10.1161/01.atv.20.10.2192. [DOI] [PubMed] [Google Scholar]
- 29.Boerth NJ, Dey NB, Cornwell TL, Lincoln TM. Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype. J Vasc Res. 1997;34:245–259. doi: 10.1159/000159231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.