Abstract
Urothelial carcinoma (UC) is the most common type of bladder cancer in Western nations. Most patients present with the non-muscle-invasive (NMIUC) form of the disease, while up to a third harbour the invasive form (MIUC). Specifically, the aetiology of NMIUC appears to be multifactorial and very different from that of MIUC. Loss of specific tumour suppressor genes as well as gain-of-function mutations in proteins within defined cellular signalling pathways have been implicated in NMIUC aetiology. The regions of chromosome 9 that harbour CDKN2A, CDKN2B, TSC1, PTCH1 and DBC1 are frequently mutated in NMIUC, resulting in functional loss; in addition, HRAS and FGFR3, which are both proto-oncogenes encoding components of the Ras–MAPK signalling pathway, have been found to harbour activating mutations in a large number of NMIUCs. Interestingly, some of these molecular events are mutually exclusive, suggesting functional equivalence. Since several of these driving changes are amenable to therapeutic targeting, understanding the signalling events in NMIUC may offer novel approaches to manage the recurrence and progression of this disease.
Globally, approximately 357 000 individuals are diagnosed with bladder carcinoma each year, with about 145 000 people dying from the disease (Ref. 1). The most common histological type of bladder cancer in Western countries has been termed urothelial carcinoma (UC) by the World Health Organization 2004 classification of bladder tumours (Ref. 2); it was formerly known as transitional cell carcinoma. Squamous cell carcinoma (SCC) of the bladder is also common worldwide, and other less common tumours include adenocarcinoma and small-cell carcinoma. It is estimated that there were approximately 71 000 new cases of UC and 14 300 deaths in the USA in 2009 as a result of the disease (Ref. 3). There are multiple risk factors for UC, which include exposure to tobacco and industrial chemicals, ingestion of arsenic-laced water, radiation therapy to organs adjacent to the bladder, therapeutic use of alkylating agents in chemotherapy regimens, and infection with the trematode Schistosoma haematobium. The latter risk factor is closely associated with SCC of the bladder (Ref. 4).
Approximately 80% of patients with UC present with non-muscle-invasive urothelial carcinoma (NMIUC; cancer stages Ta, Tis and T1), which can be successfully treated with local endoscopic resection, although a significant number of these cases exhibit recurrence with or without progression to invasive disease (Refs 5, 6). The remainder of patients present with muscle-invasive urothelial carcinoma (MIUC; cancer stages T2–4), which is commonly treated with a radical cystectomy or chemoradiotherapy. Despite aggressive treatment, approximately 50% of these MIUC patients develop subsequent metastatic disease, with most succumbing within two years of diagnosis (Refs 7, 8, 9). Each tumour stage within the NMIUC and MIUC subtypes can be associated with a grade (G1, G2, G3) that corresponds to cellular differentiation of the tumour cells in question. G1 tumours are morphologically well differentiated, whereas G3 tumours are morphologically poorly differentiated. G3 tumours are more aggressive and have a greater tendency to progress to a higher stage cancer.
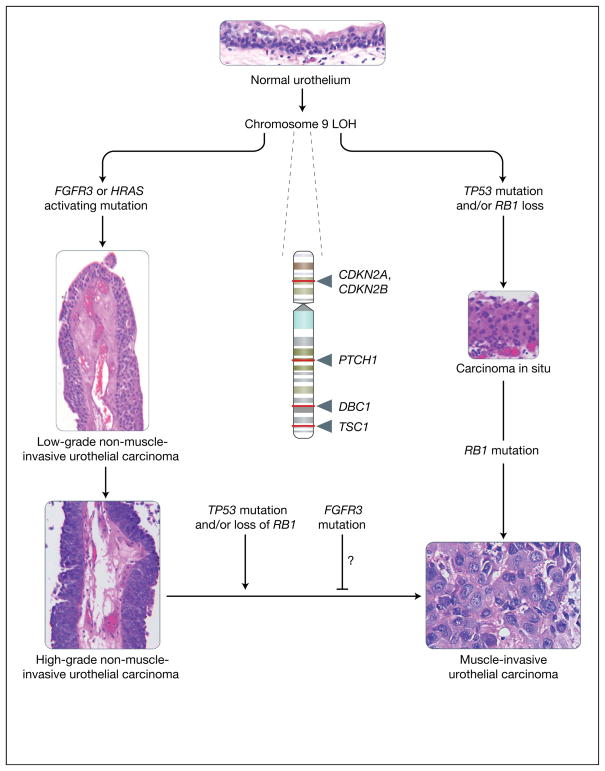
Evidence suggests that NMIUCs have a different aetiology to that of their invasive counterparts (Refs 10, 11, 12, 13, 14, 15). NMIUCs are thought to arise from localised urothelial hyperplasia, while MIUCs are thought to arise de novo from high-grade carcinoma in situ (CIS) lesions without necessarily having antecedent urothelial hyperplasia. These two types of tumours have a very different set of genetic lesions, which include activation of the intracellular GTPase HRAS and the receptor FGFR3 (fibroblast growth factor receptor 3) in the noninvasive subtype, and defects in the p53 (TP53) and RB (RB1; retinoblastoma 1) tumour suppressor pathways in the invasive subtype (Fig. 1). Loss of heterozygosity (LOH) of chromosome 9 is frequently found in both tumour types (Fig. 1). In this review, the genetic alterations that correspond to the noninvasive subtype of UCs are discussed with an emphasis on their functional relevance to pathogenesis, their value in assessment of prognosis, and their promise as therapeutic targets. For common genetic alterations in MIUC see the excellent recent review by Wu (Ref. 11).
Figure 1. Molecular lineage of non-muscle-invasive urothelial cancer versus muscle-invasive urothelial cancer.
The figure summarises the major molecular alterations that are responsible for the onset of non-muscle-invasive urothelial carcinomas (NMIUCs) and muscle invasive urothelial carcinomas (MIUCs), along with providing representative haematoxylin and eosin micrographs of these disease states. Starting from a normal urothelium five to seven cells thick (top), loss of heterozygosity (LOH) of chromosome 9 (arrows indicate relative sites of potential tumour suppressor gene loss; names of genes are given) has been associated with the majority of urothelial cancers. This is often coincident with a mutation in either FGFR3 or HRAS, which have been implicated in the genesis of NMIUCs (low- and high-grade papillary NMIUCs indicated on the left show examples of the increasing degree of architectural disorder and increasing cytologic atypia). FGFR3 and HRAS mutations are usually not present within the same cancer. By contrast, from a molecular standpoint, the MIUC class of tumours is defined by mutations in the tumour suppressor genes TP53 and RB1, which frequently coincide in the same tumours. Generally, MIUCs are thought to arise de novo from carcinoma in situ (shown on the right). Histologically, marked cytologic atypia (high ratio of nuclei to cytoplasm, mitoses, and total loss of tissue architecture) are present (bottom right) in a context of observation of definitive invasion of the muscularis propria of the bladder, in contrast to NMIUCs. As a class, NMIUCs have a high tendency to recur and rarely progress to a higher-grade carcinoma or MIUC. When this progression does occur, it is usually coincident with loss of function of the tumour suppressors TP53 or RB1. Some studies have shown that the presence of activating FGFR3 mutations correlate to a reduced frequency of progression to a higher-grade/stage cancer, but these events have not been definitively shown to be causal. CDKN2A, cyclin-dependent kinase inhibitor 2A (also known as P16); CDKN2B, cyclin-dependent kinase inhibitor 2B (also known as P15); DBC1, deleted in bladder cancer 1; FGFR3, fibroblast growth factor receptor 3; HRAS, Harvey rat sarcoma viral gene homologue; PTCH1, patched homologue 1; RB1, retinoblastoma tumour suppressor; TP53, p53 tumour suppressor; TSC1, tuberous sclerosis 1 gene. [Images: S.C. Smith. Originally photographed at 40× (NMIUCs) and 60× (MIUC, carcinoma in situ and urothelium)].
Alterations on chromosome 9
LOH is common in cancer. In NMIUC, LOH of chromosome 9 is present at the earliest stages of development and is the most frequent chromosomal deletion. One of the first studies noting this phenomenon identified that a large number of human bladder cancer specimens contained allelic loss of chromosome 9q independent of tumour grade (Ref. 16). Subsequently, multiple groups sought to identify the specific regions of mutation on chromosome 9. After analysing 252 bladder specimens, one group identified that 50% of Ta tumours contained LOH on either 9p or 9q and speculated that these regions were important in early UC development and likely to harbour tumour suppressor gene loci (Ref. 17). Later studies that employed restriction fragment length polymorphism (RFLP) mapping and microsatellite analysis of chromosome 9 implicated the region between 9p12 and 9q34.1 as potential sites for tumour suppressor genes (Refs 18, 19, 20, 21, 22, 23). Finer mapping of chromosome 9 led to the identification of specific tumour suppressor genes lost as a result of these chromosomal changes.
Chromosome 9p
The 9p chromosomal arm contains the best-characterised genetic lesions of bladder cancer, and its importance in the molecular pathogenesis of the disease is supported by similar lesions occurring in both UC and SCC despite these forms being strongly associated with different environmental risk factors (Ref. 4). Among the most common lesions are those in the gene encoding P15 (CDKN2B; also known as INK4B and MTS2) and P16 (CDKN2A; also known as INK4A and MTS1). Both P15 and P16 are important potential tumour suppressors because of their respective roles as negative regulators of the cell cycle: they inhibit the activity of the complex of cyclin D and cyclin-dependent kinase 4 (CDK4) to stop cell proliferation at the G1 phase of the cell cycle (Refs 24, 25). Mechanistically, this function is important because the CDK4 complex has been shown to catalyse the phosphorylation of RB; this in turn releases the transcription factor E2F, which is involved in regulating transcriptional targets essential for the transition from the G1 to S phase of the cell cycle (Refs 26, 27).
In the mid 1990s, several independent studies identified alterations in CDKN2A and CDKN2B in UC. A comparative multiplex polymerase chain reaction (PCR) approach revealed homozygous deletions and sequence mutations in CDKN2A in SCC that were present at three times the frequency seen in UC (Ref. 28). In addition, hemizygous and homozygous deletions were shown to be present in 92% of SCCs on chromosome 9p but in only 39% of UCs, and deletions in 9p without concomitant mutation in 9q were found at a rate of 92%, compared with only 10% of UCs having this pattern. Around the same time that the association of CDKN2A with SCC was being established, another group noted significant de novo methylation of the 5′ CpG islands at the CDKN2A locus in 67% of a cohort of 18 UC tissue samples, and suggested that this methylation might be responsible for inactivating the gene (Ref. 29). In addition, statistically significant frequencies of homozygous and hemizygous deletions in both CDKN2A and CDKN2B were reported for a cohort of patients with UC (Ref. 30). Homozygous deletions in both these genes were also found in a cohort of 13 human bladder cancer cell lines (Ref. 31), providing support for the clinical relevance of these lines in laboratory experiments as models of UC. Finally, CDKN2B was found to have decreased mRNA expression levels in NMIUC (Ref. 32). Taken together, these findings implicate the 9p21 locus, including the CDKN2A and CDKN2B tumour suppressor genes, in both SCC and UC, albeit with different frequencies of alteration in each histological subtype.
Chromosome 9q
Identification of candidate tumour suppressor genes on 9q initially proved difficult because of a lack of high-frequency subchromosomal deletions on this arm. The first indication of chromosome 9q as a possible site of tumour suppressor gene inactivation in UC was provided when a common partial deletion was found on this chromosome in a small portion of cases (5 of 70 cases) (Ref. 21). It was later shown using single-strand conformation polymorphism analysis (SSCP) that there are multiple nonsense and missense mutations in 9q34, a region that encompasses the TSC1 (tuberous sclerosis 1) gene and is frequently deleted (Ref. 33). TSC1 has been shown to be silenced in cases of tuberous sclerosis harmatomas and is speculated to act as a tumour suppressor (Refs 34, 35). When the mutations found on chromosome 9q were analysed, 90% were found to be novel and specific to bladder cancer in that they were not found in previous germline tuberous sclerosis cases (Ref. 36). It was later shown that missense mutations in the TSC1 gene induced mislocalisation of the TCS1 protein in bladder cancer cells (Ref. 37). This study also identified that when these same missense mutations were present in tuberous sclerosis, which was infrequent, they played no role in causation of tuberous sclerosis.
LOH at chromosome 9q34 was associated with decreased expression of P27, a CDK inhibitor protein encoded by CDKN1B, in a cohort of four tumours, suggesting that there might be a relationship between loss-of-function mutations in TSC1 and CDKN1B in UC (Ref. 38). In addition to TSC1, the 9q32–q33 locus was identified as having frequent partial deletions in UC (Ref. 39). This region was later found to contain a potential tumour suppressor gene, DBC1 (‘deleted in bladder cancer gene 1’; originally named DBCCR1), by searching for expressed sequence tags (ESTs) that map to yeast artificial chromosomes (YACs) spanning the region (Ref. 40). Expression of DBC1 mRNA was not detected in 50% (5 of 10) of bladder cancer cell lines evaluated. It was also found that the DBC1 gene is a frequent target for hypermethylation, suggesting that hypermethylation at this locus could be important in the early development of bladder tumour formation even in cases where deletion does not occur (Ref. 40).
An initial report of frequent deletion mutations on chromosome 9q in the region spanning q22.3–q31 (Ref. 23) was later validated using microsatellite analysis following a primer-extension preamplification technique (Ref. 41). Specifically, the 9q22.3 locus was found to harbour deletions in the region encompassing PTCH1 (patched homologue 1) and MSSE (multiple self-healing squamous epithelioma) genes, both of which are known to play important roles in diseases characterised by the development of skin tumours. These findings suggest that mutations in the q22.3–q31 region of chromosome 9 are important in UC development.
Prognostic significance of LOH on chromosome 9 for recurrence following NMIUC
As the tumour suppressor role of chromosome 9 in NMIUC became more established, a question arose of whether LOH on chromosome 9 could be used as a prognostic factor regarding the disease course of patients with NMIUC. Comparison of recurrent UCs with nonrecurrent UCs found that 54% of recurrent versus 12% of nonrecurrent tissue samples harboured LOH on chromosome 9 (Ref. 42). In this study, microsatellite markers for regions on chromosome 9p and 9q that encompass putative tumour suppressor genes CDKN2A, DBC1 and TSC1 were analysed. A significant association between risk of recurrence and LOH at the region encompassing TSC1 was noted, whereas LOH at CDKN2A or DBC1 did not show any significant relation to tumour recurrence. In addition, a later study showed that LOH of the region of chromosome 9p21 encompassing CDKN2A was not significantly associated with recurrence-free interval in NMIUC (Ref. 43). In contrast, another group, which utilised PCR analysis, identified that 33 of 56 (58.9%) patients with low-grade (G1–G2) UC had loss of CDKN2A at the 9p21 locus, and that loss was significantly associated with reduced recurrence-free probability (Ref. 44). Furthermore, a separate study noted that 12 of 33 (36%) patients who had recurrent UC demonstrated LOH on chromosome 9q12 while only one patient with nonrecurrent UC showed LOH of chromosome 9 (Ref. 45). Additional support for the positive correlation between LOH of chromosome 9 and tumour recurrence included a recent study that showed LOH at the DCB1 locus (9q32–q33) was correlated positively with tumour recurrence of NMIUC only if the LOH was located in the normal urothelium adjacent to the tumour and not in the tumour itself. This suggests that when LOH occurs at the DBC1 locus in the NMIUC itself the recurrence of that tumour is less likely (Ref. 46). In this study, it was shown that histologically normal urothelium with LOH at the DCB1 locus was associated with larger tumours than in cases without this anomaly.
In summary, the body of data regarding chromosome 9 seems to suggest that LOH at chromosome 9q has more prognostic value than LOH at chromosome 9p, although different studies employing varying technologies have made sometimes disparate findings. The presence of candidate tumour suppressor genes on each of these chromosome regions warrants further investigation into their potential use as prognostic factors for tumour recurrence following an initial diagnosis of NMIUC, and we suggest that such studies employ standardised technologies for detection of genetic lesions as well as large interinstitutional cohorts to favour findings with general applicability.
Fibroblast growth factor receptor 3
Mutation and overexpression of FGFR3 in urothelial cancer
One of the most compelling findings in recent years regarding the molecular pathogenesis of UC has been the role discovered for FGFR3. FGFR3 belongs to a five-member family of structurally related tyrosine kinase receptors, where each protein in the family is encoded by a different gene. The basic structure of the FGFR family is a glycoprotein that contains three immunoglobulin (Ig)-like domains (extracellular ligand-binding domain), a transmembrane helix domain, and an intracellular tyrosine kinase domain (Refs 47, 48, 49). FGFR3 is known to have two major splice isoforms, FGFR3b and FGFR3c, with the former being most prevalent in epithelial tissue and the latter being associated with mesenchymal tissue (Ref. 50). FGFR3 first became relevant to human disease when specific point mutations in different domains of the FGFR3 gene were discovered in various autosomal dominant human skeletal diseases including achondroplasia, hypochondroplasia, thanatophoric dysplasia I and II, and severe achondroplaisa with developmental delay and acanthosis nigrans (SADDAN) (Refs 51, 52, 53, 54, 55, 56, 57, 58, 59).
Interestingly, the mutations found to be responsible for the aforementioned debilitating autosomal dominant human skeletal diseases were found to be prevalent in human bladder cancer. In 1999, somatic missense mutations (R248C, S249C, G370/372C, K650/652E) were reported in 35% (9 of 26) of UCs and 25% (3 of 12) of cervical carcinomas examined (Ref. 60). All mutations identified in the UC and cervical carcinoma samples were identical to the mutations found in the autosomal dominant human skeletal disease thanatophoric dysplasia. It was later shown that the mutations in FGFR3 that correspond to the mutations in the autosomal dominant human skeletal diseases occur at a much higher rate in UCs than in cervical carcinomas and multiple myeloma and are virtually nonexistent in carcinomas of the upper aerodigestive tract, oesophagus, stomach, lung, rectum, colon, prostate, ovarian, breast, brain or kidneys (Refs 61, 62).
Interestingly, the R248C and K650/652E mutations had previously been implicated in constitutive activation of FGFR3 (Refs 63, 64), thus hinting at a mechanism of the oncogenic role these mutations might play in bladder cancer. The R248C mutation has been shown to constitutively activate FGFR3 through exposure of a cysteine residue that can participate in an intermolecular disulfide bond, resulting in ligand-independent dimerisation of the receptor (Ref. 64). The K650/652E mutation is thought to constitutively activate the FGFR3 receptor by altering the conformation of the activation loop in the kinase domain of the receptor (Refs 63, 64). It was later shown that the oncogenic role of mutated FGFR3 might be working through the Ras–MAPK/ERK (mitogen-activated protein kinase or extracellular-signal-regulated kinase) pathway. Constitutively active FGFR3 was able to transform NIH 3T3 cells with corresponding activation of the Ras–MAPK and phosphoinositide 3-kinase (PI3K) signalling pathways (Ref. 65); in addition, FGFR3 was shown to interact with the Ras-coupling molecules SHC1, GRB2, 80K-H (PRKCSH), SOS1 and an unidentified 66 kDa protein to modulate Ras signalling through RAF1 (Ref. 66). In fact, a recent report also indirectly hinted at the importance of the Ras pathway downstream of FGFR3 by finding that Ras mutations, also common in NMIUC (see section below), and mutations in FGFR3 are mutually exclusive in this tumour type, suggestive of functional equivalence (Ref. 67). Taken together, these findings implicate FGFR3 as an oncogene in UC that likely acts through the Ras–MAPK signalling pathway.
The association of FGFR3 alterations and tumour stage in urothelial carcinoma
The discovery of somatic missense mutations in the FGFR3 gene in human bladder cancer spawned the question of where in the pathogenesis of UC were these mutations relevant. One study found that 34 of 53 TaG1–2 bladder cancer samples had somatic missense mutations leading to amino acid changes that were identical to those found in the autosomal dominant skeletal diseases (Ref. 68). Furthermore, none of the 19 higher-stage tumours had such mutations. Another study found that the frequency of mutations was significantly higher in Ta tumours (74%; 37 of 50) than Tis (0%; 0 of 20), T1 (21%; 4 of 19) and T2–4 (16%; 7 of 43) (Ref. 69). Furthermore, when stratifying by grade, FGFR3 mutations were more prevalent in G1 (84%; 27 of 32) tumours than G2 (55%; 16 of 29) or G3 (7%; 5 of 71) tumours.
These differences in expression of mutant FGFR3 highlight an important point concerning NMIUCs. Multiple studies have shown that Ta tumours tend to exhibit a different natural history and clinical behaviour than Tis or T1 tumours: in particular, the Ta group, especially low-grade Ta, have very low rates of progression to higher-stage cancers (Refs 70, 71, 72). In tandem, genomic and transcriptomic studies of the Ta and Tis/T1 tumours suggest they have contrasting gene expression profiles (Refs 73, 74, 75). Taken together these findings support the assumption that the Ta tumours have a distinct aetiology that is separate from that of the higher-stage urothelial tumours, even when considering tumours that are technically non-muscle-invasive (i.e. Tis, T1).
Similar to the previous study that showed a higher frequency of FGFR3 mutation in noninvasive tumours, another group showed that in a cohort of 81 patients there was an inverse correlation between FGFR3 mutations and grade/stage of bladder cancer (Ref. 76). A study that looked at FGFR3 mutations in urothelial papillomas, considered the least aggressive of urothelial tumours, found that 75% (9 of 12) of urothelial papillomas evaluated harboured FGFR3 mutations (Ref. 77). This frequency was similar to the frequency of FGFR3 mutations in papillary urothelial neoplasms of low malignant potential (85%) and low-grade papillary UCs (88%). Thus, several studies validate that the FGFR3 mutation is important in the pathogenesis NMIUC, but also suggest that the FGFR3 mutation is not essential or is possibly inversely associated with progression to high-stage/grade bladder cancer.
FGFR3 and p53: divergent oncogenic pathways
As FGFR3 somatic mutations are the most common somatic mutation in NMIUC while TP53 and RB1 mutations were noted to be frequent in MIUC (Refs 70, 78), the idea of a dual-track pathway implicating alterations in FGFR3 in NMIUC versus TP53 in MIUC arose (Ref. 79). When the mutational status of FGFR3 and TP53 in a cohort of 81 newly diagnosed UCs was evaluated, a strong association of FGFR3 mutations with low-stage/grade cancers was found while mutation in TP53 was almost exclusively associated with high-stage/grade bladder cancer (Ref. 12). In a separate study that examined DNA from 260 UC tissue samples, only 5.7% had concomitant mutations in FGFR3 and overexpression of TP53 (which is generally presumed to reflect missense mutation of the TP53 gene conferring supraphysiological protein stability), while 72.7% of the samples were positive for either FGFR3 or TP53 individually (Ref. 80). Furthermore, mutations in FGFR3 and TP53 overexpression were found to be dependent on stage and grade. Mutations in FGFR3 were present in 77% of Ta, 31% of T1, and 15% of ≥T2 tumours; in relation to grade, mutations were found in 85% of urothelial neoplasia of low malignant potential, and in 71% of low-grade and 26% of high-grade cases. By contrast, overexpression of TP53 was present in 11% of Ta, 51% of T1, and 56% of ≥T2 tumours; in relation to grade, overexpression was present in 1% of LMP, and in 15% of low-grade and 54% of high-grade cases. This evidence further suggested that FGFR3 and TP53 are on two separate tracks in UC. Other work has also found an inverse relationship between mutations in FGFR3 and bladder tumour stage/grade, and a positive correlation between mutations in TP53 and bladder tumour stage/grade (Ref. 13). In 2006, a study looking at expression of FGFR3 in bladder tumours showed that loss of FGFR3 expression was positively correlated with tumour stage (Ref. 81). In addition, FGFR3+/TP53− tumours had a slower recurrence rate than the other tumours studied (FGFR3+/TP53+, FGFR3−/TP53− and FGFR3−/TP53+). Furthermore, FGFR3 mutations were found in 45% (9/20) of examined inverted papillomas (which are usually benign) while there were no TP53 mutations in this cohort (Ref. 82). Taken together, these data support distinct pathways leading to NMIUC versus MIUC (Fig. 1).
Prognostic significance of FGFR3 alterations
The inverse correlation between the presence of FGFR3 mutations and UC grade and stage that was established by various independent studies raised an intriguing question: can the mutational status of FGFR3 predict disease recurrence or progression in patients with low-stage/grade bladder cancer? One of the first studies to address this important question looked at disease recurrence in a cohort of 57 patients with bladder cancer (Ref. 68). It was noted that 14 of 23 patients with wild-type FGFR3 developed recurrent tumours within a year while only 7 of 34 patients with mutant FGFR3 developed recurrent tumours. The mean recurrence rates per year for tumours harbouring wild-type FGFR3 and mutant FGFR3 were 1.12 and 0.24, respectively. In 2003, this same group found that evaluation of FGFR3 mutational status is a better and more reproducible predictor than pathological grade of tumour recurrence rate, progression, and disease-specific survival (Ref. 83). A later study showed that in a cohort of 772 patients having NMIUCs (including TaG1, TaG2, TaG3 and T1G3 tumours) those tumours with mutations of FGFR3 trended towards reduced rates of progression but increased rates of recurrence when compared with tumours expressing wild-type FGFR3 (Ref. 84), contrasting with prior studies. Another study showed that while the mutational status of FGFR3 was generally not reliable in predicting disease recurrence in a cohort of 221 patients, in a particular subset of these patients (high-grade NMIUC) mutant FGFR3 corresponded with decreased progression when compared with high-grade NMIUC cases with wild-type FGFR3 (P = 0.009) (Ref. 85). An immunohistochemistry study reported that in papillary urothelial neoplasm of low malignant potential (PUNLMP) there was strong staining intensity of FGFR3 in nonrecurrent PUNLMP (80.5%) and weaker staining intensity in recurrent PUNLMP (56.4%), which suggests that expression of FGFR3 without regards to mutational status of the protein might be useful in defining low-grade/stage and nonrecurring tumours (Ref. 86). Interestingly, no correlation between mutational status of FGFR3 and ability to predict disease recurrence and survival was found in T1G3 tumours, leading to the suggestion that T1G3 tumours are at a crossroads between the FGFR3 and p53 oncogenic pathway and that the role of FGFR3 in this subset of tumours is diminished and less significant (Ref. 14). In summary, the preponderance of data suggests that FGFR3 mutation status might serve as a predictor of reduced progression of NMIUCs, although large, prospective interinstitutional studies will be necessary to resolve this conundrum definitively. There has been no study to date that has definitively developed a relationship between mutant FGFR3 and tumour recurrence.
These findings could potentially set the stage for evaluation of FGFR3 mutational status as a precursor to therapeutic intervention. For example, as has been noted for other receptor tyrosine kinases such as EGFR (epidermal growth factor receptor) (Ref. 87), mutational status might correlate to exquisite sensitivity of the tumour to pharmacological inhibition of the receptor, through a process that has been termed oncogene addiction (Ref. 88). In such a scenario, even though a mutant-FGFR3-harbouring tumour carries a relatively favourable prognosis, it could be treated and exhibit dramatic responses to a noninvasive, targeted modality like a small-molecule kinase inhibitor. However, given the positive prognostic associations between FGFR3 mutation and nonprogression, one could also imagine a scenario where tumours that harbour FGFR3-activating mutations might be contraindicated for FGFR3-targeted therapy, and where such therapy could paradoxically induce tumour aggression. These issues beg the question of whether FGFR3’s role in this setting is that of a marker or a mediator – that is, does it simply serve as a biomarker on low-risk tumours, or does it actually induce a signalling pathway that prevents tumour invasion that could be reactivated upon its inhibition? Clearly a deeper knowledge of this key molecule, from a clinicopathological and a molecular cell biological standpoint, will be necessary as a justification and prerequisite of implementation of future FGFR3-targeted therapies, the development of which is discussed below.
Targeted therapy against FGFR3
As the importance of FGFR3 in bladder cancer becomes better understood, recent efforts have been devoted to finding ways to disrupt FGFR3 signalling in order to treat NMIUC. There have been several in vitro studies using small-molecule inhibitors designed against FGFR3 (SU5402, SU6668, CHIR-258, PD173074) that have shown strong in vitro inhibition (Refs 89, 90, 91, 92, 93, 94) (Table 1). Many of these studies have used multiple myeloma cell lines as they have been shown to harbour activating FGFR3 mutations, although these mutations are different from those generally in NMIUC. Nevertheless, these findings suggest a potential role for the use of FGFR3 inhibitors in UC.
Table 1.
Targeted therapeutics for FGFR3, HRAS and the MAPK signalling pathway
| Molecule/class | Target | In vitro effects | In vivo effects | Refs |
|---|---|---|---|---|
| SU5402/small-molecule inhibitor | FGFR3 | Inhibits growth, reduces cell viability (dose dependently), increases apoptosis in myeloma cell lines with t(4;14)(p16.3;q32) translocation | Not reported | 91 |
| SU6668/small-molecule inhibitor | FGFR3 | Inhibits tyrosine kinase ability and mitogenesis in fibroblastic and endothelial cells, respectively | Inhibits growth of glioma, melanoma, lung, colon, ovarian and epidermoid xenograft tumours; suppresses angiogenesis in glioma animal model | 94 |
| CHIR-258/small-molecule inhibitor | FGFR3 | Inhibits growth and downstream ERK signalling; produces cytotoxic and cytostatic effects (all in myeloma cell line) | Reduces tumour growth in mouse xenograft (myeloma cell line) | 89 |
| PD173074/small-molecule inhibitor | FGFR3 | Inhibits growth, reduces cell viability (dose dependently), increases apoptosis in myeloma cell lines with t(4;14)(p16.3;q32) translocation | Delays tumour progression and increases survival in a mouse model of t(4;14)(p16.3;q32) myeloma | 91, 92 |
| FGFR3 monoclonal antibody | FGFR3 | Blocks cell proliferation in multiple urothelial cancer cell lines, attenuates FGFR kinase activity in a urotheilal cell line | Suppresses urothelial and multiple myeloma tumour xenograft models | 96, 98, 99 |
| HDACI FR901228/small-molecule inhibitor | Histone deacetylase | Increases susceptibility to apoptosis in bladder and colon cancer cell lines | Not reported | 124, 125, 126 |
| HRAS antisense oligonucleotides | HRAS | Inhibits HRAS translation in a cell-free system, reduces growth of a urothelial cell line | Decreases tumourgenicity of urothelial cancer xenografts in nude mice | 121, 122, 123 |
| Farnesyl transferase inhibitors/small-molecule inhibitor | HRAS | Antiproliferative, proapoptotic, and antiangiogenic in a variety of cancer cell lines | Inhibits tumour growth in xenografts, inhibits chemically induced lung tumours, induces tumour regression and increases longevity of mice with transgene encoding mutant HRAS, potentiates effects of radiation in xenografts | 129, 130, 131, 132, 133 |
| BRAF/MEK small-molecule inhibitors | BRAF/MEK | Reduces growth, survival, invasion and angiogenesis in various cancer cell lines | Antitumourigenic in various xenograft models | 141, 142, 143, 144, 145, 146, 147 |
| BAY 43-9006/small-molecule inhibitor | BRAF | Inhibits MEK and ERK phosphorylation in various cancer cell lines with corresponding inhibition of tumour growth | Antitumour growth activity in a variety of human tumour xenograft models | 141, 142, 143 |
| CI-1040/small-molecule inhibitor | MEK | Blocks activation of MAPK in various cancer cell lines, inhibits growth on soft agar and has antiproliferative effects on colon cancer cell lines | Inhibits growth in human colon carcinoma xenografts | 144, 145 |
| ARRY-142886/small-molecule inhibitor | MEK | Same as CI-1040; inhibits MEK more efficiently | Same as CI-1040; inhibits MEK more efficiently | 147, 148 |
Abbreviations: BRAF, v-raf murine sarcoma viral oncogene homologue B; ERK, extracellular-signal-regulated (MAP) kinase; FGFR3, fibroblast growth factor receptor 3; HDAIC, histone deacetylase inhibitor; HRAS, Harvey rat sarcoma viral gene homologue; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase (MAP2K); p, short arm of chromosome; q, long arm of chromosome; t, translocation.
Using the human combinatorial antibody library Fab 1 (HuCAL Fab-1) to screen for antibodies that complexed with FGFR3, 25 antibodies out of 37 were identified that complexed with and inhibited proliferation of FDCP-1 cells (mouse myeloid progenitor cell line) transfected with FGFR3 expression vectors (Ref. 95) (Table 1). This study was the first to show that monoclonal antibodies against FGFR3 could have significant functional blocking activity. Another group utilised human single-chain Fv antibody fragments that were generated against the extracellular domain of FGFR3α (IIIc) and showed that these antibodies blocked proliferation of RT112 cells (UC cell line) in a dose- and FGF-dependent fashion (Ref. 96). A recent study using antibodies that were generated from hybridoma clones recognised bacterially expressed fragments of FGFR3 corresponding to loops II–III of the extracellular domain (region that encompasses the S249C oncogenic mutation) (Ref. 97). These antibodies could detect bacterially expressed wild-type FGFR3 and the mutant S249C FGFR3, implying a use for this methodology in detection or therapeutics in bladder cancer. Another recent study utilising a monoclonal antibody (designated R3Mab) against FGFR3 showed, through high-resolution crystallography, blocking of ligand-binding activity and inhibition of receptor dimerisation (Ref. 98); furthermore, the R3Mab was shown to have significant antitumour activity through interruption of FGFR3 signalling and antibody-dependent cell-mediated cytotoxicty (ADCC).
In addition to the use of monoclonal antibodies directed against FGFR3 in UC, one group has attempted to enhance the effect of antibody fragments directed against FGFR3 (3C) (Ref. 96) by fusing the antibodies with recombinant gelonin toxin (rGel) (Ref. 99). These antibodies, designated 3C/rGel, were injected into SCID (severe combined immunodeficient) mice with RT112 tumour xenografts and resulted in a delay in tumour growth and a reduction in tumour size of 55–70% when compared with the control group. Furthermore, immunohistochemistry analysis showed that treatment with the 3C/rGel antibodies resulted in significant apoptotic damage when compared with controls. Although these studies are preliminary, the use of antibodies directed against FGFR3 in order to treat NMIUC might prove beneficial for the prevention of recurrence. The antibody approach is especially attractive for UC since intravesical treatment can be applied repeatedly with a reduced risk of immunity against the antibody being generated.
HRAS
Role of HRAS in urothelial cancer
HRAS is a cellular proto-oncogene that encodes a member of the Ras GTPase family. When Ras family members become activated, through physiological stimuli or through mutation, they signal to key pathways involved in cell growth, survival, differentiation and migration, including the RAF–MAPK (Ref. 100), PI3K (Ref. 101), and the RALGEF (RALGDS; ral guanine-nucleotide-dissociation stimulator) (Ref. 102) pathways. In NMIUC, HRAS has been shown to be the most commonly mutated Ras family oncogene (Refs 103, 104, 105). Several point mutations have been identified in the HRAS oncogene in UC, with the most frequent mutations occurring at codons 12, 13 and 61 (Refs 106, 107, 108, 109), causing an inability to hydrolyse GTP to GDP (Refs 110, 111, 112). This mutation is responsible for constitutive activation of the HRAS protein and the ability to transform NIH 3T3 cells.
Historically, characterisation of the exact role of HRAS mutation in UC, both in terms of epidemiology of the mutation in tumours and its functional phenotypic consequence, has proven complex, despite the early isolation of the mutant HRAS oncogene from the classic UC cell line model T24 (Ref. 111). Initial studies on the status of HRAS in bladder cancer tended to argue that its expression or mutational status was positively correlated to the invasive potential of the UC. One early study of HRAS in UC showed that by transforming a NMIUC cell line (RT4) with either normal or mutated HRAS and then orthotopically inoculating nude mice transurethrally, one could cause RT4 cells to acquire a phenotype that was more similar to cell lines derived from tumours of high grade or stage (Ref. 113). It was argued that either deregulation of HRAS expression (overexpression in this case) or expression of a mutant form of HRAS manifested as a ‘second hit’ in a two-hit pathway could lead to invasive cancer. A combination of PCR amplification and computerised analysis later identified the point mutation G to T at codon 12 in 30 of 67 tumour samples examined (Ref. 114), and when these tumour samples were stratified by grade, the overwhelming majority of HRAS mutations were found in higher-grade tumours. Furthermore, another study showed that the highly tumourigenic T24T cell line had higher expression of HRAS than its less aggressive parent T24 (Ref. 115). In contrast, evaluation of 152 bladder tumours of varying grade or stage showed that the presence of HRAS mutations had no statistically significant correlation as verified by chi-squared analysis to either grade or stage of the UC studied (Ref. 116). Interestingly, another study that evaluated DNA fragments present in urine for the most common HRAS mutations in UC found that HRAS mutation status was better able to identify low-grade (1–2) lesions (47%) than cytology alone (16%) in a cohort of 100 patients (Ref. 117).
One of the first studies to clearly define the role of HRAS in human UC evaluated the development of bladder tumours in transgenic mice expressing mutant HRAS under the regulation of the urothelial-specific uroplakin promoter (Ref. 118). The study showed that mice with low copy numbers of chromosomally integrated, mutant, uroplakin-driven rabbit HRAS developed urothelial hyperplasia followed by the onset of superficial papillary tumours. In addition, mice that had high copy numbers of mutated HRAS also exhibited urotheilal hyperplasia with accelerated-onset superficial papillary tumours. None of these tumours, in the 26 month time period in which they were evaluated, progressed to invasive tumours. Interestingly, this same group later found that mice harbouring mutant HRAS formed these superficial papillary tumours independent of the loss of function of TP53, a tumour suppressor that is frequently mutated or deleted in a large proportion of MIUCs (see above section) (Ref. 119). In this study, the group crossed mice that harboured mutant TP53 (dominant-negative mutant, driven by the mouse uroplakin II gene promoter, that lacks the DNA-binding domain but retains the tetramerisation domain, interfering with the function of the normal TP53 protein) with mice expressing activated HRAS. These mice did exhibit urothelial hyperplasia and dysplasia, but were not able to develop frank tumours. In contrast, when the mice expressing HRAS-activating mutations were crossed with TP53-knockout mice, the progeny went on to develop either low- or high-grade superficial papillary tumours, suggesting that complete loss of TP53, rather than reduction of expression or function, is requisite to synergise with mutant HRAS. Another group found that progression to papillary tumours of the bladder with regards to mutant HRAS expression was dose dependent (Ref. 120): mice that were homozygous for the activated HRAS mutation were able to develop early-onset superficial papillary tumours, while mice that were heterozygous for the activating HRAS mutant were not able to develop superficial papillary tumours, regardless of mutational status of potential tumour suppressor genes on chromosome 9. Taken together, these findings implied that activating mutations in HRAS are important in the development and progression of early urothelial tumour oncogenesis.
Targeted therapeutics for HRAS
As activated HRAS gained prominence as an early contributor in the oncogenic pathway of superficial papillary tumours of the bladder there have been efforts to disrupt its function. One of the first studies aimed at disrupting HRAS signalling involved construction of a short modified antisense nucleotide that targeted the region encompassing the activating point mutation on the 12th codon (Ref. 121) (Table 1). This antisense nucleotide caused 50% inhibition of translation in a cell-free system and also a 60% reduction in growth of T24 cells over five days. In another study, using synthetic oligonucleotides (ribozymes designed against HRAS mRNA that are capable of hydrolysing its phosphodiester bonds) it was shown that EJ cells (more aggressive variant of T24 cells) that were transfected with plasmids containing the anti-HRAS ribozymes were less effective at forming invasive tumours than EJ cells that did not express the ribozyme (Ref. 122). A similar study using a different vehicle for delivery of the anti-HRAS ribozyme (adenovirus) also showed significant reduction in the tumourigencity of EJ cells injected subcutaneously into nude mice (Ref. 123). While the studies on anti-HRAS oligonucleotides have been intriguing, the use of antisense nucleotides to treat human disease has found little footing in the clinic as of yet.
Recently, there have been connections made between the acquisition of oncogenic HRAS in cells and increased susceptibility to histone deacetylase inhibitors (HDACIs) (Table 1). In one study, J82 cells (malignant urothelial cancer cell line) that had been transfected with vectors expressing activated HRAS were much more susceptible to death by apoptosis in the presence of the HDACI FR901228 than cells that had not been transfected (Ref. 124). A similar result was shown in the colon cancer cell line HT-29 when activated HRAS was overexpressed (Ref. 125). A later study by the same group showed that the potential mechanism for apoptotic cell death in mutant HRAS transgenic J82 cells is an increase in reactive oxygen species, which induces both intrinsic and extrinsic caspase pathways (Ref. 126). In addition, suppression of the reactive oxygen species axis via the use of the antioxidant NAC (N-acetylcysteine) resulted in decreased effectiveness of the HDACI FR901228 to induce apoptosis. Taken together, these data show that HDACIs might potentially play an important role in stopping the growth of bladder tumours that harbour activating HRAS mutations. In vivo studies will need to be completed to validate the potential clinical effectiveness of this class of drugs in human bladder cancer. Very recently, genome-wide siRNA (small interfering RNA) screens have successfully identified synthetic lethal interactions with the closely related Ras-family oncogene KRAS2. In cells with mutant KRAS2, inhibition of PLK1 (polo-like kinase 1) and depletion of STK33 (serine/threonine kinase 33) result in a robust induction of cell death that does not occur in normal cells (Refs 127, 128). Such synthetic lethal screens suggest that targeting this class of genes could provide an exquisitely specific way to target mutant tumour cells with minimal toxicity to wild-type cells. This type of screening could potentially prove useful in development of modalities to target the HRAS axis in UC.
Targeting the MAPK signalling pathway
Both HRAS and FGFR3 converge on the MAPK signalling pathway (Refs 66, 67, 100). For this reason, studies have aimed at inhibiting various steps in this pathway in order to reduce the growth or recurrence of cancer (Table 1). Farnesylation is a post-translational requirement for the Ras protein. This modification leads to attachment of Ras to the plasma membrane since the protein does not have a transmembrane domain. Several farnesyl transferase inhibitors (FTIs) spanning distinct chemical classes have been developed and have shown promising anticancer effects (Refs 129, 130). Unfortunately, many of these anticancer effects did not hold up in clinical trials, where it was shown that when using FTIs as a single agent there was no therapeutic effect on lung, pancreatic, colorectal or bladder tumours (Ref. 131). Reasons that have been given for these poor results are that the FTIs might be inhibiting proteins other than farnesyl transferases or that inhibition of HRAS by these drugs could be causing a compensatory activation of other Ras family members, ultimately resulting in treatment failure (Refs 11, 132). Despite the poor results in clinical trials, in an animal model of urothelial tumours with mutationally active HRAS, treatment with FTIs significantly sensitised the tumours to radiation (Ref. 133). This finding suggests that FTIs used in combination with radiation therapy have the potential for clinical use in UC.
The Raf (ARAF, BRAF and RAF1) and MEK (MEK1/MAP2K1 and MEK2/MAP2K2) family of proteins are critical downstream mediators of the Ras and other oncogenic signalling pathways (Refs 134, 135, 136, 137, 138). The Raf family of proteins are responsible for phosphorylating MEK, which results in its activation (Refs 139, 140). Hence there was an impetus to determine if inhibition of Raf/MEK activity would have anticancer benefits. With regards to Raf and UC, clinical data have been limited because the Raf mutation, specifically in BRAF, is not a common occurrence in UC. The cancers in which BRAF mutations are prevalent (i.e. melanoma, colorectal, ovarian and thyroid) have shown some response to the small-molecule BRAF inhibitor BAY 43-9006, but it has shown limited clinical success due to its lack of specificity (Refs 141, 142, 143). MEK inhibitors (CI-1040, ARRY-142886), in contrast, have proven to be better at specifically inhibiting their target and have shown efficacy against tumours. For example, CI-1040 showed great promise in inhibition of colon carcinomas in vivo and was demonstrated to have a negative impact on the growth, survival, invasion and angiogenesis profiles of cancer cells (Refs 144, 145). However, despite promising antitumour activity in Phase I trials, CI-1040 showed limited tumour activity in Phase II trials, ultimately leading to its discontinuation (Refs 145, 146). This result prompted development of the MEK inhibitor ARRY-142886, which is much more specific than CI-1040 (Refs 147, 148). This drug is currently being used in clinical trials. At this time there are no specific clinical trials that are solely focused on the impact of Raf/MEK inhibitors with regards to UC. As the mechanism and impact of these inhibitors are better understood in the cancer models in which they are currently being studied, it is likely that these inhibitors will find some use in UC given the crucial role of Ras signalling in the pathogenesis of UC.
Summary and future directions
NMIUC is a disease with low mortality but significant morbidity due to a high recurrence rate. Understanding the various molecular signalling mechanisms that underpin this disease is crucial in developing therapies to prevent its onset and progression. Loss of heterozygosity of chromosome 9, activating mutations in FGFR3, and activating mutations in the oncogene HRAS have all been shown to be prevalent and important in NMIUC, with the latter two alterations being infrequent or absent in the invasive subtype of UC. Currently, there is much work being done in the field of inhibiting the activity and signalling of aberrant FGFR3 and HRAS, whether targeting these oncogene products themselves or their downstream mediators. Each of these tools has shown some success in vitro and in vivo. Several small-molecule inhibitors have been developed that can disrupt the function of either FGFR3 or HRAS directly or disrupt signalling at various points in the MAPK signalling pathway. Many of these inhibitors have shown some promise in clinical trials in other tumours types, suggesting that they might have some relevance in slowing UC progression. Unfortunately, no single drug has been approved for use in bladder cancer that targets the MAPK signalling pathway, FGFR3 or HRAS, although the possibility of using such drugs alone or in combination with standard of care therapies is attractive. In addition to the therapeutics discussed in this review, there are several novel therapeutic modalities that are currently being investigated, such as gene therapy using viral and nonviral vectors and direct tumouricidal viruses (Ref. 149). Further investigation of these therapeutics might prove beneficial to NMIUC patients in an adjuvant setting. Finally, NMIUC is among the most expensive tumours to treat, per person, primarily due to its frequent recurrence and necessary surveillance (Refs 3, 150). Hence, any understanding of the molecular pathogenesis of this disease that can lead to future therapies would be a great benefit to society.
Acknowledgments
We thank members of the Theodorescu lab for their suggestions and assistance and we also thank the reviewers of this manuscript for their insightful comments. This work was supported by NIH grants CA142163 to Courtney Pollard and CA075115 to Dan Theodorescu.
References
- 1.Parkin DM, et al. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: a summary and commentary. International Journal of Surgical Pathology. 2005;13:143–153. doi: 10.1177/106689690501300203. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, et al. Cancer statistics, 2009. CA: A Cancer Journal for Clinicians. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Sonny LJ, Samuel MC. Epidemiology and etiology of bladder cancer. Seminars in Surgical Oncology. 1997;13:291–298. doi: 10.1002/(sici)1098-2388(199709/10)13:5<291::aid-ssu2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Kamat AM, Lamm DL. Intravesical therapy for bladder cancer. Urology. 2000;55:161–168. doi: 10.1016/s0090-4295(99)00463-x. [DOI] [PubMed] [Google Scholar]
- 6.Pasin E, et al. Superficial bladder cancer: an update on etiology, molecular development, classification, and natural history. Reviews in Urology. 2008;10:31–43. [PMC free article] [PubMed] [Google Scholar]
- 7.Sengelov L, Kamby C, von der Maase H. Pattern of metastases in relation to characteristics of primary tumor and treatment in patients with disseminated urothelial carcinoma. Journal of Urology. 1996;155:111–114. [PubMed] [Google Scholar]
- 8.Steinberg GD, Trump DL, Cummings KB. Metastatic bladder cancer. Natural history, clinical course, and consideration for treatment. Urologic Clinics of North America. 1992;19:735–746. [PubMed] [Google Scholar]
- 9.Liebert M, Seigne J. Characteristics of invasive bladder cancers: histological and molecular markers. Seminars in Urologic Oncology. 1996;14:62–72. [PubMed] [Google Scholar]
- 10.Koss LG. Bladder cancer from a perspective of 40 years. Journal of Cellular Biochemistry (Suppl) 1992;16I:23–29. doi: 10.1002/jcb.240501305. [DOI] [PubMed] [Google Scholar]
- 11.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nature Reviews Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 12.Bakkar AA, et al. FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder. Cancer Research. 2003;63:8108–8112. [PubMed] [Google Scholar]
- 13.Lamy A, et al. Molecular profiling of bladder tumors based on the detection of FGFR3 and TP53 mutations. Journal of Urology. 2006;176:2686–2689. doi: 10.1016/j.juro.2006.07.132. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez S, et al. FGFR3 and Tp53 mutations in T1G3 transitional bladder carcinomas: independent distribution and lack of association with prognosis. Clinical Cancer Research. 2005;11:5444–5450. doi: 10.1158/1078-0432.CCR-05-0122. [DOI] [PubMed] [Google Scholar]
- 15.Karsten Z, et al. Consistent genomic alterations in carcinoma in situ of the urinary bladder confirm the presence of two major pathways in bladder cancer development. International Journal of Cancer. 2009;125:2095–2103. doi: 10.1002/ijc.24619. [DOI] [PubMed] [Google Scholar]
- 16.Tsai YC, et al. Allelic losses of chromosomes 9, 11, and 17 in human bladder cancer. Cancer Research. 1990;50:44–47. [PubMed] [Google Scholar]
- 17.Cairns P, Shaw ME, Knowles MA. Initiation of bladdercancer may involve deletion of a tumour-suppressor gene on chromosome 9. Oncogene. 1993;8:1083–1085. [PubMed] [Google Scholar]
- 18.Miyao N, et al. Role of chromosome 9 in human bladder cancer. Cancer Research. 1993;53:4066–4070. [PubMed] [Google Scholar]
- 19.Llnnenbach AJ, et al. Characterization of chromosome 9 deletions in transitional cell carcinoma by microsatellite assay. Human Molecular Genetics. 1993;2:1407–1411. doi: 10.1093/hmg/2.9.1407. [DOI] [PubMed] [Google Scholar]
- 20.Orlow I, et al. Chromosome 9 allelic losses and microsatellite alterations in human bladder tumors. Cancer Research. 1994;54:2848–2851. [PubMed] [Google Scholar]
- 21.Habuchi T, et al. Detailed deletion mapping of chromosome 9q in bladder cancer: evidence for two tumour suppressor loci. Oncogene. 1995;11:1671–1674. [PubMed] [Google Scholar]
- 22.Keen AJ, Knowles MA. Definition of two regions of deletion on chromosome 9 in carcinoma of the bladder. Oncogene. 1994;9:2083–2088. [PubMed] [Google Scholar]
- 23.Simoneau AR, et al. Evidence for two tumor suppressor loci associated with proximal chromosome 9p to q and distal chromosome 9q in bladder cancer and the initial screening for GAS1 and PTC mutations. Cancer Research. 1996;56:5039–5043. [PubMed] [Google Scholar]
- 24.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 25.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 26.Schulze A, et al. Activation of the E2F transcription factor by cyclin D1 is blocked by p16INK4, the product of the putative tumor suppressor gene MTS1. Oncogene. 1994;9:3475–3482. [PubMed] [Google Scholar]
- 27.Johnson DG. Regulation of E2F-1 gene expression by p130 (Rb2) and D-type cyclin kinase activity. Oncogene. 1995;11:1685–1692. [PubMed] [Google Scholar]
- 28.Gonzalez-Zulueta M, et al. high frequency of chromosome 9p allelic loss and CDKN2 tumor suppressor gene alterations in squamous cell carcinoma of the bladder. Journal of the National Cancer Institute. 1995;87:1383–1393. doi: 10.1093/jnci/87.18.1383. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Zulueta M, et al. Methylation of the 5′ CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Research. 1995;55:4531–4535. [PubMed] [Google Scholar]
- 30.Orlow I, et al. Deletion of the p16 and p15 genes in human bladder tumors. Journal of the National Cancer Institute. 1995;87:1524–1529. doi: 10.1093/jnci/87.20.1524. [DOI] [PubMed] [Google Scholar]
- 31.Southgate J, et al. Loss of cyclin-dependent kinase inhibitor genes and chromosome 9 karyotypic abnormalities in human bladder cancer cell lines. British Journal of Cancer. 1995;72:1214–1218. doi: 10.1038/bjc.1995.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Frere-Belda MA, et al. p15INK4b in bladder carcinomas: decreased expression in superficial tumours. British Journal of Cancer. 2001;85:1515–1521. doi: 10.1054/bjoc.2001.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hornigold N, et al. Mutation of the 9q34 gene TSC1 in sporadic bladder cancer. Oncogene. 1999;18:2567–2561. doi: 10.1038/sj.onc.1202854. [DOI] [PubMed] [Google Scholar]
- 34.Hornigold N, et al. A 1.7-megabase sequence-ready cosmid contig covering the TSC1 candidate region in 9q34. Genomics. 1997;41:385–389. doi: 10.1006/geno.1997.4681. [DOI] [PubMed] [Google Scholar]
- 35.van Slegtenhorst M, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 36.Knowles MA, et al. Mutation spectrum of the 9q34 tuberous sclerosis gene TSC1 in transitional cell carcinoma of the bladder. Cancer Research. 2003;63:7652–7656. [PubMed] [Google Scholar]
- 37.Pymar LS, et al. Bladder tumour-derived somatic TSC1 missense mutations cause loss of function via distinct mechanisms. Human Molecular Genetics. 2008;17:2006–2017. doi: 10.1093/hmg/ddn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adachi H, et al. Human bladder tumors with 2-hit mutations of tumor suppressor gene TSC1 and decreased expression of p27. Journal of Urology. 2003;170:601–604. doi: 10.1097/01.ju.0000074621.74361.10. [DOI] [PubMed] [Google Scholar]
- 39.Habuchi T, Yoshida O, Knowles MA. A novel candidate tumour suppressor locus at 9q32-33 in bladder cancer: localization of the candidate region within a single 840 kb YAC. Human Molecular Genetics. 1997;6:913–919. doi: 10.1093/hmg/6.6.913. [DOI] [PubMed] [Google Scholar]
- 40.Habuchi T, et al. Structure and methylation-based silencing of a gene (DBCCR1) within a candidate bladder cancer tumor suppressor region at 9q32–q33. Genomics. 1998;48:277–288. doi: 10.1006/geno.1997.5165. [DOI] [PubMed] [Google Scholar]
- 41.Simoneau M, et al. Four tumor suppressor loci on chromosome 9q in bladder cancer: evidence for two novel candidate regions at 9q22.3 and 9q31. Oncogene. 1999;18:157–163. doi: 10.1038/sj.onc.1202277. [DOI] [PubMed] [Google Scholar]
- 42.Joanne E, et al. Identification of loci associated with putative recurrence genes in transitional cell carcinoma of the urinary bladder. Journal of Pathology. 2002;196:380–385. doi: 10.1002/path.1052. [DOI] [PubMed] [Google Scholar]
- 43.Friedrich MG, et al. Frequent p16/MTS1 inactivation in early stages of urothelial carcinoma of the bladder is not associated with tumor recurrence. European Urology. 2001;40:518–524. doi: 10.1159/000049829. [DOI] [PubMed] [Google Scholar]
- 44.Bartoletti R, et al. Loss of P16 expression and chromosome 9p21 LOH in predicting outcome of patients affected by superficial bladder cancer. Journal of Surgical Research. 2007;143:422–427. doi: 10.1016/j.jss.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Bartlett JM, et al. Is chromosome 9 loss a marker of disease recurrence in transitional cell carcinoma of the urinary bladder? British Journal of Cancer. 1998;77:2193–2198. doi: 10.1038/bjc.1998.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez-Beltran A, et al. Loss of heterozygosity at 9q32-33 (DBC1 locus) in primary non-invasive papillary urothelial neoplasm of low malignant potential and low-grade urothelial carcinoma of the bladder and their associated normal urothelium. Journal of Pathology. 2008;215:263–272. doi: 10.1002/path.2353. [DOI] [PubMed] [Google Scholar]
- 47.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine & Growth Factor Reviews. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Karuppiah K, David G. FGF receptor mutations: dimerization syndromes, cell growth suppression, and animal models. IUBMB Life. 2000;49:197–205. doi: 10.1080/713803609. [DOI] [PubMed] [Google Scholar]
- 49.L’Hôte CGM, Knowles MA. Cell responses to FGFR3 signalling: growth, differentiation and apoptosis. Experimental Cell Research. 2005;304:417–431. doi: 10.1016/j.yexcr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Murgue B, et al. Identification of a novel variant form of fibroblast growth factor receptor 3 (FGFR3 IIIb) in human colonic epithelium. Cancer Research. 1994;54:5206–5211. [PubMed] [Google Scholar]
- 51.Rousseau F, et al. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371:252–254. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- 52.Shiang R, et al. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 53.Bellus GA, et al. A recurrent mutation in the tyrosine kinase domain of fibroblast growth factor receptor 3 causes hypochondroplasia. Nature Genetics. 1995;10:357–359. doi: 10.1038/ng0795-357. [DOI] [PubMed] [Google Scholar]
- 54.Superti-Furga A, et al. A glycine 375-to-cysteine substitution in the transmembrane domain of the fibroblast growth factor receptor-3 in a newborn with achondroplasia. European Journal of Pediatrics. 1995;154:215–219. doi: 10.1007/BF01954274. [DOI] [PubMed] [Google Scholar]
- 55.Ikegawa S, et al. Mutations of the fibroblast growth factor receptor-3 gene in one familial and six sporadic cases of achondroplasia in Japanese patients. Human Genetics. 1995;96:309–311. doi: 10.1007/BF00210413. [DOI] [PubMed] [Google Scholar]
- 56.Tavormina PL, et al. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nature Genetics. 1995;9:321–328. doi: 10.1038/ng0395-321. [DOI] [PubMed] [Google Scholar]
- 57.Tavormina PL, et al. Another mutation that results in the substitution of an unpaired cysteine residue in the extracellular domain of FGFR3 in thanatophoric dysplasia type I. Human Molecular Genetics. 1995;4:2175–2177. doi: 10.1093/hmg/4.11.2175. [DOI] [PubMed] [Google Scholar]
- 58.Fuu-Jen T, et al. Mutations in the fibroblast growth factor receptor 3 (FGFR3) cause achondroplasia, hypochondroplasia, and thanatophoric dysplasia: Taiwanese data. American Journal of Medical Genetics. 1999;86:300–301. [PubMed] [Google Scholar]
- 59.Bonaventure J, et al. Common mutations in the fibroblast growth factor receptor 3 (FGFR 3) gene account for achondroplasia, hypochondroplasia, and thanatophoric dwarfism. American Journal of Medical Genetics. 1996;63:148–154. doi: 10.1002/(SICI)1096-8628(19960503)63:1<148::AID-AJMG26>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 60.Cappellen D, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nature Genetics. 1999;23:18–20. doi: 10.1038/12615. [DOI] [PubMed] [Google Scholar]
- 61.Karoui M, et al. No evidence of somatic FGFR3 mutation in various types of carcinoma. Oncogene. 2001;20:5059–5061. doi: 10.1038/sj.onc.1204651. [DOI] [PubMed] [Google Scholar]
- 62.Sibley K, Stern P, Knowles MA. Frequency of fibroblast growth factor receptor 3 mutations in sporadic tumours. Oncogene. 2001;20:4416–4418. doi: 10.1038/sj.onc.1204543. [DOI] [PubMed] [Google Scholar]
- 63.Webster MK, Donoghue DJ. FGFR activation in skeletal disorders: too much of a good thing. Trends in Genetics. 1997;13:178–182. doi: 10.1016/s0168-9525(97)01131-1. [DOI] [PubMed] [Google Scholar]
- 64.Naski MC, et al. Graded activation of fibroblast growth factor receptor 3 by mutations causing achondroplasia and thanatophoric dysplasia. Nature Genetics. 1996;13:233–237. doi: 10.1038/ng0696-233. [DOI] [PubMed] [Google Scholar]
- 65.Agazie YM, et al. The phosphotyrosine phosphatase SHP2 is a critical mediator of transformation induced by the oncogenic fibroblast growth factor receptor 3. Oncogene. 2003;22:6909–6918. doi: 10.1038/sj.onc.1206798. [DOI] [PubMed] [Google Scholar]
- 66.Kanai M, et al. Signal transduction pathway of human fibroblast growth factor receptor 3. Journal of Biological Chemistry. 1997;272:6621–6628. doi: 10.1074/jbc.272.10.6621. [DOI] [PubMed] [Google Scholar]
- 67.Jebar AH, et al. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene. 2005;24:5218–5225. doi: 10.1038/sj.onc.1208705. [DOI] [PubMed] [Google Scholar]
- 68.van Rhijn BWG, et al. The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Research. 2001;61:1265–1268. [PubMed] [Google Scholar]
- 69.Billerey C, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. American Journal of Pathology. 2001;158:1955–1959. doi: 10.1016/S0002-9440(10)64665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spruck CH, III, et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Research. 1994;54:784–788. [PubMed] [Google Scholar]
- 71.Reznikoff CA, et al. A molecular genetic model of human bladder cancer pathogenesis. Seminars in Oncology. 1996;23:571–584. [PubMed] [Google Scholar]
- 72.Lee R, Droller MJ. The natural history of bladder cancer. Implications for therapy. Urologic Clinics of North America. 2000;27:1–13. doi: 10.1016/s0094-0143(05)70229-9. [DOI] [PubMed] [Google Scholar]
- 73.Sanchez-Carbayo M, et al. Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Research. 2002;62:6973–6980. [PubMed] [Google Scholar]
- 74.Sanchez-Carbayo M, et al. Gene discovery in bladder cancer progression using cDNA microarrays. American Journal of Pathology. 2003;163:505–516. doi: 10.1016/S0002-9440(10)63679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dyrskjøt L, et al. Identifying distinct classes of bladder carcinoma using microarrays. Nature Genetics. 2003;33:90–96. doi: 10.1038/ng1061. [DOI] [PubMed] [Google Scholar]
- 76.Takahiro K, et al. The incidence of thanatophoric dysplasia mutations in FGFR3 gene is higher in low-grade or superficial bladder carcinomas. Cancer. 2001;92:2555–2561. doi: 10.1002/1097-0142(20011115)92:10<2555::aid-cncr1607>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 77.van Rhijn BW, et al. Frequent FGFR3 mutations in urothelial papilloma. Journal of Pathology. 2002;198:245–251. doi: 10.1002/path.1202. [DOI] [PubMed] [Google Scholar]
- 78.Cordon-Cardo C, et al. p53 mutations in human bladder cancer: genotypic versus phenotypic patterns. International Journal of Cancer. 1994;56:347–353. doi: 10.1002/ijc.2910560309. [DOI] [PubMed] [Google Scholar]
- 79.Spiess PE, Czerniak B. Dual-track pathway of bladder carcinogenesis: practical implications. Archives of Pathology and Laboratory Medicine. 2006;130:844–852. doi: 10.5858/2006-130-844-DPOBCP. [DOI] [PubMed] [Google Scholar]
- 80.van Rhijn BWG, et al. FGFR3 and P53 characterize alternative genetic pathways in the pathogenesis of urothelial cell carcinoma. Cancer Research. 2004;64:1911–1914. doi: 10.1158/0008-5472.can-03-2421. [DOI] [PubMed] [Google Scholar]
- 81.Mhawech-Fauceglia P, et al. FGFR3 and p53 protein expressions in patients with pTa and pT1 urothelial bladder cancer. European Journal of Surgical Oncology. 2006;32:231–237. doi: 10.1016/j.ejso.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 82.Lott S, et al. FGFR3 and TP53 mutation analysis in inverted urothelial papilloma: incidence and etiological considerations. Modern Pathology. 2009;22:627–632. doi: 10.1038/modpathol.2009.28. [DOI] [PubMed] [Google Scholar]
- 83.van Rhijn BWG, et al. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. Journal of Clinical Oncology. 2003;21:1912–1921. doi: 10.1200/JCO.2003.05.073. [DOI] [PubMed] [Google Scholar]
- 84.Hernandez S, et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. Journal of Clinical Oncology. 2006;24:3664–3671. doi: 10.1200/JCO.2005.05.1771. [DOI] [PubMed] [Google Scholar]
- 85.Burger M, et al. Prediction of progression of non-muscle-invasive bladder cancer by WHO 1973 and 2004 grading and by FGFR3 mutation status: a prospective study. European Urology. 2008;54:835–844. doi: 10.1016/j.eururo.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 86.Francesca B, et al. Strong immunohistochemical expression of fibroblast growth factor receptor 3, superficial staining pattern of cytokeratin 20, and low proliferative activity define those papillary urothelial neoplasms of low malignant potential that do not recur. Cancer. 2008;112:636–644. doi: 10.1002/cncr.23212. [DOI] [PubMed] [Google Scholar]
- 87.Gazdar AF, et al. Mutations and addiction to EGFR: the Achilles ‘heal’ of lung cancers? Trends in Molecular Medicine. 2004;10:481–486. doi: 10.1016/j.molmed.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 88.Weinstein IB, Joe A, Felsher D. Oncogene addiction. Cancer Research. 2008;68:3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. [DOI] [PubMed] [Google Scholar]
- 89.Trudel S, et al. CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood. 2005;105:2941–2948. doi: 10.1182/blood-2004-10-3913. [DOI] [PubMed] [Google Scholar]
- 90.Mohammadi M, et al. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- 91.Grand EK, et al. Targeting FGFR3 in multiple myeloma: inhibition of t(4;14)-positive cells by SU5402 and PD173074. Leukemia. 2004;18:962–966. doi: 10.1038/sj.leu.2403347. [DOI] [PubMed] [Google Scholar]
- 92.Trudel S, et al. Inhibition of fibroblast growth factor receptor 3 induces differentiation and apoptosis in t(4;14) myeloma. Blood. 2004;103:3521–3528. doi: 10.1182/blood-2003-10-3650. [DOI] [PubMed] [Google Scholar]
- 93.Mohammadi M, et al. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO Journal. 1998;17:5896–5904. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laird AD, et al. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Research. 2000;60:4152–4160. [PubMed] [Google Scholar]
- 95.Rauchenberger R, et al. Human combinatorial Fab library yielding specific and functional antibodies against the human fibroblast growth factor receptor 3. Journal of Biological Chemistry. 2003;278:38194–38205. doi: 10.1074/jbc.M303164200. [DOI] [PubMed] [Google Scholar]
- 96.Martínez -Torrecuadrada J, et al. Targeting the extracellular domain of fibroblast growth factor receptor 3 with human single-chain Fv antibodies inhibits bladder carcinoma cell line proliferation. Clinical Cancer Research. 2005;11:6280–6290. doi: 10.1158/1078-0432.CCR-05-0282. [DOI] [PubMed] [Google Scholar]
- 97.Gorbenko O, et al. Generation of monoclonal antibody targeting fibroblast growth factor receptor 3. Hybridoma. 2009;28:295–300. doi: 10.1089/hyb.2009.0018. [DOI] [PubMed] [Google Scholar]
- 98.Qing J, et al. Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. Journal of Clinical Investigation. 2009;119:1216–1229. doi: 10.1172/JCI38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martínez-Torrecuadrada JL, et al. Antitumor activity of fibroblast growth factor receptor 3–specific immunotoxins in a xenograft mouse model of bladder carcinoma is mediated by apoptosis. Molecular Cancer Therapeutics. 2008;7:862–873. doi: 10.1158/1535-7163.MCT-07-0394. [DOI] [PubMed] [Google Scholar]
- 100.Herrera R, Sebolt-Leopold JS. Unraveling the complexities of the Raf/MAP kinase pathway for pharmacological intervention. Trends in Molecular Medicine. 2002;8:S27–S31. doi: 10.1016/s1471-4914(02)02307-9. [DOI] [PubMed] [Google Scholar]
- 101.Jiang B, et al. Chapter 2 PI3K/PTEN signaling in angiogenesis and tumorigenesis. Advances in Cancer Research. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rusanescu G, et al. Regulation of Ras signaling specificity by protein kinase C. Molecular and Cellular Biology. 2001;21:2650–2658. doi: 10.1128/MCB.21.8.2650-2658.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feinberg AP, et al. Mutation affecting the 12th amino acid of the c-Ha-ras oncogene product occurs infrequently in human cancer. Science. 1983;220:1175–1177. doi: 10.1126/science.6304875. [DOI] [PubMed] [Google Scholar]
- 104.Fujita J, et al. Ha-ras oncogenes are activated by somatic alterations in human urinary tract tumours. Nature. 1984;309:464–466. doi: 10.1038/309464a0. [DOI] [PubMed] [Google Scholar]
- 105.Fujita J, et al. Frequency of molecular alterations affecting ras protooncogenes in human urinary tract tumors. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:3849–3853. doi: 10.1073/pnas.82.11.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Visvanathan KV, Pocock RD, Summerhayes IC. Preferential and novel activation of H-ras in human bladder carcinomas. Oncogene Research. 1988;3:77–86. [PubMed] [Google Scholar]
- 107.Joyce AD, et al. Detection of altered H-ras proteins in human tumors using western blot analysis. Laboratory Investigation. 1989;61:212–218. [PubMed] [Google Scholar]
- 108.Saito S, et al. Screening of H-ras gene point mutations in 50 cases of bladder carcinoma. International Journal of Urology. 1997;4:178–185. doi: 10.1111/j.1442-2042.1997.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 109.Boulalas I, et al. Activation of RAS family genes in urothelial carcinoma. Journal of Urology. 2009;181:2312–2319. doi: 10.1016/j.juro.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 110.Tabin CJ, et al. Mechanism of activation of a human oncogene. Nature. 1982;300:143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- 111.Reddy EP, et al. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982;300:149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- 112.Taparowsky E, et al. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature. 1982;300:762–765. doi: 10.1038/300762a0. [DOI] [PubMed] [Google Scholar]
- 113.Theodorescu D, et al. Overexpression of normal and mutated forms of HRAS induces orthotopic bladder invasion in a human transitional cell carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9047–9051. doi: 10.1073/pnas.87.22.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Czerniak B, et al. Concurrent mutations of coding and regulatory sequences of the Ha-ras gene in urinary bladder carcinomas. Human Pathology. 1992;23:1199–1204. doi: 10.1016/0046-8177(92)90285-b. [DOI] [PubMed] [Google Scholar]
- 115.John JG, et al. Genetic and phenotypic changes associated with the acquisition of tumorigenicity in human bladder cancer. Genes, Chromosomes and Cancer. 2000;27:252–263. doi: 10.1002/(sici)1098-2264(200003)27:3<252::aid-gcc5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 116.Knowles MA, Williamson M. Mutation of H-ras Is infrequent in bladder cancer: confirmation by single-strand conformation polymorphism analysis, designed restriction fragment length polymorphisms, and direct sequencing. Cancer Research. 1993;53:133–139. [PubMed] [Google Scholar]
- 117.Fitzgerald JM, et al. Identification of H-ras mutations in urine sediments complements cytology in the detection of bladder tumors. Journal of the National Cancer Institute. 1995;87:129–133. doi: 10.1093/jnci/87.2.129. [DOI] [PubMed] [Google Scholar]
- 118.Zhang ZT, et al. Role of Ha-ras activation in superficial papillary pathway of urothelial tumor formation. Oncogene. 2001;20:1973–1980. doi: 10.1038/sj.onc.1204315. [DOI] [PubMed] [Google Scholar]
- 119.Gao J, et al. p53 deficiency provokes urothelial proliferation and synergizes with activated Ha-ras in promoting urothelial tumorigenesis. Oncogene. 2004;23:687–696. doi: 10.1038/sj.onc.1207169. [DOI] [PubMed] [Google Scholar]
- 120.Mo L, et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. Journal of Clinical Investigation. 2007;117:314–325. doi: 10.1172/JCI30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saison-Behmoaras T, et al. Short modified antisense oligonucleotides directed against Ha-ras point mutation induce selective cleavage of the mRNA and inhibit T24 cells proliferation. EMBO Journal. 1991;10:1111–1118. doi: 10.1002/j.1460-2075.1991.tb08051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eastham JA, Ahlering TE. Use of an anti-ras ribozyme to alter the malignant phenotype of a human bladder cancer cell line. Journal of Urology. 1996;156:1186–1188. [PubMed] [Google Scholar]
- 123.Irie A, et al. Therapeutic efficacy of an adenovirus-mediated anti-H-ras ribozyme in experimental bladder cancer. Antisense and Nucleic Acid Drug Development. 1999;9:341–349. doi: 10.1089/oli.1.1999.9.341. [DOI] [PubMed] [Google Scholar]
- 124.Choudhary S, Wang HCR. Proapoptotic ability of oncogenic H-Ras to facilitate apoptosis induced by histone deacetylase inhibitors in human cancer cells. Molecular Cancer Therapeutics. 2007;6:1099–1111. doi: 10.1158/1535-7163.MCT-06-0586. [DOI] [PubMed] [Google Scholar]
- 125.Choudhary S, Wang HC. Pro-apoptotic activity of oncogenic H-Ras for histone deacetylase inhibitor to induce apoptosis of human cancer HT29 cells. Journal of Cancer Research and Clinical Oncology. 2007;133:725–739. doi: 10.1007/s00432-007-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Choudhary S, Wang HC. Role of reactive oxygen species in proapoptotic ability of oncogenic H-Ras to increase human bladdercancer cell susceptibility to histone deacetylase inhibitor for caspase induction. Journal of Cancer Research and Clinical Oncology. 2009;135:1601–1613. doi: 10.1007/s00432-009-0608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luo J, et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137:835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Scholl C, et al. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell. 2009;137:821–834. doi: 10.1016/j.cell.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 129.Zhu K, Hamilton AD, Sebti SM. Farnesyltransferase inhibitors as anticancer agents: current status. Current Opinion in Investigational Drugs. 2004;4:1428–1435. [PubMed] [Google Scholar]
- 130.Sebti SM, Hamilton AD. Farnesyltransferase and geranylgeranyltransferase I inhibitors and cancer therapy: lessons from mechanism and bench-to-bedside translational studies. Oncogene. 2000;19:6584–6593. doi: 10.1038/sj.onc.1204146. [DOI] [PubMed] [Google Scholar]
- 131.Sebti SM, Adjei AA. Farnesyltransferase inhibitors. Seminars in Oncology. 2004;31:28–39. doi: 10.1053/j.seminoncol.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 132.Brunner TB, et al. Farnesyltransferase inhibitors: an overview of the results of preclinical and clinical investigations. Cancer Research. 2003;63:5656–5668. [PubMed] [Google Scholar]
- 133.Cohen-Jonathan E, et al. Farnesyltransferase inhibitors potentiate the antitumor effect of radiation on a human tumor xenograft expressing activated HRAS. Radiation Research. 2000;154:125–132. doi: 10.1667/0033-7587(2000)154[0125:fiptae]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 134.Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 135.Singer G, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. Journal of the National Cancer Institute. 2003;95:484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 136.Kimura ET, et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Research. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 137.Mansour SJ, et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 138.Hoshino R, et al. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 139.Katz ME, McCormick F. Signal transduction from multiple Ras effectors. Current Opinion in Genetics & Development. 1997;7:75–79. doi: 10.1016/s0959-437x(97)80112-8. [DOI] [PubMed] [Google Scholar]
- 140.Williams NG, Roberts TM. Signal transduction pathways involving the Raf proto-oncogene. Cancer and Metastasis Reviews. 1994;13:105–116. doi: 10.1007/BF00690421. [DOI] [PubMed] [Google Scholar]
- 141.Lee JT, McCubrey JA. BAY-43-9006 Bayer/Onyx. Current Opinion in Investigational Drugs. 2003;4:757–763. [PubMed] [Google Scholar]
- 142.Hotte SJ, Hirte HW. BAY 43-9006: early clinical data in patients with advanced solid malignancies. Current Pharmaceutical Design. 2002;8:2249–2253. doi: 10.2174/1381612023393053. [DOI] [PubMed] [Google Scholar]
- 143.DeGrendele H. Activity of the Raf kinase inhibitor BAY 43-9006 in patients with advanced solid tumors. Clinical Colorectal Cancer. 2003;3:16–18. doi: 10.1016/s1533-0028(11)70463-7. [DOI] [PubMed] [Google Scholar]
- 144.Sebolt-Leopold JS, et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nature Medicine. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 145.Allen LF, Sebolt-Leopold J, Meyer MB. CI-1040 ( PD184352), a targeted signal transduction inhibitor of MEK (MAPKK) Seminars in Oncology. 2003;30:105–116. doi: 10.1053/j.seminoncol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 146.Rinehart J, et al. Multicenter Phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. Journal of Clinical Oncology. 2004;22:4456–4462. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 147.Yeh TC, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clinical Cancer Research. 2007;13:1576–1583. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- 148.Davies BR, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Molecular Cancer Therapeutics. 2007;6:2209–2219. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 149.Lojo Rial C, Wilby D, Sooriakumaran P. Role and rationale of gene therapy and other novel therapies in the management of NMIBC. Expert Review of Anticancer Therapy. 2009;9:1777–1782. doi: 10.1586/era.09.106. [DOI] [PubMed] [Google Scholar]
- 150.Botteman MF, et al. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]