Abstract
Pulsed Q dissociation enables combining LTQ ion trap instruments with isobaric peptide tagging. Unfortunately, this combination lacks a technique which accurately reports protein abundance ratios and is implemented in a freely-available, flexible software pipeline. We developed and implemented a technique assigning collective reporter ion intensity-based weights to each peptide abundance ratio and calculating a protein’s weighted average abundance ratio and P value. Using an iTRAQ-labeled standard mixture, we compared our technique’s performance to the commercial software Mascot, finding that it performed better than Mascot’s non-weighted averaging and median peptide ratio techniques, and equal to its weighted averaging technique. We also compared performance of the LTQ-Orbitrap plus our technique to 4800 MALDI TOF/TOF plus Protein Pilot, by analyzing an iTRAQ-labeled stem cell lysate. We found highly correlated protein abundance ratios, indicating that the LTQ-Orbitrap plus our technique yields results comparable to the current standard. We implemented our technique in a freely available, automated software pipeline, called LTQ-iQuant, which: is mzXML-compatible; supports iTRAQ 4-plex and 8-plex LTQ data; and can be modified for and have weights trained to a user’s LTQ and other isobaric peptide tagging methods. LTQ-iQuant should make LTQ instruments and isobaric peptide tagging accessible to more proteomics researchers.
Keywords: isobaric tags, LTQ, protein quantification, open source software, weighted average
Recently, for tandem mass spectrometry (MS/MS) operation on the popular LTQ line of instruments [1–2], detecting low m/z fragments derived from isobaric tagged peptide [3–4] has been enabled by the development of pulsed Q dissociation (PQD) [5]. Several groups, including ours, have shown the effectiveness for quantitative proteomics of PQD operation using isobaric peptide tagging on the standard LTQ [2, 6–7] and on the hybrid LTQ-Orbitrap [1, 8]. Unfortunately, the use of LTQ instruments with isobaric peptide tagging has been hampered by a lack of software for automated protein quantification. Ideally, such software would meet the following criteria: 1) be compatible with centroided LTQ MS/MS data; 2) employ a technique accounting for errors introduced by low reporter ion intensities, critical for accurate protein quantification [1–2, 7–8]; and 3) be packaged in a freely-available and flexible software pipeline.
No available software currently meets all of these criteria. The Kuster group employed a script developed in-house which met the first two criteria, but was not intended as software for general use by others [1, 8]. Karp et al [9] recently described software employing a technique based on variance stabilizing transformation accounting for errors due to low reporter ion intensities; however it relies on the use of commercial software (Mascot) for quantifying LTQ data. The freely available software programs Multi-Q [10] and iTRACKER [11] are not compatible with centroided LTQ MS/MS data, while the LIBRA program [12] is constrained to those using the Trans-Proteome Pipeline. The commercially available software package Mascot, developed by Matrix Science, offers several techniques for quantifying isobaric peptide tagging data from LTQ data, but is only available to researchers who have purchased the Mascot software. The software Scaffold Q+, marketed by Proteome Software, offers compatibility to LTQ data, but unfortunately it does not account for low reporter ion intensities in its quantification technique, instead assigning protein abundance ratios based on the median abundance ratio from aggregated peptides. Finally, the current Protein Pilot software version sold by Applied Biosystems for the analysis of iTRAQ isobaric peptide tagging data [13] is not compatible with data generated from LTQ instruments.
Here we describe a software pipeline that meets the criteria described above for isobaric peptide tagging data from LTQ instruments. Supporting Information Materials and Methods describes in detail all experiments undertaken in the development of our software pipeline.
As a first step in developing our software, we needed to establish a technique for accurate protein quantification from centroided LTQ MS/MS data. Previous results indicated that relative abundance ratios calculated from lower intensity reporter ions were less accurate than those with higher intensities [1–2, 7–8]. We sought to confirm this relationship via a systematic analysis, and use the results from this analysis to develop a technique that accurately quantifies proteins. We performed mass spectrometric analysis on tryptic peptides from a yeast whole cell lysate that were labeled with iTRAQ reagents and mixed to known ratios of 10:5:2:1 for, respectively, reporter ion masses of 114, 115, 116 and 117. MS/MS scans were performed in PQD mode using optimized instrumental parameters, collecting data in centroid mode [2].
As depicted in Figure 1A, our results confirmed the relationship between reporter ion intensities and accuracy of measurement. To reach this conclusion, we first needed to devise a means to categorize each peptide according to its collected reporter ion intensities. We chose to categorize each peptide based on the product of all four reporter ion intensities, which we deemed more effective than arbitrarily categorizing based on the intensities of individual reporter ions, where it is unclear as to which of the different reporter ions should be considered. Those peptides having relatively low intensities for all four reporter ions would be expected to have a smaller product and be weighted less than those having high signal intensities for all reporter ions. In Figure 1A, the X-axis represents the product of intensities on a log2 scale, or log2(I114 × I115 × I116 × I117), where I114, I115, I116 and I117 correspond to reporter ion intensities of isobaric tags for peptides identified from our yeast standard mixture. The Y-axis represents measured abundance ratios (on a log2 scale) for I114/I115 and I116/I117, which are expected to be at ratios of 2:1 in our standard mixture. Each point represents one scan’s I114/I115 or I116/I117 ratio. The horizontal line at y = 1 represents the expected value for each measured ratio (log2 [2] = 1). Trend line 1 and Trend line 2, show that, with decreasing product of reporter ion intensities, measured peptide abundance ratios diverge from their known values. The Supporting Information Materials and Methods section describes how these trend lines were determined. Similar analysis plotting measured ratios from other combinations of iTRAQ reporter ions from our standard mixture against their expected ratios (5:1 or 10:1) showed similar results (Supporting Information Figure 1). Thus, our results indicated a consistent dependence of abundance ratio accuracy on the product of reporter ion intensities, regardless of the magnitude of the abundance ratio being measured (2:1, 5:1 or 10:1).
Figure 1. Relationship between collective reporter ion intensity and accuracy of reported peptide ratios.
A. Relationship between intensity and reported peptide ratio. X-axis is log2 (I114 × I 115 × I 116 × I 117) and Y-axis is log2 (I 114/ I 115) and log2 (I 116/ I 117) where I 114, I 115, I 116 and I 117 correspond to the intensities of 4-plex iTRAQ reporter ions. The vertical lines denote the boundaries of each bin. B. Graph showing bins on X-axis, containing 100 different peptide abundance ratios each, in order of bins containing the peptides with the lowest product of reporter ion intensity values (bin 1) to highest (bin 7). The y-axis shows the respective weights assigned to each bin and used to generate a weight matrix.
Based on these results, we sought to develop a protein quantification technique based on intensity weighting of peptide data. We used the data from the iTRAQ reagent labeled standard yeast sample above as training data to determine weights. Because accuracy varies as a function of the product of reporter ion intensities, we devised a technique that assigns to peptide abundance ratios weights proportional to this product, aggregates the peptides with their corresponding proteins, and then calculates a weighted average to obtain the protein abundance ratio (See Supporting Information Materials and Methods for details). To determine weights of reported peptide ratios, identified peptides were sorted from lowest to highest products of reporter ion intensities and grouped into bins each containing 100 identified peptides bounded by the vertical lines in Figure 1A and plotted in Figure 1B. The width of each bin is determined by the range of products of reporter ion intensities corresponding to the 100 peptides contained in the bin. We chose to assign each peptide to a bin, rather than simply rank them in linear order of intensity, because we did not want to assume that all datasets would show the same linear distribution of reporter ion intensities. In initial experiments using the standard mixture, we tried different numbers of peptide abundance ratios grouped into each bin, and found that approximately 100 peptides per bin produced the most accurate protein abundance ratios (data not shown). Given this finding, as a default our technique creates a total of N bins for any inputted dataset wherein N-1 bins contain 100 peptide ratios, and the Nth bin contains the remaining peptide ratios with the highest collective reporter ion intensity. The width of each bin in Figure 1B was determined by the range of products of reporter ion intensities corresponding to the 100 peptides contained in the bin. For example, bin 1 contained the peptides with the 100 lowest products of reporter ion intensities, bin 7 contained peptides with the highest products of reporter ion intensities. We measured how well the experimentally-determined ratio for each peptide within a bin deviated from the expected ratio to assign an error, and averaged the square of the error across all 100 peptides to calculate a variance for each bin. Bins with low variance were assigned a higher weight, while bins with a high variance were assigned a lower weight. The Y-axis in Figure 1B shows the relative weights assigned to each bin. For the yeast standard, a 2-by-7 weight matrix with the bin range in the first column and the weight of the bin in the second column was then constructed. The collective peptide identifications used to construct the weight matrix were referred to as a training data set and were used to determine default weights assigned to peptides when quantifying a sample whose protein relative abundance is unknown.
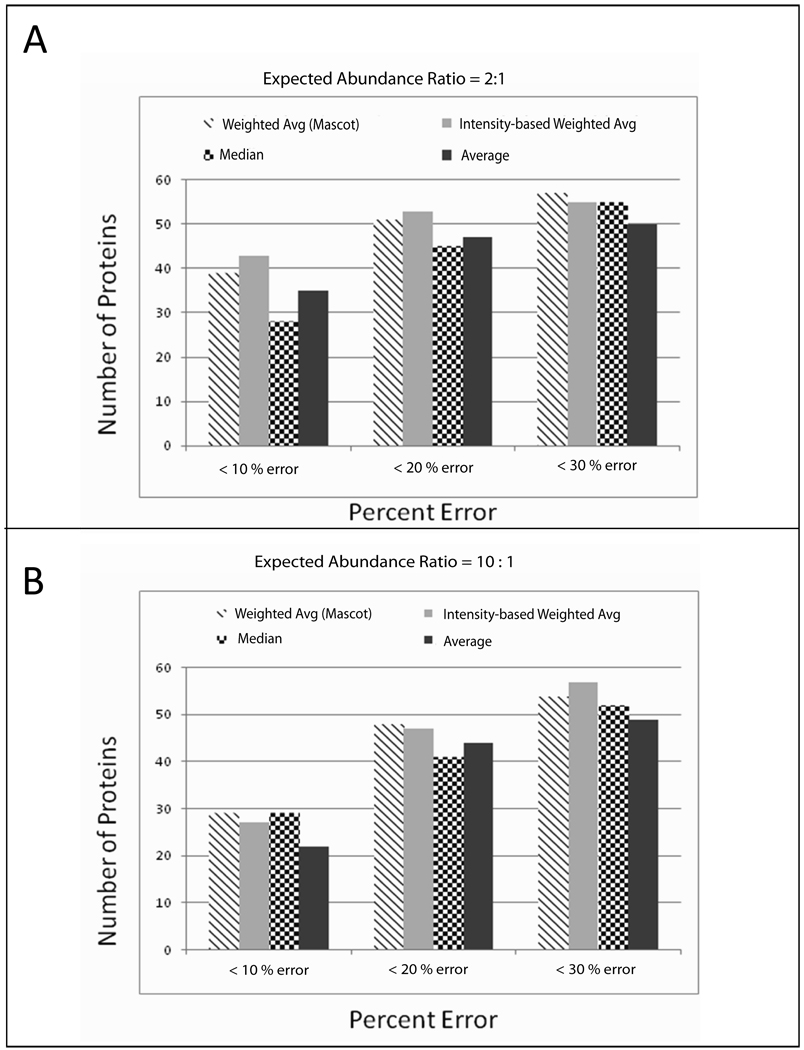
With our peptide weights in hand, we sought to compare the accuracy of using our technique to other techniques for protein quantification from isobaric peptide tagging data. We chose to compare our results to the program Mascot, because it is arguably the best commercial option for quantifying LTQ-generated data, offering several different techniques for quantifying isobaric peptide tagging data: non-weighted averaging of aggregated peptide abundance ratios, use of median peptide abundance ratio for assigning protein abundance ratio, and weighted averaging based on summation of reporter ion intensities across aggregated peptides (see Mascot online Help manual, http://www.matrixscience.com/help/quant_statistics_help.html). Using our standard yeast mixture data from the LTQ-Orbitrap, we first used Mascot for sequence database searching to match MS/MS spectra to peptide sequences (See Supporting Information Materials and Methods), which were then aggregated by protein from which they are derived. For the comparison, we only considered proteins identified by three or more unique peptides. Using the same peptide data, we then quantified each protein using the different techniques offered by Mascot and our weighted averaging technique. We compared our results only for protein abundance ratios deemed statistically significant by Mascot and high confidence proteins quantified by our technique (P value < 0.05). We then determined the number of proteins quantified by each different technique in the standard mixture at varying relative errors (≤10%, ≤20%, or ≤30%) when comparing measured abundance ratios to the expected ratios. We chose to compare results for the ratio of iTRAQ reporter ions 114:115 in our standard mixture (expected to be 2:1; Figure 2A) and the ratio of reporter ions 114:117 (expected to be 10:1; Figure 2B).
Figure 2. Comparison of our quantification technique to quantification techniques offered by Mascot.
A. Comparison of I114/ I115 abundance ratios determined by each technique (2:1 expected ratio). Our technique is denoted as “Intensity-based Weighted Avg”. The other three techniques shown are those offered by Mascot. B. Comparison of I114/ I115 abundance ratios determined by each technique (10:1 expected ratio).
Examination of the results in Figure 2A reveals that our technique quantifies about the same number of proteins with high accuracy (< 10% error) compared to Mascot’s weighted averaging technique when measuring relatively small (2-fold) abundance differences. Our technique quantifies 50% more proteins than Mascot’s median technique, and about 20% more than non-weighted averaging. When the error for quantified proteins is increased to 30%, the numbers of proteins quantified by each technique equalizes, indicating that the four techniques perform comparably when considering less accurate data.
Examination of the results in Figure 2B reveals that our technique also quantifies about the same number of proteins with high accuracy (< 10%) as Mascot’s weighting and median techniques when measuring a larger abundance difference (10-fold). The non-weighted averaging technique performs slightly worse than the other techniques at high accuracy. The 10:1 abundance ratio is the most challenging to measure accurately, due to the 10-fold less abundance from the iTRAQ 117 reporter ion, which will be detected with low signal-to-noise in many of the MS/MS spectra. Similar to the comparisons in Figure 2A, as the % error increases, all four techniques perform comparably, although both weighting techniques still quantify slightly more proteins than either the median or non-weighted averaging.
It should be noted that our technique has an advantage over the weighted average technique used by Mascot: we assign a P value as a measure of confidence of each quantified protein, while Mascot’s technique does not. Assigned P values in our software provide the user flexibility to apply different levels of stringency when interpreting their results without the need for re-analysis at different sensitivities.
Convinced of its value, we next implemented our technique in a software pipeline called LTQ-iQuant which enables automated analysis of large-scale data. Supporting Information Figure 2 details the software pipeline scheme and Supporting Information Material and Methods provides details on its construction.
Our results above demonstrated that the technique used by LTQ-iQuant performs as well as the option offered by Mascot for quantifying isobaric peptide tagging data from LTQ instruments, providing accurate quantification of proteins in complex mixtures. Next, we sought to answer an important question: how does analyzing LTQ-Orbitrap iTRAQ data with LTQ-iQuant compare to analyzing 4800 MALDI TOF/TOF data with Protein Pilot, the current de facto standard for large-scale iTRAQ-based quantitative proteomics? Because the use of the LTQ and isobaric tagging with LTQ-iQuant represents a new method for quantitative proteomics, we felt a comparison to the currently accepted method for analysis of isobaric peptide tagging was warranted to further evaluate its performance. To that end we analyzed an iTRAQ labeled mixture comparing proteins derived from un-differentiated and differentiated stem cell lysates on both instruments using comparable analysis methods (See Supporting Information Materials and Methods and Supporting Information Figure 3 for experimental details).
We first separately quantified the proteins identified on either instrument. Supporting Information Table 1 provides all relevant information on the proteins identified from either instrument. For proteins identified by two or more distinct peptides, the LTQ-Orbitrap identified 1638 proteins while the MALDI 4800 TOF/TOF identified 657 proteins. The estimated protein FDR was 0% and 0.15%, for the LTQ-Orbitrap and MALDI 4800 TOF/TOF data, respectively. 630 proteins were identified in common between the two instruments. Because we used 4-plex iTRAQ reagents for labeling stem cell samples, three separate abundance ratios relative to the I114 signal (I114/I115, I114/I116 and I114/I117) were determined for each identified protein and assigned a P value. Focusing on proteins identified by three or more peptides, the LTQ-Orbitrap plus LTQ-iQuant quantified 910 abundance ratios (P < 0.05) while the MALDI 4800 TOF/TOF plus Protein Pilot quantified 784 abundance ratios (P < 0.05).
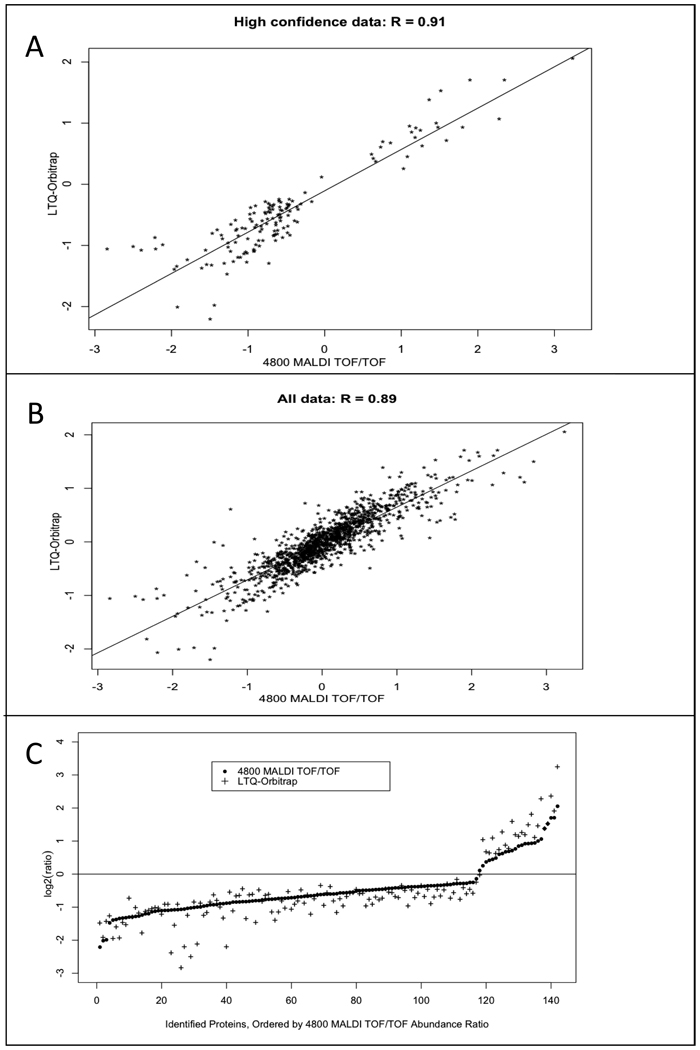
Next we assessed how well LTQ-iQuant and Protein Pilot agreed with each other when quantifying the same protein (Figure 3). We plotted a correlation graph for the common 142 protein abundance ratios that were determined with high confidence (P value < 0.05) by both Protein Pilot and LTQ-iQuant. The measured data between the two instruments and software programs showed positive correlation, with an R value of 0.91 (Figure 3A). We also plotted a correlation graph of ratios for all protein abundance ratios common to both data sets irrespective of assigned P value (Figure 3B). Even for this lower confidence data, abundance ratios obtained from the LTQ-Orbitrap and LTQ-iQuant were still largely in agreement with those same ratios from the MALDI 4800 TOF/TOF (R = 0.89).
Figure 3. Correlation analysis on proteins identified and quantified by both the LTQ-Orbitrap and 4800 MALDI TOF/TOF.
A. A correlation plot of protein abundance ratios reported with P value < 0.05 by both LTQ-iQuant and Protein Pilot. B. A correlation plot for all protein ratios identified in common between the LTQ-Orbitrap and the 4800 MALDI TOF/TOF, with no P value threshold. C. Protein abundance ratios with P value < 0.05 quantified by both our technique and Protein Pilot. Protein abundance ratios are plotted in ascending order of magnitude as measured by Protein Pilot from the 4800 MALDI TOF/TOF data (black dots) along with corresponding abundance ratio for the same protein as measured by LTQ-iQuant from LTQ-Orbitrap data (crosses).
Although the results in Figure 3A showed reasonably good positive correlation for proteins quantified by both instruments, the R value of 0.91 indicated that there was some level of disagreement between the two datasets. To better characterize the nature of this disagreement, we compared the magnitude and direction of measured relative protein abundance ratio changes between the two datasets. For abundance ratios determined with high confidence (P value < 0.05) between both instruments (Figure 3A), we plotted these in order from low to high values as determined by Protein Pilot, along with their corresponding value as determined by LTQ-iQuant (Figure 3C). The results of this plot showed that the direction of each protein abundance ratio (either increased or decreased abundance) was in agreement between the LTQ-Orbitrap and MALDI 4800 TOF/TOF for all quantified proteins, while there was some variation in the magnitude of abundance change between the two instruments contributing to the disagreement observed in the correlation graph in Figure 3A.
From this comparison, it is clear that for proteins quantified by both instruments, results obtained with the 4800 MALDI TOF/TOF and Protein Pilot were in generally good agreement to results with the LTQ-Orbitrap and LTQ-iQuant. Additionally, the LTQ-Orbitrap analysis plus our technique identified and quantified almost 2.5 times more proteins than comparable analysis on the 4800 MALDI TOF/TOF. This finding is especially significant since the 4800 instrument is the currently considered the best option for large-scale iTRAQ-based quantitative proteomic analyses [14]. Based on our collective findings from the yeast standard mixture and the comparison to the MALDI 4800, users can trust the combination of the LTQ-Orbitrap and LTQ-iQuant to provide accurate and sensitive results for quantitative proteomics using isobaric peptide tagging.
Besides automating our technique to make it amenable to large-scale quantitative proteomic studies, the LTQ-iQuant software pipeline has several characteristics that make it attractive. Instead of distributing just binary code files, we have chosen to make the source code, and documentation, freely available. The source code can be accessed at https://netfiles.umn.edu/users/onson001/www/LTQiQuant.html. The pipeline has been developed using Java, making it platform-independent. It was been tested on Windows XP, Ubuntu 8.04 - the Hardy Heron, and Mac OS × 10.4.11 platforms. LTQ-iQuant is mzXML compatible, which gives users the flexibility to employ the pipeline with different database search programs. It can be used for both 4-plex and 8-plex iTRAQ reagent labeling methods, accepts isotope purity correction factors, and is amenable to experimental data where not all four or eight iTRAQ labels are used (e.g., a binary sample comparison where only the 114 and 117 labels are used, etc.).
A key advantage of the software pipeline is the capability of users to input their own training data, enabling generation of a peptide weighting matrix customized to individual instrument performance, different isobaric tagging methods (e.g., TMT) and possibly even emerging instrumental operation methods (e.g., Orbitrap HCD [15–16] or ETD [17–18]). In our experience, the absolute intensities of reporter ions can vary between different LTQ instruments, and even on the same instrument under different tuning parameters. We would also expect that different isobaric tagging methods (e.g., TMT versus iTRAQ) would produce different absolute reporter ion intensities. Given these variations, the relationship of the product of reporter ion intensities on accuracy may differ from the relationship derived from the analysis of our standard iTRAQ labeled yeast mixture, which was used to generate our default peptide weights (Figure 1). Thus, the ability to create customized weights from user-generated training data should help ensure the most accurate quantitative measurements for users of LTQ-iQuant.
In summary, LTQ-iQuant should make the powerful combination of isobaric peptide tagging and the LTQ attractive in a wide-variety of quantitative proteomics studies.
Supplementary Material
Acknowledgments
This research was funded in part by NIH grant 1R01DE017734. We also thank members of the Center for Proteomics and Mass Spectrometry and the Minnesota Supercomputing Institute at the University of Minnesota for instrumental and computational support. We thank Kofi P. Adragni for statistical help.
Abbreviations
- PQD
pulsed Q dissociation
- SQL
Structured Query Language
- TMT
Tandem Mass Tags
Footnotes
Conflict of Interest Statement
The authors declare there are no commercial or financial conflicts of interest for the described work.
REFERENCES
- 1.Bantscheff M, Boesche M, Eberhard D, Matthieson T, et al. Robust and sensitive iTRAQ quantification on an LTQ Orbitrap mass spectrometer. Mol Cell Proteomics. 2008;7:1702–1713. doi: 10.1074/mcp.M800029-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin TJ, Xie H, Bandhakavi S, Popko J, et al. iTRAQ reagent-based quantitative proteomic analysis on a linear ion trap mass spectrometer. J Proteome Res. 2007;6:4200–4209. doi: 10.1021/pr070291b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross PL, Huang YN, Marchese JN, Williamson B, et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Thompson A, Schafer J, Kuhn K, Kienle S, et al. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem. 2003;75:1895–1904. doi: 10.1021/ac0262560. [DOI] [PubMed] [Google Scholar]
- 5.Want EJ, Cravatt BF, Siuzdak G. The expanding role of mass spectrometry in metabolite profiling and characterization. Chembiochem. 2005;6:1941–1951. doi: 10.1002/cbic.200500151. [DOI] [PubMed] [Google Scholar]
- 6.Armenta JM, Hoeschele I, Lazar IM. Differential protein expression analysis using stable isotope labeling and PQD linear ion trap MS technology. J Am Soc Mass Spectrom. 2009;20:1287–1302. doi: 10.1016/j.jasms.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Meany DL, Xie H, Thompson LV, Arriaga EA, Griffin TJ. Identification of carbonylated proteins from enriched rat skeletal muscle mitochondria using affinity chromatography-stable isotope labeling and tandem mass spectrometry. Proteomics. 2007;7:1150–1163. doi: 10.1002/pmic.200600450. [DOI] [PubMed] [Google Scholar]
- 8.Bantscheff M, Eberhard D, Abraham Y, Bastuck S, et al. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat Biotechnol. 2007;25:1035–1044. doi: 10.1038/nbt1328. [DOI] [PubMed] [Google Scholar]
- 9.Karp NA, Huber W, Sadowski PG, Charles PD, et al. Addressing accuracy and precision issues in iTRAQ quantitation. Mol Cell Proteomics. doi: 10.1074/mcp.M900628-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin WT, Hung WN, Yian YH, Wu KP, et al. Multi-Q: a fully automated tool for multiplexed protein quantitation. J Proteome Res. 2006;5:2328–2338. doi: 10.1021/pr060132c. [DOI] [PubMed] [Google Scholar]
- 11.Shadforth IP, Dunkley TP, Lilley KS, Bessant C. i-Tracker: for quantitative proteomics using iTRAQ. BMC Genomics. 2005;6:145. doi: 10.1186/1471-2164-6-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedrioli P, Keller A, King N. 2007 [Google Scholar]
- 13.ProteinPilot, Version 3.0. 2009. [Google Scholar]
- 14.Kuzyk MA, Ohlund LB, Elliott MH, Smith D, et al. A comparison of MS/MS-based, stable-isotope-labeled, quantitation performance on ESI-quadrupole TOF and MALDI-TOF/TOF mass spectrometers. Proteomics. 2009;9:3328–3340. doi: 10.1002/pmic.200800412. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Askenazi M, Jiang J, Luckey CJ, et al. A robust error model for iTRAQ quantification reveals divergent signaling between oncogenic FLT3 mutants in acute myeloid leukemia. Mol Cell Proteomics. 2009 doi: 10.1074/mcp.M900452-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Ficarro SB, Li S, Marto JA. Optimized Orbitrap HCD for quantitative analysis of phosphopeptides. J Am Soc Mass Spectrom. 2009;20:1425–1434. doi: 10.1016/j.jasms.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Han H, Pappin DJ, Ross PL, McLuckey SA. Electron transfer dissociation of iTRAQ labeled peptide ions. J Proteome Res. 2008;7:3643–3648. doi: 10.1021/pr8001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phanstiel D, Zhang Y, Marto JA, Coon JJ. Peptide and protein quantification using iTRAQ with electron transfer dissociation. J Am Soc Mass Spectrom. 2008;19:1255–1262. doi: 10.1016/j.jasms.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.