Summary
Steroid hormones are systemic signaling molecules that regulate juvenile-adult transitions in both insects and mammals. In insects, pulses of the steroid hormone 20-hydroxyecdysone (20E) are generated by increased biosynthesis followed by inactivation/clearance. Although mechanisms that control 20E synthesis have received considerable recent attention, the physiological significance of 20E inactivation remains largely unknown. We show that the cytochrome P450 Cyp18a1 lowers 20E titer during the Drosophila prepupal to pupal transition. Furthermore, this reduction of 20E levels is a prerequisite to induce βFTZ-F1, a key factor in the genetic hierarchy that controls early metamorphosis. Resupplying βFTZ-F1 rescues Cyp18a1-deficient prepupae. Since Cyp18a1 is 20E-inducible, it appears that the increased production of steroid is responsible for its eventual decline, thereby generating the regulatory pulse required for proper temporal progression of metamorphosis. The coupling of hormone clearance to βFTZ-F1 expression suggests a general mechanism by which transient signaling drives unidirectional progression through a multistep process.
Introduction
Animals develop from juveniles into sexually mature adults through successive life stages separated by temporally defined transitions. Steroid hormones are systemic signaling molecules that temporally coordinate the juvenile-adult transition in both mammals and insects through interactions with nuclear receptors (King-Jones and Thummel, 2005; McBrayer et al., 2007; Popa et al., 2008; Rewitz et al., 2009b). In insects, pulses of the steroid hormone, 20-hydroxyecdysone (20E), are responsible for this transition, a process known as metamorphosis (Riddiford, 1993).
Insight into how steroids control the genetic circuits during developmental transitions has mainly come from studies in Drosophila melanogaster, which led to a general model for gene regulation by steroid hormones in eukaryotes (Ashburner et al., 1974; Thummel, 1996, 2001, 2002). According to this model, only a pulse of 20E, i.e. a short period of high 20E titer, can trigger activation of some genes in the 20E-regulated cascade that initiates metamorphosis (Sun et al., 1994; Thummel, 1996; Woodard et al., 1994). Pulses of 20E are generated by two processes: synthesis that increases the titer and inactivation/removal that decreases the titer. Although the mechanisms that control the rise in 20E are well studied (Caldwell et al., 2005; Colombani et al., 2005; Gilbert et al., 2002; Layalle et al., 2008; McBrayer et al., 2007; Rewitz et al., 2009a; Rewitz et al., 2009b), the physiological significance of 20E inactivation is largely unexplored except for several in vitro studies that examined the importance of 20E decline during prepupal development (Fechtel et al., 1988; Richards, 1976).
One proposed route for 20E inactivation is through 26-hydroxylation catalyzed by the cytochrome P450 Cyp18a1 (Bassett et al., 1997; Guittard et al., 2010; Hurban and Thummel, 1993). Interestingly, Cyp18a1 was first identified based on its inducibility by 20E (Hurban and Thummel, 1993), consistent with the 20E-inducible 26-hydroxylase activity (Chen et al., 1994; Williams et al., 1997; Williams et al., 2000). If this is the case, inactivation is dependent on the concentration of the hormone itself, representing an elegant feedback regulation of steroid levels.
The aim of the present study was to examine the functional importance of steroid pulses during development by studying the role of Cyp18a1 in the decline of 20E levels. Here, we present evidence that Cyp18a1 is required for the decline of the 20E titer and that failure to reduce 20E levels after the late larval 20E peak disrupts metamorphic development and leads to animal death. Furthermore, we show that these animals die because elevated 20E levels repress the expression of the mid-prepupal gene βFTZ-F1, a factor necessary for providing competence to respond to 20E in a stage-specific manner during metamorphosis (Broadus et al., 1999).
Results and Discussion
Cyp18a1 overexpression yields a phenotype similar to that of ecdysone-deficient mutants
It has recently been demonstrated that Cyp18a1 hydroxylates 20E at position C26, a process believed to convert this hormone into inactive metabolites (Bassett et al., 1997; Guittard et al., 2010; Hurban and Thummel, 1993). To directly test the hypothesis that 26-hydroxylation inactivates 20E, we overexpressed this enzyme using the Gal4/UAS system during embryonic development. Mutants with reduced 20E titers during the embryonic stage show a characteristic “Halloween” phenotype that consists of a failure to secrete cuticle, a lack of head involution, and an inability of the midgut and dorsal epidermis to close (Figure 1). Ultimately these embryos die during late embryonic development and fail to hatch as first instar larvae (Chavez et al., 2000; Petryk et al., 2003; Warren et al., 2002). To examine the effects of Cyp18a1 overexpression, Gal4 drivers expressed in different tissues were used to overexpress UAS-Cyp18a1 (Table S1). Ubiquitous strong (da>Cyp18a1) or weaker (arm>Cyp18a1) expression of Cyp18a1 resulted in 100% embryonic lethality. Expression of Cyp18a1 in the CNS alone (elav-Gal4) also caused embryonic death, whereas animals expressing Cyp18a1 primarily in the fat body (pumpless-Gal4 and CG-Gal4) died in the larval and pupal stages.
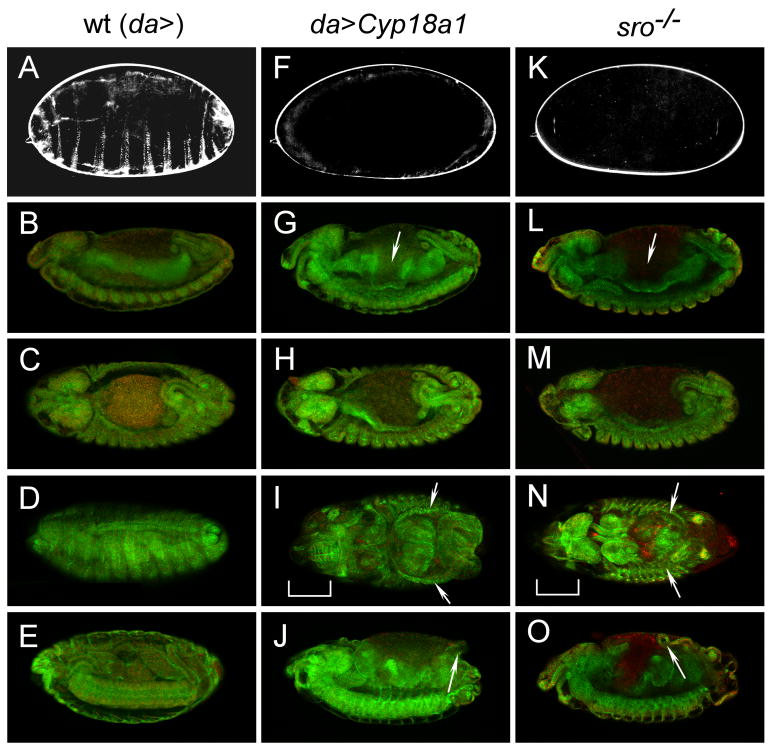
Figure 1.
The phenotype of UAS-Cyp18a1 overexpression is similar to that of ecdysone-deficient mutants. (A, F, K) Cuticle preparations of stage 17 embryos showing that embryos overexpressing Cyp18a1 (da>Cyp18a1) fail to lay down an embryonic cuticle like the low ecdysone mutant shroud1 (sro). Immunohistochemical staining with a spectrin antibody (green) and nuclear staining with DAPI (red) of embryos stage 14 lateral view (B, G, L), stage 14 dorsal view (C, H, M), stage 15-17 dorsal view (D, I, N), and stage 15-17 lateral view (E, J, O). The terminal phenotype in embryos overexpressing Cyp18a1 (I, J) is similar to homozygous sro mutants (N,O). Note the defects in midgut morphogenesis (arrows in G and L), dorsal closure (arrows in I and N), head involution (brackets in I and N) and the protruding gut as a result of the morphogenesis defect (arrows in J and O) in these embryos. Embryos are viewed with anterior to the left.
To examine the phenotype of ubiquitous Cyp18a1 overexpression, cuticles from da>Cyp18a1 embryos were prepared and compared to the Halloween mutant shroud (sro) that encodes an enzyme involved in 20E synthesis (Niwa et al., 2010). Similar to sro mutants (and all other biosynthetic enzyme mutants), da>Cyp18a1 embryos fail to produce cuticle structures such as denticle belts (compare Figures 1A, 1F and 1K). Furthermore, after stage 14, these embryos exhibit morphological defects typically observed in Halloween family mutants including abnormalities in gut morphogenesis (arrows in Figures 1G and 1L), head involution and dorsal closure (brackets and arrows in Figures 1I and 1N). In these embryos, the incomplete midgut closure leads to yolk granules that are located randomly through the dorsal opening. These animals never deposit a cuticle and die before hatching to the first larval instar. Thus, overexpression of Cyp18a1 results in a phenotype identical to that of the 20E-deficient Halloween mutants, demonstrating that Cyp18a1 inactivates 20E.
Cyp18a1-mediated inactivation of 20E is required for metamorphic development
To determine if inactivation of 20E has an important developmental function, we characterized the Cyp18a1 loss-of-function phenotype. An allele designated Cyp18a11 missing the region upstream of the translational start site including most of the 5′-untranslated region was obtained by the imprecise excision of a P-element (Figure 2A). The excision removes from -34 to -649 bp upstream of the translational start and leaves a portion of the P-element at the insertion position.
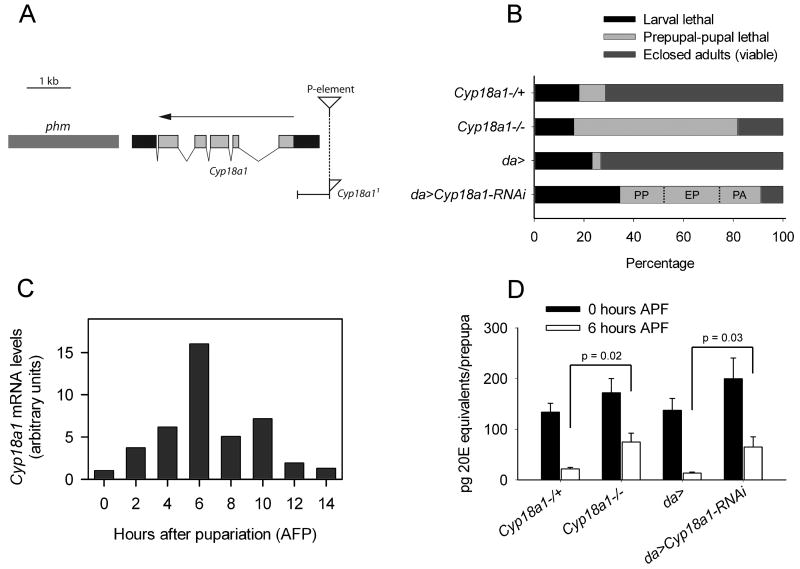
Figure 2.
Cyp18a1 is required during metamorphosis. (A) Illustration of the Cyp18a1 locus and Cyp18a11 mutant. Light gray boxes indicate protein coding sequences and black boxes show untranslated regions. An arrow indicates gene orientation and the site of the excised P-element is shown by an inverted triangle. The remaining part of the triangle in Cyp18a11 indicates the part of the P-element that is still present. phm; phantom. (B) The lethal phase was analyzed in Cyp18a11 and da>Cyp18a1-RNAi animals. Lethality during metamorphosis was scored for three phenotypic classes in da>Cyp18a1-RNAi: percentage of animals that died as prepupae (PP), early in the pupal stage (EP) or as pharate adults (PA) is shown. (C) Cyp18a1 expression during the prepupal-pupal transition detected using quantitative RT-PCR. High expression correlates with prepupal period where Cyp18a1 is necessary for development. (D) Ecdysteroid titer at puparium formation (0 hours APF) and 6 hours APF in control, Cyp18a11 and da>Cyp18a1-RNAi animals. Error bars represent SE; n = 3 batches. P values were calculated by Student's t test.
To investigate the developmental role of Cyp18a1, the lethal phase of Cyp18a11 animals was analyzed. The majority of homozygous Cyp18a11 embryos (93.2%; n=73) developed normally to the first larval instar. Analysis of post-embryonic lethality showed that most homozygous Cyp18a11 animals pupariate but fail to complete metamorphosis (Figure 2B). Cyp18a11 is a strong loss of function allele because when placed in trans over Df(1)BSC585, a deficiency covering the Cyp18a1 locus, the phenotype was indistinguishable from homozygous Cyp18a11 mutants (data not shown). A similar metamorphosis defect was observed when Cyp18a1 expression was reduced ubiquitously using UAS-Cyp18a1-RNAi (da>Cyp18a1-RNAi). During the prepupal to pupal transition, these animals die in three phenotypic classes (Figure 2B). Of those that pupariated, 72% of the animals died as prepupae or as early pupae after delayed head eversion. This shows that Cyp18a1 is important for prepupal development. A smaller proportion of the animals died as pharate adults and fewer escapers eclosed. All animals that pupate showed a malformed leg phenotype and some also displayed head eversion defects (Figure 3). These results demonstrate that Cyp18a1 activity is critical for metamorphosis. Like da-Gal4, the strong ubiquitous drivers αTub84B-Gal4 and Act5C-Gal4 produced animals that died during metamorphosis (Table S2). Weaker ubiquitous expression of Cyp18a1-RNAi using armadillo-Gal4 allowed most animals to develop to the adult stage.
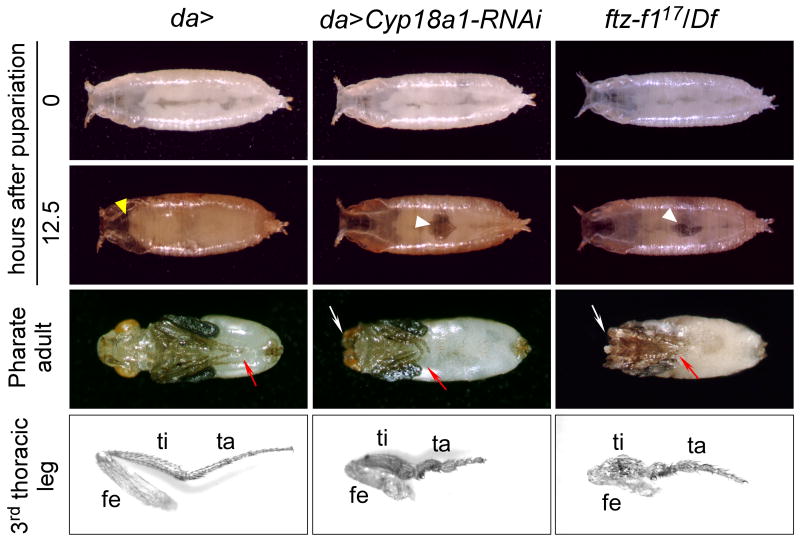
Figure 3.
Reduction of Cyp18a1 activity results in a phenotype similar to the loss of β FTZ-F1 function. Both da>CYP18-RNAi and βFTZ-F117/Df animals show failure to displace the air bubble (white arrowheads) anteriorly during prepupal development and show defects in head eversion (yellow arrowhead in da>), resulting in microcephalic and cryptocephalic phenotypes, respectively (white arrows). The third leg pair (red arrows) is malformed in all animals that reach this stage. Dissected third legs from adults are shown. fe, femur; ti, tibia; ta, tarsus.
If Cyp18a1 is critical for prepupal development, it should be expressed at high levels during this stage. Therefore, Cyp18a1 expression was analyzed using quantitative RT-PCR during the prepupal stage. After low expression at the time of pupariation when 20E peaks, expression of Cyp18a1 rapidly increases in a manner that inversely correlates with the 20E level (Figure 2C). The expression of Cyp18a1 reaches a peak in the mid-prepupal stage 6 hours after puparium formation (APF) when 20E has declined to the basal level after the late larval 20E peak at pupariation. Consistent with this expression pattern, both da>Cyp18a1-RNAi and Cyp18a11 prepupae had significantly higher 20E levels 6 hours APF (Figure 2D). These results demonstrate that Cyp18a1 has an important function in metabolizing 20E after pupariation.
It is interesting that loss of Cyp18a1 activity does not significantly arrest development at early embryonic or larval stages. Although this might indicate that the decline of the 20E level is not important during these stages, there are a few things to consider when interpreting this result. First, neither Cyp18a11 nor da>Cyp18a1-RNAi completely eliminates the transcript, so the residual Cyp18a1 activity might be high enough for the animals to make it through the embryonic and larval stages. Second, some reports indicate that other 20E inactivation routes also exist (Lafont et al., 2005; Rees, 1995), which may also explain why the 20E levels show some decline during prepupal stages even in Cyp18a11 and da>Cyp18a1-RNAi animals. Third, during larval stages, 20E can be eliminated not only by inactivation but also by excretion, whereas during metamorphosis where the animal is a closed system, a decline of 20E can only be achieved by the conversion into inactive metabolites. This might also contribute to the stage-specific sensitivity of the animal to the loss of Cyp18a1.
Reduction of Cyp18a1 activity results in a phenotype similar to the loss of βFTZ-F1
In considering the Cyp18a1 loss-of-function phenotype, we realized that it closely matched that seen in hypomorphic βFTZ-F117 mutants (Broadus et al., 1999; Sliter and Gilbert, 1992). Like animals heterozygous for βFTZ-F117 and a deletion allele Df(3L)CatDH104 that covers the βFTZ-F1 locus, da>Cyp18a1-RNAi larvae pupariate normally, but exhibit defects in pupation (Figure 3). βFTZ-F117/Df(3L)CatDH104 and da>Cyp18a1-RNAi larvae contract their bodies and evert their anterior spiracles normally at pupariation. At this stage and shortly after pupariation when they sclerotize and tan their cuticle, these animals are morphologically normal. During subsequent prepupal development, the abdominal air bubble normally translocates anteriorly in control animals and pupation occurs approximately 12 hours APF with the eversion of the head. da>Cyp18a1-RNAi and βFTZ-F117/Df(3L)CatDH104 prepupae show delayed or completely failed displacement of the abdominal air bubble and head eversion, generating microcephalic and cryptocephalic phenotypes, respectively. The third thoracic legs are also similarly short and malformed in βFTZ-F117/Df(3L)CatDH104 and da>Cyp18a1-RNAi animals (Figure 3).
The 20E-regulated genetic hierarchy that controls metamorphic development is disrupted in Cyp18a1-RNAi animals
To investigate the molecular consequences of Cyp18a1 loss during prepupal development, we examined the temporal expression profile of 20E-regulated genes including βFTZ-F1 that coordinate this particular developmental transition. Normally, βFTZ-F1 is induced in the mid-prepupal stage only after a pulse exposure to 20E, which means a period with a high level of 20E followed by a low level (Broadus et al., 1999; Woodard et al., 1994; Yamada et al., 2000). The data presented above show that Cyp18a1 loss-of-function animals experience an extended period of high 20E levels in the mid-prepupal stage that might repress βFTZ-F1 expression.
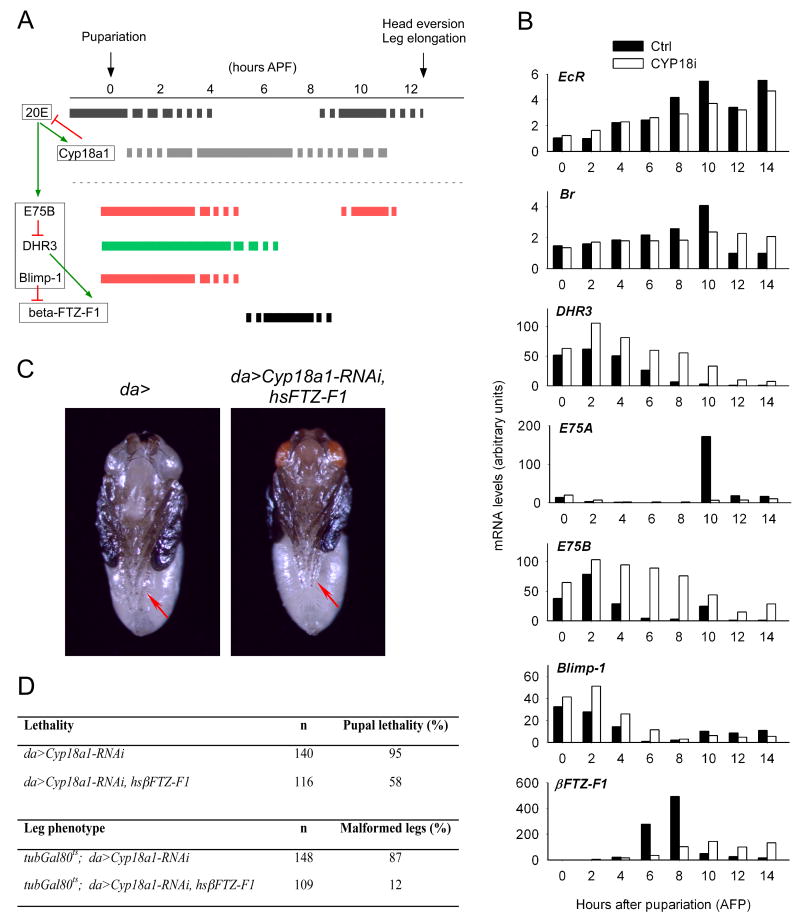
When we examined the expression of EcR which is required for the initial triggering of metamorphosis, we found that its expression is relatively normal in da>Cyp18a1-RNAi animals compared to the control (Figure 4B). In control animals, βFTZ-F1 is induced in the mid-prepupal stage 6-8 hours APF. At this point, the 20E titer drops to the basal level in these animals whereas it is still elevated in da>Cyp18a1-RNAi animals (Figure 2D). In agreement with the repressive role of 20E, reduced βFTZ-F1 expression was observed in da>Cyp18a1-RNAi animals at this stage (Figure 4B). This supports the idea that misexpression of βFTZ-F1 contributes to the phenotype of da>Cyp18a1-RNAi animals. Furthermore, increased expression of the 20E-inducible genes DHR3, E75B and Blimp-1 was observed during the early prepupal stage (Figure 4B). E75B is believed to prevent DHR3 from inducing βFTZ-F1 in the first 4 hours APF, whereas Blimp-1 is a direct repressor of βFTZ-F1 (Figure 4A) (Agawa et al., 2007; Andres et al., 1993; Broadus et al., 1999; Yamada et al., 2000). In control animals expression of E75B and Blimp-1 have declined 6 hours APF, allowing DHR3 to induce βFTZ-F1 (Figure 4B). In contrast, both repressors are expressed in da>Cyp18a1-RNAi animals 6 hours APF because these genes are induced by the higher 20E level. The prolonged expression of the βFTZ-F1 repressors E75B and Blimp-1 in da>Cyp18a1-RNAi animals provides a molecular explanation for the lack of βFTZ-F1 transcription in these animals.
Figure 4.
The ecdysone-induced genetic hierarchy necessary for metamorphic development is disrupted in Cyp18a1-RNAi prepupae. (A) A model for the regulation of βFTZ-F1 and the role of Cyp18a1 and 20E. The 20E peak at pupariation induces a set of genes including E75B, DHR3, Blimp-1 and Cyp18a1. Under these conditions, E75B represses DHR3, a transcriptional activator of βFTZ-F1, whereas Blimp-1 directly represses βFTZ-F1 expression. As Cyp18a1 lowers the 20E level, the expression of 20E-inducible genes E75B, DHR3 and Blimp-1 declines. Low expression of E75B and Blimp-1 at 6 hours APF allows residual DHR3 to induce βFTZ-F1. (B) The expression of 20E-regulated genes analyzed using quantitative RT-PCR during the prepupal-pupal transition. Data are expressed as fold changes relative to the lowest expression level for each gene, arbitrarily set to 1. (C) Expression of hsβFTZ-F1 rescues the leg phenotype (red arrows) of Cyp18a1-RNAi animals. A da>Cyp18a1-RNAi, hsβFTZ-F1 pharate adult with normally developed legs like the control (da>) is shown. (D) Ectopic expression of hsβFTZ-F1 recsues lethality and leg abnormalities of Cyp18a1-RNAi animals.
βFTZ-F1 is a nuclear receptor necessary for the stage-specific response to the prepupal 20E pulse at 10 hours APF that coordinates the prepupal-pupal transition (Broadus et al., 1999; Sliter and Gilbert, 1992). In control animals this prepupal 20E pulse induces transient high level expression of the 20E-inducible gene E75A (Figure 4B). Since the mid-prepupal expression of βFTZ-F1 is necessary for this induction, it is not observed in βFTZ-F117/Df(3L)CatDH104 animals (Broadus et al., 1999). Consistent with the reduced mid-prepupal βFTZ-F1 expression, the E75A peak at 10 hours APF was not observed in da>Cyp18a1-RNAi animals (Figure 4B). Together the results suggest that after the late larval 20E pulse that triggers larval-prepupal transition, da>Cyp18a1-RNAi animals do not acquire the βFTZ-F1-mediated competence to respond to the prepupal 20E pulse necessary for the prepupal-pupal transition.
Cyp18a1 confers the βFTZ-F1-mediated competence to respond to the prepupal ecdysone pulse
The above data suggest that the reduced βFTZ-F1 expression causes the metamorphic defect in Cyp18a1 loss-of-function animals. If this is the case, expression of βFTZ-F1 in da>Cyp18a1-RNAi animals should rescue the phenotype. Indeed, we found that ectopic βFTZ-F1 expression under control of a heat shock promoter rescued the lethality of da>Cyp18a1-RNAi animals. Forty-two percent of the da>Cyp18a1-RNAi animals containing hsβFTZ-F1 eclosed as viable adults, compared to only 5% of the control da>Cyp18a1-RNAi animals (Figure 4D). This is comparable to the percentage of βFTZ-F1 mutants rescued by expression of hsβFTZ-F1, which ranged from approximately 2-40% (Yamada et al., 2000).
Although the leg phenotype was also rescued in some of the da>Cyp18a1-RNAi, hsβFTZ-F1 flies (Figure 4C), for a better comparison we chose to quantify rescue of this phenotype in the presence of Gal80ts, a temperature sensitive Gal4 inhibitor that partially inhibits Gal4 activity at 25°C. At this temperature expression of Gal80ts in da>Cyp18a1-RNAi animals (tubGal80ts; da>Cyp18a1-RNAi) reduces the activity of the Gal4 and allows almost 100% of the pupae to eclose as adults, 87% of which exhibit the leg phenotype observed in da>Cyp18a1-RNAi animals (Figure 4D). In contrast, only 12% of the eclosed tubGal80ts; da>Cyp18a1-RNAi, hsβFTZ-F1 flies had abnormal legs. This clearly demonstrates that it is the lack of βFTZ-F1 activity that is causing the defects in leg morphogenesis in Cyp18a1-RNAi animals. Taken together, our results show that the defect in the prepupal-pupal transition in Cyp18a1 loss-of-function animals can be atrributed to the repression of βFTZ-F1, a factor necessary for the stage-specific response to 20E.
In both insects and mammals, initiation of sexual maturation is controlled by the neuroendocrine system that triggers the production of systemic steroid signals (McBrayer et al., 2007; Rewitz et al., 2009a; Rewitz et al., 2009b). To date, most insect studies have concentrated on how the rise in steroid titer induces new genetic cascades that program a developmental transition such as metamorphosis. However, as illustrated here, correct temporal progression through the metamorphic genetic program requires properly timed removal of steroid as well. Gene regulation by the consecutive rise and fall of steroids, produced by feedback regulation, may provide a general mechanism for control of temporal progression through development.
Experimental Procedures
Drosophila stocks
Flies were maintained on standard cornmeal media at 25°C under 12h/12h light/dark cycle. Drosophila stocks were obtained from the Bloomington Stock Center unless otherwise specified. The hsβFTZ-F1 (Yamada et al., 2000); βFTZ-F117 and Df(3L)CatDH104 (Broadus et al., 1999); UAS-Cyp18a1-RNAi (Vienna Drosophila RNAi Center #5602); Cyp18a11 and UAS-Cyp18a1 were gifts from Ethan Bier.
Lethal phase determination and phenotype analysis
Flies were allowed to lay eggs for 2 hours on apple juice agar plates supplemented with yeast paste. Embryonic lethality was assayed in embryos allowed to develop for 24 hours. To assay post-embryonic lethality, newly hatched first instar larvae were transferred to standard cornmeal food, with 25 larvae per vial. To examine the exact lethal phase during the prepupal-pupal stages, white prepupae were isolated and allowed to develop on moist filter paper and scored for lethality and morphological phenotype.
Quantification of ecdysteroids
Frozen prepupae (10 animals/tube) were homogenized and extracted as described previously (Warren et al., 2006). The extracts were evaporated, redissolved and subjected to ecdysteroid ELISA. The ELISA was performed in a competitive assay format using anti-20E rabbit antiserum (Cayman Chemical), acetylcholinesterase-conjugated 20E (Cayman Chemical) and standard 20E (Sigma). The acetylcholinesterase activity was quantified by Ellman's Reagent (Cayman Chemical) and the absorbance at 415 nm was detected with a Benchmark microplate reader (Bio-Rad).
Analysis of embryonic phenotypes
Cuticle from developed embryos was prepared (Rewitz et al., 2009b) and embryonic structures were visualized by staining for spectrin (a cytoskeletal protein) using a mouse anti-spectrin antibody (Developmental Studies Hybridoma Bank, University of Iowa, IA) at 1/50 dilution as described (Chavez et al., 2000), except that staining was visualized using a fluorescent conjugated secondary antibody (Molecular Probes) at 1/500 dilution. Confocal images were obtained using a Zeiss LSM 710 confocal laser-scanning microscope.
Quantitative RT-PCR
For analysis of gene expression, RNA was extracted from prepupae and pupae precisely timed from the white puparium stage. Total RNA was prepared from 6 individuals using the RNeasy Mini Kit (Qiagen), DNaseI treated on-column to remove genomic DNA and reverse transcribed using the Superscript III reverse transcriptase (Invitrogen). Quantitative RT-PCR was carried out with SYBR Green Master mix using a real time PCR LightCycler 480 (Roche) as described (Rewitz et al., 2006). To ensure homogeneity of the amplified PCR product, melting curve analysis was performed on all reactions. The Drosophila ribosomal protein L23 (rpL23) was used to normalize transcript levels (McBrayer et al., 2007). The primers used are shown in Table S3.
Rescue experiment
A hsβFTZ-F1 transgene was recombined with UAS-Cyp18a1-RNAi by standard genetic methods. In order to avoid the elevated lethality of da>Cyp18a1-RNAi animals raised at 29°C (100%), we performed the rescue experiment at 25°C where the heat shock promoter shows high enough activity to rescue mutant phenotypes (Guo et al., 2000; Qian et al., 1988; Ruaud et al., 2010).
Highlights.
Gain- and loss-of-function studies show that Cyp18a1 is a 20E inactivation enzyme.
Failure to reduce hormone levels disrupts the steroid-regulated genetic hierarchy.
Cyp18a1 loss phenocopies βFTZ-F1 mutants and can be rescued by supplying βFTZ-F1.
Both the rise and fall of steroid hormone titer are critical for animal development.
Supplementary Material
Acknowledgments
K.F.R. was supported by a postdoctoral fellowship from The Danish Natural Science Research Council (grant no. 272-07-0340). N.Y. is supported by a postdoctoral fellowship from the Japan Society for the Promotion of Science. M.B.O. was an investigator with the Howard Hughes Medical Institute for a portion of this work. Additional support was provided by NIH R01 GM093301. We are grateful to our collaborators, Lawerence Gilbert, James T. Warren and Robert Rybczynski for many stimulating discussions. We thank Ethan Bier for Cyp18a1 lines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agawa Y, Sarhan M, Kageyama Y, Akagi K, Takai M, Hashiyama K, Wada T, Handa H, Iwamatsu A, Hirose S, et al. Drosophila Blimp-1 is a transient transcriptional repressor that controls timing of the ecdysone-induced developmental pathway. Mol Cell Biol. 2007;27:8739–8747. doi: 10.1128/MCB.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres AJ, Fletcher JC, Karim FD, Thummel CS. Molecular analysis of the initiation of insect metamorphosis: a comparative study of Drosophila ecdysteroid-regulated transcription. Dev Biol. 1993;160:388–404. doi: 10.1006/dbio.1993.1315. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Chihara C, Meltzer P, Richards G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:655–662. doi: 10.1101/sqb.1974.038.01.070. [DOI] [PubMed] [Google Scholar]
- Bassett MH, McCarthy JL, Waterman MR, Sliter TJ. Sequence and developmental expression of Cyp18, a member of a new cytochrome P450 family from Drosophila. Mol Cell Endocrinol. 1997;131:39–49. doi: 10.1016/s0303-7207(97)00093-2. [DOI] [PubMed] [Google Scholar]
- Broadus J, McCabe JR, Endrizzi B, Thummel CS, Woodard CT. The Drosophila beta FTZ-F1 orphan nuclear receptor provides competence for stage-specific responses to the steroid hormone ecdysone. Mol Cell. 1999;3:143–149. doi: 10.1016/s1097-2765(00)80305-6. [DOI] [PubMed] [Google Scholar]
- Caldwell PE, Walkiewicz M, Stern M. Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr Biol. 2005;15:1785–1795. doi: 10.1016/j.cub.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Chavez VM, Marques G, Delbecque JP, Kobayashi K, Hollingsworth M, Burr J, Natzle JE, O'Connor MB. The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development. 2000;127:4115–4126. doi: 10.1242/dev.127.19.4115. [DOI] [PubMed] [Google Scholar]
- Chen JH, Kabbouh M, Fisher MJ, Rees HH. Induction of an inactivation pathway for ecdysteroids in larvae of the cotton leafworm, Spodoptera littoralis. Biochem J. 1994;301(Pt 1):89–95. doi: 10.1042/bj3010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carre C, Noselli S, Leopold P. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- Fechtel K, Natzle JE, Brown EE, Fristrom JW. Prepupal differentiation of Drosophila imaginal discs: identification of four genes whose transcripts accumulate in response to a pulse of 20-hydroxyecdysone. Genetics. 1988;120:465–474. doi: 10.1093/genetics/120.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LI, Rybczynski R, Warren JT. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol. 2002;47:883–916. doi: 10.1146/annurev.ento.47.091201.145302. [DOI] [PubMed] [Google Scholar]
- Guittard E, Blais C, Maria A, Parvy J, Pasricha S, Lumb C, Lafont R, Daborn P, Dauphin-Villemant C. CYP18A1, a key enzyme of Drosophila steroid hormone inactivation, is essential for metamorphosis. Dev Biol. 2010 doi: 10.1016/j.ydbio.2010.09.023. In press. [DOI] [PubMed] [Google Scholar]
- Guo HF, Tong J, Hannan F, Luo L, Zhong Y. A neurofibromatosis-1-regulated pathway is required for learning in Drosophila. Nature. 2000;403:895–898. doi: 10.1038/35002593. [DOI] [PubMed] [Google Scholar]
- Hurban P, Thummel CS. Isolation and characterization of fifteen ecdysone-inducible Drosophila genes reveal unexpected complexities in ecdysone regulation. Mol Cell Biol. 1993;13:7101–7111. doi: 10.1128/mcb.13.11.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Jones K, Thummel CS. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet. 2005;6:311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- Lafont R, Dauphin-Villemant C, Warren JT. Ecdysteroid chemistry and biochemistry. In: Gilbert LI, Iatrou K, Gill S, editors. Comprehensive Molecular Insect Science. Oxford: Elsevier; 2005. pp. 125–195. [Google Scholar]
- Layalle S, Arquier N, Leopold P. The TOR pathway couples nutrition and developmental timing in Drosophila. Dev Cell. 2008;15:568–577. doi: 10.1016/j.devcel.2008.08.003. [DOI] [PubMed] [Google Scholar]
- McBrayer Z, Ono H, Shimell M, Parvy JP, Beckstead RB, Warren JT, Thummel CS, Dauphin-Villemant C, Gilbert LI, O'Connor MB. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell. 2007;13:857–871. doi: 10.1016/j.devcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R, Namiki T, Ito K, Shimada-Niwa Y, Kiuchi M, Kawaoka S, Kayukawa T, Banno Y, Fujimoto Y, Shigenobu S, et al. Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the ‘Black Box’ of the ecdysteroid biosynthesis pathway. Development. 2010;137:1991–1999. doi: 10.1242/dev.045641. [DOI] [PubMed] [Google Scholar]
- Petryk A, Warren JT, Marques G, Jarcho MP, Gilbert LI, Kahler J, Parvy JP, Li Y, Dauphin-Villemant C, O'Connor MB. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci U S A. 2003;100:13773–13778. doi: 10.1073/pnas.2336088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol. 2008;70:213–238. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- Qian S, Hongo S, Jacobs-Lorena M. Antisense ribosomal protein gene expression specifically disrupts oogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1988;85:9601–9605. doi: 10.1073/pnas.85.24.9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees HH. Ecdysteroid biosynthesis and inactivation in relation to function. Eur J Entomol. 1995;92:9–39. [Google Scholar]
- Rewitz KF, Larsen MR, Lobner-Olesen A, Rybczynski R, O'Connor MB, Gilbert LI. A phosphoproteomics approach to elucidate neuropeptide signal transduction controlling insect metamorphosis. Insect Biochem Mol Biol. 2009a;39:475–483. doi: 10.1016/j.ibmb.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. Identification, characterization and developmental expression of Halloween genes encoding P450 enzymes mediating ecdysone biosynthesis in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2006;36:188–199. doi: 10.1016/j.ibmb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Rewitz KF, Yamanaka N, Gilbert LI, O'Connor MB. The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science. 2009b;326:1403–1405. doi: 10.1126/science.1176450. [DOI] [PubMed] [Google Scholar]
- Richards G. Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. IV. The mid prepupal period. Dev Biol. 1976;54:256–263. doi: 10.1016/0012-1606(76)90303-1. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Hormones and Drosophila Development. In: Bate M, Martinez-Arias M, editors. The Development of Drosophila melanogaster. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1993. pp. 899–940. [Google Scholar]
- Ruaud AF, Lam G, Thummel CS. The Drosophila nuclear receptors DHR3 and betaFTZ-F1 control overlapping developmental responses in late embryos. Development. 2010;137:123–131. doi: 10.1242/dev.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliter TJ, Gilbert LI. Developmental arrest and ecdysteroid deficiency resulting from mutations at the dre4 locus of Drosophila. Genetics. 1992;130:555–568. doi: 10.1093/genetics/130.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GC, Hirose S, Ueda H. Intermittent expression of BmFTZ-F1, a member of the nuclear hormone receptor superfamily during development of the silkworm Bombyx mori. Dev Biol. 1994;162:426–437. doi: 10.1006/dbio.1994.1099. [DOI] [PubMed] [Google Scholar]
- Thummel CS. Flies on steroids--Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet. 1996;12:306–310. doi: 10.1016/0168-9525(96)10032-9. [DOI] [PubMed] [Google Scholar]
- Thummel CS. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell. 2001;1:453–465. doi: 10.1016/s1534-5807(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Thummel CS. Ecdysone-regulated puff genes 2000. Insect Biochem Mol Biol. 2002;32:113–120. doi: 10.1016/s0965-1748(01)00112-6. [DOI] [PubMed] [Google Scholar]
- Warren JT, Petryk A, Marques G, Jarcho M, Parvy JP, Dauphin-Villemant C, O'Connor MB, Gilbert LI. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2002;99:11043–11048. doi: 10.1073/pnas.162375799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JT, Yerushalmi Y, Shimell MJ, O'Connor MB, Restifo LL, Gilbert LI. Discrete pulses of molting hormone, 20-hydroxyecdysone, during late larval development of Drosophila melanogaster: correlations with changes in gene activity. Dev Dyn. 2006;235:315–326. doi: 10.1002/dvdy.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Chen JH, Fisher MJ, Rees HH. Induction of enzymes involved in molting hormone (ecdysteroid) inactivation by ecdysteroids and an agonist, 1,2-dibenzoyl-1-tert-butylhydrazine (RH-5849) J Biol Chem. 1997;272:8427–8432. doi: 10.1074/jbc.272.13.8427. [DOI] [PubMed] [Google Scholar]
- Williams DR, Fisher MJ, Rees HH. Characterization of ecdysteroid 26-hydroxylase: an enzyme involved in molting hormone inactivation. Arch Biochem Biophys. 2000;376:389–398. doi: 10.1006/abbi.2000.1731. [DOI] [PubMed] [Google Scholar]
- Woodard CT, Baehrecke EH, Thummel CS. A molecular mechanism for the stage specificity of the Drosophila prepupal genetic response to ecdysone. Cell. 1994;79:607–615. doi: 10.1016/0092-8674(94)90546-0. [DOI] [PubMed] [Google Scholar]
- Yamada M, Murata T, Hirose S, Lavorgna G, Suzuki E, Ueda H. Temporally restricted expression of transcription factor betaFTZ-F1: significance for embryogenesis, molting and metamorphosis in Drosophila melanogaster. Development. 2000;127:5083–5092. doi: 10.1242/dev.127.23.5083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.