SUMMARY
Background
BCL-2 family proteins play a central role in regulating clonal selection and survival of lymphocytes and are frequently over expressed in lymphomas. Navitoclax (ABT-263) is a targeted high-affinity small molecule that occupies the BH3 binding groove of BCL-2 and BCL-XL and inhibits their anti-apoptotic activity. Experimentally, navitoclax kills cells in a BAX/BAK-dependent manner and results in regression of lymphoid tumors in xenograft models.
Methods
This is a phase I dose-escalation study of navitoclax in patients with relapsed or refractory lymphoid malignancies. Study endpoints included safety, maximum tolerated dose (MTD), pharmacokinetic profile and clinical activity. In addition, mechanism-based pharmacodynamic effects on platelets and lymphocytes were assessed. Navitoclax was orally administered and assessed on an intermittent schedule of once daily for 14 days followed by 7 days off (14/21 days) or on a continuous once daily schedule (21/21 days). This trial is registered with ClinicalTrials.gov, number NCT00406809.
Findings
Fifty-five patients were enrolled, (median age 59 years, IQR 51–67), of whom two did not complete the first cycle and were not evaluable for assessment of dose-limiting toxicity (DLT). Common toxicities included grade 1/2 diarrhea and fatigue in 31 and 21 patients, respectively. Thrombocytopenia and neutropenia were the serious common toxicities with grade 3/4 observed in 29 and 17 patients, respectively. On the intermittent schedule (14/21), 5 DLT’s were observed; two due to hospitalizations for bronchitis and pleural effusion, and one each due to grade 3 transaminase elevation, grade 4 thrombocytopenia and grade 3 cardiac arrhythmia. Navitoclax caused a rapid and dose-dependent decline in peripheral platelets following initial drug exposure, followed by a rebound. To reduce the platelet nadir associated with intermittent dosing, a lead-in dose followed by continuous dosing (21/21 schedule) was examined. Three DLT’s were observed on this schedule (21/21); one each due to grade 4 thrombocytopenia, grade 3 transaminase elevation and grade 3 gastrointestinal bleed. Navitoclax showed a pharmacodynamic effect on circulating platelets and T-cells. Based on these findings, a 150 mg 7-day lead-in dose followed by 325 mg dose administered on a continuous (21/21) schedule was selected for phase II study. Clinical responses occurred at all dose levels and in multiple histologies. Partial responses were observed in 10 of 46 patients with evaluable disease, and the responders had a median progression-free survival of 455 days (IQR 40-218).
INTRODUCTION
BCL-2 family proteins play a central role in lymphocyte biology where they regulate clonal selection and survival. (1–3) It is therefore not unexpected that pro-survival BCL-2 proteins are benefactors of upstream driver mutations or are themselves over expressed through translocation or amplification in many lymphoma subtypes.(4–7) The importance of these proteins in normal and malignant lymphoid biology has driven the search for inhibitors. An effective strategy to develop a highly specific inhibitor involves high-throughput NMR-based screening, parallel synthesis and structure-based design to identify small molecules that bind BCL-XL.(8, 9) This effort yielded ABT-737, which showed high affinity binding to BH3-only proteins with an affinity two to three orders of magnitude greater than previously reported compounds. Mechanistic studies showed that ABT-737 does not directly initiate apoptosis but enhances the effect of death signal and is synergistic with cytotoxic agents and radiation.(10) To overcome the low solubility and oral bioavailability of ABT-737, the ABT-263 analog (navitoclax) was developed for clinical investigation. Pre-clinical studies confirmed that like ABT-737, navitoclax had a high affinity for the anti-apoptotic BCL-2 family proteins and killed in a BAX/BAK-dependent manner.
Navitoclax demonstrated broad activity against a panel of human tumor cell lines including 11 of 23 hematological cell lines at an EC50 < 1 µmol/L.(10, 11) In vivo, navitoclax induced durable and complete tumor regressions in a murine xenograft model of acute lymphocytic leukemia and significantly improved the cure rate of rituximab plus chemotherapy in a xenograft model of mantle cell lymphoma.(11) We report the first in-human phase 1 and pharmacodynamic results of navitoclax, which induced durable responses in drug resistant lymphoid malignancies and mechanism specific pharmacodynamic adverse effects.
METHODS
Study Design
This phase 1 dose-escalation study utilized a modified Fibonacci 3+3 design to evaluate the safety, pharmacokinetics, pharmacodynamics, and preliminary efficacy of navitoclax in relapsed/refractory lymphoid malignancies. Eligibility included subjects with a histologically confirmed lymphoid malignancy as defined in the World Health Organization (WHO) classification; at least 1 prior chemotherapy regimen and relapsed or refractory disease; an Eastern Cooperative Oncology Group (ECOG) performance status 0–1; age ≥ 18 years; adequate bone marrow (platelets ≥ 100,000/µl; absolute neutrophil count ≥ 1000/µl; hemoglobin ≥ 9.0 g/dL); serum creatinine ≤ 2.0 mg/dL or calculated creatinine clearance ≥ 50; adequate hepatic function (AST and ALT ≤ 3.0 upper limit of normal (ULN); bilirubin ≤ 1.5 × ULN unless presence of Gilbert’s Syndrome); and adequate coagulation (PTT, and PT ≤ 1.2 × ULN). Initial evaluation included a history and physical examination, standard blood tests, whole body computed tomography (CT), and bone marrow biopsy. Tumor responses were evaluated by CT, bone marrow biopsy and peripheral lymphocyte counts after Cycle (C) 2 and C4, and every third cycle thereafter, and followed International Working Group criteria.(12, 13) Overall survival and progression-free survival were calculated by the Kaplan-Meier method.(14)
This study was Institutional Review Board approved, complied with the Declaration of Helsinki, and patients gave written informed consent. The study was conducted at seven sites and co-sponsored by Abbott Laboratories and Genentech, and All authors had access to the primary data and approved the manuscript.
Dose Escalation and Toxicity
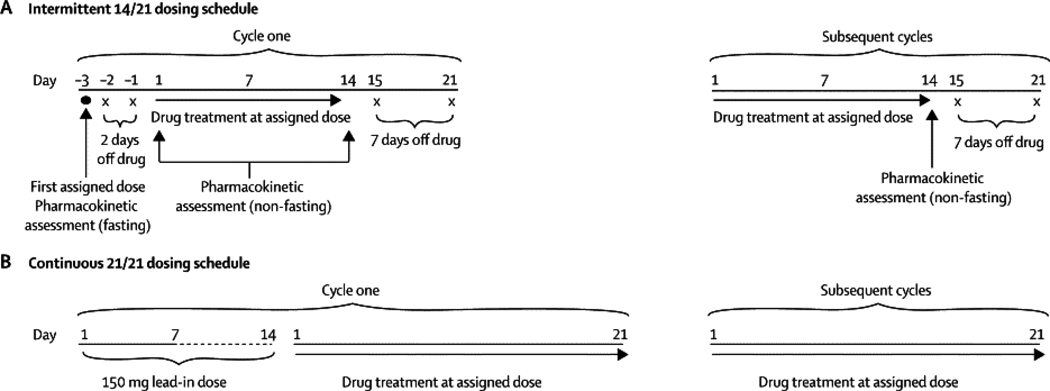
Navitoclax was orally administered over multiple dose levels (Table 1). Two schedules were evaluated: (i) an intermittent schedule on days 1–14, every 21 days (14/21) and (ii) a continuous schedule (21/21) that was preceded by a lead-in dose of 150 mg for 7–14 days to reduce acute thrombocytopenia (Figure 1). Navitoclax was escalated in cohorts of 3–9 patients. Treatment was continued until tumor progression or unacceptable toxicity as defined below. The maximum tolerated dose (MTD) was defined as the dose level at which no more than one of six patients experienced dose-limiting toxicity (DLT). DLT was defined as grade 4 thrombocytopenia, and/or at least grade 2 bleeding. Other grade 3, 4, or 5 adverse events were considered dose-limiting except grade 3 or 4 neutropenia less than or equal to 7 days without fever, grade 3 or 4 lymphopenia, and grade 3 nausea, vomiting or diarrhea unless treatment unresponsive. Unexpected grade 2 toxicity that required dose modification or delay of ≥ 1 week was also dose limiting. Adverse events were graded using NCI CTCAE V3.
Table 1.
Patient Characteristics and Dose Levels
| Variables | Number | Percent |
|---|---|---|
| Total Patients | 55 | 100% |
| Sex (Male) | 32 | 58% |
| Median Age (Range) | 59 (20–81) | |
| ECOG PS ≥ 1 | 19 | 35% |
| LDH > Normal1 | 17 | 31% |
| Stage III/IV2 | 44 | 80% |
| Histology | ||
| Diffuse Large B-cell lymphoma | 6 | 11% |
| Mantle Cell lymphoma | 4 | 7% |
| Follicular center cell lymphoma | 16 | 29% |
| “Other” Indolent B-cell lymphoma | 6 | 11% |
| CLL/SLL | 20 | 36% |
| Classical Hodgkin’s lymphoma | 2 | 4% |
| Peripheral NK/T-cell lymphoma | 1 | 2% |
| Median Prior Regimens (Range) | 4 (1–12) | |
| Dose Levels (14/21 schedule) | Evaluable (n=53) | |
| 10 mg | 3 | |
| 20 mg | 3 | |
| 40 mg | 3 | |
| 80 mg | 3 | |
| 110 mg | 1 | |
| 160 mg | 7 | |
| 225 mg | 4 | |
| 315 mg3 | 9 | |
| 440 mg | 5 | |
| Dose Levels (21/21 schedule) | ||
| 200 mg | 3 | |
| 275 mg | 6 | |
| 325 mg | 3 | |
| 425 mg | 3 | |
LDH (IU/L) above upper limit of laboratory reference.
Stage at diagnosis.
Three additional patients were enrolled pending evaluation of adverse events with the FDA before escalation could proceed.
Figure 1. Navitoclax Dosing Schedule.
(a). Intermittent (14/21) dosing schedule. For Cycle 1, navitoclax was administered on day –3 to assess pharmacokinetics (PK) and food effect. Beginning on Cycle 1 day 1, patients were dosed non-fasting for 14 consecutive days on days 1–14 of a 21- day dosing cycle. In subsequent cycles, patients receive drug on D1–14 followed by 7 days off. (b). Continuous (21/21) dosing schedule. On cycle 1, patients received a 150 mg lead-in dose for 7–14 days followed by continuous dosing at their assigned dose level.
The dose of navitoclax was interrupted for any pre-dose platelet count < 25,000/µl and could be restarted at a reduced dose level if the platelets recovered to > 50,000//µl. Navitoclax was also interrupted for any clinically significant bleeding, defined as Grade 2 or higher hemorrhage and/or a DLT and could be restarted at a reduced dose level if the toxicities resolved. The investigator and study sponsor jointly determined the reduced dose level. Patients were removed from study if they underwent more than three dose reductions and had no objective response.
Pharmacokinetic Profile
Blood samples for pharmacokinetics (PK) were collected fasting on C1, Day (D) –3 and non-fasting on D1 and 14 pre-infusion, and on C2–6 on Day 14 pre-infusion. A 24-hour urine collection was obtained after dosing on C1 D–3. Navitoclax levels were determined using a liquid chromatography method with Tandem Mass Spectrometric detection. PK parameters assessed included maximum concentration (Cmax) time to Cmax, (Tmax0), oral clearance (CL/F), and area under the concentration curve (AUC).
Statistics
Descriptive statistics of medians and ranges were calculated for continuous parameters, as well as frequencies and percentages for categorical parameters. Time to event analyses used the method of Kaplan and Meier. Progression free survival (PFS) is defined as the number of days from the date a subject started study drug to the date the subject experiences an event of disease progression, or to the date of death if disease progression is not reached and the death occurred within 2 cycles of the date of last available tumor evaluation. Subjects were censored if they had not experienced disease progression or death at their last available tumor evaluation. For overall survival, subjects were censored on the last day known to be alive. Statistical Analyses Software (SAS) version 9.2 was used for all analyses except for determination of the P-values of the Sign-test on change in CD3+ from baseline, which used SAS version 8.2.
Role of Funding Source
This trial was sponsored by Abbott Laboratories and Genentech. M06-814 was the first in human protocol submitted by Abbott with the initial IND for ABT-263. Although Abbott collaborated with advisors (including the authors) during the study design and subsequent amendments, the study was designed and data collected, analyzed and interpreted by the trial sponsor, with input from the authors and investigators in accordance with Good Clinical Practices. The initial draft provided by WHW, was reviewed and commented on by all authors and by employees of Abbott Laboratories and Genentech. WHW, the first and corresponding author, and Abbott authors HX, YLC, YC, TBB, SWE, SHR, APK, SEH and RAH had full access to the study raw data. Authors OAO, MSC, ASC, JFG, JPL, AT and KD were provided with the full data set upon request. WHW took full responsibility for the writing and final decision to submit this manuscript.
RESULTS
Patients and Toxicity
Fifty-five patients were enrolled and 53 were evaluable for DLT; two patients enrolled at the 275 mg dose level did not complete cycle one due to voluntary withdrawal and disease progression, respectively (Table 1). The patients had a median age of 59 years and most were heavily pretreated.
Two dose schedules were examined; daily for 14 days followed by a 7-day rest (14/21) and continuous dosing as described earlier (21/21). Dose cohorts ranged from 10–440 mg orally per day for the 14/21 schedule. Overall, five DLT’s were observed; one at 160 mg and two each at 315 and 440 mg dose levels. Two DLT’s due to hospitalizations for bronchitis and pleural effusion were judged unrelated to navitoclax, and one each due to grade 3 transaminase elevations, grade 4 thrombocytopenia and grade 3 atrial fibrillation were judged possibly or probably related to drug. Based on the finding that the patient with the cardiac arrhythmia had a prior history of atrial fibrillation and no other significant cardiac events were observed on this study (Table 2), it appears unlikely that navitoclax causes significant cardiac toxicity. Based on the occurrence of only one DLT in the first 6 patients treated at the 315 mg cohort, and two DLT’s in the 440 mg cohort, 315 mg was identified as the safe tolerated dose for the intermittent schedule.
Table 2.
Clinical and Laboratory Toxicity
| Events | Intermittent (14/21) Schedule All Patients (N=38) |
Continuous (21/21) Schedule All Patients (N=17) |
Intermittent (14/21) Schedule MTD 315 mg (N=9) |
Continuous (21/21) Schedule MTD 325 mg (N=5) |
||||
|---|---|---|---|---|---|---|---|---|
| Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | |
| Cardiac | ||||||||
| Atrial arrhythmia | 1 (3%) | 1 (3%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Bradycardia | 0 | 0 | 1 (6%) | 0 | 0 | 0 | 1 (20%) | 0 |
| Headache | 4 (11%) | 0 | 4 (24%) | 0 | 1 (11%) | 0 | 1 (20%) | 0 |
| Gastrointestinal | ||||||||
| Constipation | 5 (13%) | 0 | 0 | 0 | 3 (33%) | 0 | 0 | 0 |
| Diarrhea | 19 (50%) | 1 (3%) | 12 (71%) | 0 | 6 (67%) | 0 | 4 (80%) | 0 |
| Abdominal Pain | 7 (18%) | 0 | 6 (35.%) | 0 | 3 (33%) | 0 | 1 (20%) | 0 |
| Nausea | 19 (50%) | 0 | 10 (59%) | 0 | 6 (67%) | 0 | 2 (40%) | 0 |
| Vomiting | 8 (21%) | 0 | 6 (35%) | 0 | 4 (44%) | 0 | 0 | 0 |
| Dyspepsia | 6 (16%) | 0 | 2 (12%) | 0 | 1 (11%) | 0 | 0 | 0 |
| Fatigue | 15 (40%) | 0 | 6 (35%) | 0 | 3 (33%) | 0 | 1 (20%) | 0 |
| All Infections | 25 (66%) | 6 (16%) | 14 (82%) | 0 | 8 (89%) | 1 (11%) | 5 (100%) | 0 |
| Bronchitis | 1 (3%) | 1 (3%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Herpes Zoster | 0 | 1 (3%) | 1 (6%) | 0 | 1 (11%) | 0 | 1 (20%) | 0 |
| Pneumonia | 2 (5%) | 4 (11%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Hematologic | ||||||||
| Platelets | 15 (39%) | 18 (47%) | 5 (29%) | 11 (65%) | 2 (22%) | 7 (78%) | 2 (40%) | 3 (60%) |
| Neutrophils | 7 (18%) | 11 (29%) | 6 (35%) | 7 (41%) | 2 (22%) | 3 (33%) | 2 (40%) | 3 (60%) |
| Lymphocytes | 10 (26%) | 14 (37%) | 3 (18%) | 4 (24%) | 5 (56%) | 4 (44%) | 2 (40%) | 2 (40%) |
| Hemoglobin | 29 (76%) | 1 (3%) | 12 (71%) | 2 (12%) | 7 (78%) | 0 | 5 (100%) | 0 |
| Hepatic | ||||||||
| Alanine Amino- transferase |
17 (45%) | 3 (8%) | 9 (53%) | 1 (6%) | 4 (44%) | 2 (22.%) | 4 (80%) | 0 |
| Aspartate Amino- transferase |
24 (63%) | 1 (3%) | 14 (82%) | 0 | 6 (67%) | 0 | 4 (80%) | 0 |
On the intermittent schedule, significant platelet nadirs occurred with the initial doses of each cycle, followed by a modest rebound. To help reduce the acute platelet nadirs and grade 4 thormbocytopenia, a lead-in dose followed by continuous dosing (21/21 schedule) was examined in dose cohorts of 200–425 mg. Three DLT’s were observed, one at 275 mg and two at 425 mg dose levels; grade 4 thrombocytopenia, grade 3 transaminase elevation and grade 3 gastrointestinal bleed, respectively, all possibly or probably related to navitoclax. Based on these findings, a 150 mg 7-day lead-in dose followed by 325 mg dose administered on a continuous (21/21) schedule was selected for the phase II study.
When all dose levels are considered, the most common toxicities were grade 1/2 gastrointestinal complaints likely due to the drug vehicle (Table 2). Nausea and vomiting were managed with anti-emetics and diarrhea was managed with diphenoxylate and atropine (lomotil). Fatigue was relatively common but not dose limiting. Eight patients developed respiratory infections and/or bronchitis (Table 2), none of which were associated with grade 4 neutropenia. Thrombocytopenia and neutropenia were the serious common toxicities with grade 3 or 4 thrombocytopenia or neutropenia observed in 29 and 17 patients, respectively (Table 2). Unlike the thrombocytopenia, neutropenia was not clearly associated with dose level and tended to occur on the later cycles; it was also reversible upon navitoclax discontinuation. Grade 4 thrombocytopenia and neutropenia were managed by temporary suspension and dose reduction of navitoclax and filgrastim was used for persistent grade 4 neutropenia. These results indicate that navitoclax may cause unacceptable hematological toxicity in patients with limited bone marrow reserve. Overall dose levels, 11 patients required at least one dose reduction and 6 patients withdrew their consent for treatment, two of which were due to toxicity.
Pharmacokinetic Profile
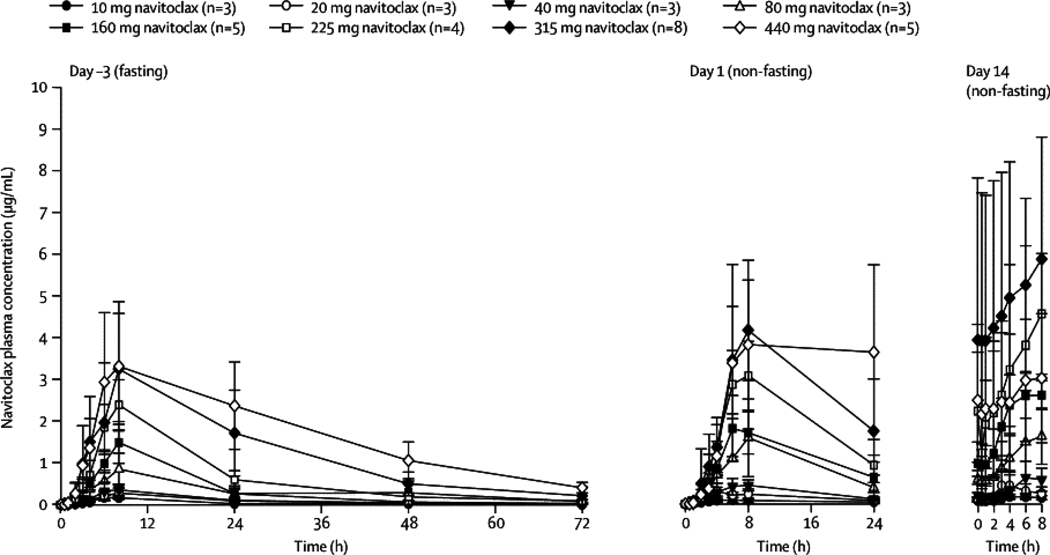
Navitoclax PK was assessed on the 14/21-day schedule (Figure 2 and Table 3). To assess food effects on absorption, navitoclax was administered fasting on day –3 and non-fasting on days 1–14 of cycle one. Overall, food increased the oral bioavailability by approximately 20% with the current lipid formulation. Exposure was dose-proportional between 10 mg and 440 mg with approximately 40% interpatient variability in the plasma AUC. Navitoclax achieved peak concentrations (Cmax) at approximately 9 hours post-dose with a half-life of approximately 17 hours, and first order elimination kinetics. The projected effective exposure of 55–88 µg·hr/mL based on animal models was achieved at dose levels of at least 315 mg/day in patients. Navitoclax could not be detected in urine, indicating negligible renal elimination.
Figure 2. Pharmacokinetic Profile of Navitoclax.
Line graph of mean (±SD) navitoclax plasma concentrations vs. time profiles in patients at Days -3 (fasting), 1 (non-fasting) and 14 (non-fasting) following oral administration of navitoclax at 10–440 mg dose levels on the 14/21-day dosing schedule.
Table 3.
Pharmacokinetic Profile of Navitoclax
| Dose | Study Daya |
N | Tmax (h) |
Cmax (µg/mL) |
AUC0–24 (µg·hr/mL) |
AUC0-inf (µg·hr/mL) |
CL/F (L/h) |
t½b (h) |
|---|---|---|---|---|---|---|---|---|
| 10 mg | −3 | 3 | 6·7 ± 1·2 | 0·18 ± 0·05 | 2·2 ± 0·4 | 3·2 ± 1·1 | 3·4 ± 1·2 | 13·8 ± 5·8 |
| 1 | 3 | 11·3 ± 11 | 0·12 ± 0·03 | 1·5 ± 0·5 | – | – | – | |
| 14 | 3 | 5·0 ± 2·6 | 0·19 ± 0·06 | – | – | – | – | |
| 20 mg | −3 | 3 | 7·3 ± 1·2 | 0·29 ± 0·13 | 3·9 ± 1·8 | 6·4 ± 3·1 | 3·7 ± 2·0 | 19·1 ± 3·3 |
| 1 | 3 | 4·0 ± 1·7 | 0·36 ± 0·18 | 4·2 ± 2·1 | – | – | – | |
| 14 | 3 | 4·3 ± 1·5 | 0·48 ± 0·31 | – | – | – | – | |
| 40 mg | −3 | 3 | 7·3 ± 1·2 | 0·37 ± 0·13 | 5·0 ± 1·6 | 8·4 ± 3·4 | 5·3 ± 2·1 | 20·2 ± 4·6 |
| 1 | 3 | 6·0 ± 2·0 | 0·50 ± 0·15 | 6·4 ± 1·2 | – | – | – | |
| 14 | 3 | 6·7 ± 1·2 | 0·65 ± 0·37 | – | – | – | – | |
| 80 mg | −3 | 3 | 8·0 ± 0·0 | 0·85 ± 0·39 | 11·9 ± 5·8 | 17·6 ± 9·3 | 5·3 ± 2·3 | 14·7 ± 1·0 |
| 1 | 2c | 7·0 ± 1·4 | 1·68 ± 0·79 | 21·1 ± 10·7 | – | – | – | |
| 14 | 3 | 7·3 ± 1·2 | 1·80 ± 0·97 | – | – | – | – | |
| 160 mg | −3 | 5d,e | 8·0 ± 0.0 | 1·49 ± 0·50 | 18·8 ± 4·4 | 32·5 ± 7·8 | 5·1 ± 1·2 | 20·2 ± 5·5 |
| 1 | 6e | 9·7 ± 7·1 | 2·05 ± 0·59 | 25·9 ± 5·5 | – | – | – | |
| 14 | 5e | 6·0 ± 1·4 | 2·78 ± 0·46 | – | – | – | – | |
| 225 mg | −3 | 4 | 7·5 ± 1·0 | 2·39 ± 0·60 | 31·7 ± 7·8 | 46·5 ± 12·4 | 5·1± 1·5 | 16·2 ± 2·8 |
| 1 | 4 | 7·5 ± 1·0 | 3·08 ± 0·82 | 43·9 ± 9v5 | – | – | – | |
| 14 | 3 | 7·3 ± 1·2 | 4·64 ± 1·57 | – | – | – | – | |
| 315 mg | −3 | 8c | 11·8 ± 7·6 | 3·56 ± 1·11 | 50·5 ± 15·6 | 91·0 ± 33·5 | 4·1 ± 2·1 | 15·1 ± 3·0 |
| 1 | 9 | 7·6 ± 1·0 | 4·35 ± 1·06 | 58·5 ± 17·5 | – | – | – | |
| 14 | 8 | 5·3 ± 2·8 | 6·44 ± 3·18 | – | – | – | – | |
| 440 mg | −3 | 5 | 10·8 ± 7·4 | 3·51 ± 1·50 | 57·6 ± 21·0 | 128·6 ± 30·4 | 3·6 ± 0·8 | 21·1 ± 2·8 |
| 1 | 5 | 17·2 ± 9·3 | 4·17 ± 2·08 | 72·5 ± 39·3 | – | – | – | |
| 14 | 5 | 4·4 ± 4·1 | 3·19 ± 1·56 | – | – | – | – | |
| All doses | −3 | 34 | 8·9 ± 4·8 | – | – | – | 4·5 ± 1·7 | 17·1 ± 4·3 |
| 1 | 35 | 9·0 ± 6·2 | – | – | – | – | – | |
CL/F = apparent oral clearance, t1/2 = half-life
Dosing under fasting (Day –3) and nonfasting (Days 1 through 14) conditions. Pharmacokinetic parameters for Day computed using concentration values adjusted for carryover for the dose on Day -3.
Harmonic mean ± pseudo standard deviation.
Pharmacodynamic Effects
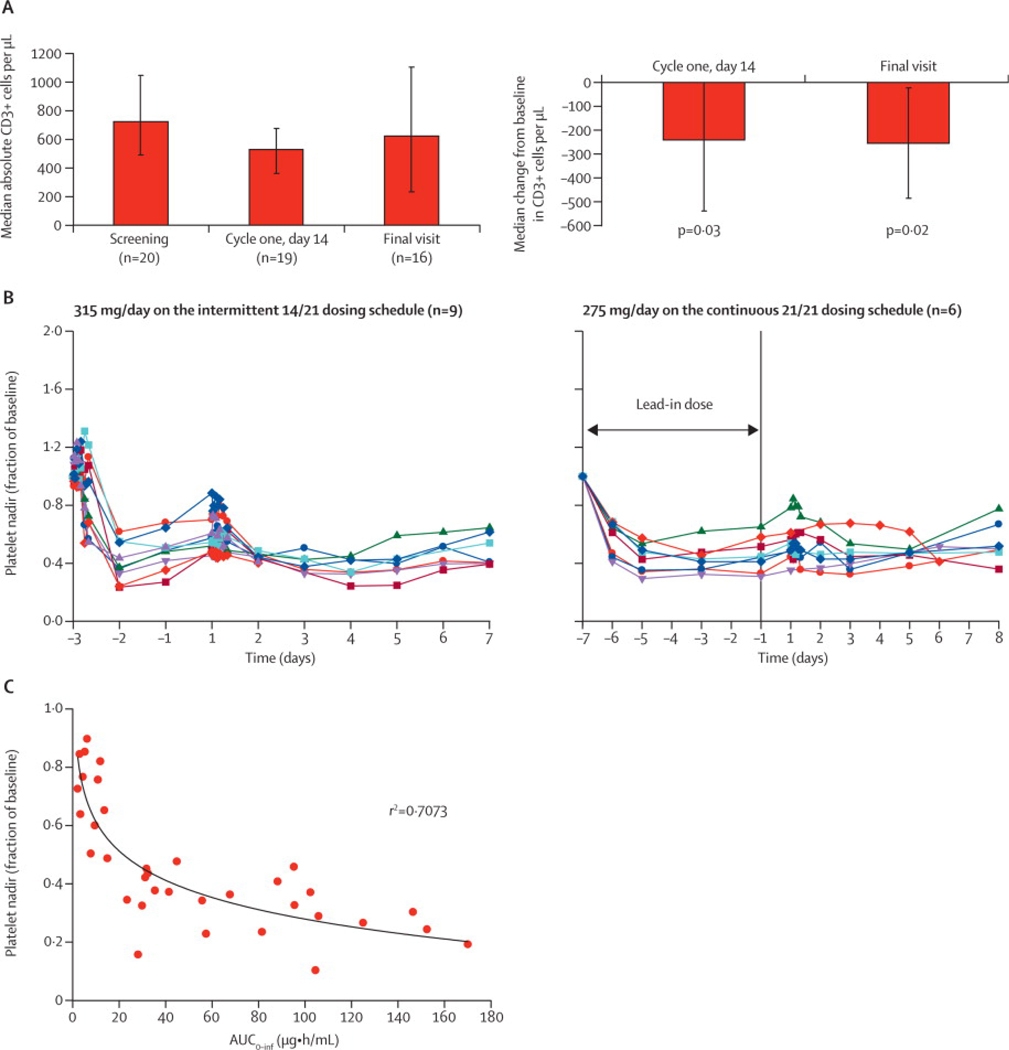
We hypothesized that if navitoclax functionally inhibited BCL-2 and BCL-XL, it should decrease the survival of normal cells in which these proteins regulate survival such as in T-cells and platelets, respectively.(15, 16) To assess this, we measured the absolute number of CD3+ cells in the circulation before, on day 14 of cycle 1, and at the end of treatment (Figure 3A). Patients showed a relatively rapid decrease in T-cells after only 14 days of drug exposure (median reduction 241 CD3+ cells/µl), which did not worsen with further treatment (Figure 3A). Importantly, the loss of T-cells was modest and not associated with opportunistic infections.
Figure 3. Pharmacodynamic Effects of Navitoclax.
(a) Bar graph of median absolute circulating CD3 cells/µl at baseline (n=20 patients), day 14 of cycle 1 (n=19 patients) and the final visit (n=16 patients) at the end of navitoclax treatment, and the median change on day 14 of cycle 1 and final visit. Patients received at least 200 mg navitoclax for at least two cycles on either the 14/21 or 21/21day schedule. The mean (range) time between baseline and final visit was 89 (29–332) days and the mean (range) daily drug exposure was 255 (137–388) mg of navitoclax. P-value was calculated using a Sign-test because the data were skewed. (b) Line graph of platelet nadirs over time at 315 mg on the 14/21-day schedule (n=9 patients) (left panel) and at 275 mg on the 21/21-day schedule (n=6 patients) (right panel). (c) Platelet nadir versus navitoclax area under the curve at multiple dose levels (n=35 patients). R-value was calculated by using SigmaPlot 9.0.
We also examined the kinetics of circulating platelets during navitoclax exposure. There was a significant reduction following a single dose of navitoclax, which was observed to begin as soon as one hour after dosing (data not shown) (Figure 3B). With continued dosing, there was a modest rise from nadir levels. To obviate the acute nadirs associated with intermittent dosing (14/21), we tested a lead-in dose followed by continuous dosing (21/21) and observed higher nadirs. Consistent with a pharmacodynamic effect, the severity of the platelet nadirs was concentration dependent (Figure 3C) and related to dose level (data not shown).
Treatment Outcome
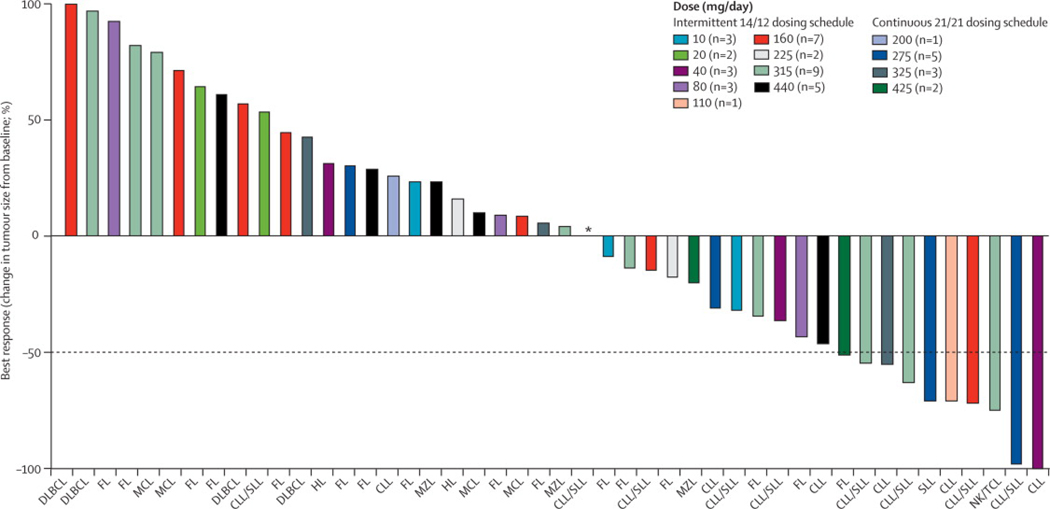
Overall, 21 of 46 patients with evaluable adenopathy showed some tumor reduction. Furthermore, 10 of these patients achieved a partial response (PR) lasting a median (range) of 455 days (IQR 40–218) (Figure 4). Responses occurred across dose levels and tumor types and were observed after a median (range) of 3.5 (2–10) cycles. Chronic lymphocytic leukemia/lymphoma (CLL/SLL), a disease of B-cell accumulation, showed the greatest sensitivity. Among the seven patients with CLL, all achieved at least a 50% reduction in their leukemia cells, and 8 of 16 patients with measurable adenopathy achieved a PR, which included patients with bulky adenopathy. Including all 20 patients with CLL/SLL, the median (range) PFS was 246 days (IQR 49-309) and the median overall survival has not been reached. Measurable tumor reduction was also observed in 6 of 16 patients with follicular lymphoma. Overall, the 16 patients with follicular lymphoma had a median PFS of 88 days (IQR 42-92). A PR was achieved in a single patient with an NK/T-cell lymphoma. The patients with CLL/SLL or follicular lymphomas had received a similar number of prior regimens with a median (range) of 4 (1–12) and 5 (1–11), respectively.
Figure 4. Antitumor Activity of Navitoclax.
Waterfall plot of maximal percent change in tumor size from baseline using standard criteria.(12, 13) Tumor subtypes are shown on the x-axis: DLBCL-diffuse large B-cell lymphoma; FL-follicular lymphoma; MCL-mantle cell lymphoma; CLL/SLL-chronic lymphocytic leukemia/small lymphocytic lymphoma; MZL-marginal zone lymphoma; HL-classical Hodgkin’s lymphoma; and NK/T-natural killer-T-cell lymphoma.
DISCUSSION
Fifty-three evaluable patients received navitoclax on two different treatment schedules. Navitoclax demonstrated a high therapeutic index with a low incidence of off-target toxicities. The major off-target toxicity was gastrointestinal, which appears likely related to the phosphatidylcholine solubilizer. Though uncommon, transaminase elevation at higher dose levels and neutropenia after prolonged drug exposure were also observed. Navitoclax also demonstrated favorable pharmacokinetic properties and therapeutic index. Due to good oral absorption, exposure was dose proportional, and the approximate 17-hour half-life allowed daily dosing. Furthermore, concentrations shown to be effective in preclinical models were achieved at the recommended phase 2 dose of 325 g/day.
The pharmacodynamic effects of navitoclax on circulating lymphocytes and platelets are novel and consistent with on-target mechanisms. Based on preclinical evidence that platelet senescence entails an apoptosis-like process mediated through BCL-XL, it is likely that intravascular apoptosis is responsible for the acute thrombocytopenia following navitoclax.(17, 18) Furthermore, the relative resistance of younger platelets to navitoclax appears to be due to their higher levels of BCL-XL, which explains the platelet kinetics observed in patients and in pre-clinical animal models using ABT-737.(18). It is also likely that navitoclax induces apoptosis of normal lymphocytes through its inhibitory effect on BCL-2. These results suggest that the pharmacodynamic effects of navitoclax are biomarkers of pharmacological inhibition of BCL-2 and BCL-XL, and should be observed with all effective inhibitors. We also observed grade 3 or 4 neutropenia in 17 patients, which raises the question of whether navitoclax may also have a pharmacodynamic effect on myeloid cells. To help assess this, we performed preliminary methylcellulose-based in vitro colony forming assays to assess the effects of ABT-737, a navitoclax analog, on human hematopoietic progenitors. In the presence of multilineage cytokines, ABT-737 inhibited the growth of both erythroid and common myeloid precursors (IC50 of 1.0 µM and 0.9 µM, respectively) (data not shown). Furthermore, BCL-2 has been shown to be necessary for the in vitro survival of myeloid progenitors under conditions of cytokine withdrawal, suggesting the neutropenia we observed in our study could be due to inhibition of BCL-2 by navitoclax.(19)
Navitoclax demonstrated clinical activity at all dose levels and across tumor types with the greatest activity seen in CLL. Letai et al. recently proposed a model, termed BH3 profiling, which may explain the differential sensitivity of lymphoma cells to BCL-2 inhibition.(20, 21) They proposed that sequestration of the activator BH3 only proteins, BIM or BID, by BCL-2 produces a “primed” state in which BCL-2 inhibition releases activator proteins and induces apoptosis. Using BH3 profiling, the group found a pattern of BCL-2 dependence in CLL with high BIM:BCL-2 complex levels and exquisite sensitivity to ABT-737.(20) Interestingly, we observed few bone marrow responses with navitoclax, even among patients with robust nodal and blood responses, which may be due to the influence of the microenvironment on increased expression of MCL-1, BCL-XL or BCL-2A1.(22) Though the activity of navitoclax was less apparent in other lymphoma subtypes, it has synergistic activity with chemotherapeutic agents in preclinical models. Given the complexity of the primed BCL-2 phenotype, and the influence of the microenvironment and upstream pathways, we hypothesize that the greatest benefit of navitoclax will be observed in combination with other agents.
Several other inhibitors of BCL-2 have undergone clinical testing. Two of these, obatoclax and gossypol, are small molecules that are reported to be pan-BCL-2 inhibitors.(23, 24) Thus far, they have shown little to no clinical activity. Furthermore, they have been shown to kill cells in a BAX/BAK-independent manner, challenging the functional significance of their weak affinity for BCL-2 family proteins and their true mechanisms of action.(25–27) A review of their toxicities also showed no on-target pharmacodynamic effects on platelets, suggesting they do not achieve effective inhibition of BCL-XL.(23, 25–28) Gossypol primarily caused gastrointestinal side effects, which were dose limiting, but caused no significant laboratory abnormalities.(23) While navitoclax also had gastrointestinal side effects, they were low grade and likely due to the drug solubilizer. Infusion-related somnolence and neurologic symptoms were the most common toxicities associated with obatoclax, and were dose limiting.(24) Modest hematological effects were also observed, including mild thrombocytopenia, which were attributed to progression of the patients’ underlying CLL.(24) A third drug, oblimersen, is an antisense oligodeoxyribonucleotide that down regulates BCL-2 translation.(29) A phase I study demonstrated limited single agent activity with only one response in 21 patients.(30) Interestingly, like navitoclax, oblimersen caused thrombocytopenia, which progressively worsened during the drug infusion and correlated with its plasma concentration.(30) While these results indicate that oblimersen has a pharmacodynamic effect on platelets, its indirect effect on BCL-2 levels through inhibition of BCL-2 mRNA is inconsistent with a direct inhibition of BCL-XL. In contrast to these other putative BCL-2 inhibitors, induction of apoptosis by navitoclax in vitro can be attributed to inhibition of BCL-2 family proteins. Coimmunoprecipitation studies show that navitoclax induced a dose-dependent decrease in BIM:BCL-2 family protein interactions in BCL-XL and BCL-2 over expressing prolymphocytic murine cell lines. Navitoclax also induced a dose-dependent decrease in cytosolic BAX and an increase in cytochrome c within 2 hours of treatment of a BCL-2 dependent human small cell lung cancer (SCLC) cell line.(11, 27)
The importance of BCL-2 family proteins in lymphoid biology and pathogenesis has driven the search for small molecule inhibitors of this pathway. With few exceptions, lymphomas express increased BCL-2, which may be physiological or pathogenetic. In follicular lymphoma and the germinal center subset of diffuse large B-cell lymphoma (DLBCL), translocation of the BCL-2 locus and immunoglobulin heavy chain promoter (t(14;18)) drives BCL-2 production, whereas in the post-germinal center subset of DLBCL, BCL-2 may be over expressed through amplification or transcriptional activation.(6, 31–33) CLL employs yet another mechanism whereby deletion or down regulation of miRNA miR-16-1 and miR-15a drives post-transcriptional increases in BCL-2.(34, 35) The occurrence of such varied pathogenetic mechanisms that increase BCL-2 expression points to the evolutionary significance of this pathway in lymphomagenesis, and the potential importance of this target in lymphoid malignancies.
Our study provides the first clinical insights to our knowledge into a pharmacologically active BCL-2 family inhibitor. We are currently investigating navitoclax in an expanded cohort of indolent and aggressive B-cell lymphomas. There are ongoing phase I studies of navitoclax with other agents including rituximab (CD20 monoclonal antibody), bendamustine and rituximab, and fludarabine, cyclophosphamide and rituximab combinations in lymphoma and CLL. Furthermore, to overcome the pharmacodynamic effect of navitoclax on circulating platelets, which will limit its ability to be combined with cytotoxic agents, a selective inhibitor of BCL-2 is under development.(36)
RESEARCH IN CONTEXT
Systematic Review
There exists an extensive literature that demonstrates apoptosis, or programmed cell death is the principal mechanism through which unwanted or damaged cells are safely eliminated.(37–40) Although cancer has historically been considered a disease of uncontrolled cell division, abnormal resistance to apoptosis is now understood to contribute to tumor initiation, progression, and resistance to chemotherapy. Defects in the apoptotic pathway confer a survival advantage that allows a net increase in tumor cell number and the accumulation of oncogenic mutations, which gives rise to highly aggressive tumors. Interactions between pro-apoptotic (pro-death) and antiapoptotic (pro-survival) BCL-2 family proteins regulate the initiation of the intrinsic apoptosis pathway. The pro-death proteins of BAX and BAK are direct mediators of apoptosis and are absolutely required for the initiation of the mitochondrial apoptosis pathway.(41) Over expression of anti-apoptotic BCL-2 family proteins (BCL-XL, BCL-2, BCL-W, A1, MCL-1) suppresses BAX and BAK and prevents the initiation of the apoptosis, thereby protecting cancer cells from responding to proapoptotic signals.
Compelling evidence for the role of BCL-2 family proteins in lymphoid biology and pathogenesis has driven the search for inhibitors.(42) With infrequent exception, lymphomas express BCL-2, which may be physiological and/or pathogenetic. The search for BCL-2 inhibitors has primarily relied on cytotoxicity screening. While such methodologies have lead to the identification of small molecules with low affinity inhibition and/or off target effects, these agents have shown relatively little single agent activity.(23, 26) An alternative strategy employed in the development of navitoclax entailed a structure-based design to identify small molecules that bind BCL-XL, which lead to the high affinity inhibitor navitoclax.(8, 10)
Interpretation
In the present study, we report that navitoclax, a high affinity inhibitor of BCL-2 family proteins, has clinical activity in lymphoid malignancies and has on-target pharmacodynamic effects on platelets and T-cells, where BCL-XL and BCL-2 regulate survival. While other putative BCL-2 inhibitors have undergone clinical testing, they have not shown significant clinical activity or targeted pharmacodynamic effects, which likely reflects low inhibition of BCL-2 family proteins. Thus, the present study provides the first proof of concept in humans, to our knowledge, that inhibition of BCL-2 family proteins leads to tumor cell death and targeted cell death of platelets and T-cells. As most cytotoxic agents induce apoptosis as a primary mechanism of cell kill, modulation of the apoptotic “threshold” with agents such as navitoclax is hypothesized to significantly increase the efficacy of current cytotoxic treatments. Presently, phase I trials are underway to assess the safety of navitoclax with cytotoxic agents. Further studies will be necessary to determine if navitoclax is safe and effective before it can be used in standard treatment.
Acknowledgements
The authors would like to thank Juliann M. Dziubinski, Katherine Papp, Lori Gressick, Michael D. Dawson and Renee Greco, for operational support; Di Li, and Joseph E. Beason for statistical analyses; Christin Tse, Morey L. Smith, Stephen K. Tahir, Kennan C. Marsh, Joy L. Bauch, Sherry J. Morgan, Joel Leverson, and Anne H. Illi-Love for their knowledge of the preclinical data referenced and their contributions during manuscript preparation, and Ai Q. Lockard for editorial assistance for the manuscript. The authors would also like to thank the contributions of the research data managers, coordinators and nurses including Margaret Shovlin, Barbara MacGregor Cortelli, Ameet Narwal, Barbara Anderson, Alice Mohr, Hazel Reynolds, Susan Twohig, Jennifer Pappanicholaou, Payal Dixit, June Greenberg, and Nancy Berman. This study was funded by Abbott Laboratories and Genentech, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Wyndham H. Wilson, Owen A. O’Connor, Myron S. Czucman, Ann S. La Casce, John F. Gerecitano, John P. Leonard, Anil Tulpule and Kieron Dunleavy were responsible for patient enrollment and collection and assembly of data. Wyndham H. Wilson, Sari H. Enschede, Andrew P. Krivoshik, Rod A. Humerickhouse, Hao Xiong, Yi-Lin Chiu, Yue Cui, Todd B. Busman, Steven W. Elmore, and Saul H. Rosenberg were responsible for data analysis and interpretation. All authors were responsible for writing, editing and final approval of the manuscript.
Conflict of Interest
WHW received funds from Abbott Laboratories to support his travel to one protocol meeting for this study. MSC receives grant and consulting fees and honorarium from Abbott Laboratories. HX, YLC, YC, TB, SWE, SHR, APK, SHE and RAH are employee of Abbott Laboratories; HX, YLC, TB, SWE, APK, SHE and RAH have stock in the company, and APK holds patents assigned to and receives funds for travel, accommodation and meeting expenses from Abbott Laboratories. JFG, JPL, KD, have no conflicts to declare. OAO, ASL and AT have yet to provide their conflict of interest and financial disclosures.
REFERENCES
- 1.Merino R, Ding L, Veis DJ, Korsmeyer SJ, Nunez G. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 1994 Feb 1;13(3):683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 3.Tsujimoto Y, Gorham J, Cossman J, Jaffe E, Croce CM. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- 4.Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010 Jan 7;463(7277):88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 7.Kondo E, Nakamura S, Onoue H, Matsuo Y, Yoshino T, Aoki H, et al. Detection of bcl-2 protein and bcl-2 messenger RNA in normal and neoplastic lymphoid tissues by immunohistochemistry and in situ hybridization. Blood. 1992 Oct 15;80(8):2044–2051. [PubMed] [Google Scholar]
- 8.Degterev A, Lugovskoy A, Cardone M, Mulley B, Wagner G, Mitchison T, et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol. 2001 Feb;3(2):173–182. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 9.Huang JW, Zhang Z, Wu B, Cellitti JF, Zhang X, Dahl R, et al. Fragment-based design of small molecule X-linked inhibitor of apoptosis protein inhibitors. J Med Chem. 2008 Nov 27;51(22):7111–7118. doi: 10.1021/jm8006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005 Jun 2;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 11.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008 May 1;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 13.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008 Jun 15;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statictical Association. 1958;53:457–481. [Google Scholar]
- 15.Akbar AN, Borthwick N, Salmon M, Gombert W, Bofill M, Shamsadeen N, et al. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993 Aug 1;178(2):427–438. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007 May;14(5):943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- 17.Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc Natl Acad Sci U S A. 2008 Dec 23;105(51):20327–20332. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007 Mar 23;128(6):1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 19.Villunger A, Scott C, Bouillet P, Strasser A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 2003 Mar 15;101(6):2393–2400. doi: 10.1182/blood-2002-07-2132. [DOI] [PubMed] [Google Scholar]
- 20.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007 Jan;117(1):112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007 Aug;12(2):171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009 Apr 30;113(18):4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Kelly WK, Wilding G, Leopold L, Brill K, Somer B. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res. 2009 May 1;15(9):3172–3176. doi: 10.1158/1078-0432.CCR-08-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009 Jan 8;113(2):299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003 Sep 25;46(20):4259–4264. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009 Jul;16(7):1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- 28.Hwang JJ, Kuruvilla J, Mendelson D, Pishvaian MJ, Deeken JF, Siu LL, et al. Phase I dose finding studies of obatoclax (GX15-070), a small molecule pan-BCL-2 family antagonist, in patients with advanced solid tumors or lymphoma. Clin Cancer Res. 2010 Aug 1;16(15):4038–4045. doi: 10.1158/1078-0432.CCR-10-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klasa RJ, Bally MB, Ng R, Goldie JH, Gascoyne RD, Wong FM. Eradication of human non-Hodgkin's lymphoma in SCID mice by BCL-2 antisense oligonucleotides combined with low-dose cyclophosphamide. Clin Cancer Res. 2000 Jun;6(6):2492–2500. [PubMed] [Google Scholar]
- 30.Waters JS, Webb A, Cunningham D, Clarke PA, Raynaud F, di Stefano F, et al. Phase I clinical and pharmacokinetic study of bcl-2 antisense oligonucleotide therapy in patients with non-Hodgkin's lymphoma. J Clin Oncol. 2000 May;18(9):1812–1823. doi: 10.1200/JCO.2000.18.9.1812. [DOI] [PubMed] [Google Scholar]
- 31.Gascoyne RD, Adomat SA, Krajewski S, Krajewska M, Horsman DE, Tolcher AW, et al. Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin's lymphoma. Blood. 1997 Jul 1;90(1):244–251. [PubMed] [Google Scholar]
- 32.Iqbal J, Sanger WG, Horsman DE, Rosenwald A, Pickering DL, Dave B, et al. BCL2 Translocation Defines a Unique Tumor Subset within the Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. Am J Pathol. 2004 July 1;165(1):159–166. doi: 10.1016/s0002-9440(10)63284-1. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson WH, Teruya-Feldstein J, Fest T, Harris C, Steinberg SM, Jaffe ES, et al. Relationship of p53, bcl-2, and tumor proliferation to clinical drug resistance in non-Hodgkin's lymphomas. Blood. 1997;89(2):601–609. [PubMed] [Google Scholar]
- 34.Aqeilan RI, Calin GA, Croce CM. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ. 2010 Feb;17(2):215–220. doi: 10.1038/cdd.2009.69. [DOI] [PubMed] [Google Scholar]
- 35.Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005 Oct 27;353(17):1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 36.Petros AM, Huth JR, Oost T, Park CM, Ding H, Wang X, et al. Discovery of a potent and selective Bcl-2 inhibitor using SAR by NMR. Bioorganic & medicinal chemistry letters. 2010 Nov 15;20(22):6587–6591. doi: 10.1016/j.bmcl.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 37.Brenner C, Kroemer G. Apoptosis. Mitochondria--the death signal integrators. Science. 2000 Aug 18;289(5482):1150–1151. doi: 10.1126/science.289.5482.1150. [DOI] [PubMed] [Google Scholar]
- 38.Arends MJ, Wyllie AH. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- 39.Reed JC. Apoptosis and cell death. Foreword. Oncogene. 2008 Oct 20;27(48):6192–6193. doi: 10.1038/onc.2008.296. [DOI] [PubMed] [Google Scholar]
- 40.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004 Jan 23;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 41.Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000 Nov;157(5):1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed JC. Apoptosis mechanisms: implications for cancer drug discovery. Oncology (Williston Park) 2004 Nov;18(13 Suppl 10):11–20. [PubMed] [Google Scholar]