Abstract
In gastrointestinal conditions such as bowel obstruction, pseudo-obstruction, and idiopathic megacolon, the lumen of affected bowel segments is distended and its motility function impaired. Our hypothesis is that mechanical stretch of the distended segments alters gene expression of cyclooxygenase-2 (COX-2), which impairs motility function. Partial obstruction was induced with a silicon band in the distal colon of rats for up to 7 days, and wild-type and COX-2 gene-deficient mice for 4 days. Mechanical stretch was mimicked in vitro in colonic circular muscle strips and in primary culture of colonic circular smooth muscle cells (SMC) with a Flexercell system. The rat colonic circular muscle contractility was significantly decreased in the distended segment oral to obstruction, but not in the aboral segment. This change started as early as day 1 and persisted for at least 7 days after obstruction. The expression of COX-2 mRNA and protein increased dramatically also in the oral, but not aboral, segment. The upregulation of COX-2 expression started at 12 h and the effect persisted for 7 days. At 24 h after obstruction, the COX-2 mRNA level in the oral segment increased 26-fold compared with controls. This was not accompanied by any significant increase of myeloperoxidase or inflammatory cytokines. Immunohistochemical studies showed that COX-2 was selectively induced in the colonic SMC. In vitro stretch of colonic muscle strips or cultured SMC drastically induced COX-2 expression. Incubation of circular muscle strips from obstructed segment with COX-2 inhibitor NS-398 restored the contractility. The impairment of muscle contractility in obstructed colon was attenuated in the COX-2 gene-deficient mice. In conclusion, mechanical stretch in obstruction induces marked expression of COX-2 in the colonic SMC, and stretch-induced COX-2 plays a critical role in the suppression of smooth muscle contractility in bowel obstruction.
Keywords: smooth muscle, prostaglandins, pseudo-obstruction, mechanotranscription, cyclooxygenase-2
bowel obstruction occurs in the small and large intestines, and may be mechanical or functional (32, 37). In functional obstructions, the lumen of the affected segments is distended without noticeable physical obstruction, such as in pseudo-obstruction, Hirschsprung's disease, ileus, and idiopathic megacolon (4, 11, 37). When the passage of lumen contents through the digestive tract is physically blocked, mechanical obstruction occurs (16, 32, 37). Mechanical bowel obstruction can occur in adults or children and may be acute or chronic, partial or complete (30). Numerous pathological conditions, including carcinomas, adhesions, and diverticulitis, result in mechanical obstruction in the gut (16, 30, 32, 37).
Regardless of the initial cause of obstruction, the consequences are largely the same: the proximal segment of the bowel is overstretched with accumulation of the luminal contents and gas, and the intraluminal pressure is increased (30). Subsequently, a series of changes occurs in the obstructed segments, including altered motility function, and increased thickness of the muscle layer (hypertrophy) (2, 10, 24, 33, 39). These changes are responsible for symptoms such as bloating, vomiting, abdominal cramps, and constipation (16, 30, 32, 33, 37) and may lead to intestinal failure (33). Although the functional and morphological changes have been well documented in the literature, the pathophysiological mechanisms underlying these changes are not known. As a result, there is no effective medical treatment for obstruction other than surgical resection or decompression (16, 30, 32). Nevertheless, even if obstruction is surgically removed, many patients have disturbed motility function in the bowel proximal to the site of resection for many years to come (17, 20).
Although mechanical overstretch is the direct and immediate consequence of obstruction and is potentially responsible for obstruction-associated pathologies, little is known regarding the signaling mechanism of stretch-initiated cellular response in the gastrointestinal tract. Our hypothesis is that obstruction-associated mechanical stretch alters smooth muscle gene expression (mechanotranscription), and the altered gene expression leads to impaired contractility. In a preliminary Affymetrix gene array screening, we found several major groups of genes whose expression is altered in the stretched segment oral to obstruction. One of the upregulated genes is cyclooxygenase-2 (COX-2). The enzyme cyclooxygenase catalyzes the major rate-limiting step of the synthesis of prostaglandins (PGs), which play important roles in cell proliferation and smooth muscle function in the gut (8, 18, 21, 34). Although COX-1 is considered a constitutive isoform of COX in most cell types, COX-2 is the inducible form within certain cells (8, 18, 21). Since COX-derived PGs are well known to affect smooth muscle contractility and promote cell proliferation (8, 18, 19, 21, 26, 34), the present study focused on stretch-induced expression of COX-2 in a rat model of partial colon obstruction. Our data demonstrate that colon obstruction leads to a dramatic increase of COX-2 gene expression selectively in the smooth muscle cells (SMCs) of the segment oral to the site of obstruction. We further identified in vitro in the smooth muscle strips and primary cultures of colonic SMCs that mechanical stretch is the direct trigger for the induction of COX-2. Using selective COX-2 inhibitors and COX-2 gene-deficient mice, we determined that stretch-induced COX-2 in the colonic SMC plays a critical role in obstruction-associated contractility impairments.
MATERIALS AND METHODS
Animal models of bowel obstruction.
Sprague-Dawley male rats weighing 200–275 g and aged between 6 and 8 wk (from Harlan Sprague Dawley, Indianapolis, IN) were used for the study. The rats were housed in a controlled environment (22°C, 12-h light-dark cycle) and allowed food and water ad libitum. The Institutional Animal Care and Use Committee at the University of Texas Medical Branch approved all procedures performed on the animals.
The rat model of partial colon obstruction was prepared by following procedures as previously described with minor modifications (2, 10, 39). Rats were anesthetized with 2% isoflurane inhalation by an E-Z Anesthesia vaporizer (Palmer, PA). After midline laparotomy, a distal colon segment 4 cm proximal to the end of colon was carefully exposed. A small mesenteric window (5 × 5 mm2) was made next to the exposed colon segment. Partial colon obstruction was induced by placing a 3-mm wide medical grade silicon ring around the colon wall through the small mesenteric window. The size of the silicon ring (∼20–21 mm in length) is ∼1–2 mm longer than the outer circumference of the colon when the colon segment is filled with fecal pellets, allowing a partial obstruction. The procedure to implement the silicon ring was completed within 2 min. The sham control rats underwent the same surgical procedure except that the ring was removed immediately after the 2-min procedure. Rats were euthanized at different time points up to 7 days following obstruction. A 3-cm-long colon segment starting at 1 cm oral to the site of obstruction was collected as stretched tissue, and a 2-cm-long colon segment starting at 0.5 cm aboral to obstruction was taken as nonstretched internal control. These tissues were used for histological, biochemical, molecular, and contractility studies.
The murine model of partial colon obstruction was prepared similarly as in rats. The B6;129S-ptgs2tmiJed/J mice with homozygous ptgs2 gene mutation (COX-2−/−) and heterozygous mutation (COX-2+/−) (5, 6) and wild-type littermate controls were purchased from the Jackson Laboratory (Bar Harbor, ME). Partial colon obstruction in male mice was induced with a 2-mm-wide silicon ring applied to the distal colon 3 cm proximal to the end of colon. The obstructed and sham control mice were euthanized for experiments in 4 days.
Tissue collection and protein extraction.
The colon segments oral and aboral to the obstruction site were collected in fresh carbogenated Krebs buffer (in mmol/l: 118 NaCl, 4.7 KCl, 2.5 CaC2, 1 NaH2PO4, 1.2 MgCl2, 11 d-glucose, and 25 NaHCO3). The segments were cleansed, opened along the mesenteric border, and pinned flat in a petri dish with Sylgard base. The mucosal/submucosal and muscularis externa layers were separated by microdissection as described previously (27–29). For protein extraction, the tissue was homogenized on ice in lysis buffer (for Western blot) or phosphate-buffered saline (PBS) solution (for ELISA or myeloperoxidase) supplemented with protease inhibitors (Sigma-Aldrich, St. Louis, MO). The compositions of lysis buffer are (in mmol/l) 20 Tris·HCl, pH 7.5, 150 NaCl, 1 EDTA, 1 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 2.5 sodium pyrophosphate, 1 β-glycerolphosphate, 1 Na3VO4, and 1% Triton X-100, and 1 μg/ml leupeptin. The compositions of PBS are (in mmol/l) 137 NaCl, 2.7 KCl, 10 Na2HPO4, KH2PO4 (pH 7.4).
In vitro stretch of colonic circular smooth muscle strips.
Colonic circular muscle strips (∼3 × 10 mm) were prepared in Krebs buffer in the presence of gadolinium (Sigma-Aldrich) to prevent stretch activated ion channels. The muscle strips were cultured in a custom silicone elastomer-bottomed plate in DMEM (Invitrogen, Carlsbad, CA). The stretched muscle strips were kept to 130% of the original length with pins on each end. Control strips were treated similarly but at their original lengths.
Primary culture of RCCSMC and in vitro stretch of RCCSMC in culture.
Rat colonic circular SMCs (RCCSMC) were isolated as described previously (27, 28). In brief, the circular muscle tissue in 0.5 × 0.5 cm2 size was incubated in sterile HEPES buffer (in mmol/l: 120 NaCl, 2.6 KH2SO4, 4 KCl, 2 CaCl2, 0.6 MgCl2, 25 HEPES, 14 glucose, and 2.1% essential amino acid mixture, pH 7.4) with 1.5 mg/ml collagenase (type II, 319 U/mg; Worthington, Freehold, NJ) and 1.0 mg/ml soybean trypsin inhibitor (Sigma-Aldrich) for 45 min at 31°C. At the end of digestion, tissue pieces were incubated in fresh buffer without digestion enzymes. The spontaneously dispersed cells were collected and cultured in DMEM supplemented with 10% fetal bovine serum in the presence of 100 U/ml of penicillin G, 100 μg/ml streptomycin sulfate, and 0.25 μg/ml amphotericin B (Invitrogen). The culture medium was changed every 3 days. Immunofluorescence staining showed that more than 95% of the cultured cells stained for smooth muscle-specific α-actin (27, 28).
Primary culture was allowed to grow for 8–10 days until it was confluent. The cells were then seeded at 8 × 104 cells/well in six-well BioFlex culture plates coated with type I collagen (Flexcell, Hillsborough, NC), grown to ∼80% confluence, and then subjected to DMEM/1% FBS for 24 h prior to stretch. Cells were subjected to stretch via a FX-4000 Flexercell Tension Plus System (Flexcell). This computer-regulated bioreactor applies multiaxial strain to cultured cells (31, 41). Through vacuum pressure, cultured cells are deformed on flexible membrane plates. Cells incubated in parallel under identical conditions but without exposure to stretch served as controls.
In some experiments, the cells were transfected with a COX-2 promoter reporter construct TIS 10L-luc, a gift from Dr. Y. S. Guo (14), for 24 h before the stretch. FuGENE 6 (Roche, Mannheim, Germany) was used to transfect the constructs in RCCSMCs. The pSEAP2 Control Vector (BD Biosciences Clontech, Palo Alto, CA) was cotransfected for normalization of the luciferase activity.
Western blotting.
The proteins in the muscularis externa and mucosa/submucosa were resolved by a standard immunoblotting method as described previously (27–29). Equal quantities (20 μg) of total protein were loaded and run on premade 8–16% Tris-glycine SDS-PAGE (Invitrogen). They were transferred to nitrocellulose membranes (Invitrogen) for incubation with primary and secondary antibodies. The following antibodies were used in the study: primary antibodies to COX-2 and COX-1 (1:1,000; Cayman Chemical, Ann Arbor, MI), secondary antibody IRDye 800-conjugated anti-mouse IgG (Rockland, Gilbertsville, PA), or Alexa Fluor 680 goat anti-rabbit IgG (Invitrogen). β-Actin (1:5,000, Sigma, St. Louis, MO) was used as loading control. The detection was done by ODYSSEY Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
RNA preparation and real-time PCR.
Total RNA was extracted from tissues by using the Qiagen RNeasy kit (Qiagen, Valencia, CA). One microgram of total RNA was reverse- transcribed by using the SuperScript III First-Strand Synthesis System (Invitrogen) for real-time quantitative PCR (27, 28). Real-time PCR was performed by use of the Applied Biosystems 7000 real-time PCR system (Foster City, CA). The assay ID for TaqMan detection of COX-2 is Rn00568225_m1. For relative quantitation of gene transcription, real-time PCR was performed with 40 ng cDNA for the target genes and the endogenous control (18S rRNA). The cycling parameters for real-time PCR were as follows: uracil N-glycosylase activation at 50°C for 2 min, AmpliTaq activation at 95°C for 10 min, denaturation at 95°C for 15 s, and annealing/extension at 60°C for 1 min (repeat 40 times) on ABI7000. Duplicate cycle threshold (CT) values were analyzed in Microsoft Excel by the comparative CT (ΔΔCT) method, as described by the manufacturer. The amount of target was obtained by normalization to endogenous reference (18S rRNA).
Enzyme immunoassay, MPO, and multiplex immunoassay of cytokines and chemokines.
Rat colonic muscularis externa and mucosa/submucosa layer were separately homogenized in cold PBS supplemented with protease inhibitors for protein extraction. LINCO rat cytokine/chemokine multiplex immunoassay kit (LINCO, St. Charles, MO) was used to quantitate cytokine/chemokine levels in the homogenates by following the manufacturer's protocols. The assay results were read and analyzed by a Bio-Rad BioPlex System powered by Luminex xMAP Technology (Bio-Rad Laboratories, Hercules, CA). PGE2 was measured with the PGE2 enzyme immunoassay kit from Cayman Chemical. The myeloperoxidase (MPO) content in the protein extract was measured by MPO ELISA kit purchased from HyCult Biotechnology (Udem, The Netherlands).
Muscle bath experiments.
Freshly obtained colon segments oral and aboral to the obstruction site were obtained, opened along the mesenteric border, cleaned, and pinned flat in a petri dish with Sylgard base in carbogenated Krebs solution. The mucosal/submucosal layers were separated and discarded by microdissection. The smooth muscle strips (4 mm × 10 mm) were mounted along the circular muscle orientation in individual muscle baths (Radnoti Glass, Monrovia, CA) filled with 10 ml carbogenated Krebs solution at 37°C. The contractile activity was recorded as previously described (29) with Grass isometric force transducers and amplifiers connected to Biopac data-acquisition system (Biopac Systems, Goleta, CA). The muscle strips were equilibrated in the muscle bath under 1 g tension for 60 min at 37°C before they were tested for contractility. The effects of obstruction and stretch were tested by obtaining concentration-response curves to ACh (10−6 to 10−2 M) in the muscle bath. The strips were left to equilibrate for at least 15 min before addition of the next concentration of ACh. The contractile response of circular muscle strips was quantified as the increase in area under contractions during 4 min after addition of ACh to the bath, over the baseline area under contractions during 4 min before the addition of ACh.
Histology and immunohistochemistry.
Full-thickness colon segments oral and aboral to obstruction were fixed in 10% buffered formalin for 48 h. Conventional hematoxylin and eosin-stained paraffin sections were prepared in the University of Texas Medical Branch Histopathology Core. With light microscopy, four fields of view were analyzed for each region of interest from sham control and obstructed animals. The thickness of circular smooth muscle layer and cell numbers per cross section were measured through a phase-contrast microscope (Nikon), fitted with a video camera (Javelin CCD), and connected to a computer. NIH Image 1.61 was used to measure the thickness of colonic circular muscle layer.
To determine where COX-2 protein was expressed in situ, immunohistochemistry staining was performed on formalin-fixed, paraffin-embedded colon segments oral and aboral to obstruction and from sham controls. Sections at 4-μm thickness were blocked with 5% normal goat serum in PBS for 20 min at room temperature, and incubated with the rabbit anti-COX-2 antibody (1:200, Cayman Chemical) and a biotin-conjugated anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA). After being incubated with avidin-biotin complex (Vector kit, Vector Laboratories), the sections were stained in diaminobenzidine tetrahydrochloride with 0.03% hydrogen peroxide. As a negative control, sections of the same specimens were processed by the same method but omitting the anti-COX-2 primary antibody.
Statistical analysis.
All data points are expressed as means ± SE. ANOVA with nonrepeated measures (by Student-Newman-Keuls test) was used for multiple comparisons and Student's test for comparisons of two means. A P value of ≤0.05 was considered statistically significant.
RESULTS
Time-dependent changes of colon smooth muscle contractility and morphological features in obstruction in rats.
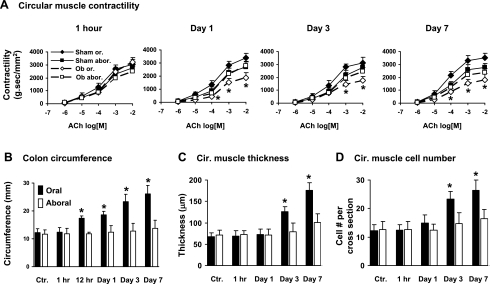
Rats with sham surgery and obstruction were euthanized in 1 h, 12 h, and 1, 3, 5, and 7 days. In the obstruction group, the colon lumen became significantly distended in the segment oral to obstruction within 12 h (Fig. 1). By 24 h, the circumference of the oral segment increased to 18.6 ± 1.2 mm from 12.2 ± 1.4 mm in controls (P < 0.05). The smooth muscle contractile response to ACh (10−6 to 10−2 M), comparing to sham control, was significantly decreased in the oral segment starting at day 1 and through all 7 days (Fig. 1A). However, the contractile response in the colon segment aboral to obstruction was not different between sham control and obstruction rats in all the time points (Fig. 1A). The response to 65 mM KCl was also significantly decreased in the oral segment, but not in the aboral segment (data not shown). The contractile response was not significantly altered in the oral segment at 1 h.
Fig. 1.
Time-dependent changes of rat colonic smooth muscle contractility and morphology for 7 days following partial colon obstruction. A: circular muscle contractility. B: colon outer circumference. C: circular (Cir.) muscle thickness. D: circular muscle cell numbers per cross section. The muscle contractility was determined in freshly isolated colonic circular muscle strips. The colon outer circumference, circular muscle thickness, and cell numbers per cross section were determined in the hematoxylin and eosin-stained tissue specimens, as described in materials and methods. N = 4 or 5 rats for each time point. *P < 0.05 vs. sham control. Ctr, sham control; Ob, obstruction; Or, oral; Abor, aboral.
Obstruction also caused smooth muscle hypertrophy and hyperplasia in the oral, but not aboral, segment (Fig. 1, C and D). The circular muscle thickness began to increase on day 3 and so did the cross-sectional SMC number of the circular muscle layer (Fig. 1, C and D). The hypertrophy and hyperplasia became more profound afterward. We did not notice significant inflammatory infiltrates in obstruction.
Induction of COX-2 expression in partial colon obstruction.
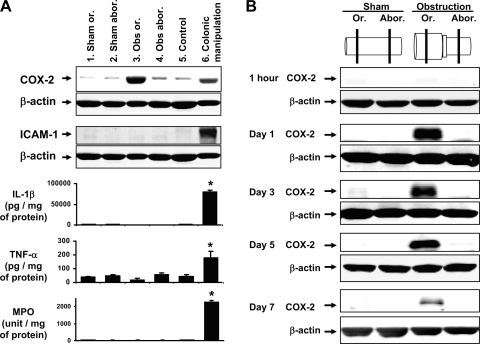
Western blotting was applied to determine whether expression of COX-2 protein was upregulated in obstruction. Our results demonstrate that COX-2 expression in the muscularis externa was dramatically increased in the stretched colon segment oral to obstruction, but not in the nonstretched aboral segment (Fig. 2). Furthermore, in the muscularis tissue taken 1 day after the laparotomy, we found no significant increase of inflammatory mediators such as intercellular adhesion molecule-1 (ICAM-1), interleukin-1β (IL-1β), or tumor necrosis factor-α (TNF-α) in the oral or aboral segments compared with sham controls (Fig. 2A). The muscularis externa MPO activity (a marker of inflammatory granule cell infiltration) was not significantly increased in either segment (Fig. 2A). The cytokine and MPO levels did not increase in the mucosa/submucosa layer in obstruction (data not shown). In contrast, if the colonic surface was rubbed with sterile moist cotton applicators as described previously as “intestinal manipulation” (18), a severe inflammatory response was induced in the colon with increased levels of COX-2, ICAM-1, IL-1β, TNF-α, and MPO in the muscularis externa (Fig. 2A).
Fig. 2.
Comparison of cyclooxygenase-2 (COX-2) expression, inflammatory mediator production and MPO activity in colonic obstruction. A: changes of COX-2, ICAM-1, IL-1β, TNF-α, and MPO activity in colon obstruction and colon manipulation compared with controls. All the samples were taken 24 h after the treatments, and the muscularis externa tissue was collected for the measurements. N = 3 or 4 rats in each group. *P < 0.05 vs. sham control. B: kinetics of COX-2 protein expression in the colonic muscularis externa. Note that COX-2 is induced only in the stretched middle colon (oral to obstruction), but not in the unstretched distal colon (aboral to obstruction) or sham control. The Western blot results were representative of at least 3 independent experiments.
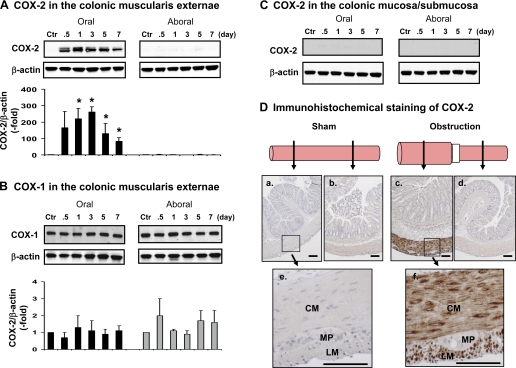
We performed a kinetics study of COX-2 expression in the muscularis tissue taken 1 h, 12 h, 1 day, 3 days, 5 days, and 7 days after surgery. The COX-2 level was not increased in the oral or aboral segment at 1 h (Fig. 2B) but drastically increased in the oral segment in 12 h to 5 days (Fig. 2B). The COX-2 level remained increased for at least 7 days (Fig. 2B). Although COX-2 was significantly induced in the stretched oral segment in obstruction from day 1 to day 7 (P < 0.05 vs. sham control) (Fig. 3A), COX-1, a constitutively expressed cyclooxygenase in gut SMC, was not significantly altered by obstruction in any of the time points (Fig. 3B).
Fig. 3.
Selective induction of COX-2 expression in the colonic SMC of obstructed colon. A: Western blot detection of COX-2 in the colonic muscularis externa in the oral (left) and aboral (right) segments. B: Western blot detection of COX-1 in the colonic muscularis externa in the oral (left) and aboral (right) segments. C: Western blot detection of COX-2 in the mucosa/submucosa in the oral (left) and aboral (right) segments. The Western blot results were representative of 3 or 4 independent experiments. D: immunohistochemical staining of COX-2 expression in the oral (a, c) and aboral (b, d) colon segments in sham control (a, b) and obstruction (c, d) for 3 days. Note that e and f are higher magnification views of the muscularis externa of oral segment from sham (e) and obstruction (f). CM, circular muscle; LM, longitudinal muscle; MP, myenteric plexus. Calibration bars represent 50 μm.
To determine the source of COX-2 expression in the obstructed colon, we first compared the COX-2 expression in muscularis externa and mucosa/submucosa tissue in Western blot and found that COX-2 expression was induced only in the muscularis externa, but not in the mucosa/submucosa (Fig. 3C). Further immunohistochemical studies in the full-thickness colon specimens showed that the increased COX-2 expression (stained brown) occurs specifically in the circular and longitudinal smooth muscle cells, but not myenteric plexus (Fig. 3D).
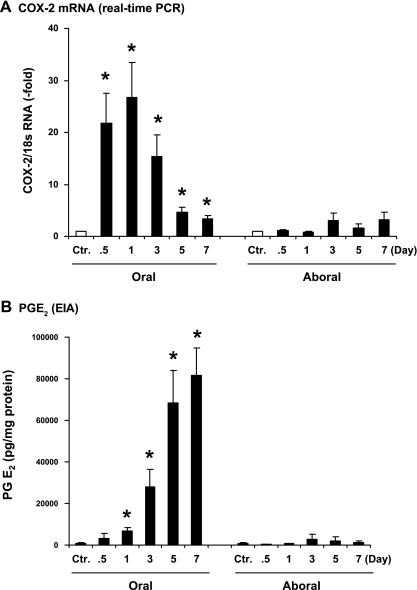
Compared with the sham control, COX-2 mRNA level increased 22 ± 6 and 26 ± 7-fold at 12 and 24 h in the stretched oral segment (P < 0.05 vs. sham) (Fig. 4A). The initial sharp increase was followed by a sustained upregulation of COX-2 message in the following days. The COX-2 mRNA level was 3.3 ± 0.7-fold by day 7 (P < 0.05 vs. sham). However, there was no statistically significant increase of COX-2 mRNA in the aboral segment at any time point (P > 0.05 vs. sham) (Fig. 4A).
Fig. 4.
Kinetic expression of COX-2 mRNA and production of PGE2 in obstruction. Expression of COX-2 mRNA was determined by real-time PCR (A) and production of prostaglandin PGE2 by EIA (B) in the muscularis externa samples of colon segments oral and aboral to obstruction. N = 3 or 4 rats for each time point. *P < 0.05 vs. sham control.
Production of prostaglandins in the obstructed colon:.
We determined whether upregulation of COX-2 in obstruction is followed by increased prostaglandin E2 (PGE2), one of the most abundant prostaglandins in the gastrointestinal tract and known to inhibit colonic SMC contractility in rats (18, 21). ELISA measurements in the colonic muscularis externa showed that the PGE2 level was dramatically increased in the oral segment time dependently (Fig. 4B). PGE2 started to increase significantly by day 1, and its level kept going up. By day 7, the PGE2 level was 120-fold higher than in sham control. In contrast, there was no significant increase of PGE2 in the aboral segment (Fig. 4B).
Induction of COX-2 expression by in vitro stretch of the primary culture of RCCSMC.
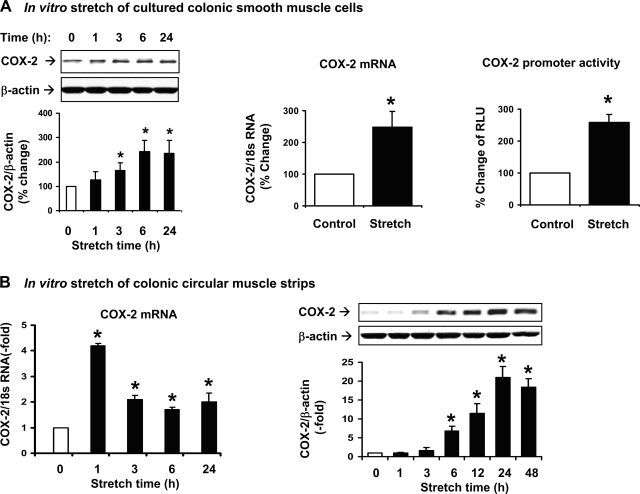
To determine whether the increase of COX-2 in obstruction is caused directly by mechanical force, primary culture of RCCSMC was stretched by the Flexercell system (25, 26) with two types of stretch: 1) cyclic stretch at 0.5 Hz and 18% elongation; and 2) static stretch at 18% elongation. We found that both cyclic stretch and static stretch time dependently induced COX-2 expression in the cultured SMC. Because static stretch mimics the in vivo stretch of obstruction, we used the mode of static stretch in the present study (Fig. 5A). We found that 18% static stretch of the cultured cells for 1 h increased the expression of COX-2 mRNA by 247 ± 50% (P < 0.05 vs. control) (Fig. 5A). In a separate experiment, the COX-2 promoter construct TIS 10L-luc (14) was transfected to the RCCSMC. Stretch at 18% for 24 h significantly increased COX-2 promoter activity by 260 ± 24% (P < 0.05 vs. control), indicating that a transcriptional mechanism is involved in stretch-induced COX-2 expression in RCCSMC (Fig. 5A).
Fig. 5.
Stretch-induced expression of COX-2 in colonic smooth muscle in two different in vitro models. A: effects of mechanical stretch on COX-2 protein and mRNA expression and COX-2 promoter activity in the primary culture of rat colon circular smooth muscle cells with the Flexercell system. Cells were statically stretched with 18% length elongation. B: effects of stretch on COX-2 mRNA and protein expression in colonic circular muscle strips in vitro. Muscle strips were stretched to 130% of the original length with pins on each of the 2 ends. The control strips were treated similarly except that the pins were put on 1 end. N = 3 or 4 for each group or time point. *P < 0.05 vs. control.
Induction of COX-2 expression and inhibition of smooth muscle contractility by in vitro stretch in colonic circular muscle strips.
In another in vitro model, rat colonic circular muscle strips were cultured in DMEM and stretched to 130% of their original length for varied time points up to 48 h. As shown in Fig. 5B, the expression of COX-2 mRNA and protein in the muscle strips was significantly induced by direct stretch in a time-dependent manner (Fig. 5B). The COX-2 mRNA increased 4.2-fold by 1-h stretch, and the COX-2 protein started to increase significantly in 6 h and remained increased for at least 48 h (Fig. 5B). We found that PGE2 level in the culture medium increased 7.1 ± 0.9-fold and 11.4 ± 1.8-fold by direct stretch of the strips for 24 and 48 h, respectively (n = 4 in each time point, P < 0.05 vs. control). The circular muscle contractility was decreased by 42 ± 3 and 38 ± 4% in response to 10−3 M ACh and 60 mM KCl, respectively, in the strips stretched for 48 h (n = 4 in each group, P < 0.05 vs. controls).
Effect of COX-2 inhibitor on contractility of colonic circular smooth muscle strips.
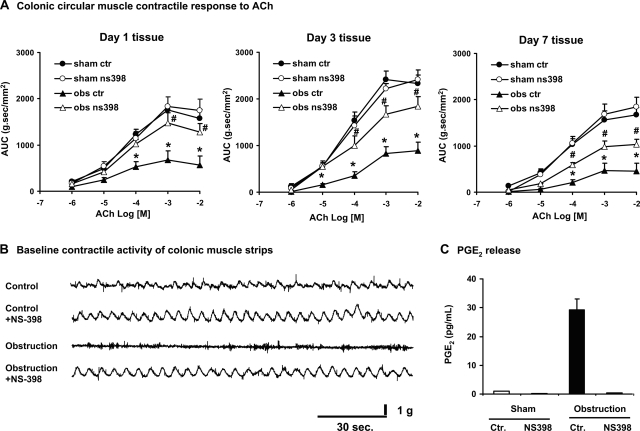
To determine whether stretch-induced COX-2 plays a role in the impairments of muscle contractility encountered in obstruction, circular muscle strips were isolated from sham and obstructed colons and incubated for 24 h with vehicle control (DMSO) and the specific COX-2 inhibitor NS-398 (10−7 M) (18, 21, 26, 34). EIA detection of PGE2 in the culture medium showed that the increase of PGE2 in the obstructed tissue was completely blocked by 10−7 M NS-398, indicating that 10−7 M of NS-398 was effective in blocking COX-2 activity (Fig. 6). The circular muscle contractility was significantly decreased in the obstructed tissue, but treatment with NS-398 10−7 M significantly restored the smooth muscle contractile response to ACh (Fig. 6A) and KCl (data not shown) in all the tissues taken on day 1, day 3, and day 7. Moreover, the spontaneous rhythmic contractions were disrupted in the muscle strips from the obstructed rats, but incubation with NS-398 restored the spontaneous contractions (Fig. 6B).
Fig. 6.
Effect of COX-2 inhibitor NS-398 in vitro. A: treatment of muscle strips in vitro with COX-2 inhibitor NS-398 (10−7 M) restored circular muscle contractile response to ACh. The tissue was collected from the rats treated for 1 day, 3 days, and 7 days and incubated in DMEM with vehicle control (DMSO) or NS-398 for 24 h. B: treatment of muscle strips with COX-2 inhibitor NS-398 (10−7 M) also restored smooth muscle spontaneous rhythmic contractions. Tracing is from the day 3 tissues, representative of 3 independent experiments. C: treatment of muscle strips in vitro with COX-2 inhibitor NS-398 (10−7 M) completely blocked PGE2 production from the day 1 tissue. N = 3 or 4. *P < 0.05 vs. sham control. #P < 0.05 vs. obstruction control. Sham ctr, sham control; obs ctr, obstruction control.
Colonic circular smooth muscle contractility in wild-type and COX-2 gene-deficient mice in obstruction.
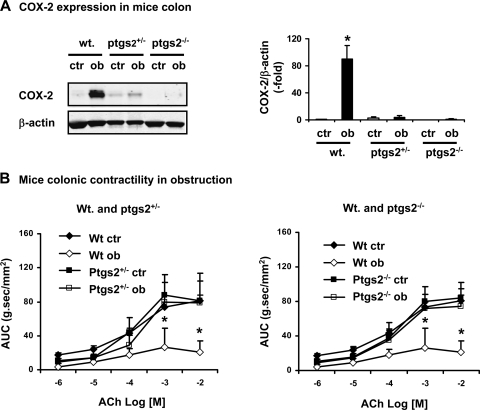
To confirm the role of COX-2 in the impairment of smooth muscle contractility in obstruction, we induced partial colon obstruction in male wild-type (COX-2+/+) and COX-2 gene-deficient mice (COX-2+/−, and COX-2−/−). The circular muscle contractility was determined in the proximal colon of mice of sham and with obstruction for 4 days. As shown in Fig. 7, obstruction caused a significant decrease of colonic circular muscle contractility to ACh in wild-type mice. However, this effect was not present in the COX-2+/− and COX-2−/− mice (Fig. 7). Western blot showed that obstruction caused drastic induction of COX-2 in the stretched colon in wild-type mice (n = 5, P < 0.05 vs. sham). Although COX-2 was barely detectable in the COX-2+/− mice, no significant increase of COX-2 was detected in the obstructed colon in the heterozygote mice (n = 4, P > 0.05 vs. sham COX-2+/−). Nevertheless, COX-2 was not detectable in the COX-2−/− mice in either sham or obstruction (Fig. 7).
Fig. 7.
Murine model of partial colon obstruction in B6;129S-ptgs2tmiJed/J mice with heterozygous (COX-2+/−) and homozygotes (COX-2−/−) ptgs2 gene mutation and wild-type (Wt) littermate controls. Note that the induction of COX-2 expression by colonic obstruction was markedly attenuated in the COX-2+/− and absent in COX-2−/− mice compared with wild-type controls (A). The obstructed colon showed a much suppressed contractility in wild-type, but not in the COX-2 gene-deficient mice (B). All the tissue samples were taken 4 days following partial colon obstruction. N = 3 or 4. *P < 0.05 vs. sham control.
DISCUSSION
Motility impairment in bowel obstruction is associated with symptoms such as bloating, vomiting, abdominal pain, and constipation, all of which compromise digestive function. The investigation into the mechanism of obstruction-associated motility impairment has continued for decades (2, 9, 24, 32, 37, 39). Studies on the acute effects of bowel obstruction showed that the motor activity in the obstructed segment increased immediately after obstruction (24, 32), whereas the motor function in the segment distal to obstruction is usually suppressed (24, 32). It is proposed that the early phase of hypermotility oral to and hypomotility aboral to obstruction result from a neuronal mechanism (24, 32) similar to the physiological peristalsis reflex (13). The lumen contents stacked oral to the occlusion stimulates mechanoreceptors in the gut wall to initiate neuronal pathways of ascending excitation and descending inhibition (13, 24). However, the motor activity oral to obstruction gradually decreases within 24 h, and remains suppressed as a long-term impact of obstruction (2, 9, 32). The reason why the smooth muscle activity in the oral segment became hypocontractile within hours is not known. Our study suggests that mechanical stretch-induced change in gene expression is the reason. We found that the expression of COX-2 peaked 12–24 h after obstruction, and the muscle contractility started to decrease in 24 h, and remained suppressed for the following 7 days. Direct stretch of colonic muscle strips in vitro mimics the effects of mechanical stretch on COX-2 gene expression and muscle contractility. The suppression of smooth muscle contractility is COX-2 dependent, as COX-2 inhibitor restores the contractile function. The impaired smooth muscle contractility in obstruction is attenuated in the COX-2 gene-deficient animals.
Induction of COX-2 leads to a dramatic increase of PGs. The PGE2 level increased more than 120-fold in the muscle layer of the obstructed colon by day 7. The huge buildup of PGs in the muscle layer exerts profound genomic and nongenomic effects on SMCs and surrounding cells in autocrine and paracrine modes (18, 21). The PGs not only affect functions of smooth muscle and surrounding cells during obstruction but may exert genomic effect on these cells for a prolonged time after obstruction. This may explain why, even if obstruction is surgically removed, many patients suffer disturbed motility function in the bowel proximal to the site of resection for many years to come (17, 20).
Although ischemia, bacteria overgrowth, or mucosal barrier dysfunction have been considered as complications in complete or strangulated bowel obstruction (7, 32, 37), these are not likely causes for the increased COX-2 expression and suppressed contractility in our model of partial obstruction. Each of those conditions would have led to massive inflammatory infiltrations in the mucosa/submucosa and muscle layers. However, there was no detectable increase of COX-2 in the mucosa/submucosa layer in our model. We did not find any significant increase of inflammatory cytokines such as IL-1β and TNF-α, or any inflammatory infiltrates in the obstructed colon in 24 h. Won et al. (39) reported in a rat model of small bowel obstruction that there were not inflammatory changes in the muscularis externa even when the tissue was collected 3 wk after induction of obstruction. The authors noticed only a marginal increase of TNF-α, but not IL-1β or IL-6, in the obstructed tissue.
Upregulation of COX-2 expression was reported in several gastrointestinal conditions including intestinal manipulation (26), TNBS-induced inflammation (19), and intestinal ischemia or necrosis (22). All these conditions are proinflammatory, and COX-2 originates in infiltrating leukocytes, activated macrophages, epithelial cells, and enteric neurons (19, 22, 26). This is not surprising because COX-2 has long been considered an inflammatory mediator. In our model, the procedure to apply an obstruction ring around the colon through a mesentery hole took less than 2 min. This procedure does not cause inflammatory infiltration to the muscle layer. If there is any surgery-associated inflammatory infiltration, there would have been increased inflammatory cytokines and COX-2 immediately. However, we did not find any significant increase of COX-2 in the obstructed colon 1 h after obstruction. The inflammatory cytokines such as TNF-α and IL-1β were not increased. In addition, we had strict sham controls (obstruction ring was applied but removed immediately) to minimize any possible effect related to laparotomy. Interestingly, colonic manipulation with cotton applicators indeed caused massive transmural inflammation and moderately increased COX-2 expression. Therefore, inflammation is not the cause of COX-2 upregulation in our model. On the contrary, our study suggests that mechanical stretch is the direct stimulus for the induction of COX-2 in the obstructed colon. First, COX-2 is induced in vivo only in the smooth muscle of stretched segment oral to occlusion, but not in the nonstretched aboral segment. Secondly, direct stretch of colonic muscle strips or cultured SMCs in vitro induces COX-2. In vitro stretch also significantly suppresses smooth muscle contractility. Furthermore, distension of the colon wall with a balloon at 30 mmHg for 40 min induces COX-2 in the colonic SMC (Y. M. Lin and X.-Z. Shi, unpublished observation). Therefore, our study demonstrates that COX-2 is not only an inflammatory mediator, but a stretch-sensitive molecule in the colon. Since cancer is the most common cause of colon obstruction in the United States (9, 16, 37), the 5-year survival rate of patients with obstructed colon cancer following surgery is significantly worse than that of their counterparts without obstruction (23, 38). This is independent of the extent of the cancer stage (38). Given that COX-2 is involved in the development of colon cancer (18, 35), our findings that the stretch-sensitive COX-2 is robustly induced in obstruction may explain why obstructed colon cancer has a worse prognosis.
Mechanical stretch-induced gene expression requires a series of signaling cascades (12, 25, 40). Studies have identified two major groups of mechanosensors: 1) the integrins and cytoskeleton; and 2) stretch-activated ion channels (1, 12, 15, 40). The muscle cells, including SMC, are very sensitive to mechanical force. In our model, although all the tissue layers and cell types in the obstructed colon wall are under stretch, obstruction induces expression of COX-2 only in the SMCs. This is in sharp contrast to other gastrointestinal conditions such as intestinal manipulation (26), gut inflammation (19), and ischemia injury (22), in which upregulation of COX-2 takes place in macrophages, infiltrating leukocytes, enteric neurons, and epithelial cells. Currently we do not know the reason for the selective induction of COX-2 in the SMCs in obstruction. However, the signaling mechanism linking stretch to induction of COX-2 in the colonic SMC may involve SMC-specific proteins, such as SMC-specific α-actin. It was recently reported that SMC-specific α-actin plays important roles in mechanotranscription through cytoskeleton-associated activation of transcription factors such as nuclear factor-κB (3, 36). Nevertheless, our data show that the mechanically sensitive SMC in the gut plays an important role in obstruction-associated pathologies through a mechanotranscription-dependent mechanism.
In summary, there is a robust and selective induction of COX-2 expression in the colonic SMCs in obstruction, and mechanical stretch is the direct stimulus of COX-2 expression in the process. COX-2 inhibition restores smooth muscle contractile function. Our study therefore demonstrates that mechanotranscription regulates gut SMC function and plays a critical role in the pathophysiology of stretch-related motility disorders such as mechanical obstruction, pseudo-obstruction, megacolon, and achalasia, in which spatial distention of the lumen is apparent. Furthermore, our study indicates that COX-2 inhibitors and mechanotranscription blockers may have therapeutic potentials in the stretch-related disorders in the gut.
GRANTS
This work was supported by National Institute of Health (R01DK082563 to X.-Z. Shi) and a John Sealy Memorial Fund grant (X.-Z. Shi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Aikawa R, Nagai T, Kudoh S, Zou Y, Tanaka M, Tamura M, Akazawa H, Takano H, Nagai R, Komuro I. Integrins play a critical role in mechanical stress-induced p38 MAPK activation. Hypertension 39: 233–238, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bertoni S, Gabella G, Ghizzardi P, Ballabeni V, Impicciatore V, Lagrasta C, Arcari ML, Barocelli E. Motor response of rat hypertrophic intestine following chronic obstruction. Neurogastroenterol Motil 16: 365–374, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Chaqour B, Yang R, Sha Q. Mechanical stretch modulates the promoter activity of the profibrotic factor CCN2 through increased actin polymerization and NF-kappaB activation. J Biol Chem 281: 20608–20622, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Connor FL, Di Lorenzo C. Chronic intestinal pseudo-obstruction: assessment and management. Gastroenterology 130: S29–S36, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, Gorry SA, Trzaskos JM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378: 406–409, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Ethridge RT, Chung DH, Slogoff M, Ehlers RA, Hellmich MR, Rajaraman S, Saito H, Uchida T, Evers BM. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology 123: 1311–1322, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Fevang J, Øvrebø K, Grong K, Svanes K. Fluid resuscitation improves intestinal blood flow and reduces the mucosal damage associated with strangulation obstruction in pigs. J Surg Res 117: 187–194, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Fornai M, Blandizzi C, Colucci R, Antonioli L, Bernardini N, Seqnani C, Baraqatti B, Baroqi S, Berti P, Spisni R, Del Tacca M. Role of cyclooxygenases 1 and 2 in the modulation of neuromuscular functions in the distal colon of humans and mice. Gut 54: 608–616, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fraser ID, Condon RE, Schulte WJ, DeCosse JJ, Cowles VE. Intestinal motility changes in experimental large bowel obstruction. Surgery 87: 677–682, 1980 [PubMed] [Google Scholar]
- 10. Gabella G. Hypertrophy of visceral smooth muscle. Anat Embryol (Berl) 182: 409–424, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Gattuso JM, Kamm MA, Talbot JC. Pathology of idiopathic megarectum and megacolon. Gut 41: 252–257, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature 413: 194–202, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Grider JR. Identification of neurotransmitters regulating intestinal peristaltic reflex in humans. Gastroenterology 97: 1414–1419, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Guo YS, Cheng JZ, Jin GF, Gutkind JS, Hellmich MR, Townsend CM., Jr Gastrin stimulates COX-2 expression in intestinal epithelial cells through multiple signaling pathways. Evidence for involvement of ERK5 kinase and transactivation of the epidermal growth factor receptor. J Biol Chem 277: 48755–48763, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Hu H, Sachs F. Stretch-activated ion channels in the heart. J Mol Cell Cardiol 29: 1511–1523, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Jordan GL., Jr The acute abdomen. Adv Surg 14: 259–315, 1980 [PubMed] [Google Scholar]
- 17. Kim HY, Kim JH, Jung SE, Lee SC, Park KW, Kim WK. Surgical treatment and prognosis of chronic intestinal pseudo-obstruction in children. J Pediatr Surg 40: 1753–1759, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Krause W, DuBois RN. Eicosanoids and the large intestine. Prostaglandins Other Lipid Mediat 61: 145–161, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Linden DR, Sharkey KA, Ho W, Mawe GM. Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J Physiol 557: 191–205, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Menezes M, Corbally M, Puri P. Long-term results of bowel function after treatment for Hirschsprung's disease: a 29-year review. Pediatr Surg Int 22: 987–990, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Mohajer B, Ma TY. Eicosanoids and the small intestine. Prostaglandins Other Lipid Mediat 61: 125–143, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Moses T, Wagner L, Fleming SD. TLR4-mediated COX-2 expression increases intestinal ischemia/reperfusion-induced damage. J Leukoc Biol 86: 971–980, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ohman U. Prognosis in patients with obstructing colorectal carcinoma. Am J Surg 143: 742–747, 1982 [DOI] [PubMed] [Google Scholar]
- 24. Prihoda M, Flatt A, Summers RW. Mechanisms of motility changes during acute intestinal obstruction in the dog. Am J Physiol Gastrointest Liver Physiol 247: G37–G42, 1984 [DOI] [PubMed] [Google Scholar]
- 25. Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res 47: 23–37, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Schwarz NT, Kalff JC, Turler A, Engel BM, Watkins SC, Billiar TR, Bauer AJ. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology 121: 1354–1371, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterology 129: 1518–1532, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Shi XZ, Sarna SK. Transcriptional regulation of inflammatory mediators secreted by human colonic circular smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 289: G274–G284, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Shi XZ, Choudhury B, Pasricha JP, Sarna SK. A novel role of VIP in colonic motility function: induction of excitation-transcription coupling in smooth muscle cells. Gastroenterology 132: 1388–1400, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Silen W. Acute intestinal obstruction. In: Harrison's Principles of Internal Medicine ( 16th ed., vol. 2), edited by Kasper DL, Braunwald E, Hauser S, Longo D, Jameson JL, Fauci AS. New York: McGraw-Hill, 2005, p. 1803–1805 [Google Scholar]
- 31. Sooranna SR, Lee Y, Kim LU, Mohan AR, Bennet PR, Johnson MR. Mechanical stretch activates type 2 cyclooxygenase via activator protein-1 transcription factor in human myometrial cells. Mol Hum Reprod 10: 109–113, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Summers RW. Approach to the patient with ileus and obstruction. In: Textbook of Gastroenterology (vol. 1), edited by Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW. Philadelphia, PA: Lippincott Williams & Wilkins, 1999, p. 842–858 [Google Scholar]
- 33. Thompson JS. Overview of etiology and management of intestinal failure. Gastroenterology 130: S3–S4, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Wallace JL. COX-2: a pivotal enzyme in mucosal protection and resolution of inflammation. ScientificWorldJournal 6: 577–588, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 29: 781–788, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang J, Zohar R, McCulloch CA. Multiple roles of alpha-smooth muscle actin in mechanotransduction. Exp Cell Res 312: 205–214, 2006. [DOI] [PubMed] [Google Scholar]
- 37. Welch JP. Bowel Obstruction. Philadelphia, PA: Saunders, 1990 [Google Scholar]
- 38. Wolmark N, Wieand HS, Rockette HE, Fisher B, Glass A, Lawrence W, Lerner H, Cruz AB, Volk H, Shibata H. The prognostic significance of tumor location and bowel obstruction in Dukes B and C colorectal cancer. Findings from the NSABP clinical trials. Ann Surg 198: 743–752, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Won KJ, Suzuki T, Hori M, Ozaki H. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol Motil 18: 53–61, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Yamaguchi O. Response of bladder smooth muscle cells to obstruction: signal transduction and the role of mechanosensors. Urology 63: 11–16, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Zampetaki A, Zhang Z, Hu Y, Xu Q. Biomechanical stress induces IL-6 expression in smooth muscle cells via Ras/Rac1-p38 MAPK-NF-κB signaling pathways. Am J Physiol Heart Circ Physiol 288: H2946–H2954, 2005 [DOI] [PubMed] [Google Scholar]