Abstract
We examined the cell-specific subcellular expression patterns for sodium- and potassium-coupled chloride (NaK2Cl) cotransporter 1 (NKCC1), Na+ bicarbonate cotransporter (NBCe1), cystic fibrosis transmembrane conductance regulator (CFTR), and Na+/H+ exchanger 3 (NHE3) to understand the functional plasticity and synchronization of ion transport functions along the crypt-villus axis and its relevance to intestinal disease. In the unstimulated intestine, all small intestinal villus enterocytes coexpressed apical CFTR and NHE3, basolateral NBCe1, and mostly intracellular NKCC1. All (crypt and villus) goblet cells strongly expressed basolateral NKCC1 (at approximately three-fold higher levels than villus enterocytes), but no CFTR, NBCe1, or NHE3. Lower crypt cells coexpressed apical CFTR and basolateral NKCC1, but no NHE3 or NBCe1 (except NBCe1-expressing proximal colonic crypts). CFTR, NBCe1, and NKCC1 colocalized with markers of early and recycling endosomes, implicating endocytic recycling in cell-specific anion transport. Brunner's glands of the proximal duodenum coexpressed high levels of apical/subapical CFTR and basolateral NKCC1, but very low levels of NBCe1, consistent with secretion of Cl−-enriched fluid into the crypt. The cholinergic agonist carbachol rapidly (within 10 min) reduced cell volume along the entire crypt/villus axis and promoted NHE3 internalization into early endosomes. In contrast, carbachol induced membrane recruitment of NKCC1 and CFTR in all crypt and villus enterocytes, NKCC1 in all goblet cells, and NBCe1 in all villus enterocytes. These observations support regulated vesicle traffic in Cl− secretion by goblet cells and Cl− and HCO3− secretion by villus enterocytes during the transient phase of cholinergic stimulation. Overall, the carbachol-induced membrane trafficking profile of the four ion transporters supports functional plasticity of the small intestinal villus epithelium that enables it to conduct both absorptive and secretory functions.
Keywords: cystic fibrosis transmembrane conductance regulator, membrane trafficking, electrogenic sodium/bicarbonate cotransporter, sodium/proton exchanger, goblet cell, pancreatic duct
the intestinal mucosa efficiently regulates absorptive and secretory functions in the intestine because of the spatial organization and functional cooperation of epithelial cells along the crypt-villus axis. While the epithelial cell types that comprise the functional units of the crypt and villus compartment are known, how individual cells cooperate to synchronize anion transport along the crypt-villus axis is not well understood (22). The previously held notion of a permanent segregation of functions between the crypt (secretory) and villus (absorptive) epithelium is clearly challenged (30, 39, 50, 59, 62), and it is increasingly recognized that anion secretion/absorption along the crypt/villus axis can vary under different physiological or pathological conditions. In common intestinal diseases, such as inflammatory bowel and celiac disease, a pathological imbalance results in diarrhea when secretion becomes dominant or unchecked. Both diseases are associated with crypt hyperplasia and villus atrophy; however, the underlying pathogenesis of ion transport defects is not understood, in part, because the presence or absence of clinically relevant transporters in a distinct intestinal cellular location remains unspecified.
There has been a recent explosion in information regarding intestinal anion transporters. Functional studies in genetically engineered animal models that examined the Cl−/HCO3− anion exchanger 2 (AE2), the SLC26A6 and SLC26A3 Cl−/HCO3− exchangers, and the sodium/bicarbonate (Na+/HCO3−) cotransporter (NBC) family members revealed critical roles for bicarbonate secretion in specific intestinal segments along the proximal-distal axis of the intestine (3, 10, 11, 36, 76). In addition to its established central role in chloride secretion, the cystic fibrosis transmembrane conductance regulator (CFTR) is now recognized as a critical regulator of intestinal bicarbonate secretion (25, 44, 67, 77). This observation has renewed interest in a long-debated but central question of the underlying pathogenesis of cystic fibrosis (CF), the genetic disease resulting from mutations in CFTR (70). In the CF intestine, the mutant CFTR protein is unable to traffic normally to the apical plasma membrane of enterocytes and thus Cl− and/or HCO3− secretion is defective. CFTR is not present in goblet cells (the second largest population of intestinal epithelial cells and the site of mucus production and secretion). Yet a central feature of CF disease is the accumulation of sticky, abnormally thick acidic mucus that obstructs the lumen of ductal structures in affected organs (86). The anion transport defects in CF were characterized in detail, but until recently, no unifying hypothesis linked goblet cell functions and CFTR-mediated anion secretion to explain the observed defects in this disease (35, 70). A model was proposed, whereby CFTR-mediated bicarbonate secretion from enterocytes could modulate mucus properties and secretion by intestinal goblet cells and thus fully account for the observed defects in CF (35). But direct evidence supporting this model has not been provided. To understand how enterocytes and goblet cells coordinate their respective anion transport functions, the current study examined the cell-specific subcellular distribution of the major anion transporters in epithelial cells along the crypt-villus axis in rat intestine. Examination of subcellular distribution patterns of transporter expression in tissues is also necessary to establish vesicle traffic as an important mechanism of regulation in physiological anion transport (6). We, therefore, used this approach to examine whether coordinated anion transport along the crypt-villus axis of the epithelium involved intracellular trafficking of CFTR, the sodium- and potassium-coupled chloride (NaK2Cl) cotransporter 1 (NKCC1), the electrogenic NBC1 (NBCe1; SLC4A4), and the sodium/proton (Na+/H+) exchanger 3 (NHE3; SLC9A3). Trafficking of NKCC1 was previously demonstrated in colonic crypt cells (71), but no study has simultaneously examined the subcellular distribution and cell-specific expression of each transporter along the crypt-villus axis.
The mucus-producing Brunner's glands of the proximal duodenum empty their contents into the crypt lumen (51). Like mucus-producing goblet cells, very little is known about the gland's role in fluid and electrolyte transport. HCO3− secretion by Brunner's glands has been assumed (23), without direct evidence to support this claim (1, 2). Accumulating evidence support a fluid transport role for Brunner's glands that may involve regulation by agonist-stimulated trafficking. The chloride bicarbonate exchanger AE2 was previously localized to the basolateral membranes, and more recently aquaporin 5 (AQP5) channels were identified on the apical and lateral domains of acinar cells of the Brunner's gland. The distribution of AQP5 suggests its presence in subapical vesicles, consistent with aquaporin's role in regulating water transport by trafficking (3, 60, 64). Both rab3D, a member of the Rab protein family, and SNARE proteins, which regulate intracellular trafficking, were identified in rat Brunner's gland (28, 84). To provide convincing evidence in support of intracellular trafficking in anion transport, we embarked on a detailed examination of the subcellular distribution of CFTR, NKCC1, and NBCe1 in rat Brunner's glands.
In human colon crypt explants cholinergic Ca2+ signals were initiated at the crypt base and spread along the crypt axis to induce rapid recruitment and activation of NKCC1 on the basolateral membranes, which was accompanied by secretory cell volume decrease, and transient fluid secretion (71). These findings also indicated that a cholinergic stimulus recruits more crypt cells to function in a syncytium in anion secretion following acute stimulation. Whether a similar mechanism can occur along the crypt/villus axis in the small intestine is unknown. We investigated this in the current study in vivo using short-term carbachol (CCh) treatment in ligated segments of rat small intestine and examined how cholinergic stimulation affected the localization and membrane trafficking of CFTR, NKCC1, NHE3, and NBCe1 in distinct epithelial cell types, and along the crypt-villus axis.
MATERIALS AND METHODS
Primary and Secondary Antibodies
Anti-CFTR.
AME4991 anti-CFTR antibody was used at 1:1,000 for light and electron microscopic immunocytochemistry and 1:2,000 for Western blot analysis (5, 41). The monoclonal mouse anti-CFTR antibody (M3A7; Chemicon International, Temecula, FL) was used in some double-label studies.
Electrogenic Na+/HCO3− cotransporter 1 (NBCe1).
The polyclonal rabbit antiserum K1A was a generous gift from Dr. Walter Boron (Case Western Reserve University). The antiserum recognizes a region of the COOH terminus common to electrogenic Na+/HCO3− cotransporter 1-A (NBCe1-A) and NBCe1-B, but different from NBCe1-C. The generation and use of K1A (anti-rb1) and B1B (anti-rb2) were reported (18).
NKCC1.
T84 is an affinity-purified polyclonal antibody against NKCC1, previously shown to recognize NKCC1 in human and rodent intestinal cells. T4 (acquired from the Developmental Hybridoma Bank) is a monoclonal antibody generated against a fusion protein encompassing the carboxy terminus (S760-S1212) of human NKCC1 (58). Both anti-NKCC1 antibodies recognize NKCC1 by immunocytochemistry and immunoblot (58) and produced identical labeling patterns in our studies.
NHE3.
The monoclonal mouse antibody (N12920/611776) (BD Biosciences, San Jose, CA) was diluted at 1:500. Two early endosome markers were used: the rabbit polyclonal antibody against the early endosomal antigen 1 (rb-EEA1; Affinity Bioreagents, Golden, CO) and the mouse monoclonal antibody m-EEA1 (BD Biosciences, San Jose, CA), which was diluted at 1:500. Recycling endosomes were visualized using the polyclonal rabbit Rme-1 antiserum (NJ177; a gift from Dr. Barth Grant, Rutgers University) diluted at 1:250, as described earlier (66). Secondary fluorescent affinity-purified goat anti-rabbit or anti-mouse IgG antibodies labeled with Alexa 488 or CY3, and the F-actin marker rhodamine-labeled phalloidin were obtained from Molecular Probes (Eugene, OR).
Animals
The Institutional Animal Care and Use Committee of Yale University School of Medicine approved the study. Male Sprague-Dawley rats (200–250 g wt, Charles River Laboratories, Wilmington, MA) were fasted overnight but allowed free access to drinking water and anesthetized with Inactin (120 mg/kg ip) injection. Body temperature was maintained with a heating pad.
Luminal Treatment of Small Intestinal Loops
Intestinal loops (∼2.5-cm length) were created with ligatures in the duodenum, proximal jejunum, and ileum. The lumen was instilled with ∼0.2 ml CCh (10 μM) prepared in normal saline (pH 7.4, at 37°C) or saline alone for 10, 15, or 20 min. The abdomen was closed, and the animal was kept warm. At the end of the experiment, the animals were euthanized by administration of intraperitoneal injection of Inactin (200 mg/kg).
Tissue Preparation
Intestinal segments were immediately removed and briefly rinsed with ice-cold PBS. Tissues were cut into ∼2-mm-thick rings, fixed in 2% paraformaldehyde in PBS, pH 7.4, for 1 h at room temperature, then rinsed with PBS and cryoprotected in 30% sucrose overnight. The tissue samples were arranged in multiple-tissue blocks (described below) and embedded in tissue-freezing medium OCT (Miles, Elkhart, IN). For identification and orientation, tissue blocks were photographed before freezing, using a camera-equipped stereomicroscope or a flat-bed scanner. OCT-embedded tissues were immediately frozen in isopentane precooled in liquid nitrogen and tissue blocks stored at −70°C until sectioning. Frozen sections (5 μm) were cut on a cryostat, mounted on Superfrost Plus slides (Fisher), and stored at −20°C. Some of the slides were stained with hematoxylin and eosin for histological assessment.
Utilization of Tissue Arrays
Multiple tissue samples were processed under identical conditions by the utilization of tissue arrays. Samples from duodenum to colon taken from the same rat were embedded in one block. Untreated tissues and tissues undergoing different experimental conditions were also embedded together. This approach allowed simultaneous analysis of multiple tissue sections. Images were taken with the same exposure time.
Immunofluorescence Labeling
All steps were carried out in a humidified chamber at room temperature, except for overnight incubation with primary antibodies, which was carried out at 4°C. Mounted frozen sections were thawed and rehydrated in PBS. To reduce autofluorescence, sections were treated with 1% sodium borohydride for 10 min, and then washed in PBS. To improve antibody labeling, some sections were exposed to 0.1 or 0.2% SDS in PBS for 10 min, then washed with PBS. Sections were incubated for 2 h in a blocking solution (BS) consisting of PBS, 10% goat serum, and 0.1% Triton-X (pH 7.4). Subsequent incubations were carried out in a BS. Sections were incubated with the primary antibodies overnight, except the NKCC1-specific T4 primary antibody (diluted 1:200) was incubated for 5–15 min at room temperature. Control sections were labeled in the absence of primary antibodies or with nonspecific IgG. Sections were washed three times in PBS and incubated for 30 min with Alexa 488 (green) or CY3 (red) conjugated secondary antibodies diluted 1:500 in BS. Filamentous actin (F-actin) staining was detected with rhodamine-phalloidin, and nuclei were visualized (blue) with 1% Hoechst dye. Slides were mounted with Slow Fade (Molecular Probes) medium prior to examination.
Immunogold Labeling
All experiments were performed on Brunner's gland tissue from nonfasted male Sprague-Dawley rats. Pentobarbital sodium anesthesia was administered intraperitoneally (60 mg/kg), and the proximal duodenum was identified. Tissue segments containing submucosal Brunner's glands were removed, cut into small pieces, and immersion fixed in 2% (wt/vol) paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4 for 12 h. Tissue was maintained in 0.2% paraformaldehyde in 0.1 M PB, pH 7.4 at 4°C until ready for use. Tissue blocks were prepared and ultrathin cryosections were immunolabeled as described previously (4, 56).
Fluorescence Image Analysis
Immunolabeled sections were stored at 4°C and examined on a Nikon Eclipse E800 epifluorescent microscope equipped with a Hamamatsu Orca R2-C10600 digital camera. The acquisition parameters were standardized in relation to the highest-intensity regions to avoid oversaturation of pixel intensity. Digital images (8 bits/channel; 1,344 × 1,024 pixels) were taken at ×40 magnification and at the same exposure time. Background-corrected fluorescence intensity levels were measured by selecting discrete regions of interest over the apical membrane (for CFTR and NHE3), the lateral membrane (for NBCe1 and NKCC1), and the intracellular apical pole. Densitometric analysis was performed using the advanced “Analysis and Record Measurements” features of Adobe Photoshop CS4 Extended. The “Record Measurements” tool provided the pixel intensities (mean gray value) of the selected areas. For background correction, pixel intensity values from the lamina propria were subtracted. For selection of regions of interests, the circular brush tool was set at 100% hardness, and ribbon-shaped areas were traced onto a new layer (set to 20% opacity, in order to see the transparent selected area and the image at the same time). For NHE3 and CFTR apical membrane labeling, ribbon-shaped areas with a thickness of ∼2.0 μm (16-pixel master diameter) were traced centered on the apical fluorescence. For NKCC1 and NBCe1 lateral membrane labeling, short ribbons with a thickness of ∼1.5 μm (12-pixel master diameter) were traced centered on the lateral membrane fluorescence. For NHE3 and CFTR intracellular apical pole labeling, short ribbons with a thickness of ∼3.0 μm (24-pixel master diameter) were traced closely below the apical membrane areas selected previously, but without overlay; the upper margin of the selected apical pole areas was ∼1.5–2 μm from the center of the apical membrane fluorescence. Similarly, for NKCC1 and NBCe1 intracellular apical pole labeling in enterocytes, short ribbons with a thickness of ∼3 μm were selected below the apical surface. For NKCC1 intracellular labeling in goblet cells, areas above the nucleus were selected. Data from 4 to 12 selected areas were averaged in each image; 6 to 8 images were analyzed for each measurement group in one animal, and data were collected from four animals.
Statistics
All measured values were presented as means ± SE. Statistical significance between two individual measurement groups was determined by unpaired t-test. Differences among groups were determined using one-way ANOVA and the Tukey's post hoc method of multiple comparisons. The level of significance was set at P < 0.05.
RESULTS
Distribution Patterns of CFTR, NBCe1, NHE3, and NKCC1 Along the Proximal-Distal Axis in Rat Intestine
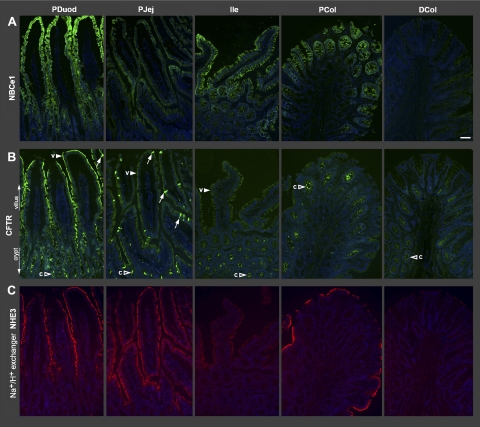
Figure 1 shows low-magnification images of the relative distribution patterns of NBCe1 (Fig. 1A), CFTR (Fig. 1B), and NHE3 (Fig. 1C) along the crypt/villus axis of five representative intestinal segments: proximal duodenum, proximal jejunum, ileum, proximal colon, and distal colon, using tissue arrays and standardized imaging as described in materials and methods. Data on quantification of fluorescence intensities (FI) of the four transporters examined in the jejunum is shown in Fig. 11. Overall, immunolabeling for all four transporters (NKCC1, NBCe1, CFTR, and NHE3) was detected in the small intestinal upper crypt and villus epithelium.
Fig. 1.
Distribution patterns of CFTR, Na+ bicarbonate cotransporter (NBCe1), and Na+/H+ exchanger 3 (NHE3) along the proximal-distal axis of rat intestine. Tissues from intestinal segments were embedded using tissue arrays. Sections were processed and imaged under standard conditions as described in materials and methods. Low-magnification images show the distribution of NBCe1 (green) (A), CFTR (green) (B), and NHE3 (red) (C). CFTR and NHE3 images were taken from a section doubled labeled for CFTR/NHE3; NBCe1 images are from a neighboring section. Villus (arrowheads, v), crypt (open arrowheads, c), CFTR High Expresser cells (arrows). PDuod, proximal duodenum; PJej, proximal jejunum; Ile, ileum; PCol, proximal colon; DCol, distal colon; Scale bar: A–C: 100 μm.
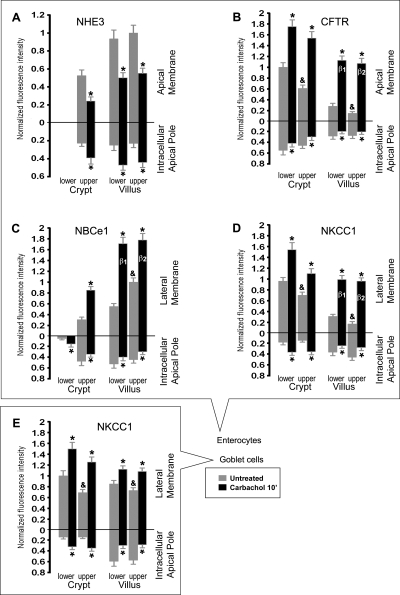
Fig. 11.
Densitometry analysis of normalized NHE3, CFTR, NBCe1 and NKCC1 fluorescence intensities (FI) at the apical or lateral membranes, and apical intracellular compartments of enterocytes and goblet cells along the crypt-villus axis in untreated and CCh-treated jejunum. Cells were analyzed from the lower and upper third of crypts, and lower and upper third of villi. *Significant difference from the untreated group. &“Upper” untreated group significantly different from “lower” untreated group. β1 and β2, no significant difference between group β1 and group β2.
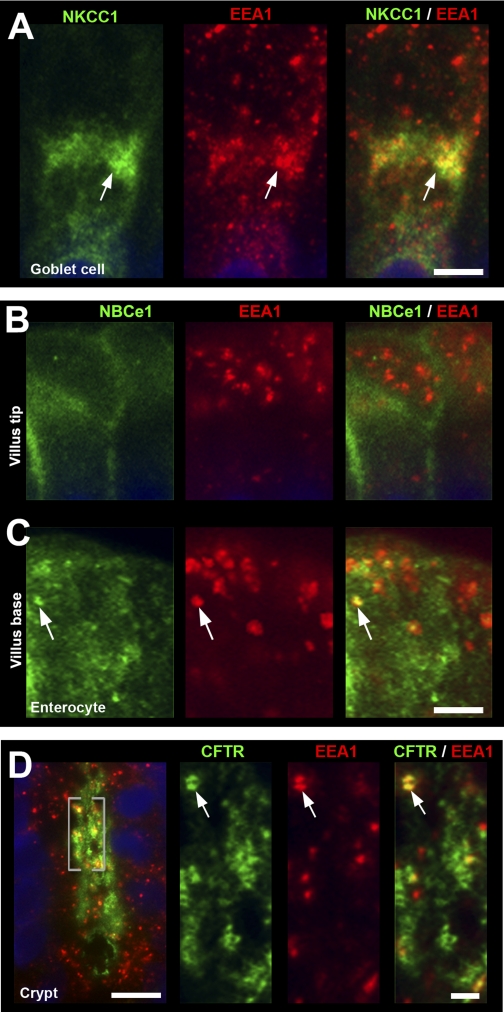
Although the FI levels varied, NBCe1, CFTR, and NHE3 were detected in the upper crypt and villus epithelia of the duodenum, jejunum, and ileum; in contrast, only CFTR was detected in the lower crypt epithelia. Proximal duodenal villi displayed high levels of NBCe1, CFTR, and NHE3; and NBCe1 exhibited an increasing gradient of expression toward the villus tip. Jejunal villi displayed moderate levels of NHE3 and CFTR but low levels of NBCe1. In the villus epithelium of the ileum, the pattern was opposite to the jejunum: NHE3 and CFTR levels were low, while NBCe1 was high. Because intracellular patterns cannot be demonstrated at low magnification, data on the localization of the fourth transporter NKCC1 are presented separately. NKCC1 labeling was strong in crypt cells (Figs. 2, 3, 11), but it was also detected in villus cells. The subcellular distribution of NKCC1 was increasingly intracellular in enterocytes from the villus base toward the villus tips, in the duodenum (Fig. 2, A and B), jejunum (Fig. 5, 10A) and ileum (data not shown). In the colon, CFTR, NKCC1, and NBCe1 were strongly coexpressed in enterocytes in the middle third of proximal colonic crypts (Fig. 8), and NHE3 was strongly expressed in surface cells (Fig. 1C). In the proximal region of the distal colon, CFTR and NKCC1 were coexpressed in crypt cells (data not shown); both NBCe1 and NHE3 levels were very weak compared with the proximal colon (Fig. 1).
Fig. 2.
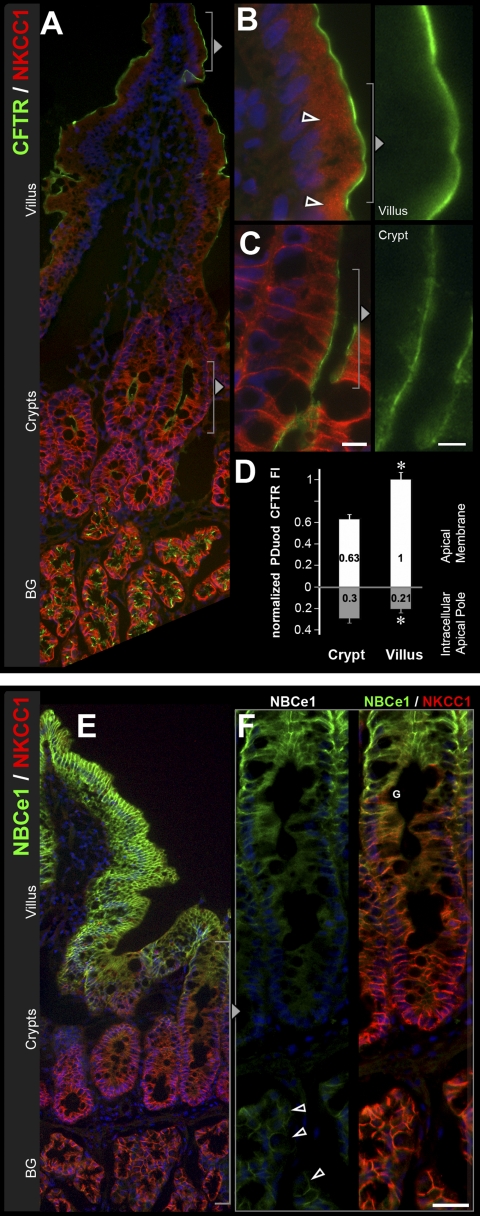
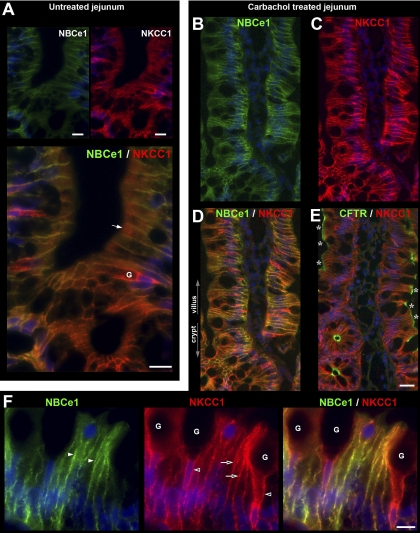
Distribution of CFTR, the sodium- and potassium-coupled chloride cotransporter 1 (NKCC1), and NBCe1 along the crypt-villus axis of proximal duodenum. Sections of rat proximal duodenum were double-labeled for CFTR (green) and NKCC1 (red) or NBCe1 (green) and NKCC1 (red). A: lower-power magnification shows distribution of CFTR and NKCC1 from the villus tip to the submucosal Brunner's glands (BG). Higher magnification of villus tip (B) shows CFTR and intracellular NKCC1 (open arrowheads) or (far right) CFTR alone. C: basolateral and some intracellular NKCC1 label in more superficial crypt cells. D: normalized CFTR fluorescence intensity (FI) levels in the crypt and villus epithelium. Data shown are means ± SE (n = 8). *P < 0.001; significantly different by Student's t-test. E: low-magnification image of NBCe1 (green) and NKCC1 (red) distribution from villus tip to the submucosal BG. F: high-magnification images of NBCe1 (left) and NBCe1/NKCC1 (right) in the crypt and BG show gradients of NBCe1 and NKCC1 expression. Weak NBCe1 label (open arrowheads) and strong NKCC1 are identified on the basolateral membrane of the submucosal BG cells. Goblet cells (G). Scale bars: B and C, left: 10 μm; C (right): 5 μm; F: 25 μm.
Fig. 3.
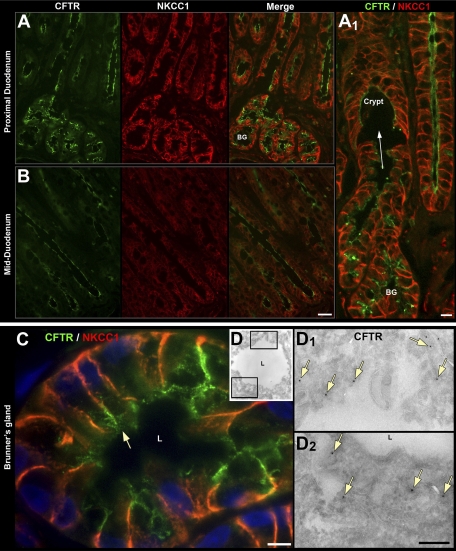
Distribution of CFTR and NKCC1 in the submucosal Brunner's glands (BG) and crypt epithelium of proximal and mid-duodenum. Distribution of CFTR (green), NKCC1 (red), and merged images in crypt (A and B) and BG in proximal duodenum (A) and mid-duodenum (B). A1: high-magnification image of site of confluence (arrow) of BG into the crypt lumen (C) High-power light micrograph of cross section of BG double labeled for NKCC1 (red) and CFTR (green) arrow, apical CFTR staining, L, lumen. D: electron micrograph of ultrathin cryosection of rat BG labeled with anti-CFTR antibody and protein A gold. D1, D2: enlarged images from D. Immunogold labeling for CFTR (arrows) on the apical membrane and subapical structures beneath the lumen (L). Scale bars: A and B, 50 μm; A1: 10 μm; C, 10 μm; D, 0.25 μm.
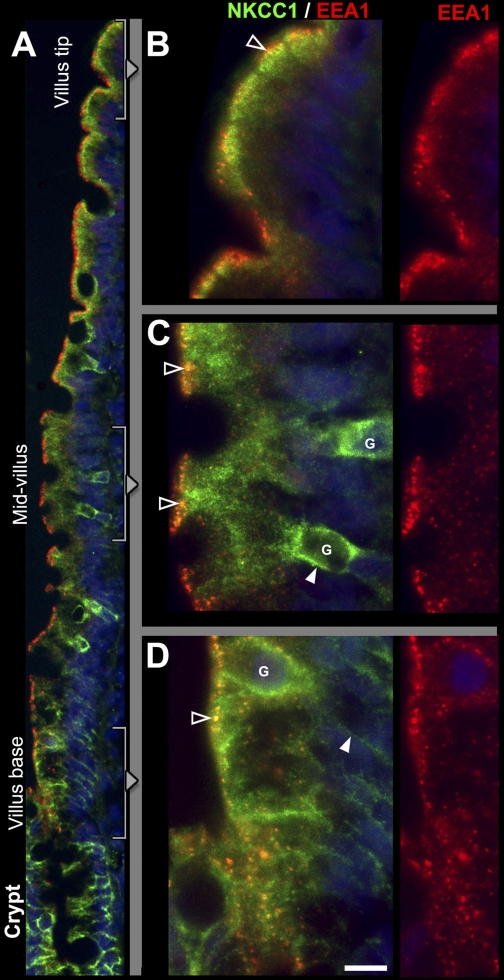
Fig. 5.
Subcellular distribution patterns of NKCC1 and EEA1-positive vesicles along the crypt-villus axis in jejunum. Sections from rat jejunum were double labeled to detect NKCC1 (green) and the early endosomal marker EEA-1 (red). A: low-power micrograph of NKCC1/EEA1 staining patterns along the crypt-villus axis. In goblet cells (G) in the mid-villus (enlarged in C) and villus base (enlarged in D), NKCC1 label is partially on the basolateral membrane (open arrowheads in C), and partially intracellular. NKCC1 is also detected in all enterocytes along the villus: at the villus base (D), enterocyte labeling is basolateral (arrowhead), but in the mid-villus (C) and villus-tip region (B), enterocyte labeling is completely intracellular. Some of the apical NKCC1 in enterocytes appear to colocalize with EEA1 (yellow; open arrowheads). Scale bar: B–D, 10 μm.
Fig. 10.
Acute carbachol-induced trafficking of CFTR, NKCC1, and NBCe1 in the jejunum. Rat jejunum was treated with carbachol (CCh) for 10 min, and tissue sections were double labeled for NBCe1 (green) or CFTR (green), and NKCC1 (red). A: images from untreated jejunum. Top: lower-magnification images of single labeling for NBCe1 and NKCC1. Bottom: enlarged merged image from superficial crypts and villus base. NKCC1 label appears largely intracellular (arrow). B–F: images of CCh treated jejunum. NBCe1 label (B), NKCC1 label (C), and merged image of NBCE1/NKCC1 double label (D). E: Neighboring section double labeled for CFTR and NKCC1. Asterisks indicate increased apical CFTR. F: high-magnification images of villus section from CCh-treated tissues double labeled for NBCe1 (green), NKCC1 (red), and merged image (right). NBCe1 (white arrowhead) and NKCC1 (open arrows) recruitment to the basolateral membranes of enterocytes is shown; NKCC1 redistribution to the basolateral membranes (open arrowhead) of goblet cells (G) is shown. Scale bars: A and F, 10 μm; B–E, 25 μm.
Fig. 8.
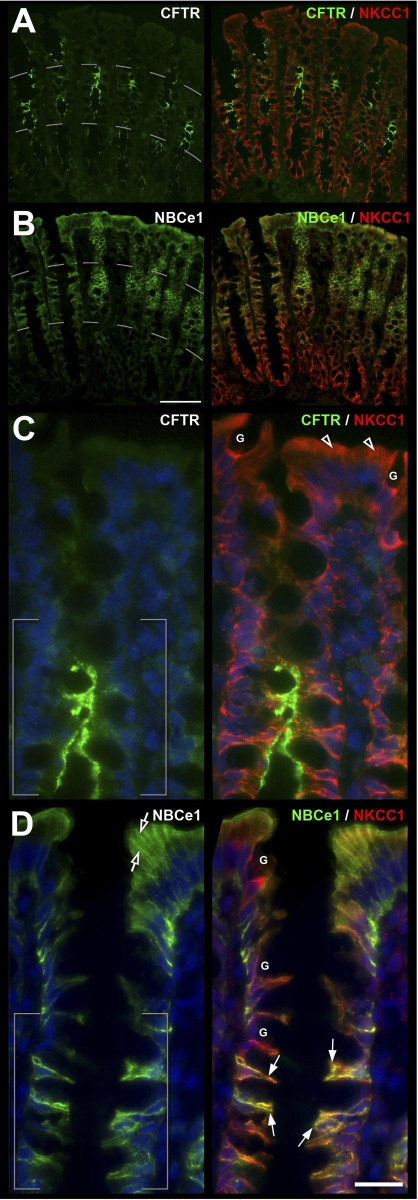
CFTR, NBCe1, and NKCC1 distribution in the proximal colon. A and B: low-magnification images of the crypt-surface epithelium double labeled for CFTR (green) or NBCe1 (green) and NKCC1 (red) shows gradients of expression. C and D: higher-magnification image of the upper two-thirds of crypt and surface epithelium shows distribution of CFTR, NBCe1, and NKCC1 in enterocytes (arrows) juxtaposed to NKCC1 expressing goblet cells (G). CFTR (C) and NBCe1 (D) appear coexpressed in the middle third crypt region (brackets). NKCC1 (open arrowheads) and NBCe1 (open arrows) staining in the upper third of the crypt and surface epithelium Scale bar: A and B: 100 μm; C and D: 25 μm.
Expression Patterns of CFTR, NBC1e, NKCC1, and NHE3 Along the Crypt-Villus Axis
CFTR.
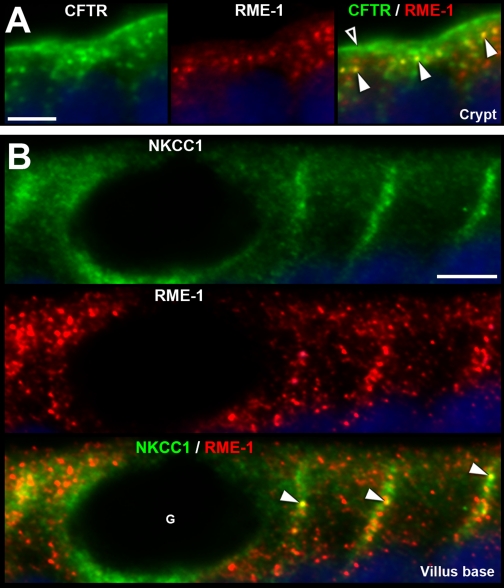
As reported previously (7, 9), the highest levels of CFTR in the intestine were found in villus CFTR High Expresser (CHE) cells (Figs. 1B, 9, A and B). In general, CFTR was detected at higher levels in crypts, and lower levels in villi along the small intestine of untreated rats. However, unexpectedly, we observed very high CFTR fluorescence intensity (FI) in the proximal duodenal villus epithelium (Figs. 1B, 2B), which appeared more intense than the crypt (Fig. 2C). To verify this observation, densitometric analysis of CFTR fluorescence intensities (FI) in proximal duodenal crypts and villi was performed. The data indicated ∼60% higher levels of CFTR on the apical membrane on the villi compared with crypts. In contrast, CFTR FI in the intracellular apical pole in villus cells was lower (∼70%) than in crypt cells (100%) (Fig. 2, B–D). Very high CFTR levels were observed in the acinar cells of the submucosal Brunner's glands (Figs. 2A, 3). Higher-magnification images of immunolabeled sections from colon revealed CFTR label was present in the crypt but not detectable in surface cells (Fig. 8, A and C). Some of the CFTR-positive subapical vesicles in villus and crypt enterocytes appeared to colocalize with EEA1, a marker of early endosomes (Fig. 6D), and with RME-1, a marker of recycling endosomes (Fig. 7A) consistent with previous observations that CFTR undergoes apical endocytosis and RME-1-dependent recycling (5, 26, 66), In contrast to the proximal duodenum, in the untreated jejunum, densitometry of CFTR fluorescence intensity (FI) on the apical membrane of enterocytes revealed a decreasing gradient from lower crypt to upper villus (Fig. 11B). The CFTR FI values (normalized to lower crypt) were as follows: lower crypt (1.0) > upper crypt (0.61) > lower villus (0.28) > upper villus (0.15).
Fig. 9.
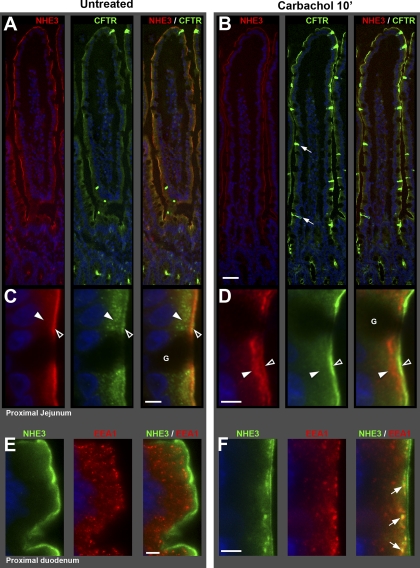
Carbachol-induced redistribution of NHE3 and CFTR in rat jejunum and duodenum. Rat proximal jejunum and proximal duodenum were treated with carbachol (CCh) for 10 min. Tissue processing and imaging were standardized (see materials and methods). A and B: NHE3 (red), CFTR (green), and NHE3/CFTR merged image taken from the villus tip to the crypts in untreated (A) and CCh-treated (B) jejunum. Small arrows point to CFTR High Expresser (CHE) cells (green). C and D: high-magnification images of apical domain of villus section from A and B. Apical brush border (open arrowheads) and subapical (solid arrowheads) localization patterns of NHE3 (red) and CFTR (green) in untreated (C) and CCh-treated (D) jejunum. Goblet cells (G). E and F: high-magnification images of villus section from proximal duodenum of untreated (E) and CCh-treated (F) double labeled for NHE3 (green) and the early endosome marker EEA1 (red); arrows denote NHE3-EEA-1 colocalization in punctate structures (yellow) below the apical brush border. Scale bars: A and B, 100 μm; C–F, 5 μm.
Fig. 6.
Subcellular distribution of NKCC1, NBCe1, and CFTR in EEA-1 positive compartments in enterocytes and goblet cells. Cryosections of proximal jejunum were double labeled with antibodies to the early endosome marker EEA1 (red) and NKCC1 (green), NBCe1 (green), or CFTR (green). A: high-magnification image of a goblet cell from the midvillus region shows intracellular staining for NKCC1 (green, arrow) and EEA1 (red, arrow) and merged image NKCC1/EEA1 colocalization (yellow, arrow). B and C: distribution of NBCe1 (green) and EEA1 (red) in enterocytes at the villus tip (B) and the villus base (C). NBCe1 staining is both membrane bound and found in EEA1-positive endosomes (arrows). D: subcellular distribution of CFTR (green) and EEA1 (red) in a jejunal crypt. High-magnification images of the highlighted region (brackets) of CFTR (green) EEA-1 (red) and merged image of EEA-1/CFTR colabel (yellow, arrows) Scale bars: A–D: 10 μm; enlarged insets in D: 5 μm.
Fig. 7.
Subcellular distribution of CFTR and NKCC1 in recycling endosomes in enterocytes. Sections of proximal jejunum were double labeled for CFTR (green) or NKCC1 (green) and the recycling endosome marker RME-1 (red). A: high-magnification images of the apical portion of a crypt show CFTR in the apical brush border (open arrowhead) and in subapical vesicles, some of which appear to colocalize with RME-1 in the subapical domain (yellow, arrowheads). B: at the villus base, NKCC1 (green) labeling is depicted in a goblet cell (G) and neighboring enterocytes. NKCC1 appears to colocalize with RME-1 recycling endosomes (yellow, arrowheads) near the lateral membrane of enterocytes (yellow, arrowheads). Scale bars: A and B, 5 μm.
NBCe1.
The distribution of NBCe1 label (green) along the crypt-villus axis of the proximal duodenum is shown in Fig. 2E. Double staining with anti-NKCC1 antibody highlights the decreasing gradient of NBCe1 distribution from the tips of the villi toward the crypt with an almost abrupt decrease in the upper third region of the crypt (Figs. 2, E and F). Lower crypt cells were generally devoid of NBCe1 label in the intestine, with the exception of the proximal duodenum, where very weak NBCe1 label was detectable (Fig. 2F). The acinar cells of the Brunner's glands exhibited very low levels of NBCe1 (Fig. 2F), compared with the robustly labeled proximal duodenal villi in the same tissue section (Fig. 1E). NBCe1 label was detected in all villus enterocytes (Figs. 1, 2E, 4, C and D, 6, B and C, 8D) and CHE cells (data not shown). NBCe1 was not detected in goblet cells and was predominantly confined to the basolateral membranes of enterocytes (Figs. 2, 4C, 6B, 10A). However, some NBCe1, particularly at the villus base, was intracellular in the apical domain of cells where it appeared to partially colocalize with early endosomes (Fig. 6C). In the upper third region of proximal colonic crypts, enterocytes exhibited strong basolateral NBCe1 labeling; in upper crypt and surface cells, NBCe1 label appeared partially intracellular, and NBCe1 label was not detected at the crypt base (Fig. 8B). Densitometry of NBCe1 FI on the lateral membrane of enterocytes in the untreated jejunum revealed an increasing gradient from lower crypt to upper villus (Fig. 11C). NBCe1 FI values (normalized to upper villus): lower crypt (0.0) < upper crypt (0.31) < lower villus (0.55) < upper villus (1.0).
Fig. 4.
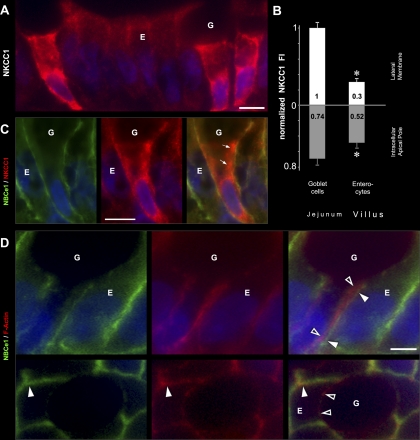
Subcellular distribution of NKCC1 and NBCe1 in villus enterocytes and goblet cells Villus sections of proximal jejunum were double labeled to detect NKCC1 (red), NBCe1 (green), and F-actin (red) using rhodamine-phalloidin. A: prominent membrane and intracellular NKCC1 staining in three goblet cells (G) among enterocytes (E). B: normalized NKCC1 fluorescence intensity (FI) at the lateral membrane and the intracellular apical pole in villus goblet cells vs. villus enterocytes. NKCC1 lateral membrane FI in enterocytes is 0.30 relative to goblet cells. Lower and upper villus cell data were combined; the individual cell group data are presented in Fig. 11E. Data shown are expressed as means ± SE. *P < 0.001; significantly different by Student's t-test. C: high-magnification image of a goblet cell (G) and neighboring enterocyte (E) double labeled for NBCe1 (green) and NKCC1 (red) and merged image. Intracellular NKCC1 in goblet cells (arrows) D: high-magnification views of double label for NBCe1/F-actin of duodenal villus enterocyte (E) and neighboring goblet cell (G) Side views (top) and en face views (bottom) show NBCe1 (green) colocalization (arrowheads) with F-Actin (red) of the enterocyte (E) but not the goblet (G) cell membrane (open arrowheads). Scale bars: A and C, 10 μm; D, 5 μm.
NKCC1 in goblet cells.
Unexpectedly, very strong label for NKCC1 was detected in all intestinal crypt and villus goblet cells (Figs. 4, 5, 8, and 10). Densitometry revealed the following relative NKCC1 FI on the lateral membrane of goblet cells (normalized to lower crypt goblet cells) in the untreated jejunum (Fig. 11E): goblet cells of lower crypt (1.0); upper crypt (0.69); lower villus (0.85); upper villus (0.73). In the villus epithelium of jejunum in untreated rats, lateral membrane NKCC1 FI was ∼3-fold higher, and intracellular NKCC1 FI was ∼1.4-fold higher in goblet cells compared with enterocytes (Figs. 4, A and B, 11E). From the villus base toward the tips, NKCC1 label appeared increasingly intracellular in goblet cells (Figs. 4C, 5C), consistent with densitometry analysis (Fig. 11E). Some intracellular NKCC1 appeared to colocalize with EEA1 in supranuclear compartments in goblet cells (Figs. 6A).
NKCC1 in enterocytes.
NKCC1 was detectable in all crypt and villus enterocytes along the intestine, including CHE cells. Densitometry of NKCC1 FI on the lateral membrane of enterocytes in the untreated jejunum, revealed a decreasing gradient from lower crypt to upper villus (Fig. 11D). The normalized NKCC1 FI values were as follows: enterocytes of lower crypt (0.96) < upper crypt (0.70) < lower villus (0.31) < upper villus (0.17). Some intracellular NKCC1 appeared to be present in EEA1-positive (Fig. 5) endosomes in the apical domain of villus enterocytes and in RME-1-positive recycling endosomes that were closely associated with the lateral cell membrane (Fig. 7B). In crypt regions and Brunner's glands, NKCC1 was mainly localized to the basolateral membrane (Figs. 2 and 3). In contrast, NKCC1 label in small intestinal villus enterocytes (Fig. 5), and colonic surface epithelium (Fig. 8) appeared predominantly in apical intracellular compartments. These intracellular localization patterns were also confirmed by densitometry from the untreated jejunum (Fig. 11D).
NHE3.
Within the crypt-villus axis of the small intestine, NHE3 label was confined to the villus epithelium and the uppermost portion of crypts (Figs. 1C and 9A). This crypt/villus pattern of distribution was remarkably similar to that of NBCe1. NHE3 label decreased sharply from the villus base toward the crypts, weak NHE3 label was detectable in upper crypt cells, but lower crypt cells were generally devoid of NHE3 label (similar to NBCe1). NHE3 was not detected in goblet cells (G; in Fig. 9, C and D), Brunner's glands, proximal and distal colon crypt cells, and CHE cells (data not shown). NHE3 immunofluorescence was detected in all villus enterocytes (Figs. 1C and 9), proximal colonic columnar cells, and at low levels, in distal colonic columnar cells (data not shown). NHE3 label was invariably confined to the apical domain (Fig. 9, A and B), where it was largely localized to the brush border membrane, but it was also present in subapical compartments (Figs. 9, C and D, and 11A). In the untreated jejunum, densitometry of relative NHE3 FI on the apical membrane of enterocytes (normalized to upper villus) was as follows: lower crypt (0.0) < upper crypt (0.52) < lower villus (0.93) < upper villus (1.0) (see Fig. 11A).
Subcellular distribution of NKCC1, CFTR, and NBCe1 in Brunner's glands.
Brunner's glands empty their contents to the crypt lumen. The site of confluence was observed within the lower third of crypts (Fig. 3A1). NKCC1 and CFTR fluorescence was very high in the Brunner's glands and the interconnected crypts (Figs. 2A and 3A). In Brunner's gland cells, NKCC1 was identified on the basolateral membranes, whereas CFTR was localized along the apical surface facing either the main lumen or the side-branches of the main lumen (Fig. 3C). In addition, punctate vesicular staining for CFTR was identified within the subapical region within cells (Fig. 3C), a feature confirmed by electron microscopy (EM) (Fig. 3D, D1, D2). At the EM level, immunogold label for CFTR was detected on apical membranes and microvilli facing the lumen and in subapical vesicles (Fig. 3D1, D2). These data confirm the high levels of apical and subapical CFTR in Brunner's glands of rat and human intestine (9, 80). NBCe1 label was very weak in Brunner's gland cells compared with villus cells of the proximal duodenum. The low level of NBCe1 in Brunner's glands (Fig. 2, E and F) contrasted with robust NKCC and CFTR staining. Staining for NHE3 was not detected (Fig. 1C).
Carbachol-Induced Redistribution of NHE3, CFTR, NBCe1, and NKCC1
Stimulation of the cholinergic gastrointestinal parasympathetic nervous system produces fluid, electrolyte, and mucus secretion (43, 69). We used the cholinergic agonist CCh to examine its effect on the subcellular distribution of the four ion transporters (Figs. 9–11). Tissues were treated with luminal CCh (10 μM) for 10, 15, or 20 min, then fixed and stained with antibodies. Within 10 min, CCh reduced apical membrane labeling of NHE3 (Fig. 9) and promoted its internalization into EEA1-labeled early endosomes (Fig. 9, E and F). In contrast, CCh increased apical membrane labeling of CFTR, and reduced CFTR label in subapical vesicles along the entire crypt-villus axis (Figs. 9, A–D, 10). Along with these changes, CCh also increased basolateral membrane labeling of NBCe1 and NKCC1 in crypt, as well as the villus epithelium (Figs. 10 and 11). CCh induced cell shrinkage (Fig. 10, A and F) in both enterocytes and goblet cells, indicating volume loss due to robust secretory activity. Densitometric analysis of fluorescence intensities (FI) for NHE3, CFTR, NKCC1, and NBCe1 (presented on the graphs of Fig. 11) revealed the following CCh-induced changes: CCh-reduced membrane NHE3 FI (relative to its FI value in untreated tissues) to 46% in upper crypt, 54% in lower villus, and 55% in upper villus (Fig. 11A). CFTR increased on the apical membrane of enterocytes ∼1.8- and ∼2.5-fold in lower and upper crypt, and ∼4- and ∼7-fold in lower and upper villus epithelium, respectively (Fig. 11B). NBCe1 was increased on the lateral membrane of enterocytes in upper crypt by ∼2.8-fold, lower villus ∼3.1-fold, and upper villus ∼1.8-fold (Fig. 11C). NKCC1 increased on the lateral membrane of enterocytes in crypt ∼1.6-fold, while in lower and upper villus ∼3.2- and 5.7-fold respectively (Fig. 11D). Finally, NKCC1 increased in goblet cell lateral membranes in lower and upper crypt by ∼1.5- and ∼1.8-fold, and in lower and upper villus ∼1.3- and ∼1.5-fold, respectively (Fig. 10E). The CCh-induced membrane recruitment of NKCC1, NBCe1, and CFTR (Figs. 9D, 10, A, D, and E), together with NHE3 internalization (Fig. 9, D and F) was observed in the same enterocytes. Notably, the CCh-induced membrane recruitment of all three transporters resulted in relatively even labeling intensities along the entire villus axis. This observation was confirmed by densitometry: in the untreated jejunum, CFTR, NBCe1, and NKCC1 FI were all significantly different in the upper villus cells (indicated by “&” on the graphs in Fig. 11) compared with the respective lower villus cells. However, in the CCh-treated jejunum, no significant differences could be detected between the lower and upper villus cell groups (indicated by “β1” and “β2” on the graphs in Fig. 11) for any of the three transporters.
As shown on the graph in Fig. 11A, the CCh-induced decrease in membrane NHE3 was accompanied by increased intracellular NHE3 in crypt and villus cell groups, providing quantitative evidence for NHE3 internalization. In a reciprocal manner, in all CFTR- and NBCe1-labeled cell groups (Fig. 11, B and C), the CCh-induced increase in membrane FI was accompanied by a concomitant decrease in intracellular FI, in support of membrane recruitment from an intracellular pool. The same membrane recruitment relationship was observed for NKCC1-labeled villus cells (Fig. 11D). However, in NKCC1-labeled crypt cells, increased membrane FI was accompanied by increased intracellular FI (Fig. 11E). As discussed later, this may indicate the ending phase of NKCC1 membrane recruitment and the beginning of NKCC1 internalization in crypt cells.
DISCUSSION
Methodological Considerations
Functional activity of CFTR, NHE3, NBCe1, and NKCC1 depends on transporter density at the cell surface that is regulated by endocytic and exocytic trafficking (8, 31, 41, 55, 65, 71, 87). Other regulatory factors include phosphorylation (29, 34, 57), protein synthesis, and short-chain fatty acids (61). Each factor is further controlled by physiological, chemical, and neural signals. While intracellular localization is valid evidence for transepithelial ion transport inactivity, membrane localization per se cannot be unconditionally viewed as a direct proof of activity. Nevertheless, membrane vs. intracellular localization has been successfully used as an indirect measure of activity for each anion transport protein examined in this study (8, 31, 41, 55, 65, 71).
NKCC1 Expression in Goblet Cells: Implications for CF
This study is the first to demonstrate that all goblet cells strongly express NKCC1 throughout the intestine. Earlier reports detected NKCC1-expressing goblet cells only in crypts (34), but recently NKCC1-expressing cells with goblet cell morphology were detected along the villus epithelium of possum ileum (15). In human airway epithelium, NKCC1 is also selectively present in goblet cells (33). As goblet cells appear abundantly equipped with the molecular machinery for basolateral Cl− uptake, and the CCh-induced membrane recruitment of NKCC1 is a morphological sign that the machinery can be switched on, the question arises what is its physiological role? The NKCC1 inhibitor bumetanide reduced (50%) the stimulatory effects of PGE2 or 5-HT on the amount of mucus released in ex vivo ileum preparations (35), suggesting that NKCC1-mediated fluid secretion from goblet cells contributes to normal mucus secretion. In HT29/B6 clone monolayers enriched (80%) in goblet cells, forskolin-induced short-circuit current increased due to Cl− secretion, and Cl− secretion was also elicited by VIP, PGE1, and dibutyryl cAMP (52). The candidate Ca2+-activated Cl− channel bestrophin 2 was not detected in small intestine, but it was expressed in colonic goblet cells and appeared to be involved in HCO3− secretion (88). The role of goblet cells in fluid secretion requires further studies. In CF goblet cells, functional NKCC1 (12) appears preserved, but stimulated NKCC1-mediated mucus release is not accompanied by CFTR-mediated fluid secretion. In the CF intestine, NKCC1-mediated overproduction and partial hydration of mucus occurs, while its CFTR/NBCe1-mediated alkalinizing mechanism is defective. This defect, accompanied by increased NHE3-mediated fluid absorption, could desiccate the overproduced mucus and account for the CF phenotype. The findings in this study support a model whereby NKCC1-expressing goblet cells can release and partially hydrate mucus, and CFTR/NKCC1/NBCe1-coexpressing enterocytes can further hydrate, alkalinize, and transform the condensed mucus to loose mucus.
Unique Ion Transporter Composition of the Proximal Duodenum and Brunner's Glands
A gradient in the rate of HCO3− secretion per unit gross area exists in the duodenum with proximal segments secreting at higher rates in rat (48) and human (47); however, its mechanism has not been clarified. The proximal duodenum (∼2 cm long in rat) is the only intestinal segment situated aborally in relation to the entrance of the alkaline pancreatic juice; thus, its epithelium together with the subepithelial Brunner's glands constitute the first lines of defense against the potentially harmful effects of stomach acid (2). Our data indicate that this unique position is reflected by a unique ion transporter composition. Among intestinal crypt regions, basolateral NKCC1 and apical CFTR levels are highest in the proximal duodenal crypts, and similarly high in the Brunner's glands confluent with these crypts. HCO3− secretion by Brunner's glands has been assumed (23), but in the absence of direct evidence (1, 2), the low levels of NBCe1 in Brunner's gland suggest a limited ability for electrogenic HCO3− secretion. The role of Brunner's gland cells in Cl− transport is currently unclear and should be tested by functional studies. However, the coordinate prominent levels of CFTR and NKCC1 in the glands and the crypts above suggest that together they can produce a robust Cl−-rich secretion to deliver the glands' mucus products to the lumen. As our data show, the villus epithelium situated above exhibits very high levels of NHE3, NBCe1, and CFTR, and this is the only villus epithelium where CFTR levels surpass the crypt. Here, high NHE3 activities can salvage excess fluid, sodium, and HCO3−. The presence of basolateral NBCe1 appears critical for supplying HCO3− to the duodenal epithelium (78). Both anion exchangers, the SLC26A3 (DRA) and the SLC26A3 (PAT), are highly expressed in proximal duodenal villus cells (75, 85, our unpublished data). In summary, the proximal duodenal villus epithelium is uniquely richly endowed with the molecular machinery to absorb fluid, as well as to produce a robust HCO3− secretion.
Ion Transporters Involved in Fluid Absorption and Stimulated Fluid Secretion are Present Together in Villus Enterocytes
This study shows that NHE3, the transporter essential for Na+ and fluid absorption, and the transporters CFTR, NKCC1, and NBCe1 involved in stimulated Cl− and HCO3− secretion are present together in all upper crypt and villus enterocytes along the small intestine. The predominantly villus localization of NHE3 in the small intestine (20, 45, 49) and NBCe1 in the duodenum (68) have been described. During basal and secretagogue-stimulated anion secretion, NBC isoforms import HCO3− (11). The relevance of electrogenic NBC (NBCe1) is supported by a HCO3− secretory defect and gross intestinal abnormalities in NBCe1-deficient mice (37), and the lack of such abnormalities in NBCn1 mice (19). The identification of CFTR in all small intestinal villus enterocytes corroborates our earlier studies (4, 7) and those of others (83), demonstrating high levels of CFTR in the mouse villus epithelium, where it can traffic to the apical membrane following secretagogue stimulation. Functional CFTR in the villus epithelium has also been shown using X-ray microanalysis of intestinal cryosections from normal and CF jejuna (63) and in BCECF studies of duodenum from normal and CF mice, demonstrating a CFTR-dependent HCO3− conductance (38). The detection of NKCC1 in all small intestinal villus enterocytes in this study is consistent with its earlier detection in jejunal villus epithelium (41) and its functional presence in villus cells (59, 62), where it contributes to cAMP- and ACh-dependent Cl− secretion (79).
CFTR and NHE3 can physically associate in the same cell, and their interaction may augment a switch to transform the cell's function from absorption to HCO3− secretion (53, 73). Indeed cAMP-induced inhibition of NHE3 activity and stimulation of HCO3− secretory activity of CFTR occurs in parallel in duodenal midvillus epithelium (38). Both apical SLC26A3 and SLC26A6 Cl−/HCO3− exchangers involved in the CFTR-dependent switch to HCO3− secretion (76) are predominantly localized to cells in the villi (75, 85). One implication of our study is that the molecular machinery (NHE3, CFTR, NBCe1, SLC26A3, and SLC26A6) essential for the dual function of fluid absorption/HCO3− secretion appears to be present in small intestinal villus enterocytes. This may be relevant for diseases that produce villus atrophy and crypt hyperplasia, such as Crohn's disease and diarrhea, which result in reduced HCO3− secretion and fluid absorption (17, 24). Considering the detection of NKCC1 in villus cells and the reciprocal carbachol effect of NHE3 internalization and CFTR/NKCC1 membrane recruitment, the other implication of the results of this study is that small intestinal villus enterocytes also appear to have the molecular machinery required for the dual function of fluid absorption/Cl− secretion. Finally, the CFTR/NKCC1/NBCe1 coexpression in proximal colonic midcrypt cells, and NKCC1/NBCe1 in colonic surface cells are consistent with previous findings that colonic crypt, as well as surface cells can perform Cl−/HCO3− secretory functions (39, 40).
Intestinal epithelial cells may retain their protein pool through their short life cycle Contrary to the detection of NKCC1 and CFTR along the entire villus epithelium in the current study, Mathews et al. (61) failed to detect NKCC1 mRNA in isolated enterocytes from the midvillus and villus tip in rat jejunum, and Trezise and Buchwald (82) reported that CFTR mRNA levels decrease along the crypt-villus axis. Limitations in sensitivity of detection systems and/or degradation of mRNA in already low-expressing epithelia may explain the lack of detection of mRNA in villus cells. However, similar discrepancies between mRNA and protein distribution for other transport proteins within the crypt-villus axis have been documented in the small intestine (13, 46, 54). It is an emerging concept that epithelial cells leaving the crypt may reduce transcribing mRNA but retain protein throughout their short (2–5 days) life cycle (14). Our data suggest that epithelial cells may retain anion transporter expression as they migrate up the villus: CFTR and NBCe1 partially, and NKCC1 predominantly in intracellular compartments, for rapid recruitment to the membrane upon stimulation.
Carbachol-Induced Membrane Trafficking
CCh can stimulate the release of ACh from mucosal axon terminals (21, 42) and can act through muscarinic (M1, M3, and M5) and nicotinic receptors on mucosal axon plexuses or basolateral muscarinic receptors in epithelial cells (27, 71, 81). This study did not resolve whether CCh's luminal actions were direct (via receptors on mucosal cells or cholinergic axons) or indirect (via receptors on mucosal noncholinergic axons). However, in vivo actions of luminal cholinergic agents have been reported: luminal CCh stimulated gastric mucus release (69); oral administration of CCh induced cholinergic symptoms (72); and luminal pilocarpin altered intestinal transmucosal potential difference (16). The lack of observed responses to luminally applied CCh in vitro (32) may be explained by dysfunction of the severed mucosal axon plexuses under in vitro experimental conditions.
The observed increase in membrane-bound anion transporters following acute CCh stimulation represent regulated intracellular trafficking, because the 10- to 20-min treatment intervals were shorter than the time (4 to 6 h) required for new protein synthesis (71). In the unstimulated intestine CFTR, NBCe1, NHE3, and NKCC1 partially localized to intracellular vesicles, including early and recycling endosomes, consistent with regulation by recycling (74). Similar to our in vivo findings, in isolated ileal cell membrane preparations, CCh rapidly decreased NHE3 in the brush border and increased its association with early endosomes, indicating acute regulation by trafficking (55).
Using human colon explants, Reynolds et al. (71) demonstrated that ACh-induced membrane trafficking of NKCC1 is a rapid, short-lived biphasic event: its membrane recruitment is detectable after 1 min in lower crypt and progresses upward, but its internalization starts in lower crypt after 10 min. In our tissues treated with CCh for 10 min, the approximately twofold increase of intracellular NKCC1 in crypt cells (Fig. 11, D and E) may also reflect the onset of NKCC1 internalization. The observed increase in membrane NKCC1 FI in association with NKCC1 internalization in crypt cells is unexpected but likely reflects the limitation of sensitivity of the methodology in precisely distinguishing membrane localization from internalized NKCC1 that is close to the lateral membrane as observed (see Fig. 7B) for NKCC1-containing recycling endosomes.
In contrast, in villus cells, NKCC1 internalization was not evident after 10 min; increased membrane levels and reduced intracellular levels were observed (Fig. 11, D and E). However, NKCC1 internalization was observed in villus cells after 20 min (unpublished data). Because ACh is a more labile molecule than CCh, the time-interval required for CCh's effects to be detected may be longer compared with that for endogenous cholinergic responses. In the colon, upon cholinergic stimulation, intercellular Ca2+ waves recruited and synchronized the activity of epithelial cells (72). The densitometry analysis in the untreated jejunum (Fig. 11) indicated significantly different levels of NKCC1, CFTR, and NBC in lower vs. upper villus. However, after CCh stimulation, all three anion transporter levels became relatively even along the villi, implying that a similar synchronization may occur along the villus axis. Further studies will be necessary to elucidate the intracellular and intercellular pathways of cholinergic anion transporter regulation.
The results from the current study provide an improved understanding of the functional synchrony of epithelial cell organization and ion transport along the crypt-villus axis. The data support a prominent role for cell-specific endocytic recycling in acutely regulating anion transport, functional cooperation between goblet cells and enterocytes, and differentiation of anion transport functions between crypt and villus epithelium that work together to maintain intestinal homeostasis. These studies also provide further elucidation of the link between anion transport and goblet cell dysfunction in CF and other intestinal diseases.
GRANTS
This study was supported by National Institutes of Health R01 DK 077065 grant to N. Ameen and a DK 34989 grant to the Digestive Diseases Research Core at Yale University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Dmitri Kravstov for reviewing the manuscript.
REFERENCES
- 1. Ainsworth M, Koss MA, Hogan DL, Isenberg JI. Higher proximal duodenal mucosal bicarbonate secretion is independent of Brunner's glands in rats and rabbits. Gastroenterology 109: 1160–1166, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol 288: C1–C19, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Alper SL, Rossmann H, Wilhelm S, Stuart-Tilley AK, Shmukler BE, Seidler U. Expression of AE2 anion exchanger in mouse intestine. Am J Physiol Gastrointest Liver Physiol 277: G321–G332, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Ameen N, Alexis J, Salas P. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem Cell Biol 114: 69–75, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998–1006, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Ameen N, Silvis M, Bradbury NA. Endocytic trafficking of CFTR in health and disease. J Cyst Fibros 6: 1–14, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ameen NA, Ardito T, Kashgarian M, Marino CR. A unique subset of rat and human intestinal villus cells express the cystic fibrosis transmembrane conductance regulator. Gastroenterology 108: 1016–1023, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Ameen NA, Martensson B, Bourguinon L, Marino C, Isenberg J. CFTR channel insertion to the apical surface in rat duodenal villus epithelial cells is upregulated by VIP in vivo. J Cell Sci 112: 887–894, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Ameen NA, van Donselaar E, Posthuma G, de Jonge H, McLaughlin G, Geuze HJ, Marino C, Peters PJ. Subcellular distribution of CFTR in rat intestine supports a physiologic role for CFTR regulation by vesicle traffic. Histochem Cell Biol 114: 219–228, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Bachmann O, Reichelt D, Tuo B, Manns MP, Seidler U. Carbachol increases Na+-HCO3− cotransport activity in murine colonic crypts in a M3-, Ca2+/calmodulin-, and PKC-dependent manner. Am J Physiol Gastrointest Liver Physiol 291: G650–G657, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Bachmann O, Rossmann H, Berger UV, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. cAMP-mediated regulation of murine intestinal/pancreatic Na+/HCO3− cotransporter subtype pNBC1. Am J Physiol Gastrointest Liver Physiol 284: G37–G45, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Bachmann O, Wuchner K, Rossmann H, Leipziger J, Osikowska B, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. Expression and regulation of the Na+-K+-2Cl− cotransporter NKCC1 in the normal and CFTR-deficient murine colon. J Physiol 549: 525–536, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balen D, Ljubojevic M, Breljak D, Brzica H, Zlender V, Koepsell H, Sabolic I. Revised immunolocalization of the Na+-d-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol 295: C475–C489, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev 22: 1856–1864, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bartolo RC, Harfoot N, Gill M, McLeod BJ, Butt AG. Secretagogues stimulate electrogenic HCO3− secretion in the ileum of the brushtail possum, Trichosurus vulpecula: evidence for the role of a Na+/HCO3− cotransporter. J Exp Biol 212: 2645–2655, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Baxter PS, Wilson AJ, Read NW, Hardcastle J, Hardcastle PT, Taylor CJ. Abnormal jejunal potential difference in cystic fibrosis. Lancet 1: 464–466, 1989 [DOI] [PubMed] [Google Scholar]
- 17. Bell S, Kamm MA. Antibodies to tumour necrosis factor alpha as treatment for Crohn's disease. Lancet 355: 858–860, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na+-HCO3− cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol Cell Physiol 278: C1200–C1211, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Bok D, Galbraith G, Lopez I, Woodruff M, Nusinowitz S, BeltrandelRio H, Huang W, Zhao S, Geske R, Montgomery C, Van Sligtenhorst I, Friddle C, Platt K, Sparks MJ, Pushkin A, Abuladze N, Ishiyama A, Dukkipati R, Liu W, Kurtz I. Blindness and auditory impairment caused by loss of the sodium bicarbonate cotransporter NBC3. Nat Genet 34: 313–319, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB. Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine. Expression and localization. J Clin Invest 93: 106–113, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown JH, Taylor P. Muscarinic receptor agonists and antagonists. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics, edited by Brunton LL. New York: McGraw-Hill, 2006, p. 183–200 [Google Scholar]
- 22. Chang EB, Rao MC. Intestinal water and electrolyte transport: mechanisms of physiological and adaptive responses. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven Press, 1994, p. 2027–2081 [Google Scholar]
- 23. Charney AN, Donowitz M. Gastrointestinal influences on hydrogen ion balance. In: Acid-Base Disorders and Their Treatment, edited by Gennari FJ AH, Galla JH, Madias NE. Boca Raton: Taylor & Francis, 2005, p. 209–240 [Google Scholar]
- 24. Ciancio MJ, Chang EB. Epithelial secretory response to inflammation. Ann NY Acad Sci 664: 210–221, 1992 [DOI] [PubMed] [Google Scholar]
- 25. Clarke LL, Stien X, Walker NM. Intestinal bicarbonate secretion in cystic fibrosis mice. JOP 2: 263–267, 2001 [PubMed] [Google Scholar]
- 26. Collaco A, Marathe J, Hoknke H, Kravstov D, Ameen NA. Syntaxin 3 is necessary for cAMP and cGMP-regulated exocytosis of CFTR: implications for enterotoxigenic diarrhea. Am J Physiol Cell Physiol 299: C1450–C1460, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cooke HJ. Neurobiology of the intestinal mucosa. Gastroenterology 90: 1057–1081, 1986 [DOI] [PubMed] [Google Scholar]
- 28. Cosen-Binker LI, Morris GP, Vanner S, Gaisano HY. Munc18/SNARE proteins' regulation of exocytosis in guinea pig duodenal Brunner's gland acini. World J Gastroenterol 14: 2314–2322, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D'Andrea L, Lytle C, Matthews JB, Hofman P, Forbush B, 3rd, Madara JL. Na:K:2Cl cotransporter (NKCC) of intestinal epithelial cells. Surface expression in response to cAMP. J Biol Chem 271: 28969–28976, 1996 [DOI] [PubMed] [Google Scholar]
- 30. de Jonge HR. The response of small intestinal villus and crypt epithelium to cholera toxin in rat. Evidence against a specific role of the crypt cells in choleragen induced secretion. Biochim Biophys Acta 381: 128–143, 1975 [DOI] [PubMed] [Google Scholar]
- 31. Del Castillo IC, Fedor-Chaiken M, Song JC, Starlinger V, Yoo J, Matlin KS, Matthews JB. Dynamic regulation of Na+-K+-2Cl− cotransporter surface expression by PKC-ε in Cl−–secretory epithelia. Am J Physiol Cell Physiol 289: C1332–C1342, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest 77: 348–354, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dolganov GM, Woodruff PG, Novikov AA, Zhang Y, Ferrando RE, Szubin R, Fahy JV. A novel method of gene transcript profiling in airway biopsy homogenates reveals increased expression of a Na+-K+-Cl− cotransporter (NKCC1) in asthmatic subjects. Genome Res 11: 1473–1483, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B. Activation of the Na-K-Cl cotransporter NKCC1 detected with a phospho-specific antibody. J Biol Chem 277: 37551–37558, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119: 2613–2622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gawenis LR, Bradford EM, Alper SL, Prasad V, Shull GE. AE2 Cl−/HCO3− exchanger is required for normal cAMP-stimulated anion secretion in murine proximal colon. Am J Physiol Gastrointest Liver Physiol 298: G493–G503, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3− cotransporter. J Biol Chem 282: 9042–9052, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Gawenis LR, Franklin CL, Simpson JE, Palmer BA, Walker NM, Wiggins TM, Clarke LL. cAMP inhibition of murine intestinal Na/H exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology 125: 1148–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Geibel JP. Secretion and absorption by colonic crypts. Annu Rev Physiol 67: 471–490, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Geibel JP, Singh S, Rajendran VM, Binder HJ. HCO3− secretion in the rat colonic crypt is closely linked to Cl− secretion. Gastroenterology 118: 101–107, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Golin-Bisello F, Bradbury NA, Ameen NA. Heat stable enterotoxin (STa) and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am J Physiol Cell Physiol 289: C708–C716, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Harrington AM, Lee M, Ong SY, Yong E, Farmer P, Peck CJ, Chow CW, Hutson JM, Southwell BR. Immunoreactivity for high-affinity choline transporter colocalises with VAChT in human enteric nervous system. Cell Tissue Res 341: 33–48, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Hirota CL, McKay DM. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol 149: 463–479, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA. CFTR mediates cAMP and calcium-activated duodenal epithelial bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol 272: G872–G878, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol Gastrointest Liver Physiol 270: G29–G41, 1996 [DOI] [PubMed] [Google Scholar]
- 46. Hwang ES, Hirayama BA, Wright EM. Distribution of the SGLT1 Na+/glucose cotransporter and mRNA along the crypt-villus axis of rabbit small intestine. Biochem Biophys Res Commun 181: 1208–1217, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Isenberg J, Hogan D, Koss MA, Selling J. Human duodenal mucosal bicarbonate secretion. Gastroenterology 91: 370–378, 1986 [PubMed] [Google Scholar]
- 48. Isenberg JI, Flemström G, Johansson C. Mucosal bicarbonate secretion is significantly greater in the proximal versus distal duodenum in the in vivo rat. In: Mechanisms of Mucosal Protection in the Upper Gastrointestinal Tract, edited by Allen A, Flemstrom G, Garner A, Silen W, Turnberg LA. New York: Raven Press, 1984, p. 175–180 [Google Scholar]
- 49. Knickelbein RG, Aronson PS, Dobbins JW. Membrane distribution of sodium-hydrogen and chloride-bicarbonate exchangers in crypt and villus cell membranes from rabbit ileum. J Clin Invest 82: 2158–2163, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kockerling A, Fromm M. Origin of cAMP-dependent chloride secretion from both crypts and surface epithelia of rat intestine. Am J Physiol Cell Physiol 264: C1294–C1300, 1993 [DOI] [PubMed] [Google Scholar]
- 51. Krause WJ. Brunner's glands: a structural, histochemical and pathological profile. Prog Histochem Cytochem 35: 259–367, 2000 [PubMed] [Google Scholar]
- 52. Kreusel KM, Fromm M, Schulzke JD, Hegel U. Cl− secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6). Am J Physiol Cell Physiol 261: C574–C582, 1991 [DOI] [PubMed] [Google Scholar]
- 53. Lamprecht G, Seidler U. The emerging role of PDZ adapter proteins for regulation of intestinal ion transport. Am J Physiol Gastrointest Liver Physiol 291: G766–G777, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Lee WS, Kanai Y, Wells RG, Hediger MA. The high affinity Na+/glucose cotransporter. Re-evaluation of function and distribution of expression. J Biol Chem 269: 12032–12039, 1994 [PubMed] [Google Scholar]
- 55. Li X, Zhang H, Cheong A, Leu S, Chen Y, Elowsky CG, Donowitz M. Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent. J Physiol 556: 791–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liou W, Geuze HJ, Slot JW. Improving structural integrity of cryosections for immunogold labeling. Histochem Cell Biol 106: 41–58, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Lytle C, Forbush B., 3rd Regulatory phosphorylation of the secretory Na-K-Cl cotransporter: modulation by cytoplasmic Cl. Am J Physiol Cell Physiol 270: C437–C448, 1996 [DOI] [PubMed] [Google Scholar]
- 58. Lytle C, Xu JC, Biemesderfer D, Forbush B., 3rd Distribution and diversity of Na-K-Cl cotransport proteins: a study with monoclonal antibodies. Am J Physiol Cell Physiol 269: C1496–C1505, 1995 [DOI] [PubMed] [Google Scholar]
- 59. MacLeod RJ, Hamilton JR. Regulatory volume increase in mammalian jejunal villus cells is due to bumetanide-sensitive NaKCl2 cotransport. Am J Physiol Gastrointest Liver Physiol 258: G665–G674, 1990 [DOI] [PubMed] [Google Scholar]
- 60. Marinelli RA, Tietz PS, Pham LD, Rueckert L, Agre P, LaRusso NF. Secretin induces the apical insertion of aquaporin-1 water channels in rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 276: G280–G286, 1999 [DOI] [PubMed] [Google Scholar]
- 61. Matthews JB, Hassan I, Meng S, Archer SY, Hrnjez BJ, Hodin RA. Na-K-2Cl cotransporter gene expression and function during enterocyte differentiation. Modulation of Cl- secretory capacity by butyrate. J Clin Invest 101: 2072–2079, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McNicholas CM, Brown CDA, Turnberg LA. Na-K-CL cotransport in villus and crypt cells from rat duodenum. Am J Physiol Gastrointest Liver Physiol 267: G1004–G1011, 1994 [DOI] [PubMed] [Google Scholar]
- 63. O'Loughlin EV, Hunt DM, Gaskin KJ, Steil D, Bruzuszcak IM, Martin HCO, Bambach C, Smith R. X-ray microanalysis of cell elements in normal and cystic fibrosis Jejunum: evidence for chloride secretion in villi. Gastroenterology 110: 411–418, 1996 [DOI] [PubMed] [Google Scholar]
- 64. Parvin M, Kurabuchi S, Murdiastuti K, Yao C, Kosugi-Tanaka C, Akamatsu T, Kanamori N, Hoso K. Subcellular redistribution of AQP5 by vasoactive intestinal polypeptide in the Brunner's gland of the rat duodenum. Am J Physiol Gastrointest Liver Physiol 288: G1283–G1291, 2005 [DOI] [PubMed] [Google Scholar]
- 65. Perry C, Quissell DO, Reyland ME, Grichtchenko II. Electrogenic NBCe1 (SLC4A4), but not electroneutral NBCn1 (SLC4A7), cotransporter undergoes cholinergic-stimulated endocytosis in salivary ParC5 cells. Am J Physiol Cell Physiol 295: C1385–C1398, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Picciano JA, Ameen N, Grant BD, Bradbury NA. Rme-1 regulates the recycling of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Cell Physiol 285: C1009–C1018, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Poulsen J, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capabilty of cystic fibrosis transmembrane conductance regulator. Physiology 91: 5340–5344, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Praetorius J, Hager H, Nielsen S, Aalkjaer C, Friis UG, Ainsworth MA, Johansen T. Molecular and functional evidence for electrogenic and electroneutral Na+-HCO3− cotransporters in murine duodenum. Am J Physiol Gastrointest Liver Physiol 280: G332–G343, 2001 [DOI] [PubMed] [Google Scholar]
- 69. Price KJ, Hanson PJ, Whittle BJ. Stimulation by carbachol of mucus gel thickness in rat stomach involves nitric oxide. Eur J Pharmacol 263: 199–202, 1994 [DOI] [PubMed] [Google Scholar]
- 70. Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 372: 415–417, 2008 [DOI] [PubMed] [Google Scholar]
- 71. Reynolds A, Parris A, Evans LA, Lindqvist S, Sharp P, Lewis M, Tighe R, Williams MR. Dynamic and differential regulation of NKCC1 by calcium and cAMP in the native human colonic epithelium. J Physiol 582: 507–524, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schulz M, Graefe T, Stuby K, Andresen H, Kupfermann N, Schmoldt A. Case report: acute unintentional carbachol intoxication. Crit Care 10: R84, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Seidler U, Singh AK, Cinar A, Chen M, Hillesheim J, Hogema B, Riederer B. The role of the NHERF family of PDZ scaffolding proteins in the regulation of salt and water transport. Ann NY Acad Sci 1165: 249–260, 2009 [DOI] [PubMed] [Google Scholar]
- 74. Silvis MR, Bertrand CA, Ameen N, Golin-Bisello F, Butterworth MB, Frizzell RA, Bradbury NA. Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol Biol Cell 20: 2337–2350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007 [DOI] [PubMed] [Google Scholar]
- 76. Singh AK, Riederer B, Chen M, Xiao F, Krabbenhoft A, Engelhardt R, Nylander O, Soleimani M, Seidler U. The switch of intestinal Slc26 exchangers from anion absorptive to HCO3− secretory mode is dependent on CFTR anion channel function. Am J Physiol Cell Physiol 298: C1057–C1065, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Singh AK, Sjoblom M, Zheng W, Krabbenhoft A, Riederer B, Rausch B, Manns MP, Soleimani M, Seidler U. CFTR and its key role in in vivo resting and luminal acid-induced duodenal HCO3− secretion. Acta Physiol (Oxf) 193: 357–365, 2008 [DOI] [PubMed] [Google Scholar]
- 78. Sjoblom M, Singh AK, Zheng W, Wang J, Tuo BG, Krabbenhoft A, Riederer B, Gros G, Seidler U. Duodenal acidity “sensing” but not epithelial HCO3− supply is critically dependent on carbonic anhydrase II expression. Proc Natl Acad Sci USA 106: 13094–13099, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Stewart CP, Turnberg LA. A microelectrode study of responses to secretagogues by epithelial cells in villus and crypt of rat small intestine. Am J Physiol Gastrointest Liver Physiol 257: G334–G343, 1989 [DOI] [PubMed] [Google Scholar]
- 80. Strong TV, Boehm K, Collins FS. Localization of the cystic fibrosis conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest 93: 347–354, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tobin G, Giglio D, Lundgren O. Muscarinic receptor subtypes in the alimentary tract. J Physiol Pharmacol 60: 3–21, 2009 [PubMed] [Google Scholar]
- 82. Trezise AEO, Buchwald M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature 353: 434–436, 1991 [DOI] [PubMed] [Google Scholar]
- 83. Tuo B, Wen G, Zhang Y, Liu X, Wang X, Dong H. Involvement of phosphatidylinositol 3-kinase in cAMP- and cGMP-induced duodenal epithelial CFTR activation in mice. Am J Physiol Cell Physiol 297: C503–C515, 2009 [DOI] [PubMed] [Google Scholar]
- 84. Valentijn JA, van Weeren L, Ultee A, Koster AJ. Novel localization of Rab3D in rat intestinal goblet cells and Brunner's gland acinar cells suggests a role in early Golgi trafficking. Am J Physiol Gastrointest Liver Physiol 293: G165–G177, 2007 [DOI] [PubMed] [Google Scholar]
- 85. Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology 136: 893–901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut 56: 1153–1163, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yu H, Riederer B, Stieger N, Boron WF, Shull GE, Manns MP, Seidler UE, Bachmann O. Secretagogue stimulation enhances NBCe1 (electrogenic Na+/HCO3− cotransporter) surface expression in murine colonic crypts. Am J Physiol Gastrointest Liver Physiol 297: G1223–G1231, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yu K, Lujan R, Marmorstein A, Gabriel S, Hartzell HC. Bestrophin-2 mediates bicarbonate transport by goblet cells in mouse colon. J Clin Invest 120: 1722–1735, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]