Abstract
Major liver resection is associated with impaired intestinal perfusion and intestinal ischemia, resulting in decreased mucosal integrity, increased bacterial translocation, and an increased risk of postoperative sepsis. However, the mechanism by which ischemia impairs intestinal mucosal integrity is unclear. We therefore evaluated the role of Ca2+-sensitive, intermediate-conductance (IKCa) basolateral potassium channels in enhanced intestinal permeability secondary to chemical hypoxia. The effects of chemical hypoxia induced by 100 μM dinitrophenol (DNP) and 5 mM deoxyglucose (DG) on basolateral IKCa channel activity and whole cell conductance in intact human colonic crypts, and paracellular permeability (GS) in isolated colonic sheets, were determined by patch-clamp recording and transepithelial electrical measurements, respectively. DNP and DG rapidly stimulated IKCa channels in cell-attached basolateral membrane patches and elicited a twofold increase (P = 0.004) in whole cell conductance in amphotericin B-permeabilized membrane patches, changes that were inhibited by the specific IKCa channel blockers TRAM-34 (100 nM) and clotrimazole (CLT; 10 μM). In colonic sheets apically permeabilized with nystatin, DNP elicited a twofold increase (P = 0.005) in GS, which was largely inhibited by the serosal addition of 50 μM CLT. We conclude that, in intestinal epithelia, chemical hypoxia increases GS through a mechanism involving basolateral IKCa channel activation. Basolateral IKCa channel inhibition may prevent or limit increased intestinal permeability during liver surgery.
Keywords: Ca2+-sensitive intermediate-conductance basolateral potassium channels
major liver resection is associated with postoperative sepsis in up to 31% of patients (15, 17, 19, 45). Subphrenic and perihepatic abscesses are the commonest form of sepsis (3, 7, 27, 33, 45), but bacteremia, biliary fistulae, wound infections, and pneumonia may also occur (19, 33). These complications increase postoperative mortality and may also reduce long-term survival (19, 33, 45). Impaired intestinal perfusion, gut barrier failure, and increased bacterial translocation are recognized events following liver resection in humans and animals (10, 16, 25, 26, 39, 42, 46). Intestinal ischemia has therefore been proposed as the key event underlying increased mucosal permeability and bacterial translocation, resulting in sepsis and multiorgan failure (36).
Epithelial barrier function depends on tight junction (TJ) integrity and the adherens junction, the two forming the apical junctional complex (AJC), a continuous circumferential cell-to-cell belt restricting diffusion of hydrophilic molecules (20). The complexes are formed by juxtaposition of transmembrane protein subunits from adjacent cells, linking in zipper-fashion to form a continuous barrier. TJ proteins include the claudins, occludin, and JAM, whereas the AJC is made up of cadherins. These integral membrane proteins are associated with cytosolic proteins such as zonula occludens (ZO)-1, -2, and -3 and α- and β-catenin that form a cytoplasmic protein plaque, stabilized by connection to the actin cytoskeleton. The phosphoamino acid content and junctional localization of occludin and ZO-1 are regulated by Rho-GTPase and increase with constitutive Rho signaling. Constitutive Rho signaling also protects against ischemia-induced changes in TJ integrity, whereas inhibition has the opposite effect, suggesting that inhibition of Rho is directly involved in ischemia-induced injury (13).
The first clue that intestinal permeability might be linked to basolateral membrane K+ channel activity arose from a study using confluent monolayers of cultured T84 colonic adenocarcinoma cells, in which the G protein agonist mastoparan caused a fourfold increase in paracellular conductance (permeability) (GS), rearrangement of F-actin, and stimulation of basolateral K+ channels (1). These changes were inhibited by the “serosal” addition of Ba2+ ions, which suggested that the mastoparan-induced increase in paracellular permeability reflected basolateral K+ channels activation. Furthermore, mastoparan caused sequestration and a reduction in the activity of Rho, similar to the changes seen during ischemia. Ischemia also stimulates K+ channel activity in a variety of nonintestinal tissues (14, 22, 34, 40, 41). The most abundant type of basolateral K+ channel in human colonic crypt cells is Ca2+-sensitive, intermediate-conductance K+ (IKCa) channels (21, 31). Here we show that chemical hypoxia activates basolateral IKCa channels in human colonic crypts, while simultaneously increasing the permeability of the colonic epithelium. Furthermore, the increase in colonic permeability induced by chemical hypoxia is prevented by IKCa channel inhibition, which points to a novel approach for preventing the detrimental effects of enhanced intestinal permeability during major liver surgery.
METHODS
Isolation of human colonic crypts.
After obtaining informed, written consent, we isolated colonic crypts from four to six endoscopic biopsies removed from the sigmoid colon during routine colonoscopy or flexible sigmoidoscopy in patients being investigated for abdominal pain or iron deficiency anemia, in whom there were no macroscopic mucosal abnormalities and routine histology of the mucosa was subsequently found to be normal. The study was approved by the Leeds Teaching Hospitals Ethics Committee. Biopsies were processed to provide isolated, intact colonic crypts by a Ca2+ chelation technique, as described previously (2).
Patch-clamp studies.
Recordings were made from basolateral membrane patches of cells in the mid third of the crypts in the cell-attached and perforated whole cell configurations. Patch pipettes were fabricated from borosilicate glass microhematocrit tubes by using a two-stage pipette puller (Narashige) and had tip resistances of 2–5 MΩ when filled with a solution containing (in mM) 145 KCl, 1.2 CaCl2, 1.2 MgCl2, and 10 HEPES. The bathing solution contained (in mM) 4.5 KCl, 140 NaCl, 1.2 CaCl2, 1.2 MgCl2, 10 HEPES, and 5 glucose. Experiments were carried out at room temperature (20–22°C) to maintain cellular viability (35). Single channel and whole cell currents were recorded by use of a patch-clamp amplifier (Axopatch 200B, Axon Instruments). Currents were low-pass filtered at 2 kHz, sampled at 5 kHz, displayed on computer monitor, and saved to hard drive for later analysis using pCLAMP version 9.0 software (Axon Instruments).
Baseline cell-attached recordings were made until steady-state channel activity was observed. Chemical hypoxia was then induced by superfusion of crypts with the bath solution containing 100 μM dinitrophenol (DNP) to inhibit oxidative phosphorylation and 5 mM deoxyglucose (DG) to inhibit glycolysis. Channel open probability (NPo, where N was the maximum number of channels observed in a patch and Po was the single-channel open probability) was calculated by using patch-clamp software (pCLAMP 8.0, Axon Instruments). The effects of metabolic inhibitors on K+ channel activity were evaluated by comparing steady-state NPo values under baseline conditions and following the addition of metabolic inhibitors.
Whole cell K+ currents were measured after permeabilization of the basolateral membrane to allow electrical access to the cell interior, by using the pore-forming agent amphotericin B in the pipette solution (final concentration 240 μg/ml) (8). Whole cell recordings were obtained while crypts were superfused with bath solution, first in the absence and then in the presence of 100 μm DNP and 5 mM DG. The effects of the specific IKCa channel inhibitors 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34) and clotrimazole (CLT) on whole cell K+ currents were also investigated by adding 100 nM TRAM-34 or 10 μM CLT to the bath solution containing 100 μM DNP and 5 mM DG. Linear regression analysis (Microsoft Excel) was used to determine the whole cell conductance from the slope of the linear portion of the relationship between the whole cell current (nA) and the command voltage (mV). The reversal potential of the cell was determined from the x-axis intercept of the current-voltage plot.
Measurement of colonic permeability.
We obtained sigmoid colon from patients undergoing resection for colonic cancer after first obtaining their informed, written consent. Segments of tissue 10 cm in length were taken at least 10 cm from the cancer, and sheets of mucosa stripped of their underlying serosa and muscle were prepared as described previously (30). Each mucosal sheet was divided into two equal parts and mounted in paired Ussing chambers. Tissues (area 0.5 cm2) were bathed on the mucosal side with a solution containing (in mM) 114 K+ gluconate, 25 KHCO3, 1.2 KH2PO4, 10 Ca2+ methane sulfonate, 1.2 MgSO4, and 11 glucose, and on the serosal side with a solution containing 113 Na+ gluconate, 25 NaHCO3, 5.8 K+ gluconate, 1.2 KH2PO4, 10 Ca2+ methane sulfonate, 1.2 MgSO4, and 11 glucose. Both solutions were gassed with 95% O2 and 5% CO2 and maintained at 37°C (30). Under open-circuit conditions, transepithelial voltage (VT) was monitored with KC1–4% agar bridges, and Ag-AgCl electrodes placed at opposite ends of the chamber were used to pass 2.5-s rectangular current pulses (I; = 50 μA) across the tissue. Measurements of VT, total tissue conductance (GT; = I/ΔVT), and calculated short-circuit current (Isc; = VT·GT) were obtained when they had reached constant values. Chemical hypoxia was induced in one of the paired tissues by adding 100 μM DNP to the nongassed mucosal and serosal solutions. The other tissue acted as a “control” tissue and was gassed constantly with 95% O2 and 5% CO2.
After VT and Isc had reached steady-state values, the paracellular permeability of both tissues was determined by the nystatin technique (43). Nystatin (Sigma Chemical, St. Louis, MO; final concentration 500 U/ml) was added to the mucosal solution to permeabilize the apical membrane to monovalent ions, resulting in marked increases in VT, Isc, and GT, which were monitored at 15-s intervals until steady-state values were achieved. Under these experimental conditions, the increased values of VT and calculated Isc after apical membrane permeabilization reflected current flow as K+ moved across the basolateral membrane down its concentration gradient. The relationship between GT and Isc was linear, as described by the equation
where EBl was the electromotive force across the basolateral membrane and GS was the shunt or paracellular conductance. GS was estimated from the y-axis intercept of the linear relationship between GT and Isc (43).
In separate series of experiments, we investigated the effects of the IKCa channel blockers TRAM-34 and CLT on paracellular permeability during chemical hypoxia using the nystatin permeabilization method described above, having first added 100 nM TRAM-34 or 20–50 μM CLT to the serosal solution of both control tissues (gassed with 95% O2 and 5% CO2) and tissues exposed to 100 μM DNP (nongassed).
Statistics.
Results are shown as means ± SE. Comparisons were made using Student's t-test for paired data, P < 0.05 indicating a statistical difference between means.
RESULTS
Effect of chemical hypoxia on IKCa channel activity.
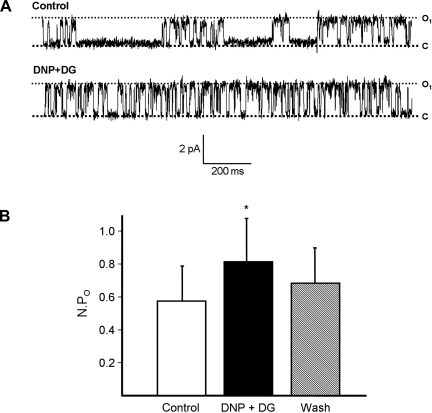
IKCa channels dominate the basolateral membrane conductance of human colonic crypt cells and have previously been characterized in detail (21, 31). Initial experiments were done to determine the response of IKCa channels to chemical hypoxia. In cell-attached basolateral membrane patches on cells in the mid third of crypts (n = 10 crypts from 4 patients), chemical hypoxia induced by adding 100 μM DNP and 5 mM DG stimulated IKCa channel activity (Fig. 1A), NPo increasing from 0.58 ± 0.21 to a new steady-state value of 0.81 ± 0.26 within 2–3 min (P = 0.04), and the subsequent washout of DNP and DG resulted in partial reversal of the effect, NPo decreasing to 0.70 ± 0.22 (Fig. 1B).
Fig. 1.
Chemical hypoxia activates basolateral intermediate-conductance (IKCa) channels. A: representative recording showing channel activation by 100 μM dinitrophenol (DNP) and 5 mM deoxyglucose (DG) [command voltage = 100 mV; channel openings indicated by current transitions between closed channel current (C) and open state (O1)]. B: summary of effect of DNP and DG, and subsequent washout (Wash), on channel activity [channel open probability (NPo)] (n = 10, *P = 0.04 compared with control).
Effect of chemical hypoxia on whole cell conductance.
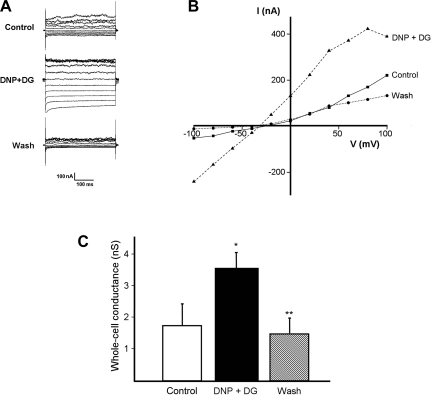
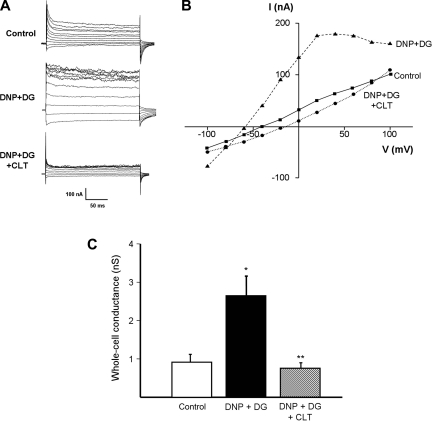
In whole cell perforated basolateral membrane patches (n = 7 crypts from 7 patients), the addition of 100 μM DNP and 5 mM DG increased whole cell conductance from 1.74 ± 0.63 to 3.54 ± 0.47 nS (P = 0.004; Fig. 2C) within 2–3 min (Fig. 3), which was accompanied by a shift in the cell reversal potential from −58 ± 8 to −74 ± 4 mV (P = 0.03) consistent with K+ channel activation; results from a representative experiment are shown (Fig. 2, A and B). These electrical changes reversed completely after washout of DNP and DG (Fig. 2, B and C).
Fig. 2.
Chemical hypoxia increases whole cell conductance. A: representative experiment showing effect of 100 μM DNP and 5 mM DG, and subsequent washout, on whole cell currents. B: current (I)-voltage (V) relationship using data from A, showing that DNP and DG increased whole cell conductance (I/V) and hyperpolarized the cell membrane. C: summary of effect of DNP and DG, and subsequent washout, on whole cell conductance (n = 7; *P = 0.005 compared with control, **P = 0.05 compared with DNP and DG).
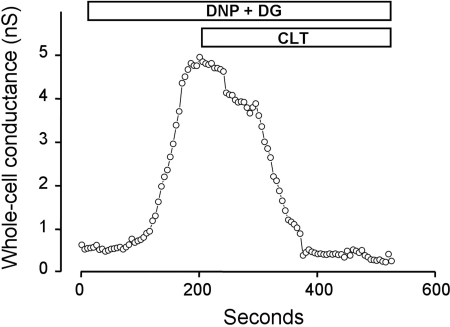
Fig. 3.
Representative data showing time course of stimulation of whole cell conductance by 100 μM DNP and 5 mM DG and subsequent inhibitory effect of 10 μM clotrimazole (CLT).
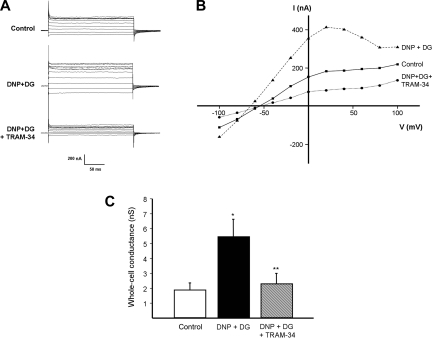
In further experiments (n = 6 crypts from 6 patients), we studied the effects of the highly specific IKCa channel blocker TRAM-34 (100 nM) on the increase in whole cell conductance induced by DNP and DG. As before (Fig. 2), the addition of DNP and DG increased whole cell conductance from 1.88 ± 0.5 to 5.43 ± 1.2 nS (P = 0.03; Fig. 4C), whereas cells hyperpolarized from −69 ± 4 to −80 ± 1 mV (P = 0.006); results from a representative experiment are shown (Fig. 4, A and B). The subsequent addition of TRAM-34 decreased whole cell conductance from 5.43 ± 1.2 to 2.28 ± 0.7 nS (P = 0.01; Fig. 4C) and depolarized cells from −80 ± 1 to −69 ± 4 mV (P = 0.04).
Fig. 4.
1-[(2-Chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34) inhibits increase in whole cell conductance induced by chemical hypoxia. A: representative experiment showing effect of 100 μM DNP and 5 mM DG, and subsequent addition of 100 nM TRAM-34, on whole cell currents. B: current-voltage relationship using data from A, showing that DNP and DG increased whole cell conductance and hyperpolarized the cell membrane, whereas these effects were completely inhibited by TRAM-34. C: summary of effect of DNP and DG, and subsequent addition of 100 nM TRAM-34, on whole cell conductance (n = 6; *P = 0.03 compared with control, **P = 0.01 compared with DNP and DG alone).
We also studied the effect of CLT (10 μm), an IKCa channel blocker with potential clinical efficacy. In this set of experiments (n = 7 crypts from 7 patients), the addition of DNP and DG increased whole cell conductance from 0.91 ± 0.2 to 2.65 ± 0.5 nS (P = 0.02; Fig. 5C), whereas the cell hyperpolarized from −55 ± 7 to −74 ± 3 mV (P = 0.01); results from a representative experiment are shown (Fig. 5, A and B). The addition of CLT completely inhibited the DNP and DG-enhanced whole cell conductance (Fig. 3), which decreased from 2.65 ± 0.5 to 0.75 ± 0.15 nS (P = 0.006, Fig. 5C), whereas there was an accompanying cell depolarization from −74 ± 3 to −54 ± 10 mV (P = 0.07).
Fig. 5.
CLT inhibits increase in whole cell conductance induced by chemical hypoxia. A: representative experiment showing effect of 100 μM DNP and 5 mM DG, and subsequent addition of 10 μM CLT, on whole cell currents. B: current-voltage relationship using data from A, showing that DNP and DG increased whole cell conductance and hyperpolarized the cell membrane, whereas these effects were completely inhibited by CLT. C: summary of effect of DNP and DG, and subsequent addition of 10 μM CLT, on whole cell conductance (n = 7; *P = 0.02 compared with control, **P = 0.006 compared with DNP and DG alone).
Effect of chemical hypoxia on colonic paracellular permeability.
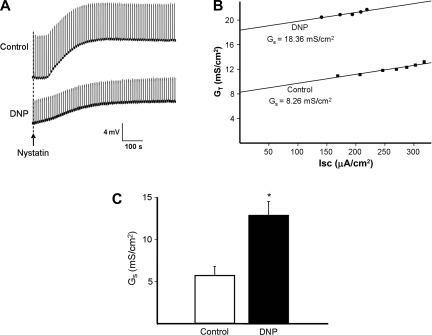
Under open-circuit conditions, colonic paracellular permeability in pairs of tissue was evaluated by estimating GS from the y-intercept of the linear plot of the relationship between GT and Isc following the addition of nystatin (43). As shown in a representative experiment (Fig. 6A), addition of nystatin to the mucosal chamber (containing K+ gluconate solution) resulted in a marked and sustained increase in VT in the control tissue, whereas the increase in the paired DNP-pretreated tissue was less pronounced. In both cases, the steady-state postnystatin VT reflected K+ current across the basolateral membrane (Na+ gluconate solution in the serosal chamber). It should be noted that transient deflections in the VT traces (corresponding to the 2.5 s rectangular current pulses) were smaller in the DNP-pretreated tissue than in its paired control, consistent with a DNP-induced increase in GT (and, by inference, GS), as confirmed by the relationship between GT and Isc (Fig. 6B). In six such experiments, pretreatment with DNP resulted in a twofold increase in GS from 5.7 ± 1.1 to 12.8 ± 1.7 mS/cm2 (P = 0.005).1
Fig. 6.
Chemical hypoxia increases paracellular conductance (GS). A: representative experiment showing increases in transepithelial voltage (VT) after nystain-induced apical membrane permeabilization in control and paired DNP-treated colon. B: plots of total tissue conductance (GT) against short-circuit current (Isc; corrected for tissue area) obtained from A, the y-intercepts providing estimates of GS. C: summary of effect of DNP on GS (n = 6 pairs of tissue, *P = 0.005 compared with control).
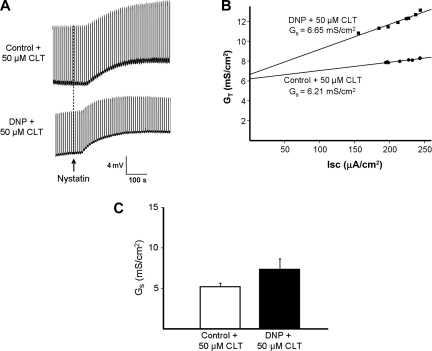
To determine whether IKCa channel blockade attenuates the increase in colonic paracellular permeability induced by chemical hypoxia, a similar experimental protocol was used, except that 50 μM CLT was added to the serosal solution bathing control and DNP-pretreated tissues. In the presence of CLT, the increase in VT following nystatin was similar in the control and DNP-pretreated tissues (Fig. 7A), and CLT prevented the increase in GS evoked by DNP pretreatment (compare Figs. 7B and 6B). In five experiments in which serosal CLT was present throughout, there was only a small and insignificant difference in GS between control and DNP-pretreated tissues (5.2 ± 0.45 and 7.4 ± 1.2 mS/cm2, P = 0.22).2 Similar results were obtained with use of 20 μM CLT (n = 4, P = 0.99). These results demonstrate that IKCa channel blockade by CLT largely prevents the increase in colonic paracellular permeability induced by chemical hypoxia.
Fig. 7.
CLT inhibits increase in paracellular conductance (GS) induced by chemical hypoxia. A: representative experiment showing increases in VT after nystain-induced apical membrane permeabilization in control and paired DNP-treated colon in the presence of 50 μM CLT. B: plots of GT against Isc (corrected for tissue area) obtained from A, the y-intercepts providing estimates of GS. CLT inhibited the DNP-induced increase in GS shown in Fig. 6. C: summary showing that CLT largely prevented the increase in GS induced by DNP (n = 5 pairs of tissue, P = 0.22 compared with control).
DISCUSSION
To the best of our knowledge, the present study is the first to report that metabolic stress secondary to chemical hypoxia elicits a rapid increase in IKCa channel activity in the basolateral membranes of native human intestinal epithelial cells. Metabolic stress has previously been shown to activate different types of K+ channel in cholangiocytes and hepatocytes (40, 41), vascular cells (22, 34), and airway cells (14). However, the magnitude of the effects we observed in human colonic crypt cells (45% and ∼2-fold increases in IKCa channel activity and whole cell K+ conductance, respectively) were much smaller than those reported previously in human hepatoma and biliary cell lines, in which there were 40- to 70-fold increases in whole cell K+ conductance (40, 41). On the other hand, we observed more sustained responses to chemical hypoxia in human colonic crypt cells compared with those seen in the cell lines. These differences may reflect differences in the inherent transport functions between these cells, since cholangiocytes are primarily secretory (40) whereas colonocytes are primarily absorptive (29). There may also be cell-specific differences between the intracellular regulatory pathways that determine K+ channel responses to chemical hypoxia.
The ability of K+ channel activity to regulate intestinal permeability has been demonstrated previously in cultured monolayers of T84 human colonic adenocarcinoma cells and airway epithelia (1, 44). Other ion channels also appear to modulate intestinal permeability, since the ability of ischemia to increase the permeability of porcine ileum was largely reversed by adding the ClC-2 (Cl−) channel agonist lubiprostone to the mucosal surface (23). In addition, the Na+/H+ exchanger isoform 2 (NHE2) has been implicated in the regulation of intestinal barrier function, the mucosal application of HOE-694 (a specific NHE2 inhibitor) to ischemic tissue resulting in rapid and significant increases in transepithelial resistance (24).
The studies with the nystatin-permeabilized human colonic sheets indicate that chemical hypoxia resulted in an increase in paracellular permeability, as judged by the increase in estimated GS. Furthermore, the hypoxia-induced increase in GS was almost completely prevented by serosally applied CLT, an inhibitor of IKCa channels. It should be emphasized that in the presence of a serosally directed K+ gradient, transepithelial but not microelectrode estimates of GS will be influenced by an electromotive force in the paracellular shunt pathway if this is K+ selective (43). However, in nystatin-treated rabbit distal colon, there was no significant difference between the transepithelial and microelectrode estimates of GS in the presence of a serosally directed K+ gradient (43). Furthermore, similar studies performed in human distal colon indicated identical transepithelial and microelectrode estimates of GS (1.6 ± 0.2 and 1.6 ± 0.3 mS/cm2, respectively) (30). These two sets of observations indicate that the paracellular shunt pathway of colonic epithelium has no significant K+ selectivity and therefore there would be no significant paracellular electromotive force under the experimental conditions used in the present study. Thus our data provide strong evidence that chemical hypoxia increases paracellular conductance (or permeability) and presumably decreases TJ integrity, through a mechanism involving IKCa channel activation. Although the exact relationship between chemical hypoxia, IKCa channel activity and TJ integrity is unclear, it is possible that TJ conformation in native human colonic epithelium changes in response to cell membrane hyperpolarization following the activation of IKCa channels. However, any link between cell membrane potential and TJ integrity may be tissue specific, given that cell membrane depolarization activated the Rho-ROK signaling pathway leading to MLC phosphorylation in LLC-PK1 kidney tubular cells (37) and MLC phosphorylation increased TJ permeability in malignantly transformed Caco-2 intestinal monolayers (38). Additional studies are therefore required to determine whether cell membrane potential and MLC phosphorylation have interdependent roles in TJ regulation during chemical hypoxia in human intestinal epithelia.
In the context of the present study, it is important to distinguish between two entirely separate types of intestinal barrier dysfunction (11). The first involves translocation of particulate antigens (including bacteria) via the transcellular route. The second involves an increase in paracellular permeability (as elicited by chemical hypoxia in our experiments), which raises the question of whether such a change could lead to increased bacterial migration across the intestinal epithelium. This possibility is supported by several studies; for example, Salmonella typhi Ty2 crossed Caco-2 enterocyte monolayers via the paracellular route, whereas other Salmonella crossed transcellularly (18). In addition, Clostridium difficile toxin A and toxin B both changed cytoskeletal actin, decreased transepithelial resistance, and increased paracellular migration of Salmonella typimurium, Escherichia coli, and Proteus mirabilis across Caco-2 monolayers (9). The importance of enhanced paracellular access as a possible route for bacterial invasion of the intestinal epithelium has also been highlighted by studies in human colonic T84 cell monolayers, which showed that apically applied Shigella flexneri moved across monolayers via paracellular pathways, but only after they had been opened by the migration of basally applied polymorphonuclear leukocytes (28).
From a clinical standpoint, it is noteworthy that CLT inhibited both the increase in IKCa channel activity and the increase in paracellular conductance elicited by chemical hypoxia in human colon. This raises the possibility that IKCa channel blockade may be a novel and effective therapeutic approach for limiting increased intestinal permeability secondary to intraoperative intestinal ischemia. CLT is commonly used to treat systemic and cutaneous fungal infections and is relatively free of side effects. It has also been shown to act as an antisickling agent by inhibiting erythrocyte IKCa channels in healthy volunteers and in patients with sickle cell disease (4–6). However, parenteral administration of CLT in the surgical setting may have unpredictable and possibly hazardous effects, particularly on liver function (6). On the other hand, somatostatin peptides produce rapid, substantial inhibition of basolateral IKCa channels in human colonic crypt cells (32), and parenteral octreotide (a synthetic analog of somatostatin) is already widely used to control secretory diarrhea (12). Octreotide may therefore be worthy of evaluation as a safe and convenient means of limiting or preventing ischemia-induced increases in intestinal permeability during major liver resection.
GRANTS
This research was supported by a project grant from the Leeds Teaching Hospitals National Health Service Trust Charitable Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Footnotes
The value of GS in control tissues obtained from transepithelial measurements under open-circuit conditions (5.7 ± 1.1 mS/cm2) was greater than that previously reported in human sigmoid colon by using intracellular microelectrode measurements, also under open-circuit conditions (1.6 ± 0.3 mS/cm2) (30). The reason for this discrepancy is unclear, but under open-circuit conditions part of the transepithelial potential could be dissipated by backleakage of cations across TJs, resulting in estimates of GS higher than those obtained under short-circuit conditions (where there is no current flow through TJs). This hypothesis was tested in 11 pairs of tissues by estimating GS from transepithelial measurements under short-circuit conditions in the absence and in the presence of DNP. GS in the absence of DNP was 4.3 ± 0.4 mS/cm2 and increased twofold (to 8.7 ± 0.9 mS/cm2; P = 0.00025) in the presence of DNP. Using two pairs of tissues from each of three colons, we also studied the effect of DNP on GS under short-circuit and open-circuit conditions. Under short-circuit conditions, estimated values of GS were greater in the presence than in the absence of DNP (12.9 ± 1.8 vs. 4.8 ± 0.3 mS/cm2; P = 0.047) and were lower than those obtained under open-circuit conditions (19.5 ± 1.8 vs. 13.8 ± 1.1 mS/cm2; P = 0.013). Taken together, these data indicate that, although estimates of GS under short-circuit conditions were generally lower than those under open-circuit conditions, DNP-induced chemical hypoxia resulted in significant increases in GS in both cases.
Data from individual tissues were analyzed to estimate basolateral membrane conductance (Gbl). Assuming that nystatin fully permeabilized the apical membrane, then GT = Gbl + GS and Gbl can be estimated as the difference between GS and the maximum values of GT (see Figs. 6B and 7B). There were no significant differences in the mean values of Gbl between control and DNP-treated tissues, either in the absence of CLT (4.84 ± 1.1 and 3.96 ± 2.0 mS/cm2, respectively) or in the presence of CLT (4.24 ± 0.8 and 5.1 ± 1.3 mS/cm2, respectively). Although the single-channel data (Fig. 1) showed that chemical hypoxia clearly activated basolateral IKCa channels, it would appear that this was insufficient to register as a change in estimated Gbl obtained from the transepithelial data.
REFERENCES
- 1. Blumenstein I, Gerhard R, Ries J, Kottra G, Stein J. Regulation of mastoparan-induced increase of paracellular permeability in T84 cells by RhoA and basolateral potassium channels. Biochem Pharmacol 65: 1151–1161, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bowley KA, Morton MJ, Hunter M, Sandle GI. Non-genomic regulation of intermediate conductance potassium channels by aldosterone in human colonic crypt cells. Gut 52: 854–860, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brancatisano R, Isla A, Habib N. Is radical hepatic surgery safe? Am J Surg 175, 161–163, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Brugnara C, Armsby CC, Sakamoto M, Rifai N, Alper SL, Platt O. Oral administration of clotrimazole and blockade of human erythrocyte Ca2+-activated K+ channel: the imidazole ring is not required for inhibitory activity. J Pharmacol Exp Ther 273: 266–272, 1995 [PubMed] [Google Scholar]
- 5. Brugnara C, De Franceschi L, Alper SL. Inhibition of Ca2+-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J Clin Invest 92: 520–526, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brugnara C, Gee B, Armsby CC, Kurth S, Sakamoto M, Rifai N, Alper SL, Platt O. Therapy with oral clotrimazole induces inhibition of the Gardos channel and reduction of erythrocyte dehydration in patients with sickle cell disease. J Clin Invest 97: 1227–1234, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doci R, Gennari L, Bignami P, Montalto F, Morabito A, Bozzetti F, Bonalumi MG. Morbidity and mortality after hepatic resection of metastases from colorectal cancer. Br J Surg 82: 377–381, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors. Proc Natl Acad Sci USA 92: 9298–9302, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feltis BA, Kim AS, Kinneberg KM, Lyerly DL, Wilkins TD, Erlandsen SL, Wells CL. Clostridium difficile toxins may augment bacterial penetration of intestinal epithelium. Arch Surg 134: 1235–1242, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Ferri M, Gabriel S, Gavelli A, Franconer IP, Huguet C. Bacterial translocation during portal clamping for liver resection. A clinical study. Arch Surg 132: 162–165, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Fink MP. Effect of critical illness on microbial translocation and gastrointestinal mucosal permeability. Semin Respir Infect 9: 256–260, 1994 [PubMed] [Google Scholar]
- 12. Geoghegan J, Pappas TN. Clinical uses of gut peptides. Ann Surg 225: 145–154, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gopalakrishnan S, Raman N, Atkinson SJ, Marrs JA. Rho GTPase signalling regulates tight junction assembly and protects tight junctions during ATP depletion. Am J Physiol Cell Physiol 275: C798–C809, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Groschner K, Silberberg SD, Gelband CH, Van Breemen C. Ca2+-activated K+ channels in airway smooth muscle are inhibited by cytoplasmic adenosine triphosphate. Pflügers Arch 417: 517–522, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Jarnagin WR, Gonen M, Fong Y, Dematteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 236: 397–406, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kasravi FB, Wang L, Wang XD, Molin G, Bengmark S, Jeppson B. Bacterial translocation in acute liver injury induced by d-galactosamine. Hepatology 23: 97–103, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Kim ST, Kim KP. Hepatic resections for primary liver cancer. Cancer Chemother Pharmacol 33: S18–S23, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Kops SK, Lowe DK, Bement WM, West AB. Migration of Salmonella typhi through intestinal epithelia monolayers: an in vitro study. Microbiol Immunol 40: 799–811, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Laurent C, Sa Cunha A, Couderc P, Rullier E, Saric J. Influence of postoperative morbidity on long-term survival following liver resection for colorectal metastases. Br J Surg 90: 1131–1136, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Lee DBN, Huang E, Ward HJ. Tight junction biology and kidney dysfunction. Am J Physiol Renal Physiol 290: F20–F34, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Lomax RB, Warhurst G, Sandle GI. Characteristics of two basolateral potassium channel populations in human colonic crypts. Gut 38: 243–247, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller AL, Morales E, Leblanc NR, Cole WC. Metabolic inhibition enhances Ca2+-activated K+ current in smooth muscle cells of rabbit portal vein. Am J Physiol Heart Circ Physiol 265: H2184–H2195, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Moeser AJ, Nighot PK, Engelke KJ, Ueno R, Blikslager AT. Recovery of mucosal barrier function in ischemic porcine ileum, and colon is stimulated by a novel agonist of the CIC-2 chloride channel, lubiprostone. Am J Physiol Gastrointest Liver Physiol 292: G647–G656, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Moeser AJ, Nighot PK, Ryan KA, Wooten JG, Blikslager AT. Prostaglandin-mediated inhibition of Na+/H+ exchanger isoform 2 stimulates recovery of barrier function in ischemia-injured intestine. Am J Physiol Gastrointest Liver Physiol 291: G885–G894, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Narioka J, Nishi M, Ogata Y, Kuwahara T, Nakayama H, Tashiro S, Ohnishi Y. Promotion of bacterial translocation by major liver resection in obstructive jaundice in rats colonised predominantly with indigenous Escherichia coli. J Med Microbiol 51: 687–694, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Okay E, Karadenizl A, Muezzinoglu B, Zeybek U, Arzu Ergen H, Isbir T. N-acetylcysteine attenuates bacterial translocation after partial hepatectomy in rats. J Surg Res 127: 164–170, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Pace RF, Blenkharn JI, Edwards WJ, Orloff M, Blumgart LH, Benjamin IS. Intra-abdominal sepsis after hepatic resection. Ann Surg 209: 302–306, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perdomo JJ, Gounon P, Sansonetti PJ. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J Clin Invest 93: 633–643, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sandle GI. Salt and water absorption in the human colon: a modern appraisal. Gut 43: 294–299, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandle GI, McGlone F. Segmental variability of membrane conductances in rat and human colonic epithelia. Implications for Na, K and Cl transport. Pflügers Arch 410: 173–180, 1987 [DOI] [PubMed] [Google Scholar]
- 31. Sandle GI, McNicholas CM, Lomax RB. Potassium channels in colonic crypts. Lancet 343: 23–25, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Sandle GI, Warhurst G, Butterfield I, Higgs NB, Lomax RB. Somatostatin peptides inhibit basolateral potassium channels in human colonic crypts. Am J Physiol Gastrointest Liver Physiol 277: G967–G975, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Shigeta H, Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, Hayakawa N, Kanai M, Nimura Y. Bacteremia after hepatectomy: an analysis of a single-center, 10-year experience with 407 patients. Langenbecks Arch Surg 387: 117–124, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Silberberg SD, Van Breemen C. A potassium current activated by lemakalim and metabolic inhibition in rabbit mesenteric artery. Pflügers Arch 420: 118–120, 1992 [DOI] [PubMed] [Google Scholar]
- 35. Sträter J, Wedding U, Barth TFE, Koretz K, Elsing C, Moller P. Rapid onset of apoptosis in vitro follows disruption of β1-integrin/matrix interactions in human colonic crypt cells. Gastroenterology 110: 1776–1784, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg 20: 411–417, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Szaszi K, Sirokmany G, Di Ciano-Oliveira C, Rotstein OD, Kapus A. Depolarization induces Rho-Rho kinase-mediated myosin light chain phosphorylation in kidney tubular cells. Am J Physiol Cell Physiol 289: C673–C685, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol Cell Physiol 273: C1378–C1385, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Wang X, Andersson R, Soltesz V, Guo W, Bengmark S. Water-soluble ethylhydroxyethyl cellulose prevents bacterial translocation induced by major liver resection in the rat. Ann Surg 217: 155–167, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Y, Roman R, Schlenker T, Hannun YA, Raymond J, Fitz JG. Cytosolic Ca2+ and protein kinase Cα couple cellular metabolism to membrane K+ permeability in a human biliary cell line. J Clin Invest 99: 2890–2897, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Sostman A, Roman R, Stribling S, Vigna S, Hannun Y, Raymond J, Fitz JG. Metabolic stress opens K+ channels in hepatoma cells through a Ca2+- and protein kinase Cα-dependent mechanism. J Biol Chem 271: 18107–18113, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Welte M, Pichler B, Groh J, Anthuber M, Jauch KW, Pratschke E, Lenhart FP, Haller M, Frey L, Peter K. Perioperative mucosal pH and splanchnic endotoxin concentration in orthotopic liver transplantation. Br J Anaesth 76: 90–98, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Wills NK, Lewis SA, Eaton DC. Active and passive properties of rabbit descending colon: a microelectrode and nystatin study. J Membr Biol 45: 81–108, 1979 [DOI] [PubMed] [Google Scholar]
- 44. Winter MC, Carson MR, Sheldon RA, Shasby DM. Mastoparan activates apical chloride and potassium conductances, decreases cell volume, and increases permeability of cultured epithelial cell monolayers. Am J Respir Cell Mol Biol 6: 583–593, 1992 [DOI] [PubMed] [Google Scholar]
- 45. Yanaga K, Kanematsu T, Takenaka K, Sugimachi K. Intraperitoneal septic complications after hepatectomy. Ann Surg 203: 148–152, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yeh DC, Wu CC, Ho WM, Cheng SB, Lu IY, Liu TJ, P'eng FK. Bacterial translocation after cirrhotic liver resection: a clinical investigation of 181 patients. J Surg Res 111: 209–214, 2003 [DOI] [PubMed] [Google Scholar]