Abstract
Novel protein kinase C isoforms (PKC δ and ε) mediate early events in acute pancreatitis. Protein kinase D (PKD/PKD1) is a convergent point of PKC δ and ε in the signaling pathways triggered through CCK or cholinergic receptors and has been shown to activate the transcription factor NF-κB in acute pancreatitis. For the present study we hypothesized that a newly developed PKD/PKD1 inhibitor, CRT0066101, would prevent the initial events leading to pancreatitis. We pretreated isolated rat pancreatic acinar cells with CRT0066101 and a commercially available inhibitor Gö6976 (10 μM). This was followed by stimulation for 60 min with high concentrations of cholecystokinin (CCK, 0.1 μM), carbachol (CCh, 1 mM), or bombesin (10 μM) to induce initial events of pancreatitis. PKD/PKD1 phosphorylation and activity were measured as well as zymogen activation, amylase secretion, cell injury and NF-κB activation. CRT0066101 dose dependently inhibited secretagogue-induced PKD/PKD1 activation and autophosphorylation at Ser-916 with an IC50 ∼3.75–5 μM but had no effect on PKC-dependent phosphorylation of the PKD/PKD1 activation loop (Ser-744/748). Furthermore, CRT0066101 reduced secretagogue-induced zymogen activation and amylase secretion. Gö6976 reduced zymogen activation but not amylase secretion. Neither inhibitor affected basal zymogen activation or secretion. CRT0066101 did not affect secretagogue-induced cell injury or changes in cell morphology, but it reduced NF-κB activation by 75% of maximal for CCK- and CCh-stimulated acinar cells. In conclusion, CRT0066101 is a potent and specific PKD family inhibitor. Furthermore, PKD/PKD1 is a potential mediator of zymogen activation, amylase secretion, and NF-κB activation induced by a range of secretagogues in pancreatic acinar cells.
Keywords: PKD/PKD1 inhibitor CRT0066101, zymogen activation, amylase secretion, cell injury
acute pancreatitis is characterized by a series of early events including premature activation and retention of digestive zymogens, decreased apical secretion, inflammatory responses, edema, and cell death. A wealth of evidence points to the pivotal role played by the serine-threonine kinase protein kinase C (PKC) in these events, particularly the so-called “novel” isoforms PKC-δ and PKC-ε (30–34). Recently, a distinct serine-threonine kinase, protein kinase D (PKD/PKD1), has emerged as a key downstream target of novel PKC isoforms in pancreatic acinar cells during secretagogue-induced models of acute pancreatitis (2, 4, 42).
PKD/PKD1 (initially known as PKC μ) has structural and regulatory properties that distinguish it from typical members of the PKC family. It is the founding member of a broader PKD family including PKD/PKD1, PKD2, and PKD3. The PKD/PKD1 isoform can be activated by a wide spectrum of stimuli including diacylglycerol, phorbol esters, growth factors, and downstream mediators of G protein-coupled receptors including PKC (2, 27, 35, 42). PKD/PKD1 is largely activated by the novel PKC isoforms that phosphorylate residues Ser-744 and Ser-748 of the PKD/PKD1 activation loop (2, 25, 37, 38). PKD/PKD1 further undergoes autophosphorylation at residue Ser-916 during its activation process (22). PKD/PKD1 isoforms are involved in an extensive range of intracellular functions including secretion (4, 6, 18), phosphorylation of histone deacetylase (21), and heat shock proteins (21), apoptosis (21), and proliferation (26). Furthermore, PKD/PKD1 isoforms are involved in initiation of gene transcription via activation of transcription factors such as c-Jun (11, 40), cAMP-response element binding protein (40), and, most notably, NF-κB (42).
In isolated rat pancreatic acinar cells, a number of secretagogues have been shown to activate PKD/PKD1 (2). The hormone cholecystokinin (CCK) and the cholinergic agonist carbachol (CCh) induced a dose-dependent rapid activation of PKD/PKD1 as measured by in vitro kinase assay and by phosphorylation at the activation loop (Ser-744/748) or autophosphorylation site (Ser-916). This activation of PKD/PKD1 correlated with increased NF-κB activity (42). Inhibition of novel PKCs with isoform-specific peptide inhibitors resulted in a decrease in PKC activity and subsequently PKD/PKD1 activity, highlighting the downstream signaling events. Since primary pancreatic acinar cells are difficult to keep responsive and viable in long-term culture for transfection, the AR42J cell line, a well-established surrogate model for studying intracellular mechanisms of pancreatitis, was used (19, 24, 30). Knockdown of PKD/PKD1 resulted in decreased NF-κB activity whereas overexpression of PKD/PKD1 caused increased NF-κB activation, confirming the critical role of PKD/PKD1 in NF-κB activation (42). The data from this study convincingly argue that PKD/PKD1 is involved in one of the main stages found in experimental pancreatitis but no data are currently available regarding the role of PKD/PKD1 in premature zymogen activation. We have previously shown that inhibition of PKC with specific inhibitors does reduce zymogen activation in isolated pancreatic acini, but we cannot be certain that this effect is PKC/PKD/PKD1 mediated (33, 34). It may occur via another pathway.
In our present study, we have used a newly developed small molecule pharmacological inhibitor, CRT0066101, isolated from a high-throughput screen to identify PKD family-specific inhibitors. Recently, this PKD family-specific inhibitor was shown to block pancreatic cancer growth both in vitro and in vivo (9). We have highlighted the role PKD/PKD1 plays in another pancreatic disease process, acute pancreatitis, by showing that this drug reduces zymogen activation, amylase secretion, and NF-κB activation without affecting cell injury or morphology.
MATERIALS AND METHODS
Preparation of isolated pancreatic acini.
Acini were isolated as previously described (3). Briefly, fasted male Sprague-Dawley rats 50–150 g (Charles River Laboratories, Wilmington, MA) were euthanized by CO2 via a protocol approved by the Yale University and the Veterans Administration Animal Care and Use Committees at both the Connecticut and Greater Los Angeles Health Care Systems. Acinar medium was prepared as follows (in mM): 10 HEPES (pH 7.4), 95 NaCl, 4.7 KCl, 0.6 MgCl2, 1 NaH2PO4, 10 glucose, 2 glutamine, plus 0.1% BSA, 1 × MEM-amino acids (GIBCO-BRL, San Jose, CA) and 1.3 mM CaCl2. The pancreas was collected in 15 ml of calcium-free acinar media. The pancreas was then minced in a minimal volume of calcium-free medium for 5 min, washed three times with calcium-free medium. The minced tissue was then placed into a 50-ml flask with 12 ml of acinar medium containing 50 U/ml of type-4 collagenase (Worthington, Freehold, NJ) for 60 min at 37°C with shaking (120 rpm). The digest was filtered through a 300- to 400-μm mesh (Sefar American, Depew, NY) and washed with acinar medium. Isolated acini (groups of 20–100 acinar cells) were distributed among the 24 wells (0.5 ml suspension/well) of a 24-well Falcon tissue culture plate (Becton Dickinson, Franklin Lakes, NJ). All reagents were purchased from Sigma (St. Louis, MO) unless otherwise noted.
Acinar experimental protocol.
Acini were recovered for 120 min at 37°C under constant O2 with shaking (90 rpm). Medium was changed at 60 min. At 120 min acini were treated without changing medium. Acini were treated with CCK (0.1 μM), CCh (1 mM), or bombesin (10 μM) for 60 min (unless otherwise noted). Samples were collected, placed in 1.5-ml centrifuge tubes (USA Scientific, Waltham, MA), and centrifuged for 1 min at 30 g, and 50 μl of the resulting cell-free supernatant was removed to a 0.5-ml microcentrifuge tube to assay for secreted amylase. The remaining 450 μl of cells + media was retained for zymogen activation assays and determination of total amylase. All samples were stored at −80°C.
PKD/PKD1 inhibitors.
Acinar cells were treated with 10 μM of either Gö6976, a general PKD/PKD1 inhibitor, or CRT0066101 (obtained from Cancer Research Technology, London, UK) for 120 min prior to secretagogue stimulation.
Immunoblot analysis.
Materials included antibodies against PKD1/PKD2 C-20 were from Santa Cruz Biotechnology (Santa Cruz, CA); phosphoserine 744/748 PKD/PKD1 antibody that detects primarily the phosphorylated state of Ser 744 and the phosphoserine 916 PKD/PKD1 antibody were obtained from Cell Signaling Technology (Beverly, MA). The latter antibody also detects the autophosphorylated form of PKD2. After samples were adjusted for protein concentration, equal amounts of protein were fractionated by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. The membranes were blocked at 4°C overnight or at room temperature for 60 min in Tris-buffered saline, supplemented with 5% nonfat dry milk, and probed with primary antibody (1:500; overnight). Then the membranes were incubated with secondary antibodies conjugated with horseradish peroxidase for 60 min at room temperature. Blots were developed by use of the enhanced chemiluminescence detection kit (Pierce, Rockford, IL).
Kinase activity assays.
ATP and [γ-32P] ATP were from Perkin Elmer (Torrance, CA). Protein-A-agarose was from Roche Applied Science (Mannheim, Germany), and the PKD/PKD1 substrate syntide-2 was from Bachem (Chicago, IL). PKD/PKD1 in pancreatic acinar cells or pancreatic tissue lysates was immunoprecipitated at 4°C for 180 min with the PKD-specific C-20 antibody (1:100) and protein A-agarose as previously described (42). Exogenous substrate syntide-2 phosphorylation by immunoprecipitated PKD/PKD1 was carried out by mixing 20 μl of the washed immunocomplexes with 10 μl of a phosphorylation mixture containing 100 μM ATP (including [γ-32P]ATP at 2 μCi/assay or with specific activity, 400–600 cpm/pmol) and 2.5 mg/ml syntide-2 (PLARTLSVAGLPGKK) in kinase buffer. After 10 min of incubation at 30°C, the reaction was stopped by adding 100 μl of 75 mMH3PO4, and 75 μl of the supernatant was spotted on P-81 phosphocellulose paper. Free [γ-32P] ATP was separated from the labeled substrate by washing the P-81 paper four times for 5 min in 75 mM H3PO4. The papers were dried, and the radioactivity incorporated into syntide-2 was determined by Cerenkov counting.
Enzymatic activity assays.
Enzyme activities were carried out as previously described (3). Briefly, samples were thawed, homogenized, and centrifuged. To each well of a 24-well plate (Greiner Bio-one Cellstar TC-Plate) the following were added: 100 μl of postnuclear supernatant, 350 μl of assay buffer [50 mM Tris (pH 8.1), 150 mM NaCl, 1 mM CaCl2, 0.01% BSA]. The assay was initiated by the addition of 50 μl of 400 μM enzyme substrate (fluorometric trypsin substrate: Boc-Gln-Ala-Arg-MCA catalog no. 3135-v; Peptides International, Louisville, KY and fluorometric chymotrypsin substrate: Suc-Ala-Ala-Pro-Phe-AMC catalog no. 230914; Calbiochem, San Diego, CA; fluorogenic cathepsin B substrate III: Z-Arg-Arg-AMC, 2HCl catalog no. 219392; Calbiochem) diluted in assay buffer (40 μM final). The plate was read by using a fluorometric microtiter plate reader (model HTS 7000; Perkin-Elmer Analytical Instruments, Shelton, CT; 380-nm excitation; 440-nm emission; 20 reads/10 min).
Lactate dehydrogenase assay.
The assay was performed by using the commercially available Cytotox 96 nonradioactive cytotoxicity assay kit according to the manufacturer's instructions (Promega, Madison, WI). In brief, acinar cells were pretreated with CRT0066101 for 120 min followed by stimulation with cholecystokinin (CCK, 0.1 μM), CCh (1 mM), or bombesin (10 μM) for a further 120 min. Samples were collected and centrifuged at 30 g for 30 s. A 50-μl aliquot of medium was then removed to measure lactate dehydrogenase (LDH) release from the cells. A manufacturer-provided lysis reagent was then added to the remaining 450 μl of cells and medium to determine total LDH. Both cell and medium samples were assayed. The results were expressed as the percent LDH released into the medium.
Amylase assay.
Amylase activity was determined by using a commercial kit (Phaebadas kit; Pharmacia Diagnostic, Rochester, NY) as described (3). Amylase secretion was calculated as the percent total cellular amylase released into the medium [medium/(medium + cells)].
Morphological analysis.
Acini were isolated as above and treated with or without CRT0066101 (10 μM) for 120 min followed by treatment with CCK (100 nM), CCh (1 mM), or bombesin (10 μM) for an additional 60 min. In some experiments cells were preincubated with Gö6976 (10 μM) or the trypsin inhibitor N-α-p-tosyl-l-lysine chloromethyl ketone (TLCK, 200 μM). Acini were centrifuged at 30 g for 5 min and medium was removed. To the acinar pellets 1 ml of PLP fixative (10 mM NaIO4, 75 mM lysine, 37.5 mM NaPO4, 2% paraformaldehyde) was added and placed on ice for 60 min. Pellets were then washed two times with PBS and postfixed in 1.0% osmium tetroxide (Polysciences, Warrington, PA), dehydrated in ethanol in propylene oxide, embedded in 100% EPON resin, and sectioned by use of an ultramicrotome and then stained with hematoxylin. Sections were examined for cytosolic vacuole formation and plasma membrane blebbing by use of an Olympus BX51 microscope.
Preparation of nuclear extracts and NF-κB DNA binding activity measurement.
Nuclear protein extracts were prepared by using ActiveMotif nuclear extract kit (Carlsbad, CA) following the manufacturer's instructions. NF-κB DNA binding activities were measured with EMSA as described previously (42) or with ELISA (for pancreas tissue) using ActiveMotif NF-κB p65 Transcription factor assay kit, following the manufacturer's instructions.
Statistical analysis.
Data represent means ± SE of at least three individual experiments unless otherwise noted, with each experiment being performed in at least duplicate. A Student's t-test analysis was used to determine statistical significance and P values of < 0.05 were assigned significance.
RESULTS
Secretagogue-induced zymogen activation but not amylase secretion is inhibited by the pharmacological PKD/PKD1 inhibitor Gö6976 (10 μM).
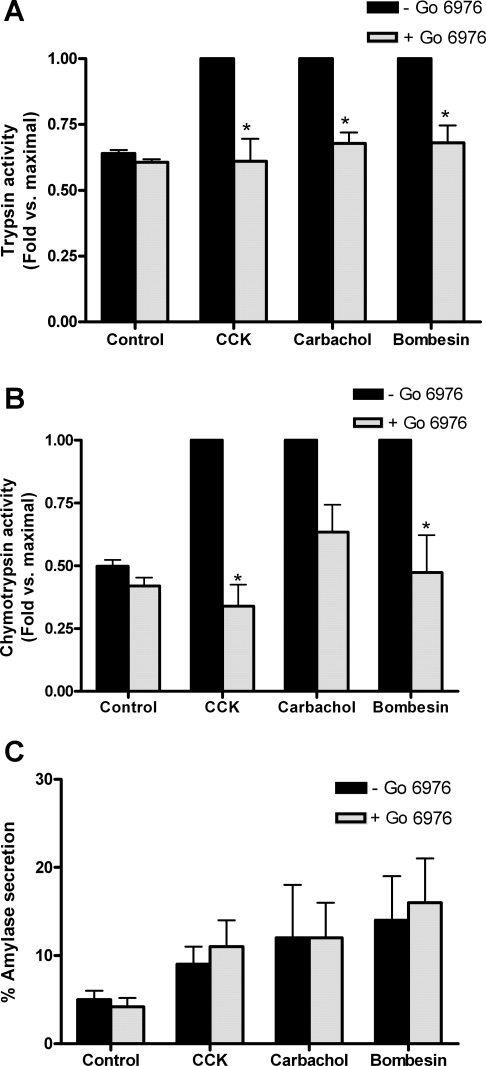
Initially we examined the role of PKD/PKD1 in pancreatitis using a commercially available inhibitor in an experimental model of acute pancreatitis induced by treating isolated acinar cells with high concentrations of secretagogues (the hormone CCK, the muscarinic agonist CCh, and the gastrin receptor agonist bombesin). Pretreatment of pancreatic acini with Gö6976 (10 μM), a pharmacological PKD/PKD1 inhibitor that does not inhibit novel PKC isoforms, reduced secretagogue-induced zymogen activation (Fig. 1). Trypsin and chymotrypsin activities were measured as markers of zymogen activation. Trypsin activities were completely inhibited in CCK-stimulated cells and reduced by 80% of maximal in CCh- and bombesin-stimulated cells that had been pretreated with Gö6976 (Fig. 1A). Chymotrypsin (Fig. 1B) activities followed a similar pattern with complete inhibition for CCK stimulated cells and a 62 and 89% reduction in activity for CCh- and bombesin-stimulated cells, respectively. Amylase secretion was unaffected by Gö6976 pretreatment for all secretagogues (Fig. 1C). At the concentrations used in this study, Gö6976 (10 μM) has been shown to inhibit PKD/PKD1 autophosphorylation at Ser-916 by 43% of maximal with a small inhibition at Ser-744/748 (2). Recently, however, the specificity and efficacy of this inhibitor has been questioned (1, 8, 28); therefore we developed a more specific PKD/PKD1 inhibitor to assess the role of PKD/PKD1 in pancreatitis.
Fig. 1.
PKD/PKD1 inhibitor Gö6976 (10 μM) inhibits secretagogue-induced zymogen activation but not amylase secretion. Trypsin (A) and chymotrypsin activities (B) were measured in cells that had been stimulated with 100 nM CCK, 1 mM carbachol (CCh), or 10 μM bombesin for 60 min either with (speckled bars) or without (black bars) pretreatment with PKD/PKD1 inhibitor Gö6976 (10 μM; 2 h). Data were normalized to amylase content and expressed as fold vs. maximal for each secretagogue. Amylase secretion (C) was expressed as % total. *P < 0.05 vs. maximal secretagogue; N = 3.
Secretagogue-stimulated autophosphorylation and kinase activity of PKD/PKD1 is inhibited by CRT0066101 (1–10 μM).
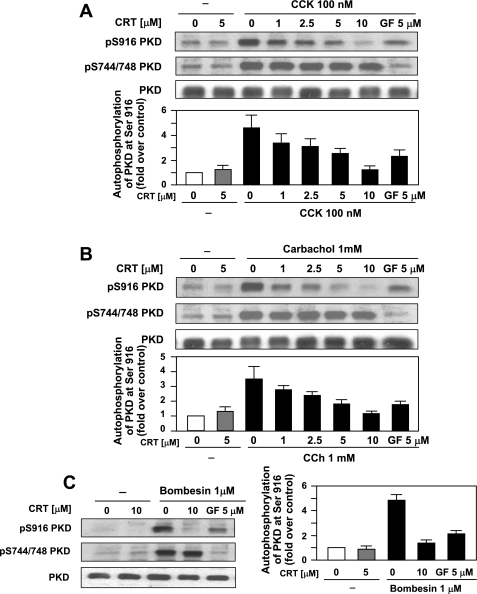
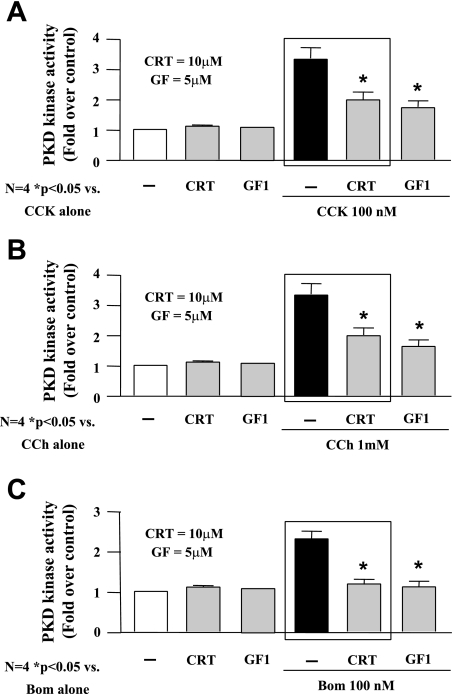
In a recent study, we have developed a novel, small-molecule PKD/PKD1 inhibitor, CRT0066101, that has a greater specificity for PKD/PKD1 (9). This compound has been shown to reduce growth of tumors in both subcutaneous and orthotopic models of pancreatic cancer by inhibiting autophosphorylation and activation of PKD/PKD1. The widely accepted model of PKD/PKD1 activation involves a series of phosphorylation events, although this has recently been questioned by a couple of studies (14, 28). Generally, phosphorylation of PKD/PKD1 occurs via a PKC-dependent mechanism at Ser-744/748 and autophosphorylation at Ser-916, leading to activation of the kinase (13, 26, 39, 43). Treatment of isolated acinar cells with hyperstimulatory concentrations of secretagogue (CCK 100 nM; CCh 1 mM; bombesin 1–10 μM) for 60 min caused phosphorylation of PKD/PKD1 at both Ser-744/748 and Ser-916 and increased its kinase activity by approximately threefold (Figs. 2 and 3, respectively). Pretreatment of acinar cells, prior to secretagogue stimulation, with increasing concentrations of CRT0066101 (1–5 μM) reduced PKD/PKD1 autophosphorylation at Ser-916, with complete inhibition at 10 μM (Fig. 2). PKC-dependent phosphorylation at Ser-744/748 was unaffected by CRT0066101 pretreatment. For comparison, pretreatment with GF109203X (GF, 5 μM), a classic and novel PKC inhibitor, inhibited PKC-dependent phosphorylation at Ser-744/748 and reduced autophosphorylation at Ser-916. Pretreatment with CRT0066101 reduced PKD/PKD1 kinase activity induced by hyperstimulatory CCK, CCh, or bombesin by 63, 63, and 88%, respectively (Fig. 3). By comparison, pretreatment with the novel PKC inhibitor GF reduced PKD/PKD1 activity 70, 74, and 94% of maximal with CCK, CCh, and bombesin, respectively. In summary, CRT0066101 inhibited PKD/PKD1 autophosphorylation at Ser-916 and reduced secretagogue-stimulated kinase activity.
Fig. 2.
CRT0066101 (CRT; 1–10 μM) inhibits secretagogue-stimulated autophosphorylation of PKD/PKD1. Rat pancreatic acinar cells were incubated with 100 nM CCK, 1 mM CCh, or 10 μM bombesin for 60 min. Some samples were pretreated for 120 min with increasing concentrations of PKD/PKD1 inhibitor CRT0066101, as indicated, or PKC inhibitor GF109203X (GF; 5 μM). Western blotting analyses of CCK (A)-, CCh (B)-, and bombesin (C)-induced PKD/PKD1 1 phosphorylation by use of PKD/PKD1 1 pS744/748 antibody or PKD/PKD1 1 pS916 antibody is shown. Blots were reblotted for PKD/PKD1 1 expression with PKD/PKD1 C-20 antibody to verify equal protein loading. All Western blots shown are representative of 3 independent experiments. Densitometry measurements of CCK (A)-, CCh (B)-, and bombesin (C)-induced PKD/PKD1 1 phosphorylation at S744/748 and S916 are shown beneath blots (N = 3).
Fig. 3.
CRT0066101 (1–10 μM) inhibits secretagogue-stimulated kinase activity. Rat pancreatic acinar cells were incubated with 100 nM CCK (A), 1 mM CCh (B), or 10 μM bombesin (C) for 60 min. Some samples were pretreated for 120 min with increasing concentrations of PKD/PKD1 inhibitor CRT0066101, as indicated, or PKC inhibitor GF (5 μM). The acinar cell lysates were immunoprecipitated with PKD/PKD1 C-20 antibody, and PKD/PKD1 catalytic activity in the immunocomplexes was determined by in vitro kinase assay. *P < 0.05 vs. secretagogue alone, N = 3. Boxes emphasize reduction of PKD activity by CRT 0066101.
Secretagogue-induced zymogen activation and amylase secretion is reduced by PKD/PKD1 inhibition with CRT0066101 (10 μM).
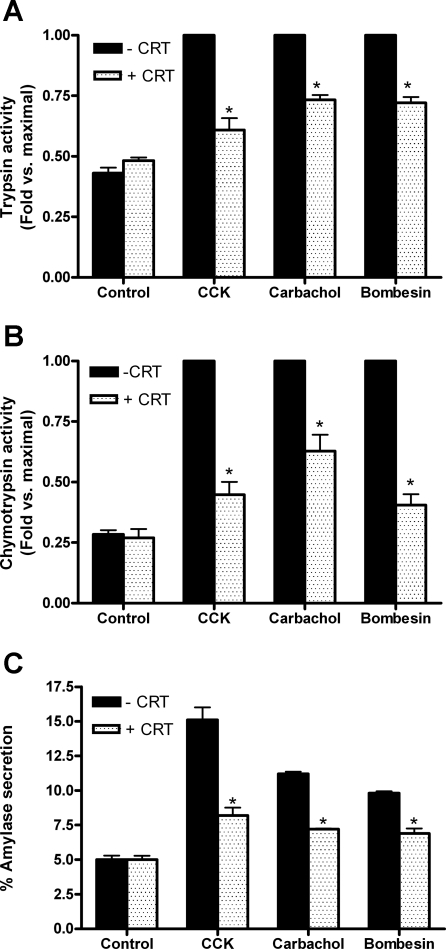
We assessed whether CRT0066101-dependent inhibition of PKD/PKD1 autophosphorylation and reduction in PKD/PKD1 activity affected zymogen activation and amylase secretion. Trypsin activities were reduced by 75, 52, and 53% of maximal for CCK-, CCh-, and bombesin-stimulated cells, respectively, which had been pretreated with CRT0066101 (Fig. 4A). Chymotrypsin (Fig. 4B) activities followed a similar pattern with 75, 50, and 81% reduction in activity for CCK, CCh, and bombesin-stimulated cells, respectively. In contrast to studies with Gö6976, amylase secretion was significantly reduced for all secretagogues following CRT0066101 pretreatment (CCK: reduced from 15 to 8%; CCh: 11 to 7%; bombesin: 10 to 7%, Fig. 4C). CRT0066101 pretreatment did not reduce amylase secretion induced by physiological concentrations of CCK or CCh (Table 1). Although a reduction in physiological bombesin-induced amylase secretion was seen, the reduction was not statistically significant.
Fig. 4.
CRT0066101 (10 μM) inhibits secretagogue-induced zymogen activation and amylase secretion. Trypsin (A) and chymotrypsin activities (B) were measured in cells that had been stimulated with 100 nM CCK, 1 mM CCh, or 10 μM bombesin for 60 min either with (speckled bars) or without (black bars) pretreatment with PKD/PKD1 inhibitor CRT0066101 (10 μM; 2 h). Data were normalized to amylase content and expressed as fold vs. maximal for each secretagogue. Amylase secretion (C) was expressed as % total. *P < 0.05 vs. maximal secretagogue; N = 6 for CCK; N = 4 for CCh and bombesin.
Table 1.
CRT does not reduce secretagogue-stimulated amylase secretion at physiological concentrations of secretagogue
| % Amylase Secretion |
||
|---|---|---|
| Secretagogue | −CRT | +CRT |
| No secretagogue | 4.0 ± 1.0 | 5.0 ± 1.0 |
| CCK (0.1 nM) | 13.0 ± 2.0 | 12.0 ± 4.0 |
| Carbachol (1 μM) | 12.0 ± 2.0 | 12.0 ± 1.0 |
| Bombesin (10 nM) | 18.0 ± 5.0 | 11.0 ± 2.0 |
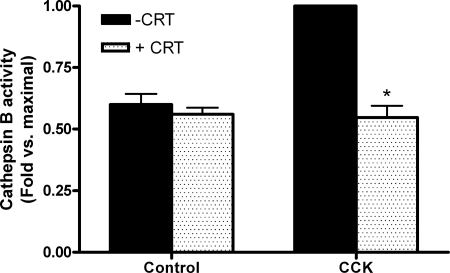
Secretagogue-induced cathepsin B activity is reduced by PKD/PKD1 inhibition with CRT0066101 (10 μM).
We further explored the potential mechanism by which PKD/PKD1 influences zymogen activation. The lysosomal hydrolase cathepsin B is largely responsible for activation of trypsinogen, and we assessed whether CRT0066101-dependent inhibition of PKD/PKD1 reduced cathepsin B activity. CCK-induced cathepsin B activity was inhibited by CRT0066101, indicating that PKD regulates cathepsin B-induced trypsinogen activation (Fig. 5).
Fig. 5.
CRT0066101 (10 μM) inhibits secretagogue-induced cathepsin B activity. Cathepsin B activity was measured in cells, which had been stimulated with 100 nM CCK for 60 min either with (speckled bars) or without (black bars) pretreatment with PKD/PKD1 inhibitor CRT0066101 (10 μM; 120 min). Data were normalized to amylase content and expressed as fold vs. maximal.
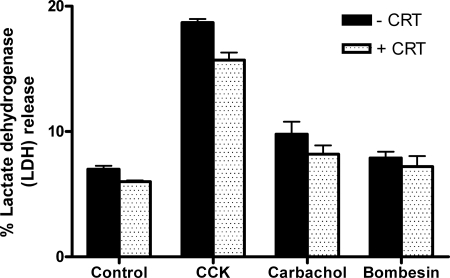
Secretagogue-induced cell injury is unaffected by CRT0066101 (10 μM).
The effects of CRT0066101 on cellular injury were evaluated by LDH release as an index of cell damage (Fig. 6). Acini were treated with CCK, CCh, or bombesin in the presence or absence of CRT0066101 for 120 min, and the percent LDH release into the cell culture medium was determined. After treatment with 100 nM CCK, LDH release rose from a control level of 7% to 18%, with 1 mM CCh it increased to 10%, and with 10 μM bombesin there was no significant increase. Stimulated cells pretreated with CRT0066101 exhibited a slight reduction in injury, but this was not statistically significant. To further evaluate effects of CRT0066101 on cellular injury caused by supraphysiological doses of CCK, CCh, and bombesin, morphological indicators of injury were assessed.
Fig. 6.
CRT0066101 (10 μM) does not cause cellular injury or affect secretagogue-induced cellular injury as measured by lactate dehydrogenase (LDH) release. Rat pancreatic acinar cells were incubated with (speckled bars) or without (black bars) CRT0066101 (10 μM) for 120 min prior to 100 nM CCK, 1 mM CCh, or 10 μM bombesin treatment for an additional 120 min. Samples were assayed for LDH release as a percentage of total LDH (N = 3).
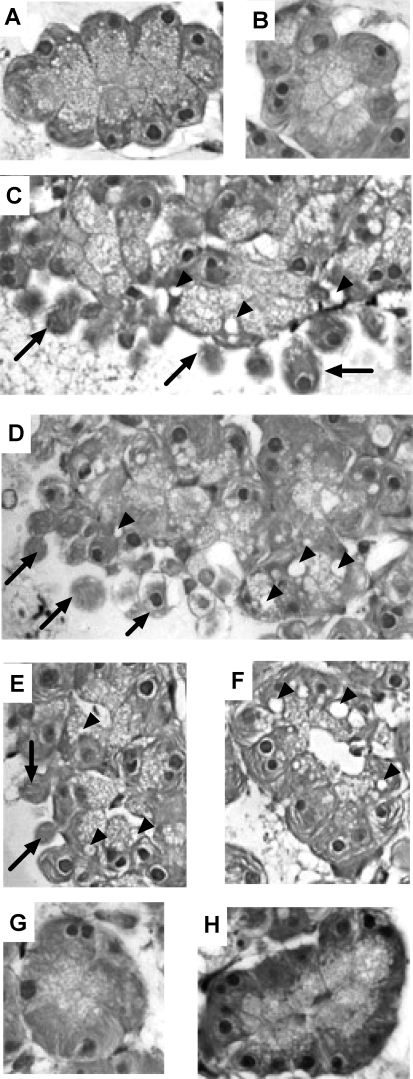
Secretagogue-induced morphological changes are unaffected by CRT0066101 (10 μM).
Effects of CRT0066101 on secretagogue-stimulated changes in cell morphology were assessed by light microscopy. Untreated cells showed none of the hallmark indicators of cell injury (Fig. 7A). Cells pretreated with 10 μM CRT0066101 did not exhibit any signs of cell injury either (Fig. 7B). Cells treated with CCK 100 nM (Fig. 7C) showed both plasma membrane blebbing (arrows) and formation of cytosolic vacuoles (arrowheads). Pretreatment with CRT0066101 prior to CCK stimulation had no effect on CCK-induced membrane blebbing and cytosolic vacuole formation (Fig. 7D). Treatment of acinar cells with 1 mM CCh caused plasma membrane blebbing and vacuolization (Fig. 7E); compared with CCh-stimulated cells pretreated with CRT0066101, a similar degree of vacuolization was observed but blebbing was reduced (Fig. 7F). Bombesin-treated cells (10 μM bombesin) with or without CRT0066101 pretreatment showed no signs of cellular injury, most likely because activated zymogens have been secreted (7).
Fig. 7.
CRT0066101 (10 μM) does not cause morphological changes or alter secretagogue-induced morphological changes. Acini were incubated with or without CRT0066101 (10 μM) for 120 min prior to 100 nM CCK, 1 mM CCh, or 10 μM bombesin treatment for an additional 60 min. Cells were then embedded and assessed for cytosolic vacuole formation and membrane blebbing. A, control; B, control + CRT0066101 (10 μM); C, CCK; D, CCK + CRT0066101; E, CCh; F, CCh + CRT0066101; G, bombesin; H, bombesin + CRT0066101. Arrows indicate membrane blebbing and arrowheads indicate vacuoles. Images were taken at ×40 magnification and are representative of each treatment group.
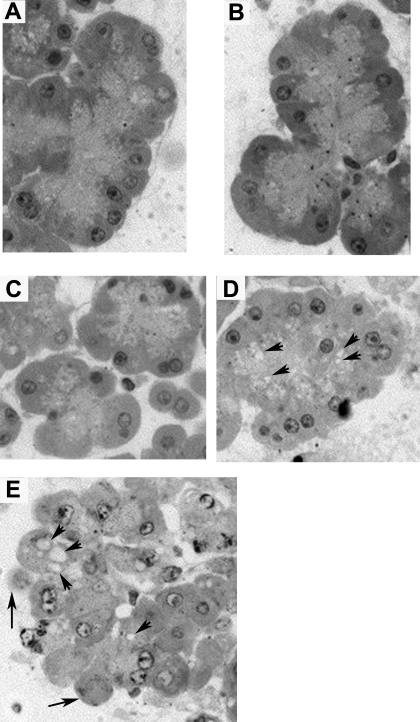
Secretagogue-induced morphological changes are reduced by Gö6976 (10 μM) but not by trypsin inhibitor TLCK (200 μM).
Given that CRT0066101 reduces zymogen activation but also secretion, there may be sufficient levels of residual activated enzyme to maintain acinar cell injury. Since Gö6976 reduces zymogen activation but not secretion, we investigated whether morphological evidence of acinar cell injury was reduced in the presence of Gö6976. Cells pretreated with 10 μM Gö6976 did not exhibit any signs of cell injury (Fig. 8A) and cells pretreated with Gö6976 followed by stimulation with 100 nM CCK lacked evidence of morphological injury (Fig. 8B), suggesting that secretion of active enzymes reduces injury. To further verify the contribution of activated trypsin to acinar cell injury, we pretreated cells with CRT0066101 and a trypsin inhibitor (TLCK) to see whether inhibition of residual trypsin reduced morphological injury. TLCK pretreatment did not alter cell morphology (Fig. 8C) in unstimulated cells, nor did it reduce morphological injury induced by CCK (100 nM), with or without CRT0066101 pretreatment (Fig. 8, D and E).
Fig. 8.
Secretagogue-induced morphological changes are reduced by Gö6976 (10 μM) but not by trypsin inhibitor N-α-p-tosyl-l-lysine chloromethyl ketone (TLCK; 200 μM). Acini were incubated with or without Gö6976 (10 μM), CRT0066101 (10 μM), or TLCK (200 μM) for 120 min prior to 100 nM CCK for an additional 60 min. Cells were then embedded and assessed for cytosolic vacuole formation and membrane blebbing. A, control + Gö6976 (10 μM); B, CCK + Gö6976 (10 μM); C, control + TLCK (200 μM); D, CCK + TLCK (200 μM); E, CCK + TLCK (200 μM) + CRT0066101 (10 μM). Arrows indicate membrane blebbing and arrowheads indicate vacuoles. Images were taken at ×40 magnification and are representative of each treatment group.
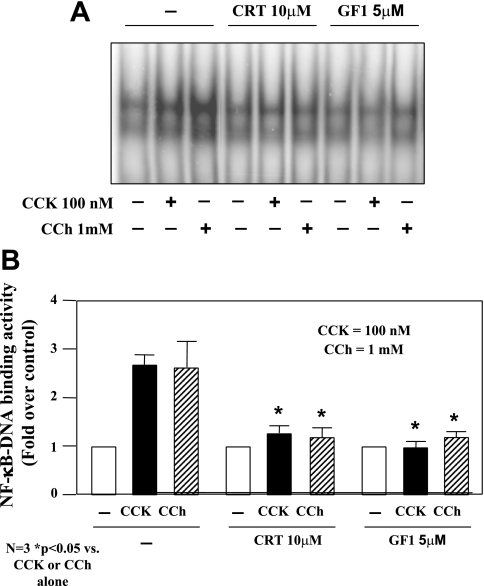
Secretagogue-induced NF-κB activation is inhibited by CRT0066101 (10 μM).
To determine the role of PKD/PKD1 activation in secretagogue-induced NF-κB activation, we pretreated the isolated pancreatic acini with CRT0066101 (10 μM) for 120 min before stimulation with a pathological dose of CCK (100 nM) or CCh (1 mM). NF-κB-DNA binding activity in nuclear extracts was measured by EMSA. As illustrated in Fig. 9, CRT0066101 (10 μM) markedly reduced CCK- and CCh-stimulated NF-κB activation. These results are consistent with our previous findings achieved in rat pancreatic acinar AR42 J cells by using a molecular approach to upregulate or downregulate PKD/PKD1 expression (42).
Fig. 9.
CRT0066101 (10 μM) inhibits secretagogue-induced NF-κB activation. Rat pancreatic acinar cells were incubated with or without CRT0066101 (10 μM) for 120 min prior to 100 nM CCK or 1 mM CCh treatment for an additional 60 min. A: NF-κB binding activity was measured in nuclear extracts by EMSA. B: NF-κB band intensities were quantified in the PhosphorImager and normalized on the band intensity in unstimulated control acinar cells. Values are means ± SE (N = 3).
DISCUSSION
Previous work from our laboratories has demonstrated a role for the novel protein kinase C isoforms, PKCδ and PKCε, in the early stages of acute pancreatitis, including zymogen activation, secretion, and NF-κB activation (30–34). More recently the focus has centered on downstream targets of these PKC isoforms, most notably protein kinase D (PKD/PKD1, 4, 2, 42). Because of the lack of effective specific PKD/PKD1 inhibitors and the difficulties associated with genetic manipulation of primary acinar cell culture, our prior study has used indirect approaches to address the role of PKD/PKD1 in acute pancreatitis (42). Such techniques include inhibition of PKD/PKD1 through pharmacological inhibition of PKC and small interfering RNA knockdown or overexpression of PKD/PKD1 in a pancreatic AR42J cell model. Although these approaches are fine for determining effects of PKD/PKD1 on ubiquitous events such as NF-κB activation, they cannot be effectively used to assess the role of PKD/PKD1 in acinar cell-specific processes such as premature zymogen activation.
Exciting new findings have recently shown that a potent and selective inhibitor of PKD/PKD1, CRT0066101, can inhibit the growth of pancreatic tumors in vivo (9). This drug not only has the potential to treat cancer by blocking PKD/PKD1-mediated mitogenic signaling pathways but can be used to investigate the role of PKD/PKD1 in other pathological processes. Therefore, in our present study, we have employed this inhibitor to determine the function of PKD/PKD1 in pathological responses elicited during acute pancreatitis.
Initially, we induced early pancreatitis responses in isolated pancreatic acini by treatment with high (pathological) concentrations of diverse secretagogues (CCK, CCh, and bombesin) and measured zymogen activation and amylase secretion. In cells that had been pretreated with a commercially available PKD/PKD1 inhibitor, Gö6976 (10 μM), we saw a significant reduction in trypsin and chymotrypsin activities for all three secretagogues, with no change in amylase secretion (Fig. 1). Gö 6976, however, has also been shown to inhibit other kinases including PKCα and PKCβ1 as well as the TrkA, TrkB, JAK2, and JAK3 tyrosine kinases (1, 8, 20). This should not pose a problem in the context of zymogen activation since previous studies have concluded that PKCα does not mediate zymogen activation (33). The results presented here indicate that PKD/PKD1 mediates zymogen activation although nonspecific effects of Gö6976 cannot be ruled out (1, 8). Therefore, we complemented these initial findings with studies using a specific PKD/PKD1 inhibitor, CRT0066101.
Prior to using CRT0066101 in our studies we characterized its efficacy in pancreatic acinar cells. CRT0066101 inhibited secretagogue-induced autophosphorylation of PKD/PKD1 at Ser-916 but did not affect phosphorylation at Ser-744/748, the PKC-dependent transphosphorylation sites in the activation loop, underscoring the specificity of CRT0066101 as a PKD/PKD1 inhibitor. To further complement this, similar treatment groups were carried out in the presence of GF109203X, a classic and novel PKC inhibitor, in place of CRT0066101. As can be seen from Fig. 2, when PKC is inhibited, phosphorylation at Ser-744/748 and Ser-916 are dramatically reduced, indicating that autophosphorylation at Ser-916 is partly dependent on PKC phosphorylating PKD/PKD1 at Ser-744/748. As a further assessment of the efficacy of CRT0066101, we looked at in vitro kinase activity of PKD/PKD1. Regardless of stimuli, CRT0066101 pretreatment significantly reduced kinase activity. The fact that activity is not completely inhibited could be due to the fact that PKD/PKD1 is still being phosphorylated by PKC and therefore may partially retain some activity. Furthermore, when acinar cells are pretreated with PKC inhibitor GF, activity is reduced by a similar degree. Again, since some PKD/PKD1 autophosphorylation is taking place at Ser-916 this may account for the partial activity seen (Fig. 3).
CRT0066101 inhibited not only secretagogue-induced PKD/PKD1 autophosphorylation and activity, but zymogen activation as well (Fig. 4). Coupled with the Gö6976 data, this indicates that PKD/PKD1 is a mediator of zymogen activation. However, unlike with Gö6976 pretreatment, CRT0066101 also reduced amylase secretion (Fig. 4C). A possibility for this discrepancy could be the degree of efficacy when comparing Gö6976 with CRT0066101. In previous studies, Gö6976 has been shown to reduce Ser-916 phosphorylation, although only by 43% of maximal (2). In our study, CRT0066101 reduces Ser-916 phosphorylation by >95% of maximal. It is possible that the partial inhibition by Gö6976 still leaves enough active kinase to facilitate secretion but not zymogen activation. PKD/PKD1 has previously been shown to promote hormone-induced amylase secretion in mouse exocrine pancreas (4). We have seen no effect of PKD/PKD1 inhibition by CRT0066101 on amylase secretion induced with physiological concentrations of secretagogue (Table 1), although PKD/PKD1 promotes secretion at hyperstimulatory secretagogue levels (Fig. 4). These findings raise the intriguing possibility that pathological secretion may proceed via a PKD/PKD1-mediated mechanism, whereas physiological secretion may not.
Inhibition of zymogen activation by CRT0066101 raises the interesting question of how PKD is influencing zymogen activation. One possibility is that PKD regulates the upstream activator of trypsinogen, cathepsin B. Another possibility is that it influences the activity of endogenous trypsin inhibitors in the cell. We pretreated cells with CRT0066101 and then stimulated with CCK (100 nM) and assayed for cathepsin B activity. We found that inhibition of PKD with CRT0066101 inhibits cathepsin B activity completely (Fig. 5), strongly indicating that this is the predominant mechanism leading to PKD-mediated zymogen activation.
Reduction in PKD/PKD1-mediated zymogen activation by CRT0066101 would likely result in diminished cellular injury, although this was not the case when measuring LDH release as a marker of cellular injury (Fig. 6). Furthermore, when conducting studies of morphological injury, we observed that CRT0066101 alone does not cause injury to the acinar cell (Fig. 7, A vs. B), nor does it prevent secretagogue-induced injury, as depicted by plasma membrane blebbing and/or vacuolization (Fig. 7, C and E vs. D and F, respectively for CCK and CCh). Little injury is seen for bombesin-treated cells with or without CRT0066101 (Figs. 6 and 7, G and H), probably because bombesin is known to induce zymogen activation but it also causes secretion of activated enzymes (7). In addition, the level of zymogen activation for CCK and CCh stimulation is greater than that seen for bombesin (not shown), and hence a smaller amount of active enzyme will be present in bombesin-treated cells.
Given that CRT0066101 reduces zymogen activation it seems strange that it does not protect acinar cells from injury. A potential reason for this could be that secretion is reduced by CRT0066101 as well, and this might result in a net amount of active enzyme being retained within the acinar cell that is sufficient to cause cell injury. We assessed whether residual activated enzyme levels due to reduced amylase secretion may be the factor contributing to morphological injury. Pretreatment of cells with Gö6976, which reduced zymogen activation but not secretion, prevented CCK-induced morphological injury (Fig. 8B), supporting this theory. To confirm this we took another approach and pretreated cells with CRT0066101 and the trypsin inhibitor TLCK to completely inhibit any residual active trypsin. Surprisingly, TLCK did not reduce CCK-induced morphological injury in the presence of CRT0066101 (Fig. 8, D and E). Thus the protective effects conferred by Gö6976 may be due to inhibition of another pathway, given the nonspecific nature of this inhibitor. The lack of protective effect of CRT0066101 (with or without TLCK) indicates that morphological changes associated with acinar cell injury might not be related to zymogen activation (15).
Our previous study using a molecular approach to upregulate or downregulate PKD/PKD1 expression level in rat pancreatic acinar AR42J cells indicated that PKD/PKD1 mediates CCK- or CCh-induced NF-κB activation (42). Here the use of CRT0066101 to inhibit PKD/PKD1 catalytic activity enabled us to verify the earlier data achieved in AR42J cells and demonstrated again that PKD/PKD1 is required in NF-κB activation induced by pathological doses of secretagogues in pancreatic acinar cells.
The work outlined in this study clearly defines a role for PKD/PKD1 in acute pancreatitis and raises the question as to what its intracellular targets may be. Sustained increases in cytosolic calcium and intraluminal acidification of zymogen-containing compartments are two processes that optimize premature zymogen activation (23, 29, 41). It is possible that PKD/PKD1 may phosphorylate target proteins involved in these events. For sustained calcium elevations such targets include the inositol (1,4,5) trisphosphate receptor or the calcium-sensitive release channel ryanodine receptor (12, 23). In addition, mitochondrial calcium uptake is inhibited by a concerted action of p38MAPK and PKD/PKD1 and may provide another pathway for sustaining cytosolic calcium levels (16). For intraluminal acidification of compartments, an ATP-dependent proton pump, vacuolar ATPase (vATPase), has been shown to be pivotal in facilitating premature zymogen activation (15, 41). Little is known about its regulation in mammalian systems but phosphorylation by PKD/PKD1 could potentially affect vATPase assembly and function. Furthermore, acidification of compartments by vATPase could optimize cathepsin B activity, and our data show that cathepsin B activity is PKD sensitive. In terms of amylase secretion, PKD/PKD1 is known to play a role in zymogen granule trafficking and exocrine secretion (4). In Madin-Darby canine kidney cells, a PKD/PKD1 isoform has been shown to promote cargo-specific transport via a basolateral route as opposed to an apical route (10). Therefore, it seems plausible that, in acinar cells, PKD/PKD1 may also contribute to pathological secretion via a basolateral route. This would then imply cross talk between PKD/PKD1 and some of the already-identified members of the basolateral secretory pathway, including PKCα and Munc18c (5, 17). Finally, we and others have shown that PKD/PKD1 mediates NF-κB activation via phosphorylation of IκBα (42). It is interesting to note that activation of zymogens and NF-κB, although both affected by PKD/PKD1, are independently regulated events and are not sequential (36).
In summary, we have used a newly developed, selective PKD/PKD1 inhibitor, CRT0066101, to demonstrate that PKD/PKD1 mediates zymogen activation, amylase secretion, and NF-κB activation in acute pancreatitis. Furthermore, these studies reveal PKD/PKD1 as the convergent point of three different receptor-mediated pathways and underscores its pivotal role in the disease process.
GRANTS
This work was supported by a National Institutes of Health (NIH) Grant R21 (DK69702 to E. C. Thrower) USC-UCLA Research Center for Alcoholic Liver and Pancreatic Injury Grant (5 P60 AA11999 from the National Institute on Alcohol Abuse and Alcoholism; Tsukamoto; to S. J. Pandol), MD Anderson Cancer Center Physician Scientist Program Award (to S. Guha), McNair Foundation Scholar Award (to S. Guha), and NIH 5P30CA16672 (Career Development Award to S. Guha).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Tom Gordon, Meghan Kelly, SueAnn Mentone, and Shao Song for expert technical assistance, Fred Gorelick MD and Thomas Kolodecik for critical reading of this manuscript, and Dr. Christopher R. Ireson and his group at the Cancer Research Technology Discovery Laboratories, London, UK for providing us with CRT0066101.
REFERENCES
- 1. Behrens MM, Strasser U, Choi DW. Go 6976 is a potent inhibitor of neurotrophin-receptor intrinsic tyrosine kinase. J Neurochem 72: 919–924, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Berna MJ, Hoffmann KM, Tapia JA, Thill M, Pace A, Mantey SA, Jensen RT. CCK causes PKD1 activation in pancreatic acini by signaling through PKC-delta and PKC-independent pathways. Biochim Biophys Acta 1773: 483–501, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaudhuri A, Kolodecik TR, Gorelick FS. Effects of increased intracellular cAMP on carbachol-stimulated zymogen activation, secretion, and injury in the pancreatic acinar cell. Am J Physiol Gastrointest Liver Physiol 288: G235–G243, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen LA, Li J, Silva SR, Jackson LN, Zhou Y, Watanabe H, Ives KL, Hellmich MR, Evers BM. PKD3 is the predominant protein kinase D isoform in mouse exocrine pancreas and promotes hormone-induced amylase secretion. J Biol Chem 284: 2459–2471, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cosen-Binker LI, Lam PP, Binker MG, Reeve J, Pandol S, Gaisano HY. Alcohol/cholecystokinin-evoked pancreatic acinar basolateral exocytosis is mediated by protein kinase C alpha phosphorylation of Munc18c. J Biol Chem 282: 13047–13058, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Fugmann T, Hausser A, Schoffler P, Schmid S, Pfizenmaier K, Olayioye MA. Regulation of secretory transport by protein kinase d-mediated phosphorylation of the ceramide transfer protein. J Cell Biol 178: 15–22, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grady T, Mah'moud M, Otani T, Rhee S, Lerch MM, Gorelick FS. Zymogen proteolysis within the pancreatic acinar cell is associated with cellular injury. Am J Physiol Gastrointest Liver Physiol 275: G1010–G1017, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Grandage VL, Everington T, Linch DC, Khwaja A. Go6976 is a potent inhibitor of the JAK 2 and FLT3 tyrosine kinases with significant activity in primary acute myeloid leukaemia cells. Br J Haematol 135: 303–316, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Harikumar KB, Kunnumakkara AB, Ochi N, Tong Z, Deorukhkar A, Sung B, Kelland L, Jamieson S, Sutherland R, Raynham T, Charles M, Bagherazadeh A, Foxton C, Boakes A, Farooq M, Maru D, Diagaradjane P, Matsuo Y, Sinnett-Smith J, Gelovani J, Krishnan S, Aggarwal BB, Rozengurt E, Ireson CR, Guha S. A novel small-molecule inhibitor of protein kinase D blocks pancreatic cancer growth in vitro and in vivo. Mol Cancer Ther 9: 1136–1146, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hausser A, Storz P, Martens S, Link G, Toker A, Pfizenmaier K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex [see comment]. Nat Cell Biol 7: 880–886, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hurd C, Waldron RT, Rozengurt E. Protein kinase D complexes with C-jun N-terminal kinase via activation loop phosphorylation and phosphorylates the C-jun N-terminus. Oncogene 21: 2154–2160, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Husain SZ, Prasad P, Grant WM, Kolodecik TR, Nathanson MH, Gorelick FS. The ryanodine receptor mediates early zymogen activation in pancreatitis. Proc Natl Acad Sci USA 102: 14386–14391, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iglesias T, Waldron RT, Rozengurt E. Identification of in vivo phosphorylation sites required for protein kinase D activation. J Biol Chem 273: 27662–27667, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Jacamo R, Sinnett-Smith J, Rey O, Waldron RT, Rozengurt E. Sequential protein kinase C (PKC)-dependent and PKC-independent protein kinase D catalytic activation via Gq-coupled receptors: differential regulation of activation loop Ser(744) and Ser(748) phosphorylation. J Biol Chem 283: 12877–12887, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kolodecik T, Gorelick FS, Thrower EC. Genetic and pharmacologic manipulation of vacuolar ATPase: effects on zymogen activation in pancreatic acini. Open Access Animal Physiol 1: 1–11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koncz P, Szanda G, Fulop L, Rajki A, Spat A. Mitochondrial Ca2+ uptake is inhibited by a concerted action of p38 MAPK and protein kinase D. Cell Calcium 46: 122–129, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Lam PP, Cosen Binker LI, Lugea A, Pandol SJ, Gaisano HY. Alcohol redirects CCK-mediated apical exocytosis to the acinar basolateral membrane in alcoholic pancreatitis. Traffic 8: 605–617, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Li J, Chen LA, Townsend CM, Jr, Evers BM. PKD1, PKD2, and their substrate Kidins220 regulate neurotensin secretion in the BON human endocrine cell line. J Biol Chem 283: 2614–2621, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malka D, Vasseur S, Bodeker H, Ortiz EM, Dusetti NJ, Verrando P, Dagorn JC, Iovanna JL. Tumor necrosis factor alpha triggers antiapoptotic mechanisms in rat pancreatic cells through pancreatitis-associated protein I activation. Gastroenterology 119: 816–828, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem 268: 9194–9197, 1993 [PubMed] [Google Scholar]
- 21. Matthews SA, Liu P, Spitaler M, Olson EN, McKinsey TA, Cantrell DA, Scharenberg AM. Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol Cell Biol 26: 1569–1577, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matthews SA, Rozengurt E, Cantrell D. Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/protein kinase Cmu. J Biol Chem 274: 26543–26549, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Petersen OH, Sutton R. Ca2+ signaling and pancreatitis: effects of alcohol, bile and coffee. Trends Pharmacol Sci 27: 113–120, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Piiper A, Leser J, Lutz MP, Beil M, Zeuzem S. Subcellular distribution and function of Rab3A-D in pancreatic acinar AR42J cells. Biochem Biophys Res Commun 287: 746–751, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Rey O, Reeve JR, Jr, Zhukova E, Sinnett-Smith J, Rozengurt E. G protein-coupled receptor-mediated phosphorylation of the activation loop of protein kinase D: dependence on plasma membrane translocation and protein kinase Cε. J Biol Chem 279: 34361–34372, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem 280: 13205–13208, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Rozengurt E, Sinnett-Smith J, Van Lint J, Valverde AM. Protein kinase D (PKD): a novel target for diacylglycerol and phorbol esters. Mutat Res 333: 153–160, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Rybin VO, Guo J, Steinberg SF. Protein kinase D1 autophosphorylation via distinct mechanisms at Ser744/Ser748 and Ser916. J Biol Chem 284: 2332–2343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saluja AK, Donovan EA, Yamanaka K, Yamaguchi Y, Hofbauer B, Steer ML. Cerulein-induced in vivo activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology 113: 304–311, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Satoh A, Gukovskaya AS, Edderkaoui M, Daghighian MS, Reeve JR, Jr, Shimosegawa T, Pandol SJ. Tumor necrosis factor-alpha mediates pancreatitis responses in acinar cells via protein kinase C and proline-rich tyrosine kinase 2. Gastroenterology 129: 639–651, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR, Jr, Shimosegawa T, Pandol SJ. PKC-δ and -ε regulate NF-κB activation induced by cholecystokinin and TNF-α in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 287: G582–G591, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Satoh A, Gukovskaya AS, Reeve JR, Jr, Shimosegawa T, Pandol SJ. Ethanol sensitizes NF-κB activation in pancreatic acinar cells through effects on protein kinase Cε. Am J Physiol Gastrointest Liver Physiol 291: G432–G438, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Thrower EC, Osgood S, Shugrue CA, Kolodecik TR, Chaudhuri AM, Reeve JR, Jr, Pandol SJ, Gorelick FS. The novel protein kinase C isoforms -δ and -ε modulate caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 294: G1344–G1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thrower ECP, Wang JM, Cheriyan S, Lugea AP, Kolodecik TR, Yuan JP, Reeve JR, Jr, Gorelick FS, Pandol SJ. Protein kinase Cδ-mediated processes in cholecystokinin-8-stimulated pancreatic acini. Pancreas 38: 930–935, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci USA 91: 8572–8576, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Acker GJ, Weiss E, Steer ML, Perides G. Cause-effect relationships between zymogen activation and other early events in secretagogue-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 292: G1738–G1746, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Vertommen D, Rider M, Ni Y, Waelkens E, Merlevede W, Vandenheede JR, Van Lint J. Regulation of protein kinase D by multisite phosphorylation. Identification of phosphorylation sites by mass spectrometry and characterization by site-directed mutagenesis. J Biol Chem 275: 19567–19576, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Waldron RT, Rey O, Iglesias T, Tugal T, Cantrell D, Rozengurt E. Activation loop Ser744 and Ser748 in protein kinase D are transphosphorylated in vivo. J Biol Chem 276: 32606–32615, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Waldron RT, Rozengurt E. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. J Biol Chem 278: 154–163, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Waldron RT, Whitelegge JP, Faull KF, Rozengurt E. Identification of a novel phosphorylation site in c-jun directly targeted in vitro by protein kinase D. Biochem Biophys Res Commun 356: 361–367, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Waterford SD, Kolodecik TR, Thrower EC, Gorelick FS. Vacuolar ATPase regulates zymogen activation in pancreatic acini. J Biol Chem 280: 5430–5434, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuan J, Lugea A, Zheng L, Gukovsky I, Edderkaoui M, Rozengurt E, Pandol SJ. Protein kinase D1 mediates NF-κB activation induced by cholecystokinin and cholinergic signaling in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 295: G1190–G1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yuan J, Slice L, Walsh JH, Rozengurt E. Activation of protein kinase D by signaling through the alpha subunit of the heterotrimeric G protein Gq. J Biol Chem 275: 2157–2164, 2000 [DOI] [PubMed] [Google Scholar]