Abstract
Atherogenesis is associated with elevated levels of low-density lipoprotein (LDL) and its oxidized form (oxLDL) in the blood. The liver is an important scavenger organ for circulating oxLDLs. The present study aimed to examine endocytosis of mildly oxLDL (the major circulating form of oxLDLs) in liver sinusoidal endothelial cells (LSECs) and the involvement of the scavenger receptors stabilin-1 and stabilin-2 in this process. Freshly isolated LSECs, Kupffer cells (KCs), and stabilin-1- and stabilin-2-transfected human embryonic kidney cells were incubated with fluorescently labeled or radiolabeled oxLDLs [oxidized for 3 h (oxLDL3), 6 h, or 24 h (oxLDL24)] to measure endocytosis. The intracellular localization of oxLDLs and stabilins in LSECs was examined by immunofluorescence and immunogold electron microscopy. Whereas oxLDL24 was endocytosed both by LSECs and KCs, oxLDL3 (mildly oxLDL) was taken up by LSECs only. The LSEC uptake of oxLDLs was significantly inhibited by the scavenger receptor ligand formaldehyde-treated serum albumin. Uptake of all modified LDLs was high in stabilin-1-transfected cells, whereas stabilin-2-transfected cells preferentially took up oxLDL24, suggesting that stabilin-1 is a more important receptor for mildly oxLDLs than stabilin-2. Double immunogold labeling experiments in LSECs indicated interactions of stabilin-1 and stabilin-2 with oxLDL3 on the cell surface, in coated pits, and endocytic vesicles. LSECs but not KCs endocytosed mildly oxLDL. Both stabilin-1 and stabilin-2 were involved in the LSEC endocytosis of oxLDLs, but experiments with stabilin-transfected cells pointed to stabilin-1 as the most important receptor for mildly oxLDL.
Keywords: endocytosis, scavenger receptors, scavenger endothelial cells, mildly oxidized low-density lipoprotein, stabilin
low-density lipoprotein (LDL), the main cholesterol carrier in blood, can undergo in vivo oxidation in the arterial walls (58) and plasma (2, 19). This modification transforms LDL to a proinflammatory, immunogenic, and cytotoxic oxidized LDL (oxLDL) that is generally held as a key component in atherosclerosis development (48, 57). oxLDL is also associated with ageing (6) and pathologies such as Alzheimer's disease (25), glomerulosclerosis (28), and diabetes (31).
The oxidation of LDL renders it a scavenger receptor (SR) ligand (11, 20). In the early events of atherosclerosis, arterial wall intima macrophages take up oxLDL via SR pathways, resulting in cholesterol accumulation and subsequent foam cell formation (48, 57). In patients with cardiovascular disease (acute myocardial infarction), plasma levels of oxLDL have been reported to be approximately fourfold higher than in healthy subjects (19), and it has been suggested that an efficient mechanism of oxLDL clearance, maintaining low levels of oxLDL in the circulation, is essential to avoid cardiovascular complications from this ligand (19, 22).
The extent of oxidation of the LDL particle affects a wide spectrum of biological properties of oxLDL, for instance, the composition of the LDL particle and the affinity for macrophage SRs (27, 49). Mildly oxLDL is the major form of oxLDL found in blood (7, 18, 19), whereas heavily oxLDL is present mainly in atherosclerotic plaques (58). However, also mildly oxidized forms of LDL have proatherogenic properties (3, 56, 57). Plasma clearance studies performed in rodents by intravenous injection of radiolabeled heavily oxLDL (i.e., LDL oxidized for 20–24 h) showed that the ligand was rapidly removed from blood by uptake in Kupffer cells (KCs, resident liver macrophages) and liver sinusoidal endothelial cells (LSECs) (30, 54). Approximately 50% of the injected ligand was removed by KCs, whereas one-third of the ligand was recovered in LSECs (54). Mildly oxLDL (LDL oxidized for 3 h) was removed from the circulation at a markedly slower rate (54), and the role of KCs and LSECs in elimination of mildly oxLDL has not been fully elucidated.
Eliminating a wide range of potentially injurious particles and molecules from the blood, KCs and LSECs together constitute the largest scavenger cell system in the body. Particulate matter (>200 nm in diameter) is phagocytosed by the KCs, whereas LSECs mediate clearance of soluble macromolecules and colloids <200 nm in diameter via receptor-mediated endocytosis (45). Our hypothesis is that mildly oxLDLs are more susceptible to endocytic uptake in the LSECs, whereas the heavily oxLDL, which tends to aggregate (39), is more prone to phagocytic uptake in KCs.
LSECs express several different SRs that have been suggested as possible mediators of oxLDL uptake. These include SR-A (21, 33), SR-B (SR-B1 and CD36) (33), and SR-H (stabilin-1/FEEL-1/CLEVER-1 and stabilin-2/FEEL-2/HARE) (1, 35, 40, 41, 61). Several reports point to a minor role of SR-A and -B in the clearance of oxLDL and other SR ligands in LSECs. Studies in SR-A knockout mice showed normal blood clearance of oxLDL, and cultured LSECs from these mice endocytosed and degraded acetylated LDL (another model ligand for SRs) equally well as wild-type LSECs (14, 30, 55). Of the class B SRs, the expression of SR-B1 in LSECs was found to be rather low compared with hepatocytes (33), and studies in SR-B1 knockout mice showed no difference in oxLDL blood clearance compared with wild types (5). The finding that an antibody to CD36 that inhibits CD36-mediated uptake of SR ligands in other cell types had no effect on the LSEC uptake of SR ligands (37) also suggests a minor role of this SR receptor in LSECs.
These findings suggest that the “classical” SRs (SR-A, SR-B1, and CD36) are unimportant in the LSEC-mediated uptake of SR ligands. Instead, it has been suggested that the recently discovered SRs stabilin-1 and stabilin-2 that are highly expressed in the LSECs (34, 35, 40) play an important role in the elimination of blood-borne macromolecular SR ligands (15, 16, 35). However, their role in the LSEC endocytosis of oxLDLs has not been elucidated.
The present study was carried out to examine the LSEC-mediated endocytosis of LDL with different degrees of oxidation and the involvement of stabilin-1 and -2 in this process.
MATERIALS AND METHODS
Chemicals and Reagents
Formaldehyde-treated bovine serum albumin (FSA) was prepared as described (36). Rabbit nonimmune IgG, mouse serum, goat serum, monensin, and EDTA were from Sigma Chemical (St. Louis, MO). Carrier-free Na125I was from Perkin-Elmer Norge (Oslo, Norway), and 1,3,4,6-tetrachloro-3α,6α-diphenylglycoluril (Iodogen) was from Pierce Chemical (Rockford, IL). Protein A Hi Trap columns and Gelatin Sepharose 4B were from GE Healthcare (Uppsala, Sweden). Human fibronectin was purified from human plasma by affinity chromatography on Gelatin Sepharose 4B as described by the manufacturer. Culture medium RPMI 1640 and DMEM were from PAA Laboratories (Pasching, Austria), and endothelial cell growth medium was from Medprobe (Oslo, Norway). Human serum albumin (HSA) was from Octapharma (Ziegelbrucke, Switzerland) and fetal calf serum (FCS) was from Bio Whittaker. Hyaluronan (14 kDa) was a kind gift of Dr. Staffan Johansson (Uppsala University), and Healon (1,900–3,900 kDa) was from Pharmacia (Uppsala, Sweden). Rabbit anti-human Cu2+ oxidized LDL IgG was from Abcam (Cambridge, UK), and rabbit anti-fluorescein isothiocyanate (FITC) was from Dako (Denmark). The antibodies against different SRs are listed in Table 1. Nuclear dye Draq 5 was from Biostatus Limited (Leicestershire, UK). Alexa-488 goat anti-rabbit, Alexa-546 and Alexa-488 goat anti-mouse-antibodies, and the fluorescent probe 3,3-dioctadecylindocarbocyanine (DiI) were from Molecular Probes (Eugene, OR).
Table 1.
Antibodies targeting scavenger receptors
| Dilution |
|||||
|---|---|---|---|---|---|
| Antibody | Raised Against | Species Specificity | Confocal microscopy | Electron microscopy | Ref. No./Manufacturer |
| Anti-hS1 | COOH-terminal portion of recombinant human stabilin-1 | Human, pig, rat, mouse | 1:500 (antiserum) | 15,39 | |
| WS-1 | Whole recombinant human stabilin-1 | Human, pig, rat, mouse | 180 μg/ml (serum IgG) | 15 | |
| Anti-rS2 | Whole rat liver stabilin-2, SDS denatured | Human, pig, rat, mouse | 1:400 (antiserum) | 50 μg/ml (serum IgG) | 34,39 |
| Anti-LOX-1 | Oxidized low-density lipoprotein receptor 1 (LOX-1, clone T20) | Rat, bovine | 5 μg/ml | 43 | |
| Anti-CD36 | CD36 (clone FA6-152) | Human, rat | 2 μg/ml | Abcam (Cambridge, UK) | |
| Anti-CD163 | Rat macrophages | Rat | 10 μg/ml | AbD Serotec (Oxford, UK) | |
h, Human; r, rat.
Animals
Sprague Dawley male rats (Scanbur BK, Sollentuna, Sweden) were kept under standard conditions and fed standard chow ad libitum (Scanbur, Nittedal, Norway). The experimental protocols were approved by the ethics committee of the Norwegian Animal Research Authority in accordance with the Norwegian Animal Experimental and Scientific Purposes Act of 1986.
LDL Isolation and Oxidation
LDL (density = 1.019–1.063 g/ml) was isolated from fresh human plasma by density gradient ultracentrifugation (42) and preserved with 10% sucrose in 150 mM NaCl with 0.24 mM EDTA (pH 7.4) at −80°C for ≤6 mo (43). Before the experiments, sucrose was removed by dialysis at 4°C against phosphate-buffered saline (PBS). The protein concentration was determined by Bio-Rad protein assay (Bio-Rad, Oslo, Norway). LDL (0.2 mg/ml) was oxidized by copper sulfate (CuSO4, 10 μM) at 37°C for 3, 6, or 24 h (oxLDL3, oxLDL6, and oxLDL24, respectively). The oxidation process was stopped by adding EDTA to 305 μM after which samples were stored under a nitrogen atmosphere at 4°C for ≤1 wk before use to avoid further oxidation. CuSO4 and EDTA were removed by extensive dialysis against PBS at 4°C, and oxLDLs were centrifuged (12,000 g) for 20 min before use.
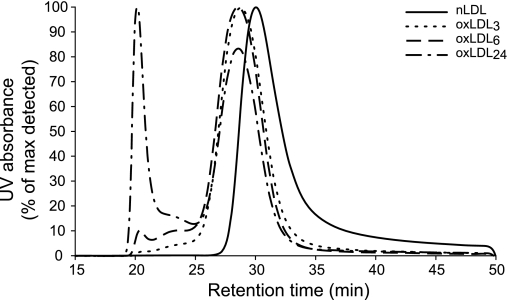
The chromatographic profile of LDL and oxLDLs was evaluated by size-exclusion chromatography on a Superose-6 10/300 column (Amersham Pharmacia Biotech) using a DIONEX HPLC system (Fig. 1). The LDL showed a main peak corresponding to 2,100 kDa while oxLDL3 and oxLDL6 had a similar molecular mass, 3,000 and 3,100 kDa, respectively. oxLDL24 showed two peaks, one corresponding to 3,100 kDa and the other representing molecules ≥32,000 kDa. The relative agarose gel electrophoresis mobility (REM) (38) of oxLDL3, oxLDL6, and oxLDL24 compared with native LDL was 1.46 ± 0.17, 1.80 ± 0.26, and 2.16 ± 0.23 respectively, in 6–12 experiments (means ± SD). Our oxLDL3 is closely similar to the oxLDL fraction (REM = 1.3) isolated from plasma of patients with transplant-associated coronary artery disease (18).
Fig. 1.
Size-exclusion chromatography of oxidized low-density lipoprotein (oxLDL). Separation of native LDL (nLDL) and oxLDL with different degrees of oxidation was performed by size-exclusion chromatography using the DIONEX HPLC system and Superose-6 10/300 column (Amersham Pharmacia Biotech). Low-density lipoprotein (LDL) retention time was detected by continuous ultraviolet (UV) absorbance (280 nm) measurement of the eluent. LDL relative molecular mass (Mr) values were calculated from the standard curve generated by plotting the retention time of protein standards against their corresponding Mr. Native LDL eluted as a single peak corresponding to 2,100 kDa. Oxidation induced a shift of the main peak toward a higher-molecular-size region [3,000 kDa for LDL oxidized by copper sulfate (CuSO4) for 3 h (oxLDL3), 3,100 kDa for LDL oxidized by CuSO4 for 6 (oxLDL6) and 24 (oxLDL24) h]. Moreover, with increased oxidation time, the additional peaks of intermediately (13,000 kDa) and heavily (32,000 kDa) aggregated material began to appear and became very pronounced in the sample oxidized for 24 h.
Labeling of Ligands
Native LDL, oxLDLs, and FSA in PBS were labeled with carrier-free Na125I in a direct reaction using Iodogen as described by the manufacturer and separated from unbound 125I by dialysis against PBS with 1 mM EDTA and 0.02% azide at 37°C overnight, and then against PBS at 4°C for 2 h before use. The resulting specific radioactivity was ∼1–2 × 106 cpm/μg protein. DiI-oxLDLs were prepared by incubating oxLDL and DiI in a protein-dye ratio of 1,000:1 at 37°C for 16 h under nitrogen atmosphere. FITC-labeled FSA (FITC-FSA) was prepared as described (29).
Endocytosis of Radiolabeled Ligands
Rat LSECs were isolated and purified as described (47), seeded (0.5 × 106 cells/cm2) in 1- or 2-cm2 collagen-coated tissue culture wells, and maintained in serum-free RPMI 1640 medium for 1–2 h before use. Human embryonic kidney 293 cells (HEK) stably transfected with mouse stabilin-1 (mS1-HEK), mouse stabilin-2 (mS2-HEK) (15) and nontransfected HEK were a kind gift from Dr. Sophie Johansson (Uppsala University). These cell lines were grown in 2-cm2 fibronectin-coated wells in DMEM/10% FCS until confluent and then incubated with RPMI 1640/1% HSA for 8 h before use in experiments. 125I-labeled oxLDL endocytosis (1–8 h) experiments were done as previously described (16). Antibody inhibition studies were performed as described (16, 29).
Immunofluorescence Microscopy
Primary cultures of rat nonparenchymal liver cells (NPCs) were obtained from the NPC-enriched liver cell suspension after removal of hepatocytes by low-speed differential centrifugation (47). Purified LSEC cultures (>95% LSECs) and NPC cultures containing 10–24% KCs (CD163 positive cells) and 75–91% LSECs (stabilin-2 positive cells) and very few (≤1%) stellate cells (identified by their content of autofluorescent vitamin A) were established on collagen-coated glass cover slips and incubated with ligands at indicated conditions, washed, and then fixed in 4% formaldehyde for 15 min at 4°C. The cells were permeabilized in 0.01% Triton X-100 for 4 min and immune labeled by antibodies against stabilins, CD36, or CD163 (see Table 1) as described (15, 29). Phagocytosis of 2-μm latex beads was used as a functional marker of KCs. Specimens were examined in a Zeiss Laser Scanning Microscope 510 Meta (Carl Zeiss, Obercochen, Germany) with an immersion-oil Plan-Apochromat ×63/1.4 objective lens (pinholes = 1 airy disc; optical section <0.8 μm). The weighted colocalization coefficients (WCC) were calculated using the Zeiss LSM software.
Immunogold Electron Microscopy
LSEC cultures were incubated with 40 μg/ml oxLDL3 or 10 μg/ml FITC-FSA in RPMI 1640 medium for 15 min, then washed in ice-cold PBS and fixed in 4% formaldehyde in 200 mM HEPES buffer, pH 7.5, for 1 h. Cryosections were prepared, and immunocytochemical labeling was performed as described (13, 52). Antibodies against oxLDL (30 μg/ml), FITC (37 μg/ml), and stabilins (see Table 1) were detected by protein A-gold complexes. Double labeling was performed in a sequential manner, using a fixative block (1% glutaraldehyde in H2O) between the first and second marker pair. The dried sections were examined in a JEOL JEM 1010 transmission electron microscope (JEOL, Tokyo, Japan) operating at 80 kV. Control experiments were routinely included in parallel by omission of the primary antibodies.
SDS-PAGE and Western Blot Analysis
Rat LSECs, bovine aorta endothelial cells (AECs, obtained by gentle scraping of the endothelial lining of bovine aorta within 4 h postmortem), and rat liver extract (Wistar female) were solubilized in RIPA buffer with proteinase inhibitors, subjected to SDS-PAGE (4–12% gels; Invitrogen), and then transferred to nitrocellulose membranes. The target protein on the membranes was detected with anti-CD36 (1:300) or anti-LOX-1 (10 μg/ml) and visualized with luminol.
Statistical Analysis
The statistical analysis of endocytosis results was performed with one-way ANOVA and Bonferroni post hoc test, using SPSS software (version 15.00; SPSS, Chicago, IL).
RESULTS
Mildly oxLDL is Endocytosed by LSECs but not by KCs
Previous studies have shown that heavily oxLDL (20 h oxLDL) is taken up by KCs and LSECs, with the highest uptake in KCs (54). To test if this is also true for mildly oxLDL (oxLDL3), freshly established NPC cultures containing both KCs (10–24%) and LSECs (75–91%) were incubated with 10 μg/ml DiI-labeled oxLDL3 for 30 min and then with 0.5 × 106/ml latex beads (ø = 2 μm) for another 30 min; fixed and labeled with antibodies against stabilin-2, CD36, or the macrophage marker CD163; and subjected to confocal microscopy (Fig. 2, A–C). This revealed massive uptake of oxLDL3 in LSECs (stabilin-2-positive cells), but no detectable uptake in KCs, distinguished by their active phagocytosis of latex beads. Only KCs showed positive CD163 and CD36 staining. Parallel cultures incubated with 10 μg/ml DiI-labeled oxLDL24 for 30 min showed active uptake of this ligand in both LSECs and KCs (Fig. 2, D–F). Rat hepatocytes in primary culture showed no uptake of either of the two oxLDLs (data not shown).
Fig. 2.
Confocal microscopy analysis of oxLDL uptake in nonparenchymal liver cells (NPCs). Rat NPCs were incubated with 10 μg/ml of 3,3-dioctadecylindocarbocyanine (DiI)-oxLDL3 (A–C, red fluorescence) or DiI-oxLDL24 (D–F, red fluorescence) at 37°C for 30 min; washed and incubated with 0.5 × 106/ml latex beads (ø = 2 μm) for another 30 min; and fixed and immune labeled with antibodies against stabilin-2 (A and D), CD36 (B and E), or the macrophage marker CD163 (C and F). Positive immune labeling was visualized with Alexa-488 goat anti-rabbit/anti-mouse antibodies (green fluorescence), respectively. Cell nuclei were visualized with Draq 5 (blue fluorescence). The images presented are overlays of the triple-fluorescence channels and bright field images. Arrows point to latex beads in Kupffer cells (KCs).
Endocytosis of oxLDLs in LSECs
The time course of oxLDL and LDL endocytosis in LSECs was examined by incubating primary LSEC cultures with radiolabeled ligand (0.1 μg/ml) for 1, 2, and 4 h at 37°C (Fig. 3A). The cultures did not recognize LDL, whereas the uptake of 125I-oxLDL3, 125I-oxLDL6, and 125I-oxLDL24 was 15, 25, and 29%, respectively, after 1 h and increased twofold from 1 to 4 h, indicating a specific uptake mechanism for oxLDLs in LSECs.
Fig. 3.
Endocytosis of oxLDLs in liver sinusoidal endothelial cells (LSECs). A: time course endocytosis in rat LSEC cultures incubated with 0.1 μg/ml of radiolabeled ligands for various time periods at 37°C. Results (means ± SD) are averages of triplicate measurements representing the sum of cell-associated and degraded ligand (details in materials and methods). Corresponding results were found in two other experiments. B: LSEC capacity for endocytosis of oxLDLs. Rat LSEC cultures were incubated with radiolabeled ligand (0.1 μg/ml) alone (control) or together with the indicated amounts of homologous nonlabeled molecules for 1 h at 37°C. The results are means of 4 experiments, representing cells from 4 different animals. Error bars represent SE. C: specificity of endocytosis of oxLDLs in rat LSEC cultures incubated with 0.1 μg/ml of indicated radiolabeled ligands alone (control) or together with excess amounts of nonlabeled formaldehyde-treated serum albumin (FSA) or LDL (100 μg/ml) for 2 h at 37°C. The results shown represent an average of 4 independent experiments. *Statistically significant (P < 0.01) difference in uptake compared with the other two treatments.
The LSEC oxLDL endocytic capacity was investigated by incubating the cells with 0.1 μg/ml of 125I-oxLDLs together with nonlabeled oxLDL at 0, 5, 10, 20, or 40 μg/ml at 37°C for 1 h (Fig. 3B). The LSEC endocytosis of oxLDL3 was saturated at lower ligand concentrations than the endocytosis of LDL oxidized for 6 or 24 h (Fig. 3B).
The specificity of oxLDL endocytosis in rat LSECs was investigated by competitive inhibition experiments (Fig. 3C). Coincubation of radiolabeled oxLDL3, oxLDL6, and oxLDL24 (0.1 μg/ml) with excess amounts (100 μg/ml) of the SR ligand FSA (4) for 2 h almost completely blocked the LSEC uptake of 125I-oxLDL3 and 125I-oxLDL6, whereas the endocytosis of 125I-oxLDL24 was inhibited by 35% (P < 0.01; n = 4). These findings confirm previous reports that uptake of oxLDLs in LSECs is via an SR-mediated process. Nonmodified LDL had no significant inhibitory effect on the endocytosis of any of the oxLDLs in these cells. Hyaluronan (100 μg/ml) of high or low molecular weight had no inhibitory effect on the LSEC uptake of oxLDLs or FSA (data not shown).
Antibody inhibition experiments where LSEC cultures were preincubated with IgG [1 mg/ml; dose as described (16, 29)] purified from anti-rat (r) S2 antiserum, for 30 min before a 2-h incubation of the cells with 125I-FSA, 125I-oxLDL3, 125I-oxLDL6, or 125I-oxLDL24 (0.1 μg/ml) at 37°C, exhibited a slight but not significant inhibitory effect of the stabilin-2 antibody on the endocytosis of the oxLDLs (n = 5, data not shown), whereas the uptake of 125I-FSA was inhibited by 45% (n = 3, P < 0.01) compared with control cultures treated with nonimmune IgG.
Endocytosis of oxLDL in Stabilin-1- and Stabilin-2-Transfected HEK
The uptake of oxLDLs in mS1-HEK and mS2-HEK was investigated by time course endocytosis of radioiodinated ligands. Confluent cultures were incubated with 0.1 μg/ml of 125I-LDL or 125I-oxLDLs for 1–8 h (Fig. 4). A basal level of LDL endocytosis was measured both in nontransfected and stabilin-transfected HEK (Fig. 4A). In the mS1-HEK, the time course of oxLDL3, oxLDL6, and oxLDL24 endocytosis was similar and markedly higher than the LDL uptake (Fig. 4B). In contrast, in the mS2-HEK, the oxLDL3 and oxLDL6 uptake was only slightly higher than the LDL uptake, whereas the uptake of oxLDL24 was significantly higher (Fig. 4C). This suggests that stabilin-2 has a higher affinity for highly oxLDL compared with mildly oxLDL, whereas stabilin-1 shows similar affinity for all oxLDLs.
Fig. 4.
Time course endocytosis of oxLDLs in stabilin-1- and stabilin-2-transfected cells. Confluent nontransfected human embryonic kidney 293 cell (HEK) cultures (A) and cultures of HEK cells stably transfected with mouse stabilin-1 (mS1-HEK, B) and stabilin-2 (mS2-HEK, C) were incubated with 0.1 μg/ml of radiolabeled ligands as indicated at 37°C for various time periods. Results (means ± SD) are averages of triplicate measurements representing the sum of cell-associated and degraded ligand (details in materials and methods). Corresponding results were found in two other experiments.
Competitive inhibition experiments in the transfected cell lines were performed using the same protocol as used for rat LSEC cultures. In mS1-HEK (Fig. 5A), FSA (100 μg/ml) significantly inhibited the uptake of all oxLDLs (P < 0.05; n = 4), whereas the uptake of 125I-LDL was not affected. Excess amounts of nonlabeled LDL only inhibited the endocytosis of 125I-LDL in the mS1-HEK (Fig. 5A). This indicates that the uptake of all oxLDLs in mS1-HEK is mainly through stabilin-1. The competitive inhibition of FSA and LDL was different in the mS2-HEK (Fig. 5B). In these cells, FSA (100 μg/ml) inhibited the uptake of 125I-oxLDL6 and 125I-oxLDL24 (P < 0.05; n = 4) but not the 125I-LDL and 125I-oxLDL3 uptake, which in turn were inhibited by LDL (84 and 49% for 125I-LDL and 125I-oxLDL3, respectively), suggesting that a significant part of the uptake of oxLDL3 in mS2-HEK is through the LDL receptor.
Fig. 5.
Specificity of endocytosis of oxLDLs in stabilin-1- and stabilin-2-transfected cells. Confluent mS1-HEK cultures (A) and mS2-HEK cultures (B) were incubated with 0.1 μg/ml of the indicated radiolabeled ligands alone (control) or together with excess amounts of nonlabeled FSA or LDL (100 μg/ml) for 2 h at 37°C. The results presented are an average of three independent experiments. *Statistically significant (P < 0.01) difference in uptake compared with the other two treatments. **Statistically significant (P < 0.01) difference in uptake compared with control only.
Cross competition experiments between oxLDL3 and oxLDL24 in the stabilin-transfected HEK cells showed that excess amounts (100 μg/ml) of oxLDL24 could inhibit endocytosis of 125I-oxLDL3 (0.1 μg/ml) in both cell lines (84 and 66% inhibition in mS1-HEK and mS2-HEK, respectively; P < 0.05, n = 3), suggesting that part of the oxLDL3 uptake in the mS2-cells also goes via stabilin-2. Interestingly, oxLDL3 was also able to significantly inhibit the uptake of 125I-oxLDL24 (P < 0.05, n = 3). However, the inhibitory effect of oxLDL3 on 125I-oxLDL24 endocytosis was more pronounced in the stabilin-1-transfected cells (48 vs. 28% inhibition in mS1- and mS2-HEK, respectively), suggesting a stronger affinity of mildly oxLDL for stabilin-1. Hyaluronan (100 μg/ml) (high or low molecular weight) did not inhibit the uptake of any forms of oxLDLs, LDL, or FSA in these cells (data not shown).
In HEK, LDL significantly inhibited the uptake of 125I-LDL, 125I-oxLDL3, and 125I-oxLDL6, but not 125I-oxLDL24; FSA had no significant effect on the uptake of any of the ligands (data not shown), indicating that some of the uptake of oxLDLs is via the LDL receptor in HEK.
Intracellular Localization of Mildly oxLDL and Stabilins in LSECs
The cellular localization of endocytosed oxLDL3, stabilin-1, and stabilin-2 in primary rat LSEC was investigated by confocal laser scanning microscopy and immunogold electron microcopy.
Confocal microscopy.
Following incubation for 1 h at 4°C in the presence of 40 μg/ml DiI-oxLDL3, LSEC cultures were washed, and the incubation continued for another 20 min at 37°C in ligand-free medium. In parallel incubations, LSECs were pulsed (10 min at 37°C) with DiI-oxLDL3 (10 μg/ml) in serum-free medium before the incubation was continued for another 50 min in the presence of 10 μM monensin (a vacuolar-type H+-ATPase inhibitor that inhibits vesicular traffic from early endosomes). The cells were then fixed and immune stained for stabilin-1 and stabilin-2.
Colocalization of stabilin-1 and DiI-labeled oxLDL3 in intracellular vesicles of non-monensin-treated LSECs is shown in Fig. 6, A–C (WCCs: 80.8 ± 8.8% for stabilin-1 and 57.3 ± 14.1% for oxLDL3, n = 13). Control cultures labeled with preimmune serum at similar concentrations showed insignificant staining for stabilin-1 (data not shown). With monensin treatment, DiI-oxLDL3 and stabilin-1 accumulated in the same enlarged vesicles (Fig. 6, D–F) (WCCs: 83.7 ± 6.1% for stabilin-1 and 84.0 ± 6.4% for oxLDL3, n = 15), indicating that stabilin-1 colocalizes with oxLDL3 in the endocytic pathway of the ligand.
Fig. 6.
Confocal microscopy analysis of stabilin-1 and oxLDL colocalization in LSECs. A–C: pulse-chase experiment. Rat LSEC cultures were incubated with 40 μg/ml of DiI-oxLDL3 at 4°C for 1 h and then washed and incubated in medium only at 37°C for 20 min. D–F: cells were incubated with 10 μg/ml of DiI-oxLDL3 at 37°C for 10 min and then incubated in the presence of 10 μM monensin for another 50 min to inhibit intracellular traffic and recycling of receptor. The cells were immune labeled with anti-human S1 antiserum and visualized with Alexa-488 goat anti-rabbit antibody. Arrows point to colocalized DiI-oxLDL3 (red fluorescence) and stabilin-1 (green fluorescence) labeling in corresponding channels. Cell nuclei are visualized with Draq 5 (blue fluorescence) in the overlays.
Immune staining of LSECs incubated with DiI-oxLDL3, with stabilin-2 antiserum (anti-rS2), also showed colocalization of stabilin-2 with endocytosed ligand (Fig. 7, A–C) (WCCs: 95.5 ± 4.7% for stabilin-2 and 54.8 ± 11.9% for oxLDL3, n = 13). Preimmune serum gave insignificant staining (data not shown). In monensin-treated LSECs, the colocalization of DiI-oxLDL3 with stabilin-2 (Fig. 7, D–F) was enhanced (WCCs: 72.6 ± 4.5% for stabilin-2 and 91.9 ± 4.5% for oxLDL3, n = 12).
Fig. 7.
Confocal microscopy analysis of stabilin-2 and oxLDL colocalization in LSECs. A–C: pulse-chase experiment. Rat LSEC cultures were incubated with 40 μg/ml of DiI-oxLDL3 at 4°C for 1 h and then washed and incubated in medium only at 37°C for 20 min. D–F: rat LSEC cultures were incubated with 10 μg/ml of DiI-oxLDL3 at 37°C for 10 min and then incubated in the presence of 10 μM monensin for another 50 min. The cells were fixed and immune-labeled with anti-rat S2 antiserum and visualized with Alexa-488 goat anti-rabbit antibody. Arrows point to colocalized DiI-oxLDL3 (red) and stabilin-2 (green) labeling in corresponding channels, and cell nuclei are visualized with Draq 5 (blue fluorescence).
Electron microscopy.
The colocalization of stabilin-1 and -2 with oxLDL3 in LSECs was examined in greater detail by electron microscopy of double immunogold-labeled cryosections of cells incubated with 40 μg/ml oxLDL3 for 15 min at 37°C (Fig. 8). Because FSA significantly inhibited uptake of oxLDL3 in stabilin-1- and stabilin-2-transfected cells, similar types of studies were also performed on LSECs incubated with 10 μg/ml FITC-FSA for 15 min [Supplemental Fig. 1 (Supplemental data for this article may be found on the American Journal of Physiology: Gastrointestinal and Liver Physiology website.)]. The electron micrographs were analyzed by counting the number of gold particles and measuring the distance between differently sized gold markers. The distances regarded as indicative of direct receptor-ligand interaction were as follows: FITC-FSA and stabilins, <30 nm; oxLDL and stabilins, <50 nm (the larger distance is allowable because of the larger size of the oxLDL particle).
Fig. 8.
Double immunogold labeling for stabilin-1/-2 and oxLDL3 in LSECs. Rat LSECs were incubated with 40 μg/ml oxLDL3 for 15 min at 37°C, fixed, and processed for immunogold labeling as described in materials and methods. Arrows point to close colocalization of small and large gold particles, indicating direct receptor-ligand interaction. Arrowheads point to the plasma membrane. N, cell nucleus; CP, coated pits. Scale bars = 200 nm. A and B: close colocalization of oxLDL3 (5 nm gold) and stabilin-1 (10 nm gold) is seen in an endocytic vesicle and at the plasma membrane surface in A and in a CP in B. C–E: close colocalization of stabilin-2 (5 nm gold) and oxLDL3 (10 nm gold) is seen in larger endosomes (C), coated vesicles (D), and in CP (E).
Colocalization of stabilin-1 with oxLDL3 was seen mostly in larger endosomes but also in coated pits and occasionally at the cell surface (Fig. 8, A and B). Image analysis (n = 15) indicated that ∼32% of the stabilin-1 molecules were bound to oxLDL3. Colocalization of stabilin-1 with FITC-FSA was seen in similar types of structures as with oxLDL3 (Supplemental Fig. 1, A–C).
Colocalization of stabilin-2 with oxLDL3 (Fig. 8, C–E) and FITC-FSA (Supplemental Fig. 1, D and E) was also seen in larger endosomes, in coated vesicles, and occasionally at the cell surface. Image analysis (n = 15) indicated that ∼6% of the stabilin-2 molecules were bound to oxLDL3.
Expression of LOX-1 and CD36 in LSECs
CD36 and LOX-1 are two important receptors for oxLDL in macrophages and endothelial cells in atherosclerotic plaques (27, 44). The expression of CD36 and LOX-1 in rat LSECs was examined by SDS-PAGE and Western blotting of RIPA buffer lysates of freshly isolated cells and by immunofluoresence of freshly isolated liver NPCs. Anti-CD36 antibodies stained KCs but not LSECs (Fig. 2, B and E), whereas the LOX-1 staining was negative in both cell types (data not shown). Rat LSECs were negative for both CD36 and LOX-1 in Western blots, whereas protein extracts of rat whole liver and bovine AECs showed positive bands for CD36 and anti-LOX-1, respectively (Supplemental Fig. 2).
DISCUSSION
Atherogenic blood-borne oxLDL is removed mainly by cells lining the liver sinusoids (30, 54). Although heavily oxLDL (oxLDL24) was taken up both by KCs and LSECs (54), we demonstrated that the more “physiological” oxLDL3 (mildly oxLDL) (7, 18, 19) was recognized only by LSECs, with no detectable uptake in KCs (Fig. 2). This suggests an important role for LSECs in plasma elimination of oxLDLs and thus in prevention of atherosclerosis.
Oxidation of LDL for 20–24 h induces excessive modification of the LDL, including chemical modifications and aggregation and fusion of the molecules (39). This results in an increased net negative charge and size of the LDL particle (Fig. 1). Therefore, the clearance mechanism of mildly oxLDL and heavily oxLDL and their affinity to receptors may vary, since both aggregation and degree of modification exhibit profound effects on receptor/ligand interaction (8, 27, 50).
KCs and LSECs constitute the liver reticuloendothelial system. KCs remove particles ≥200 nm by phagocytosis, and LSECs eliminate soluble macromolecules and colloids <200 nm (45). In the present study, heavily oxLDL exhibited (at least) 10-fold higher molecular mass compared with mildly oxLDLs and native LDL (Fig. 1), probably because of aggregation and fusion, rendering it susceptible to KC phagocytosis. Mildly oxLDLs showed only a minor increase in size compared with native LDL, rendering it prone to endocytosis by LSECs rather than phagocytosis by KCs. This notion is in agreement with findings that very slight degrees of oxidation of LDL led to removal from plasma (24) but were not sufficient to increase the in vitro uptake in macrophages (49). Furthermore, pretreatment of rats with gadolinium chloride, which selectively removes KCs from the liver, reduced the hepatic uptake of intravenously injected mildly oxLDL only from 80 to 60% compared with control animals (53). These reports, along with the present findings, suggest that LSECs play an important role in the blood clearance of mildly oxLDL.
Various SRs can bind and/or mediate endocytosis of oxLDL and acetylated LDL as reviewed elsewhere (20). Among them SR-A (21, 33), SR-B1 (33), CD36 (9, 32), stabilin-1, and stabilin-2 (35, 40) have been reported to be present on LSECs. It is worth noting that oxLDL plasma clearance studies, and in vitro studies in SR-A and SR-B1 mouse knockout models, suggest a minor importance of these receptors in the LSEC elimination of oxLDLs (5, 14, 27, 30). Consistent with a previous report (9), we found no protein expression in rat LSECs of LOX-1, which is an important oxLDL receptor in atherosclerotic plaques (44). The rat LSECs were also negative for CD36. This finding is in contrast with studies in another rat strain (Wistar), which detected CD36 mRNA both in LSECs, KCs, and hepatocytes (9, 32), suggesting strain differences in the LSEC expression of this receptor. Strain and gender differences in CD36 protein expression were reported in rat hepatocytes (60). CD36 has been suggested as an important oxLDL receptor on macrophages (27), but our results indicate a minor role of this receptor in uptake of mildly oxLDL since none of the CD36 positive KCs in the NPC cultures endocytosed oxLDL3 (Fig. 2B).
Of the different SRs expressed in LSECs, the stabilins have been suggested as the most important receptors for blood clearance of macromolecular waste materials (15, 16). Stabilin-1 and -2 are highly expressed in the LSECs (34, 35, 40), and stabilin-2 is reported to be a major clearance receptor for several SR ligands (advanced glycation end products, hyaluronan, NH2-terminal propeptide of type I procollagen, and FSA) in the LSECs (16, 35). In addition, indirect evidence suggests that stabilin-1 is an important SR receptor in LSECs as well (15).
In the present study, stabilin-1- and stabilin-2-transfected HEK actively took up oxLDLs. Whereas the stabilin-2-transfected cells favored more heavily oxLDL (Fig. 4C), the stabilin-1-transfected cells took up mildly and heavily oxLDLs (Fig. 4B) at a similar rate. Furthermore, FSA, which is a specific ligand for LSEC SRs (10), inhibited uptake of mildly oxLDL in mS1-HEK but not in mS2-HEK (Fig. 5). These findings suggest that stabilin-1 is more important than stabilin-2 for endocytosis of mildly oxLDL. Notably, the inhibitory effect of FSA toward heavily oxLDL was lower than for mildly oxLDL in both stabilin-transfected cells. This is probably because of the higher net negative charge of heavily oxLDL, which increases the binding affinity of a given SR ligand (8).
A polyclonal stabilin-2 antibody, which is reported to inhibit the LSEC uptake of hyaluronan by 80% (35), failed to inhibit the uptake of any of the oxLDLs in LSECs, whereas the endocytosis of FSA was inhibited by 45%. These observations, along with the fact that FSA did not inhibit endocytosis of oxLDL3 in mS2-HEK, suggest that stabilin-2 is not the main receptor for mildly oxLDL. The more negatively charged heavily oxLDL was more actively endocytosed by the mS2-HEK, suggesting that its affinity to stabilin-2 may be very high, preventing effective binding competition by stabilin-2 antibodies. Stabilin-2 has an X-link hyaluronan-binding domain (17) and also several BX7B motifs that may bind this ligand (40). However, hyaluronan (high or low molecular weight) did not inhibit uptake of FSA or any of the oxLDLs in LSEC or in mS2-HEK cells, which suggests that the stabilin-2-binding domain of oxLDL (and FSA) is not the hyaluronan-binding region. To our knowledge, no functional inhibitory antibody is available for rat stabilin-1.
Immunofluorescence studies showed that oxLDL3 frequently colocalized with both stabilin-1 and -2 in endocytic compartments in monensin-treated LSECs (Figs. 5 and 6). Also by pulse-chase studies in nontreated cells, we found, when chasing for 20 min, that oxLDL3 accumulated and colocalized with stabilins in vesicles of LSECs. Stabilin-1 and -2 are transmembrane receptors that constitutively recycle between the plasma membrane and the compartments of endocytic pathways irrespective of ligand binding (15, 41), and it has been suggested that stabilin-1 internalizes its ligand by extremely rapid cycling between the cell surface and early endosome compartments (41). This explains the presence of stabilin-1- and stabilin-2-positive structures with little or no oxLDL3 cargo in the non-monensin-treated cells.
Immunofluorescence studies have too low resolution to show close colocalization, indicating direct receptor-ligand interactions, and we therefore performed double immunogold labeling experiments on LSEC cryosections for more detailed studies. Close colocalization of stabilin-1, and to a minor extent stabilin-2, with oxLDL3 was observed at the cell membrane surface, in coated pits, and in small and large vesicles of LSECs. Stabilin-2 has been previously reported to internalize other ligands via coated pits in LSEC cultures (16), and both stabilin-1 and -2 have been found in the clathrin- and adaptin-associated endocytic pathway (15). These reports further indicate that stabilin-mediated endocytosis of mildly oxLDL in LSECs is via a clathrin-mediated pathway, which is the main endocytic pathway in these cells (12, 26). Conversely, CD36 endocytosis has been shown to occur via a lipid raft pathway rather than the clathrin-mediated pathway (59). This is in agreement with the notion that CD36 may not be involved in the oxLDL3 uptake in LSECs.
oxLDL3 consists mainly of mildly oxLDL as shown by its agarose gel mobility. However, Chang et al. (7) found that a small proportion of heavily oxLDL also exists in oxLDL3. We observed small amounts of intermediately and even heavily aggregated material in the oxLDL3 preparation (Fig. 1), which is in keeping with the report by Chang et al. This may explain why FSA failed to inhibit oxLDL3 uptake in stabilin-2-transfected HEK, whereas stabilin-2 receptors colocalized with oxLDL3 as shown by electron microscopy of LSECs.
The mildly oxidized form of LDL is reported to be the major circulating oxLDL (7), whereas the heavily oxLDL detected in atherosclerotic lesions (58) is rarely found in the circulation of healthy human subjects, probably because of the many antioxidants present in plasma (51, 57). In addition, any heavily oxLDL that gains access to the circulation would be rapidly removed by uptake in liver (30, 54). Notably, mildly oxLDLs also exhibit pathogenic properties and are regarded as the physiological proatherogenic molecule (3, 56). Therefore, an effective LSEC clearance of circulating mildly oxLDL appears to be important in the prevention of atherosclerosis. Mildly oxLDL is removed from the circulation faster than LDL, but at a much slower rate than heavily oxLDL (54). This may, in part, be due to the relatively lower endocytic capacity of LSECs for this ligand compared with heavily oxLDL (Fig. 3B). We found that the saturation level of oxLDL3 uptake in rat LSECs was lower than its plasma concentration measured in cardiovascular disease (19). This could be because of the relatively lower amounts of stabilin-1 at the cell surface: stabilin-1 has cytoplasmic endosomal localization domains leading to its predominantly endosomal location (40). This saturation level is yet to be tested in human LSECs but presumably is not very high. In the aging liver, the endocytic function of LSECs was suggested to decrease, which, together with age-related endothelial thickening and defenestration (23, 46), may lead to ineffective clearance of oxLDL from the blood and increase the risk of lipid plaque formation in the arterial wall.
This study addresses the issue of plasma clearance of mildly oxLDL that represents physiological blood-borne oxLDL. Our findings lend support to the hypothesis that LSECs but not KCs are the most important scavenger cells in removing this mildly oxLDL from the circulation. Although both stabilin-1 and stabilin-2 are involved in the LSEC endocytosis of oxLDL, stabilin-1 appears to be more important for the uptake of mildly oxLDL.
GRANTS
This study was supported by Tromsø University Research Foundation, Norway; Basque Government, Spain (grant no. BFI 05.525); Tom Wilhelmsen's, Nansen's, and Inger Holm's Memorial Foundations, Norway; and National Institute on Ageing Grant 1.R21 AG-026582-01A1.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Helga-Marie Bye and Cristina Ionica Øie for technical assistance and Dr. Tatsuya Sawamura (National Cardiovascular Center Research Institute, Suita, Osaka, Japan) for kindly donating the anti-LOX-1 antibody.
REFERENCES
- 1. Adachi H, Tsujimoto M. FEEL-1, a novel scavenger receptor with in vitro bacteria-binding and angiogenesis-modulating activities. J Biol Chem 277: 34264–34270, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Avogaro P, Bon GB, Cazzolato G. Presence of a modified low density lipoprotein in humans. Arteriosclerosis 8: 79–87, 1988 [PubMed] [Google Scholar]
- 3. Berliner JA, Territo MC, Sevanian A, Ramin S, Kim JA, Bamshad B, Esterson M, Fogelman AM. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest 85: 1260–1266, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blomhoff R, Eskild W, Berg T. Endocytosis of formaldehyde-treated serum albumin via scavenger pathway in liver endothelial cells. Biochem J 218: 81–86, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bourret G, Brodeur MR, Luangrath V, Lapointe J, Falstrault L, Brissette L. In vivo cholesteryl ester selective uptake of mildly and standardly oxidized LDL occurs by both parenchymal and nonparenchymal mouse hepatic cells but SR-BI is only responsible for standardly oxidized LDL selective uptake by nonparenchymal cells. Int J Biochem Cell Biol 38: 1160–1170, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Brinkley TE, Nicklas BJ, Kanaya AM, Satterfield S, Lakatta EG, Simonsick EM, Sutton-Tyrrell K, Kritchevsky SB. Plasma oxidized low-density lipoprotein levels and arterial stiffness in older adults: the health, aging, and body composition study. Hypertension 53: 846–852, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang YH, Abdalla DS, Sevanian A. Characterization of cholesterol oxidation products formed by oxidative modification of low density lipoprotein. Free Radic Biol Med 23: 202–214, 1997 [DOI] [PubMed] [Google Scholar]
- 8. De Rijke YB, Biessen EA, Vogelezang CJ, van Berkel TJ. Binding characteristics of scavenger receptors on liver endothelial and Kupffer cells for modified low-density lipoproteins. Biochem J 304: 69–73, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duryee MJ, Freeman TL, Willis MS, Hunter CD, Hamilton BC, 3rd, Suzuki H, Tuma DJ, Klassen LW, Thiele GM. Scavenger receptors on sinusoidal liver endothelial cells are involved in the uptake of aldehyde-modified proteins. Mol Pharmacol 68: 1423–1430, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Elvevold K, Smedsrod B, Martinez I. The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. Am J Physiol Gastrointest Liver Physiol 294: G391–G400, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Eskild W, Henriksen T, Skretting G, Blomhoff R, Berg T. Endocytosis of acetylated low-density lipoprotein, endothelial cell-modified low-density lipoprotein, and formaldehyde-treated serum albumin by rat liver endothelial cells. Evidence of uptake via a common receptor. Scand J Gastroenterol 22: 1263–1269, 1987 [DOI] [PubMed] [Google Scholar]
- 12. Falkowska-Hansen B, Falkowski M, Metharom P, Krunic D, Goerdt S. Clathrin-coated vesicles form a unique net-like structure in liver sinusoidal endothelial cells by assembling along undisrupted microtubules. Exp Cell Res 313: 1745–1757, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Griffiths G. Fine Structure Immunocytochemistry. Berlin, Germany: Springer-Verlag, 1993 [Google Scholar]
- 14. Hansen B, Arteta B, Smedsrod B. The physiological scavenger receptor function of hepatic sinusoidal endothelial and Kupffer cells is independent of scavenger receptor class A type I and II. Mol Cell Biochem 240: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Hansen B, Longati P, Elvevold K, Nedredal GI, Schledzewski K, Olsen R, Falkowski M, Kzhyshkowska J, Carlsson F, Johansson S, Smedsrod B, Goerdt S, McCourt P. Stabilin-1 and stabilin-2 are both directed into the early endocytic pathway in hepatic sinusoidal endothelium via interactions with clathrin/AP-2, independent of ligand binding. Exp Cell Res 303: 160–173, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Hansen B, Svistounov D, Olsen R, Nagai R, Horiuchi S, Smedsrod B. Advanced glycation end products impair the scavenger function of rat hepatic sinusoidal endothelial cells. Diabetologia 45: 1379–1388, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Harris EN, Weigel JA, Weigel PH. The human hyaluronan receptor for endocytosis (HARE/Stabilin-2) is a systemic clearance receptor for heparin. J Biol Chem 283: 17341–17350, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holvoet P, Stassen JM, Van Cleemput J, Collen D, Vanhaecke J. Oxidized low density lipoproteins in patients with transplant-associated coronary artery disease. Arterioscler Thromb Vasc Biol 18: 100–107, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation 98: 1487–1494, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Horiuchi S, Sakamoto Y, Sakai M. Scavenger receptors for oxidized and glycated proteins. Amino Acids 25: 283–292, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Hughes DA, Fraser IP, Gordon S. Murine macrophage scavenger receptor: in vivo expression and function as receptor for macrophage adhesion in lymphoid and non-lymphoid organs. Eur J Immunol 25: 466–473, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Itabe H. Oxidized low-density lipoproteins: what is understood and what remains to be clarified. Biol Pharm Bull 26: 1–9, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Ito Y, Sorensen KK, Bethea NW, Svistounov D, McCuskey MK, Smedsrod BH, McCuskey RS. Age-related changes in the hepatic microcirculation in mice. Exp Gerontol 42: 789–797, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Juul K, Nielsen LB, Munkholm K, Stender S, Nordestgaard BG. Oxidation of plasma low-density lipoprotein accelerates its accumulation and degradation in the arterial wall in vivo. Circulation 94: 1698–1704, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Kankaanpaa J, Turunen SP, Moilanen V, Horkko S, Remes AM. Cerebrospinal fluid antibodies to oxidized LDL are increased in Alzheimer's disease. Neurobiol Dis 33: 467–472, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Kjeken R, Mousavi SA, Brech A, Gjoen T, Berg T. Fluid phase endocytosis of [125I]iodixanol in rat liver parenchymal, endothelial and Kupffer cells. Cell Tissue Res 304: 221–230, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem 277: 49982–49988, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Lee HS. Oxidized LDL, glomerular mesangial cells and collagen. Diabetes Res Clin Pract 45: 117–122, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Li R, McCourt P, Schledzewski K, Goerdt S, Moldenhauer G, Liu X, Smedsrod B, Sorensen KK. Endocytosis of advanced glycation end-products in bovine choriocapillaris endothelial cells. Microcirculation 16: 640–655, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Ling W, Lougheed M, Suzuki H, Buchan A, Kodama T, Steinbrecher UP. Oxidized or acetylated low density lipoproteins are rapidly cleared by the liver in mice with disruption of the scavenger receptor class A type I/II gene. J Clin Invest 100: 244–252, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lopes-Virella MF, Virella G, Orchard TJ, Koskinen S, Evans RW, Becker DJ, Forrest KY. Antibodies to oxidized LDL and LDL-containing immune complexes as risk factors for coronary artery disease in diabetes mellitus. Clin Immunol 90: 165–172, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Maeno Y, Fujioka H, Hollingdale MR, Ockenhouse CF, Nakazawa S, Aikawa M. Ultrastructural localization of CD36 in human hepatic sinusoidal lining cells, hepatocytes, human hepatoma (HepG2-A16) cells, and C32 amelanotic melanoma cells. Exp Parasitol 79: 383–390, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Malerod L, Juvet K, Gjoen T, Berg T. The expression of scavenger receptor class B, type I (SR-BI) and caveolin-1 in parenchymal and nonparenchymal liver cells. Cell Tissue Res 307: 173–180, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Martens JH, Kzhyshkowska J, Falkowski-Hansen M, Schledzewski K, Gratchev A, Mansmann U, Schmuttermaier C, Dippel E, Koenen W, Riedel F, Sankala M, Tryggvason K, Kobzik L, Moldenhauer G, Arnold B, Goerdt S. Differential expression of a gene signature for scavenger/lectin receptors by endothelial cells and macrophages in human lymph node sinuses, the primary sites of regional metastasis. J Pathol 208: 574–589, 2006 [DOI] [PubMed] [Google Scholar]
- 35. McCourt PA, Smedsrod BH, Melkko J, Johansson S. Characterization of a hyaluronan receptor on rat sinusoidal liver endothelial cells and its functional relationship to scavenger receptors. Hepatology 30: 1276–1286, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Mego JL, Bertini F, McQueen JD. The use of formaldehyde-treated 131-I-albumin in the study of digestive vacuoles and some properties of these particles from mouse liver. J Cell Biol 32: 699–707, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakajou K, Horiuchi S, Sakai M, Hirata K, Tanaka M, Takeya M, Kai T, Otagiri M. CD36 is not involved in scavenger receptor-mediated endocytic uptake of glycolaldehyde- and methylglyoxal-modified proteins by liver endothelial cells. J Biochem 137: 607–616, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Noble RP. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res 9: 693–700, 1968 [PubMed] [Google Scholar]
- 39. Oorni K, Pentikainen MO, Ala-Korpela M, Kovanen PT. Aggregation, fusion, and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactions. J Lipid Res 41: 1703–1714, 2000 [PubMed] [Google Scholar]
- 40. Politz O, Gratchev A, McCourt PA, Schledzewski K, Guillot P, Johansson S, Svineng G, Franke P, Kannicht C, Kzhyshkowska J, Longati P, Velten FW, Goerdt S. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem J 362: 155–164, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prevo R, Banerji S, Ni J, Jackson DG. Rapid plasma membrane-endosomal trafficking of the lymph node sinus and high endothelial venule scavenger receptor/homing receptor stabilin-1 (FEEL-1/CLEVER-1). J Biol Chem 279: 52580–52592, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Redgrave TG, Roberts DC, West CE. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem 65: 42–49, 1975 [DOI] [PubMed] [Google Scholar]
- 43. Rumsey SC, Galeano NF, Arad Y, Deckelbaum RJ. Cryopreservation with sucrose maintains normal physical and biological properties of human plasma low density lipoproteins. J Lipid Res 33: 1551–1561, 1992 [PubMed] [Google Scholar]
- 44. Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature 386: 73–77, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Seternes T, Sorensen K, Smedsrod B. Scavenger endothelial cells of vertebrates: a nonperipheral leukocyte system for high-capacity elimination of waste macromolecules. Proc Natl Acad Sci USA 99: 7594–7597, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simon-Santamaria J, Malovic I, Warren A, Oteiza A, Le Couteur D, Smedsrod B, McCourt P, Sorensen KK. Age-related changes in scavenger receptor-mediated endocytosis in rat liver sinusoidal endothelial cells. J Gerontol A Biol Sci Med Sci 65: 951–960, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smedsrod B, Pertoft H, Eggertsen G, Sundstrom C. Functional and morphological characterization of cultures of Kupffer cells and liver endothelial cells prepared by means of density separation in Percoll, and selective substrate adherence. Cell Tissue Res 241: 639–649, 1985 [DOI] [PubMed] [Google Scholar]
- 48. Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem 272: 20963–20966, 1997 [DOI] [PubMed] [Google Scholar]
- 49. Steinbrecher UP, Witztum JL, Parthasarathy S, Steinberg D. Decrease in reactive amino groups during oxidation or endothelial cell modification of LDL. Correlation with changes in receptor-mediated catabolism. Arteriosclerosis 7: 135–143, 1987 [DOI] [PubMed] [Google Scholar]
- 50. Svistounov DN, Berg TJ, McCourt PA, Zykova SN, Elvevold KH, Nagai R, Horiuchi S, Smedsrod BH. Lack of recognition of Nepsilon-(carboxymethyl)lysine by the mouse liver reticulo-endothelial system: implications for pathophysiology. Biochem Biophys Res Commun 309: 786–791, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Tanigawa H, Miura S, Zhang B, Uehara Y, Matsuo Y, Fujino M, Sawamura T, Saku K. Low-density lipoprotein oxidized to various degrees activates ERK1/2 through Lox-1. Atherosclerosis 188: 245–250, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Tokuyasu KT. Application of cryoultramicrotomy to immunocytochemistry. J Microsc 143: 139–149, 1986 [DOI] [PubMed] [Google Scholar]
- 53. Usynin IF, Khar'kovsky AV, Balitskaya NI, Panin LE. Gadolinium chloride-induced Kupffer cell blockade increases uptake of oxidized low-density lipoproteins by rat heart and aorta. Biochemistry (Mosc) 64: 620–624, 1999 [PubMed] [Google Scholar]
- 54. Van Berkel TJ, De Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem 266: 2282–2289, 1991 [PubMed] [Google Scholar]
- 55. Van Berkel TJ, Van Velzen A, Kruijt JK, Suzuki H, Kodama T. Uptake and catabolism of modified LDL in scavenger-receptor class A type I/II knock-out mice. Biochem J 331: 29–35, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem 272: 13597–13607, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 88: 1785–1792, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest 84: 1086–1095, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zeng Y, Tao N, Chung KN, Heuser JE, Lublin DM. Endocytosis of oxidized low density lipoprotein through scavenger receptor CD36 utilizes a lipid raft pathway that does not require caveolin-1. J Biol Chem 278: 45931–45936, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Zhang X, Fitzsimmons RL, Cleland LG, Ey PL, Zannettino AC, Farmer EA, Sincock P, Mayrhofer G. CD36/fatty acid translocase in rats: distribution, isolation from hepatocytes, and comparison with the scavenger receptor SR-B1. Lab Invest 83: 317–332, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Zhou B, Weigel JA, Fauss L, Weigel PH. Identification of the hyaluronan receptor for endocytosis (HARE). J Biol Chem 275: 37733–37741, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.