Abstract
Male Sprague-Dawley rats were chronically fed a high-unsaturated-fat diet for 130 days by using total enteral nutrition (TEN), or the same diet in which ethanol (EtOH) isocalorically replaced carbohydrate calories. Additional groups were supplemented with the antioxidant N-acetylcysteine (NAC) at 1.7 g·kg−1·day−1. Relative to an ad libitum chow-fed group, the high-fat-fed controls had three- to fourfold greater expression of fatty acid transporter CD36 mRNA and developed mild steatosis but little other hepatic pathology. NAC treatment resulted in increased somatic growth relative to controls (4.0 ± 0.1 vs. 3.1 ± 0.1 g/day) and increased hepatic steatosis score (3.5 ± 0.6 vs. 2.7 ± 1.2), associated with suppression of the triglyceride hydrolyzing protein adiponutrin, but produced no elevation in serum alanine aminotransferase (ALT). Chronic EtOH treatment increased expression of fatty acid transport protein FATP-2 mRNA twofold, resulting in marked hepatic steatosis, oxidative stress, and a twofold elevation in serum ALT. However, no changes in tumor necrosis factor-α or transforming growth factor-β expression were observed. Fibrosis, as measured by Masson's trichrome and picrosirius red staining, and a twofold increase in expression of type I and type III collagen mRNA, was only observed after EtOH treatment. Long-term EtOH treatment increased hepatocyte proliferation but did not modify the hepatic mRNAs for hedgehog pathway ligands or target genes or genes regulating epithelial-to-mesenchymal transition. Although the effects of NAC on EtOH-induced fibrosis could not be fully evaluated, NAC had additive effects on hepatocyte proliferation and prevented EtOH-induced oxidative stress and necrosis, despite a failure to reverse hepatic steatosis.
Keywords: antioxidant, alcohol, necrosis, fibrosis, hedgehog signaling
alcohol-induced hepatic fibrosis and cirrhosis are the main causes of mortality in alcoholics. However, a reasonable model of alcohol-induced fibrotic injury that does not involve genetic manipulation is lacking. Oral feeding of ethanol (EtOH) as part of the Lieber-DeCarli diet results in steatosis but little further progression of injury in either rats or mice (21). Previous studies by Tsukamoto et al. (35) in which high levels of EtOH were infused intragastrically to rats over a 16-wk period have reported the development of fibrosis. However, there have been few mechanistic studies examining the progression of liver injury from simple steatosis to steatohepatitis and on to fibrosis by using this model.
It has been suggested that, in addition to hepatocyte proliferation (23), liver regeneration following injury may involve a ductular reaction representing expansion of the progeny of resident liver progenitor cells (immature cholangiocytes and oval cells) residing along the canals of Hering (9). This ductular reaction has been suggested to involve increased signaling through the hedgehog (Hh) pathway (12, 17). Recent studies using a short-term intragastric infusion model to produce alcoholic liver injury in mice have suggested the involvement of Hh signaling in recruiting liver progenitor cells (17). In addition, Hh-mediated signals have been implicated in the stimulation of epithelial-to-mesenchymal transition (EMT) between quiescent hepatic stellate cells and myofibroblastic hepatic stellate cells contributing to development of fibrosis (9, 10). Limited immunohistochemical studies in livers from patients with alcoholic liver disease have also suggested increased Hh signaling (17). A growing body of experimental evidence suggests that oxidative stress promotes hepatic inflammation and immune responses after chronic alcohol consumption contributing to hepatic injury (20, 24, 34). We have previously reported that development of alcoholic steatohepatitis in the total enteral nutrition (TEN) model after 45 days of EtOH exposure was partly blocked by the dietary antioxidant N-acetylcysteine (NAC). NAC prevented the development of hepatic oxidative stress and appearance of autoantibodies against proteins adducted with lipid peroxidation products, and partly blocked EtOH-induced increases in TNF-α mRNA expression and necrotic injury (29). In the present study, the ability of NAC to prevent additional pathology after 130 days of EtOH treatment was examined.
MATERIALS AND METHODS
Animals and experimental design.
All the animal studies described below were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory animals” at an American Association for Accreditation of Laboratory Animal Care-approved animal facility at Arkansas Children's Hospital Research Institute. Groups of male Sprague-Dawley rats, 350 g were either fed chow ad libitum or had an intragastric cannula surgically implanted and were infused (166 kcal·kg−3/4·day−1) with isocaloric liquid diets by using TEN as described previously (3, 28, 29). Control TEN diets contained 16% protein (whey peptides), 39% carbohydrate (dextrose and maltodextrin), and 45% fat (corn oil) together with National Research Council-recommended levels of vitamins and minerals (3, 19, 28, 29). In the EtOH-treated groups, EtOH substituted isocalorically for carbohydrate calories and the diet composition was 16% protein, 5% carbohydrate, 45% fat, and 34% EtOH calories as described previously (19, 28, 29). In addition, TEN and EtOH groups were treated with NAC (NAC, EtOH + NAC) at 1.7 g·kg−1·day−1 added to the diets. Urine EtOH concentrations (UEC) were measured daily via an Analox Instruments GL5 analyzer. After 130 days, the animals were euthanized.
Pathological evaluation.
Liver samples were fixed in 10% neutral buffered formalin, processed, and paraffin-embedded sections were stained with hematoxylin and eosin (H&E). H&E-stained liver sections were scored for macro- and microsteatosis, inflammation (macrophage infiltration) and necrosis by a veterinary pathologist (L. Hennings) with no prior knowledge of the treatment groups. A modified Kleiner score was assigned as previously reported (6). Steatosis was scored as the percentage of parenchymal cells containing fat (micro- or macrosteatosis) as <5% = 0, 5–33% = 1, >33–66% = 2; >66% = 3. Presence of contiguous patches of microsteatosis was given a weighted score of 1. The presence of inflammation based on infiltration by polymorphonuclear cells, leukocytes, and mononuclear cells was evaluated by using a scale on which no inflammation = 0; <2 foci of inflammatory cells per ×200 field = 1, 2–4 foci per ×200 field = 2, >4 foci per ×200 field = 3. For scoring of necrosis, ballooning degeneration was assessed by using a scale on which 0 = no ballooning degeneration, 1 = few balloon cells, and 2 = prominent ballooning degeneration. An additional necrosis score = 1 was added for presence or absence of serum markers of necrosis [serum alanine aminotransferase (ALT) values > 55]. Total pathology score was determined by summing the scores for steatosis, inflammation, and necrosis. Therefore the maximum nonfibrotic pathology score = 9. Fibrosis was detected histologically by Masson's trichrome (33) and picrosirius red staining of collagen (7) by using digital slide scanning (Aperio, Scanscope T2, Aperio, Vista, CA) and associated image analysis software. Staining of picrosirius red was evaluated by using densitometric methods and color deconvolution and assigned a score ranging from 0 to 300, where score is computed as (% area strongly stained × 3) + (% moderately stained × 2) + (% weakly stained × 1). In addition, fibrosis was assessed biochemically by measurement of hepatic hydroxyproline content after acid hydrolysis of proteins (37) by use of a rat hydroxyproline ELISA kit (Cusabio Biotech, Newark, DE). Fibrosis was determined at the molecular level by real-time RT-PCR analysis of collagen mRNA expression (10). Apoptosis was assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining using the In Situ Cell Death Detection Kit from Roche Diagnostics (Indianapolis, IN). Endonuclease G (EndoG) is a proapoptotic mitochondrial enzyme that has been shown to be highly expressed in liver (1) and to translocate to cell nucleus and induce apoptotic DNA fragmentation during toxic liver injury (4). EndoG staining was conducted by using polyclonal anti-EndoG antibody (dilution 1:400) (Millipore, Bedford, MA) as described previously (1). The primary antibodies were detected with anti-rabbit IgG-Alexa Fluor 594 (Invitrogen, Carlsbad, CA). The sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI), mounted under coverslips by using the Antifade kit (Invitrogen), and analyzed under an Olympus IX-81 microscope (Olympus, Center Valley, PA). Acquisitions were done by using a Hamamatsu-ORCA digital camera (Hamamatsu, Bridgewater, NJ) and software SlideBook 4.2 (Intelligent Imaging Innovations, Denver, CO). Images were developed, masked, and analyzed by using the SlideBook software. Expression of EndoG was quantified by evaluation of the integral optical density of every available cell in the given field of view. At least 10 different fields of view per sample replicate were analyzed. Hepatic cellular proliferation was measured by immunohistochemical analysis of proliferating cell nuclear antigen (PCNA) expression as described by Greenwell et al. (15). Nuclei of S-phase cells were stained dark brown.
Biochemical analysis.
Blood EtOH concentration (BEC) at euthanasia was measured by Analox and serum ALT levels assessed as a measure of liver damage by using the Infinity ALT liquid stable reagent (Thermo Electron, Waltham, MA) according to manufacturer's protocols. Liver microsomes were prepared by differential ultracentrifugation, and p-nitrophenol hydroxylation was measured as described previously (18). Liver lipid peroxidation was assessed as a measure of oxidative stress as described by Ohkawa et al. (25). Hepatic GSH concentrations were quantified in liver by using a commercially available kit (703002) from Cayman Chemical (Ann Arbor, MI). Triglycerides were extracted from liver homogenates with chloroform/methanol (2:1 vol/vol) and triglycerides and cholesterol concentrations assayed by using commercially available reagents (Synermed, Westfield, IN).
Autoantibodies against MDA and lipid peroxide (LOOH) protein adducts.
Bovine serum albumin adducts with MDA and LOOH were prepared as described by Mottaran et al. (24). Antibodies to adducted proteins in rat serum were measured in microwell plates coated with modified or native BSA as described previously (24).
Analysis of gene expression.
Expression of mRNAs coding for the cytokines TNF-α and TGF-β for α type I and type III collagen, matrix metallopeptideases (MMPs) and the MMP inhibitor TIMP1 were assessed by real-time RT-PCR by using primers described previously (6, 7, 10, 11). Primer pairs used for real-time RT-PCR analysis of mRNAs for genes involved in hepatic lipid homeostasis, hedgehog signaling target genes, and genes regulating EMT are shown in Table 1. Total RNA was isolated from tissue by using SV total RNA Isolation System (Promega, Madison, WI) according to the manufacturer's protocol. The integrity of the RNA was confirmed by denaturing gel electrophoresis. RNA samples were quantified by using the RiboGreen quantitation assay, after DNase digestion, according to procedures developed by the manufacturer (Molecular Probes, Eugene, OR). Reverse transcription reaction was performed with 0.5 μg of total RNA in a final volume of 10 μl, by using M-MLV Reverse Transcriptase (Invitrogen, Stockholm, Sweden) essentially according to procedures developed by the manufacturer. Each PCR reaction contained 2–10 μl of cDNA template, 1× buffer, 1.25–1.5 mM MgCl2, 0.2 mM dNTPs, 0.25 μM of each primer, and 0.625 units Taq DNA polymerase (ABGene, Epsom, UK). Each PCR consisted of 95°C for 1 min followed by 25–40 cycles of 95°C for 15 s, 52°C or 60°C for 20 s, and 72°C for 1 min. PCR products were separated on ethylene bromide-containing agarose gels. Identity of PCR products was verified by DNA sequencing, by using BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems) according to the manufacturer's instructions. Real-time PCR was performed by using 5 μl of diluted cDNA template in a 25-μl reaction containing 0.25 μM of each primer and 12.5 μl SYBR Green real-time PCR MasterMix (Applied Biosystems). Each run consisted of 50°C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 s, 60°C for 20 s, and 72°C for 60 s and was followed by a melt curve analysis and separation on ethylene bromide containing agarose gels. PCR was performed by using the Applied Biosystems ABI PRISM 7700 Sequence Detection System. All samples were run blind, and reactions were performed in at least duplicates from separate cDNA reactions. All primers were designed to span at least one intron. Expression of gene products was normalized against mRNA for 18S, expression of which did not change significantly across groups.
Table 1.
Primer sequences utilized for real-time RT-PCR to assess gene expression associated with fatty acid homeostasis, matrix remodeling, Hh signaling, and EMT
| FATP-2-F | 5′-AGT ACA TCG GTG AAC TGC TTC GGT-3′ |
| FATP-2-R | 5′-TGC CTT CAG TGG AAG CGT AGA ACT-3′ |
| FATP-5-F | 5′-TTC AGG GAC CAC TGG ACT TCC AAA-3′ |
| FATP-5-R | 5′-ACC ACA TCA TCA GCT GTT CTC CCA-3′ |
| CD36-F | 5′-CGA AGG CTT GAA TCC TAA CGA A-3′ |
| CD36-R | 5′-TGT TGA CCT GCA GTC GTT TTG-3′ |
| SREBP-1c-F | 5′-CTC ATC AAC AAC CAA GAC AGT G-3′ |
| SREBP-1c-R | 5′-GAG AAG CAG GAG AAG AGA AGC-3′ |
| FASN-F | 5′-GAG GTG GTG ATA GCC GGT ATG T-3′ |
| FASN-R | 5′-GAC CGC TTA GGC AAC CCA-3′ |
| CPT-1-F | 5′-AGA TAC TGT GAG CAG GTA CC-3′ |
| CPT-1-R | 5′-TCC GAG GTT GAC AGC AAA-3′ |
| PPAR-α-F | 5′-TTG TGA CTG GTC AAG CTC AGG ACA-3′ |
| PPAR-α-R | 5′-TCC ACC ATG TTG AAT GGT TGT GGC-3′ |
| ACO-F | 5′-CGT CCC AAG AAC TCC AGA TA-3′ |
| ACO-R | 5′-CAG TAG GGC TGT TGA GAA TG-3′ |
| CYP4A1-F | 5′-GAC TCC ATT CGA CTG ATG CTA GAC-3′ |
| CYP4A1-R | 5′-AAT TTC CAT CCA CCT GAA CAC TG-3′ |
| PNPLA3 (adiponutrin)-F | 5′-GTG TGC CCG AAT GAC CAT GT-3′ |
| PNPLA3 (adiponutrin)-R | 5′-GCC TTG GGG TTT GTG GAG AG-3′ |
| Perilipin-F | 5′-AAA AGA TCC CGG CTC TTC AAT AC-3′ |
| Perilipin-R | 5′-GCA CGC TGA TGC TGT TCC T-3′ |
| MTP-F | 5′-ATT AAG GCT CTG GAT ACA TGC AAA A-3′ |
| MTP-R | 5′-GCA AAA GCC CTG GTC TCT TCT-3′ |
| ApoB100-F | 5′-ACC AGG ATG GAA TGC TGT CTG TCA-3′ |
| ApoB100-R | 5′-TCC AGC TTG TTG GAA GCA GCA AAG-3′ |
| Rab3D-F | 5′-ATC TGA CAT GCT TGG TCC AGG TGA-3′ |
| RAb3D-R | 5′-TGC TCA GTA GGG CTG CAG AAT GAT-3′ |
| MMP3-F | 5′-ATT GGC ACA AAG GTG GAT GCT GTC-3′ |
| MMP3-R | 5′-ACG ACG CCT TCC ATG GAT CTT CTT-3′ |
| MMP13-F | 5′-GCA TGA AAA CTG TGG GGA GT-3′ |
| MMP13-R | 5′-AGC TGA AAT CTT GCC TTG GA-5′ |
| Timp-1F | 5′-TCC CTT GCA AAC TGG AGA GTG ACA-3′ |
| Timp-1R | 5′-CCA AGG TAT TGC CAG GTG CAC AAA-3′ |
| Gli 2-F | 5′-ATC AAG AGG GAG CTA CAC GCA ACA-3′ |
| Gli 2-R | 5′-ATC CAC TGA CGA CGT TTG GAC TCA-3′ |
| mmp-9F | 5′-TTC TCG AAT CAC GGA GGA AGC CAA-3′ |
| mmp-9R | 5′-AAG GCT GAG TTC AAC TTT GCA GGC-3′ |
| bmp 7-F | 5′-ATG TTA GCT TCC GAG ACC TTG GCT-3′ |
| bmp 7-R | 5′-TGG TGG CGT TCA TGT AGG AGT TCA-3′ |
| krt 7-F | 5′-ACC CTC AAC AAC AAA TTC GCC TCC-3′ |
| krt 7-R | 5′-TGC TCT TGG CTG ACT TCT GTT CCT-3′ |
| Hip-F | 5′-TGT GCC GTG GAT CGA C-3′ |
| Hip-R | 5′-GAT CTC CGA ACA CGT AGC TT-3′ |
| Desmoplakin-F | 5′-GGA AGT CAG CCA AGC AAA AC-3′ |
| Desmoplakin-R | 5′-GGC TCT CCT TTT CAC ACT GC-3′ |
| S100A4-F | 5′-ATA CTC AGG CAA CGA GGG TG-3′ |
| S100A4-R | 5′-CTT CCG GGG CTC CTT ATC-3′ |
| Id 2-F | 5′-ATG GAA ATC CTG CAG CAC GTC ATC-3′ |
| Id 2-R | 5′-ACG TTT GGT TCT GTC CAG GTC TCT-3′ |
| Shh-F | 5′-ACA AGA AAC TCC GAA CGA TT-3′ |
| Shh-R | 5′-GCC CTC AGT CAC TCG AAG-3′ |
EMT, epithelial-to-mesenchymal transition.
Statistics.
Data are expressed as means ± SE. In most cases, groups were compared statistically by one- or two-way ANOVA followed by Student-Newman-Keuls post hoc analysis for specific comparisons between means by using SigmaStat for Windows (Jandal Scientific Software). In the case of the liver pathology scores that were not normally distributed, median values were compared by one-way ANOVA of ranks followed by Dunn's test.
RESULTS
Effects of alcohol and NAC on body composition and EtOH metabolism.
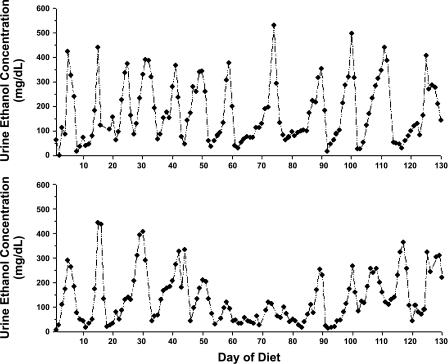
Weight gain and body composition data are presented in Table 2. Compared with ad libitum chow feeding, high-fat TEN controls gained more weight and had increased abdominal fat (P < 0.05) but liver weight was lower when expressed as % body weight (P < 0.05). Addition of NAC to the control TEN or EtOH diet increased weight gain and liver weight (P < 0.05) but resulted in no further increase in fat mass. EtOH treatment reduced weight gain in TEN rats, despite isocaloric feeding, in both the presence or absence of NAC (P < 0.05), and increased relative liver weight (P < 0.05) without significant effects on adiposity. In agreement with previous studies (3, 19, 28, 29), UECs demonstrated a characteristic pulsatile pattern of peaks around 300–400 mg/dl and nadirs below 50 mg/dl with a periodicity of 6–7 days, despite continuous infusion of the same dose of EtOH throughout the study (Fig. 1). Pulse characteristics were maintained during the entire period of infusion and as previously reported (29) diet supplementation with NAC had no effect on UEC pulses or mean UEC values (Table 2).
Table 2.
Effects of EtOH and NAC on growth, body composition, and UEC
| Group | BW at Euthansia, g | Weight Gain, g/day | Abdominal Fat, % BW | Liver Weight, g | Liver Weight/BW, % | Mean UEC, mg/dl |
|---|---|---|---|---|---|---|
| Chow | 565 ± 10a | 1.65 ± 0.08a | 2.14 ± 0.18a | 17.14 ± 2.26a | 3.00 ± 0.13b | |
| Control | 758 ± 11d | 3.13 ± 0.08d | 3.81 ± 0.25b | 21.66 ± 3.47a,b | 2.68 ± 0.04a | |
| NAC | 864 ± 15e | 3.95 ± 0.09e | 3.62 ± 0.46b | 29.16 ± 6.91b | 3.24 ± 0.34b | |
| EtOH | 623 ± 15b | 2.10 ± 0.11b | 3.24 ± 0.26b | 26.25 ± 4.10b | 4.08 ± 0.06c | 196 ± 35 |
| EtOH + NAC | 689 ± 18c | 2.61 ± 0.13c | 3.51 ± 0.25b | 31.71 ± 7.88b | 4.25 ± 0.16c | 173 ± 33 |
Data are means ± SE for 7–11 rats/group.
Chow, ad libitum chow-fed rats; Control, rats fed high-fat diets via total enteral nutrition; NAC, rats fed control diets supplemented with 1.7 g·kg−1·day−1 N-acetylcysteine; EtOH, rats fed isocaloric diets with ethanol (EtOH) at 12 g·kg−1·day−1; EtOH + NAC, rats fed EtOH diet +1.7 g·kg−1·day−1 NAC; see materials and methods. BW, body weight; UEC, urine ethanol concentration. Means without a common superscript letter are significantly different (P < 0.05) by 1-way ANOVA followed by Student-Newman-Keuls post hoc analysis.
Fig. 1.
Representative urine ethanol (EtOH) concentration profiles of male rats treated with EtOH as part of total enteral nutrition (TEN) diets for 130 days.
Effects of alcohol and NAC on hepatic pathology.
Chronic consumption of the high-fat TEN control diet resulted in significant accumulation of hepatic triglycerides and development of mild steatosis compared with chow-fed rats (P < 0.05) (Fig. 2, Table 3). Analysis of expression of hepatic genes known to regulate hepatic lipid homeostasis revealed that the high-fat TEN groups had greater expression of mRNA coding for the fatty acid transporter CD36 (P < 0.05) and a trend toward increased expression of fatty acid synthase (FASN) (P = 0.08) (Table 4). In addition, the high-fat TEN diet increased expression of mRNA for adiponutrin (patatin-like phospholipase domain containing 3 protein, PNPLA3), a protein involved in triglyceride hydrolysis, nearly threefold (P < 0.05). Unexpectedly, addition of NAC to the control diet by itself actually increased triglyceride accumulation (P < 0.05) and steatosis. Appearance of steatosis in the NAC group was accompanied by a dramatic reduction in expression of adiponutrin mRNA compared with the high-fat TEN control (P < 0.05) (Table 4). NAC treatment also increased expression of the mRNA encoding the mitochondrial fatty acid import protein carnitine palmitoyl transferase (CPT-1). NAC treatment increased apoptosis as determined by TUNEL staining (P < 0.05) and increased the inflammation/degeneration score. However, no increase was observed in serum ALT values above chow or TEN controls. EtOH-treated rats accumulated hepatic triglycerides and developed significantly greater steatosis than high-fat TEN controls (Table 3, Fig. 3) (P < 0.05). EtOH treatment had no effects on mRNAs encoding genes involved in fatty acid synthesis: sterol regulatory element binding protein (SREBP-1c) and FASN and actually increased mRNAs encoding for genes involved in fatty acid degradation: CPT-1 and cytochrome P-450 (CYP)4A1 (P < 0.05) (Table 4). EtOH also increased expression of mRNA of the major hepatic fatty acid transporter FATP-2 and expression of mRNA for Rad3D, a GTPase involved in lipid droplet trafficking (P < 0.05) (Table 4). However, no effects of EtOH were observed on expression of mRNA for either ApoB100 or the mitochondrial triglyceride transfer protein involved in VLDL secretion (Table 4). EtOH-treated rats exhibited the greatest degree of steatohepatitis and overall nonfibrotic pathology scores (Table 3) but had no significant increases in expression of mRNA for either of the cytokines TNF-α (1.00 ± 0.18 vs. 1.22 ± 0.18; control vs. EtOH) or TGFβ (1.00 ± 0.14 vs. 1.27 ± 0.15; control vs. EtOH). The EtOH group had the greatest level of apoptosis as measured by TUNEL staining, although the % of apoptotic cells was very small. This was accompanied by increased EndoG staining (P < 0.05). The EtOH group also had increased serum ALT values compared with all the other groups, indicating significant necrosis (P < 0.05) (Fig. 2, Table 3). In some cases, EndoG colocalized with TUNEL-positive DAPI-negative nuclei, suggesting that the DNA fragmentation was, at least partially, produced by this endonuclease (Fig. 3). These pathological changes were accompanied by induction of CYP2E1-dependent p-nitrophenol hydroxylase activity (P < 0.05) and evidence of oxidative stress: decreased hepatic levels of the antioxidant glutathione (GSH) and increased antibody titers directed against proteins adducted with lipid peroxides and the lipid peroxidation product malondialdehyde (MDA) (P < 0.05). In contrast, no significant effect of EtOH was observed on overall levels of liver lipid peroxidation as measured by thiobarbituric acid-reactive products (TBARS; Table 5). Addition of NAC to the EtOH diets prevented the appearance of oxidative stress. Liver GSH and antibody titers against lipid peroxide and MDA-adduced proteins were restored to control values (P < 0.05), (Table 5). NAC treatment also partially ameliorated the inflammation/necrosis score and prevented EtOH-induced increases in serum ALT and apoptosis (P < 0.05) despite a lack of effect on EndoG expression. However, addition of NAC to the diet had no significant effect on triglyceride accumulation or steatosis score in EtOH-treated rats. Compared with the EtOH group, EtOH + NAC treated rats had reduced expression of FATP-2 mRNA (P < 0.02) but also had reduced levels of adiponutrin (P < 0.05) (Table 4).
Fig. 2.
Representative hematoxylin and eosin-stained liver sections from ad libitum chow-fed rats (chow), rats fed high-fat diets via total enteral nutrition (Control); fed control diets supplemented with 1.7 g·kg−1·day−1 N-acetylcysteine (NAC); fed isocaloric diets with EtOH at 12 g·kg−1·day−1 (EtOH), or fed EtOH diet + 1.7 g·kg−1·day−1 NAC (EtOH + NAC) as described in materials and methods. Magnification ×200. NAC rats exhibited predominantly microsteatosis accompanied by appearance of scattered ballooning hepatocytes (arrowhead). EtOH and EtOH + NAC rats exhibited predominantly macrosteatosis (arrows).
Table 3.
Effects of EtOH and NAC on nonfibrotic liver pathology
| Pathology Score |
|||||||
|---|---|---|---|---|---|---|---|
| Group | Steatosis | Inflammation/Necrosis | Total | Liver Triglycerides, μg/g liver | Serum ALT, U/l | Apoptosis, % TUNEL-positive cells | Endonuclease G, staining intensity/cell |
| Chow | 1.08 ± 0.02a | 2.86 ± 0.34a | 3.82 ± 0.35a | 10.20 ± 1.50a | 42.50 ± 4.10a | 0.055 ± 0.05a | 5,500 ± 1,000a |
| Control | 2.65 ± 1.21b | 3.72 ± 1.16b | 5.31 ± 1.93b | 83.73 ± 14.37b | 45.40 ± 3.40a | 0.135 ± 0.03b | 8,000 ± 1,000a,b |
| NAC | 3.54 ± 0.55b | 3.86 ± 0.90b | 6.54 ± 1.19b | 219.31 ± 54.63c | 48.88 ± 6.90a | 0.160 ± 0.04b | 7,800 ± 3,000a,b |
| EtOH | 3.62 ± 0.84b | 4.47 ± 1.06b | 7.50 ± 1.55b | 227.86 ± 23.02c | 105.37 ± 23.0b | 0.197 ± 0.05b | 15,100 ± 8,100b |
| EtOH + NAC | 3.92 ± 0.30b | 3.35 ± 0.65a,b | 6.20 ± 0.80b | 279.90 ± 25.70c | 64.20 ± 16.80a | 0.070 ± 0.02a | 12,000 ± 3,500b |
Data are means ± SE for n = 7–11 rats/group.
Pathology score was assigned as defined under materials and methods; normal pathology score is steatosis = 0 and inflammation/necrosis = 0. Total possible pathology score: steatosis 4 plus inflammation/necrosis 5 for total possible score of 9. ALT, alanine aminotransferase activity; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
Means without a common superscript letter are significantly different (P < 0.05) by 1-way ANOVA followed by Student-Newman-Keuls post hoc analysis.
Table 4.
Effects of EtOH and NAC on hepatic pathways regulating lipid homeostasis
| Group | Chow | Control | NAC | EtOH | EtOH + NAC |
|---|---|---|---|---|---|
| Fatty acid import | |||||
| FATP-2 | 1.00 ± 0.11a | 1.28 ± 0.10a | 1.26 ± 0.10b | 2.35 ± 0.41b | 1.37 ± 0.18a |
| FATP-5 | 1.00 ± 0.16a | 1.25 ± 0.18a | 0.62 ± 0.12a | 1.48 ± 0.37a | 0.77 ± 0.17a |
| CD36 | 1.00 ± 0.12a | 3.77 ± 0.57b | 3.18 ± 0.43b | 2.92 ± 0.41b | 3.21 ± 0.62b |
| Fatty acid synthesis | |||||
| SREBP-1c | 1.00 ± 0.08a | 1.13 ± 0.11a | 0.88 ± 0.11a | 1.34 ± 0.21a | 0.89 ± 0.14a |
| FASN | 1.00 ± 0.20a | 2.80 ± 0.51a | 2.11 ± 0.46a | 1.73 ± 0.52a | 1.47 ± 0.44a |
| Fatty acid degradation | |||||
| CPT-1 | 1.00 ± 0.15a | 1.30 ± 0.28a | 2.07 ± 0.28b | 2.14 ± 0.31b | 1.80 ± 0.24a,b |
| PPAR-α | 1.00 ± 0.17a | 0.81 ± 0.10a | 0.65 ± 0.10a | 0.94 ± 0.10a | 0.91 ± 0.19a |
| ACO | 1.00 ± 0.12a | 1.35 ± 0.11a | 1.16 ± 0.19a | 1.59 ± 0.25a | 1.42 ± 0.26a |
| CYP4A1 | 1.00 ± 0.14a | 1.68 ± 0.22a | 1.57 ± 0.25a | 3.02 ± 0.56b | 2.51 ± 0.69a,b |
| Triglyceride associated | |||||
| PNPLA3 (adiponutrin) | 1.00 ± 0.30a | 3.73 ± 0.94b | 0.61 ± 0.25a | 2.00 ± 0.89a,b | 0.79 ± 0.27a |
| Perilipin | 1.00 ± 0.23a | 1.41 ± 0.27a | 0.89 ± 0.33a | 1.10 ± 0.33a | 2.47 ± 0.62a |
| VLDL secretion | |||||
| MTP | 1.00 ± 0.09a | 1.24 ± 0.08a | 1.33 ± 0.32a | 1.61 ± 0.13a | 1.81 ± 0.32a |
| ApoB100 | 1.00 ± 0.09a | 1.01 ± 0.06a | 1.04 ± 0.09a | 1.26 ± 0.18a | 1.14 ± 0.11a |
| Rab3D | 1.00 ± 0.12a | 0.81 ± 0.08a | 0.86 ± 0.08a | 1.60 ± 0.14b | 1.90 ± 0.17b |
Real-time RT-PCR data are expressed as gene expression normalized to expression of 18S and expressed relative to Chow = 1.0. Data are presented as mean ± SE for n = 7–11 rats/group. Means without a common superscript letter are significantly different (P < 0.05) by 1-way ANOVA followed by Student-Newman-Keuls post hoc analysis.
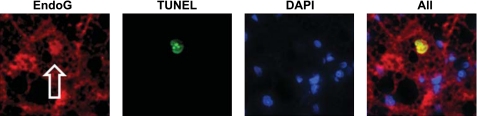
Fig. 3.
Colocalization of endonuclease G staining (EndoG, left) with terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)-stained apoptotic hepatocytes (2nd panel) in the liver of EtOH-treated rats. 3rd panel: 4,6-diamidino-2-phenylindole (DAPI) staining of nuclei. Right: merged staining.
Table 5.
Effects of EtOH and NAC on hepatic CYP2E1 activity and oxidative stress
| Group | p-Nitrophenol Hydroxylase (CYP2E1-Dependent Activity), pmol·mg microsomal protein−1·min−1 | Glutathione, nmol GSH/mg protein | TBARS, nmol/g liver | BSA-MDA | BSA-LOOH |
|---|---|---|---|---|---|
| Chow | 161.00 ± 10.00a | 27.34 ± 4.54b | 0.59 ± 0.30 | 0.30 ± 0.02b | 0.19 ± 0.02a |
| Control | 124.00 ± 5.80a | 22.50 ± 2.24b | 0.55 ± 0.50 | 0.22 ± 0.01a | 0.24 ± 0.01a |
| NAC | 135.60 ± 22.58a | 26.59 ± 6.70b | 0.70 ± 0.50 | 0.24 ± 0.02a | 0.22 ± 0.02a |
| EtOH | 250.90 ± 18.75b | 18.20 ± 0.78a | 0.75 ± 0.80 | 0.44 ± 0.02c | 0.42 ± 0.03b |
| EtOH + NAC | 233.35 ± 43.5b | 31.44 ± 3.35b | 0.74 ± 0.40 | 0.34 ± 0.01b | 0.29 ± 0.02a |
Data are means ± SE for n = 7–11 rats/group.
TBARS, thiobarbituric acid-reactive products; BSA-MDA, antibody titer (OD 460 nm, ELISA value) against BSA adducted with malondialdehyde (MDA); BSA-LOOH, antibody titer (OD 460 nm, ELISA value) against BSA adducted with lipid peroxide (LOOH).
Column means without a common superscript letter are significantly different (P < 0.05) by 1-way ANOVA followed by Student-Newman-Keuls post hoc analysis.
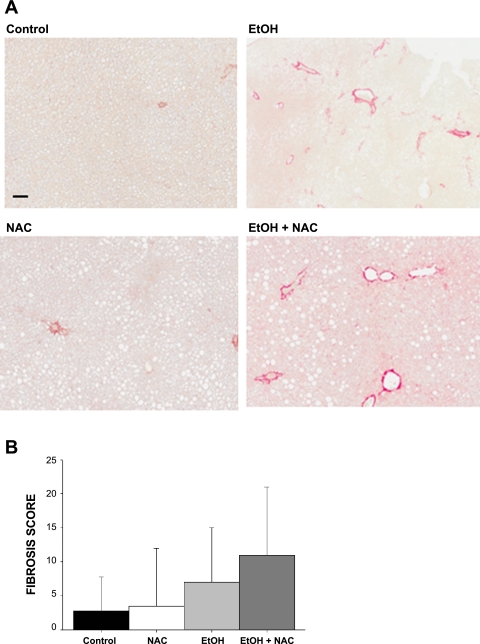
Parenchymal collagen deposition as assessed histologically by Masson's trichrome (Fig. 4) and picrosirius red staining (Fig. 5A) was only observed in the rat groups treated with EtOH. However, even after 130 days of EtOH exposure, fibrosis was marginal in all but a few rats in each of the EtOH and EtOH + NAC groups and was highly variable. Median fibrosis score was based on Picosirius red staining, P = 0.08 (2-way ANOVA) (Fig. 5B). Biochemical analysis of hydroxyproline content revealed highest mean values in the EtOH group, but this did not achieve statistical significance (Table 6). At the molecular level the expression of α1 collagen type I and type III mRNAs was increased two- to threefold in the EtOH-treated groups (P < 0.05). There was a trend for MMPs involved in matrix remodeling to be elevated in both EtOH and NAC groups (P < 0.1). Diet supplementation with NAC in addition to EtOH had no significant effect on histological fibrosis score, hydroxyproline content, or collagen mRNA expression relative to the EtOH group. However, increased expression of mRNAs coding for MMPs was observed in the EtOH + NAC group, relative to the TEN and chow groups (P < 0.05) (Fig. 5, Table 6). No significant effects of diet, NAC, or EtOH were observed on expression of mRNA encoding the MMP inhibitor TIMP1 (Table 5).
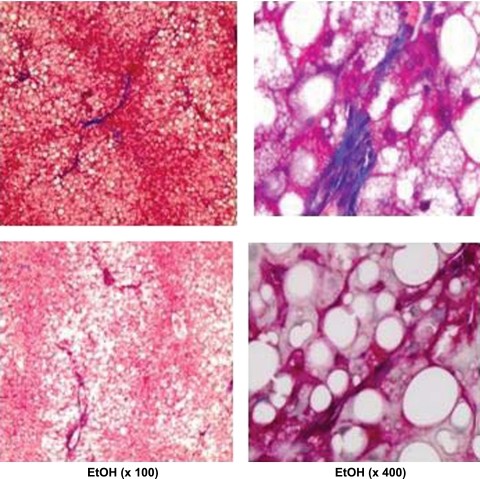
Fig. 4.
Masson's trichrome staining of collagen fibers demonstrating the appearance of fibrosis in liver of some EtOH-treated rats after 130 days of feeding via TEN. Magnification ×100 left, ×400 right.
Fig. 5.
Top: picrosirius red staining of collagen in rats fed high-fat diets via TEN (Control), fed control diets supplemented with 1.7 g·kg−1·day−1 NAC, fed isocaloric diets with EtOH at 12 g·kg−1·day−1, or fed EtOH diet + 1.7 g·kg−1·day−1 NAC as described in materials and methods. Magnification ×120; bar = 100 μM. Increased portal staining was observed in EtOH and EtOH + NAC groups. Bottom: fibrosis score based on image quantitation of red staining.
Table 6.
Effects of EtOH and NAC on hepatic hydroxyproline content and expression of mRNAs associated with fibrosis and matrix remodeling
| Group | Hydroxyproline, % hydroxyproline/total protein content | α1 Collagen Type I mRNA | α1 Collagen Type III mRNA | MMP-3 mRNA | MMP9 mRNA | MMP-13 mRNA | TIMP-1 mRNA |
|---|---|---|---|---|---|---|---|
| Chow | 1.70 ± 0.40 | 1.00 ± 0.25a | 1.00 ± 0.07a | 1.00 ± 0.14a | 1.00 ± 0.50a | 1.00 ± 0.20a | 1.00 ± 0.43 |
| Control | 2.32 ± 0.50 | 1.29 ± 0.11a | 1.23 ± 0.09a | 1.67 ± 0.27a | 1.42 ± 0.07a | 3.20 ± 0.62a | 1.30 ± 1.27 |
| NAC | 2.02 ± 0.40 | 1.30 ± 0.12a | 1.34 ± 0.27a | 5.72 ± 1.97a | 5.17 ± 2.32a | 3.47 ± 2.21a | 1.21 ± 0.44 |
| EtOH | 2.97 ± 1.15 | 2.62 ± 0.65b | 1.97 ± 0.21b | 4.82 ± 1.42a,b | 7.51 ± 3.25a,b | 5.60 ± 1.98a | 2.24 ± 0.96 |
| EtOH + NAC | 1.96 ± 0.60 | 2.59 ± 0.70b | 2.82 ± 0.49b | 6.52 ± 1.47b | 2.78 ± 3.14b | 13.80 ± 3.36b | 1.18 ± 0.23 |
Real-time RT-PCR data are expressed as gene expression normalized to expression of 18S and expressed relative to Chow = 1.0. Data are presented as mean ± SE for n = 7–11 rats/group. Column means without a common superscript letter are significantly different (P < 0.05) by 1-way ANOVA followed by Student-Newman-Keuls post hoc analysis.
Effects of EtOH and NAC on regenerative repair.
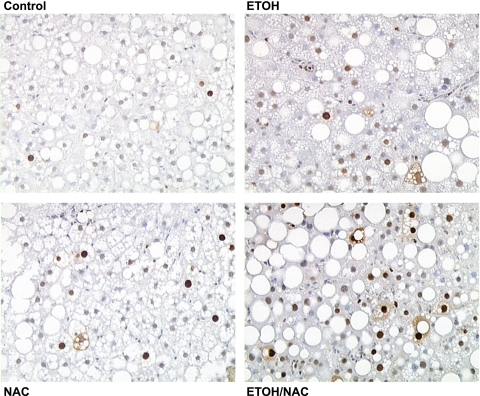
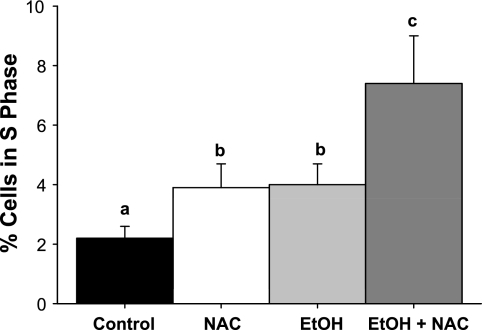
Proliferative status of liver was assessed in TEN-fed rats with or without EtOH and NAC treatment using PCNA staining (Fig. 6). Both NAC and EtOH treatment increased the number of cells in S phase (P < 0.05), and the combination of EtOH and NAC had additive effects on proliferation (P < 0.05 by 2-way ANOVA, Fig. 7). However, even though livers from EtOH-treated groups appeared to be engaged in regenerative repair, we found no evidence for Hh signaling or EMT (Table 7). mRNA expression of Shh ligand was undetectable in all groups and expression of another Hh target gene, Gli2 did not differ statistically between groups. mRNA expression of the Hh antagonist Hip was unaffected by EtOH and was actually increased (P < 0.05) by the combination of EtOH and NAC. Desmoplakin, BMP-7, and Id2 have all been shown to inhibit EMT and to be reduced in other rodent models of fibrosis such as carbon tetrachloride treatment (9, 10). Both NAC and EtOH alone suppressed desmoplakin mRNA expression (P < 0.05). However, no effects of EtOH or NAC alone were observed on BMP-7 or Id2 mRNA expression. The combination of EtOH and NAC, while further stimulating repair, had no effect on desmoplakin or Id2 mRNA and actually increased expression of BMP-7 mRNA (P < 0.05). In addition, no effect of EtOH was observed on expression of mRNA for the pro-EMT factor S100A4 relative to TEN controls, whereas NAC treatment suppressed S100A4 mRNA expression in both the presence and absence of EtOH (P < 0.05) (Table 7).
Fig. 6.
Representative proliferating cell nuclear antigen (PCNA) staining of hepatocyte proliferation in rats fed high-fat diets via total enteral nutrition (Control), fed control diets supplemented with 1.7 g·kg−1·day−1 NAC, fed isocaloric diets with EtOH at 12 g·kg−1·day−1, or fed EtOH diet + 1.7 g·kg−1·day−1 NAC as described in materials and methods. Magnification ×200.
Fig. 7.
Quantitation of liver cells in S phase in rats fed high-fat diets via TEN (Control), fed control diets supplemented with 1.7 g·kg−1·day−1 NAC, fed isocaloric diets with EtOH at 12 g·kg−1·day−1, or fed EtOH diet + 1.7 g·kg−1·day−1 NAC as described in materials and methods, based on cell counting after PCNA staining. Data are means ± SE, % cells in S phase. Bars with different letters differ P < 0.05 by 2-way ANOVA followed by Student-Newman-Keuls post hoc analysis for multiple pairwise comparisons.
Table 7.
Effects of EtOH and NAC on hepatic Hedgehog pathway activation and EMT
| Group | Chow | Control | NAC | EtOH | EtOH + NAC |
|---|---|---|---|---|---|
| Genes involved in Hedgehog activation | |||||
| Shh | nd | nd | nd | nd | nd |
| Gli2 | 1.00 ± 0.61 | 4.17 ± 1.35 | 1.71 ± 0.52 | 0.48 ± 0.15 | 3.78 ± 1.31 |
| Hip | 1.00 ± 0.20a | 1.85 ± 0.29a,b | 1.56 ± 0.27a | 1.34 ± 0.18a | 2.94 ± 0.44b |
| Genes involved in EMT | |||||
| Desmoplakin | 1.00 ± 0.08a | 2.15 ± 0.23b | 0.49 ± 0.06a | 1.25 ± 0.28a | 0.58 ± 0.13a |
| BMP-7 | 1.00 ± 0.20a | 1.90 ± 0.36a | 2.09 ± 0.95a | 1.36 ± 0.20a | 4.28 ± 1.02b |
| Id 2 | 1.00 ± 0.15 | 1.35 ± 0.28 | 0.67 ± 0.25 | 0.70 ± 0.17 | 0.73 ± 0.24 |
| S100A4 | 1.00 ± 0.10a | 6.00 ± 1.43b | 1.78 ± 0.41a | 7.59 ± 3.4b | 1.98 ± 0.04 |
Real-time RT-PCR data are expressed as gene expression normalized to expression of 18S and expressed relative to Chow = 1.0. Data are presented as means ± SE for n = 7–11 rats/group. nd, Not determined. Means within a row without a common superscript letter are significantly different (P < 0.05) by 1-way ANOVA followed by Student-Newman-Keuls post hoc analysis.
DISCUSSION
Compared with ad libitum feeding of a standard commercially available rat diet, 130 days of feeding the TEN control diet resulted in increased weight gain and some hepatic fat accumulation. This is not surprising since liquid diets are more easily absorbed than solid diets and the TEN diet is higher in fat. Triglyceride accumulation appeared to be primarily associated with increased fatty acid import via CD36. This is consistent with previous data from our laboratory in which diets high in fat elicited a similar increase in CD36 expression when overfed via TEN (6, 22). Interestingly, TEN controls also had a three- to fourfold increase in expression of adiponutrin mRNA relative to chow-fed rats. Adiponutrin has been suggested to play a role in triglyceride hydrolysis, and mutations in this enzyme have been shown to promote hepatic triglyceride accumulation in humans (16, 30). Thus the increase in adiponutrin expression may have served to limit hepatic triglyceride accumulation after high-fat feeding in the present study.
Although the development of steatosis in the TEN controls resulted in small increases in apoptosis, there was no evidence of necrotic injury in male rats at this age, at this % of dietary fat and level of caloric intake.
Unexpectedly, chronic dietary supplementation with NAC increased somatic growth in isocalorically fed rats both in the presence and absence of EtOH and by itself produced rather extensive liver steatosis without any evidence of further injury. Increased triglyceride accumulation was associated with a dramatic reduction of adiponutrin expression in NAC-fed groups relative to the TEN controls. Coadministration of EtOH and NAC did not reduce triglyceride accumulation relative to either treatment alone. This is probably because NAC treatment also suppressed adiponutrin mRNA expression in EtOH-treated rats. NAC treatment also increased cellular proliferation over TEN controls. It is unclear whether these metabolic and cell cycle effects of NAC are related its antioxidant properties or are specific to NAC. This requires further chronic feeding studies with other dietary antioxidants.
As has been described in many previous reports, EtOH treatment produced extensive hepatic steatosis. However, the molecular mechanisms underlying this effect remain in some dispute. This is in part because so many possible pathways or combinations of pathways may be involved and because the effects of EtOH are dependent on the dietary context in which EtOH exposure occurs. In mice fed EtOH and high-carbohydrate diets, it has been reported that EtOH stimulates fatty acid synthesis through the SREBP-1c/FASN pathway and inhibits fatty acid degradation via PPARα-mediated pathways and that these actions are secondary to inhibition of adiponectin secretion and inhibition of downstream AMP kinase and sirtuin deacetylase pathways (31, 38, 39). In contrast, in previous studies in our laboratory and in the present study, we have shown that when EtOH is administered as part of TEN diets high in polyunsaturated fats such as corn oil, steatosis occurs despite unaltered or decreased fatty acid synthesis and increases in fatty acid degradation (5). Data from the present study suggest that EtOH-induced triglyceride accumulation is mediated at least in part via increases in fatty acid import into the liver since mRNA of the major fatty acid transport protein FATP-2 (13) was significantly unregulated in the EtOH group compared with chow or TEN controls. Other investigators have suggested that impaired VLDL secretion also contributes to development of alcoholic steatosis (8, 32). Sugimoto et al. (32) have reported decreased mRNA expression and activity of microsomal triglyceride transfer protein (MTP), a protein that catalyses the transfer of triglycerides between membranes and the lipoproteins that make up VLDL, in rats fed EtOH via Lieber-DeCarli liquid diets. However, we observed no effects of EtOH or any other treatment on MTP mRNA expression in the present study. More recent studies have suggested that EtOH impairs lipid droplet trafficking as a result of reduced expression of Rab ATPases such as Rab3D (8). In contrast, we found significant increases in Rab3D mRNA expression after EtOH and EtOH + NAC treatment in the present study. However, effects of EtOH on other aspects of lipoprotein packaging and VLDL export cannot be ruled out. Although we observed no effects on ApoB100 mRNA expression, much of the regulation of this major component of VLDL is posttranscriptional (14, 26) and the degradation of ApoB100 protein via post-Endoplasmic Reticulum presecretory proteolysis has been shown to be stimulated by oxidative stress (27).
Although the source of reactive oxygen species or other short-lived radical species in the liver following EtOH treatment remains the subject of debate (2), it is clear that production of lipid-derived radicals results in depletion of antioxidant defenses is linked to the development of inflammation and contributes to development of hepatic injury (2, 20, 24, 29). In addition, protein adducts with lipid peroxidation products are known to stimulate the host immune response and result in an autoimmune-like disease (24, 34, 36). Antibodies to MDA- and lipid peroxide-adducted proteins have been demonstrated in both animal models of alcoholic liver disease and human alcoholics and correlate with the degree of injury (24, 29, 36) as well as with inflammatory responses (36). Our present data suggest that dietary NAC acts as an effective antioxidant, preventing EtOH-induced depletion of hepatic GSH and development of an immune response to lipid peroxidation product-adducted proteins. These responses are in agreement with an earlier experiment conducted in the TEN-rat model in which EtOH treatment was continued for only 45 days compared with 130 days in the present study (29). Also, in agreement with the previous study, we observed that prevention of EtOH-induced increases in serum ALT values when EtOH and NAC were administered together, accompanied by a reduction in apoptotic DNA fragmentation and necroinflammatory injury. The decrease in apoptotic injury, despite a lack of effect on EndoG expression in EtOH-exposed rats after NAC treatment, seems to indicate that in this case, NAC restricts EndoG access to nuclear DNA, perhaps by preventing oxidative damage of mitochondrial membranes.
Surprisingly, 130 days of EtOH feeding resulted in less inflammation and necrosis than we have previously reported for 45-day EtOH treatment and there was no deterioration in the cyclic patterns of EtOH clearance revealed by monitoring BECs. It appears that under the present conditions of good nutrition, the liver adapts to high levels of EtOH over a long period by increasing hepatocyte proliferation to replace damaged apoptotic and necrotic cells and acute increases in inflammatory cytokines normalize with time. The relatively small induction of CYP2E1 activity observed in the present study relative to previous shorter term EtOH studies in the same model (29) may also reflect long-term adaptation to alcohol. The protective effects of NAC on hepatic pathology appear to be explained by additive effects of NAC and EtOH on hepatocyte proliferation. It has been suggested that, during chronic liver injury, activation of Hh-responsive progenitor cells can repopulate the liver with immature hepatocytes in an attempt to compensate for the impaired proliferation of mature hepatocytes (9, 10, 12). In our hands, EtOH-stimulated regenerative activity does not appear to involve Hh signaling, indicating that mature hepatocyte proliferation rather that the recruitment of liver progenitor cells is responsible for replacing cell loss. This is in contrast with recent data in other models indicating that 1) EtOH-induced oxidative stress decreases the proliferative activity of mature hepatocytes (12) and 2) Hh-responsive cells increase in the liver of EtOH-treated mice and patients with severe alcoholic hepatitis (10). It appears that long-term adaptation to alcoholic injury occurs in the present model and involves, among other factors, the capacity of mature hepatocytes to replace cell losses when animals are well nourished. This is relevant to the human situation since it is well known that a significant proportion of alcohol abusers can tolerate heavy drinking without appreciable signs of hepatic injury.
Consistent with earlier studies by Tsukamoto et al. (35), we observed increases in subendothelial collagen deposition in some of the EtOH and EtOH + NAC rats and no evidence of fibrosis in TEN controls or after NAC treatment alone despite the presence of steatosis. Perisinusoidal fibrosis in EtOH-treated groups was accompanied by an increase in both collagen and MMP mRNA expression, indicating effects on matrix remodeling. However, fibrosis was marginal and highly variable even after 130 days of EtOH treatment and the degree of variability made it impossible to fully assess the effects of NAC on development of fibrosis.
In conclusion, the data presented suggest that chronic feeding of high-fat diets results in development of significant hepatic pathology as a result of increased fatty acid import and development of steatosis. Additional consumption of alcohol further enhances fatty acid transport and results in additional oxidative stress and necroinflammatory injury. However, in well-nourished animals, a regenerative response occurs characterized by increased hepatocyte proliferation. Understanding the mechanisms underlying this adaptation to high alcohol intake might help to elucidate interindividual variability in the susceptibility to alcoholic liver disease among alcohol abusers. Dietary antioxidants such as NAC have been proposed as treatments for alcoholic liver injury. Although this experiment was not designed to determine whether NAC would reverse alcoholic liver pathology, NAC treatment did prevent necrotic injury and further stimulated repair in EtOH-treated animals. However, NAC treatment by itself also produced steatosis as a result of inhibited adiponutrin expression. It is unclear whether this effect is related to the antioxidant properties of NAC or is specific to NAC itself.
GRANTS
This research was supported in part by R01 AA009300 (D. R. Petersen), R01 AA18282 (M. J. Ronis), R01 AA08645 (T. M. Badger), and R01 DK078908 (A. G.Basnakian).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the following people for technical assistance: Matt Ferguson, Tammy Dallari, Courtney Reynolds, Jamie Badeaux, Neha Sharma, Crystal Combs, Alena Savenka, Nicholas Braman, and Cristina Mombello.
REFERENCES
- 1. Apostolov EO, Wang X, Shah SV, Basnakian AG. Role of EndoG in development and cell injury. Cell Death Differ 14: 1971–1974, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 124: 779–790, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Badger TM, Ronis MJJ, Lumpkin CK, Jr, Valentine CK, Shahare M, Irby D, Huang J, Mercado C, Thomas PE, Ingelman-Sundberg M, Crouch J. Effects of chronic ethanol on growth hormone secretion and hepatic P450 isozymes of the rat. J Pharmacol Exp Ther 264: 438–447, 1993 [PubMed] [Google Scholar]
- 4. Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci 94: 217–225, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Baumgardner JN, Shankar K, Korourian S, Badger TM, Ronis MJ. Undernutrition enhances alcohol-induced hepatocyte proliferation in the liver of rats fed via total enteral nutrition. Am J Physiol Gastrointest Liver Physiol 293: G355–G364, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Baumgardner JN, Shankar K, Hennings L, Badger TM, Ronis MJJ. A new rat model for non alcoholic steatohepatitis utilizing overfeeding of diets high in polyunsaturated fat by total enteral nutrition. Am J Physiol Gastrointest Liver Physiol 294: G27–G38, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Baumgardner JN, Shankar K, Hennings L, Albano E, Badger TM, Ronis MJJ. N-acetyl-cysteine attenuates progression of liver pathology in a rat model of non-alcoholic steatohepatitis. J Nutr 138: 1872–1880, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chi S, Schroeder BS, McNiven MA. Alcohol-induced impairments in hepatocellular Rab content and function: role in impaired lipid droplet trafficking after ethanol administration (Abstract). Alcohol Clin Exp Res 34: S122, 2010. [Google Scholar]
- 9. Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology 50: 2007–2013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, Sudan DL, Sicklick JK, Michelotti GA, Rojkind M, Diehl AM. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol 297: G1093–G1106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Del Signore A, De Sanctis V, Di Mauro E, Negri R, Perrone-Capano C, Paggli P. Gene expression pathways induced by axotomy and decentralization of rat superior cervical ganglion neurons. Eur J Neurosci 23: 65–74, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Diehl AM. Recent events in alcoholic liver disease. V. Effect of ethanol on liver regeneration. Am J Physiol Gastrointest Liver Physiol 288: G1–G6, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Falcon A, Doege H, Fluitt A, Tsang B, Watson N, Kay MA, Stahl A. FATP2 is a hepatic fatty acid transporter and peroxisomal very long chain acyl Co-A synthase. Am J Physiol Endocrinol Metab 299: E384–E393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fisher EA, Zhou M, Mitchell DM, Wu X, Omura S, Wang H, Goldberg AL, Ginsberg HN. The degradation of apoprotein B100 is mediated by the ubiquitin-proteasome pathway and involves heat shock protein 70. J Biol Chem 272: 20427–20434, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Greenwell A, Foley JF, Maronpot RR. An enhancement method for immunohistochemical staining of proliferating cell nuclear antigen in archival rodent tissues. Cancer Lett 59: 251–256, 1991 [DOI] [PubMed] [Google Scholar]
- 16. He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, Hobbs HH. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem 285: 6706–6715, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jung Y, Brown KD, Witek RP, Omenetti A, Yang L, Vandongen M, Milton RJ, Hines IN, Rippe RA, Spahr L, Rubbia-Brabt L, Diehl AM. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology 134: 1532–1543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koop DR. Hydroxylation of p-nitrophenol by rabbit ethanol-inducible cytochrome P450 isozyme 3a. Mol Pharmacol 29: 399–404, 1986 [PubMed] [Google Scholar]
- 19. Korourian S, Hakkak R, Ronis MJJ, Shelnutt SR, Waldran J, Ingelman-Sundberg M, Badger TM. Diet and risk of ethanol-induced hepatotoxicity: carbohydrate-fat relationships in rats. Toxicol Sci 47: 110–117, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol 13: 389–396, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Lieber CS, DeCarli LM. Hepatotoxicity of ethanol. J Hepatol 12: 394–401, 1991. [DOI] [PubMed] [Google Scholar]
- 22. Marecki JC, Shankar K, Ronis MJJ, Badger TM. Ectopic fat disposition and insulin resistance develop after overfeeding of a high fat diet to prepubertal rats despite increased adiponectin signaling. J Nutr Biochem 2010. April 30 [Epub ahead of print]. [Google Scholar]
- 23. Michalopoulos GK. Liver regeneration. J Cell Physiol 213: 286–300, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mottaran E, Stewart SF, Rolla R, Vay D, Cipriani V, Moretti MG, Vidali M, Sartori M, Rigamonti V, Day CP, Albano E. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic Biol Med 32: 38–45, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358, 1979 [DOI] [PubMed] [Google Scholar]
- 26. Pan M, Liang JS, Fisher EA, Ginsberg HN. The late addition of core lipids to nascent apolipoprotein B100, resulting in the assembly and secretion of triglyceride-rich lipoproteins, is independent of both microsomal triglyceride transfer protein activity and new triglyceride synthesis. J Biol Chem 277: 4413–4421, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Pan M, Cederbaum AI, Zhang YL, Ginsberg HN, Williams KJ, Fisher EA. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J Clin Invest 113: 1277–1287, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ronis MJJ, Hakkak R, Korourian S, Albano E, Yoon S, Ingelman-Sundberg M, Lindros KO, Badger TM. Alcoholic liver disease in rats fed ethanol as part of oral or intragastric low carbohydrate diets. Exp Biol Med (Maywood) 229: 351–360, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Ronis MJJ, Butura A, Sampey BP, Shankar K, Prior RL, Korourian S, Albano E, Ingelman-Sundberg M, Petersen DR, Badger TM. Effects of N-acetylcysteine on ethanol-induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic Biol Med 39: 619–630, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ; NASH CRN The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic liver disease. Hepatology 52: 894–903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponeutrin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 298: G364–G374, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sugimoto T, Yamashita S, Ishigami M, Sakai N, Hirano K, Tahara M, Matsumoto K, Nakamura T, Matsuzawa Y. Decreased microsomal triglyceride transfer protein activity contributes to initiation of alcoholic liver steatosis in rats. J Hepatol 36: 157–162, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Tahan G, Tarcin O, Tahan V, Eren F, Gedik N, Sahan E, Biberoglu N, Guzel S, Bozbas A, Tozum N, Yucel O. The effects of N-acetylcysteine on bile duct ligation-induced liver fibrosis in rats. Dig Dis Sci 52: 3348–3354, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Thiele GM, Freeman TL, Klassen LW. Immunologic mechanisms of alcoholic liver injury. Semin Liver Dis 24: 273–287, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Tsukamoto H, Towner SJ, Ciofalo LM, French SW. Ethanol-induced liver fibrosis in rats fed high fat diets. Hepatology 6: 814–822, 1986 [DOI] [PubMed] [Google Scholar]
- 36. Vidali M, Vietala J, Occhino G, Ivaldi A, Sutti S, Niemelä O, Albano E. Immune responses against oxidative stress-derived antigens are associated with increased circulating tumor necrosis factor-α and accelerated liver damage in heavy drinkers. Free Radic Biol Med 45: 306–311, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Yang FG, Fang BW, Lou JS. Effects of Haobie Yangyin decoction on hepatic fibrosis induced by carbon tetrachloride. World J Gastroenterol 16: 1458–1464, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol 294: G892–G898, 2008 [DOI] [PubMed] [Google Scholar]
- 39. You M, Rogers CQ. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood) 234: 850–859, 2009 [DOI] [PubMed] [Google Scholar]