Abstract
The importance of Niemann-Pick C1 Like-1 (NPC1L1) protein in intestinal absorption of dietary sterols, including both cholesterol and phytosterols, is well documented. However, the exact mechanism by which NPC1L1 facilitates cholesterol transport remains controversial. This study administered 22-(N(-7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-23,24-bisnor-5-cholen-3β-ol (NBD-cholesterol) and [3H]cholesterol to Npc1l1+/+ and Npc1l1−/− mice to determine whether NPC1L1 facilitates dietary sterol uptake by enterocytes and/or participates in intracellular sterol delivery to the endoplasmic reticulum (ER) for lipoprotein assembly before secretion into plasma circulation. Results showed that [3H]cholesterol absorption was reduced but not abolished in Npc1l1−/− mice compared with Npc1l1+/+ mice. In the presence of Pluronic L-81 to block pre-chylomicron exit from the ER, significant amounts of [3H]cholesterol were found to be associated with lipid droplets in the intestinal mucosa of both Npc1l1+/+ and Npc1l1−/− mice, and the intracellular [3H]cholesterol can be esterified to cholesteryl esters. These results provided evidence indicating that the main function of NPC1L1 is to promote cholesterol uptake from the intestinal lumen but that it is not necessary for intracellular cholesterol transport to the ER. Surprisingly, NBD-cholesterol was taken up by intestinal mucosa, esterified to NBD-cholesteryl esters, and transported to plasma circulation to similar extent between Npc1l1+/+ and Npc1l1−/− mice. Ezetimibe treatment also had no impact on NBD-cholesterol absorption by Npc1l1+/+ mice. Thus, NBD-cholesterol absorption proceeds through an NPC1L1-independent and ezetimibe-insensitive sterol absorption mechanism. Taken together, these results indicate that NBD-cholesterol can be used to trace the alternative cholesterol absorption pathway but is not suitable for tracking NPC1L1-mediated cholesterol absorption.
Keywords: intestinal cholesterol absorption, Niemann-Pick C1 Like-1, intracellular cholesterol transport, fluorescent sterol, ezetimibe
the relationship between high plasma cholesterol levels, particularly the cholesterol associated with low-density lipoprotein (LDL), and increased risk of cardiovascular disease (CVD) is well established. Several studies have shown that lowering plasma and LDL cholesterol levels reduces the risk of CVD (12, 16, 18, 23). Plasma cholesterol is derived from endogenous synthesis, primarily by the liver, and from the diet after absorption through the gastrointestinal tract. Although plasma cholesterol homeostasis is exquisitely regulated with an inverse relationship between hepatic cholesterol production and dietary cholesterol intake under normal conditions, excessive cholesterol biosynthesis or cholesterol intake will lead to perturbed cholesterol homeostasis and elevation of plasma cholesterol and LDL levels (20). Therefore, enormous resources and efforts have been spent over the past three decades to develop cholesterol synthesis and absorption inhibitors to reduce hypercholesterolemia. The most effective cholesterol synthesis inhibitors are the statins that target 3-hydroxy-3-methylglutaryl-CoA reductase, the rate-limiting enzyme in the cholesterol biosynthetic pathway (7). The effectiveness of statin therapy in decreasing plasma cholesterol levels and reducing the incidence of coronary artery disease has led to its recommendation as the primary strategy in lipid management (15, 27). Unfortunately, a substantial number of patients on statin therapy still experienced cardiovascular events. Moreover, a significant number of individuals failed to reach the targeted goal of plasma and LDL cholesterol reduction (6). The effectiveness of statins also decreases over time. Finally, some individuals cannot tolerate statin therapy and develop severe adverse effects such as muscle pain and weaknesses that may eventually progress to rhabdomyolysis (5, 17, 22). These studies demonstrated the need to identify additional cholesterol reduction mechanisms as alternative or secondary prevention strategies to achieve maximal cardioprotective benefits.
Studies aimed at reducing plasma cholesterol levels through inhibition of dietary cholesterol absorption led to the development of ezetimibe, which specifically decreases total cholesterol absorption by 54% and leads to a 15% decrease in plasma LDL-cholesterol (26). Studies designed to determine the mechanism whereby ezetimibe inhibits cholesterol absorption identified Niemann-Pick C1-Like 1 (NPC1L1) as one of the targets of ezetimibe (8). The importance of NPC1L1 in the cholesterol absorption pathway and its inhibition by ezetimibe was demonstrated by experiments showing that Npc1l1−/− mice have reduced cholesterol absorption and that the residual cholesterol absorption is not sensitive to ezetimibe inhibition (1). Additionally, human variations in the NPC1L1 expression pattern correlate not only with their cholesterol absorption efficiency, but also with their varied response to ezetimibe therapy (2, 24).
These studies documented the importance of NPC1L1 in the entire cholesterol absorption pathway, a process that includes cholesterol uptake from the intestinal lumen to the mucosa, cholesterol esterification and assembly into chylomicrons within the enterocytes, and secretion to the lymph. However, it is not clear whether NPC1L1 is required for cholesterol uptake, as originally proposed (1), and/or necessary for intracellular cholesterol transport to the endoplasmic reticulum (ER) for lipoprotein assembly, as suggested by other studies (3, 21). The goal of this study is to elucidate the role of NPC1L1 in the cholesterol absorption process, testing the hypothesis that NPC1L1 facilitates cholesterol uptake but is not required for the transport of the internalized cholesterol to the ER for lipoprotein assembly.
MATERIALS AND METHODS
Materials.
Cholesterol was purchased from Sigma (St. Louis, MO). The radioactive [1,2-3H]cholesterol, [4-14C]cholesterol, and [1,2-3H]cholesterol ether were purchased from American Radiolabeled Chemicals (St. Louis, MO). 22-(N(-7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-23,24-bisnor-5-cholen-3β-ol (NBD-cholesterol) was purchased from Invitrogen Molecular Probes (Eugene, OR). Ezetimibe was obtained as Zetia tablets purchased from Merck Schering Plough (Whitehouse Station, NJ). NCS II Tissue Solubilizer was purchased from GE Healthcare (Pittsburgh, PA).
Animals and diets.
The Npc1l1−/− mice were generated as previously described by targeting the Npc1l1 gene (3) and transferred to the University of Cincinnati, where they were bred in house. Animals, except where noted otherwise, were maintained on a regular chow diet. For animals placed on an ezetimibe therapy, Zetia tablets were ground into powder and then mixed with food at a dosage of 10 mg Zetia/100 g. All mice were maintained under a 12:12-h light-dark cycle in a temperature- and humidity-controlled room at the University of Cincinnati Laboratory Animal Medical Services, with free access to food and water. All procedures used in this study were approved and authorized by the Institutional Animal Care and Use Committee at the University of Cincinnati. Only 10- to 15-wk-old male animals were used in these studies.
Cholesterol uptake.
Mice fasted overnight were orally fed with 3.45 mg Pluronic L-81 or saline by stomach gavage (25). A second dose of Pluronic L-81 was administered after 1 h and was followed immediately by an oral gavage of 0.4 mg NBD-cholesterol, 1 μCi [3H]cholesterol, or 1 μCi [3H]cholesterol ether plus 1 μCi [14C]cholesterol in 100 μl olive oil. The total mass of gavaged cholesterol was maintained at 0.4 mg per mouse. The mice were euthanized after 3 h, a time at which NBD-cholesterol absorption has been shown to be at steady state (25). The intestine was excised and separated into three equal 7-cm sections representing the proximal, middle, and distal intestine. Tissues from mice that received NBD-cholesterol were placed in 0.4% paraformaldehyde overnight, embedded in optimal cutting temperature medium, and frozen. The tissue samples were sectioned into 10-μm sections with a cryostat, fixed with 3.7% paraformaldehyde, and viewed at 488 nm on a fluorescence microscope. Representative 100-mg tissues from mice that received [3H]cholesterol or [3H]cholesterol ether plus [14C]cholesterol were placed in tissue solubilizer and then incubated overnight at 37°C before liquid scintillation counting to determine the amount of radiolabeled cholesterol associated with the intestinal tissues. The data are reported as a percentage of the total amount of radiolabeled cholesterol or cholesterol ether administered to each animal.
Thin-layer chromatography analysis of intestine samples.
Lipids in the intestinal samples of mice administered [3H]cholesterol were extracted by chloroform/methanol (2:1, vol:vol) and then separated on silica gel plates using a petroleum ether-ethyl ether-acetic acid (300:150:1.5) solvent system. The samples were visualized along with the comigrating reference standards with iodine vapor. Cholesteryl ester (CE) and free cholesterol (FC) bands were scraped and counted. The percentage of cholesterol esterified to CE was determined using the following equation: [CE counts/(FC counts + CE counts)] × 100. The NBD-cholesterol in mouse intestine after oral administration was extracted by hexane-water (1:1, vol/vol) and then separated on silica gel plates using a petroleum ether-ethyl ether-acetic acid (300:150:1.5, vol/vol/vol) solvent system. The samples were visualized by measuring fluorescence at 450 nm and quantified by densitometry. The percentage of NBD-cholesterol esterified was determined using the following equation: [CE fluorescence/(FC fluorescence + CE fluorescence)] × 100.
Plasma sterol quantification.
NBD-cholesterol quantification was adapted from a previously described method (25). Briefly, mice fasted overnight were injected with 10% poloxamer (10 μl/g ip) to inhibit lipolysis and clearance of circulating lipoproteins and were then fed immediately with an oral gavage of [14C]cholesterol and 0.4 mg NBD-cholesterol in olive oil. The mice were euthanized by isoflurane overdose after 4 h. Blood was collected by cardiac puncture and then centrifuged for plasma isolation. Absorption of [14C]cholesterol was quantified by scintillation counting of 10-μl plasma samples. NBD-cholesterol was quantified by extracting a 50-μl plasma sample with hexane and water (1:1, vol/vol). The extracted lipids were solubilized in 500 μl of 10 mM taurocholate for fluorescence measurement at an excitation of 485 nm and emission of 535 nm. Total NBD-cholesterol concentration in each sample was determined by comparing fluorescent intensity to a standard curve.
Statistical analysis.
Data are presented as means ± SD. Statistical significance (P < 0.05) was determined by one-way or two-way ANOVA with post test analysis using the Bonferroni post hoc test or Student's unpaired t-test with Prism 4 software (GraphPad).
RESULTS
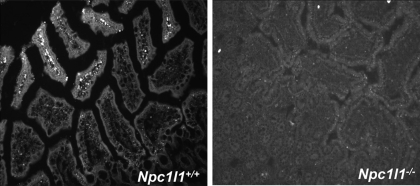
Previous studies indicated that NBD-cholesterol behaves like cholesterol when administered to hamsters in vivo and is internalized by enterocytes, esterified, and secreted to the plasma in a cholesterol absorption inhibitor-sensitive manner (25). Therefore, NBD-cholesterol was utilized to study the role of NPC1L1 in intestinal cholesterol absorption and transport. The Npc1l1+/+ and Npc1l1−/− mice were orally gavaged with a bolus meal of olive oil supplemented with NBD-cholesterol and were then euthanized after 3 h to assess NBD-cholesterol uptake and transport in the intestine. In Npc1l1+/+ mice, the orally administered NBD-cholesterol was found primarily in the lamina propria of the proximal and middle sections of the intestine (Fig. 1). In contrast, NBD-cholesterol was not observed throughout the intestinal tract of Npc1l1−/− mice (Fig. 1).
Fig. 1.
Uptake of 22-(N(-7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-23,24-bisnor-5-cholen-3β-ol (NBD-cholesterol) into the enterocytes of Niemann-Pick C1 Like-1 (NPC1L1) protein-null (Npc1l1−/−) and Npc1l1+/+ mice. Representative images are shown of 20 intestinal sections from Npc1l1+/+ (left) and Npc1l1−/− (right) mice (n = 10 mice per group) 3 h after gavaged with 0.4 mg of NBD-cholesterol in 100 μl olive oil. The intestine was fixed with 0.4% paraformaldehyde and embedded in optimal cutting temperature (OCT) medium before fluorescent microscopy. NBD-cholesterol appears to be localized to the lamina propria in Npc1l1+/+ mice but is not visible in Npc1l1−/− mice.
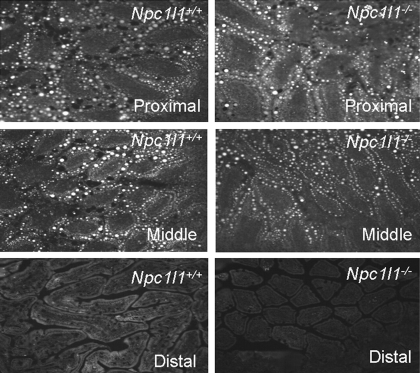
The lack of NBD-cholesterol in the intestine of Npc1l1−/− mice may be due to the lack of cholesterol uptake by NPC1L1-deficient enterocytes. Alternatively, since intestinal absorption of NBD-cholesterol is more rapid compared with cholesterol (25), the NBD-cholesterol taken up by enterocytes may be rapidly secreted from the cells, thereby limiting its visualization within enterocytes at a single time point. To differentiate these possibilities, Pluronic L-81, which has been shown previously to inhibit pre-chylomicron exit from the ER (28–30), was used to determine whether NBD-cholesterol can be taken up by enterocytes and transported to the ER in the absence of NPC1L1. Both Npc1l1+/+ and Npc1l1−/− mice treated with Pluronic L-81 before gastric gavage of NBD-cholesterol in olive oil showed the association of NBD-cholesterol with the large lipid droplets within the enterocytes of the proximal and middle sections, but not the distal sections of the intestine (Fig. 2). These results indicate that NPC1L1 is not necessary for cholesterol trafficking to the ER once it is taken up by the enterocytes. This conclusion was confirmed by additional results showing that [3H]cholesterol administered to Pluronic L-81-treated Npc1l1+/+ and Npc1l1−/− mice was esterified intracellularly to a similar extent (Fig. 3).
Fig. 2.
Uptake of NBD-cholesterol into the enterocytes of Npc1l1+/+ and Npc1l1−/− mice in the presence of Pluronic L-81. Representative images are shown of 20 intestinal sections from the proximal (top), middle (middle), and distal (bottom) intestines of Npc1l1+/+ (left) and Npc1l1−/− (right) mice (n = 10 mice per group) 3 h after gavaged with 3.45 mg Pluronic L-81 followed by 0.4 mg of NBD-cholesterol in 100 μl olive oil. The intestine was fixed with 0.4% paraformaldehyde and embedded in OCT medium before fluorescent microscopy. NBD-cholesterol is contained within intestinal lipid droplets of both Npc1l1+/+ and Npc1l1−/− mice.
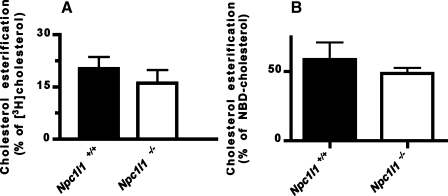
Fig. 3.
Cholesterol is esterified in Npc1l1+/+ and Npc1l1−/− mice. A: the Npc1l1+/+ (solid bars; n = 6) and Npc1l1−/− (open bars; n = 7) mice were fed a diet of 1 μCi [3H]cholesterol in 100 μl olive oil, and intestine was collected after 3 h. Lipids were extracted, and the amount of cholesterol esterification was determined by thin-layer chromatography. Percent cholesterol esterification was determined by [CE counts/(FC counts + CE counts)] × 100. B: the Npc1l1+/+ (solid bars; n = 4) and Npc1l1−/− (open bars; n = 5) mice were fed 0.4 mg NBD-cholesterol in 100 μl olive oil, and blood was collected after 4 h. Plasma lipids were extracted for thin-layer chromatography separation of cholesterol and cholesteryl esters. The amount of NBD-cholesterol present in each band was determined by measuring fluorescent intensity at 488 nm. Percent esterification was determined as [CE fluorescence/(FC fluorescence + CE fluorescence)] × 100.
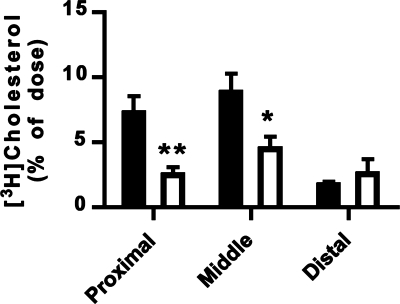
The presence of orally administered NBD-cholesterol and [3H]cholesterol in the intestine of Npc1l1−/− mice suggested that dietary sterols may be taken up by enterocytes in the absence of NPC1L1. In the next experiment, Npc1l1+/+ and Npc1l1−/− mice were fed [3H]cholesterol in olive oil after Pluronic L-81 treatment to compare the amount of sterol uptake by Npc1l1+/+ and Npc1l1−/− enterocytes. Although less [3H]cholesterol was found in proximal and middle intestine of Npc1l1−/− mice compared with Npc1l1+/+ mice (Fig. 4), significant amounts of [3H]cholesterol was present in the intestine of Npc1l1−/− mice (Fig. 4). Moreover, the amount of [3H]cholesterol in the distal intestine was similar between Npc1l1+/+ and Npc1l1−/− mice (Fig. 4). Importantly, when the nonabsorbable [3H]cholesterol ether was fed to Npc1l1+/+ mice along with [14C]cholesterol and Pluronic L-81, minimal uptake of [3H]cholesterol ether compared with [14C]cholesterol was observed (Fig. 5A). Additionally, the residual amount of [3H]cholesterol ether taken up by the intestine was similar in the presence or absence of Pluronic L-81 (Fig. 5B). These latter observations indicated that the NBD-cholesterol and [3H]cholesterol observed in the intestine of Npc1l1−/− mice was not due to nonspecific membrane permeabilizing effects of Pluronic L-81. Taken together, the data provide strong indication that whereas NPC1L1 enhances dietary sterol uptake by the enterocytes, additional NPC1L1-independent mechanism(s) exist(s) for intestinal uptake of dietary sterols.
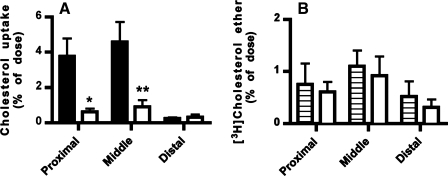
Fig. 4.
Uptake of [3H]cholesterol into the intestine of Npc1l1+/+ and Npc1l1−/− mice. The Npc1l1+/+ (solid bars) and Npc1l1−/− (open bars) mice were given a dose of 3.45 mg Pluronic L-81 followed by an oral gavage of 1 μCi [3H]cholesterol in 100 μl olive oil. The intestine was removed, cut into 100-mg sections, and dissolved in tissue solubilizer before radioactivity determinations. Data represent means ± SE from 13 Npc1l1+/+ and 10 Npc1l1−/− mice. *P < 0.05, **P < 0.01, statistically significant differences from the Npc1l1+/+ mice.
Fig. 5.
Effect of Pluronic L-81 on intestinal uptake of [3H]cholesterol ether and [14C]cholesterol. A: the Npc1l1+/+ mice were given a dose of 3.45 mg Pluronic L-81 followed by an oral gavage of 1 μCi [14C]cholesterol (solid bars) and [3H]cholesterol ether (open bars). The intestine was collected, cut into 100-mg sections, and dissolved in tissue solubilizer before radioactivity determinations. B: the Npc1l1+/+ mice were given a dose of saline (hatched bars) or 3.45 mg Pluronic L-81 (open bars) followed by an oral gavage of 1 μCi [3H]cholesterol ether. The intestine was collected, cut into 100-mg sections, and dissolved in tissue solubilizer before radioactivity determinations. Data represent means ± SE from 3 mice in each group. *P < 0.05, **P < 0.01, statistically significant differences from [3H]cholesterol absorption data.
The detection of NBD-cholesterol in the intestine of Npc1l1−/− mice is a surprising finding, suggesting that NBD-cholesterol may be transported independent of NPC1L1. To test this possibility, we compared the amount of NBD-cholesterol absorbed and transported to plasma in Npc1l1+/+ and Npc1l1−/− mice. Results showed that similar amounts of NBD-cholesterol were absorbed and transported to plasma between Npc1l1−/− mice and Npc1l1+/+ mice (Fig. 6, A and B). As expected on the basis of the retention of NBD-cholesterol in enterocytes upon inhibition of chylomicron secretion with Pluronic L-81, the NBD-cholesterol in plasma of Npc1l1+/+ and Npc1l1−/− mice was associated with lipoproteins as assessed by density ultracentrifugation (data not shown). The absorption of NBD-cholesterol was also similar between Npc1l1+/+ mice with or without ezetimibe treatment (Fig. 6, A and B). Interestingly, the total amount of NBD-cholesterol absorbed in all three groups of animals was similar to the amount of [3H]cholesterol absorbed in Npc1l1−/− and ezetimibe-treated Npc1l1+/+ mice (Fig. 6B). Furthermore, the NBD-cholesterol absorbed by the intestine and transported to the plasma was esterified to a similar extent in Npc1l1+/+ and Npc1l1−/− mice (Fig. 3B). Taken together, these results indicate that NBD-cholesterol absorption is independent of NPC1L1.
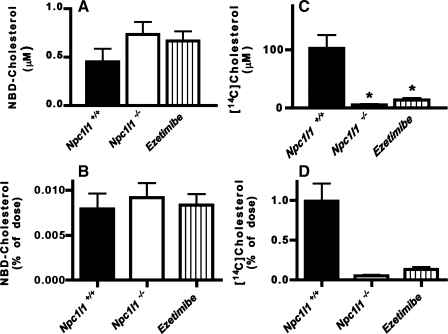
Fig. 6.
Absorption of NBD-cholesterol and [14C]cholesterol in Npc1l1+/+, Npc1l1−/−, and ezetimibe-treated Npc1l1+/+ mice. Npc1l1+/+ (solid bars; n = 5), Npc1l1−/− (open bars; n = 3), and ezetimibe treated Npc1l1+/+ (hatched bars; n = 4) mice were given an oral gavage of 0.4 mg NBD-cholesterol and 1 μCi [14C]cholesterol in olive oil. Blood was collected after 4 h via cardiac puncture. Plasma was extracted with hexane, and fluorescence was measured to quantify NBD-cholesterol absorption (A and B). A 10-μl sample of plasma was counted for radioactivity determinations (C and D). Data represent means ± SE of 5 Npc1l1+/+, 3 Npc1l1−/−, and 4 ezetimibe-treated Npc1l1+/+ mice. *P < 0.05, statistically significant difference from Npc1l1+/+ group.
DISCUSSION
The importance of NPC1L1 in intestinal absorption of dietary sterols, including both cholesterol and phytosterols, is well documented in the literature (1, 4). However, the exact mechanism by which NPC1L1 facilitates cholesterol transport remains controversial. Earlier studies using histochemical techniques localized NPC1L1 on the brush border membranes of the proximal intestine, suggesting that NPC1L1 is a cell surface transporter responsible for cholesterol uptake by the enterocytes (1). Chinese hamster ovary cells transfected with epitope-tagged rat Npc1l1 cDNA also revealed plasma membrane localization of the epitope-tagged protein (11), thus providing additional evidence that NPC1L1 is a cell surface transporter protein. However, other studies have failed to substantiate cell surface localization of NPC1L1, but instead showed its presence in intracellular vesicular compartments, consistent with a potential role in intracellular cholesterol transport (3). This controversy was partially resolved by more recent studies demonstrating that NPC1L1 shuttles between plasma membrane and intracellular compartments in a cholesterol-dependent manner, with cholesterol deprivation stimulating NPC1L1 translocation to the plasma membrane to promote cholesterol uptake (19, 32). Moreover, ezetimibe inhibits cholesterol absorption by suppressing sterol-induced internalization of NPC1L1 (9).
Consistent with results reported previously, the current study showed significant attenuation of dietary [3H]cholesterol absorption into plasma lipoproteins in Npc1l1−/− and ezetimibe-treated Npc1l1+/+ mice. Nevertheless, a significant amount of dietary [3H]cholesterol was absorbed and transported to plasma circulation of Npc1l1−/− mice. The reduced [3H]cholesterol absorption in Npc1l1−/− mice coincided with decreased [3H]cholesterol in the proximal intestine when chylomicron secretion was suppressed with Pluronic L-81. The decreased intestinal uptake and absorption of dietary cholesterol in the absence of NPC1L1 is consistent with previous observations showing reduction but not absence of cholesterol absorption in Npc1l1−/− and ezetimibe-treated mice (1). Importantly, results of the current study revealed that [3H]cholesterol internalized into enterocytes in the absence of NPC1L1 can be esterified to [3H]cholesteryl esters and was found in association with pre-chylomicrons in the ER of Pluronic L-81-treated mice. Taken together, these results provide strong evidence that the main function of NPC1L1 in the intestine is to promote cholesterol uptake from the lumen but that it is not necessary for the transport of internalized cholesterol to intracellular compartments where lipoprotein assembly occurs.
The current study also used NBD-cholesterol to visualize sterol uptake by intestines of Npc1l1+/+ and Npc1l1−/− mice. Unlike another fluorescent sterol, BODIPY-cholesterol, which is poorly esterified intracellularly (10), NBD-cholesterol has been shown to be absorbed, esterified, and present in plasma when fed to hamsters (25). Intestinal absorption of NBD-cholesterol is also similar to cholesterol absorption with specificity for enterocytes instead of globlet cells, and it is inhibitable by the cholesterol absorption inhibitors L166,143 and L165,313 (25). Our results showed that NBD-cholesterol was also rapidly absorbed by enterocytes and secreted into the lamina propria of Npc1l1+/+ mice. However, in the presence of dietary fat and Pluronic L-81 to retain pre-chylomicrons in the ER, the NBD-cholesterol was found to be associated with lipid droplets in intracellular compartments of the intestine in both Npc1l1+/+ and Npc1l1−/− mice. The intracellular retention of NBD-cholesterol in the intestine of Pluronic L-81-treated Npc1l1−/− mice further illustrated that this fluorescent sterol can be transported to the ER and assembled into pre-chylomicrons in the absence of NPC1L1. The presence of NBD-cholesterol in the intestine of Npc1l1−/− mice was not an artifact of membrane permeabilization induced by Pluronic L-81 because cholesteryl ether was not absorbed under similar conditions. Rather, the presence of NBD-cholesterol in intracellular compartments of Npc1l1−/− mouse intestines was consistent with the ability of these animals to absorb dietary sterols to a limited extent. Moreover, the NBD-cholesterol was found to be absorbed and was transported to plasma circulation in Npc1l1−/− mice. Surprisingly, the amount of NBD-cholesterol absorbed by Npc1l1−/− mice was similar to that observed in Npc1l1+/+ mice and was similar to the amount of [14C]cholesterol absorbed by Npc1l1−/− and ezetimibe-treated Npc1l1+/+ mice. Taken together, these results indicate that, whereas NBD-cholesterol can be used to track cholesterol absorption through the gastrointestinal tract in vivo, as suggested by others previously (25), NBD-cholesterol absorption proceeds through a NPC1L1-independent and ezetimibe-insensitive manner.
In summary, results of the current study revealed a minor but potentially physiologically significant pathway of dietary sterol absorption that is independent of NPC1L1. This alternative sterol absorption pathway is not sensitive to ezetimibe inhibition but can be suppressed by treatment with other cholesterol absorption inhibitors such as L-166,143 or L-165,313 (25). The presence of an alternative ezetimibe-insensitive cholesterol absorption pathway is consistent with recent data showing that significant amounts of cholesterol were secreted into the lymph in ezetimibe-treated rats (31). The effectiveness of the latter inhibitors at low concentrations to inhibit both cholesterol and NBD-cholesterol absorption suggests that this ezetimibe-insensitive and NPC1L1-independent alternative pathway of cholesterol absorption may also be protein mediated. Whether cholesterol-binding proteins identified previously in brush border membranes (13, 14) participate in this alternative cholesterol absorption pathway remains to be determined. In any event, the demonstration that NBD-cholesterol tracks dietary sterols through the alternative instead of NPC1L1-dependent pathway will facilitate the identification of the protein(s) involved. Although the current study showed that this alternative cholesterol absorption pathway is minor and that NPC1L1 is responsible for most of the cholesterol absorption in mice, recent studies have demonstrated that NPC1L1-independent pathway may account for up to 46% of the cholesterol absorbed in humans (26). Thus, the identification of exact mechanism and the protein(s) involved with this alternative cholesterol absorption pathway will facilitate design of therapeutics that target both NPC1L1-dependent and NPC1L1-independent pathways of absorption to maximally inhibit cholesterol absorption in prevention of hypercholesterolemia.
GRANTS
This work was supported by National Institutes of Health Grant RO1 DK-076907.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Altmann SW, Davis HR, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Lyer SPN, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303: 1201–1204, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Cohen JC, Pertsemlidis A, Fahmi S, Esmail S, Vega GL, Grundy SM, Hobbs HH. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low density lipoprotein levels. Proc Natl Acad Sci USA 103: 1810–1815, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem 280: 12710–12720, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Davis HR, Jr, Zhu Lj, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SPN, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem 279: 33586–33592, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Degreef LE, Opdam FL, Teepe-Twiss IM, Jukema JW, Guchelaar HJ, Tamsma JT. The tolerability and efficacy of low-dose simvastatin in statin-intolerant patients. Eur J Intern Med 21: 293–296, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Donnelly LA, Palmer CN, Whitley AL, Lang CC, Doney AS, Morris AD, Donnan PT. Apolipoprotein E genotypes are associated with lipid-lowering responses to statin treatment in diabetes: a Go-DARTS study. Pharmacogenet Genomics 18: 279–287, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Endo A, Kuroda M, Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett 72: 323–326, 1976 [DOI] [PubMed] [Google Scholar]
- 8. Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, MacIntyre DE, Ogawa A, O'Neill KA, Iyer SPN, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci USA 102: 8132–8137, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ge L, Wang J, Qi W, Miao HH, Cao J, Qu YX, Li BL, Song BL. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab 7: 508–519, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Holtta-Vuori M, Uronen RL, Repakova J, Salonen E, Vattulainen I, Panula P, Li Z, Bittman R, Ikonen E. BODIPY-cholesterol: a new tool to visualize sterol trafficking in living cells and organisms. Traffic 9: 1839–1849, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Iyer SPN, Yao X, Crona JH, Hoos LM, Tetzloff G, Davis HR, Graziano MP, Altmann SW. Characterization of the putative native and recombinant rat sterol transporter Niemann-Pick C1 Like 1 (NPC1L1) protein. Biochim Biophys Acta 1722: 282–292, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C. Metabolic syndrome, low-density lipoprotein cholesterol, and risk of cardiovascular disease: a population-based study. Atherosclerosis 189: 369–374, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Knopfel M, Davies JP, Duong PT, Kvaerno L, Carreira EM, Phillips MC, Ioannou YA, Hauser H. Multiple plasma membrane receptors but not NPC1L1 mediate high affinity, ezetimibe-sensitive cholesterol uptake into the intestinal brush border membrane. Biochim Biophys Acta 1771: 1140–1147, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Labonté ED, Howles PN, Granholm NA, Rojas JC, Davies JP, Ioannou YA, Hui DY. Class B type I scavenger is responsible for the high affinity cholesterol binding activity of intestinal brush border membrane vesicles. Biochim Biophys Acta 1771: 1132–1139, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma PTS, Gil G, Sudhof TC, Bilheimer DW, Goldstein JL, Brown MS. Mevinolin, an inhibitor of cholesterol synthesis, induces mRNA for low density lipoprotein receptor in livers of hamsters and rabbits. Proc Natl Acad Sci USA 83: 8370–8374, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 349: 1269–1276, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Pasternak RC, Smith SC, Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 40: 567–572, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Pekkanen J, Linn S, Heiss G, Suchindran CM, Leon A, Rifkind BM, Tyroler HA. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med 322: 1700–1707, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Petersen NH, Faergeman NJ, Yu L, Wustner D. Kinetic imaging of NPC1L1 and sterol trafficking between plasma membrane and recycling endosomes in hepatoma cells. J Lipid Res 49: 2023–2037, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Quintao E, Grundy SM, Ahrens EH., Jr Effects of dietary cholesterol on the regulation of total body cholesterol in man. J Lipid Res 12: 233–247, 1971 [PubMed] [Google Scholar]
- 21. Sane AT, Sinnett D, Delvin E, Bendayan M, Marcil V, Menard D, Beaulieu JF, Levy E. Localization and role of NPC1L1 in cholesterol absorption in human intestine. J Lipid Res 47: 2112–2120, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Schmitz G, Drobnik W. Pharmacogenomics and pharmacogenetics of cholesterol-lowering therapy. Clin Chem Lab Med 41: 581–589, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. Atheroscler Suppl 5: 91–97, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Simon JS, Karnoub MC, Devlin DJ, Arreaza MG, Qiu P, Monks SA, Severino ME, Deutsch P, Palmisano J, Sachs AB, Bayne ML, Plump AS, Schadt EE. Sequence variation in NPC1L1 and association with improved LDL-cholesterol lowering in response to ezetimibe treatment. Genomics 86: 648–656, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Sparrow CP, Patel S, Baffic J, Chao YS, Hernandez M, Lam MH, Montenegro J, Wright SD, Detmers PA. A fluorescent cholesterol analog traces cholesterol absorption in hamsters and is esterified in vivo and in vitro. J Lipid Res 40: 1747–1757, 1999 [PubMed] [Google Scholar]
- 26. Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 106: 1943–1948, 2002 [DOI] [PubMed] [Google Scholar]
- 27. The Lovastatin Group Therapeutic response to lovastatin (mevinolin) in nonfamilial hypercholesterolemia. A multicenter study. The Lovastatin Study Group II. JAMA 256: 2829–2834, 1986 [DOI] [PubMed] [Google Scholar]
- 28. Tso P, Balint JA, Bishop MB, Rodgers JB. Acute inhibition of intestinal lipid transport by Pluronic L-81 in the rat. Am J Physiol Gastrointest Liver Physiol 241: G487–G497, 1981 [DOI] [PubMed] [Google Scholar]
- 29. Tso P, Drake DS, Black DD, Sabesin SM. Evidence for separate pathways of chylomicron and very low-density lipoprotein assembly and transport by rat small intestine. Am J Physiol Gastrointest Liver Physiol 247: G599–G610, 1984 [DOI] [PubMed] [Google Scholar]
- 30. Tso P, Gollamudi SR. Pluronic L-81: a potent inhibitor of the transport of intestinal chylomicrons. Am J Physiol Gastrointest Liver Physiol 247: G32–G36, 1984 [DOI] [PubMed] [Google Scholar]
- 31. Yang L, Li X, Ji Y, Kohan AB, Wang DQH, Howles PN, Hui DY, Lai J, Tso P. Effect of ezetimibe on incretin secretion in response to the intestinal absorption of a mixed meal. Am J Physiol Gastrointest Liver Physiol 299: G1003–G1011, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu L, Bharadwaj S, Brown JM, Ma Y, Du W, Davis MA, Michaely P, Liu P, Willingham MC, Rudel LL. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J Biol Chem 281: 6616–6624, 2006 [DOI] [PubMed] [Google Scholar]