Abstract
Hypersensitivity in inflammatory/irritable bowel syndrome is contributed to in part by changes in the receptive properties of colorectal afferent endings, likely including mechanically insensitive afferents (MIAs; silent afferents) that have the ability to acquire mechanosensitivity. The proportion and attributes of colorectal MIAs, however, have not previously been characterized. The distal ∼3 cm of colorectum with either pelvic (PN) or lumbar splanchnic (LSN) nerve attached was removed, opened longitudinally, pinned flat in a recording chamber, and perfused with oxygenated Krebs solution. Colorectal receptive endings were located by electrical stimulation and characterized as mechanosensitive or not by blunt probing, mucosal stroking, and circumferential stretch. MIA endings were tested for response to and acquisition of mechanosensitivity by localized exposure to an inflammatory soup (IS). Colorectal afferents were also tested with twin-pulse and repetitive electrical stimulation paradigms. PN MIAs represented 23% of 211 afferents studied, 71% (30/42) of which acquired mechanosensitivity after application of IS to their receptive ending. LSN MIAs represented 33% of 156 afferents studied, only 23% (11/48) of which acquired mechanosensitivity after IS exposure. Mechanosensitive PN endings uniformly exhibited significant twin-pulse slowing whereas LSN endings showed no significant twin-pulse difference. PN MIAs displayed significantly greater activity-dependent slowing than LSN MIAs. In conclusion, significant proportions of MIAs are present in the colorectal innervation; significantly more in the PN than LSN acquire mechanosensitivity in an inflammatory environment. This knowledge contributes to our understanding of the possible roles of MIAs in colon-related disorders like inflammatory/irritable bowel syndrome.
Keywords: afferent fiber, mechanically insensitive afferent, irritable bowel syndrome, sensitization, visceral pain
visceral afferent fibers conveying sensory information into the central nervous system have drawn constant research interest since the 1930s (44). Although chemo/nutrient and even thermosensitivity have been documented (e.g., 29, 30, 46), mechanosensitive afferents have been most thoroughly studied because the most common search strategy to localize visceral afferent endings has employed mechanical stimulation (e.g., luminal distension, probing, stretch; Refs. 9, 18, 22, 28, 40, 41). The viscera are also innervated by an unknown proportion of so-called silent or sleeping nociceptors that are more accurately designated as mechanically insensitive afferents (MIAs) (31).
MIAs were first documented in the medial/posterior articular nerve innervating the knee joint of the cat (17, 38). These endings were electrically excitable but insensitive to mechanical stimulation. However, when the knee joint was experimentally inflamed, the previously “silent” afferents became spontaneously active and mechanosensitive. MIAs thus normally do not respond [or have very high response thresholds (20)] to mechanical stimulation but become active and mechanosensitive in pathophysiological conditions where they contribute to the development of hyperalgesia. MIAs have been best characterized in human microneurographic studies (e.g., 39, 49), where they constitute 6–24% of cutaneous C-fiber afferents.
MIAs have also been reported as present in the visceral innervation. However, mechanical search stimuli were used in these studies and the designation MIA was inferred post hoc following an inflammatory or chemical insult to the organ (e.g., 11, 22, 27, 33). Visceral MIAs have never been systematically studied and their number/proportion is thus incorrectly estimated to be from 35% to over 90% (18, 19, 40). Visceral MIAs are important because of their likely contribution to visceral discomfort, pain, and hypersensitivity that characterize functional gastrointestinal disorders such as irritable bowel syndrome (IBS) (8).
We undertook a prospective, systematic study of MIAs in the mouse colorectum using an unbiased electrical stimulation search strategy to locate excitable receptive endings. The objectives of the study were to determine the proportions and characteristics of mechanically sensitive and insensitive afferents in the pelvic and lumbar splanchnic innervations of the mouse colorectum. Portions of these data have been reported in abstract form (13).
METHODS
All experiments were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
In vitro mouse colorectum-nerve preparation.
Male mice (C57BL/6) 6–8 wk old (20–30 g; Taconic, Germantown, NY) were euthanized via CO2 inhalation followed by exsanguination after perforation of the right atrium. As described previously (4, 6, 23, 24), the distal colorectum (∼3 cm) along with either the lumbar splanchnic nerve (LSN) or pelvic nerve (PN) was transferred to ice-cold Krebs solution (in mM: 117.9 NaCl, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4·7H2O, 2.5 CaCl2, 11.1 d-glucose, 2 butyrate, and 20 acetate at ∼34°C) bubbled with carbogen (95% O2-5% CO2) to which was added the L-type calcium channel antagonist nifedipine (4 μM; to block spontaneous muscle contractions) and indomethacin (3 μM; cyclooxygenase inhibitor to block endogenous prostaglandin synthesis, which occurs during the surgical procedure of isolating the colorectum and PN or LSN nerves). The distal colorectum was opened longitudinally along the antimesenteric border and pinned flat mucosal side up in a tissue chamber superfused with 34°C Krebs solution. In the adjacent recording chamber, the PN or LSN was laid on a mirror and covered with paraffin oil. Under a dissection microscope, the nerve trunk was teased into fine bundles ∼10 μm thick that were individually placed onto a platinum-iridium recording electrode. A reference electrode was placed in the tissue chamber.
Afferent identification by electrical stimulation.
A round-tipped concentric electrode (external Φ0.55 mm, internal Φ0.125 mm; FHC, Bowdoin, ME) was placed perpendicular to the mucosal surface to electrically excite afferent endings (0.5-ms current pulse duration at 0.3 Hz). The large external diameter of the electrode and its hemispherical tip prevented damage to the colorectum; the electrode was connected to a micromanipulator by a compliant bridge that imposed a modest mechanical force to the mucosal surface (∼100 mg). A suprathreshold stimulus intensity (10 mA) producing a ∼2-mm radius of current spread was used to excite afferent endings. The electrode was moved systematically (∼1.5 mm steps) to examine the entire colorectal surface. When an afferent ending was activated, electrode position was adjusted to pinpoint the site of activation requiring minimum stimulus intensity (i.e., stimulus threshold). Endings with a stimulus threshold > 3 mA were discarded. Conduction velocity (CV) was calculated from the conduction delay and minimum distance between the stimulating cathode at the electrical receptive field and recording site. The minimum distance consisted of two straight lines that intersect at either the major pelvic ganglion (pelvic afferents) or inferior mesenteric ganglion (splanchnic), which represent the axon length within the colorectum tissue and in the nerve bundle, respectively.
Afferent categorization by mechanical stimulation.
After location by electrical stimulation, receptive endings were tested with mechanical stimuli for classification as serosal, muscular, mucosal or muscular/mucosal as previously described (6, 24): probing with calibrated von Frey-like nylon monofilaments (0.4, 1, and 1.4 g force), mucosal stroking with calibrated nylon filaments (10 mg force) and circumferential stretch. As recently described (12), stretch was produced by a servo-controlled force actuator (Aurora Scientific, Aurora, Ontario, Canada). With the colorectum firmly pinned to the chamber base, custom-made claws (1-mm interval) were placed along the antimesenteric edge of the colorectum to permit uniform, circumferential stretch using a slow ramped force (0 to 170 mN at 5 mN/s). This allowed correlation of circumferential force (0–170 mN) to intraluminal pressure (0–45 mmHg) by the following equation: Pressure = 2πForce/(LD), where L is colorectal length and D is circumference.
All afferent endings were located by electrical stimulation except mesenteric afferents, a class of mechanosensitive endings in the LSN pathway difficult to activate electrically because of location, which were instead located by mechanical stroking with a brush (6).
Chemical stimulation of MIAs.
After verifying mechanical insensitivity by absence of response to mechanical stimuli, we tested MIAs for chemical activation and acquisition of mechanosensitivity. Bronze tubing (1 cm high × 4 mm square) was placed over the receptive ending, the Krebs solution was removed, and 150 μl of an inflammatory soup (IS) (25) was applied directly to the receptive ending for 3 min. Subsequently, the IS and the tubing were removed and responses to probing (1.4 g), mucosal stroking (10 mg), and circumferential stretch (0–170 mN) were tested. Following the same procedure as above, the receptive ending was then exposed to capsaicin (3 μM) for 3 min and tested for response to the same three mechanical stimuli. For MIAs that acquired mechanosensitivity, mechanical stimulation was repeated at 15 and 30 min. Electrical stimulation was repeated at the end of testing to verify viability of the afferent ending.
IS was prepared in aliquots of 20 μl by combining bradykinin, serotonin, and histamine dissolved in distilled water with prostaglandin E2 dissolved in dimethylsulfoxide, frozen and stored at −20°C. On the day of an experiment, an aliquot was diluted to 10 μM (each mediator) in freshly oxygenated Krebs solution (25) and adjusted to pH 6.0 by hydrochloric acid. Capsaicin aliquots of 100 mM were prepared by dissolving capsaicin powder in ethanol anhydrous, frozen, and then diluted to 3 μM in freshly oxygenated Krebs solution on the day of an experiment. All chemicals were purchased from Sigma-Aldrich, St. Louis, MO.
Twin-pulse and repetitive stimulation.
Before application of IS, some endings were studied after a stimulus-free interval of at least 10 min with use of constant-current stimulation (2× activation threshold, 0.5 ms duration) delivered to the receptive ending in twin-pulse and repetitive stimulation paradigms. 1) A twin-pulse stimulus at an interpulse interval of 50 ms was used to assess whether the conduction latency of the second action potential was shorter (twin-pulse speeding) or longer (twin-pulse slowing) than the first. The twin pulse difference was quantified as percentage change in the second conduction latency relative to the first. 2) A repetitive train of pulses (2 Hz for 3 min) was delivered to colorectal endings to investigate activity-dependent slowing of conduction, which was quantified as the percentage increase in conduction latency of the last action potential relative to the first. A few fibers were also stimulated with a brief high-frequency train of pulses (10 Hz for 10 s) to evaluate conduction failure.
Data recording and analysis.
Electrical signals from afferent endings were filtered (0.3–10 kHz), differentially amplified (×10,000; DAM 80, World Precision Instruments, New Haven, CT), digitized at 20 kHz via a 1401 interface (Cambridge Electronic Design, Cambridge, UK), stored on a PC, and monitored online by an audio monitor. Action potentials were discriminated on the basis of principal component analysis using Spike2 software (Cambridge Electronic Design). Data are presented as means ± SE. One- or two-way ANOVA and Dunn's or Holm-Sidak post hoc pairwise multiple comparisons were performed with repeated measures as appropriate by use of SigmaPlot v9.0 (Systat Software, San Jose, CA). Differences in means and proportions were assessed by t-tests and χ2 tests, respectively. P < 0.05 was considered significant.
RESULTS
Electrical activation thresholds and conduction velocities of colorectal endings.
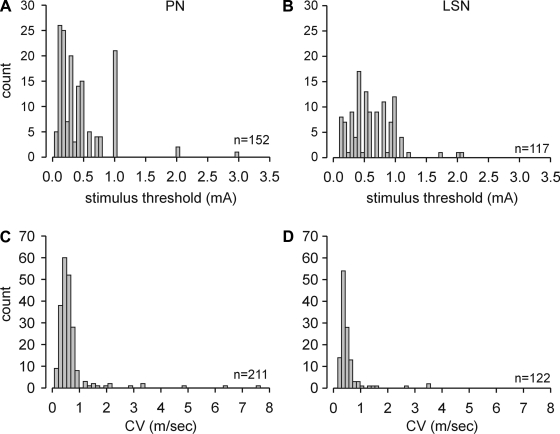
A total of 211 PN and 156 LSN colorectal endings were investigated in detail. All 211 PN and 112 LSN endings were identified by suprathreshold electrical stimulation of the colorectal surface; 44 LSN endings located on/along the mesentery (i.e., mesenteric afferents) were found by mechanically stroking the mesenteric attachment (some of which were subsequently stimulated electrically). Electrical activation thresholds of 152 PN and 117 LSN colorectal endings (including 10 mesenteric endings) and their respective distributions are illustrated in Fig. 1, A and B; electrical activation thresholds of 59 PN and 5 LSN afferents located by electrical stimulation were not precisely determined. Typically, the electrical stimulus threshold to evoke an action potential increased abruptly as the electrode was moved away from the center of the electrical receptive field. When the electrode was moved ∼3 mm away, an action potential could no longer be evoked even at maximum stimulus strength (10 mA). The mean activation threshold of PN endings (0.44 ± 0.03 mA) was significantly lower than that of LSN endings (0.61 ± 0.03 mA; P < 0.001); 96% of all electrical activation thresholds were ≤1.0 mA.
Fig. 1.
Distributions of electrical stimulus thresholds (A and B) and conduction velocities (C and D) of pelvic nerve (PN) and lumbar splanchnic nerve (LSN) endings in the mouse colorectum. CV, conduction velocity.
The CVs of 211 PN and 122 LSN (including 10 mesenteric) endings were calculated and are presented in Fig. 1, C and D. The mean CVs in the pelvic (0.68 ± 0.06 m/s) and splanchnic (0.53 ± 0.04 m/s) nerves did not differ (P = 0.07); 93% of all colorectal afferent fibers had CVs <1 m/s and 23 fibers (16 PN and 7 LSN) had CV >1 m/s.
Distribution of colorectal ending subtypes.
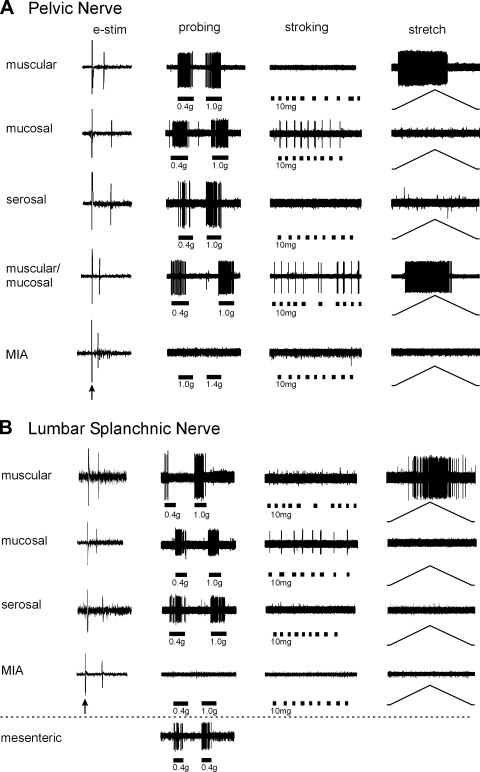
Colorectal endings were categorized on the basis of their response or inability to respond to three distinct mechanical stimuli: von Frey probing (0.4, 1.0, and 1.4 g), mucosal stroking (10 mg), and circumferential stretch (0–170 mN). Receptive endings were divided into five mechanosensitive classes (muscular/mucosal, muscular, mucosal, serosal, and mesenteric) (6, 24) and one mechanically insensitive class (MIAs) as illustrated in Fig. 2. Muscular/mucosal receptive endings are unique to the PN whereas mesenteric endings are only found in the LSN (6); both the pelvic and lumbar splanchnic pathways contain MIAs.
Fig. 2.
Mouse colorectal pelvic nerve (A) and lumbar splanchnic nerve (B) endings identified by electrical stimulation (e-stim; leftmost column; arrow indicates stimulus artifact) and classified based on responses to mechanical stimuli. All mechanosensitive endings responded to probing (0.4–1.4 g); muscular endings were also activated by circumferential stretch (0–170 mN), mucosal endings also by stroking (10 mg), and muscular/mucosal endings also by both stretch and stroking. Serosal endings were activated only by probing. Mechanically insensitive afferents (MIA) did not respond to mechanical stimuli. Muscular/mucosal endings were unique to the pelvic pathway; mesenteric endings were unique to the splanchnic pathway (0.4 g probing).
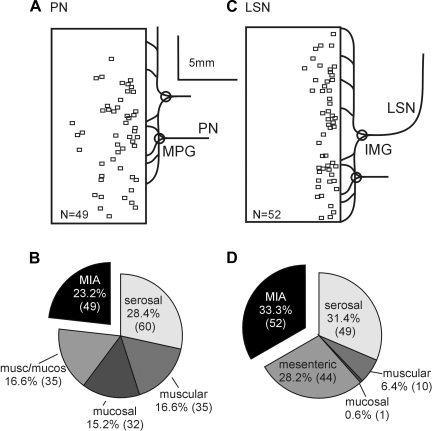
Mechanosensitive endings localized electrically in the present study have a similar topographical distribution as previously reported (6, 12, 21) and thus are not presented here. To summarize, mechanosensitive PN receptive endings were most densely distributed throughout the distal 20 mm of the colorectum whereas those in the LSN were concentrated on and near the mesenteric attachment; none were found along the antimesenteric border. As illustrated in Figs. 3, A and C, the topographical distributions of MIA endings were similar to the distributions of their mechanosensitive counterparts (6, 12, 21). The proportions of the different endings in pelvic and splanchnic pathways are presented in Fig. 3, B and D, respectively. Excluding MIAs from the distribution, the relative proportions of mechanosensitive afferents in this study are comparable to previous reports (6, 21) (Table 1). Overall, MIAs comprise ∼27.5% of the mouse colorectal innervation. They are the second most common class (23.2%) in the PN pathway and most common (33.3%) in the LSN pathway.
Fig. 3.
Distributions of MIA receptive endings (A and C) and proportions of afferent classes (B and D) of PN and LSN endings innervating the mouse colorectum. Squares represent the location of MIA endings. The proportions of MIAs (exploded black pie segments) did not differ between the pelvic and lumbar splanchnic nerve innervations. MPG, major pelvic ganglion; IMG, inferior mesenteric ganglion.
Table 1.
Comparison of proportions of mechanosensitive colorectal afferents
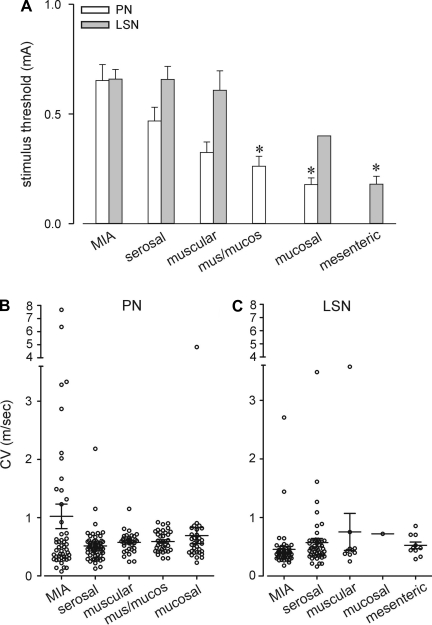
Figure 4 presents electrical activation thresholds and CVs for fibers based on classification. Electrical activation thresholds in both pathways were greatest for MIAs and least for mucosal (PN) and mesenteric (LSN) endings (P < 0.01 vs. respective MIAs). In general, stimulation activation intensity was ordered with presumed histological location of mechanosensitive ending (serosal > muscular > muscular/mucosal > mucosal = mesenteric). There were no significant differences in CVs either within pathways or between receptive ending types, although MIAs in the PN pathway exhibited the greatest variability in CV (e.g., 12 of 23 total endings in both pathways with CVs greater than 1 m/s were PN MIAs).
Fig. 4.
Electrical stimulus thresholds (A) and conduction velocities (B and C) of colorectal endings in the PN and LSN pathways. *Significantly different (P < 0.05, 1-way ANOVA with Dunn's post hoc multiple comparison) from respective MIAs. B and C: scatterplot of conduction velocities; horizontal line represents mean conduction velocity for each class of ending.
Sensitization/activation of MIAs.
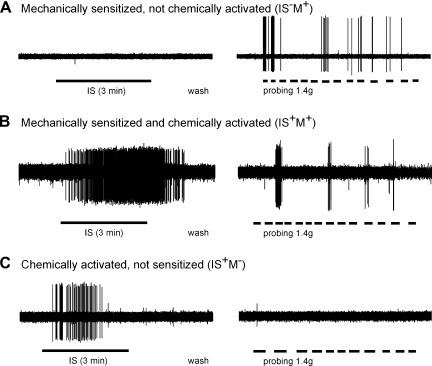
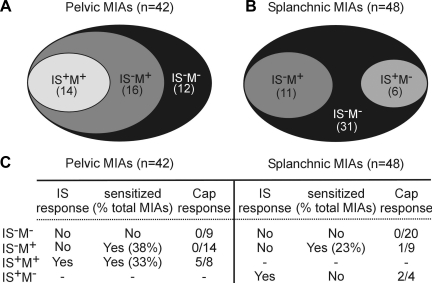
Activation of and acquisition of mechanosensitivity of MIAs was tested by application of an IS to the receptive ending; responses to capsaicin (Cap; 3 μM) were subsequently tested on some endings after IS application. Examples of the effect of exposure of MIAs to IS are illustrated in Fig. 5. Most (∼66%) MIAs that acquired mechanosensitivity did not respond to IS (Fig. 5A). Mechanosensitivity was apparent only for probing (1.4 g) and not to either stroking or circumferential stretch and typically lasted ∼15 min (and was no longer present 30 min after washout). As summarized in Fig. 6, 42 PN and 48 LSN MIAs were exposed to IS. The majority of PN MIAs (30/42, 71%) acquired mechanosensitivity to probing after IS application, 16 of which were not activated directly by IS. Of 31 PN MIAs also exposed to Cap, only 5 responded (and all 5 also responded to IS). In contrast, significantly fewer LSN MIAs (11/48; 23%; P < 0.001 vs. PN, χ2 test) acquired mechanosensitivity after exposure to IS. Of 33 LSN MIAs exposed to Cap, only 3 responded (2 of which also responded to IS, but did not acquire mechanosensitivity). Among MIAs that did acquire mechanosensitivity, the mean number of action potentials generated by 10 consecutive von Frey stimuli (∼1 s duration, ∼2 s interval) was significantly greater for PN (65.1 ± 8.3) than LSN (20.6 ± 9.9; P < 0.01) MIA endings.
Fig. 5.
Representative examples of responses of MIAs to chemical stimuli and acquisition of mechanosensitivity. A: most MIAs that acquired mechanosensitivity (23/31) did not respond to direct application of inflammatory soup (IS) or capsaicin (Cap) but responded to 1.4 g probing. B: some MIAs (8/31) that acquired mechanosensitivity also responded directly to the application of IS and/or Cap. C: a small proportion of MIAs (4/64 studied and exclusive to the splanchnic pathway) responded to chemical stimuli (IS or Cap) but did not acquire mechanosensitivity. Subgroups of MIAs are activated (IS+) or not (IS−) by IS and acquired (M+) or not (M−) mechanosensitivity to probing.
Fig. 6.
Summary of activation and/or acquisition of mechanosensitivity of MIAs by IS in pelvic (A) and lumbar splanchnic (B) pathways. Responses of MIAs to IS and/or Cap are summarized in C.
Fiber responses to electrical stimulation paradigms.
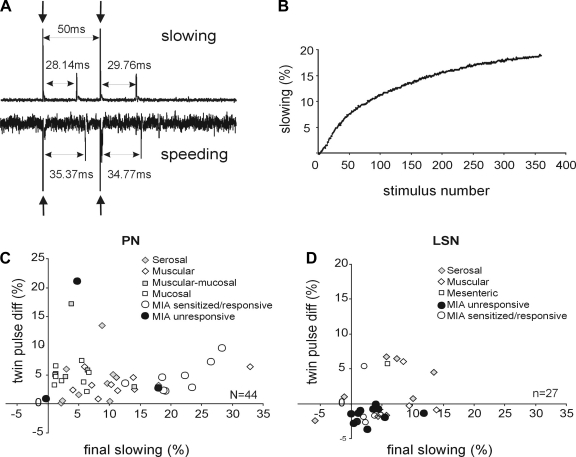
Displayed in Fig. 7, A and B are typical data recorded using the twin-pulse and repetitive stimulation paradigms. Previous investigators have used these stimulation paradigms to further characterize MIAs and to distinguish afferent from efferent endings in cutaneous tissue (36, 50). Twin pulse differences are plotted against final slowing percentage for both pelvic (Fig. 7C) and lumbar splanchnic (Fig. 7D) endings. All 44 PN endings tested, including 11 MIAs, showed twin-pulse slowing (i.e., positive twin pulse differences, z-test, P < 0.001). PN MIAs that acquired mechanosensitivity after exposure to IS showed significantly (P < 0.001) greater activity-dependent slowing (21.2 ± 1.8%) relative to their mechanosensitive PN counterparts (7.5 ± 1.1%). In contrast, the 27 LSN endings studied did not exhibit significant twin-pulse slowing or speeding (z-test, P = 0.79); specifically, neither the 12 mechanosensitive LSN endings (z-test, P = 0.054) nor the 4 LSN MIAs that acquired mechanosensitivity after exposure to IS (z-test, P = 0.89) showed significant twin-pulse differences. LSN MIAs that acquired mechanosensitivity after exposure to IS did not differ from their mechanosensitive LSN counterparts in either activity-dependent slowing (P = 0.2) or twin-pulse difference (P = 0.3). However, the twin-pulse difference (−1.68 ± 0.3%) of LSN MIAs that did not acquire mechanosensitivity after exposure to IS differed significantly (P < 0.02) from all other LSN endings (1.44 ± 0.91%).
Fig. 7.
Afferent responses to twin-pulse and repetitive stimulation paradigms. A: typical recordings showing slowing (top) and speeding (bottom) following an electrical twin-pulse stimulation (50-ms interpulse interval) at the colorectal receptive field. The second conduction latency is 1.6 ms longer than the first in the top trace and 0.6 ms shorter in the bottom trace, corresponding to twin-pulse slowing of 5.8% and speeding of 1.7%, respectively. Arrows indicate stimulus artifacts. B: typical change of latency in an afferent with repetitive stimulation of 2 Hz for 3 min. The final slowing is quantified as percentage increase of the last conduction latency relative to the first one. C and D: twin-pulse differences plotted against final slowing for the 44 PN (C) and 27 LSN (D) afferents tested. diff, Difference.
DISCUSSION
The present study provides new information about the proportion of MIAs in the colorectal innervation of the mouse and their ability to acquire mechanosensitivity after brief exposure to a mixture of inflammatory mediators. Assuming that visceral MIAs in pathophysiological states (e.g., postinfectious/postinflammatory IBS) sensitize to mechanical and/or other stimuli, they likely contribute significantly to visceral hypersensitivity. Reports that the discomfort, pain, and cutaneous hypersensitivity in IBS patients rely in large part on persistent afferent drive (35, 48) suggest that sensitized MIAs (and/or sensitized mechanosensitive afferents) may contribute to that persistent drive.
To the best of our knowledge, this is the first systematic investigation of visceral MIAs. We developed an electrical stimulation search strategy that bypasses transduction processes tuned to mechanical, thermal, and/or chemical energies, thus localizing excitable endings with dense voltage-gated sodium channels (i.e., the spike initiation zone). It is, accordingly, unbiased with respect to activation of receptive endings. We focused on MIAs in the present study and did not examine thermal or chemoselective sensitivity. Thus we cannot exclude the possibility that those MIAs (29% in the PN and 77% in the LSN) that did not acquire mechanosensitivity or respond to IS or Cap represent thermo- or chemoselective endings. In addition, the proportion of unresponsive MIAs (IS−M− in Fig. 6) might be overestimated since 3 min application may not be adequate for IS to diffuse through the tissue to activate or sensitize MIA endings. That a significant proportion of MIAs acquired mechanosensitivity, however, is consistent with the existing literature on MIAs in other tissues. MIAs comprised significant and similar proportions of both the PN and LSN innervations of the colorectum, although acquisition of mechanosensitivity after exposure to IS was more common and more robust in the PN (71%) relative to the LSN (23%).
In conjunction with previous studies of mechanosensitive afferents innervating the mouse colorectum (6) and bladder (51), the present results provide further evidence that pelvic and splanchnic innervations of pelvic organs have different functions, one of which appears to relate to MIAs and their ability to acquire mechanosensitivity. It is possible, however, that the “sensitizing” stimulus in the present study was not optimal. IS was used because it contains inflammatory mediators and protons commonly found in inflammatory exudates (7, 8, 25). IS has been widely used in studies of nociceptor sensitization, including sensitization of mouse colorectal and bladder afferents (24, 51), is hydrophilic with rapid onset and washout, and has been a reliable acute sensitizer.
Although developed to search for MIAs, the electrical search strategy also provided additional information beyond this principal focus. By systematically repositioning the electrode around a receptive ending, we verified that the 10-mA suprathreshold search intensity reliably activated receptive endings within a 2-mm radius of the electrode tip. At a distance ≥3 mm from the receptive ending, 10-mA stimulation did not evoke an action potential unless the electrode was on an axon. Activation stimulus intensity sharply dropped at the receptive ending and 96% of activation thresholds were <1 mA. The mean activation intensity for LSN endings was greater than for their PN counterparts, consistent with a previous finding relative to mechanosensitive LSN and PN afferents (6). Also consistent with previous studies, the activation intensity of mechanosensitive endings followed nicely the presumed anatomical location of their terminals (2, 45); serosal endings had the highest and mucosal (PN) and mesenteric (LSN) endings had the lowest mean activation thresholds. Interestingly, the mean stimulus intensity for activation of MIAs was greater than for mechanosensitive endings and did not differ between pelvic and splanchnic pathways. This implies that either the sensory terminals of MIAs are located relatively deep to the mucosal surface or their spike initiation zone is less excitable (i.e., lower density of voltage-gated sodium channels or greater membrane capacitance).
Although in many ways an ideal search stimulus, electrical stimulation does not selectively activate only sensory afferents. Recto- and colospinal neurons are “intrinsic” primary afferent neurons with cell bodies in the colorectum and projections to L6/S1 spinal cord segments (i.e., via the pelvic pathway) (32, 47) that could be activated orthodromically. They represent a small proportion (∼6%) of the rat colonic (enteric) neuron population. In five MIAs that did not respond to chemical stimulation (2 PN and 3 LSN), we stimulated at 10 mA, 10 Hz and noted robust conduction delays in all five MIAs. This limited sample suggests that chemically unresponsive MIAs found by electrical stimulation are unlikely to be recto- or colospinal neurons. Alternatively, electrical stimulation may antidromically activate autonomic (sympathetic and parasympathetic) efferent terminals that could be mistakenly identified as MIAs. To examine this possibility, we conducted twin-pulse and repetitive stimulation of 71 colorectal endings to assess time-dependent excitability changes in the axonal membrane following an action potential.
This strategy is based on previous results from stimulation of cutaneous peripheral nerves showing that autonomic efferent fibers exhibit an increase in conduction velocity at interspike intervals less than 150 ms regardless of mean stimulation frequency (3, 16). Using a twin-pulse stimulus (50–70 ms interpulse interval), Ringkamp and colleagues (36) recently found in cutaneous nerves of monkeys that twin-pulse speeding occurs only in sympathetic efferents whereas twin-pulse slowing is typically exhibited by afferent fibers. We applied this stimulus strategy for the first time to visceral nerves and found that all PN MIAs identified with electrical stimulation exhibited twin-pulse slowing. This was not unexpected in that PN single-fiber recordings in this in vitro preparation are proximal to the major pelvic ganglion. With stimulus intensity tuned to activate excitable afferent terminals, it is unlikely that parasympathetic efferents contained in the PN transmit action potentials transganglionically. In contrast, mechanosensitive LSN endings as well as LSN MIAs that acquired mechanosensitivity after exposure to IS did not exhibit significant twin-pulse slowing or speeding. Eleven LSN MIAs that did not acquire mechanosensitivity after exposure to IS, however, exhibited twin-pulse speeding, suggesting that they were not afferent, but efferent endings.
We doubt this could be accounted for by inconsistent identification of receptive endings by the electrical search paradigm, which localized endings in the PN pathway with results in full agreement with findings from the cutaneous nervous system literature (36). In this in vitro colorectal preparation, the receptive ending localized by electrical stimulation of the mucosal surface is significantly more excitable than the nerve trunk, which enters the colorectum from the serosal side. To avoid activating serosal nerve bundles, we set a cutoff stimulus threshold of 3 mA; 96% of the endings studied had activation thresholds below 1 mA. Regardless, most mechanosensitive LSN endings did not exhibit significant twin-pulse slowing or speeding. Some (5 of 12) tended to exhibit speeding, as did 3 (of 4) MIAs that acquired mechanosensitivity after exposure to IS, suggesting perhaps that this stimulation protocol may not reliably segregate afferents from efferents in the LSN innervation. Alternatively, because LSN MIAs that did not acquire mechanosensitivity after exposure to IS exhibited significant twin-pulse speeding relative to other LSN endings, it may be a matter of degree in the magnitude of slowing/speeding that differs between the PN and LSN colorectal innervations and not the stimulation protocol per se. Accordingly, it is likely that most LSN MIAs that did not acquire mechanosensitivity after exposure to IS are in fact sympathetic efferents. The applicability of this strategy to the visceral innervation, however, requires further investigation.
With respect to mechanosensitive endings in the PN and LSN pathways, their proportions and topographical locations did not differ from two other reports in which the search stimulus was mechanical (Table 1). This outcome further validates the use of the electrical search strategy and similarly validates use of a mechanical search strategy when studying mechanosensitivity. However, use of a noninjurious brush search stimulus potentially introduces a bias toward low-threshold mechanosensitive afferents, which is consistent with the finding here of a slightly greater proportion of serosal afferent endings (i.e., those with the highest activation intensity among mechanosensitive afferents) relative to previous studies. In the present study, the size of mechanosensitive receptive fields were roughly quantified by von Frey probing and the majority were 0.5–1 mm2, consistent with a previous report (6).
The documented and estimated proportions of MIAs innervating different tissues varies widely (6–97%; Refs. 17–20, 22, 26, 31, 39). Afferents innervating skin have been systematically investigated using an electrical search strategy both in vivo and in vitro [e.g., skin-nerve preparation (20, 26, 34, 52) similar to the colorectum-nerve preparation used here], but visceral afferents have not heretofore been studied systematically in a similar manner. In studies using hollow organ distension as the stimulus, afferent fibers unresponsive to distension have led to estimates of MIAs between 34% (rat colorectum and bladder) and 97% (cat bladder) MIAs (18, 19, 40). Subsequent reports considered as MIAs activity (action potentials) that appeared in the record after application of a chemical stimulus (11, 27, 33). In the present study, MIAs were defined as electrically excitable endings that did not respond to 1.4-g von Frey punctate probing, which exceeds most, if not all, response thresholds of serosal endings reported previously (4, 6, 21, 23, 24). Endings with thresholds to probing >1.4 g are highly unlikely to be discovered by mechanically brushing the colorectal mucosal surface or by hollow organ distension except at suprathreshold intensities. Moreover, we noted that repeated punctate stimulation ≥1.4 g is damaging to the colorectum. Perhaps not surprisingly, colorectal MIAs have topological distributions similar to their mechanosensitive counterparts (6, 24) but represent a novel group of endings unlike mechanosensitive endings described previously (4, 6, 21, 23, 24).
MIAs in general constitute a significant proportion of the afferent innervation, acquire mechanosensitivity after tissue insult, and are considered important contributors to the development and maintenance of hypersensitive states. In the present study, PN MIAs were significantly more excitable than LSN MIAs and exhibited an average activity-dependent slowing of over 20%, consistent with studies of nociceptive afferents in rats (15) and human (42, 43). In contrast, the average slowing of LSN MIAs was 3.1%. Despite the relatively brief exposure to IS in the present study, significant proportions of PN and LSN MIAs acquired mechanosensitivity equivalent to (LSN) or greater than (PN) the 38–50% of cutaneous MIAs described above that acquired mechanosensitivity in a protocol similar to that employed here.
Transient receptor potential vanilloid type 1 (TRPV1) receptors are expressed in a significant proportion of colorectal sensory neurons (10, 37), and we used the TRPV1 agonist Cap to assess TRPV1 presence in MIAs. Cap has been reported to activate 47% PN and 61% LSN colorectal mechanosensitive endings (4). In the present study, Cap activated only 12.5% of MIAs (5/31 PN and 3/33 LSN), suggesting that TRPV1 is not as widely expressed in MIAs as in other colorectal endings and thus that MIAs are chemotypically as well as functionally different than their mechanosensitive counterparts. Consistent with this interpretation are reports that transient receptor potential channels TRPV1 (24) and TRPA1 (5), which exhibit significant colocalization in dorsal root ganglion neurons (1), play important roles in normal colorectal mechanotransduction (where MIAs would not be expected to contribute).
The physiological function of visceral MIAs remains to be established. Neither pelvic nor splanchnic MIAs are likely to be activated by physiological levels of colon distention or contraction in situ but would be expected to contribute to visceral discomfort and pain in the presence of colorectal insult. MIAs in the present study acquired mechanosensitivity when exposed briefly to IS, but none responded to circumferential stretch equivalent to 45 mmHg intraluminal distension or mucosal stroking. The IS to which MIAs were exposed produced short-lasting, reversible effects and thus does not replicate a pathophysiological circumstance (e.g., tissue inflammation). In a previous study, we examined the afferent innervation of the hindpaw 24 h after inflammation induced by plantar incision, finding that the proportion of MIAs was significantly reduced, which suggests they had acquired mechanosensitivity at reduced thresholds for activation (34). Preliminary data support the prediction that the proportions of PN and LSN MIAs studied after colon inflammation would similarly be reduced (14).
This study documented the presence of significant proportions of MIAs in colorectal pelvic and lumbar splanchnic pathways and established their ability to acquire mechanosensitivity under brief inflammatory conditions. The pelvic pathway contained a greater proportion of MIAs that acquired mechanosensitivity and exhibited a greater magnitude of response to mechanical stimulation. These findings introduce another mechanism that might be involved in development and/or maintenance of visceral hypersensitivity in irritable/inflammatory bowel syndromes.
GRANTS
This research was supported by National Institutes of Health award NS 19912.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Michael Burcham for preparation of figures.
B. Feng contributed concept and design; acquisition, analysis, and interpretation of data; and drafting of the manuscript. G. F. Gebhart contributed concept and design; obtaining funding; study supervision; and review of the manuscript.
REFERENCES
- 1. Anand U, Otto WR, Facer P, Zebda N, Selmer I, Gunthorpe MJ, Chessell IP, Sinisi M, Birch R, Anand P. TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci Lett 438: 221–227, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil 16, Suppl 1: 28–33, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bostock H, Campero M, Serra J, Ochoa J. Velocity recovery cycles of C fibres innervating human skin. J Physiol 553: 649–663, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brierley SM, Carter R, Jones W, III, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol 567: 267–281, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O'Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology 137: 2084–2095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brierley SM, Jones RC, III, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Bueno L, Fioramonti J. Effects of inflammatory mediators on gut sensitivity. Can J Gastroenterol 13, Suppl A: 42A–46A, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Bueno L, Fioramonti J. Visceral perception: inflammatory and non-inflammatory mediators. Gut 51, Suppl 1: i19–i23, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cervero F, Sann H. Mechanically evoked responses of afferent fibres innervating the guinea-pig's ureter: an in vitro study. J Physiol 412: 245–266, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience 140: 247–257, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Coutinho SV, Su X, Sengupta JN, Gebhart GF. Role of sensitized pelvic nerve afferents from the inflamed rat colon in the maintenance of visceral hyperalgesia. Prog Brain Res 129: 375–387, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Feng B, Brumovsky PR, Gebhart GF. Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am J Physiol Gastrointest Liver Physiol 298: G402–G409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feng B, Gebhart GF. Silent afferents in the pelvic nerve innervation of the mouse colon (Abstract). Gastroenterology 136: A723, 2009. [Google Scholar]
- 14. Feng B, La JH, Tanaka T, Gebhart GF. Silent afferents and colorectal hypersensitivity. Program No. 682.19/VV3. In: 2010 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience [Google Scholar]
- 15. Gee MD, Lynn B, Cotsell B. Activity-dependent slowing of conduction velocity provides a method for identifying different functional classes of C-fibre in the rat saphenous nerve. Neuroscience 73: 667–675, 1996 [DOI] [PubMed] [Google Scholar]
- 16. George A, Serra J, Navarro X, Bostock H. Velocity recovery cycles of single C fibres innervating rat skin. J Physiol 578: 213–232, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grigg P, Schaible HG, Schmidt RF. Mechanical sensitivity of group III and IV afferents from posterior articular nerve in normal and inflamed cat knee. J Neurophysiol 55: 635–643, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Habler HJ, Janig W, Koltzenburg M. A novel type of unmyelinated chemosensitive nociceptor in the acutely inflamed urinary bladder. Agents Actions 25: 219–221, 1988 [DOI] [PubMed] [Google Scholar]
- 19. Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425: 545–562, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Handwerker HO, Kilo S, Reeh PW. Unresponsive afferent nerve fibres in the sural nerve of the rat. J Physiol 435: 229–242, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitization: different subtypes, different pathways, and different time-courses. Gut 58: 1333–1341, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Janig W, Koltzenburg M. On the function of spinal primary afferent fibres supplying colon and urinary bladder. J Auton Nerv Syst 30 Suppl: S89–S96, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Jones RC, III, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology 133: 184–194, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Jones RC, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25: 10981–10989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kessler W, Kirchhoff C, Reeh PW, Handwerker HO. Excitation of cutaneous afferent nerve endings in vitro by a combination of inflammatory mediators and conditioning effect of substance P. Exp Brain Res 91: 467–476, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol 68: 581–595, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Lynn PA, Blackshaw LA. In vitro recordings of afferent fibres with receptive fields in the serosa, muscle and mucosa of rat colon. J Physiol 518: 271–282, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McMahon SB, Morrison JF. Spinal neurones with long projections activated from the abdominal viscera of the cat. J Physiol 322: 1–20, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mei N. Intestinal chemosensitivity. Physiol Rev 65: 211–237, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Mei N, Lucchini S. Current data and ideas on digestive sensitivity. J Auton Nerv Syst 41: 15–18, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Meyer RA, Davis KD, Cohen RH, Treede RD, Campbell JN. Mechanically insensitive afferents (MIAs) in cutaneous nerves of monkey. Brain Res 561: 252–261, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Neuhuber WL, Appelt M, Polak JM, Baier-Kustermann W, Abelli L, Ferri GL. Rectospinal neurons: cell bodies, pathways, immunocytochemistry and ultrastructure. Neuroscience 56: 367–378, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol 87: 2095–2103, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. J Neurophysiol 87: 721–731, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Price DD, Craggs JG, Zhou Q, Verne GN, Perlstein WM, Robinson ME. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. Neuroimage 47: 995–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ringkamp M, Johanek LM, Borzan J, Hartke TV, Wu G, Pogatzki-Zahn EM, Campbell JN, Shim B, Schepers RJ, Meyer RA. Conduction properties distinguish unmyelinated sympathetic efferent fibers and unmyelinated primary afferent fibers in the monkey. PLoS One 5: e9076, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robinson DR, Gebhart GF. Inside information: the unique features of visceral sensation. Mol Interv 8: 242–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schaible HG, Schmidt RF. Responses of fine medial articular nerve afferents to passive movements of knee joints. J Neurophysiol 49: 1118–1126, 1983 [DOI] [PubMed] [Google Scholar]
- 39. Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork E, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci 15: 333–341, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol 71: 2046–2060, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol 72: 2420–2430, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Serra J, Campero M, Bostock H, Ochoa J. Two types of C nociceptors in human skin and their behavior in areas of capsaicin-induced secondary hyperalgesia. J Neurophysiol 91: 2770–2781, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Serra J, Campero M, Ochoa J, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J Physiol 515: 799–811, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sheehan D. The afferent nerve supply of the mesentery and its significance in the causation of abdominal pain. J Anat 67: 233–249, 1933 [PMC free article] [PubMed] [Google Scholar]
- 45. Song X, Chen BN, Zagorodnyuk VP, Lynn PA, Blackshaw LA, Grundy D, Brunsden AM, Costa M, Brookes SJ. Identification of medium/high-threshold extrinsic mechanosensitive afferent nerves to the gastrointestinal tract. Gastroenterology 137: 274–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol 80: 2632–2644, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Suckow SK, Caudle RM. Identification and immunohistochemical characterization of colospinal afferent neurons in the rat. Neuroscience 153: 803–813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain 105: 223–230, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjork HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci 19: 10184–10190, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weidner C, Schmidt R, Schmelz M, Hilliges M, Handwerker HO, Torebjork HE. Time course of post-excitatory effects separates afferent human C fibre classes. J Physiol 527: 185–191, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol 99: 244–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zimmermann K, Hein A, Hager U, Kaczmarek JS, Turnquist BP, Clapham DE, Reeh PW. Phenotyping sensory nerve endings in vitro in the mouse. Nat Protoc 4: 174–196, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]