Abstract
Gut apical Na+-glucose cotransporter 1 (SGLT1) activity is high at the birth and during suckling, thus contributing substantially to neonatal glucose homeostasis. We hypothesize that neonates possess high SGLT1 maximal activity by expressing apical SGLT1 protein along the intestinal crypt-villus axis via unique control mechanisms. Kinetics of SGLT1 activity in apical membrane vesicles, prepared from epithelial cells sequentially isolated along the jejunal crypt-villus axis from neonatal piglets by the distended intestinal sac method, were measured. High levels of maximal SGLT1 uptake activity were shown to exist along the jejunal crypt-villus axis in the piglets. Real-time RT-PCR analyses showed that SGLT1 mRNA abundance was lower (P < 0.05) by 30–35% in crypt cells than in villus cells. There were no significant differences in SGLT1 protein abundances on the jejunal apical membrane among upper villus, middle villus, and crypt cells, consistent with the immunohistochemical staining pattern. Higher abundances (P < 0.05) of total eukaryotic initiation factor 4E (eIF4E) protein and eIE4E-binding protein 1 γ-isoform in contrast to a lower (P < 0.05) abundance of phosphorylated (Pi) eukaryotic elongation factor 2 (eEF2) protein and the eEF2-Pi to total eEF2 abundance ratio suggest higher global protein translational efficiency in the crypt cells than in the upper villus cells. In conclusion, neonates have high intestinal apical SGLT1 uptake activity by abundantly expressing SGLT1 protein in the epithelia and on the apical membrane along the entire crypt-villus axis in association with enhanced protein translational control mechanisms in the crypt cells.
Keywords: intestinal glucose absorption, enterocyte proliferation and differentiation, gene expression, eukaryotic protein translational initiation and elongation factors
gut mucosal apical Na+-glucose cotransporter 1 (SGLT1) and lactase activities are programmed to be high at birth and during neonatal suckling (41, 51, 56). This high glucose- and lactose-processing capacity in coupling with hepatic glucose production through glycogenolysis and by postnatal increases in gluconeogenesis capacity is essential to maintain whole body glucose homeostasis in neonates (23). However, biological mechanisms of high apical SGLT1 uptake activity in neonates are not clear.
Intestinal glucose absorption is proposed to occur via both paracellular and transcellular routes. The transcellular route is viewed to be the major pathway under normal physiological conditions (7). Being a secondary active hexose transporter, SGLT1 is known to play an important role in gut apical glucose uptake (53). The high-capacity and low-affinity facilitated glucose transporter 2 (GLUT2) is originally known as the sole hexose transporter on the basolateral membrane (6). GLUT2 has been shown to play an important role for apical hexose transport under high substrate concentrations in rats (25). However, lack of contribution of GLUT2 to apical absorption of hexose was reported in mice (2, 37). Expression of GLUT2 on the apical membrane does not extend to humans (37). More recently, GLUT2 has been shown not to exist and function on the small intestinal apical membrane in the pig (37). On the other hand, whereas the newly identified high-affinity facilitated glucose transporter 7 (GLUT7) is shown to recognize d-glucose as a substrate in the Xenopus oocyte expression system, the physiological role of GLUT7 in the apical glucose uptake remains to be defined (8). The small intestinal mucosa is compartmentalized into villi bearing differentiated mature epithelial cells that arise from multipotent stem cells and undergo proliferation and differentiation, as they migrate from the crypt compartment (9). Enterocytes constitute up to 90% of epithelial cells in the crypt and more than 95% of villus cells (9). Thus investigations designed for understanding biology of gut mucosal apical glucose transport via SGLT1 have focused on the expression of SGLT1 during enterocyte proliferation and differentiation.
Research regarding the regulation of SGLT1 expression has been primarily conducted by using cell lines and in animals. Expression of SGLT1 is regulated at the levels of transcription (29), mRNA stability (33), posttranslational intracellular trafficking (10, 52), and activity (50). However, little is known about the role of protein translational control in SGLT1 expression during enterocyte proliferation and maturation. Furthermore, current understanding of the SGLT1 expression during enterocyte proliferation and differentiation along the crypt-villus axis is largely established with adult animals. Tracer and phlorizin-binding kinetics demonstrated that SGLT1 was initially synthesized in crypt cells and migrated up the villus with much higher activity in the villus cells in adult rodents (16) and rabbits (36). Immunohistochemical staining studies showed that SGLT1 protein was restricted to the apical membrane of villus cells and was absent or negligible on the apical membrane in crypt cells in adult rats (1, 48) and rabbits (24) and in weanling pigs (37). Although the Weiser cell isolation-based research for studying intestinal gene expression along the crypt-villus axis has been effectively carried out in more recent years (12, 34), in situ hybridization has been primarily used for examining SGLT1 expression along the crypt-villus axis in adult animals in the past in showing that SGLT1 mRNA abundance was undetectable or very low in the crypt but high in villus cells (24, 45). On the other hand, plasticity of patterns of SGLT1 expression along the crypt-villus axis, as affected by diets and physiological condition, is also evident from previous studies (3, 11, 15, 37). Thus transcription of SGLT1 gene and expression of SGLT1 protein and activity on the apical membrane are enterocyte differentiation dependent along the crypt-villus axis in adult animals. However, there is a scarcity of reports on intestinal SGLT1 expression along the crypt-villus axis in neonates. Puchal and Buddington (41) proposed that neonatal pigs might possess high mucosal apical Na+-glucose cotransport activity by expressing the sugar transporter along the entire crypt-villus axis. This speculation was based on a much earlier autoradiographic analysis of intestinal Na+-alanine uptake in the neonate (43). In neonatal pigs, gut mucosa possesses both fetal and adult types of enterocytes (44). Thus expression of intestinal SGLT1 in neonatal pigs is likely to be regulated through unique mechanisms. Understanding the pattern of SGLT1 expression along the crypt-villus axis and mechanisms associated with its regulation would reveal how the neonatal gut is programmed to possess high mucosal apical SGLT1 activity.
Therefore, the objectives of this study were to firstly test the hypothesis that the neonatal gut mucosal SGLT1 was expressed abundantly along the entire jejunal crypt-villus axis, thereby leading to high SGLT1 protein density and activity per villus unit or per unit of mucosa, and secondly to investigate how the pattern of jejunal SGLT1 expression was maintained in the neonate. To understand the dissociation between apical SGLT1 activity-protein abundance and SGLT1 mRNA abundance in the epithelia along the jejunal crypt-villus axis, changes in abundances of some of the key eukaryotic protein translational pathway initiation and elongation factors, including eukaryotic initiation factor 4E (eIF4E) protein, eIE4E-binding protein 1 (eIE4E-BP1) isoforms, and eukaryotic elongation factor 2 (eEF2), were also measured in the neonatal pigs.
MATERIALS AND METHODS
Animals and tissue preparation.
The experimental protocol was approved by the institutional animal care and use committees. A total of 36 commercial crossbred piglets, taken from different sows at the age of 7 days, were individually housed in metabolic cages equipped with feeders in an environmentally controlled room with the inside cage local temperature maintained at ∼28°C. The piglets were fed three times daily a liquid commercial formula containing (%) 25 lactose, 25 protein, and 10 fat (Litter Life, Merricks, Middleton, WI) (14). At the age of 16 days, the piglets were euthanized and jejunal segments were collected for isolation of epithelial cells and immunohistochemical analyses.
Sequential isolation of epithelial cells along the gut crypt-villus axis.
The Weiser distended intestinal sac technique for sequentially isolating small intestinal epithelial cells along the crypt-villus axis from the piglets was used in this study (14). The divided intestinal segments were immediately used in preincubation to remove mucus by following our previous procedures (14). After the preincubation, the intestinal segments were filled with an isolation buffer (1.5 mM Na2EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 0.5 mM DTT, and 2 mM l-glutamine at pH 7.4, oxygenated with the O2-CO2 mixture and warmed at 37°C) for sequential isolation of 12 epithelial cell fractions (F1 through F12) from the villus tip to the crypt bottom by following our previous procedures (14). Isolated cell fractions were also pooled between the proximal and the distal jejunal segments for each piglet (12). Each collected cell fraction was washed twice with 150 ml of an oxygenated cell suspension buffer (155 mM KCl at pH 7.4) and the cells were retained through centrifugation at 400 g for 4 min at 4°C (12). The washed cells were sampled for measuring cell viability by Trypan blue exclusion and the rest of the cells were immediately frozen at −80°C for further preparation of apical membrane vesicles to be used in glucose transport measurements.
For Western blot analyses of target proteins, including SGLT1 protein and other protein synthetic pathway initiation and elongation factors, and real-time RT-PCR analysis of SGLT1 mRNA abundance in epithelial cells along the crypt-villus axis in the neonatal pig intestine, three major epithelial cell fractions, consisting of the upper villus (F1–F4), the middle villus (F5–F8), and the crypt (F9–F12) epithelial cells, were sequentially isolated through three consecutive incubations of the isolated small intestinal segments for 40, 50, and 60 min, respectively, after the initial preincubation from six additional piglets by using the distended intestinal sac technique described above. Isolated epithelial cell fractions from each piglet were washed, retained by the same procedures, and immediately frozen in liquid nitrogen. The frozen cells were further pulverized under liquid nitrogen with a mortar and pestle and stored at −80°C for further analyses.
Preparation of apical membrane vesicles.
Apical membrane vesicles were prepared by Mg2+ precipitation and differential precipitation according to our previously established procedures (12). The crude apical membrane pellets were then suspended in a suitable amount of a membrane suspension buffer (150 mM KSCN, 180 mM d-mannitol at pH 7.4) and centrifuged at 39,000 g for 30 min to generate the final apical membrane vesicle pellets. The final pellets were resuspended with a 25-gauge needle in a suitable volume of the same membrane suspension buffer. The final membrane vesicle suspension was assayed for protein content and diluted with the same buffer to contain 4 mg protein/ml for subsequent transport measurements. Several aliquots of the final membrane vesicle suspension were taken for the assays of marker enzyme activities.
Protein and marker enzyme assays.
Protein content was determined by using a commercial kit (Bio-Rad Laboratories, Hercules, CA) with bovine serum albumin (fraction V) as a standard. All the following enzyme assays were carried out under the conditions when enzyme reactions were linear with time. Aminopeptidase N activity was assayed according to the procedure of Maroux et al. (35) with further adaptations by Fan et al. (12). The K+-stimulated p-nitrophenyl phosphatase activity was measured according to Murer et al. (38) with revisions Fan et al. The succinate dehydrogenase activity was measured according to King (27) and Fan et al. The d-glucose-6-phosphatase activity was measured according to Hübscher and West (22) and Fan et al. The acid phosphatase activity was measured according to Hübscher and West and Fan et al.
In vitro transport measurements.
In vitro d-glucose transport experiments were carried out with tracer d-[6-3H]glucose (specific activity: 0.96–1.22 TBq/mmol, Amersham, Arlington Heights, IL) by the rapid filtration procedure (12). This was conducted on the same day as the apical membrane vesicle preparation. Uptake buffers are described in details in the legends to Fig. 1 and Fig. 2. A total of 11 gradient levels of d-glucose below 1 mM were used with eight levels of glucose between 2.3–92 mM in the uptake media, as shown in Fig. 2. To correct for the d-glucose simple diffusion under a Na+-gradient condition for the experimental results reported in Fig. 1, a batch of apical membrane vesicle suspension was mixed with HgCl2 stock solution at the dosage of 0.40 μmol HgCl2/mg protein. Our previous studies have shown that HgCl2 at the dosage of 0.165 μmol/mg protein could completely block carrier-mediated nutrient transport (13). d-Glucose simple diffusion was then measured with the HgCl2-treated vesicles with the buffer (2.4 μM d-[6-3H]glucose, 180 mM d-mannitol, 150 mM NaSCN, and 10 mM Trizma·HCl at pH 7.4).
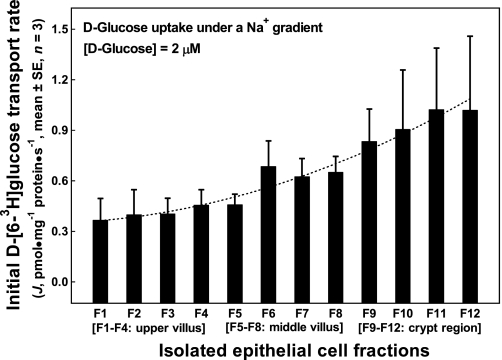
Fig. 1.
Initial rates of d-glucose uptake under a Na+ gradient into the apical membrane vesicles prepared from epithelial cells isolated along the jejunal crypt-villus axis in the liquid formula-fed neonatal pigs. Apical membrane vesicles were preloaded with a buffer containing 180 mM d-mannitol, 150 mM KSCN, 10 mM Trizma·HCl at pH 7.4. Uptake buffer contained 2.4 μM d-[6-3H]glucose, 180 mM d-mannitol, 150 mM NaSCN, and 10 mM Trizma·HCl at pH 7.4. The uptake media (60 μl), resulting from mixing 50 μl uptake buffer with 10 μl apical membrane vesicles, contained 2.0 μM d-[6-3H]glucose, 180 mM d-mannitol, 125 mM NaSCN, 25 mM KSCN, and 10 mM Trizma·HCl. Each point represents mean and SE (n = 3) of measurements from 3 uptake experiments (duplicate observations in each experiment) using 3 separate batches of apical membrane vesicle suspension prepared from 12 epithelial cell fractions (F1 through F12). Each batch of epithelial cell fractions was collected and pooled from the proximal and the distal jejunal segments of 2 piglets; thus a total of 6 piglets were used in these uptake experiments. J, rate of d-[6-3H]glucose tracer uptake into apical membrane vesicles.
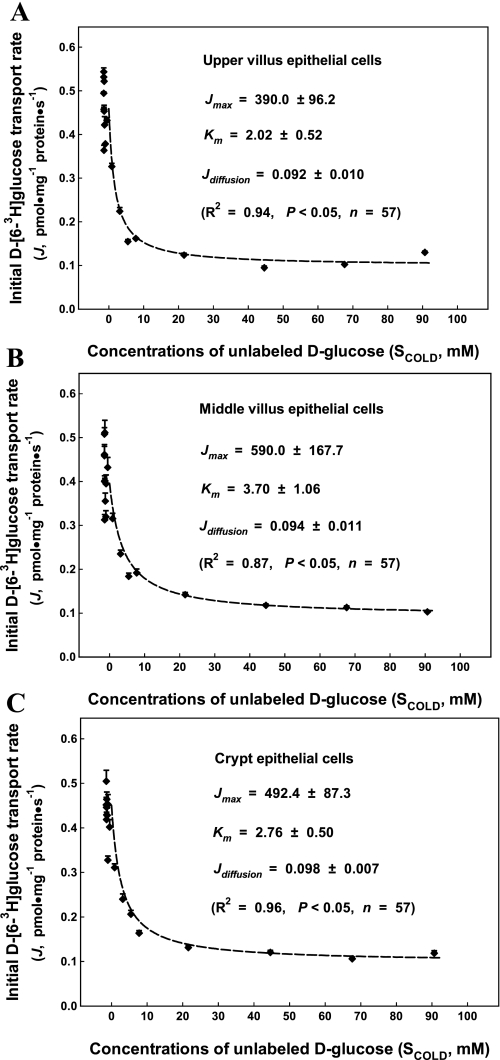
Fig. 2.
Kinetics of sodium-d-glucose cotransporter 1 (SGLT1) glucose uptake into the apical membrane vesicles prepared from the upper villus (A), the middle villus (B), and the crypt epithelial cells (C) isolated along the jejunal crypt-villus axis in the neonatal pig. Initial rates of the d-[6-3H]glucose tracer uptake as a function of extravesicular concentrations of nonlabeled d-glucose as shown. Apical membrane vesicles were preloaded with a buffer containing 180 mM d-mannitol, 150 mM KSCN, 10 mM Trizma·HCl at pH 7.4. A total of 19 uptake buffers contained 2.4 μM d-[6-3H]glucose, 150 mM NaSCN, and 10 mM Trizma·HCl at pH 7.4 and differed in the concentrations of d-glucose (0–110 mM) and d-mannitol (180–70 mM). The uptake media (60 μl), resulting from mixing 50 μl uptake buffer and 10 μl apical membrane vesicles, contained 2.0 μM d-[6-3H]glucose, 125 mM NaSCN, 25 mM KSCN, 0–92 mM d-glucose, 180–88 mM d-mannitol, and 10 mM Trizma·HCl. Each point represents mean and SE (n = 3) of measurements from 3 uptake experiments (duplicate observations in each experiment) using 3 separate batches of apical membrane vesicle suspension prepared from 3 epithelial cell fractions (the upper villus cell, F1–F4; the middle villus cell, F5–F8; and the crypt cell, F9–F12). Each batch of epithelial cell fractions was collected and pooled from the proximal and the distal segments of two piglets; thus a total of 18 piglets were used in these experiments. Jmax, maximal rate of d-glucose uptake into the apical membrane vesicles; Km, the SGLT1 affinity; Jdiffusion, apparent transmembrane diffusion rate of d-glucose into the apical membrane vesicles; Scold, extracellular concentration of unlabeled d-glucose.
After the protein assay, the final apical membrane vesicle suspension was then equilibrated for an additional 30 min on ice before transport measurements. Based on our preliminary measurements, 6-s incubations were used to measure the initial tracer d-[6-3H]glucose transport rate at 2 μM. After 20-s of equilibrating the prepared membrane vesicle suspension to room temperature (24°C), uptake incubation of 10 μl of apical membrane vesicle suspension with 50 μl of uptake buffer was initiated by a foot switch-activated vibromixer (12). The process was terminated by the addition of 1.125 ml of ice-cold wash solution (180 mM d-mannitol, 150 mM NaSCN, 10 mM Trizma·HCl, 0.1 mM HgCl2 at pH 7.4). Timing was performed with an electronic GraLab model 545 timer/intervalometer (12). To eliminate contributing effects from differences in membrane potential among the cell fractions, a membrane-permeable anion, SCN−, was included in the membrane suspension buffer and also in glucose uptake buffers to clamp membrane potential (12). To minimize nonspecific binding to the filter membrane by d-[6-3H]glucose, 0.25-μm cellulose acetate filters were presoaked with 20 mM d-glucose buffer at pH 7.4 and mounted in a manifold filtration unit that was connected to a vacuum source (12). Composition of uptake buffers is described in detail in legends to the figures.
Western blotting analyses of target protein abundances.
SGLT1 protein abundance was determined in the three epithelial cell fractions of both their cellular homogenate and apical membrane by using procedures described by Kles and Tappenden (28). Briefly, a linear range, from 0 to 15 μg protein, was established before samples were analyzed, and 3 μg protein was loaded into each well. Samples were boiled for 4 min to denature the proteins and the proteins were separated by 12.5% SDS-PAGE. Proteins were then transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad) by using a semidry transfer apparatus (Bio-Rad). Western blotting for SGLT1 was performed by using a polyclonal rabbit anti-human SGLT1 (1:5,000 dilution in 1% BSA and PBS-Tween; Chemicon, Temecula, CA), and a secondary antibody (Bio-Rad), conjugated with horseradish peroxidase (HRP) and diluted to 1:10,000 in 1% BSA and PBS-Tween. Membranes were developed using the Opti-4CN kit (Bio-Rad). Photographs of the membranes were taken using the Kodak Image Station 440, and densitometry was performed with Kodak 1D Network software (Eastman Kodak, New Haven, CT).
Total eIF4E protein, eIE4E-BP1, total eEF2 protein, and phosphorylated (Thr56) eEF2 protein abundances were determined by Western blotting as previously reported (26). Specifically, 0.5 g of frozen cell fractions were homogenized in 1 ml of a lysis buffer (20 mM Tris·HCl, 2 mM EGTA, 0.2 mM Na2EDTA, 50 mM NaF, 50 mM β-glycerophosphate, 1 mM benzamidine, 1 mM DTT, 0.5 mM Na3VO4, 0.1 mM PMSF, and 10 μg/ml each of aprotinin, leupeptin, and pepstatin A at pH 7.4) on ice for 2 min. Then the sample was incubated on ice for 30 min with gentle inversion of the tube every 5 min and then centrifuged at 12,000 g for 20 min at 4°C. The resulting supernatants were sampled for the analyses of their protein concentration prior to Western blotting of the target proteins.
A suitable amount of the previously prepared supernatants were diluted with Milli-Q H2O and boiled in 2× SDS loading buffer (250 mM Tris·HCl, 500 mM β-mercaptoethanol, 2% SDS, 0.1% phenol blue, 10% glycerol at pH 6.8) for 5 min. Aliquots (20 to 100 μg protein) were subjected to 10% SDS-PAGE and transferred to PVDF membrane (Millipore, Billerica, MA) via semidry transfer apparatus (Bio-Rad). The PVDF membranes were blocked at room temperature for 1 h with 6% nonfat dry milk powder dissolved in 1 × TBS (25 mM Tris·HCl, 0.15 M NaCl at pH 7.4) and then incubated at 4°C with a primary antibody overnight. A primary mouse anti-human eIF4E (25 kDa) monoclonal antibody (1:1,000 dilution in 6% skim milk powder, Sc-9976, Santa Cruz Biotechnology, Santa Cruz, CA) was used for detection of porcine eIF4E. A primary rabbit anti-human eIE4E-BP1 (20 kDa) polyclonal antibody (1:1,000 dilution in 6% skim milk powder, A300-501A, Bethyl Laboratories, Montgomery, TX) was used for detection of porcine eIE4E-BP1. A primary rabbit anti-human eEF2 (100 kDa) polyclonal antibody (1:1,000 dilution in 6% skim milk powder, Sc-25634, Santa Cruz Biotechnology) was used for detection of total porcine eEF2. A primary phosphor-eEF2 (Thr56) polyclonal antibody (1:1,000 dilution in 6% skim milk powder, Cell Signaling Technology, Danvers, MA) was used for detection of phosphorylated porcine eEF2. A mouse anti-chicken β-actin monoclonal antibody (1:10,000 dilution, Cedarlane Laboratories, Burlington, NC) was used for detection of porcine β-actin. Following the overnight incubation, membranes were washed (8 × 5 min) in 1 × TBS with 0.1% Tween-20 and incubated at room temperature for 1 h with a secondary antibody. A secondary donkey anti-rabbit HRP-conjugated IgG (1:10,000 dilution in 6% skim milk powder, Promega, Madison, WI) was used for eIE4E-BP1, total eEF2, and phosphor-eEF2. A secondary donkey anti-mouse HRP-conjugated IgG (1:10,000 dilution in 6% skim milk powder, Promega) was used for eIF4E and β-actin. Blots were visualized by using the enhanced chemiluminescence detection system (Sigma-Aldrich, St. Louis, MO). Photographs of the film were scanned and densitometry was performed with Scion Imaging software (Scion, Frederick, MD). β-Actin was used to normalize the abundance of the target proteins. Western blot analyses were all performed in duplicate for each sample.
Immunohistochemical localization of SGLT1 protein along the crypt-villus axis.
Formalin-fixed and paraffin-embedded sections of both the proximal and the distal jejunal segments were subjected to automated immunohistochemistry procedures for SGLT1 protein by use of a Dako autostainer (Dako, Carpinteria, CA). Processed 4-μm sections were mounted on charged slides, deparaffinized and rehydrated, and treated with 3% hydrogen peroxide for 10 min to quench endogenous tissue peroxidases. Heat-induced antigen retrieval was accomplished by using Tris-EDTA buffer, pH 9 (Target Retrieval Solution, Dako) and a pressure cooker (Decloaking Chamber, BioCare Medical, Concord, CA). Following 10-min incubation with universal blocker (Dako), duplicate tissue sections were incubated with anti-SGLT1 rabbit polyclonal antibody (Chemicon, Temecula, CA) at a dilution of 1:600 for 30 min at room temperature. Peroxidase-labeled goat anti-mouse/anti-rabbit polymer detection system was used (UltraVision ONE, Lab Vision, Fremont, CA) with Nova Red (Vector Laboratories, Burlingame, CA) as chromogen. Tissues were counterstained with Harris hematoxylin (Fisher Scientific, Pittsburgh, PA). For negative control sections, nonimmune rabbit serum diluted to a protein concentration similar to that of the diluted SGLT1 antibody was substituted for the primary antibody. Slide sections were photographed at ×10 magnification with an Olympus BX41 microscope and Olympus Q Color 5 digital camera (Olympus, Melville, NY) using Q Capture Pro software (QImaging, Surrey, BC, Canada).
Primer design, RNA extraction, and real-time RT-PCR analysis.
Primers for SGLT1 and β-actin were designed with Primer 3 (http://frodo.wi.mit.edu/primer3/primer3_code.html) based on a porcine sequence to produce an amplification product that spanned at least two exons. The SGLT1 and β-actin PCR primers used were 5′-GGCTGGACGAAGTATGGTGT-3′ (forward) and 5′-ACAACCACCCAAATCAGAGC-3′ (reverse), and 5′-GGATGCAGAAGGAGATCACG-3′ and 5′-ATCTGCTGGAAGGTGGACAG-3′, respectively. The expected size of the SGLT1 RT-PCR product was 153 bp (corresponding to bp 9 to 161 of porcine SGLT1 sequence, GenBank accession no. M34044). The expected size of the β-actin RT-PCR product was 130 bp (corresponding to bp 569 to 698 of the previously reported porcine β-actin sequence, GenBank accession no. U07786).
Total RNA was isolated from 100 mg of each of the three major epithelial cell fractions by use of TRIzol (Invitrogen, Carlsbad, CA) and treated with DNase I (Invitrogen) according to the manufacturer's instructions. The RNA quality was checked by 1% agarose gel electrophoresis, stained with 10 μg/ml ethidium bromide. The RNA had an OD260-to-OD280 ratio of 1.8 to 2.0. Real-time PCR was performed by using one-step SYBR Green RT-PCR Mix (Qiagen, Valencia, CA), containing MgCl2, dNTP, reverse transcriptase, and Hotstar Taq polymerase. Equal amount of DNase I-treated RNA (100 ng) from all samples was added to a total volume of 25 μl containing 12.5 μl SYBR Green mix, 0.25 μl RT mix, and 1 μM each of forward and reverse primers. We used the following protocol: 1) reverse transcription program (30 min at 50°C); 2) denaturation program (15 min at 95°C); 3) amplification and quantification program, repeated 45 cycles (15 s at 95°C, 15 s at 54°C, 15 s at 72°C); and 4) melting curve program (60–99°C with a heating rate of 0.1°C/s and fluorescence measurement). We used β-actin as the internal control to normalize the amount of starting RNA used for RT-PCR for all samples. Amplification and melt curve analysis were performed in Smart Cycler (Cepheid, Sunnyvale, CA). Melt curve analysis was conducted to confirm the specificity of each product, and the size of products was verified on ethidium bromide-stained 2% agarose gels in Tris acetate-Na2EDTA buffer. The identity of each product was confirmed by dideoxy-mediated chain termination sequencing at the University of Guelph Molecular Supercenter.
Calculations, transport kinetics, and statistical analyses.
The initial rates of the tracer glucose uptake under a Na+-gradient condition were calculated according to our previously established procedure (12). Kinetic parameter estimates, including glucose transporter affinity, maximal transport activity, and apparent diffusion rate, were determined according to a previously established model (12) as expressed in Eq. 1:
| (1) |
In Eq. 1, J is initial rate of the tracer glucose uptake into membrane vesicles (pmol·mg−1 protein·s−1), Jmax is the maximal rate of glucose transport into membrane vesicles to be estimated (pmol·mg−1 protein·s−1), Stracer is extravesicular concentrations of the radioactively labeled tracer d-[6-3H]glucose concentration (2 μM), Km is SGLT1 affinity to be estimated (mM), Scold is extravesicular concentrations of unlabeled d-glucose (mM), and Jdiffusion is d-glucose apparent diffusion rate in the membrane vesicles (pmol·mg−1 protein·s−1). All kinetic parameter estimates of the SGLT1 activities and patterns of the initial glucose uptake activities were analyzed by using the Fig.P curve-fitting program (Fig.P, 1993, Biosoft, Cambridge, UK).
The relative SGLT1 mRNA abundance ratio (R) in the upper villus, the middle villus, and the crypt epithelial cell fractions was calculated by using the value obtained for the crypt cell fraction as the control according to the delta-delta method (32) according to Eq. 2:
| (2) |
where R is the relative expression ratio value of the target gene. −ΔΔCt(sample − control) = (Ct of SGLT1 − Ct of β-actin)sample − (Ct of SGLT1 − Ct of β-actin)control. Ct value is the cycle number at which both target and housekeeping genes are amplified beyond the threshold of 30 fluorescence units. Real-time PCR efficiencies were acquired by amplification of dilution series of RNA according to the formula 10(−1/slope) and were consistent between SGLT1 and β-actin. Negative controls were performed in which water was substituted for RNA.
Data for the epithelial cell fractions obtained from Western blotting analyses and real-time RT-PCR analyses were subjected to the analysis of variance using SAS (the SAS Institute, Cary, NC). Comparison of the kinetic parameter estimates was conducted by the pooled two-tailed Student's t-test (4). Comparisons of the molecular end points among the epithelial cell fractions were conducted by using Tukey's multiple comparisons (SAS). Comparison of all the end point differences with a level of P < 0.05 was considered significant.
RESULTS
The liquid formula-fed neonatal piglets at the age of 16 days at the end of this study had a mean ± SE (n = 30) of body weight 4.7 ± 0.1 kg, small intestinal fresh weight of 209.0 ± 8.0 g, and small intestinal length of 7.23 ± 1.54 m. Freshly isolated and retained small intestinal epithelial cells had a cell viability of 90–95% by Trypan blue exclusion prior to being frozen in liquid nitrogen for storage.
The apical membrane marker, aminopeptidase N specific activity, was enriched between 6- and 12-fold in the prepared apical membrane fraction compared with their cell homogenate samples. Furthermore, the enterocyte basolateral membrane marker, K+-stimulated phosphatase specific activity, was enriched less than onefold in the apical membrane vesicles compared with their corresponding cell homogenate, suggesting little contamination of the apical membrane vesicle preparation with the enterocyte basolateral membrane. Other intracellular membrane markers, including the mitochondrial membrane marker succinate dehydrogenase, the endoplasmic reticulum marker d-glucose-6-phosphatase, and the lysosomal membrane marker acid phosphatase specific activities, were below twofold in the prepared apical membrane vesicle fraction compared with their corresponding cell homogenate samples, indicating little contamination of the apical membrane vesicle preparations with these intracellular membrane fractions.
Initial rates of d-glucose transport across the apical membrane, under a Na+ gradient, exhibited a quadratic pattern of progressive increases (P < 0.05) from the tip of the villus cells (F1) to the bottom crypt epithelial cells (F12) at the tracer d-[6-3H]glucose concentration of 2.0 μM (Fig. 1). Glucose concentration of 2.0 μM used in this transport experiment was far below a concentration needed for reaching glucose transport saturation kinetics. Thus the pattern of initial rates of d-glucose transport activity presented in Fig. 1 would not necessarily reflect changes in abundances of the apical membrane-bound SGLT1 in the isolated gut epithelial cells.
We used a well-defined kinetic model to obtain glucose uptake kinetic parameter estimates in this study. This kinetic model and associated measurements allowed simultaneous determination of the component of apparent diffusive component. This kinetic model uses initial tracer d-[6-3H]glucose transport rate as an independent variable, concentrations of unlabeled d-glucose as a dependent variable, and the tracer d-[6-3H]glucose concentration (2 μM) as an input parameter. Thus, unlike a conventional relationship between initial nutrient uptake rates and substrate concentrations, kinetic curves associated with this model, as described in Eq. 1, visually resemble competitive inhibition kinetics, namely, the inhibition of tracer d-[6-3H]glucose uptake by unlabeled d-glucose (Fig. 2). However, we were unable to partition high-affinity d-glucose transport kinetics for representing the GLUT7 uptake component by using the initial rates measured with the first 10–12 sets of uptake media of glucose concentrations corresponding to the reported GLUT7 Km value range. Furthermore, we could not demonstrate a saturable d-glucose uptake component for representing typical low-affinity GLUT2 kinetics with d-glucose concentration in the uptake media for up to ∼92 mM as shown in Fig. 2. In fact, kinetics of the measured d-glucose uptake activity under the Na+-gradient conditions in the apical membrane vesicles prepared from the upper, the middle, and the crypt epithelial cells were best fitted by a single transporter system (Fig. 2). Thus kinetics of the partitioned d-glucose uptake activity were primarily mediated via SGLT1 and were determined with the apical membrane vesicles prepared from the upper villus (F1 through F4), the middle villus (F5 through F8), and the crypt (F9 through F12) epithelial cells under the condition that the membrane potential in the apical membrane vesicles was clamped with SCN−. Kinetics of SGLT1-mediated d-glucose uptake activity were determined with the apical membrane vesicles prepared from the upper villus (Fig. 2A), the middle villus (Fig. 2B), and the crypt (Fig. 2C) epithelial cells.
Kinetic parameter estimates of SGLT1 uptake activity analyzed with the tracer inhibition kinetic model, including Jmax, Km, and Jdiffusion, are summarized and compared in Table 1. There were differences (P < 0.05) in Jmax between the three epithelial cell fractions. Jmax was highest in the middle villus, intermediate in the crypt, and lowest in the upper epithelial cells. There were also differences (P < 0.05) in Km between the three epithelial cell fractions. Km was highest in the middle villus, intermediate in the crypt, and lowest in the upper villus epithelial cells. On the other hand, the partitioned apparent d-glucose diffusion Jdiffusion would represent simple diffusion due to the leaky nature of the membrane vesicles under the in vitro culture condition. There were differences (P < 0.05) in Jdiffusion between the upper and the crypt epithelial cells; however, no significant difference was observed in Jdiffusion between the upper and the middle villus epithelial cells or between the middle villus and crypt epithelial cells.
Table 1.
Kinetic parameter estimates of Na+-d-glucose cotransport into apical membrane vesicles prepared from the upper villus, the middle villus, and crypt epithelial cells isolated along the jejunal crypt-villus axis in the liquid formula-fed neonatal pig
| Kinetic Parameters |
|||
|---|---|---|---|
| Cell Types | Jmax | Km | Jdiffusion |
| Upper villus epithelial cells | 390.0 ± 96.2a | 2.02 ± 0.52a | 0.092 ± 0.010a |
| Middle villus epithelial cells | 590.0 ± 167.7b | 3.70 ± 1.06b | 0.094 ± 0.011ab |
| Crypt epithelial cells | 492.4 ± 87.3c | 2.76 ± 0.50c | 0.098 ± 0.007b |
Jmax, maximal transport rate (pmol·mg−1 protein·s−1); Km, SGLT1 affinity (mM). Jdiffusion, apparent transmembrane diffusion rate of d-glucose (pmol·mg−1 protein·s−1). Parameter estimates were derived with P < 0.05 (parameter estimates ± SE, n = 57). a,b,cMeans in the same column with different superscript letters differ (P < 0.05).
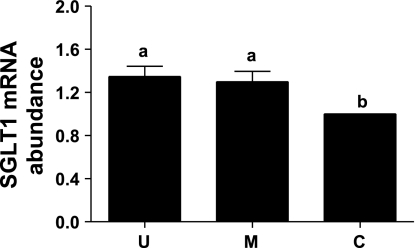
To determine whether the changing pattern of SGLT1 mRNA abundances paralleled those of the SGLT1 Jmax data described in the above section and SGLT1 protein abundance data in the epithelial cells in the next section, relative abundances of SGLT1 mRNA were analyzed by real-time RT-PCR using β-actin as a housekeeping gene. The Ct values for the amplification of β-actin cDNA were similar and were not significantly different among the three major epithelial cells (data not shown). There were no significant differences in the SGLT1 mRNA abundance between the upper villus and the middle villus epithelial cells. However, SGLT1 mRNA abundance was increased (P < 0.05) by 35 and 30% in the upper villus and the middle villus epithelial cells, respectively, compared with the crypt epithelial cells (Fig. 3).
Fig. 3.
Real-time RT-PCR analysis of SGLT1 mRNA abundances in the upper villus (U), the middle villus (M), and crypt (C) epithelial cells isolated along the jejunal crypt-villus axis in the liquid formula-fed neonatal pigs. Results were normalized using β-actin expression as a housekeeping control gene in each real-time RT-PCR. Data are expressed relative to the crypt cell value (value = 1) and are presented as means ± SE (n = 6) in arbitrary units. Bars with a different superscript letters differ (P < 0.05).
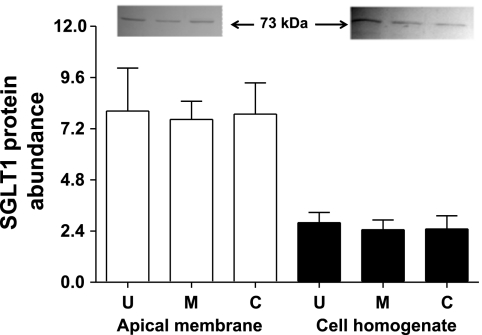
Western blot analyses showed the presence of a 73-kDa SGLT1 protein band in total cell homogenate and the apical membrane preparations from the isolated upper villus, the middle villus, and the crypt epithelial cells (Fig. 4). There were no significant differences in SGLT1 protein abundances in both the total cell homogenate and the apical membrane preparation among the upper villus, the middle villus, and the crypt epithelial cells (Fig. 4). Thus there is a discrepancy in the pattern of change for SGLT1 mRNA and SGLT1 protein abundances, suggesting that SGLT1 protein abundance in the crypt epithelial cells was additionally maintained by posttranscriptional mechanisms, likely through protein translational control mechanisms.
Fig. 4.
Western blot analysis of SGLT1 protein abundances in the upper villus, the middle villus, and crypt epithelial cells isolated along the jejunal crypt-villus axis in the liquid formula-fed neonatal pigs. Data are in arbitrary units and are presented as means ± SE (n = 6).
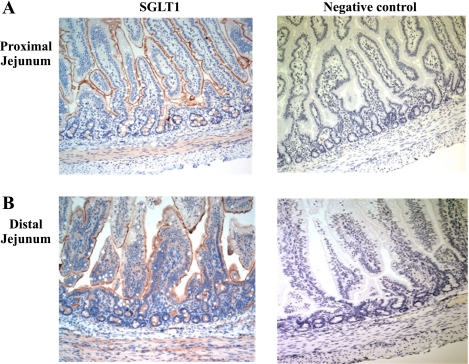
To further visually reveal SGLT1 localization in the epithelial cells along the entire jejunal crypt-villus in the neonatal pigs, immunohistochemical staining was performed. As was shown in Fig. 5, SGLT1 protein expression was specific to the apical membrane of enterocytes along the entire crypt-villus axis in both the proximal (Fig. 5A) and the distal (Fig. 5B) jejunum. The SGLT1 staining was not observed, when the primary antibody was excluded in the negative control staining (Fig. 5). Furthermore, the upper villus region of the distal jejunal epithelia was highly vacuolated (Fig. 5B).
Fig. 5.
Immunohistochemical localization of SGLT1 along the jejunal crypt-villus axis in the liquid formula-fed neonatal pigs. Expression of SGLT1 (brown) was observed on the apical membrane along the entire crypt-villus axis in both the proximal (A) and the distal (B) jejunal regions (left). The staining was not observed when the primary antibody was excluded (right). All images are shown at ×10 magnification.
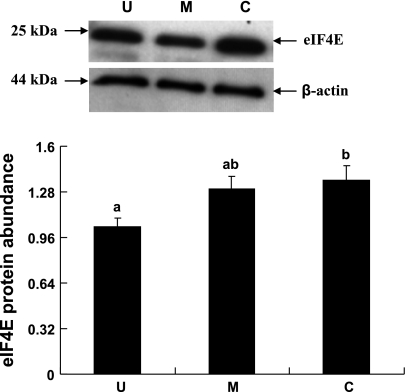
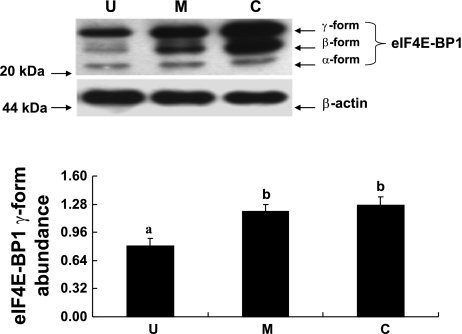
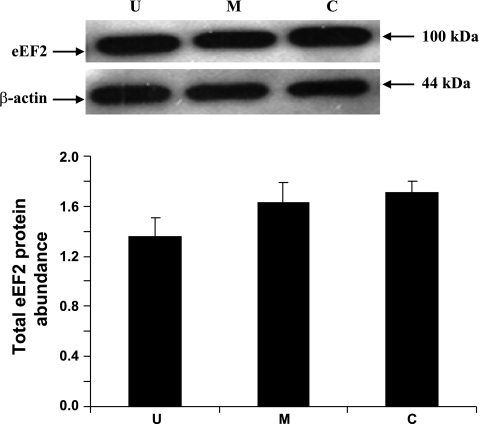
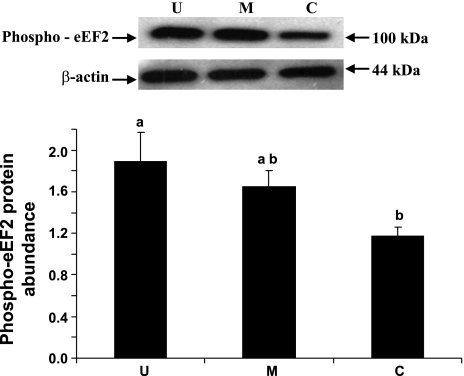
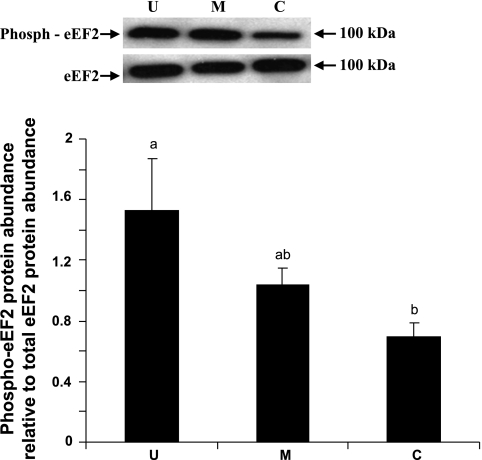
eIF4E, eIF4E-BP1, and eEF2 are some of the well recognized cellular protein translational initiation and elongation factors. The abundances of eIF4E, eIF4E-BP1, and eEF2 were measured by immunoblot analyses. When expressed relative to β-actin, total eIF4E abundance was lower by 31% (P < 0.05) in the upper villus epithelial cell compared with the crypt cells. However, no significant differences in eIF4E abundances were observed between the middle villus and the crypt epithelial cells (Fig. 6). eIF4E-BP1 was also clearly resolved into α-, β-, and γ-isoforms in all three major epithelial cells (Fig. 7). It was apparent that a very small proportion of eIF4E-BP1 was in the α-isoform, whereas the majority of the eIF4E-BP1 protein was in the γ-isoform and/or β-isoform (Fig. 7). Furthermore, the eIF4E-BP1 γ-isoform abundance was lower by 40% (P < 0.05) in the upper villus compared with the crypt epithelial cells. However, no significant difference in eIF4E-BP1 γ-isoform abundance was observed between the middle villus and the crypt epithelial cells (Fig. 8). On the other hand, there were no significant differences in abundances of the total eEF2 protein between the upper villus, the middle villus, and the crypt epithelial cells (Fig. 7). Abundance of phosphorylated-eEF2 protein (Thr56) and the ratio of the phosphorylated-eEF2 protein to total eEF2 abundances were lower by 37% and 1.1-fold (P < 0.05), respectively, in the crypt epithelial cells compared with the upper villus epithelial cells (Figs. 9 and 10). However, no significant differences in abundances of phosphorylated-eEF2 protein (Thr56) and the ratio of the phosphorylated-eEF2 protein to total eEF2 abundances were observed between the upper villus and the middle villus epithelial cells (Figs. 9 and 10).
Fig. 6.
Western blot analysis of eukaryotic initiation factor 4E (eIF4E) protein abundances in the upper villus, the middle villus, and crypt epithelial cells isolated along the jejunal crypt-villus axis in the liquid formula-fed neonatal pigs. Data were normalized with β-actin as the housekeeping control gene protein in arbitrary units and are presented as means ± SE (n = 6). Bars with different superscript letters differ (P < 0.05).
Fig. 7.
Western blot analysis of eIF4E-binding protein 1 (eIF4E-BP1) γ-isomer abundances in the upper villus, the middle villus, and crypt epithelial cells isolated along the jejunal crypt-villus axis in the liquid formula-fed neonatal pig. Data were normalized with β-actin as the housekeeping protein in arbitrary units and are presented as means ± SE (n = 6). Bars with different superscript letters differ (P < 0.05).
Fig. 8.
Western blot analysis of total eukaryotic elongation factor 2 (eEF2) protein abundances in the upper villus, the middle villus, and crypt epithelial cells isolated along the jejunal crypt-villus axis in the liquid formula-fed neonatal pig. Data were normalized with β-actin as the housekeeping protein in arbitrary units and are presented as means ± SE (n = 6).
Fig. 9.
Western blot analysis of phosphorylated eEF2 (phospho-eEF2) protein abundances in the upper villus, the middle villus, and crypt epithelial cells isolated along the jejunal crypt-villus axis in the liquid-formula-fed neonatal pig. Data were normalized with β-actin as the housekeeping protein in arbitrary units and are presented as means ± SE (n = 6). Bars with different superscript letters differ (P < 0.05).
Fig. 10.
Phospho-eEF2 protein abundance to total eEF2 protein abundance ratio in the upper villus, the middle villus, and crypt epithelial cells isolated along the jejunal crypt-villus axis in the liquid formula-fed neonatal pigs. Data are presented as means ± SE (n = 6). Bars with a different superscript letters differ (P < 0.05).
DISCUSSION
Our major objective of this study was to understand the biological mechanisms of possessing high apical SGLT1 activity by neonates through examining the expression of SGLT1 uptake activity kinetics by epithelia along the small intestinal crypt-villus axis in the neonatal piglets. Results from this study suggested that apical SGLT1 activity in terms of Jmax was highly expressed in the epithelial cells along the entire jejunal crypt-villus axis, leading to high apical SGLT1 maximal uptake activity per villus unit or per unit of mucosa in the neonatal piglets. There is a scarcity of literature reports regarding the expression of apical SGLT1 activity along the crypt-villus axis in neonates. These observations in the neonatal pigs from this study were in contrast to the findings previously reported in weanling pigs and other species of adult animals and in cell line studies in showing that enterocytic expression of apical SGLT1 activity was cell differentiation dependent, being high in mature and differentiated villus epithelia but very low or absent in proliferative crypt cells (10, 16, 36). Therefore, these results suggest that expression of apical SGLT1 maximal uptake activity in the epithelia occurs along the entire small intestinal crypt-villus axis and is uniquely regulated in neonates.
The apical SGLT1 affinity estimates (Km, 2.02–3.70 mM) determined in the epithelia along the crypt-villus axis in the neonatal pigs from this study are within the range of the Km values (0.78–7.10 mM) reported in postnatal developmental pigs (41). Although GLUT7 has been characterized to recognize d-glucose as a substrate with a high affinity (Km < 0.500 mM) in the Xenopus oocyte expression system (8), the physiological importance of GLUT7 in the apical glucose uptake is unclear. Our tracer d-glucose uptake kinetic analyses measured with isolated jejunal apical membrane vesicles did not reveal a putative GLUT7-like high-affinity d-glucose uptake kinetic component. This suggests that the GLUT7 does not likely contribute to the absorption of luminal glucose in the pig. In addition, our kinetic analyses of tracer d-glucose uptake in jejunal apical membrane vesicles did not reveal a putative GLUT2-like low-affinity d-glucose uptake kinetic component with Km at ∼56 mM, which was reported in rats (25). This suggests that the GLUT2 is unlikely to play a role for the absorption of luminal glucose in the pig. This observation is consistent with a recent study in weanling pigs in concluding that GLUT2 protein is not expressed on the apical membrane in the pig (37), in contrast to the earlier studies in rats (25). Thus SGLT1 is the major apical hexose transporter responsible for the intestinal absorption of luminal glucose in the neonatal pig.
The dissociation between SGLT1 Jmax values and SGLT1 mRNA abundances along the crypt-villus axis particularly in the crypt cells from this study suggested that the apical SGLT1 Jmax in the crypt epithelial cell was not limited by the SGLT1 mRNA abundance in the neonatal pigs. Whereas a significant difference in SGLT1 mRNA abundance between the villus and the crypt epithelial cells was observed, SGLT1 mRNA was significantly expressed in the crypt cells in the neonatal pigs shown from this study. Results in the neonatal pigs from this study are not consistent with the pattern of SGLT1 gene expression previously reported in adult animals in showing that transcriptional expression of SGLT1 gene was enterocyte differentiation dependent and SGLT1 mRNA abundance was undetectable or very low in the crypt cells (24, 30, 45). On the other hand, our data of dissociation between SGLT1 Jmax and mRNA abundance in the crypt cells from this study are in line with previous studies conducted with adult animals and in the HT-29-D4 cell line in showing that apical SGLT1 activity was regulated, at least in part, at the posttranscriptional level (10, 31). Thus our results indicate that a significant level of SGLT1 mRNA was transcribed in the crypt epithelial cells, partially contributing to the high levels of the apical SGLT1 protein abundance and its maximal d-glucose uptake activity in the crypt cells in the neonatal pigs.
Western blot analysis showed that SGLT1 protein evenly distributed in the epithelial cell homogenate and on the isolated apical membrane along the entire jejunal crypt-villus axis in the neonatal pigs from this study. Our immunohistochemical staining of SGLT1 further supported the observation of SGLT1 specific expression on the apical membrane along the entire crypt-villus axis in both the proximal and the distal jejunum in the liquid formula-fed neonatal pig. The existence of highly vacuolated upper villus epithelia in the distal jejunum, as shown in Fig. 5B, was consistent with much earlier study in showing that suckling neonatal gut possessed vacuolated fetal enterocytes till 19 days of age (44). Furthermore, the consistent expression patterns between the high apical SGLT1 maximal d-glucose uptake activity and the apical SGLT1 protein abundances observed from this study suggest that the expression of the apical SGLT1 protein is not enterocyte differentiation dependent and occurs along the entire crypt-villus axis in these neonatal pigs. Reciprocally, literature reports suggest that expression of the apical SGLT1 protein abundance is enterocyte differentiation dependent, being high in villus cells and very low or negligible in crypt cells in weanling pigs (37) and adult animals (1, 24, 48). Therefore, expression of SGLT1 on the apical membrane occurs along the entire jejunal crypt-villus axis in the neonatal pig.
Western blot analysis identified the porcine immune-reactive SGLT1 protein band at ∼73 kDa. This is consistent with a similar size of the mature SGLT1 protein at 75 kDa reported in the weanling pig (37) and in adult animals of other species (19, 20). Furthermore, observations from previous SGLT1 biosynthetic studies (19, 20) are in line with in situ phlorizin binding kinetic studies in adult mice in showing that biosynthesis of premature SGLT1 isoform was conceived and induced primarily in the crypt enterocytes (15). Based on the reported porcine SGLT1 coding cDNA sequence (GenBank accession no. NM_001164021), the theoretical porcine SGLT1 protein was calculated to be at ∼73 kDa, consistent with our Western analysis (Fig. 4). Our Western analyses suggested that SGLT1 protein expressed in the neonatal porcine gut mucosa was not likely glycosylated at a significant level. The lack of differences in the SGLT1 abundances in the cell homogenates and the apical membrane preparations among the three epithelial cell fractions from this study would suggest that intracellular trafficking did not seem to play a role in regulation of the apical SGLT1 expression in the neonates. In contrast, intracellular trafficking was shown to play a major role in expressing apical SGLT1 in studies with cell lines (10, 50, 52). In theory, the relative SGLT1 abundances in the cell homogenates and the apical membrane preparations in the neonatal pigs from this study, as analyzed by Western blotting, are largely the net balance between the biosynthesis of SGLT1 and SGLT1 degradation. Considering that the level of the intracellular SGLT1 degradation might be very low, changes in SGLT1 abundances in the cell homogenates and the apical membrane preparations isolated along the crypt-villus axis likely reflected changes in de novo synthesis of SGLT1 protein, which needs to be further explored in future in vivo SGLT1 labeling and biosynthesis studies in neonates.
An intriguing question is how the apical SGLT1 protein abundance was maintained despite significantly low abundance of SGLT1 mRNA observed in the crypt cells in the neonatal piglets from this study. Under this context, it has been well documented that translational control is an important strategy and the most determinant by which eukaryotic cells regulate gene expression and control target gene protein levels (47, 49). The eukaryotic protein synthetic pathway components, including initiation, elongation, and termination, have been well established with rate-limiting steps, and their regulatory mechanisms being continuously elucidated (47, 49). Several initiation and elongation factors have been shown to play essential roles in eukaryotic protein translational control, including eIF4E, eIF4E-BP1, and eEF2. eIF4E is a major target for the control of cap-dependent translational initiation and plays very important roles in the regulation of protein synthesis by involving the formation of eIF4F complex (18, 46). The availability of eIF4E for initiating protein synthesis is further regulated by the eIF4E-BP1, which, in its hyperphosphorylated state, reduces its binding to eIF4E and releases more eIF4E for the formation of eIF4F complex for enhanced engaging to mRNA and protein translation (54, 55). The polypeptide elongation process occurs after protein translation initiation and is mediated by eEF2 that couples to GTP hydrolysis (40, 55). The phosphorylation of eEF2 at Thr56, catalyzed by eEF2 kinase, inactivates eEF2 and, therefore, substantially inhibits protein synthesis (42). So far, very little has been reported regarding the contribution of protein translational control mechanisms to the expression of SGLT1 during enterocyte proliferation and differentiation. Although in vivo SGLT1 protein synthesis and total cellular protein synthetic efficiency were not measured in this study, changes in abundances and/or phosphorylation of these important protein translational initiation and elongation factors, including eIF4E, eIF4E-BP1, and eEF2, were examined in the epithelial cells isolated along the crypt-villus axis in the neonatal pigs from this study. It is noteworthy that eIF4E-BP1 immune-reactive bands were clearly resolved into α-, β-, and γ-isoform in all the three major epithelial cell fractions in this study, consistent with previous studies on the eIF4E-BP1 protein in porcine muscle (26). Earlier studies showed that when eIF4E-BP1 was resolved by SDS-PAGE, it was separated into three isoforms with the fastest migration band characterized to be α-isoform being the least phosphorylated isoform and the slowest migration band designated to be γ-isoform being the most highly phosphorylated isoform (39). Significantly higher abundances of eIF4E and eIE4E-BP1 γ-isoform in combination with a lower phosphorylated eEF2 abundance and phosphorylated eEF2-to-total eEF2 abundance ratio observed in the crypt cells compared with the upper villus cells in this study would suggest that global protein translational efficiency was higher in crypt cells than in the upper villus cells in the neonates. This observation is in general agreement with a proteomic study by Chang et al. (5), who showed that intestinal transcription and translation related proteins were predominantly expressed in crypts in mice. Accordingly, microarray studies revealed that genes with a role in protein translation, folding, and ribosome biogenesis were significantly upregulated in crypt cells from mouse and human intestines (17, 34). On the other hand, the expression of eIF4E, eIF4E-BP1, and eEF2 and/or their phosphorylation along with other translational initiation and elongation factors are known to be modulated through mammalian target of rapamycin signaling pathway by a wide range of extracellular stimuli such as nutrient availability, intracellular energy status, hormonal factors, and physiological status (54, 55). Of these influencing factors, it will be important to understand how availability of luminal nutrients specifically regulates the biosynthesis of SGLT1 protein in the epithelial cells along the crypt-villus axis in neonates in future research. Therefore, our results showed that global cellular protein translation efficiency was higher in crypt cells than in the upper villus cells and this may be involved in maintaining high SGLT1 protein abundances in the crypt cells and on the apical membrane of the cells in the neonates.
In conclusion, SGLT1 protein was abundantly expressed in the epithelia and on the apical membrane of these cells along the entire jejunal crypt-villus axis, thereby leading to high levels of apical SGLT1 maximal uptake activity per villus unit and in per unit of gut mucosa in the neonates. Despite relatively low SGLT1 mRNA abundance, higher cellular global protein translational efficiency, as supported by comparative examination of eIF4E, eIF4E-BP1, and eEF2E abundances, may be contributing to maintaining the abundant expression of the SGLT1 protein and its activity in the crypt cells of the neonatal piglets.
GRANTS
This project has been supported from the Natural Sciences and Engineering Research Council of Canada Discovery Program and the Ontario Ministry of Agriculture, Food and Rural Affairs-the University of Guelph Partnership Program (to M. Z. Fan), the Natural Science Foundation of China (30828025; to Y. L. Yin), and USDA/ARS under Cooperative Agreements 58-6250-6-001/the National Institute of Health R01-HD33920 (to D. G. Burrin). C. Yang was supported by a visiting scholarship from the Chinese Academy of Sciences and Z. Wang was supported by a visiting scholarship from the Chinese Ministry of Education while participating in related research activities in Guelph, ON, Canada. M. Z. Fan was supported by a USDA/ARS postdoctoral research fellowship when the project was initiated at the Baylor College of Medicine, Houston, TX.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We are grateful to Tania Archbold, Zhixiang Wang, Qi Wang, and Erin Hayes for tissue harvesting and to Josepha DeLay and Susan Lapos for immunohistochemical analyses.
REFERENCES
- 1. Balen D, Ljubojevic M, Breljak D, Brzica H, Zlender V, Koepsell H, Sabolic I. Revised immunolocalization of the Na+-d-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol 295: C475–C489, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Barone S, Fussell SL, Singh AK, Lucas F, Xu J, Kim C, Wu X, Yu Y, Amlal H, Seidler U, Zuo J, Soleimani M. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem 284: 5056–5066, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burant CF, Flink S, DePaoli AM, Chen J, Lee WS, Hediger MA, Buse JB, Chang EB. Small intestine hexose transport in experimental diabetes. Increased transporter mRNA and protein expression in enterocytes. J Clin Invest 93: 578–585, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Byrkit DR. Statistics Today—A Comprehensive Introduction. Menlo Park, CA: Benjamin/Cummings, 1987 [Google Scholar]
- 5. Chang J, Chance MR, Nicholas C, Ahmed N, Guilmeau S, Flandez M, Wang D, Byun D, Nasser S, Albanese JM, Corner GA, Heerdt BG, Wilson AJ, Augenlicht LH, Mariadason JM. Proteomic changes during intestinal cell maturation in vivo. J Proteomics 71: 530–546, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheeseman CI. GLUT2 is the transporter for fructose across the rat intestinal basolateral membrane. Gastroenterology 105: 1050–1056, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Cheeseman CI. Intestinal hexose absorption: transcellular or paracellular fluxes. J Physiol 544: 336, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheeseman C. GLUT7: a new intestinal facilitated hexose transporter. Am J Physiol Endocrinol Metab 295: E238–E241, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat 141: 461–480, 1974 [DOI] [PubMed] [Google Scholar]
- 10. Delézay O, Baghdiguian S, Fantini J. The development of Na+-dependent glucose transport during differentiation of an intestinal epithelial cell clones is regulated by protein kinase C. J Biol Chem 270: 12536–12541, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Dudeja PK, Wali RK, Klitzke A, Brasitus TA. Intestinal d-glucose transport and membrane fluidity along crypt-villus axis of streptozocin-induced diabetic rats. Am J Physiol Gastrointest Liver Physiol 259: G571–G577, 1990 [DOI] [PubMed] [Google Scholar]
- 12. Fan MZ, Matthews J, Etienne NMP, Stoll B, Lackeyram D, Burrin DG. Expression of brush border l-glutamate transporters in epithelial cells along the crypt-villus axis in the neonatal pig. Am J Physiol Gastrointest Liver Physiol 287: G385–G398, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Fan MZ, Adeola O, Asem EK. Estimation of apparent l-amino acid diffusion in porcine jejuna enterocyte brush border membrane vesicles. Physiol Res 50: 373–381, 2001 [PubMed] [Google Scholar]
- 14. Fan MZ, Stoll B, Jiang R, Burrin DG. Enterocyte digestive enzyme activity along the crypt-villus and longitudinal axes in the neonatal pig small intestine. J Anim Sci 79: 371–381, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Ferraris RP, Diamond J. Crypt-villus site of glucose transporter induction by dietary carbohydrate in mouse intestine. Am J Physiol Gastrointest Liver Physiol 262: G1069–G1073, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Freeman HJ, Johnston G, Quamme GA. Sodium-dependent d-glucose transport in brush-border membrane vesicles from isolated rat small intestinal villus and crypt epithelial cells. Can J Physiol Pharmacol 65: 1213–1219, 1987 [DOI] [PubMed] [Google Scholar]
- 17. Gassler N, Newrzella D, Bohm C, Lyer S, Li L, Sorgenfrei O, van Laer L, Sido B, Mollenhauer J, Poustka A, Schirmacher P, Gretz N. Molecular characterisation of non-absorptive and absorptive enterocytes in human small intestine. Gut 55: 1084–1089, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem 68: 913–963, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Hediger MA, Mendlein J, Lee HS, Wright EM. Biosynthesis of the cloned intestinal Na+/glucose cotransporter. Biochim Biophys Acta 1064: 360–364, 1991 [DOI] [PubMed] [Google Scholar]
- 20. Hirayama BA, Wright EM. Glycosylation of the rabbit intestinal brush border Na+-glucose cotransporter. Biochim Biophys Acta 1103: 37–44, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Hirsch JR, Loo DDF, Wright EM. Regulation of Na+/glucose cotransporter expression by protein kinases in Xenopus laevis oocytes. J Biol Chem 271: 14740–14746, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Hübscher G, West GR. Specific assays of some phosphatases in subcellular fractions of small intestinal mucosa. Nature 205: 799–800, 1965 [DOI] [PubMed] [Google Scholar]
- 23. Hume R, Burchell A, Williams FL, Koh DK. Glucose homeostasis in the newborn. Early Hum Dev 81: 95–101, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Hwang E, Hirayama BA, Wright EM. Distribution of the SGLT1 Na+/glucose cotransporter and mRNA along the crypt-villus axis of rabbit small intestine. Biochem Biophys Res Commun 181: 1208–1217, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J 350: 155–162, 2000 [PMC free article] [PubMed] [Google Scholar]
- 26. Kimball SR, Jefferson LS, Nguyen HV, Suryawan A, Bush JA, Davis TA. Feeding stimulates protein synthesis in muscle and liver of neonatal pigs through an mTOR-dependent process. Am J Physiol Endocrinol Metab 279: E1080–E1087, 2000 [DOI] [PubMed] [Google Scholar]
- 27. King TE. Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. Methods Enzymol 10: 322–331, 1967 [Google Scholar]
- 28. Kles KA, Tappenden KA. Hypoxia differentially regulates nutrient transport in rat jejunum regardless of luminal nutrient present. Am J Physiol Gastrointest Liver Physiol 283: G1336–G1342, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Korn T, Kühlkamp T, Track C, Schatz I, Baumgarten K, Gorboulev V, Koepsell H. The plasma membrane-associated protein RS1 decreases transcription of the transporter SGLT1 in confluent LLC-PK1 cells. J Biol Chem 276: 45330–45340, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Lee WS, Kanai Y, Wells RG, Hediger MA. The high affinity Na+/glucose cotransporter. Re-evaluation of function and distribution of expression. J Biol Chem 269: 12032–12039, 1994 [PubMed] [Google Scholar]
- 31. Lescale-Matys L, Dyer J, Scott D, Freeman TC, Wright EM, Shirazi-Beechey SP. Regulation of the ovine Na+/glucose cotransporter (SGLT-1) is dissociated from mRNA abundance. Biochem J 291: 435–440, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time PCR quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Loflin P, Lever JE. HuR binds a cyclic nucleotide-dependent, stabilizing domain in the 3′ untranslated region of Na+/glucose cotransporter (SGLT1) mRNA. FEBS Lett 509: 267–271, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Mariadason JM, Nicholas C, L'Italien KE, Zhuang M, Smartt HJ, Heerdt BG, Yang W, Corner GA, Wilson AJ, Klampfer L, Arango D, Augenlicht LH. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology 128: 1081–1088, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Maroux S, Louvard D, Baratti J. The aminopeptidase from hog intestinal brush border. Biochim Biophys Acta 321: 282–295, 1973 [DOI] [PubMed] [Google Scholar]
- 36. Meddings JB, DeSouza D, Goel M, Thiesen S. Glucose transport and microvillus membrane physical properties along the crypt-villus axis of the rabbit. J Clin Invest 85: 1099–1107, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Ionescu C, Bravo D, Shirazi-Beechey SP. Expression of Na+/glucose co-transporter 1 (SGLT1) in the intestine of piglets weaned to different concentrations of dietary carbohydrate. Br J Nutr 13: 1–9, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Murer H, Ammann E, Biber J, Hopfer U. The surface membrane of the small intestinal epithelial cell. 1. Localization of adenyl cyclase. Biochim Biophys Acta 433: 509–519, 1976 [DOI] [PubMed] [Google Scholar]
- 39. Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator 5'-cap function. Nature 371: 762–767, 1994 [DOI] [PubMed] [Google Scholar]
- 40. Proud CG. Role of mTOR signaling in the control of translation initiation and elongation by nutrients. Curr Top Microbiol Immunol 279: 215–244, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Puchal AA, Buddington RK. Postnatal development of monosaccharide transport in pig intestine. Am J Physiol Gastrointest Liver Physiol 262: G895–G902, 1992 [DOI] [PubMed] [Google Scholar]
- 42. Ryazanov AG, Shestakova EA, Natapov PG. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature 334: 170–173, 1988 [DOI] [PubMed] [Google Scholar]
- 43. Smith MW. Autoradiographic analysis of alanine uptake by newborn pig intestine. Experientia 37: 868–869, 1981 [DOI] [PubMed] [Google Scholar]
- 44. Smith MW, Peacock MA. Anomalous replacement of foetal enterocytes in the neonatal pig. Proc R Soc Lond B Biol Sci 206: 411–420, 1980 [DOI] [PubMed] [Google Scholar]
- 45. Smith MW, Turvey A, Freeman TC. Appearance of phloridzin-sensitive glucose transport is not controlled at mRNA level in rabbit jejunal enterocytes. Exp Physiol 77: 525–528, 1992 [DOI] [PubMed] [Google Scholar]
- 46. Sonenberg N. eIF4E, the mRNA cap-binding protein: from basic discovery to translational research. Biochem Cell Biol 86: 178–183, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Sonenberg N, Hinnerbush AG. Regulation of translation initiation in eukaryotes: mechanism and biological targets. Cell 136: 731–745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H. Immunohistochemical localization of Na+-dependent glucose transporter in rat jejunum. Cell Tissue Res 267: 3–9, 1992 [DOI] [PubMed] [Google Scholar]
- 49. Van Der Kelen K, Beyaert R, Inzé D, De Veylder L. Translational control of eukaryotic gene expression. Crit Rev Biochem Mol Biol 44: 143–168, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Vayro S, Silverman M. PKC regulates turnover rate of rabbit intestinal Na+-glucose transporter expressed in COS-7 cells. Am J Physiol Cell Physiol 276: C1053–C1060, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Vega YM, Puchal AA, Buddington RK. Intestinal amino acid and monosaccharide transport in suckling pigs fed milk replaces with different sources of carbohydrate. J Nutr 122: 2430–2439, 1992 [DOI] [PubMed] [Google Scholar]
- 52. Veyhl M, Keller T, Gorboulev V, Vernaleken A, Koepsell H. RS1 (RSC1A1) regulates the exocytotic pathway of Na+-d-glucose cotransporter SGLT1. Am J Physiol Renal Physiol 291: F1213–F1223, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Wright EM, Hirayama BA, Loo DDF, Turk E, Hager K. Intestinal sugar transport. In: Physiology of the Gastrointestinal Tract (3rd ed.), edited by Johnson LR. New York: Raven, 1994, p. 571–610 [Google Scholar]
- 54. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471–484, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Yang X, Yang C, Farberman A, Rideout TC, de Lange CFM, France J, Fan MZ. The mammalian target of rapamycin-signaling pathway in regulating metabolism and growth. J Anim Sci 86: E36–E50, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Zhang H, Malo C, Buddington RK. Suckling induces rapid intestinal growth and changes in brush border digestive functions of newborn pigs. J Nutr 127: 418–426, 1997 [DOI] [PubMed] [Google Scholar]