Abstract
The dorsal motor nucleus of the vagus (DMV) is pivotal in the regulation of upper gastrointestinal functions, including motility and both gastric and pancreatic secretion. DMV neurons receive robust GABA- and glutamatergic inputs. Microinjection of the GABAA antagonist bicuculline (BIC) into the DMV increases pancreatic secretion and gastric motility, whereas the glutamatergic antagonist kynurenic acid (KYN) is ineffective unless preceded by microinjection of BIC. We used whole cell patch-clamp recordings with the aim of unveiling the brain stem neurocircuitry that uses tonic GABA- and glutamatergic synapses to control the activity of DMV neurons in a brain stem slice preparation. Perfusion with BIC altered the firing frequency of 71% of DMV neurons, increasing firing frequency in 80% of the responsive neurons and decreasing firing frequency in 20%. Addition of KYN to the perfusate either decreased (52%) or increased (25%) the firing frequency of BIC-sensitive neurons. When KYN was applied first, the firing rate was decreased in 43% and increased in 21% of the neurons; further perfusion with BIC had no additional effect in the majority of neurons. Our results indicate that there are several permutations in the arrangements of GABA- and glutamatergic inputs controlling the activity of DMV neurons. Our data support the concept of brain stem neuronal circuitry that may be wired in a finely tuned organ- or function-specific manner that permits precise and discrete modulation of the vagal motor output to the gastrointestinal tract.
Keywords: electrophysiology, vagus, brain stem
sensory information from the gastrointestinal (GI) tract is encoded by chemo- and mechanoreceptors with different modalities, responses, and properties (reviewed in Ref. 11). Regardless of their function(s) or modalities, however, primarily nonnoxious visceral sensory information is transmitted through the afferent vagus into the brain stem via a glutamatergic synapse at the level of the nucleus tractus solitarius (NTS) (2, 3, 8, 54). The NTS comprises various subnuclei that appear to be organized viscerotopically (1, 9, 57), such that the vagal sensory input from each visceral organ is concentrated in a particular subnucleus. The subnucleus centralis, for example, receives inputs almost exclusively from the esophagus.
The motor output to the GI tract is provided by the spontaneously firing neurons of the dorsal motor nucleus of the vagus (DMV), which, in contrast to NTS neurons, are organized in neuronal “columns” that project to the viscera through each of the five subdiaphragmatic vagal branches (reviewed in Ref. 57). DMV neurons within each column, however, are not identical but comprise heterogeneous neuronal populations that differ in their neurochemical, morphological, and electrophysiological properties (13, 16, 30, 34, 37, 40, 55).
The vagal output to the GI tract is involved in the regulation of several different functions related to digestive processes, which include reflex control of GI motility, gastric, pancreatic, and duodenal secretion, as well as homeostatic adaptations to external stimuli and challenges. All of these functions require a fine modulatory control that is achieved by the vast array of afferent inputs from multiple regions of the central nervous system (CNS), which monitor the physiological state of the animal and convey this information to the brain stem nuclei. NTS and DMV neurons, as well as their synaptic connections, represent the final CNS locus for the integrated modulation of the sensory-motor brain-gut axis to synchronize the activity of the GI tract.
NTS and DMV neurons are connected through a dense synaptic network that uses mainly GABA, glutamate, and catecholamines as neurotransmitters (23, 31, 49, 56, 59). In recent years, many in vitro studies have reported the modulation of these synapses by various substances (14, 15, 18, 21, 24, 25, 28, 33, 58). A large array of in vivo studies have also correlated the effects of microinjections of neurotransmitters and neuromodulators in the dorsal vagal complex (DVC; i.e., DMV, NTS, and area postrema) with responses in the various GI organs. More specifically, a series of studies focused on the GI responses to microinjections of the GABA- and glutamate-related substances in the brain stem. Microinjections of the specific GABAA antagonist bicuculline methiodide (BIC) into the DMV increase gastric acid secretion, intragastric pressure, and exocrine pancreatic secretions (27, 44, 53), suggesting that DMV neurons innervating the stomach and the exocrine pancreas receive tonic GABAergic inputs. Glutamate, in contrast, appears to exert site-specific effects on esophageal and gastric motility. Activation of rostral DMV neurons by glutamate microinjection elicits gastric contractions and increases tone of the lower esophageal sphincter, whereas glutamate microinjections in the caudal DMV have the opposite effect (45, 50, 61). Moreover, microinjections of the specific ionotropic glutamate antagonist kynurenic acid (KYN) alone have no effect on gastric motility, but abolish the effect of BIC (53). These observations suggest that tonically active GABAergic synapses exert their effect on gastric motility via actions involving the activation of glutamate synapses. Taken together, it is evident that GABA- and glutamatergic inputs to DMV neurons play a fundamental role in the regulation of vagal output; their role in the modulation of the firing rate of DMV neurons, however, has not been investigated yet.
This study used a brain stem slice preparation to investigate whether 1) the firing rate of DMV neurons is modulated by tonic GABAergic and glutamatergic inputs and 2) all the GABA- and glutamatergic inputs onto DMV neurons induce similar responses.
MATERIALS AND METHODS
All experiments were conducted with the approval of the Institutional Animal Care and Use Committee and according to National Institutes of Health regulations.
Retrograde tracing.
A subgroup of Sprague-Dawley rats (14 days old) of both sexes were anesthetized with a solution of isoflurane with air (400–600 ml/min). Once a suitable depth of anesthesia was obtained (checked via the foot pinch withdrawal reflex), the abdominal and thoracic areas were shaved and cleaned with disinfectant and a laparotomy was performed. Crystals of the fluorescent tracer 1,1′-dioctadecyl-3,3,3′3′-tetra-methyl-indo-carbocyanine perchlorate (DiI; Molecular Probes, Eugene, OR) were applied to either the major curvature of the gastric fundus or corpus. To confine the dye to the gastric areas of interest, the application site was embedded in a fast-hardening epoxy compound. The epoxy compound was allowed to dry, and the area was examined visually to ensure that the dye had remained affixed to the organ surface and not made contact with other tissues. The surgical area was washed with warm sterile saline solution and blotted with cotton tips, and the laparotomy was closed. The animals were allowed to recover for at least 7 days.
Electrophysiology.
The brain stems were removed as described previously (15, 16). Briefly, rats were anesthetized with isoflurane and euthanized via administration of bilateral pneumothorax prior to removal of the brain stem, which was then placed in oxygenated Krebs solution at 4°C. Five to six coronal sections (300 μm thick) through the DVC were cut on a vibratome and incubated in oxygenated Krebs solution at 30°C for at least 90 min. A single slice was transferred to a custom-made perfusion chamber (volume 500 μl) and kept in place with a nylon mesh. The chamber was maintained at 32 ± 1°C by perfusion with warmed, oxygenated Krebs solution at a rate of 2.5–3.0 ml/min.
In the subgroup of rats that underwent placement of DiI on the corpus or fundus, prior to electrophysiological recording, DiI-labeled neurons were identified by using a Nikon E600-FN microscope equipped with tetramethylrhodamine isothiocyanate epifluorescent filters. Once labeled neurons were identified, whole cell recordings were made under bright-field illumination using DIC (Nomarski) optics.
Whole cell recordings were made with patch pipettes (2–5 MΩ tip resistance) filled with a potassium gluconate solution using an Axopatch 2B amplifier (Molecular Devices, Sunnyvale, CA). Recordings of nonlabeled DMV neurons were conducted only in the medial portions of the nucleus, i.e., the area that comprises exclusively gastric projecting DMV neurons (29, 46, 52) as well as from gastric-projecting DMV neurons labeled unequivocally with DiI. Data were filtered at 2 kHz, digitized via a Digidata 1320A interface (Molecular Devices), acquired, stored, and analyzed on a personal computer utilizing pClamp9 software (Molecular Devices). Recordings were accepted only if the series resistance was <20 MΩ. The neuronal firing rate was set to ∼1 pulse/s via injection of direct current.
BIC (50 μM; Sigma Aldrich, St. Louis, MO) and KYN (1 mM; Sigma Aldrich) were applied to the bath via a series of manually operated valves. Following a 1-min baseline period, each antagonist, i.e., BIC or KYN, was applied for 7 min in random order. After 7 min, the second drug, i.e., KYN or BIC, was added to the perfusate for a further 7 min, after which all drugs were washed out until the firing rate recovered toward baseline values, usually up to 20 min. In one group of cells, the GABAA receptor antagonist SR95531 (gabazine; 20 μM; Sigma Aldrich) was applied instead of BIC. To assess the effects of drugs, each cell served as its own control. Only one cell per slice was tested with each drug.
Data were analyzed by averaging the number of action potentials in control conditions, during drug perfusion and washout periods.
Statistical analysis.
Data were analyzed by comparing action potential frequency at baseline and during treatment by paired Student's t-test, intergroup comparisons were analyzed with the χ2 test. Significance was defined as P < 0.05.
RESULTS
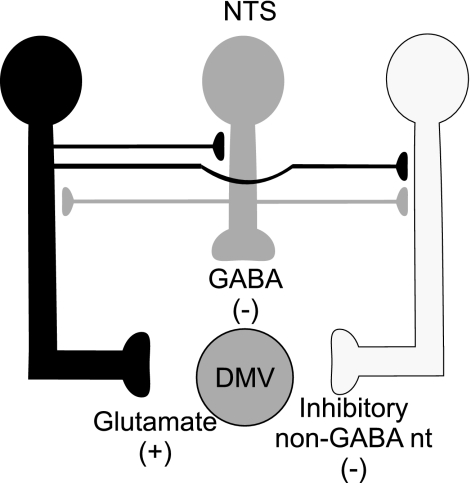
Throughout this article we use a simplified graphic representation of the NTS-DMV synaptic connections as depicted in Fig. 1 as a template for the potential synaptic circuit. In each subsequent figure, the putative site of action of the drug tested is highlighted. Since the slice was perfused with specific antagonists, a variation in the neuronal firing rate indicates a site of action on a synapse that is tonically active.
Fig. 1.
Schematic diagram showing glutamate (black), GABA (gray), and inhibitory non-GABAergic (white) neurons impinging on the dorsal motor nucleus of the vagus (DMV). Note that we do not mean to imply that the synaptic contacts occur specifically on the dendrites, axons, or synaptic terminals, but they are depicted in this manner for schematic clarity. NTS, nucleus tractus solitarius.
BIC modulates the firing rate of the majority of DMV neurons.
Effects of 50 μM BIC on the firing rate were tested in 62 DMV neurons. In these neurons, the basal firing rate was 1.03 ± 0.03 action potentials/s. Perfusion of the slice with BIC altered the firing rate in 44 of the 62 (i.e., 71%) neurons tested, suggesting that the vast majority of DMV neurons receive a tonic GABAergic input. In the remaining 18 (i.e., 29%) neurons, perfusion with BIC did not alter their firing rate, indicating that this neuronal subpopulation is not under a tonic GABAergic influence. In one group of cells, the GABAA receptor antagonist SR95531, which inhibits phasic but not tonic GABA currents (4, 31), was applied instead of BIC. Of the 22 cells tested, 5 (23%) increased their firing rate from 1.0 ± 0 action potentials/s to 1.8 ± 0.25 action potentials/s, 8 (36%) decreased their firing rate from 1.0 ± 0 to 0.18 ± 0.07 action potentials/s, and the remaining 9 (41%) did not respond to SR 95531. Since a greater percentage of neurons responded to BIC than to SR 95531, and since BIC blocks both tonic and phasic GABAergic currents (4, 31), we used BIC for experiments in which effects of KYN were tested.
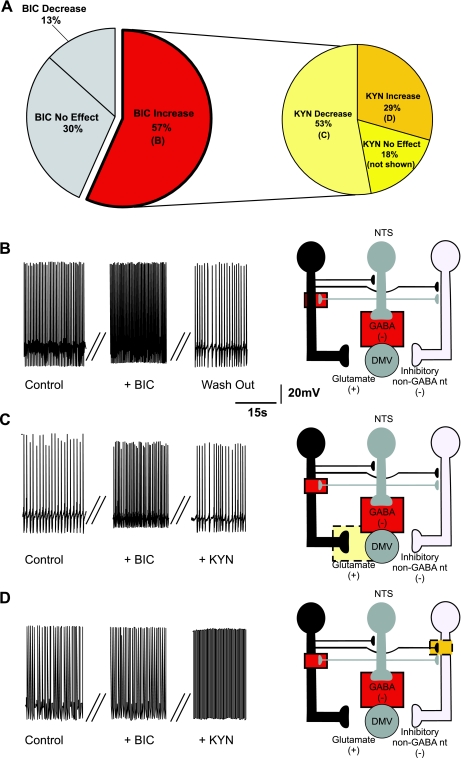
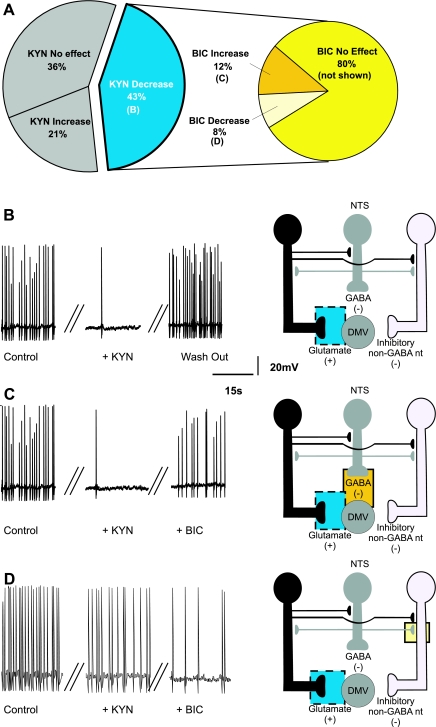
BIC increased the firing rate from 1.02 ± 0.04 to 3.13 ± 0.52 action potentials/s in 35 of the 44 (i.e., 80%) responsive neurons (Fig. 2, A and B). In these neurons, perfusion with 1 mM KYN in the continuous presence of BIC either decreased (from 4.8 ± 0.25 to 1.67 ± 0.06 action potentials/s; N = 16) or blocked (0 ± 0 action potentials/s; N = 3) the firing rate in 19 (i.e., 54%; Fig. 2, A and C) and increased the firing rate in 10 (i.e., 29%; from 2.29 ± 0.27 to 3.1 ± 0.3 action potentials/s; Fig. 2D) neurons. The firing rate of the remaining 6 (i.e., 17%) neurons was unaffected by KYN perfusion (from 2.0 ± 0.40 to 2 ± 0.38 action potentials/s). Data are summarized in Table 1, and the potential sites of action of BIC and KYN are depicted in Fig. 2, B–D.
Fig. 2.
Pie charts (A), representative traces (B–D, left), and the proposed neuronal circuits (B–D, right) in neurons in which perfusion with bicuculline (BIC) increased the firing rate of DMV neurons. Perfusion with BIC increased the firing frequency of DMV neurons (N = 35), and perfusion with kynurenic acid (KYN) decreased the firing frequency of the majority of these neurons. Note that the pie chart on the right represents subgroups of neurons in which BIC increased the firing frequency. The increase in firing rate induced by BIC is likely due to antagonism of GABAA receptors located either on the membrane of the DMV neuron or on tonically active glutamatergic neurons that impinge on the DMV neuron. Perfusion with KYN in the presence of BIC decreased the firing rate of DMV neurons (N = 19). This response was likely due to KYN-mediated inhibition of a tonic glutamatergic input onto DMV neurons. Perfusion with KYN in the presence of BIC further increased the firing rate of DMV neurons (N = 10), suggesting the presence of tonic glutamatergic synapses impinging onto non-GABAergic neurons. In all figures, the proposed site of action of bicuculline is indicated by a solid-line red square and the proposed site of action of kynurenic acid is indicated by a dotted-line yellow square.
Table 1.
Effect of bicuculline and kynurenic acid on the firing rate of DMV neurons
| Bicuculline |
||||||
|---|---|---|---|---|---|---|
| All neurons |
Gastric-projecting neurons |
|||||
| Increase | No effect | Decrease | Increase | No effect | Decrease | |
| Kynurenic acid | ||||||
| Increase 17 (28%) | 10 | 6 | 1 | 3 | 3 | 0 |
| No effect 16 (27%) | 6 | 7 | 4 | 2 | 1 | 1 |
| Decrease 27 (45%) | 19 | 5 | 4 | 3 | 4 | 2 |
| Total | 35 (56%) | 18 (29%) | 9 (14%) | 8 (42%) | 8 (42%) | 3 (16%) |
Bicuculline was applied first, followed by kynurenic acid. Numbers represent the number of neurons that exhibited given responses to bicuculline (columns) and kynurenic acid (rows). DMV, doral motor nucleus of the vagus.
These data suggest that the majority of DMV neurons receive tonic GABAergic and glutamatergic synaptic inputs.
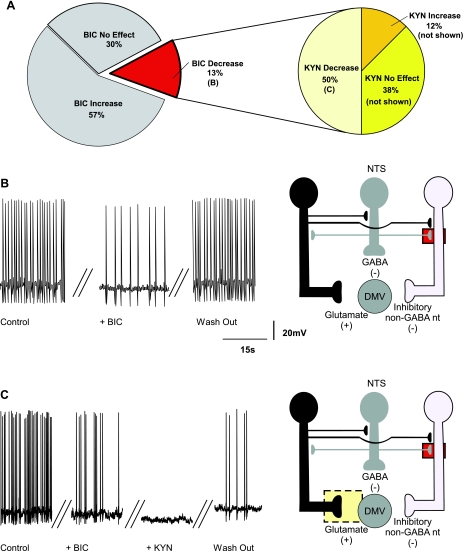
BIC decreased the firing rate in 9 (i.e., 20%) neurons from 1.2 ± 0.13 to 0.48 ± 0.15 action potentials/s, indicating that a small population of DMV neurons do not receive a direct tonic GABAergic input but, rather, receive inputs from inhibitory nonGABAergic neurons that are themselves subject to a tonic GABAergic influence (Fig. 3). In these neurons, perfusion with KYN, in the presence of BIC, further decreased or blocked the firing rate in 4 (i.e., 44%; from 0.22 ± 0.13 to 0.12 ± 0.12 action potentials/s; Fig. 3, A and C), suggesting that some DMV neurons receive a direct tonic glutamatergic input (Fig. 3C).
Fig. 3.
Pie charts (A), representative traces (B and C, left), and the proposed neuronal circuits (B and C, right) in neurons in which perfusion with BIC decreased the firing rate of DMV neurons. Perfusion with BIC decreased the firing frequency of DMV neurons (N = 9), and KYN further decreased the firing frequency in majority of these neurons. The pie chart on the right represents subgroups of neurons in which BIC decreased the firing frequency. The decrease in firing rate induced by BIC is likely due to antagonism of GABAA receptors located on the membrane of non-GABAergic inhibitory neurons. Perfusion with KYN in the presence of BIC further decreased the firing rate of DMV neurons (N = 4). This response was likely due to KYN-mediated inhibition of a tonic glutamatergic input onto DMV neurons.
In the remaining five neurons in which BIC decreased the firing rate, perfusion with KYN increased in 1 (from 0.77 to 0.9 action potentials/s) and had no effect on the firing rate of the 4 remaining (i.e., 44%) neurons (Fig. 3A).
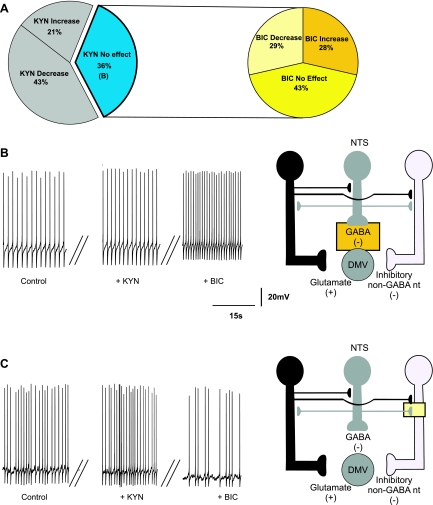
The remaining 18 (i.e., 29% of all the neurons tested with BIC) neurons tested did not respond to perfusion with BIC with an alteration in firing rate (Fig. 4), suggesting that these neurons are not under tonic GABAergic influence. The firing rate of these neurons was 0.99 ± 0.04 and 1.0 ± 0.04 action potentials/s before and after application of BIC, respectively. In these 18 neurons, application of KYN decreased the firing rate in 5 (i.e., 28%; from 0.95 ± 0.05 to 0.68 ± 0.18 action potentials/s; Fig. 4B), increased it in 6 (i.e., 33%, from 1.05 ± 0.07 to 2.0 ± 0.33 action potentials/s; Fig. 4C), and had no effect on the firing rate of 7 (i.e., 39%; not shown) neurons. These data indicate that the majority of neurons that are not under tonic GABAergic influence are modulated by tonic glutamatergic inputs and that some of these inputs impinge on an inhibitory non-GABAergic interneuron (Fig. 4, B and C).
Fig. 4.
Pie charts (A), representative traces (B and C, left), and the proposed neuronal circuits (B and C, right) in neurons in which perfusion with BIC did not affect the firing rate (N = 18) of DMV neurons. A: perfusion with KYN in the presence of BIC decreased the firing rate of DMV neurons (N = 5). B: the decrease in the firing rate in response to KYN was likely due to KYN-mediated inhibition of a tonic glutamatergic input onto DMV neurons. C: perfusion with KYN in the presence of BIC increased the firing rate of DMV neurons (N = 6), suggesting the presence of tonic glutamatergic synapses impinging onto non-GABAergic neurons.
Nineteen of the neurons that were tested with BIC and KYN were identified by fluorescence labeling as gastric-projecting neurons. The percentage of gastric-projecting neurons that had their firing rate modulated in response to perfusion with BIC and KYN was not different from those of the unidentified neurons (data not shown, but see Table 1; P > 0.05).
In summary, these data suggest that the firing rate of the majority of DMV neurons is modulated by tonic GABAergic inputs and that the firing rate of a large percentage of DMV neurons is also modulated by tonic glutamate inputs.
KYN modulates the firing rate of the majority of DMV neurons.
Basal firing rate of neurons that were tested with KYN was 1.04 ± 0.03 action potentials/s. Perfusion of the slice with 1 mM KYN altered the firing rate in 37 of 58 (64%) tested neurons, indicating that the firing rate of these DMV neurons is modulated by tonic glutamatergic inputs. In the remaining 21 (36%) neurons, perfusion with KYN did not alter their firing rate, indicating that this neuronal subpopulation is not under a tonic glutamatergic influence.
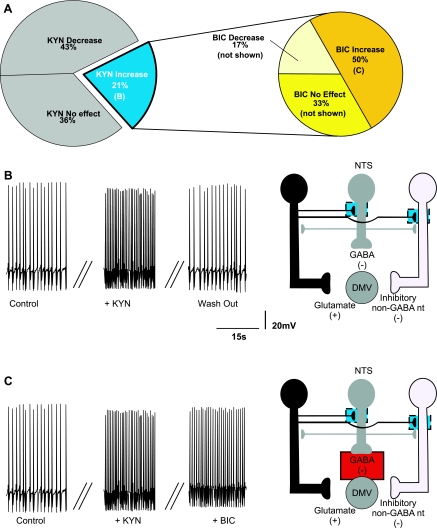
KYN decreased the firing rate in 9 (i.e., 24%; from 1.06 ± 0.07 to 0.3 ± 0.06 action potentials/s) and abolished it in 16 of the 37 (i.e., 43%; from 1.03 ± 0.07 to 0 ± 0 action potentials/s) responsive neurons (Fig. 5, A and B), indicating that tonic excitatory glutamate inputs affect, perhaps even control, the firing rate of DMV neurons. In the continuous presence of KYN, application of BIC increased the firing rate in 3 (i.e., 12%; from 0.3 ± 0.06 to 0.53 ± 0.08 action potentials/s; Fig. 5C), decreased it in 2 (i.e., 8%; from 0.33 ± 0.13 to 0.13 ± 0.13 action potentials/s Fig. 5D), and had no additional effect on 20 neurons (i.e., 80%; not shown). The proposed sites of action of KYN and BIC are depicted in Fig. 5, B–D (Table 2).
Fig. 5.
Pie charts (A), representative traces (B–D, left), and the proposed neuronal circuits (B–D, right) in neurons in which perfusion with KYN decreased the firing rate of DMV neurons. A: perfusion with KYN decreased (or abolished) the firing frequency of DMV neurons (N = 25), and BIC had no additional effect on the firing frequency in majority of these neurons. B: the decrease in firing rate induced by KYN is likely due to antagonism of glutamatergic receptors located on the membrane of the DMV neuron. C: perfusion with BIC in the presence of KYN increased the firing rate of DMV neurons (N = 3). This response was likely due to BIC-mediated inhibition of a tonic GABAergic input onto DMV neurons. D: perfusion with BIC in the presence of KYN further decreased the firing rate of DMV neurons (N = 2), suggesting the presence of tonic GABAergic synapses impinging onto non-GABAergic neurons.
Table 2.
Effect of kynurenic acid and bicuculline on the firing rate of DMV neurons
| Kynurenic Acid |
||||||
|---|---|---|---|---|---|---|
| All neurons |
Gastric-projecting neurons |
|||||
| Increase | No effect | Decrease | Increase | No effect | Decrease | |
| Bicuculline | ||||||
| Increase 15 (26%) | 6 | 6 | 3 | 2 | 3 | 1 |
| No effect 33 (57%) | 4 | 9 | 20 | 2 | 4 | 5 |
| Decrease 10 (22%) | 2 | 6 | 2 | 0 | 4 | 1 |
| Total | 12 (21%) | 21 (36%) | 25 (43%) | 4 (18.2%) | 11 (50.0%) | 7 (31.8%) |
Kynurenic acid was applied first, followed by bicuculline. Numbers represent the number of neurons that exhibited given responses to kynurenic acid (columns) and bicuculline (rows).
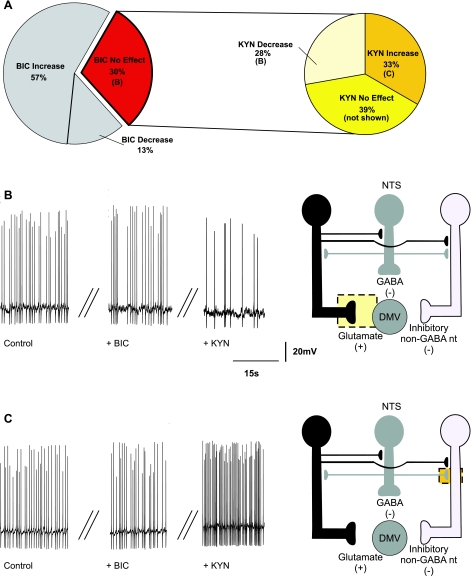
Perfusion with KYN increased the firing rate in 12 (32%) neurons (from 1.09 ± 0.04 to 2.03 ± 0.17 action potentials/s), suggesting that the firing rate of a subpopulation of DMV neurons is modulated by tonic glutamatergic inputs impinging on inhibitory synapses (Fig. 6). In these neurons, application of BIC further increased the firing rate in 6 (i.e., 50%; from 2.39 ± to 4.90 ± 0.1 action potentials/s; Fig. 6B), decreased it in 2 (i.e., 18%; from 1.77 ± 0.37 to 1.18 ± 0.08 action potentials/s), and did not affect the firing rate in 4 neurons (i.e., 32%). These data indicate that many of these DMV neurons are also under tonic GABAergic influence, whereas the BIC-induced decrease in firing rate suggests the presence of a non-GABAergic inhibitory neuron controlled by a glutamate synapse. The proposed sites of action of KYN and BIC are depicted in Fig. 6, B and C.
Fig. 6.
Pie charts (A), representative traces (B and C, left), and the proposed neuronal circuits (B and C, right) in neurons in which perfusion with KYN increased the firing rate of DMV neurons. A: perfusion with KYN increased the firing frequency of DMV neurons (N = 12) and perfusion with BIC further altered the firing frequency of 8 of these neurons. B: the increase in firing rate induced by KYN is likely determined by antagonism of tonically active glutamatergic receptors located on the membrane of either GABAergic or inhibitory non-GABAergic neurons. C: perfusion with BIC in the presence of KYN further increased the firing rate of DMV neurons (N = 6). This response was likely due to a BIC-mediated inhibition of a tonic GABAergic input onto DMV neurons.
Perfusion with KYN had no effect on the firing rate of 21 (i.e., 36%) DMV neurons (Fig. 7A), indicating that these neurons do not receive tonic glutamatergic inputs. The firing rate of these neurons was 0.98 ± 0.03 action potentials/s in control conditions and 0.99 ± 0.05 action potentials/s after application of KYN. In these neurons, further perfusion with BIC increased the firing rate in 6 (i.e., 29%; from 0.93 ± 0.13 to 2.23 ± 0.53 action potentials/s; Fig. 7B), decreased it in 6 (i.e., 29%; from 1.17 ± 0.18 to 0.72 ± 0.08 action potentials/s; Fig. 7C), and had no effect on the firing rate of the 9 remaining neurons (i.e., 42%; data not shown). These data suggest that the firing rate of neurons that do not receive tonic glutamatergic input is modulated by tonic GABA inputs impinging on either excitatory or inhibitory synapses. The proposed sites of action of BIC and KYN are depicted in Fig. 7, B and C.
Fig. 7.
Pie charts (A), representative traces (B and C, left), and the proposed neuronal circuits (B and C, right) in neurons in which perfusion with KYN did not affect the firing rate (N = 21) of DMV neurons. Perfusion with BIC in the presence of KYN either increased (N = 6) (B) or decreased (N = 6) (C) the firing rate of DMV neurons (N = 6). The increase in the firing frequency was likely due to BIC-mediated inhibition of a tonic GABAergic input onto DMV neurons. Perfusion with BIC in the presence of KYN decreased the firing rate of DMV neurons (N = 6), suggesting the presence of tonic GABAergic synapses impinging onto non-GABAergic neurons.
Twenty-two neurons that were tested with KYN and BIC were identified as gastric-projecting neurons. The percentage of gastric-projecting neurons that have their firing frequency modulated in response to KYN or BIC was not different from that of the unidentified neurons (P > 0.05).
In summary, these data demonstrated that the firing rate of the majority of DMV neurons is modulated by both tonic GABAergic and glutamatergic inputs. The finding that prior application of KYN decreased the percentage of neurons affected by BIC suggests that tonic GABAergic inputs affect DMV neurons through excitatory, glutamatergic interneurons. Moreover, the finding that KYN increases the firing rate of a subpopulation of DMV neurons, but that this increased activity was not affected by BIC, suggests that this subpopulation receives a tonic glutamatergic input that controls an inhibitory, non-GABAergic neuron, most likely utilizing catecholamines activating an α2 adrenoreceptor (unpublished data).
DISCUSSION
In this study, we have shown that, in a brain stem slice preparation, 1) the firing rate of most DMV neurons is modulated by tonic GABAergic and glutamatergic inputs and 2) there are several permutations in the arrangements of the GABA- and glutamatergic inputs onto DMV neurons.
These data support the concept of a brain stem neuronal circuitry that is arranged in a possibly organ- or function-specific, as well as in a finely tuned manner, which permits a precise modulation of the vagal motor output to the GI tract.
Our conclusions are based on the observation that, using an approach as simple as the sequential treatment with the GABAA receptor antagonist BIC and the nonselective ionotropic glutamate receptor antagonist KYN, we uncovered the presence of several different neuronal circuits in which BIC and KYN have excitatory, inhibitory, or no effects on DMV activity.
The dense synaptic network between the NTS and the DMV uses mainly GABA and glutamate, as well as catecholamines, as neurotransmitters (23, 31, 49, 56, 59) and serves as the final CNS locus for the control of vagal output to the subdiaphragmatic viscera. The complexity of the adaptive tasks necessary to provide an adequate control of bodily homeostasis is achieved by integrated levels of modulation that require coordinated actions at many sites along the brain-gut axis. The diverse arrangements of these synaptic connections between GABAergic and glutamatergic neurons in NTS-DMV circuits ensure that the majority of parasympathetic homeostatic functions can be regulated by DMV neurons by use of only two simple neurotransmitters. We hypothesize that subtle differences, such as those obtained by variations in synapses impinging on DMV neurons, e.g., tonic vs. phasic, glutamate vs. GABA, represent a metabolically inexpensive means by which vagal output to the GI tract can be precisely tuned to ever-changing physiological or environmental cues.
Indeed, it is not too far-reaching to assume that autonomic brain stem circuits may have multiple levels of pathway specificity, from the sensory neurons and terminals, to second-order NTS and motoneurons of the DMV to the effector organ, encompassing all aspects of the vagovagal circuit. Such a concept implies that every autonomic function is controlled by distinct sets of neurons, each of which is committed to one particular type of response and comprises a “dedicated” neuronal circuit. This type of neuronal organization may represent specialization or segregation into specific functional lines and implies a “task matching” capability in which subsets of parasympathetic (and sympathetic) brain stem neurons integrate vital cardiac, respiratory, and GI functions. This concept of task matching organization is supported by solid evidence, particularly with regard to NTS neurons that receive inputs from baroreceptor sensitive afferent fibers of the aortic depressor nerve (5–7, 26, 39, 43). With respect to specifically GI circuits and mechanisms, this concept is starting to receive attention and is supported by both anatomical and physiological data (reviewed in Refs. 20, 57).
The in vivo observations of Sivarao and colleagues (53), in which the significant increase of gastric motility obtained by microinjection of BIC in the DVC was blocked by microinjection of KYN, was essential in providing evidence of the glutamatergic modulation of a tonic GABAergic input to DMV neurons. Furthermore, Mussa and Verberne (44) reported that, as with vagal control of gastric tone, pancreatic exocrine secretion is also held in check by GABAergic tonic inputs onto brain stem vagal motoneurons. When transposed to the brain stem slice preparation, these in vivo observations predict that 1) perfusion of the slice with BIC increases the firing rate of DMV neurons; 2) KYN perfusion has no effect on the firing rate of DMV neurons; and, 3) BIC would have been ineffective when perfused in the presence of KYN.
Our results suggest that these hypothetical scenarios can, indeed, be reproduced in the brain stem slice preparation. Our results also suggest, however, that the aforementioned scenarios should not be taken as the only potential circuitry arrangements, as we have uncovered the presence of synaptic arrangements that would not explain the in vivo results of Sivarao et al. (53) and Mussa and Verberne (44). In particular, we should point out that the data obtained from labeled gastric-projecting and those obtained from unlabeled, but most likely gastric-projecting, DMV neurons have revealed a similar synaptic arrangement between the two groups. This observation would then argue in favor of the generalized use of the brain stem slice preparation as a model to study the synaptic arrangement of the GI vagal circuits.
Although several differences between the in vivo and in vitro experimental approaches have to be taken into consideration, the most likely explanation of the several permutations in the slice preparation is that DMV neurons with different functions, for example those devoted to the modulation of gastric motility vs. those devoted to the control of gastric acid secretion, respond differently to BIC and KYN because distinct circuits serve diverse functions. As yet, neuronal tracing techniques cannot distinguish between different physiological functions such as, for example, gastric motility vs. secretion or pancreatic endocrine vs. exocrine secretion; therefore most likely the labeled DMV neurons represent a functionally mixed neuronal population.
It has also to be taken into consideration that in vivo studies report the physiological outcome of effects on several neuronal populations, which may include inhibitory, excitatory, and unaffected single neurons. Conversely, the present study measured the firing rate of single DMV neurons identified as per their target organ, but not their function. The correlation between neuronal responses to BIC and KYN with their GI function has still to be investigated.
In the present study, we demonstrated that tonic GABA and glutamate inputs have different effects on subpopulations of DMV neurons, suggesting that these neurons contain distinct complements of GABAA and glutamate receptors, as suggested previously (12, 31, 40, 41). Note that we examined only ionotropic receptors; the presence of GABA and glutamate metabotropic receptors in vagal brain stem circuits (17, 21, 35, 38, 47, 48, 60) adds another potentially larger level of complexity. Indeed, in the present study we did not examine the contribution of metabotropic receptors to the basal firing rate of DMV neurons and thus we cannot exclude their activation; however, we did not observe any significant effect on the DMV membrane induced by the GABAB antagonist saclofen (17) or the metabotropic group II and III antagonists EGLU and AP-4 (21). Evidence from several groups implicates catecholamines, particularly norepinephrine (NE), as an important neurotransmitter between NTS and DMV (10, 36, 42, 49). Acting via α2 adrenoreceptors, NE may well be the non-GABAergic inhibitory neurotransmitter proposed in the present study. In fact, perfusion with the α2 adrenoceptor antagonist yohimbine partially decreased the firing rate in a subset of DMV neurons pretreated with KYN (unpublished observations). These α2 adrenoceptor-mediated effects, however, were not observed in DMV neurons perfused with yohimbine only (42), indicating that catecholaminergic neurotransmission onto the DMV may be driven through other, yet undefined, synapses.
The diverse arrangements of these GABA and glutamate ionotropic circuits may also contribute to modulate sensitivity of DMV neurons to other neurotransmitters. Indeed, several neurotransmitters preferentially affect either excitatory or inhibitory currents in the DMV. Met-enkephalin and pancreatic polypeptides, for example, alter the glutamatergic currents onto DMV neurons but have no effect on GABAergic inputs (14, 19), unless the appropriate conditions ensue (15, 22). Similarly, perfusion of DMV neurons with cannabinoids appear to produce a depolarization-induced suppression of inhibitory GABAergic but not excitatory glutamatergic currents (51). The presence or absence of GABAA and/or glutamate receptors on a particular neuron could, therefore, dictate whether that neuron would respond to other neurotransmitters. Since both GABA and glutamate inputs converge and modulate the activity of single DMV neurons, this implies that the activity of a given brain stem circuit is also differentially regulated by the discrete distribution of the several neurotransmitters' receptors.
The convergence of tonic or phasic GABA and glutamate inputs onto DMV neurons when combined with the different expression of receptor subtypes may, therefore, provide a metabolically inexpensive basis for the integration of inputs from multiple areas of the CNS or directly from the visceral organs onto single DMV neurons.
Although there is undisputable evidence of glutamatergic synapses impinging on GABAergic neurons in NTS (5, 32), these glutamatergic inputs from the tractus solitarius onto the NTS neurons are unlikely to be those responsible for the observed effects of KYN. In fact, contrary to cardiovascular afferent fibers comprising the baroreflex, GI vagal fibers impinging onto NTS neurons are rarely observed to have a baseline tonic activity (11) sufficient to drive the activity of NTS-DMV connections and fully activate vagovagal reflexes.
In summary, we have demonstrated that, in a brain stem slice preparation, tonic excitatory (glutamate) and inhibitory (GABA) inputs modulate the firing rate of the majority of DMV neurons via actions at ionotropic receptors. Furthermore, different combinations of excitatory and inhibitory synaptic inputs results in dramatically different effects of glutamate and GABA on DMV neuronal excitability.
The present data support the hypothesis that brain stem circuitry may be organized, possibly in an organ-specific or function-specific manner, in such a way as to permit precise modulation of vagal motor output to discrete visceral areas. Such a neuronal arrangement strongly supports the application of the concept of task matching organization to the functional brain stem circuits that control GI functions.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank the National Institutes of Health (DK 55530) and National Science Foundation (IBN-08-18736) for their support. We also thank Cesare M. and Zoraide Travagli, and W. Nairn Browning for support and encouragement.
REFERENCES
- 1. Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283: 248–268, 1989 [DOI] [PubMed] [Google Scholar]
- 2. Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol Heart Circ Physiol 259: H1307–H1311, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Aylwin ML, Horowitz JM, Bonham AC. NMDA receptors contribute to primary visceral afferent transmission in the nucleus of the solitary tract. J Neurophysiol 77: 2539–2548, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol 59: 814–824, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS). J Neurophysiol 99: 1712–1722, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Bailey TW, Hermes SM, Whittier KL, Aicher SA, Andresen MC. A-type potassium channels differentially tune afferent pathways from rat solitary tract nucleus to caudal ventrolateral medulla or paraventricular hypothalamus. J Physiol 582: 613–628, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailey TW, Jin YH, Doyle MW, Andresen MC. Vanilloid-sensitive afferents activate neurons with prominent A-type potassium currents in nucleus tractus solitarius. J Neurosci 22: 8230–8237, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baptista V, Zheng ZL, Coleman FH, Rogers RC, Travagli RA. Characterization of neurons of the nucleus tractus solitarius pars centralis. Brain Res 1052: 139–146, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barraco R, El-Ridi M, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull 29: 703–765, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Bertolino M, Vicini S, Gillis RA, Travagli RA. Presynaptic α2-adrenoceptors inhibit excitatory synaptic transmission in rat brain stem. Am J Physiol Gastrointest Liver Physiol 272: G654–G661, 1997. [DOI] [PubMed] [Google Scholar]
- 11. Beyak MJ, Bulmer DCE, Jiang W, Keating C, Rong W, Grundy D. Extrinsic sensory afferent nerves innervating the gastrointestinal tract. In: Physiology of the Gastrointestinal Tract (4th ed.), edited by Johnson LR. Burlington, MA: Elsevier Academic, 2006, chapt. 25, p. 685–726 [Google Scholar]
- 12. Broussard DL, Li H, Altschuler SM. Colocalization of GABA A and NMDA receptors within the dorsal motor nucleus of the vagus nerve (DMV) of the rat. Brain Res 763: 123–126, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Browning KN, Coleman FH, Travagli RA. Characterization of pancreas-projecting rat dorsal motor nucleus of the vagus neurons. Am J Physiol Gastrointest Liver Physiol 288: G950–G955, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Browning KN, Kalyuzhny AE, Travagli RA. Opioid peptides inhibit excitatory but not inhibitory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Neurosci 22: 2998–3004, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Browning KN, Kalyuzhny AE, Travagli RA. Mu-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci 24: 9344–9352, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J Physiol 517: 521–532, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Browning KN, Travagli RA. Mechanism of action of baclofen in rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 280: G1106–G1113, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Browning KN, Travagli RA. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol 531: 425–435, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Browning KN, Travagli RA. Neuropeptide Y and peptide YY inhibit excitatory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Physiol 549: 775–785, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Browning KN, Travagli RA. Short-term receptor trafficking in the dorsal vagal complex: an overview. Auton Neurosci 126–127: 2–8, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Browning KN, Travagli RA. Functional organization of presynaptic metabotropic glutamate receptors in vagal brainstem circuits. J Neurosci 27: 8979–8988, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Browning KN, Travagli RA. Modulation of inhibitory neurotransmission in brainstem vagal circuits by NPY and PYY is controlled by cAMP levels. Neurogastroenterol Motil 21: 1309–e126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res 1017: 208–217, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis SF, Williams KW, Xu W, Glatzer NR, Smith BN. Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: implications for autonomic regulation. J Neurosci 23: 3844–3854, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Derbenev AV, Stuart TC, Smith BN. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J Physiol 559: 923–938, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fan W, Andresen MC. Differential frequency-dependent reflex integration of myelinated and nonmyelinated rat aortic baroreceptors. Am J Physiol Heart Circ Physiol 275: H632–H640, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Feng HS, Lynn RB, Han J, Brooks FP. Gastric effects of TRH analogue and bicuculline injected into dorsal motor vagal nucleus in cats. Am J Physiol Gastrointest Liver Physiol 259: G321–G326, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Ferreira M, Jr, Browning KN, Sahibzada N, Verbalis JG, Gillis RA, Travagli RA. Glucose effects on gastric motility and tone evoked from the rat dorsal vagal complex. J Physiol 536: 141–152, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fox EA, Powley TL. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res 341: 269–282, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Fox EA, Powley TL. Morphology of identified preganglionic neurons in the dorsal motor nucleus of the vagus. J Comp Neurol 322: 79–98, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Gao H, Smith BN. Tonic GABAA receptor-mediated inhibition in the rat dorsal motor nucleus of the vagus. J Neurophysiol 103: 904–914, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glatzer NR, Derbenev AV, Banfield BW, Smith BN. Endomorphin-1 modulates intrinsic inhibition in the dorsal vagal complex. J Neurophysiol 98: 1591–1599, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Grabauskas G, Zhou SY, Das S, Lu Y, Owyang C, Moises HC. Prolactin-releasing peptide affects gastric motor function in rat by modulating synaptic transmission in the dorsal vagal complex. J Physiol 561: 821–839, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo JJ, Browning KN, Rogers RC, Travagli RA. Catecholaminergic neurons in rat dorsal motor nucleus of vagus project selectively to gastric corpus. Am J Physiol Gastrointest Liver Physiol 280: G361–G367, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Hay M, McKenzie H, Lindsley K, Dietz N, Bradley SR, Conn PJ, Hasser EM. Heterogeneity of metabotropic glutamate receptors in autonomic cell groups of the medulla oblongata of the rat. J Comp Neurol 403: 486–501, 1999 [PubMed] [Google Scholar]
- 36. Herman MA, Niedringhaus M, Alayan A, Verbalis JG, Sahibzada N, Gillis RA. Characterization of noradrenergic transmission at the dorsal motor nucleus of the vagus involved in reflex control of fundus tone. Am J Physiol Regul Integr Comp Physiol 294: R720–R729, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Jarvinen MK, Powley TL. Dorsal motor nucleus of the vagus neurons: a multivariate taxonomy. J Comp Neurol 403: 359–377, 1999 [PubMed] [Google Scholar]
- 38. Jin YH, Bailey TW, Andresen MC. Cranial afferent glutamate heterosynaptically modulates GABA release onto second-order neurons via distinctly segregated metabotropic glutamate receptors. J Neurosci 24: 9332–9340, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci 24: 4709–4717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kessler JP, Baude A. Distribution of AMPA receptor subunits GluR1–4 in the dorsal vagal complex of the rat: a light and electron microscope immunocytochemical study. Synapse 34: 55–67, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Laccasagne O, Kessler JP. Cellular and subcellular distribution of the amino-3-hydroxy-5-metyl-4-isoxazole propionate receptor subunit GluR2 in the rat dorsal vagal complex. Neuroscience 99: 557–563, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Martinez-Pena y Valenzuela IM, Rogers RC, Hermann GE, Travagli RA. Norepinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 286: G333–G339, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McDougall SJ, Peters JH, Andresen MC. Convergence of cranial visceral afferents within the solitary tract nucleus. J Neurosci 29: 12886–12895, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mussa BM, Verberne AJ. Activation of the dorsal vagal nucleus increases pancreatic exocrine secretion in the rat. Neurosci Lett 433: 71–76, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Niedringhaus M, Jackson PG, Evans SR, Verbalis JG, Gillis RA, Sahibzada N. Dorsal motor nucleus of the vagus: a site for evoking simultaneous changes in crural diaphragm activity, lower esophageal sphincter pressure, and fundus tone. Am J Physiol Regul Integr Comp Physiol 294: R121–R131, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol 273: 207–223, 1988 [DOI] [PubMed] [Google Scholar]
- 47. Page AJ, Young RL, Martin CM, Umaerus M, O'Donnell TA, Cooper NJ, Coldwell JR, Hulander M, Mattsson JP, Lehmann A, Blackshaw LA. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology 128: 402–410, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Partosoedarso ER, Young RL, Blackshaw LA. GABAB receptors on vagal afferent pathways: peripheral and central inhibition. Am J Physiol Gastrointest Liver Physiol 280: G658–G668, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol 285: R479–R489, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rossiter CD, Norman WP, Jain M, Hornby PJ, Benjamin SB, Gillis RA. Control of lower esophageal sphincter pressure by two sites in dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 259: G899–G906, 1990 [DOI] [PubMed] [Google Scholar]
- 51. Roux J, Wanaverbecq N, Jean A, Lebrun B, Trouslard J. Depolarization-induced release of endocannabinoids by murine dorsal motor nucleus of the vagus nerve neurons differentially regulates inhibitory and excitatory neurotransmission. Neuropharmacology 56: 1106–1115, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol 238: 473–488, 1985 [DOI] [PubMed] [Google Scholar]
- 53. Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil 10: 305–313, 1998 [DOI] [PubMed] [Google Scholar]
- 54. Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol 512: 149–162, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Travagli RA, Gillis RA. Hyperpolarization-activated currents IH and IKIR, in rat dorsal motor nucleus of the vagus neurons in vitro. J Neurophysiol 71: 1308–1317, 1994 [DOI] [PubMed] [Google Scholar]
- 56. Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 260: G531–G536, 1991 [DOI] [PubMed] [Google Scholar]
- 57. Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wan S, Browning KN, Travagli RA. Glucagon-like peptide-1 modulates synaptic transmission to identified pancreas-projecting vagal motoneurons. Peptides 28: 2184–2191, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Willis A, Mihalevich M, Neff RA, Mendelowitz D. Three types of postsynaptic glutamatergic receptors are activated in DMNX neurons upon stimulation of NTS. Am J Physiol Regul Integr Comp Physiol 271: R1614–R1619, 1996 [DOI] [PubMed] [Google Scholar]
- 60. Young RL, Cooper NJ, Blackshaw AL. Anatomy and function of group III metabotropic glutamate receptors in gastric vagal pathways. Neuropharmacology 54: 965–975, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Zhou SY, Lu YX, Yao H, Owyang C. Spatial organization of neurons in the dorsal motor nucleus of the vagus synapsing with intragastric cholinergic and nitric oxide/VIP neurons in the rat. Am J Physiol Gastrointest Liver Physiol 294: G1201–G1209, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]