Abstract
Heme oxygenase-1 (HO-1) induction by hemin or Panhematin protects against experimental pancreatitis. As a preclinical first step toward determining whether HO-1 upregulation is a viable target in acute pancreatitis (AP) patients, we tested the hypothesis that HO-1 expression in peripheral blood mononuclear cell (PBMC) subsets of hospitalized patients with mild AP is upregulated then normalizes upon recovery and that cells from AP patients have the potential to upregulate their HO-1 ex vivo if exposed to Panhematin. PBMCs were isolated on days 1 and 3 of hospitalization from the blood of 18 AP patients, and PMBC HO-1 levels were compared with PMBCs of 15 hospitalized controls (HC) and 7 volunteer healthy controls (VC). On day 1 of hospitalization, AP patients compared with VCs had higher HO-1 expression in monocytes and neutrophils. Notably, AP monocyte HO-1 levels decreased significantly upon recovery. Panhematin induced HO-1 in ex vivo cultured AP PBMCs more readily than in HC or VC PBMCs. Furthermore, PBMCs from acutely ill AP patients on day 1 were more responsive to HO-1 induction compared with day 3 upon recovery. Similarly, mouse splenocytes had enhanced HO-1 inducibility as their pancreatitis progressed from mild to severe. In conclusion, AP leads to reversible PBMC HO-1 upregulation that is associated with clinical improvement and involves primarily monocytes. Leukocytes from AP patients or mice with AP are primed for HO-1 induction by Panhematin, which suggests that Panhematin could offer a therapeutic benefit.
Keywords: Panhematin
acute pancreatitis (AP) can follow a severe course that leads to 10–30% mortality in high-risk patients and, in the United States alone, it accounts for over 220,000 hospital admission every year (34). The most common risk factors for developing AP include alcohol and gallstones. Other risk factors include diagnostic and therapeutic instrumentation of the biliary and/or pancreatic ducts such as endoscopic retrograde cholangiopancreatography (ERCP) (34). To date, despite the significant morbidity, mortality, and cost incurred, no effective active treatment aside from supportive therapy (e.g., fluid resuscitation) exists. Thus better understanding of the mechanism(s) of disease and the characterization of potential therapeutic targets are of high priority in the field.
Heme oxygenase-1 (HO-1) is a rate-limiting enzyme that catabolizes heme and is important for the maintenance of iron homeostasis, regulation, and suppression of inflammatory responses (9, 15, 19, 22, 23, 28, 29, 32). Induction of HO-1 expression using hemin or Panhematin (PH), the latter being an approved hemin formulation for the treatment of porphyrias (1, 14), protects mice from pancreatic injury in experimental pancreatitis models (21). Intravenous hematin has been used clinically to treat patients with acute porphyrias for the past four decades, and PH was the first Food and Drug Administration agency-approved formulation in the United States (27). Other formulations, such as heme arginate, have been used in Europe to treat patients with acute porphyrias and other hematological disorders such as myelodysplastic syndromes (10, 20, 26, 33). More recently, parenteral hemin administration to healthy individuals was shown to induce plasma HO-1 levels (5).
HO-1 induction correlates with the suppression of inflammatory responses (4, 16, 19, 22, 25). The anti-inflammatory effects of HO-1 are believed to be mediated by carbon monoxide and biliverdin, the end products of heme degradation through HO-1-mediated catalysis (22). We previously showed that hemin upregulates HO-1 expression in monocytes and ameliorates pancreatic injury in mouse models of AP (21). Although HO-1 upregulation occurs in the context of experimental pancreatitis, the extent of upregulation is significantly more robust upon administration of hemin (21) or PH (unpublished observations) which is likely contributing to the protective effect. More recently, Chen et al. (6) showed that a carbon monoxide releasing compound protected rats from sodium taurocholate-induced pancreatitis. In addition, induction of HO-1 was shown to improve pancreatic graft survival in a rat model of pancreatic transplantation (4). On the basis of these findings, we reasoned that HO-1 induction in leukocyte populations may play a role in the resolution of human AP and investigated the expression of HO-1 over time in patients with AP compared with controls.
As a preclinical first step toward targeting HO-1 as a potential therapeutic intervention in human AP, we investigated the expression and ex vivo inducibility of HO-1 in PMBCs of hospitalized patients with AP compared with hospitalized control (HC) patients who were admitted to the hospital because of etiologies unrelated to pancreatic disease. We also included healthy volunteer controls (VC) as an additional study group. HO-1 levels were assessed in various leukocyte subsets as patients recovered from their AP. In addition, using an experimental model of AP we examined the ex vivo inducibility of mouse splenocyte HO-1 during the course of pancreatitis in response to PH.
MATERIALS AND METHODS
Patients.
A total of 33 patients (18 AP and 15 HC) admitted to either Stanford Hospital, Palo Alto Veteran Affairs Hospital, or Santa Clara Valley Medical Center, and 7 volunteer controls (VC) of similar age and sex were recruited. The VC group consisted of healthy nonhospitalized volunteers. Patients with past history of AP or chronic pancreatitis were excluded. The diagnosis of AP was confirmed by biochemical assessment (serum lipase levels) and in some with additional abdominal imaging by computed tomography (CT). Clinical severity of AP was evaluated by use of Ranson's criteria, a clinical and laboratory method that assesses inflammation or organ failure (e.g., Ranson's score ≥3 indicates a likelihood of severe pancreatitis) (24, 34). CT-based scoring (Balthazar) that takes into account extent of pancreatic inflammation and necrosis was also used in those with CT scans (2). The patient-related arm of the study was approved by the involved institutional review boards.
Reagents.
Antibodies directed to CD19, CD3, CD14, and CD11b were purchased from Becton Dickinson (San Diego, CA). HO-1-FITC for intracellular HO-1 staining and polyclonal HO-1 and HO-2 antibodies were purchased from Stressgen (Victoria, BC, Canada). PH was obtained from Ovation Pharmaceuticals (Deerfield, IL).
Cell isolation and in vitro cultures.
PBMCs were isolated by density centrifugation from blood samples of patients or volunteer controls using Histopaque-1119 (Sigma; St. Louis, MO). For in vitro cultures, cells were resuspended in RPMI-1640 medium supplemented with 10% fetal bovine serum and incubated at 37 °C for 16, 24, or 48 h. Cells were either treated with vehicle alone or with PH, equivalent to the optimal concentration of 100 μM hemin. (7)
Induction of experimental pancreatitis.
Female FVB/n mice (purchased from Taconic; 15–19 g) were fasted for 12 h and then fed a choline-deficient diet (Harlan Teklad) supplemented with 0.5% dl-ethionine (Sigma-Aldrich) (CDE) or normal chow (control group) for 1, 2, or 3 days. Blood was collected from mice by cardiac puncture, and then serum was isolated for subsequent lipase determination using Becton Dickinson Microtainer Serum Separator Tubes. To assess pancreatic injury, mouse pancreata were fixed in 10% neutral buffered formalin. Pancreata were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The histological score and grading were based on edema, inflammation, hemorrhage, and parenchymal necrosis as described previously (21). The animal protocols were approved by the Institutional Animal Care and Use Committee.
Flow cytometry.
PBMCs were stained with antibodies to CD19 (B cells), CD3 (T cells), and CD14 and CD11b (monocytes). PBMCs were subsequently fixed and permeabilized by use of Becton Dickinson Cytofix/Cytoperm and stained for intracellular HO-1. Stained samples were analyzed by flow cytometry using a Becton Dickinson LSRII (Supplementary Fig. S1; the online version of this article contains supplemental data). Rainbow Calibration Particles from Spherotech (Lake Forest, IL) were run after each flow cytometry session. HO-1 mean fluorescence intensity (MFI) values for leukocyte subsets were normalized by using Rainbow Calibration Particles to allow for the comparison of data from multiple flow cytometry sessions. Analysis of flow cytometry raw data was performed via Flowjo software (Treestar, Ashland, OR).
Western blotting.
PBMCs were lysed in a homogenization buffer (187 mM Tris, 3% SDS, 5 mM EDTA) at a cell density of 1 × 107 cells/50 μl. Protein concentration was determined by the BCA method. Equal amounts of protein were loaded followed by SDS-PAGE then transfer to polyvinylidene difluoride membranes. Western blotting was performed, and membranes were incubated with antibodies to HO-2 (which is constitutively expressed) as a loading control or to HO-1. Equal loading was also confirmed by Coomassie staining of the membranes.
Statistical analysis.
Data were analyzed with SAS version 9.1 (Cary, NC). To account for small sample sizes and nonnormal distribution of data, Fisher's exact test, the Mann-Whitney U-test, and the Wilcoxon's signed rank test were used for categorical data, continuous data, and paired data, respectively. All P values are two tailed, and a value of 0.05 was considered statistically significant.
RESULTS
Demographics of patients and healthy volunteers.
There were no differences in sex, age, or ethnicity between the AP (n = 18) and HC (n = 15) patients and the VC group (n = 7) (Table 1). AP patients were admitted with abdominal pain and elevated lipase levels that were at least three times the upper normal limit. In addition, some of the patients had CT evaluation as part of their admission workup with findings consistent with AP. The majority of the pancreatitis cases were thought to be due to gallstones, and, based on the CT Severity Index (Balthazar Score; mean score of 2.4, Table 2) and clinical status, the pancreatitis was categorized as mild. The CT severity index correlates well with the development of local complications and mortality associated with AP (2). Patients with a past history of pancreatic disease (e.g., previous AP or chronic pancreatitis) were excluded. The diagnoses of the HC group are described in Table 3, and only a minority of the patients had “inflammatory” conditions.
Table 1.
Patient demographics
| VC | HC | AP | P Value | |
|---|---|---|---|---|
| Number of subjects | 7 | 15 | 18 | Not applicable |
| Sex, male/female | 4/3 | 9/6 | 8/10 | 0.54 |
| Age, yr | 47 ± 10 | 47 ± 12 | 46 ± 16 | 0.95 |
| Ethnicity, White/Nonwhite | 3/4 | 11/4 | 9/9 | 0.53 |
VC, volunteer controls; HC, hospitalized controls; AP, acute pancreatitis patients. Statistical analysis is based on Fisher's Exact Test for sex and ethnicity and Mann-Whitney U-Test for age.
Table 2.
Characteristics of AP patients
| Cause of pancreatitis (n = 18) | |
| Gallstone, no. of patients | 10 |
| Alcohol, no. of patients | 3 |
| Hypertriglyceridemia, no. of patients | 1 |
| Post-ERCP, no. of patients | 1 |
| Unknown, no. of patients | 3 |
| Disease severity | |
| CT severity index (Balthazar score) | 2.4 (0–6) |
| Biochemical data | |
| Lipase (normal range: 13-63) | 2,660 (200–10,200) |
ERCP, endoscopic retrograde cholangiopancreatography; CT, computed tomography.
Table 3.
Diagnosis of HC patients
| Diagnosis | Number of Patients |
|---|---|
| Upper gastrointestinal bleed | 3 |
| Congestive heart failure | 2 |
| Cellulitis | 2 |
| Amyotrophic lateral sclerosis | 2 |
| Metastatic renal cell carcinoma | 1 |
| Ischial ulcer | 1 |
| Neurosyphilis | 1 |
| Drug overdose | 1 |
| Liver abscess | 1 |
| Cholelithiasis | 1 |
AP patients' PBMCs express higher levels of HO-1 compared with the HC and VC groups.
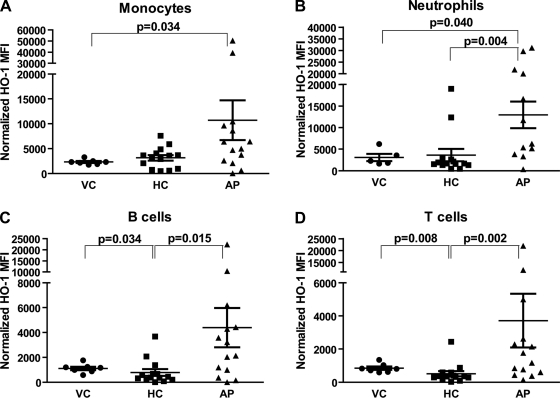
Patients with AP had higher level of circulating monocyte (mean 10,718; range 44–50,400 arbitrary normalized MFI units) and neutrophil (mean 12,951; range 333–31,200 arbitrary normalized MFI units) HO-1 expression compared with monocytes (mean 2,311; range 1,747–3,281 arbitrary normalized MFI units, P = 0.034) and neutrophils (mean 3,085; range 1,616–3,575 arbitrary normalized MFI units, P = 0.040) of VCs (Fig. 1, A and B). AP patients also had higher level of neutrophil, T cell (mean 3,716; range 149–21,990 arbitrary normalized MFI units), and B cell (mean 4,387; range 0–22,440 arbitrary normalized MFI units) HO-1 levels compared with neutrophils (mean 3,634; range 465–18,979 arbitrary normalized MFI units, P = 0.004), T cells (mean 514; range 19–2,443 arbitrary normalized MFI units, P = 0.002), and B cells (mean 786; range 0–3,680 arbitrary normalized MFI units, P = 0.015) from HC patients (Fig. 1, B–D). Although monocyte HO-1 levels were higher in AP (mean 10,718; range 44–50,400 arbitrary normalized MFI units) as opposed to monocytes (mean 3,153; range 465–7,575 arbitrary normalized MFI units, P = 0.051) from HC patients, it did not reach statistical significance. VC patients, compared with HCs, had higher T and B cell but not monocyte or neutrophil HO-1 levels (Fig. 1, A–D).
Fig. 1.
Leukocyte subset expression of heme oxygenase-1 (HO-1) using fluorescence-activated cell sorting (FACS) analysis. Peripheral blood mononuclear cells (PBMCs; obtained from patients on the first day of admission) were stained for leukocyte-specific [monocytes (A), neutrophils (B), B cells (C), T cells (D)] surface markers as described in materials and methods and then stained for intracellular HO-1. The cells were analyzed by FACS and corrected for isotype control antibody and normalized by using Spherotech beads to allow comparison between patients. Statistical analysis was done via the Mann-Whitney U-test. VC, volunteer controls; HC, hospitalized controls; AP, acute pancreatitis; MFI, mean fluorescence intensity.
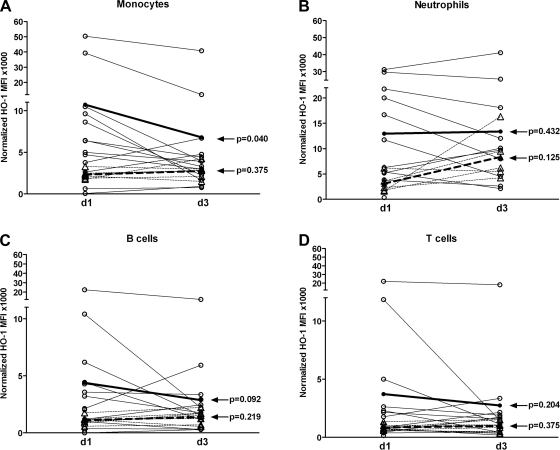
Clinical improvement in AP patients is associated with a significant decrease in monocyte HO-1 levels.
PBMCs HO-1 levels were assayed at day 3 following admission to determine whether there were changes in HO-1 expression over time. At day 3, no change in neutrophil, T cell, or B cell HO-1 levels were detected in AP patients or VCs (Fig. 2, B–D). However, there was significant decrease in circulating monocyte HO-1 levels from day 1 to day 3 in AP (mean 6,797; range 754–40,800 arbitrary normalized MFI units, P = 0.040) patients as opposed to levels from day 1 to day 3 in VCs (mean 2,745; range 1,495–2,978 arbitrary normalized MFI units, P = 0.038) (Fig. 2A). This decrease in HO-1 level was associated with recovery, since all patients with AP recovered by day 3 as determined by improvement of lipase levels and clinical symptoms.
Fig. 2.
Leukocyte subset expression of HO-1 over time. PBMCs were obtained from VC and AP patients on day 1 (d1) and day 3 (d3) and stained for leukocyte surface markers and intracellular HO-1. The cells were then analyzed by FACS and corrected for isotype control antibody and normalized by use of Spherotech beads to allow comparison between individuals and over time. Samples from VC are shown in broken lines with mean values shown in bold broken bold line; samples from AP are shown in solid lines with mean values shown in bold solid line. Statistical analysis (day 1 vs. day 3) was based on the Wilcoxon's signed-rank test. A: monocytes. B: neutrophils. C: B cells. D: T cells.
Clinical presentation and correlation with HO-1 levels.
AP patients reported nonspecified and vague abdominal pain ranging in duration between 2 days to 3 mo prior to the day of admission. The patients presented to hospital as their abdominal pain either worsened or had additional associated symptoms such as nausea or vomiting. The only patient we could definitively determine the onset of pancreatitis-related pain with relative accuracy was the patient with post-ERCP pancreatitis. This patient had the highest level of HO-1. Consistent with our cohort of AP patients having mild disease, only one and two of the patients met three and two of admission's Ranson criteria, respectively. The remaining AP patients met either one or none of Ranson's criteria. We did not find a correlation between admission Ranson's criteria and leukocyte HO-1 levels (data not shown). In addition, we did not find a correlation between lipase and leukocyte HO-1 levels (Spearman correlation coefficient 0.26–0.34, P = 0.23–0.36). The two highest HO-1 levels in the HC patients were in a patient with neurosyphilis and a patient with metastatic renal cell carcinoma, likely reflecting some degree of inflammatory response.
HO-1 inducibility in response to PH is greater in PBMCs of AP patients compared with HC patients and VC individuals.
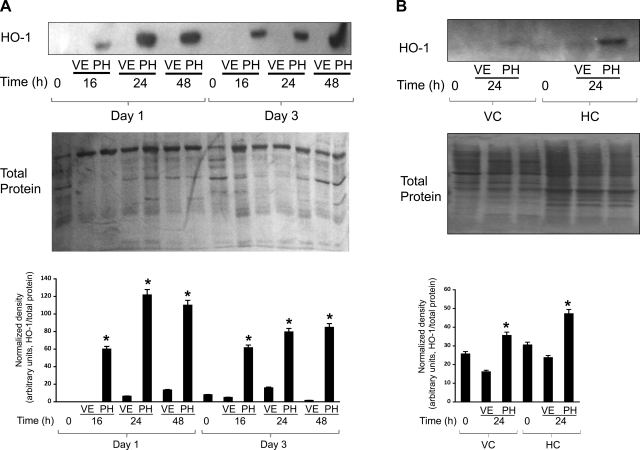
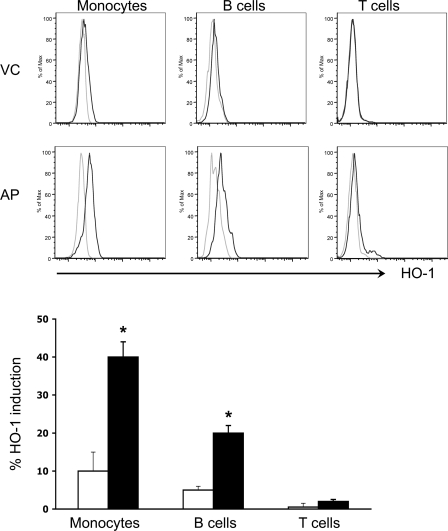
Hemin treatment in mice induces HO-1 in various tissues such as the liver, spleen, colon, and pancreas (21). HO-1+ monocytes are recruited to the pancreas following hemin treatment and confer protection against experimental pancreatitis (21). Thus, as a first step toward considering the targeting of HO-1 as a potential therapeutic intervention in human AP, we investigated whether HO-1 in PBMCs can be induced ex vivo upon treatment with PH. If so, this approach may offer a potential therapy because it suggests that an infusion of PH in this setting would result in further upregulation of HO-1 or, alternatively, that isolation of leukocytes from patients followed by priming with PH to upregulate HO-1 may offer a potential autologous cell-based therapy. PBMCs from AP patients at day 1 readily upregulated HO-1 following 16-, 24-, and 48-h culture with PH (Fig. 3A). Maximal HO-1 upregulation was seen after 24-h culture. The inducibility of HO-1 in response to PH was less during recovery or at day 3 (Fig. 3A). In contrast, minimal HO-1 induction by PH was seen in HC patients or VCs at day 1 following 24-h culture (Fig. 3B). Similarly, analysis by flow cytometry revealed higher HO-1 induction by PH in cultured PBMCs of AP patients as opposed to those of VCs (Fig. 4). This increase in HO-1 expression was highest in monocyte followed by B cell subsets. There was no change in T cell HO-1 levels.
Fig. 3.
Panhematin (PH)-mediated HO-1 induction in AP patients, HC patients, and VC PBMCs. A: PBMCs from AP patient blood samples obtained on hospital admission day 1 and day 3 were isolated by density centrifugation. PBMCs were cultured for 16, 24, or 48 h with PH or vehicle (VE) alone. B: PBMCs were isolated from VC healthy individuals and HC patients and cultured for 24 h as in A. For both A and B, cultured PBMCs were lysed in a homogenization buffer as described in materials and methods, and 10 μg total protein from each sample was separated by SDS-PAGE for Western blotting to compare HO-1 levels. Duplicate gels were also stained with Coomassie blue to confirm equal protein loading. Bar graphs show HO-1 density quantification normalized to protein loaded. *P < 0.05 compared with VE.
Fig. 4.
PH-mediated HO-1 induction in leukocyte subsets from AP patients and healthy VC. PBMCs from AP patients at the time of admission and from VC donors were isolated by density centrifugation. PBMC were cultured for 24 h with either PH or VE alone. The cells were subsequently stained for leukocyte surface markers followed by staining for intracellular HO-1. The immune-stained cells were then analyzed by FACS. Histograms with light and dark lines are from VE- and PH-treated cells, respectively. Bar graph shows % HO-1 induction as determined from the FACS histograms. Solid and open bars represent results from AP and VC donors, respectively. *P < 0.05.
HO-1 induction in leukocytes in response to PH correlates with severity of experimental pancreatitis.
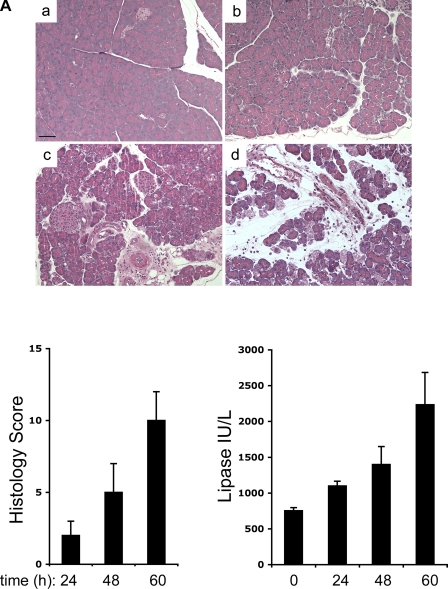
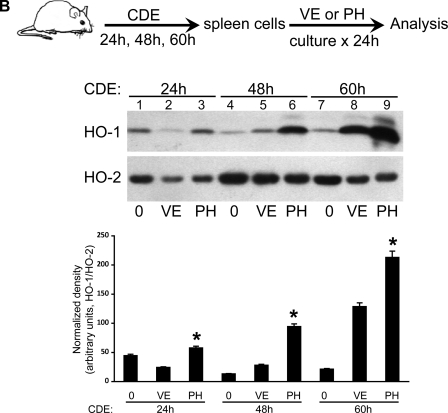
To determine whether similar ex vivo induction of human HO-1 by PH (as shown in Figs. 3 and 4) can be seen in an experimental mouse model of pancreatitis, we added PH ex vivo for 24 h to cultured spleen cells isolated from mice with mild, moderate, and severe pancreatitis. To induce experimental pancreatitis, mice were fed a CDE diet. In this model, disease severity increases over time with 100% mortality after 5 day of CDE feeding, and severe pancreatic injury with significant hemorrhage and necrosis is observed after 3 days of CDE feeding (17, 18). We used the CDE model of pancreatitis because it offers an opportunity to study mild to severe disease over time as shown by the progression of pancreatitis based on histological and biochemical assessment (Fig. 5A). Similar to the finding for PBMCs from AP patients at day 1, greater splenocyte PH-mediated HO-1 induction was seen after 60 h (severe disease, lane 9) as opposed to 24 (mild disease, lane 3) or 48 h (moderate disease, lane 6) of CDE feeding (Fig. 5B). In addition, culturing splenocytes from severely ill (lane 8) but not moderately (lane 5) or mildly (lane 2) ill mice with vehicle alone can induce HO-1 (Fig. 5B). Thus splenocyte HO-1 inducibility increases with the severity of experimental pancreatitis. As expected, very little change is observed in HO-2, a heme oxygenase isoenzyme that is constitutively expressed.
Fig. 5.
PH-mediated HO-1 induction in mouse splenocytes during experimental pancreatitis. A: FVB/n mice were fed regular chow (a) or CDE for 24 (b), 48 (c), or 60 h (d) to induce mild, moderate, and severe pancreatitis, respectively. Pancreata were isolated and fixed in 10% formalin, and sections were stained with hematoxylin and eosin and scored. Serum was used for determination of lipase levels. Scale bar, 50 μm. B: leukocytes from mouse spleens of the FVB/n mice fed CDE for 24, 48, or 60 h were isolated and cultured for 24 h with either PH or VE alone. Cultured leukocytes were lysed in a homogenization buffer as described in materials and methods, and 10 μg total protein from each sample was separated by SDS-PAGE followed by Western blot analysis to compare HO-1 protein expression levels. Equal protein loading was confirmed by assessing levels of the constitutively-expressed HO-2 isoform. Bar graphs show the density of HO-1 relative to HO-2. *P < 0.05 compared with VE.
DISCUSSION
We show here that HO-1 levels are significantly lower in circulating monocytes and neutrophils of VCs and neutrophils, B cells, and T cells of HC patients compared with AP patients at the time of admission. These findings are consistent with the fact that HO-1, also known as heat shock protein 32, is induced during oxidative stress (3). Although our AP population was comprised of mildly ill patients, significant upregulation of HO-1 was detected, which is consistent with the findings of Takaki and coworkers (30) of increased MFI monocyte HO-1 levels in patients with septic shock compared with healthy volunteers and less ill patients. However, unlike the Takaki et al. study, we used HO-1 MFI normalized to Spherobeads so we can more accurately compare different patient samples as well as differences over time (8). The findings by Takaki and coworkers as well as others using various models of sepsis support the conclusion that HO-1 upregulation is associated with less severe damage and a better outcome (12, 30, 31).
Monocyte HO-1 levels were highest at the onset of pancreatitis (defined by hospital admission) and decreased significantly with clinical improvement, since all of the AP patients were recovering by day 3. Although only one of the patients had ERCP-induced pancreatitis, the observation that this patient had the highest level of HO-1 (and not lipase), and an acute presentation supports the hypothesis that HO-1 levels are likely to be highest early on during the disease course. We did not assess HC patient HO-1 levels at the later date. A limitation of our study is that the HC patient population comprised a heterogeneous group with different nonpancreatic disease diagnoses and different degrees of disease severity. However, in contrast to AP patient monocytes, VC monocyte HO-1 levels remained constant and AP patient neutrophil, T and B cell HO-1 levels also did not change. We are not able to discern the exact time of pancreatitis onset prior to the time of admission to the hospital since the majority of our patients had gallstones with nonspecific pain from a few days to several weeks in duration prior to admission. However, our results suggest that early HO-1 upregulation in leukocytes and particularly in monocytes may help limit or dampen the inflammatory response associated with pancreatic injury. Given the animal data showing that HO-1+ monocytes are rapidly recruited to the pancreas and confer protection against experimental pancreatitis (21), high-HO-1-expressing monocytes may be able to access the inflamed pancreas and could contribute to resolution of disease and patient recovery. However, this latter hypothesis and whether there is a cause and an effect between HO-1 upregulation and recovery from AP remain to be proven in humans. Presently, we do not know whether monocyte HO-1 levels remain the same, increase, or decrease in patients who fail to improve since all our AP patients recovered. However, rodent studies show that splenocyte HO-1 levels in response to exogenous PH continue to increase as the severity of pancreatitis increases (e.g., Fig. 5B).
Aside from the beneficial effects of upregulating HO-1 and/or carbon monoxide in animal models, intensive-care-admitted septic survivors are found to have higher arterial blood carbon monoxide levels as opposed to septic nonsurvivors (30). Thus, one postulate is that a relatively higher degree of HO-1 inducibility is associated with resolution of AP. As such, we tested whether PBMCs could respond to exogenous PH, an approved drug that induces HO-1, and whether differences in induction exist between the three cohorts we studied. Findings herein showed that PBMCs from AP patients respond more readily to PH-mediated HO-1 upregulation as opposed to HC and VC PBMCs, thereby suggesting that leukocytes from AP patients are primed for HO-1 induction and may respond readily to treatment with agents such as PH.
Analysis of leukocyte subsets in cultured PBMCs showed that monocytes followed by B cells had higher HO-1 induction in response to exogenous PH. Unlike the role of HO-1 in monocytes, HO-1's role in B cells is not well studied, and the significance of HO-1 induction in B cells here is not clear. We did not observe significant HO-1 induction in T cells, which suggests that T cells are less likely to play a role. Alternatively, a subset of T cells such as HO-1+ regulatory T cells (Tregs), which have been shown to suppress airway inflammation (35), may have been missed in our study given their low number and the use of the pan-T cell marker, CD3. However, a more recent study showed that HO-1 is neither required for mouse Treg development or function (37). Our results are also consistent with a previous study that showed significant HO-1 induction in cultured monocytes but not lymphocytes in acutely ill children compared with healthy controls (36). Overexpression of HO-1 by macrophages (by adenovirus-mediated transfer) leads to increased macrophage-derived IL-10 and attenuated lung inflammation (11) whereas macrophages from HO-1-deficient mice were shown to release increased amounts of proinflammatory cytokines upon stimulation (13). Thus, AP patients are likely to have therapeutic benefit from PH-mediated monocyte induction of HO-1.
The studies herein were performed in patients with mild self-limited disease to show proof of principle that HO-1 is upregulated in relatively mild, acute, self-limited pancreatitis, resolves with recovery, and could be induced further by PH in monocytes from these patients. This adds support to the potential of testing PH therapy in patients with more severe AP, which might be performed in future clinical studies.
Although the AP group we studied had mild and self-limited disease that may not require nor benefit from hemin therapy, an important finding herein is the differential responsiveness to PH depending on the stage of illness, since PBMCs from acutely ill patients (day 1) had more enhanced HO-1 induction than PBMCs from recovering patients (day 3). Additional studies in more severe AP patients will be required, but the findings in experimental pancreatitis covering the range of mild to severe involvement are supportive since, splenocytes from mice experiencing severe AP had higher responsiveness to PH-mediated HO-1 induction compared with those with less severe disease. These findings suggest that leukocytes from acute or severely ill AP patients have the capacity to respond to pharmacological induction of HO-1, which may render cell-based therapy a viable option. In support of this, we previously showed that transfer of in vivo hemin-primed cells ameliorates the disease in mice with pancreatitis (21). Our study does not explain why monocytes are differentially affected. However, the animal data (21) indicates that monocytes during the early phase of disease are the primary cells that migrate to the pancreas and upregulate their HO-1 levels either before or upon migration.
In conclusion, our data suggest that differential HO-1 upregulation in leukocyte subpopulations is observed in patients admitted with AP. Further upregulation of HO-1 could be induced by ex vivo treatment with PH. As patients recovered from AP, HO-1 expression in monocytes decreased as well as PBMC responsiveness to exogenous PH. PBMCs from acutely ill AP patients, as well as splenocytes from mice with severe pancreatitis, had enhanced PH-mediated HO-1 induction compared with the corresponding cells from recovering patients and mice with less severe pancreatitis, respectively, indicating that PH may offer a therapeutic benefit in patients with AP. The potential effectiveness of PH, as shown in mouse models of pancreatitis (21), awaits testing in clinical trials.
GRANTS
This work was supported by National Institutes of Health (NIH) grants DK073909 and DK47918 and by Ovation Pharmaceuticals (M. B. Omary) and NIH P30 Center grants DK56339 to Stanford University and DK34933 to the University of Michigan.
DISCLOSURES
The supplier of Panhematin (Ovation Pharmaceuticals) provided support for this study and has a pending patent application for the possible use of Panhematin in human pancreatitis.
Supplementary Material
REFERENCES
- 1. Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, Desnick RJ. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med 142: 439–450, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 223: 603–613, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bauer M, Huse K, Settmacher U, Claus RA. The heme oxygenase-carbon monoxide system: regulation and role in stress response and organ failure. Intensive Care Med 34: 640–648, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Becker T, ZuVilsendorf AM, Terbish T, Klempnauer J, Jorns A. Induction of heme oxygenase-1 improves the survival of pancreas grafts by prevention of pancreatitis after transplantation. Transplantation 84: 1644–1655, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bharucha AE, Kulkarni A, Choi KM, Camilleri M, Lempke M, Brunn GJ, Gibbons SJ, Zinsmeister AR, Farrugia G. First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin Pharmacol Ther 87: 187–190, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen P, Sun B, Chen H, Wang G, Pan S, Kong R, Bai X, Wang S. Effects of carbon monoxide releasing molecule-liberated CO on severe acute pancreatitis in rats. Cytokine 49: 15–23, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Devadas K, Dhawan S. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J Immunol 176: 4252–4257, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Dhein S, Mohr FW, Delmar M. (editors) Practical Methods in Cardiovascular Research. Berlin: Springer, 2005 [Google Scholar]
- 9. Durante W. Heme oxygenase-1 in growth control and its clinical application to vascular disease. J Cell Physiol 195: 373–382, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Herrick AL, McColl KE, Moore MR, Cook A, Goldberg A. Controlled trial of haem arginate in acute hepatic porphyria. Lancet 1: 1295–1297, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Inoue S, Suzuki M, Nagashima Y, Suzuki S, Hashiba T, Tsuburai T, Ikehara K, Matsuse T, Ishigatsubo Y. Transfer of heme oxygenase 1 cDNA by a replication-deficient adenovirus enhances interleukin 10 production from alveolar macrophages that attenuates lipopolysaccharide-induced acute lung injury in mice. Hum Gene Ther 12: 967–979, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Jao HC, Lin YT, Tsai LY, Wang CC, Liu HW, Hsu C. Early expression of heme oxygenase-1 in leukocytes correlates negatively with oxidative stress and predicts hepatic and renal dysfunction at late stage of sepsis. Shock 23: 464–469, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol 165: 1045–1053, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lamon JM, Frykholm BC, Bennett M, Tschudy DP. Prevention of acute porphyric attacks by intravenous haematin. Lancet 2: 492–494, 1978 [DOI] [PubMed] [Google Scholar]
- 15. Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med 8: 240–246, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Li X, Schwacha MG, Chaudry IH, Choudhry MA. Heme oxygenase-1 protects against neutrophil-mediated intestinal damage by down-regulation of neutrophil p47phox and p67phox activity and O2− production in a two-hit model of alcohol intoxication and burn injury. J Immunol 180: 6933–6940, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lombardi B, Estes LW, Longnecker DS. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by dl-ethionine fed with a choline-deficient diet. Am J Pathol 79: 465–480, 1975 [PMC free article] [PubMed] [Google Scholar]
- 18. Lombardi B, Rao NK. Acute hemorrhagic pancreatic necrosis in mice. Influence of the age and sex of the animals and of dietary ethionine, choline, methionine, and adenine sulfate. Am J Pathol 81: 87–100, 1975 [PMC free article] [PubMed] [Google Scholar]
- 19. Morse D, Pischke SE, Zhou Z, Davis RJ, Flavell RA, Loop T, Otterbein SL, Otterbein LE, Choi AM. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem 278: 36993–36998, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Mustajoki P, Seppalainen AM. Neuropathy in latent hereditary hepatic porphyria. Br Med J 2: 310–312, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakamichi I, Habtezion A, Zhong B, Contag CH, Butcher EC, Omary MB. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J Clin Invest 115: 3007–3014, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol 24: 449–455, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA 94: 10919–10924, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 139: 69–81, 1974 [PubMed] [Google Scholar]
- 25. Rushworth SA, MacEwan DJ, O'Connell MA. Lipopolysaccharide-induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J Immunol 181: 6730–6737, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruutu T, Volin L, Tenhunen R. Haem arginate as a treatment for myelodysplastic syndromes. Br J Haematol 65: 425–428, 1987 [DOI] [PubMed] [Google Scholar]
- 27. Siegert SW, Holt RJ. Physicochemical properties, pharmacokinetics, and pharmacodynamics of intravenous hematin: a literature review. Adv Ther 25: 842–857, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol Renal Physiol 286: F425–F441, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RB, Choi AM, Poss KD, Bach FH. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med 4: 1073–1077, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Takaki S, Takeyama N, Kajita Y, Yabuki T, Noguchi H, Miki Y, Inoue Y, Nakagawa T. Beneficial effects of the heme oxygenase-1/carbon monoxide system in patients with severe sepsis/septic shock. Intensive Care Med 36: 42–48, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Tamion F, Richard V, Renet S, Thuillez C. Protective effects of heme-oxygenase expression against endotoxic shock: inhibition of tumor necrosis factor-alpha and augmentation of interleukin-10. J Trauma 61: 1078–1084, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 61: 748–755, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Volin L, Ruutu T, Knuutila S, Tenhunen R. Heme arginate treatment for myelodysplastic syndromes. Leuk Res 12: 423–431, 1988 [DOI] [PubMed] [Google Scholar]
- 34. Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med 354: 2142–2150, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Xia ZW, Zhong WW, Xu LQ, Sun JL, Shen QX, Wang JG, Shao J, Li YZ, Yu SC. Heme oxygenase-1-mediated CD4+CD25high regulatory T cells suppress allergic airway inflammation. J Immunol 177: 5936–5945, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Yachie A, Toma T, Mizuno K, Okamoto H, Shimura S, Ohta K, Kasahara Y, Koizumi S. Heme oxygenase-1 production by peripheral blood monocytes during acute inflammatory illnesses of children. Exp Biol Med (Maywood) 228: 550–556, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Zelenay S, Chora A, Soares MP, Demengeot J. Heme oxygenase-1 is not required for mouse regulatory T cell development and function. Int Immunol 19: 11–18, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.