Abstract
In developing countries many enteric infections are caused by acid-sensitive pathogens. Failure of the gastric acid barrier to infection has been reported in cholera but gastric acid secretion has been little studied in other enteric infections. We therefore studied basal and stimulated gastric acid in 185 Bangladeshi men admitted to hospital for the treatment of enteric infection. Patients with dysentery (amoebiasis, n=24 and shigellosis, n= 19) and culture-negative diarrhoea (n=69) had similar mean gastric acid levels (basal, 3–5 mmol/h; stimulated, 11–17 mmol/h), which remained stable in those patients studied throughout 12 weeks of convalescence. In contrast, patients with secretory diarrhoea caused by cholera or enterotoxigenic Escherichia coli (ETEC) had low gastric acid levels (P<O.05 compared with other groups) (cholera, n=34: basal mean 1·8 mmol/h [SD=2·2], stimulated mean 7·9 mmol/h [SD=6·4]; etec, n=39: basal mean 2·7 mmol/h [SD=2·8], stimulated mean 9·4 mmol/h [SD=7·5]). Cholera patients' gastric acid level rose during convalescence to similar levels to the dysentery patients'. Low gastric acid level was associated with severe disease in patients with cholera (P<0·02) or etec (P<0·05). Gastric acid level fell with increasing age (P<0·007) but this did not account for the differences between groups. Gastric acid levels were not associated with Giardia duodenalis or Strongyloides stercoralis co-infection, fever, use of tobacco, or chewing betel nut. Cholera and secretory diarrhoea caused by etec may, therefore, partly result from a reduction in gastric acid level which does not occur during dysentery. Factors which impair gastric acid secretion may predispose to diarrhoeal disease in developing countries.
Keywords: hypochlorhydria, diarrhoeal disease, amoebiasis, shigellosis, cholera, Escherichi coli, etec, gastric acid secretion, Bangladesh
Introduction
Diarrhoeal diseases are important causes of morbidity and mortality world-wide. Gastric acid can provide an effective barrier against enteric infection (Howden & Hunt, 1987), killing ingested pathogens within minutes (Giannella et al., 1973). Susceptibility to enteric infection is increased by failure of gastric acid secretion due to acid-suppressing drugs (Larner & Hamilton, 1994). pernicious anaemia (Hunt, R. H., 1988). gastrectomy, or vagotomy (Gitelson, 1971). Intermittent failure of acid production, so-called tropical hypochlorhydria (Nalin et al., 1978a), is common in poor communities (Sarker & Gyr, 1992) and gastric acid level is frequently low in patients with cholera (Desai et al., 1971), or in those recently recovered from it (Sarker & Gyr, 1992). Little is known about gastric acid in other enteric infections and co-factors which may cause this failure of gastric acid secretion have been suggested but not systematically studied. We therefore tested the ability of patients to secrete gastric acid during and after unselected enteric infections and investigated the effect of proposed modulators of gastric acid secretion: co-infection with Giardia duodenalis (=G. lamblia) (see Haas & Bucken, 1967) or Strongyloides stercoralis (see Jones, 1950). the use of betel nut or cigarettes (Lanas & Hirschowitz, 1992), patient age (Weaver, 1995), anaemia (Davidson & Markson, 1955), and fever (Chang, 1933).
Methods
Patients
One hundred and ninety adult men with severe diarrhoea admitted consecutively to the International Centre for Diarrhoeal Disease Research Hospital, Dacca, Bangladesh, were invited to take part in the study. All but 5 gave informed consent and took part in the study. The study was approved by the ethics committees of the Bangladesh Medical Research Council and The Johns Hopkins University.
Investigations, diagnosis and treatment
A full clinical history and examination included recording if each patient chewed betel nut or smoked tobacco. Investigations included height, daily weight, blood count, urea, creatinine, plasma specific gravity, stool examination or rectal swab. The daily volume of unformed stool was recorded and stool emulsified in saline was examined for protozoa, including haematophagous trophozoites of Entamoeba histolytica, and helminth ova. Shigellosis was diagnosed by culture on MacConkey and Shigella–Salmonella agar. Vibrio cholerae (E1 Tor biotype in all cases) was isolated on gelatine and thiosulphate–citrate–bile sucrose agars, and the duration of carriage was assessed by daily rectal swabs. To test for enterotoxigenic Escherichia coli (ETEC), 5-lactose positive colonies were selected for heat-labile toxin adrenal cell assay and heat-stable toxin infant mouse assay (Donta et al., 1974). Salmonella typhi was not isolated and enteropathogenic E. coli and Campylobacter jejuni were not studied. On the fourth day, patients swallowed a string capsule which was retrieved 12 h later and examined for G. duodenalis and S. stercoralis (see Beal et al., 1970).
Rehydration and antimicrobial therapy were not influenced by inclusion in the study. Treatment of amoebiasis was with metronidazole, 1·5 g in 3 divided doses: for shigellosis, a single dose oi ampicillin, 4·0 g; and for cholera, patients were given tetracycline.
Gastric acid studies
Gastric acid studies were done the morning after admission using a standard procedure (Sack et al., 1972), after at least 12 h intravenous rehydration. In brief, fasting patients swallowed a 2 mm bore weighted polyvinyl tube and were placed in the left recumbent position. Patients received intravenous 5% dextrose throughout. Twenty mL of water were injected through the tube and sucked back; if the fluid volume was low or pH was high, the tube position was checked with fluoroicopy. Thirty min later, ‘basal’ samples were aspirated every 15 min for one hour. Fifty mg ametazole hydrochloride (betazole hydrochloride, Histalog®) was then given intramuscularly in order to stimulate gastric acid secretion. ‘Stimulated’ samples were then aspirated every 15 min for another hour, allowing calculation of basal and stimulated rates of secretion (mL/h). Each sample was titrated to pH 7 with 0·1 M NaOH to determine the acid concentration (mmol/L), allowing calculation of basal and stimulated gastric acid (mmol/h). Patients with severely limited stimulated gastric acid levels (<5 mmol/h) were classified as hypochlorhydric.
Convalescent gastric acid studies were also performed on the 136 patients for whom diarrhoeal aetiology was established. These convalescent studies were done one week after admission and after full recovery, 4 and 12 weeks after admission, so that each patient acted as his own control.
Statistical analysis
Statistical analysis was performed using SPSS® statistical software (Chicago, Illinois, USA). Normally distributed data were analysed using one-way analysis of variance and the two-tailed t test. The Kruskal–Wallis test was used for non-parametric data.
Results
Clinical features
Characteristics of the study population are shown in the Table.
Table.
Characteristics of 185 patients with severe diarrhoea who participated in the study of gastric acid secretiona
| Culture-negative | Dysentery | Secretory diarrhoea | |||
|---|---|---|---|---|---|
| diarrhoea | Amoebiasis | Shigellosis | Cholera | ETECb | |
| Clinical features | |||||
| Age (years) | 33·0 (12·1) | 46·7 (13·5) | 35·3 (14·5) | 31·1 (14·7) | 40·4 (15·1) |
| Admission weight (kg) | 43·6 (5·1) | 40·0 (5·3) | 46·0 (6·9) | 41·6 (5·7) | 43·2 (6·3) |
| In-patient weight gain (kg) | 2·2 (1·8) | 1·2 (2·5) | 0·6 (2·2) | 3·2 (1·9) | 2·4 (2·5) |
| Stool volume (L) | 1·9 (1·5) | 0·4 (0·6) | 0·4 (0·4) | 18·5 (20·2) | 2·0 (2·9) |
| Duration of diarrhoea | |||||
| Pre-admission (d) | 1·7 (2·6) | 40·9 (44·8) | 4·3 (1·7) | 0·9 (0·9) | 0·8 (0·7) |
| Post-admission (h) | 21·5 (21·8) | 30·3 (24·4) | 26·1 (21·6) | 89·6 (43·1) | 45·1 (20·3) |
| Laboratory features | |||||
| Leucocyte count (day 1) | 13·4 (5·6) | 18·1 (10·3) | 12·9 (8·1) | 22·8 (7·6) | 16·0 (6·5) |
| Plasma specific gravity (day 1) | 270·7 (32·3) | 242·3(20·0) | 265·4(19·1) | 299·3(49·3) | 275·7 (28·5) |
| Haematocrit (day 1) | 41·8 (6·3) | 35·8 (5·4) | 42·0 (2·6) | 46·3 (6·3) | 40·2 (5·9) |
| Haematocrit (day 7) | 40·0 (6·3) | 35·9 (5·8) | 40·8 (2·7) | 37·9 (4·2) | 37·0 (7·0) |
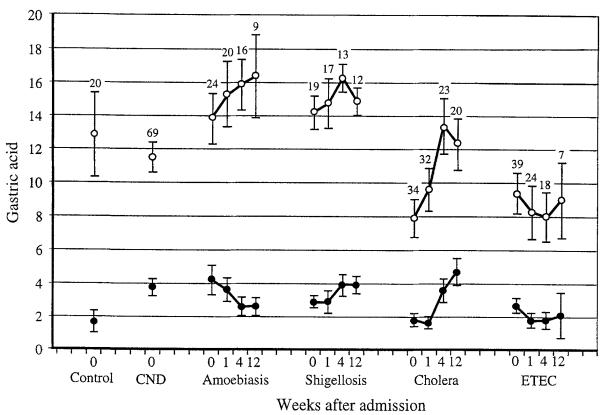
Values in the Table are means with standard deviations in parentheses. The number of patients studied at each time is given in Fig. 1.
Enterotoxigenic E. coli.
Amoebiasis patients were older than those with culture-negative diarrhoea, shigellosis and cholera (all P<0·05). They had suffered more prolonged diarrhoea before admission and weighed less on discharge than all other groups (all P<0·05), although there was no significant difference in height. Amoebiasis patients had lower haematocrits on admission than all other groups (all P<0·05) and the haematocrit did not change significantly, remaining low over 12 weeks. Amoebiasis patients also had lower plasma specific gravity on admission and on day 7 than other groups (all P<0·005), but this rose significantly (days 1 or 7 vs. week 4, P<O·05) so that specific gravity was similar for all groups at 4 and 12 weeks.
Patients with cholera or etec had more severe metabolic acidosis (P<0·001, data not shown) and more prolonged diarrhoea (P<0·05) than the other groups. Duration and volume of diarrhoea were higher in cholera patients than in other groups (all P<0·05) and only cholera patients were sufficiently dehydrated to cause elevated urea and creatinine levels (data not shown), specific gravity, and haematocrit on admission compared with convalescent values (all P<0·002). The mean admission temperature was elevated in all groups except those with cholera, none of whom was febrile.
Gastric acid studies
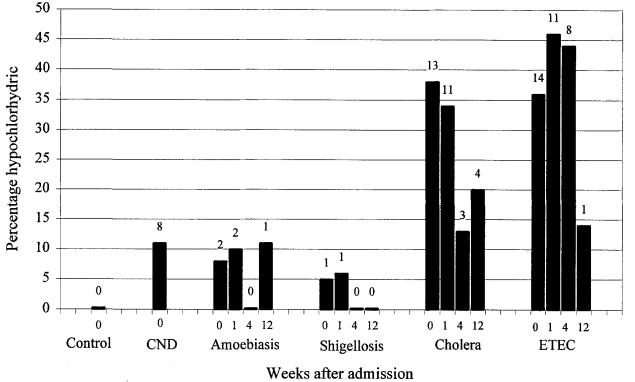
Gastric acid data are shown in Fig. 1 and the number of hypochlorhydric patients in Fig. 2. Culture-negative diarrhoea, amoebiasis and shigellosis patients on admission had similar gastric acid levels which remained stable for those patients studied up to 12 weeks later: fewer than 12% were hypochlorhydric at any time. Neither basal nor stimulated gastric acid correlated with disease severity (as indicated by weight gain in hospital, admission haematocrit or plasma specific gravity, stool volume, and diarrhoea duration before or after admission).
Fig. 1.
Basal (closed circles) and stimulated (open circles) gastric acid levels for each diagnostic group on admission (week 0) and 1, 4 and 12 weeks later. Patients with culture-negative diarrhoea (CND) were studied only on admission. The number of patients attending at each time is shown above the standard error bars for stimulated gastric acid. etec: enterotoxigenic E. coli; Control: data my controls given by Sack et al. (1972) are plotted for comparison. Values are means with standard error bars, in mmolh.
Fig. 2.
Percentage of patients who were hypochlorhydric on admission (week 0) and 1,4 and 12 weeks later. The number of hypochlorhydric patients is shown above each bar. The number of patients studied at each time is shown in Fig. 1. Patients with culture-negative diarrhoea (CND) were studied only on admission. Hypochlorhydria was defined as stimulated gastric acid less than 5 mmol/h. etec: enterotoxigenic E. coli; Control: data from healthy controls given by Sack et al. (1972) are plotted for comparison.
Cholera patients on admission had lower basal and stimulated gastric acid levels than the culture-negative diarrhoea, amoebiasis and shigellosis groups (all P<0·05). On day 7, cholera patients' basal gastric acid remained lower than that in the amoebiasis group and stimulated acid remained lower than in the amoebiasis and shigellosis groups (all P<0·05). Four weeks after admission, gastric acid had risen significantly (P<0·05 compared with days 1 or 7), so that after 4 and 12 weeks of convalescence there was no significant difference in gastric acid between the cholera, amoebiasis or shigellosis groups. On days 1 and 7, more than one-third of the cholera patients were hypochlorhydric and this proportion fell during convalescence. The relatively low gastric acid level on days 1 and 7 was due to a significant reduction in both volume and concentration of gastric acid (basal and stimulated, data not shown).
In the cholera patients, low gastric acid on admission was associated with severe disease: volume of diarrhoea correlated inversely with basal (r=−0·31, P<0·02) and stimulated (r=−0·36. P<0·01) gastric acid; and the duration of V. cholerae excretion (mean=5·45 d, sd=2·25, n=33) correlated with total volume of diarrhoea (r=0·57, P<0·0001) and correlated inversely with basal (r=−0·28, P<0.06) but not stimulated gastric acid.
Patients with etec had lower stimulated gastric acid levels than culture-negative diarrhoea patients on admission (P<0.004) and than cholera patients in week 4 (P<0·001). etec uatients also had lower stimulated gastric acid levels ihan amoebiasis and shigellosis patients on admission and in weeks 1. 4 and 12 (P<0·05). Basal gastric acid was low, but significantly suppressed compared with cholera and shigellosis groups only in week 4 (P<0·05). More than one-third of patients with etec were hypochlorhydric on days 1 and 7 and week 4, but this persisted in only one of the 7 who attended in week 12. This reduction in gastric acid was due to near normal volumes of relatively dilute acid being secreted (basal and stimulated, data not shown).
In the etec patients, low gastric acid on admission was also associated with disease severity: the duration of diarrhoea correlated inversely with basal (r=−0·34, P<0·03) and stimulated (r=−0·27, P<0·07) gastric acid; and diarrhoea volume correlated inversely with stimulated gastric acid (r=−0·27, P<0·05). Both types of etec toxin were associated with hypoacidity and there was no significant difference between them: 31 patients were infected with etec that produced both heat-labile and heat-stable toxins (mean basal acid=2·7 mmolih, sd=2·8; stimulated=9·2 mmol/h, sd=7·0), while 7 had etec which produced heat-stable toxin alone (mean basal acid=2·1 mmol/h, sd=2·3; stimulated=7·5 mmol/h, sd=7·6).
Thirty-three per cent of patients were co-infected with G. duodenalis and 36% with S. stercoralis, but infection with these parasites was not associated with gastric acid level on days 1 or 7 (data not shown) or with volume of diarrhoea. Seventy-three per cent of patients chewed betel nut regularly and 72% smoked cigarettes. There was no association between gastric acid level on admission and use of betel nut, tobacco, or severity or duration of pyrexia in any patient group or in all groups combined. Stimulated gastric acid was inversely proportional to age for all patient groups except those With cholera (all P<0·007) and there was a similar trend for basal secretion, which was significant for etec patients only (P<0·003).
Discussion
Gastric acid secretion was suppressed in patients with secretory diarrhoea (cholera and etec) compared with those with dysentery (amoebiasis and shigellosis) or culture-negative diarrhoea. Patients acted as their own controls, and 4 to 12 weeks after admission gastric acid secretion was similar in patients with amoebiasis, shigellosis and cholera, implying that these were normal values. This was supported by the similar levels reported in healthy Bengali people of low socioeconomic class used as controls in another study (see Figs 1 and 2); their mean age was 34 years, and their mean weight was 43 kg (Sack et al., 1972).
Patients with amoebiasis and shigellosis had similar and stable gastric acid levels throughout diarrhoea and convalescence. The cyst of E. histolytica is resistant to acid (Ravdin & Petri, 1995), so low gastric acid levels would not be expected to predispose to this infection. Shigellosis may occur irrespective of normal gastric acid because this pathogen is relatively resistant to acid and as few as 10 organisms may cause disease (Peterson et al., 1989). Our results imply that amoebiasis and shigellosis do not affect gastric acid, they occur mainly in people with normal gastric acid, and gastric acid does not influence susceptibility to these pathogens.
Patients with secretory diarrhoea (cholera or etec) had low gastric acid levels. Previous cholera studies have reported similar hypoacidity (Gitelson, 1971; van Loon et al., 1990). including a studv in a Bennali vovulation (Sack et al., 1972). The hypoacidity associated with cholera resolved within 4 weeks of diarrhoea and, although mean gastric acid levels remained low in patients with etec, only one patient remained hypochlorhydric throughout convalescence. Interpretation of this convalescent etec data is limited because only a few, self-selected patients attended.
Could secretory diarrhoea have caused hypoacidity? In a studv of vrison volunteers (Nalin et al., 1978a), experimentally induced cholera was not found to affect gastric acid, but the volunteers were not severely dehydrated. In canine experiments, dehvdration alone may stimulate rather than suppress gastric acid (Hunt, J. N: & Wan, 1967). Dehydration is unlikely to have caused hypoacidity in our study because patients with etec showed no laboratory evidence of dehydration but exhibited more prolonged hypoacidity than the dehydrated cholera patients. Also, patients with culture-negative diarrhoea maintained gastric acid levels despite passing similar volumes of stool to etec patients. Furthermore, cholera toxin is immunologically similar to heat-labile etec enterotoxin and they both stimulate cyclic adenosine monophosphate. Gastric acid secretion was similar in etec patients with heat-labile toxin and the functionally unrelated heat-stable toxins. An effect of enterotoxin or dehydration on gastric acid therefore seems less likely than the possibility that gastric hypoacidity predisposed to enteric infection. Ingestion of 1×108 etec (see Desai et al., 1971; Howden & Hunt, 1987) or V. cholerae (see Cash et al., 1974) is needed to cause diarrhoea, but this dose is reduced by several log units if alkali is given to neutralize gastric acid. Furthermore, susceptibility to cholera and diarrhoea severity are inversely related to pre-infection gastric acid level (Hornick et al., 1971). Hypoacidity may, therefore, have preceded and increased susceptibility to V. cholerae and etec by allowing bacteria to survive passage through the stomach to infect the intestines.
The populations of many developing countries have lower gastric acid levels than are typical in developed countries (Sarker & Gyr, 1992), but measurement is not standardized. We used 50 mg of ametazole hydrochloride to allow comparison with another study in this population (Sack et al., 1972). Larger doses may cause more acid secretion (Zaterka & Neves, 1964), but this could not explain our results. Severe malnutrition (Gracey et al., 1977; Gilman et al., 1988) or anaemia (Davidson & Markson, 1955) can impair gastric acid secretion, but amoebiasis patients had normal acid levels despite their age, hypoproteinaemia and anaemia, implying that these factors alone did not impair the gastric barrier to infection. Chewing betel nut and tobacco use were also unrelated to gastric acid level. Cannabis use may suppress gastric acid secretion (Nalin et al., 1978b), but this was uncommon in our population. In contrast with artificial elevation of body temperature which suppresses gastric acid (Bandes et al., 1948), fever did not correlate with gastric acid level, which was lowest in cholera patients who were afebrile. There are reports of hypoacidity in symptomatic giardiasis (Haas & Bucken, 1967) and strongyloidiasis (Jones, 1950), but we found that co-infection with these parasites was not related to gastric acid level. None of these factors which may influence gastric acid secretion therefore appears to explain the results of our study.
A more likely explanation for low gastric acid level is infection with Helicobacter pylori, which is associated with chronic atrophic gastritis (Hunt, 1994) and chronic hypoacidity (Morris & Nicholson, 1987) and may be the main cause of idiopathic tropical hypochlorhydria (Cater, 1992). Chronic atrophic gastritis would not explain transient suppression of gastric acid secretion but primary infection, re-activation or re-infection are possible causes (Klein et al., 1994). We did not test for H. pylori. but in Banaladesh 85% of individuals are seropogitive by the age if 39 years and this seropositivity is an independent risk factor for cholera (Clemens et al., 1995).
This study implied that secretory diarrhoea caused by V. cholerae and etec may result partly from a reduction in the gastric acid barrier to infection which neither occurs during, nor predisposes to, dysentery and other common diarrhoeal diseases. Research into the cause of tropical hypochlorhydria may have implications for the prevention of enteric infections. Screening for gastric hypoacidity has been proposed to focus scarce preventive resources on those at greatest risk (Weisse et al., 1995), or to allow dietarv vrovhvlaxis with citrus fruits (Gitelson, 1971) or alcohol (Weisse et al., 1995). Faecal contamination of the environment alone does not explain the high incidence of diarrhoeal diseases: intermittent failure of gastric acid secretion may predispose to these infections in the developing world.
Acknowledgements
This study was supported by NIH grant 1-UOl-A135894-01. We are grateful to Dr G. Hall. Dr R. Black and Dr J. Friedland for editoria1 help and to Dr G. Madico for computer assistance.
References
- Bandes J, Hollander F, Bierman W. Effect of physically induced pyrexia on gastric acidity. Gastroenterolopy. 1948;10:697–707. [PubMed] [Google Scholar]
- Beal GB, Viens P, Grant RGL, Hughes JM. A new technique for sampling duodenal contents: demonstration of upper small bowel pathogens. American Journal of Tropical Medicine and Hygiene. 1970;19:349–352. doi: 10.4269/ajtmh.1970.19.349. [DOI] [PubMed] [Google Scholar]
- Cash RA, Music SI, Libonati JP, Craig JP, Pierce NF, Hornick RB. Response of man to infection with Vibrio cholerae. I. Clinical, serologic and bacteriologic responses to a known inoculum. Journal of Infectious Diseases. 1974;129:45–52. doi: 10.1093/infdis/129.1.45. [DOI] [PubMed] [Google Scholar]
- Cater RE. Helicobacter pylori as the major causal factor in chronic hypochlorhydria. Medical Hypotheses. 1992;39:367–374. doi: 10.1016/0306-9877(92)90064-j. [DOI] [PubMed] [Google Scholar]
- Chang HC. Gastric secretion in fever and infectious diseases. Journal of Clinical Investigation. 1933;12:155–169. doi: 10.1172/JCI100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J, Albert MJ, Rao M, Qadri F, Huda S, Kay B, van Loon PL, Sack D, Pradhan BA, Sack RB. Impact of infection by Helicobacter pylori on the risk and severity of endemic cholera. Journal of Infectious Diseases. 1995;171:1653–1656. doi: 10.1093/infdis/171.6.1653. [DOI] [PubMed] [Google Scholar]
- Davidson WMB, Markson JL. The gastric mucosa in iron deficiency anaemia. Lancet. 1955;ii:639–643. doi: 10.1016/s0140-6736(55)92481-8. [DOI] [PubMed] [Google Scholar]
- Desai HG, Zaveri MP, Antia FP. Spontaneous and persisting decrease in maximal acid output. British Medical Journal. 1971;i:313–315. doi: 10.1136/bmj.2.5757.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta ST, Moon HW, Whipp SC. Detection of heat-labile Escherichia coli enterotoxin with the use of adrenal cells in tissue culture. Science. 1974;183:334. doi: 10.1126/science.183.4122.334. [DOI] [PubMed] [Google Scholar]
- Giannella RA, Broitman SA, Zamcheck N. Influence of gastric acidity on bacterial and parasitic infections. A perspective. Annals of Internal Medicine. 1973;78:271–276. doi: 10.7326/0003-4819-78-2-271. [DOI] [PubMed] [Google Scholar]
- Gilman RH, Partanen R, Brown KH, Spira WM, Khanam S, Greenberg B, Bloom SR, Ali A. Decreased gastric acid secretion and bacterial colonization of the stomach in severely malnourished Bangladeshi children. Gastroenterology. 1988;94:1308–1314. doi: 10.1016/0016-5085(88)90668-3. [DOI] [PubMed] [Google Scholar]
- Gitelson S. Gastrectomy, achlorhydria and cholera. Israeli Journal of Medical Science. 1971;7:663–667. [PubMed] [Google Scholar]
- Gracey M, Cullity GJ, Suharjong Sunoto. The stomach in malnutrition. Archives of Disease in Childhood. 1977;52:325–327. doi: 10.1136/adc.52.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas J, Bucken EW. Pathogenicity of the lamblia infections. Deutsche Medizinische Wochenschrif. 1967;92:1869–1871. doi: 10.1055/s-0028-1106056. [DOI] [PubMed] [Google Scholar]
- Hornick RB, Music SI, Wenzel R, Cash R, Libonti JP, Snyder MJ, Woodward TE. The Broad Street pump revisited: response of volunteers to ingested cholera vibrios. Bulletin of the New York Academy of Medicine. 1971;47:1181–1191. [PMC free article] [PubMed] [Google Scholar]
- Howden CW, Hunt RH. Relationship between gastric secretion and infection. Gut. 1987;28:96–107. doi: 10.1136/gut.28.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JN, Wan B. Electrolytes of mammalian gastric juice. In: Code CF, editor. Handbook of Physiology, Section 6: Alimentary Canal. 2nd edition American Physiological Society; Washington, DC: 1967. [Google Scholar]
- Hunt RH. The protective role of gastric acid. Scandinavian Journal of Gastroenterology. 1988;23S:34–49. doi: 10.3109/00365528809099128. [DOI] [PubMed] [Google Scholar]
- Hunt RH. HP and pH–relevance of gastric acid to the treatment of Helicobacter pylori infection. Journal of Gastroenterology. 1994;29(supplement 7):128–133. [PubMed] [Google Scholar]
- Jones CA. Clinical studies in human strongyloidiasis. Gastroenterology. 1950;16:743–756. [PubMed] [Google Scholar]
- Klein PD, Gilman RH, Leon-Barua RL, Diaz F, Smith EO, Graham DY. The epidemiology of Helicobacter pylori in Peruvian children between 6 and 30 months of age. American Journal of Gastroentmology. 1994;89:2196–2200. [PubMed] [Google Scholar]
- Lanas A, Hirschowitz BI. Influence of smoking on basal and on vagally and maximally stimulated gastric acid and pepsin secretion. Scandinavian Journal of Gastroenterology. 1992;27:208–212. doi: 10.3109/00365529208999950. [DOI] [PubMed] [Google Scholar]
- Larner AJ, Hamilton MIR. Infective complications of therapeutic gastric acid inhibition. Alimentary Pharmacology and Therapeutics. 1994;8:579–584. doi: 10.1111/j.1365-2036.1994.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting pH. American Journal of Gastroenterology. 1987;82:192–199. [PubMed] [Google Scholar]
- Nalin DR, Levine RJ, Levine MM, Hoover D, Bergquist E, McLaughlin J, Libonati J, Alam J, Hornick RB. Cholera, non-vibrio cholera and stomach acid. Lancet. 1978a;ii:856–859. doi: 10.1016/s0140-6736(78)91568-4. [DOI] [PubMed] [Google Scholar]
- Nalin DR, Levine MM, Rhead J, Bergquist E, Rennels M, Hughes T, O'Donnell S, Hornick RB. Cannabis, hypochlorhydria, and cholera. Lancet. 1978b;ii:859–862. doi: 10.1016/s0140-6736(78)91569-6. [DOI] [PubMed] [Google Scholar]
- Peterson WL, Mackowiak PA, Barnett CC, Marling-Cason M, Haley ML. The human gastric bactericidal barrier: mechanisms of action, relative antibacterial activity, and dietary influences. Journal of Infectious Diseases. 1989;159:979–983. doi: 10.1093/infdis/159.5.979. [DOI] [PubMed] [Google Scholar]
- Ravdin JI, Petri WA. Entamoeba histolytica (amebiasis) In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 4th edition Churchill Livingstone; New York: 1995. pp. 2395–2408. [Google Scholar]
- Sack GH, Pierce NF, Hennessey KN, Mitra RC, Sack RB, Mazumder DNG. Gastric acid in cholera and non-cholera diarrhoea. Bulletin of the World Health Organization. 1972;47:31–36. [PMC free article] [PubMed] [Google Scholar]
- Sarker SA, Gyr k. Non-immunological defense mechanisms of the gut. Gut. 1992;33:987–993. doi: 10.1136/gut.33.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon FPL, Clemens JD, Shahrier M, Sack DA, Stephensen CB, Khan MR, Rabbani GH, Rao MR, Banik AK. Low gastric acid as a risk factor for cholera transmission: application of a new non-invasive gastric acid field test. Journal of Clinical Epidemiology. 1990;12:1361–1367. doi: 10.1016/0895-4356(90)90103-v. [DOI] [PubMed] [Google Scholar]
- Weaver LT. Helicobacter pylori infection, nutrition and growth of West African infants. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1995;89:347–350. doi: 10.1016/0035-9203(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Weisse ME, Eberly B, Pearson DA. Wine as a digestive aid: comparative antimicrobial effects of bismuth salicylate and red and white wine. British Medical Journal. 1995;311:1657–1660. doi: 10.1136/bmj.311.7021.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaterka S, Neves DP. Maximal gastric secretion in human subjects after Histalog stimulation. Gastroenterology. 1964;47:251–257. [PubMed] [Google Scholar]