Abstract
Pressure and temperature are important environmental variables that influence living systems. However, while they vary over a considerable range on Earth and other planets, it has hardly been addressed how straightforwardly and to what extent cellular life can acquire resistance to extremes of these parameters within a defined genomic context and a limited number of generations. Nevertheless, this is a very pertinent question with respect to the penetration of life in allegedly inhospitable environments. In this study, directed evolution was used to reveal the potential of the nonsporulating and mesophilic model bacterium Escherichia coli to develop the ability to survive exposure to high temperature or pressure. While heat resistance could only marginally be increased, our data show that piezoresistance could readily and reproducibly be extended into the GPa range, thereby greatly exceeding the currently recognized maximum for growth or survival.
IMPORTANCE
While extremophilic microorganisms generally serve as the reference for microbial survival capacities in inhospitable environments, we set out to examine how readily a mesophilic model bacterium such as Escherichia coli could build up resistance to extremes of temperature or pressure within a very short evolutionary time scale. Both heat and high pressure constitute ecologically important physical stresses that are able to irrevocably penetrate the entire cell. Our results for the first time establish that cellular life can acquire resistance to pressures extending into the GPa range.
INTRODUCTION
The known edges of our biosphere are typically delineated by extremophilic bacteria and archaea that manage to resist the severe physical and/or chemical conditions encountered in these environments. As such, studies of microorganisms from marine hydrothermal vents show that the currently recognized upper temperature and pressure limits for growth are 121°C (1) and 120 MPa (2), respectively. Besides the existence of extremophiles, however, another and less documented reflection of bacterial adaptability is the capacity to develop resistance against inhospitable environments within a defined genomic content and a limited number of generations. Such directed evolution of bacterial resistance could yield clues as to the possibility of the existence or persistence of microbial life in extreme environments that so far have been inaccessible or unexplored by current observational techniques.
In this context, we have examined how rapidly and to what extent a mesophilic and nonsporulating model bacterium such as Escherichia coli might adapt to lethal heat or high pressure (HP), two of the most important and general physical constraints on life. We followed a directed-evolution strategy in which an E. coli culture was iteratively challenged with progressively increasing temperatures or pressures. Between consecutive exposures to heat or HP, survivors were allowed to resuscitate and grow under standard conditions and thus automatically became enriched in the resulting population. After a number of such cycles, a single clone was purified from the population in order to compare its resistance profile to that of the original parent strain. The outcomes of these selection regimens are depicted in Fig. 1A and B for development of heat and HP resistance, respectively.
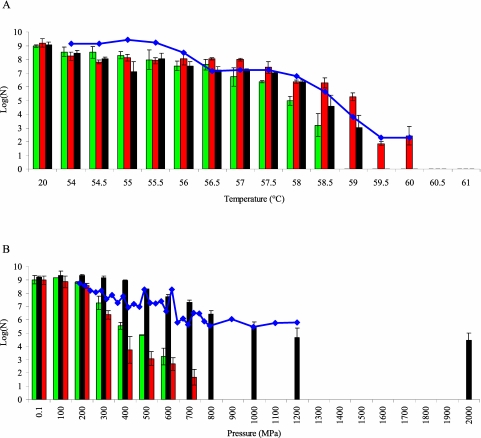
FIG 1 .
Directed evolution of E. coli K-12 MG1655 towards heat (at atmospheric pressure) (A) or HP (at ambient temperature) (B) resistance. An axenic culture of E. coli MG1655 was iteratively exposed to progressively intensifying temperature or pressure shocks (15 min each), with a resuscitation and growth step between consecutive treatments. Survival after each treatment was determined (blue diamonds) and expressed as log(N), in which N represents the number of survivors determined by plate counts and expressed as CFU per milliliter. At the end of both selection regimens, the most heat (red bars)- and pressure (black bars)-resistant clone that had enriched in the respective cultures was isolated and its acquired heat (A) and HP (B) resistance was determined and compared to that of the original parental strain (green bars). It should be noted that these selection profiles are representative of 20 and 4 independent trials for heat and HP resistance development, respectively. Further experimental details can be found in the supplemental material (Text S1).
The results immediately reveal that the developed heat resistance was only marginal, shifting the upper survival temperature by approximately 1.03-fold from 58.5 to 60.0°C (Fig. 1A, compare green and red bars). However, the developed piezoresistance was extraordinary, and the upper pressure limit for survival readily increased at least 3-fold from 600 MPa to 2 GPa (Fig. 1B, compare green and black bars). In fact, as many as ca. 1 in 104 cells of the HP-resistant population readily survived a 2-GPa treatment. While signs of alleged enzymatic activity in E. coli cells at 1.4 GPa have been reported previously (3, 4), these results provide the first irrevocable evidence of microbial survival at ≥2 GPa.
It is also worth noting that the HP- and heat-resistant mutants displayed only a marginal cross-resistance against heat and HP, respectively (Fig. 1), indicating that the acquired adaptations are very specific for each stress and precluding any a priori causal link between HP and heat resistance.
Importantly, GPa-HP-resistant E. coli mutants have been obtained in all four independent HP evolution experiments performed. Moreover, in addition to this reproducibility, the relative ease with which GPa resistance seems able to evolve in E. coli is further underscored by the fact that none of these independently isolated mutants displayed any obvious growth defects at atmospheric pressure (data not shown). Together, these observations seem to indicate that this bacterium has the genetic blueprint to acquire extreme levels of HP resistance when selection forces it to do so. This apparent predisposition might also be reflected by the fact that natural isolates of E. coli display a great intrinsic variability with regard to HP resistance in the 200- to 700-MPa range studied so far (5, 6, 7).
To the best of our knowledge, the level of piezoresistance that E. coli developed during this study by far exceeds the currently recognized maximum for growth (i.e., 120 MPa) (2) or survival (i.e., 800 to 900 MPa) (8, 9) for any vegetative microorganism. In fact, it was generally assumed that vegetative microorganisms would never be able to survive exposures in the GPa range, as such pressures would irreversibly denature the proteome and disrupt membrane-related processes (10). Indeed, during in vitro studies most proteins unfold at pressures of 400 to 800 MPa and protein-protein and protein-nucleic acid interactions are disrupted. Furthermore, lipid model systems of cell membranes already undergo unfavorable fluid-gel transitions in the 100- to 300-MPa range (10). However, the extent to which these processes are reversible in a cellular context is not known.
Surprisingly, as shown in Fig. 1B, the evolved HP-resistant E. coli mutant displayed similar levels of survival when exposed to 1.2 and to 2 GPa. This could indicate that, despite the significant difference in their intensities, the two pressures have similar impacts on the cell that can be countered by the resistance mechanism. However, when we compared inactivation kinetics of the HP-resistant mutant at 800 MPa with that at 2 GPa, we found that the inactivation rate at 2 GPa was significantly lower than that at 800 MPa (see Fig. S1 in the supplemental material). The latter result is clearly reflected in the corresponding decimal reduction times: D2 GPa = 16 min > D800 MPa = 6.5 min (Fig. S1). It should be noted that within the 1.2- to 2-GPa range, water undergoes subsequent transitions from liquid into ice-VI and ice-VII crystal forms, as predicted by its pressure-temperature phase diagram. However, it is unclear whether or not such phase transitions could somehow contribute to the resilience of the HP-resistant mutant at these pressures.
In summary, we report on the extraordinary adaptive potential of a mesophilic nonsporulating bacterium to GPa-high pressures. These findings can have major implications in exploring the ecological boundaries for survival or adaption of cellular life with regard to HP as a limiting factor on Earth and perhaps other celestial bodies. Further analysis of the GPa-HP-resistant bacteria artificially evolved in this study will shed new light on how cells can safeguard the integrity of their proteins and membranes at these pressures and eventually how organisms can adapt to or have evolved from HP environments.
SUPPLEMENTAL MATERIAL
ACKNOWLEDGMENTS
This work was supported by a doctoral fellowship of the Flemish Agency for Innovation by Science and Technology (IWT-Vlaanderen, to D.V.), a postdoctoral fellowship of the Research Foundation - Flanders (FWO-Vlaanderen, to F.M.), a Senior Research Fellowship award of the Engineering and Physical Sciences Research Council (EPSRC EP/D07357X, to P.F.M.), and a grant from the Research Fund of the Katholieke Universiteit Leuven (STRT1/10/036, to A.A.).
Footnotes
Citation Vanlint, D., R. Mitchell, E. Bailey, F. Meersman, P. F. McMillan, et al. 2011. Rapid acquisition of gigapascal-high-pressure resistance by Escherichia coli. mBio 2(1):e00130-10. doi:10.1128/mBio.00130-10.
REFERENCES
- 1. Kashefi K., Lovley D. R. 2003. Extending the upper temperature limit for life. Science 301:934 [DOI] [PubMed] [Google Scholar]
- 2. Zeng X., Birrien J. L., Fouquet Y., Cherkashov G., Jebbar M., Querellou J., Oger P., Cambon-Bonavita M. A., Xiao X., Prieur D. 2009. Pyrococcus CH1, an obligate piezophilic hyperthermophile: extending the upper pressure-temperature limits for life. ISME J. 3:873–876 [DOI] [PubMed] [Google Scholar]
- 3. Sharma A., Scott J. H., Cody G. D., Fogel M. L., Hazen R. M., Hemley R. J., Huntress W. T. 2002. Microbial activity at gigapascal pressures. Science 295:1514–1516 [DOI] [PubMed] [Google Scholar]
- 4. Yayanos A. A. 2002. Are cells viable at gigapascal pressures? Science 297:295 [DOI] [PubMed] [Google Scholar]
- 5. Vanlint D., Michiels C. W., Aertsen A. 2011. Piezophysiology of the model bacterium Escherichia coli, p. 669-686. In: K. Horikoshi (Ed.), Extremophiles Handbook, Springer, Japan. [Google Scholar]
- 6. Alpas H., Kalchayanand N., Bozoglu F., Sikes A., Dunne C. P., Ray B. 1999. Variation in resistance to hydrostatic pressure among strains of food-borne pathogens. Appl. Environ. Microbiol. 65:4248–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robey M., Benito A., Hutson R. H., Pascual C., Park S. F., Mackey B. M. 2001. Variation in resistance to high hydrostatic pressure and rpoS heterogeneity in natural isolates of Escherichia coli O157:H7. Appl. Environ. Microbiol. 67:4901–4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hauben K. J., Bartlett D. H., Soontjens C. C., Cornelis K., Wuytack E. Y., Michiels C. W. 1997. Escherichia coli mutants resistant to inactivation by high hydrostatic pressure. Appl. Environ. Microbiol. 63:945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jofré A., Aymerich T., Bover-Cid S., Garriga M. 2010. Inactivation and recovery of Listeria monocytogenes, Salmonella enterica and Staphylococcus aureus after high hydrostatic pressure treatments up to 900 MPa. Int. Microbiol. 13:105–112 [DOI] [PubMed] [Google Scholar]
- 10. Daniel I., Oger P., Winter R. 2006. Origins of life and biochemistry under high-pressure conditions. Chem. Soc. Rev. 35:858–875 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.