Abstract
A seven-valent pneumococcal conjugate vaccine (PCV7) introduced in the United States in 2000 has been shown to reduce invasive pneumococcal disease (IPD) in both vaccinated children and adults through induction of herd immunity. We assessed the impact of infant immunization on pneumococcal pneumonia hospitalizations and mortality in all age groups using Health Care Utilization Project State Inpatient Databases (SID) for 1996 to 2006 from 10 states; SID contain 100% samples of ICD9-coded hospitalization data for the selected states. Compared to a 1996–1997 through 1998–1999 baseline, by the 2005–2006 season, both IPD and pneumococcal pneumonia hospitalizations and deaths had decreased substantially in all age groups, including a 47% (95% confidence interval [CI], 38 to 54%) reduction in nonbacteremic pneumococcal pneumonia (ICD9 code 481 with no codes indicating IPD) in infants <2 years old and a 54% reduction (CI, 53 to 56%) in adults ≥65 years of age. A model developed to calculate the total burden of pneumococcal pneumonia prevented by infant PCV7 vaccination in the United States from 2000 to 2006 estimated a reduction of 788,838 (CI, 695,406 to 875,476) hospitalizations for pneumococcal pneumonia. Ninety percent of the reduction in model-attributed pneumococcal pneumonia hospitalizations occurred through herd immunity among adults 18 years old and older; similar proportions were found in pneumococcal disease mortality prevented by the vaccine. In the first seasons after PCV introduction, when there were substantial state differences in coverage among <5-year-olds, states with greater coverage had significantly fewer influenza-associated pneumonia hospitalizations among children, suggesting that PCV7 use also reduces influenza-attributable pneumonia hospitalizations.
IMPORTANCE
Pneumonia is the world’s leading cause of death in children and the leading infectious cause of death among U.S. adults 65 years old and older. Pneumococcal conjugate vaccination of infants has previously been shown to reduce invasive pneumococcal disease (IPD) among seniors through prevention of pneumococcal transmission from infants to adults (herd immunity). Our analysis documents a significant vaccine-associated reduction not only in IPD but also in pneumococcal pneumonia hospitalizations and inpatient mortality rates among both vaccinated children and unvaccinated adults. We estimate that fully 90% of the reduction in the pneumonia hospitalization burden occurred among adults. Moreover, states that more rapidly introduced their infant pneumococcal immunization programs had greater reductions in influenza-associated pneumonia hospitalization of children, presumably because the vaccine acts to prevent the pneumococcal pneumonia that frequently follows influenza virus infection. Our results indicate that seven-valent pneumococcal conjugate vaccine use has yielded far greater benefits through herd immunity than have previously been recognized.
INTRODUCTION
Pneumonia is not only the world’s leading cause of death among children (1), but it is also the leading infectious cause of death among adults (2). Immunization of infants using pneumococcal conjugate vaccines has reduced invasive pneumococcal disease (IPD) and hospitalization for pneumonia in children in randomized trials (3–5). Since its introduction in the United States in 2000, seven-valent conjugate pneumococcal vaccine (PCV7) has reduced IPD dramatically, including in unvaccinated age groups, through induction of herd immunity (6–10).
Most of the burden of pneumococcal disease, however, is not from IPD but nonbacteremic pneumonia in adults 65 years old and older, that is, pneumococcal pneumonia without a positive culture from a sterile site. It is biologically plausible that interruption of transmission of vaccine-type Streptococcus pneumoniae can reduce this burden. A groundbreaking prospective observational study through 2004 on the Health Care Cost and Utilization Project (HCUP) Nationwide Inpatient Sample, a 20% sample of hospitalizations nationwide, found a significant reduction in ICD9-coded pneumococcal pneumonia in infants <2 years old and a nonsignificant trend toward such a reduction in both pneumococcal and all-cause pneumonia in persons ≥65 years old (11). De Wals et al. have reported a significant decline in pneumococcal (lobar) pneumonia in Canadian children <5 years of age after Canada introduced the vaccine for all children in December 2004 (12), and Jardine et al. very recently reported significant reductions in pneumococcal pneumonia in all age groups in Australia since the vaccine was introduced there in 2005 (13).
Prevention of pneumococcal infection may also reduce hospitalization following influenza. Much of the pneumonia hospitalization burden in all age groups occurs during the winter, a fact that has been attributed in part to bacterial pneumonia occurring as a complication of influenza virus infection (14–17). Influenza virus and S. pneumoniae have been shown to demonstrate a “lethal synergy” in animal models (18–20), and an analysis of an investigational nine-valent conjugate pneumococcal vaccine in a double-blind randomized trial (21) indicated that the rate of laboratory-confirmed influenza-associated pneumonia hospitalization was 45% lower among vaccine recipients than among controls.
In this paper, we describe a multipart investigation into the effects of the introduction of PCV7 on pneumonia putatively caused by S. pneumoniae infection in all age groups in the United States. We used National Immunization Survey data from the Centers for Disease Control and Prevention (CDC) to estimate the growing portion of the under-5-year-old age cohort that had been immunized in each season. For outcome data, we obtained complete (100%) hospitalization data available from 10 states that were part of the HCUP State Inpatient Databases (SID) for the entire period of 1996 through 2006. Examining ICD9-coded SID data, we quantified rate reductions coded as IPD, pneumococcal pneumonia, and all-cause pneumonia after PCV7 was introduced. Because specific pathogens responsible for pneumonia hospitalizations are rarely confirmed, ICD9-coded data only capture a fraction of the actual number of cases of pneumococcal pneumonia. We therefore adapted modeling techniques used by influenza epidemiologists (16, 22) to estimate the fraction of all-cause pneumonia attributable to S. pneumoniae, influenza virus, and respiratory syncytial virus (RSV) infections. We then used Poisson regression modeling to estimate the vaccine-associated reductions in the burden of IPD, pneumococcal pneumonia (directly coded and model attributed), and all-cause pneumonia in the United States. Finally, we used differences in coverage rates between states to assess the effect of the vaccine on influenza-related pneumonia. Our findings show that PCV7 has directly and indirectly reduced both IPD and pneumococcal pneumonia—including nonbacteremic pneumococcal pneumonia—in children and adults.
RESULTS
Reductions in hospitalization rates after PVC7 introduction.
For each of 10 SID states (Arizona, Colorado, Iowa, Massachusetts, New Jersey, New York, Oregon, Utah, Washington, and Wisconsin), we compared age-specific hospitalization rates for seasons after vaccine introduction to a baseline from the three seasons immediately before vaccine introduction (1996–1997 through 1998–1999). The SID are 100% samples of all hospitalizations at all types of hospitals. The 10 states we analyzed contain 24% of the U.S. population, and the SID data from these states contain records of over 1 million pneumonia cases per year.
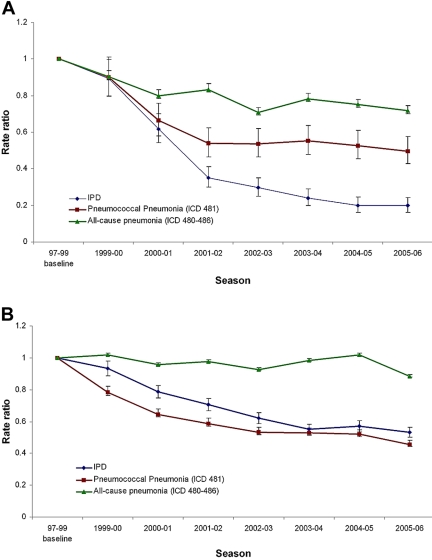
Plots of rate ratios (RRs) over time before and after the introduction of PCV7 show a pattern of reductions from the baseline for all age groups for IPD and pneumococcal pneumonia (ICD9 code 481) and for most age groups for all-cause pneumonia (ICD9 codes 480 to 486). Figure 1 shows the RRs over time for infants <2 years old and adults ≥65 years old. Table 1 shows the average rates and RRs of IPD, pneumococcal pneumonia, and all-cause pneumonia in the 10 states in the 2005–2006 season compared to the baseline for six age groups. Figure S1 in the supplemental material shows a representative plot of monthly pneumococcal pneumonia hospitalizations versus season over the entire period, in this case, for adults ≥65 years old (seniors) in New Jersey.
FIG 1 .
Rate ratio versus season, comparing a three-season pre-PCV7 introduction baseline outcome rate to those of subsequent seasons for all 10 study states combined and for (A) children under 2 years of age and (B) adults ≥65 years old.
TABLE 1 .
Annual hospitalization rates per 100,000 in the pre-PCV7 period in the 10 study states at baselinea and in the 2005–2006 season
| Age (yr) | Rate of IPD cases |
RR (95% CI) | Rate of pneumococcal (lobar) pneumonia cases (ICD9 481) |
RR (95% CI) | Rate of nonbacteremic pneumococcal pneumonia cases (ICD9 481 with no IPD code |
RR (95% CI) | Rate of all-cause pneumonia cases |
RR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2005–2006 | Baseline | 2005–2006 | Baseline | 2005–2006 | Baseline | 2005–2006 | |||||
| 0-1 | 27.8 | 5.5 | 0.20 (0.16–0.24) | 25.9 | 13.4 | 0.49 (0.43–0.58) | 22.1 | 12.4 | 0.53 (0.46–0.62) | 1,026.5 | 753.7 | 0.72 (0.70–0.73) |
| 2–4 | 5.4 | 2.0 | 0.35 (0.27–0.47) | 11.8 | 9.1 | 0.83 (0.72–0.96) | 10.5 | 8.5 | 0.88 (0.76–1.02) | 307.5 | 303.4 | 0.99 (0.96–1.01) |
| 5–17 | 1.4 | 0.7 | 0.47 (0.36–0.60) | 4.9 | 2.6 | 0.52 (0.46–0.59) | 4.4 | 2.4 | 0.54 (0.47–0.62) | 76.1 | 67.0 | 0.87 (0.85–0.90) |
| 18–39 | 3.5 | 1.4 | 0.38 (0.34–0.43) | 11.6 | 4.3 | 0.37 (0.35–0.40) | 9.4 | 3.5 | 0.39 (0.36–0.42) | 88.5 | 62.6 | 0.68 (0.67–0.70) |
| 40–64 | 8.9 | 6.0 | 0.64 (0.60–0.68) | 30.2 | 16.6 | 0.56 (0.54–0.58) | 24.8 | 13.6 | 0.56 (0.54–0.58) | 250.9 | 234.6 | 0.92 (0.91–0.93) |
| ≥65 | 30.6 | 17.1 | 0.53 (0.50–0.56) | 144.9 | 64.7 | 0.46 (0.44–0.47) | 126.1 | 55.9 | 0.46 (0.44–0.47) | 1,875. 2 | 1672. 6 | 0.88 (0.87–0.89) |
1996–1997 through 1998–1999 seasons.
We conducted a substudy that counted patients with the 481 code but with no mention of IPD codes, indicating hospitalization for lobar pneumonia or nonbacteremic pneumococcal pneumonia. The patients in this category accounted for ~85% of the total number of all patients with the 481 code for pneumococcal pneumonia, which suggests that the great majority of cases in this category were coded on the basis of X-ray findings rather than a blood culture growing a pneumococcus. We observed very similar temporal age patterns of reduction in both outcomes (Table 1). Note that the average incidence of cases at the baseline and in 2005–2006 are averages of the data from all 10 states, while the RRs are the means of the average RR reductions within individual states; thus, the rates in 2005–2006 cannot be multiplied by the RR given in Table 1 to reach the exact baseline rate.
PCV7 coverage among children <5 years old.
Using an age cohort method similar to that of Nuorti et al. (23), we analyzed CDC National Immunization Survey data (24) to estimate the seasonal PCV7 coverage among all children <5 years old who either (i) had received three or more vaccine doses, with the first dose occurring at less than 1 year of age, or (ii) had been given “catch-up” immunization of one or more doses, with the first dose occurring after 1 year of age. After the vaccine was introduced, the total proportion of vaccinated children increased steadily in all 10 states in our study, although the rate at which coverage increased varied among these states (see Fig. S2 in the supplemental material). We generated PCV7 coverage estimates for each season as the geometric mean of the coverage rates in the surrounding years (thus, the 1999–2000 value may be slightly overestimated, as most doses were given in the second half of the year 2000). By the 2005–2006 season, the coverage of children <5 years old was close to 80% in all 10 study states.
Monthly pneumonia hospitalizations attributable to S. pneumoniae and influenza virus.
Most pneumonia patients are not tested to determine the specific pathogen causing their illness. The numbers of patients given discharge diagnoses for RSV infection, influenza, and pneumococcal pneumonia thus substantially underestimate the true numbers of such events, although these values do reflect the epidemic patterns for these pathogens. We used all-age monthly time series of hospitalizations ICD9 coded as any mention influenza, RSV infection, and pneumococcal pneumonia as explanatory variables to estimate the proportions of all pneumonia hospitalizations attributable to the three pathogens responsible in each age group in each of the 10 states. We included RSV in the model to control for the effect of this respiratory pathogen, which is especially important among children; we do not further analyze RSV-related hospitalization trends here.
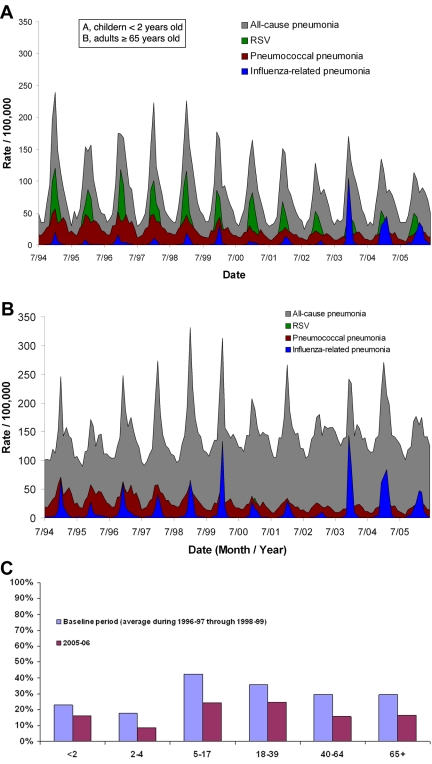
Representative time series for attributed rates of RSV infection- and influenza-related and pneumococcal pneumonia, as well as the proportions of all-cause pneumonia attributed by the model to the pneumococcus are shown in Fig. 2; representative model fits are shown in Fig. S3 in the supplemental material. As expected, our model found greater attributions of RSV in children (Fig. 2A) and influenza virus in seniors in terms of incidence rates (Fig. 2B).
FIG 2 .
Representative plots of rates of all-cause pneumonia (ICD9 codes 480 to 486, grey) and model attributions for RSV-related pneumonia (green), pneumococcal pneumonia (red), and influenza virus-related pneumonia (light blue). Data shown are from New Jersey for (A) children under 2 years of age and (B) adults ≥65 years old; panel C shows the proportion of all-cause pneumonia attributed by our model to the pneumococcus.
PCV7-associated reductions in the burden of hospitalizations for pneumococcal disease.
We applied Poisson regression modeling techniques to monthly time series of hospitalization data from 10 SID states for the 1996–1997 through 2005–2006 seasons and found statistically significant associations of PCV7 coverage with decreases in hospitalization rates for IPD, International Statistical Classification of Diseases and Related Health Problems (ICD)-coded pneumococcal pneumonia, model-attributed pneumococcal pneumonia, and all-cause pneumonia in all age groups. We then used U.S. census data and the modeling results—specifically, our estimates of the reduction in hospitalizations associated with incremental increases in vaccine coverage—to estimate the total reductions in the pneumococcal disease hospitalization burden associated with PCV7 vaccine use in the United States since the vaccine was introduced (Table 2). In percentage terms, the IPD burden declined the most sharply. The burdens of pneumococcal pneumonia and nonbacteremic pneumococcal pneumonia also declined significantly in all age groups. We found statistically significant reductions in all-cause pneumonia hospitalization in three age groups (infants <2 years old, 5 to 17 year olds, and 18 to 39 year olds) and no significant reduction in the population as a whole. Note that the relative rates given in Tables 1 and 2 are not directly comparable. Table 1 provides rates in the 2005–2006 season alone relative to the baseline, whereas the modeled reductions are cumulative across all of the study years and thus less sensitive to variability in individual study years, explaining, for example, the apparent discrepancy between the 40- to 64-year-old age group results in the two tables.
TABLE 2 .
Estimated PCV7-associated reductionsa in the U.S. burden of hospitalizations for various pneumococcal pneumonia outcomes, 1999–2000 through 2005–2006 seasons
| Age group | Estimated IPD reduction | 95% CI | Estimated pneumococcal (lobar) pneumonia (ICD9 481) reduction | 95% CI | Estimated nonbacteremic pneumococcal pneumonia (ICD9 481 with no IPD codes) reduction | 95% CI | Estimated attributed (model-derived) pneumococcal pneumonia reduction | 95% CI | Estimated all-cause pneumonia reduction | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| <2 | 8,440 | 8,101–8,752 | 4,437 | 3,550–5,216 | 3,399 | 4,119–2,572 | 36,442 | 31,928–40,667 | 80,494 | 56,295–103,022 |
| 2–4 | 2,025 | 1,766–2,244 | 1,442 | 741–2,067 | 934 | 1,590–188 | 2,540 | 2,156–2,899 | −3,920 | −16,325–7,818 |
| 5–17 | 1,528 | 1,257–1,772 | 4,544 | 3,863–5,170 | 3,977 | 4,595–3,305 | 38,211 | 35,064–41,187 | 8,879 | 368–17,121 |
| 18–39 | 8,592 | 7,658–9,432 | 28,344 | 26,324–30,249 | 21,808 | 23,411–20,094 | 86,925 | 74,592–97,881 | 82,299 | 47,421–113,857 |
| 40–64 | 7,270 | 5,428–9,004 | 30,536 | 27,469–33,450 | 24,614 | 26,976–22,169 | 122,239 | 104,095–139,195 | −35,071 | −71,505–−530 |
| ≥65 | 20,046 | 16,851–23,014 | 113,789 | 101,965–124,905 | 99,415 | 110,428–87,565 | 502,482 | 447,571–553,546 | 62,987 | −50,803–173,014 |
| Total | 47,899 | 41,060–54,218 | 183,093 | 163,911–201,057 | 154,147 | 171,118–135,893 | 788,838 | 695,406–875,376 | 195,668 | −34,550–414,303 |
Cumulative number of cases prevented since vaccine introduction.
PCV7-associated reductions in the burden of in-hospital mortality with pneumococcal disease.
To assess the mortality burden changes, we used the discharge status variable to find events where the patient had died while hospitalized. Using a similar Poisson modeling approach but seasonal rather than monthly data (because the numbers of in-hospital deaths were much smaller), we found significant associations of increases in PCV7 coverage with reductions in in-hospital mortality for IPD, pneumococcal pneumonia, and all-cause pneumonia diagnoses (Table 3). Because in-hospital deaths due to pneumococcal, influenza virus, and RSV infections are rare outcomes in most age groups, we did not have sufficient data to model the monthly mortality time series, a necessary step in estimating attributed pneumococcal pneumonia mortality.
TABLE 3 .
Estimated PCV7-associated reductionsa in the U.S. burden of mortality while hospitalized for IPD, pneumococcal pneumonia, or all-cause pneumonia, 1999–2000 through 2005–2006 seasons
| Age group | Estimated IPD reduction | 95% CI | Estimated pneumococcal pneumonia (ICD9 481) reduction | 95% CI | Estimated all-cause pneumonia reduction | 95% CI |
|---|---|---|---|---|---|---|
| <2 | 212 | 81–275 | 34 | –66–67 | 548 | 425–646 |
| 2–4 | 46 | 12–62 | 26 | –3–38 | 191 | 136–235 |
| 5–17 | 103 | 65–130 | 13 | –14–29 | 350 | 252–431 |
| 18–39 | 810 | 674–922 | 1,088 | 888–1,245 | 2,495 | 2,220–2,754 |
| 40–64 | 1,045 | 709–1,349 | 2,304 | 1,990–2,596 | 8,513 | 7,674–9,312 |
| ≥65 | 4,007 | 3,246–4,693 | 10,895 | 9,772–11,934 | 71,556 | 61,644–81,013 |
| Total | 6,222 | 4,788–7,431 | 14,360 | 12,568–15,908 | 83,653 | 72,351–94,391 |
Number of deaths prevented.
PCV7-associated reductions in all-cause pneumonia hospitalizations attributable to influenza virus.
The burden of influenza virus-attributable pneumonia varies substantially from season to season, depending on the characteristics of the strains that dominate each season. The seasons 1999–2000 through 2002–2003 were dominated by seasonal A/H1N1 or influenza virus B strains and were relatively mild compared to the baseline seasons. However, A/H3N2 strains dominated the 2003–2004 and 2004–2005 seasons, resulting in a sharp increase in the number of influenza-related pneumonia hospitalizations; these increases are reflected in Fig. 2. This variability, unrelated to the PCV7 vaccination program, hampered our ability to model time trends in influenza-related hospitalizations that were attributable to PCV7 use.
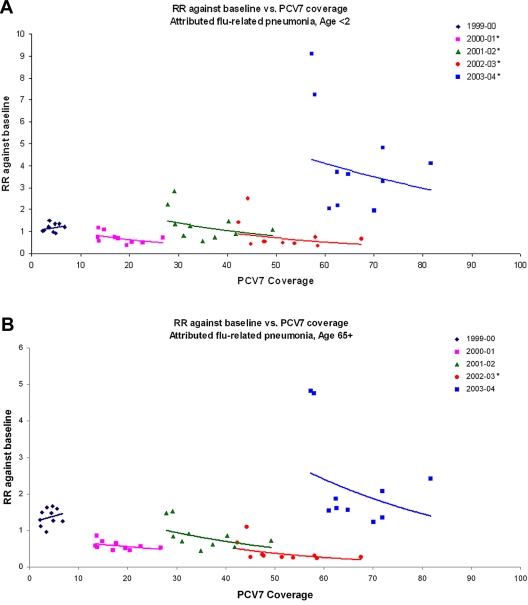
So, instead, we looked for single-season associations between state PCV coverage rates and changes in IPD and attributed influenza-related pneumonia hospitalization rates, for each of the seasons 2000–2001 through 2003–2004 and compared to a prevaccine baseline. For attributable influenza-related pneumonia in infants <2 years old, higher rates of coverage by state were significantly associated with a reduced disease burden in all four seasons (Fig. 3); among seniors, we found negative associations in all seasons, although these were significant only in the 2002–2003 season (Table 4). For IPD, we found a negative association between incidence and PCV7 coverage by state in all four seasons for children <2 years of age; however, this negative association was significant only for the 2001–2002 and 2002–2003 seasons; among seniors, the trend was negative in all seasons except 2000–2001 but was not significant in any season (see Fig. S4 in the supplemental material).
FIG 3 .
Scatterplots of state hospitalization RRs (season/baseline) versus state PCV coverage for the 1999–2000 through the 2003–2004 seasons for (A) attributed influenza-related pneumonia among those <2 years old and (B) attributed influenza-related pneumonia among those ≥65 years old. The lines represent exponential fits to the data from each season, showing the trend. An asterisk next to the year in each panel indicates a significant trend.
TABLE 4 .
Modeled rate reductions per 10 percentage point increase in PCV7 coverage for two age groups
| Season | Estimated reduction (95% CI) | |||
|---|---|---|---|---|
| IPD | Model-attributed influenza-related pneumonia | |||
| <2-yr-old group | ≥65-yr-old group | <2-yr-old group | ≥65-yr-old group | |
| 2000–2001 | 0.84 (0.51–1.39) | 1.09 (0.87–1.35) | 0.50 (0.29–0.85) | 0.91 (0.75–1.12) |
| 2001–2002 | 0.77 (0.60–0.98) | 0.94 (0.76–1.15) | 0.61 (0.39–0.96) | 0.85 (0.66–1.11) |
| 2002–2003 | 0.75 (0.62–0.90) | 0.96 (0.83–1.12) | 0.53 (0.36–0.78) | 0.80 (0.67–0.96) |
| 2003–2004 | 0.82 (0.62–1.09) | 0.93 (0.78–1.11) | 0.59 (0.37–0.92) | 0.68 (0.41–1.15) |
Residual hospitalization and mortality burden in 2006.
In the 2005–2006 season, the residual hospitalization burden among children less than 2 years old was 5.5 IPD cases per 100,000, 12.4 nonbacteremic ICD9 481 cases per 100,000, and 115 model-attributed pneumonia cases per 100,000. The remaining burdens among persons 65 years old and older were, of course, considerably higher: 17.1 IPD cases per 100,000, 55.9 noninvasive ICD9-coded cases per 100,000, and 271.7 model-attributed pneumococcal pneumonia cases per 100,000. For in-hospital mortality, the residual burden among infants <2 years old was 0.2 IPD and 0.2 noninvasive ICD9 code 481 cases per 100,000. The remaining burdens among persons 65 years old and older were 2.8 IPD and 3.5 noninvasive ICD9-coded cases per 100,000.
DISCUSSION
It has been demonstrated repeatedly that PCV7 has substantially reduced IPD among vaccinated children. Our data extend these observations to show significant reductions in IPD and pneumococcal pneumonia in all age groups. Our modeling results indicate that the vaccine has prevented nearly 800,000 hospitalizations for pneumococcal pneumonia in the United States during the period from 2000 to 2006. We note that 90% of the model-attributed pneumococcal pneumonia and 95% of the nonbacteremic pneumococcal pneumonia reductions were in adults ≥18 years old. These data show that indirect effects were responsible for most of the burden of pneumococcal disease prevented, although a substantial burden of both lobar and all-cause pneumonia remains.
The reductions in IPD we found in 10 states correspond closely to observations based on data from the CDC Active Bacterial Core (ABC) surveillance sites (9). By 2006, we found reductions of 80% and 65% in all-serotype IPD among infants <2 years old and children 2 to 4 years old, respectively, while Pilishvili et al. found a reduction of 76% among all children <5 years old. Similarly, for adults ≥65 years old, we found a reduction of all-serotype IPD of 47% by 2006, while Pilishvili et al. reported a reduction of 37% by 2007. This gives us confidence that using ICD-coded data can produce results similar to those of studies using laboratory-confirmed cases only. Moreover, Pilishvili et al. also observed that the declines in IPD rates were specific to events caused by the seven serotypes of S. pneumoniae in the vaccine; this key observation provides strong evidence that the vaccine in fact caused the reductions demonstrated here. Our analysis, however, cannot separate vaccine-type reductions from non-vaccine-type disease because ICD9 codes are not strain specific. Although the pattern of reductions in IPD incidence in our study reflects that observed in the CDC’s population-based ABC surveillance data, the baseline rates in our study (27.8 cases/100,000 in infants <2 years old and 30.6 cases/100,000 in adults ≥65 years old) are lower than those reported in the ABC study (56.8 and 60 cases/100,000, respectively) for the same time period (6). Unlike the ABC sites, no audit is possible in our study to identify missed cases. Our estimates of hospitalized patients with IPD therefore represent an underestimate of the true burden due to this condition.
Grijalva et al. (11) studied trends in pneumococcal and all-cause pneumonia rates before and after the year 2000. They attributed the changing trends in these outcomes before and after PCV7 introduction to the benefits of the vaccine (11, 25). They found that introduction of PCV7 vaccine coincided with declines in pneumococcal pneumonia rates in infants <2 years old. They also found a trend toward a reduction in pneumococcal pneumonia rates in adults ≥65 years old, although it was not statistically significant. However, while they did observe a reduction in pneumococcal pneumonia hospitalizations in infants <2 years old, among children 2 to 4 years old, this reduction was significant in 2004 (11) but no longer so in 2006 (25), even though this age group had achieved high PCV7 coverage by 2006. We found the lowest reduction (17%) in pneumococcal pneumonia in this age group and a nonsignificant reduction of 12% when bacteremic cases were excluded. The pneumococcal types included in PCV7 decrease in frequency after 2 years of age, and the proportion of nonvaccine types is therefore greater above that age (26). Children who get pneumonia at 2 to 4 years of age tend to have a greater frequency of risks, such as group child care. These risks continue to make them susceptible to non-PCV7 types, so that replacement disease has a larger impact on this age group than on infants <2 years of age (27). Nelson et al. conducted a comprehensive, population-based evaluation of trends in pneumonia and influenza (P&I) rates in a health maintenance organization population and found a nonsignificant 40% decline in the rates of hospitalization of infants for community-acquired pneumonia from 1998 to 2004, with no consistent evidence of a decline in adult rates and thus no evidence of indirect PCV7 benefits (28). However, this study was of a smaller population (~800,000), and the severe 2003–2004 Fujian A/H3N2 influenza season likely led to increased P&I rates in the post-PCV period and an underestimation of vaccine program benefits.
Our study extends these findings using a longer time series, 100% state inpatient data, and all identified (not just primary diagnosis) cases. It also introduces a novel modeling approach to overcome the nonspecificity of the all-cause pneumonia endpoint, with the result that we find significant reductions in pneumococcal pneumonia in all age groups after the PCV7 launch in the United States. A primary goal of our investigation was to tease out the specific effects of PCV7 vaccination on pneumococcal pneumonia rates. In particular, we wanted to look at the age-specific effect of the vaccine on the total pneumococcal pneumonia burden, including both the “tip of the iceberg” that is indicated by the ICD9 481 code and the much larger “under-the-waterline” portion that, although caused by S. pneumoniae infection, is not diagnosed as such. We believe that model-attributed pneumococcal pneumonia, being more specific to S. pneumoniae infection, is a more telling outcome than all-cause pneumonia, which is affected by many viral and bacterial infections that PCV7 cannot prevent.
To attribute the pneumococcal pneumonia portion of all-cause pneumonia, we adapted statistical methods that influenza epidemiologists have long used to attribute a portion of the winter rise in pneumonia hospitalizations and deaths to influenza (29–31). Specifically, we modified the approach of Thompson et al. (16), which relied on the time pattern of laboratory-confirmed illness due to RSV and influenza virus to estimate the portion of hospitalizations related to influenza (16). We, however, used ICD-coded hospitalizations specific to respiratory pathogens and included hospitalizations due not only to influenza virus and RSV infections but also to pneumococcal pneumonia. In this regard, we followed Pitman et al. (22) in that we included time patterns for multiple pathogens to attribute a portion of the observed all-cause pneumonia pattern to each pathogen. Doing so allowed us to estimate the proportion of all-cause pneumonia attributable to S. pneumoniae infection while controlling for pneumonia associated with the other two pathogens.
Regarding our modeling of the reductions in burden, with the exception of all-cause pneumonia, we found significant reductions in hospitalizations for each age group and for each outcome (IPD and observed and model-attributed pneumococcal pneumonia) across all age groups, with patterns very much like those estimated from ABC data derived from laboratory-confirmed IPD cases. For all-cause pneumonia, however, we found a significant burden reduction only among infants <2 years old and a nonsignificant reduction of 200,000 hospitalizations across all age groups (Table 2). We attribute this result to the fact that all-cause pneumonia is the least specific outcome analyzed and particularly susceptible to variability in influenza severity between seasons. For example, across all age groups at the baseline (1996–1997 through 1998–1999), our model attributes ~30% of what is coded as all-cause pneumonia to pneumococcal infection and 7% to influenza across all age groups.
Another goal of our investigation was to assess the effect of PCV7 on influenza-related pneumonia. The variability of influenza season severity confounded our time series modeling efforts. We therefore studied state differences in influenza-attributed pneumonia within single seasons, taking advantage of between-state differences in PCV7 coverage up through the 2003–2004 season, when the recommendation for influenza immunization was extended to children and <10% of young children were fully immunized against influenza (32). We used a Poisson regression technique to evaluate whether, for each age group and season, states with higher coverage had significantly greater reductions than those with lower coverage. Because we included the baseline of each state and age group as a covariate, each acted as its own control for bias resulting from interstate differences in socioeconomic status and other potential confounders.
In these single-season analyses, we found that reductions in model-attributed influenza-related pneumonia hospitalization were significantly associated with higher PCV7 coverage for the first four seasons after vaccine use began; although the trend was toward reduction among seniors, the results were not significant (Table 4). The window of time within which there was significant variance between states in PCV coverage and before there was widespread herd immunity to the PCV7 types in the United States was limited to these seasons. During this time, we see an association of 10% PCV7 coverage variance with significant 39 to 50% reductions in influenza-associated pneumonia in infants <2 years old. This is similar in magnitude to the observation of a 45% reduction in influenza-associated pneumonia hospitalization among African infants <2 years old who received PCV9 vaccination in an environment in which the PCV9 types accounted for only half of the types causing disease in that age group (21). These data suggest that the majority of influenza-associated pneumonia hospitalizations in this age group may be due to pneumococcal coinfection.
Although the associated reductions with influenza are encouraging, we also note that the model failed to detect significant reductions for IPD in seniors (column 2, Table 4) although ABC data have shown it and we have already demonstrated it in Table 1 and Figure 1. This was likely the result of instability of the model as only few datapoints were available for single-year analysis. Although the estimates for rate reductions in IPD produced by our single-year analysis have wide confidence intervals (CIs), the estimates agree reasonably well with those produced by our multiyear model, namely, 0.81 and 0.93 for the youngest and oldest age groups, respectively, per 10 percentage point increase in PCV7 coverage. This helps to increase our confidence in our model-attributed influenza-related pneumonia results.
We recognize at least three potential limitations of our study. First, we relied on ICD9 codes for the status of the inpatients we studied. The cases were not ascertained by medical chart review, and thus, our approach assumes that physicians assigned ICD9-coded diagnoses correctly. Specifically, since ICD9 code 481 is indicated for cases of S. pneumoniae or lobar pneumonia, we cannot know whether the doctor reached that conclusion from laboratory testing, X-ray results, or clinical judgment; however, because the temporal trend resembles that of IPD in our study, we can argue that the 481 code is capturing the “ground truth” reduction in pneumococcal disease. Moreover, because only a small subset (10 to 20%) of all records with ICD9 code 481 also have IPD codes, we can assume that in most cases ICD9 code 481 represents clinical lobar pneumonia rather than microbiologically defined pneumococcal pneumonia.
Second, the method we used to attribute a portion of all-cause pneumonia to S. pneumoniae infection (Poisson regression modeling) assumes that the trends and patterns in the ICD-coded explanatory variables changed over time only as a result of vaccine effectiveness. If, for example, the PCV7 program had caused physicians to become less likely to diagnose S. pneumoniae infection and use ICD9 code 481—because they submitted samples for testing less frequently after the vaccine was introduced, for example—then the model would tend to overestimate the benefits of the PCV7 program. Although this phenomenon may have occurred in outpatient settings, we think it less likely for the diagnosis of inpatients, because hospital physicians may be more responsive to medical and economic imperatives to order laboratory testing to determine the etiology of their patients’ illnesses.
Third, we did not take into account aging within the senior age group; an increasing percentage of seniors are over 80 years of age, and the risk of pneumonia hospitalization increases with age. This may explain, in part, the finding that the all-cause pneumonia hospitalization rate did not decrease over time in the oldest age group (Fig. 1B). Thus, by not controlling tightly for age in persons over 65, we may have underestimated the true benefits of the vaccine program for seniors in terms of all-cause pneumonia reduction. For the more specific outcomes like IPD and S. pneumoniae pneumonia (ICD9 code 481), this is less of a problem in that the estimated reduction is profound.
In early 2010, a new 13-valent version of the vaccine (PCV13) was introduced in the United States with the promise of further reducing the residual burden of pneumococcal disease. According to our study, in the 2005–2006 season, there was a residual hospitalization burden of 5.5 IPD cases, 12.4 noninvasive ICD9 481 cases, and 115 model-attributed pneumococcal pneumonia cases per 100,000 among children less than 2 years of age, with considerably higher residual burdens among adults. We plan to continue to follow these rates in years to come, in order to assess the magnitude and sustainability of the disease burden reduction following PCV13 introduction.
The ability of PCV7 to induce herd immunity invites a discussion of how best to protect seniors, a group that does not respond as well to vaccination because of immune senescence or underlying illness. Although PCV7-vaccinated children accounted for only ~5% of the U.S. population by 2006, our study suggests that this was sufficient to reduce the burden of pneumococcal disease substantially in all age groups. Our results further suggest that more than 90% of the prevention of hospitalizations and deaths due to pneumonia attributable to the vaccine occurred among patients ≥18 years old, and most of this occurred among those ≥65 years old. There is evidence that this group has not been well protected by direct immunization with either the 23-valent pneumococcal vaccine (33) or influenza vaccine (34–37). It is possible that indirect protection (herd immunity) through vaccination of children will more effectively protect seniors against pneumococcal disease.
Immunization with PCV7 in the United States has led to profound reductions in the disease burden among adults in the first 7 years of the program. Whether this continues to be the case in the future should be monitored carefully due to the possibility of strain replacement. Whether the U.S. experience can be extrapolated to other countries remains to be seen. The different patterns of social mixing, overcrowding, and residual carriage of vaccine types among HIV-infected infants (38) all demand caution in extrapolating these observations to developing countries, where studies on herd immunity induced by PCV introduction will be an essential part of vaccination impact measurement.
MATERIALS AND METHODS
Data sources.
The HCUP maintains SID from many U.S. states (39). The annual state-specific SID files contain data elements in a uniform format, and most files contain data on a state’s total hospital discharges coded according to the ICD. We based our analysis on available SID data from 10 states for 1996 to 2006 that provided the admission month and the age in years. Because SID data have no personal identifiers, this study is exempt from Institutional Review Board consideration.
The CDC National Immunization Survey (NIS) collects vaccination histories for children 19 to 35 months old to estimate national and state level vaccination coverage for many vaccines, including PCV7. We used NIS public use SAS data files available at the CDC website to estimate vaccine coverage in children <5 years old in each state and for each study season (24).
We used U.S. Census Bureau estimates of yearly U.S. age- and state-specific populations to denominate the number of hospitalizations for each outcome. We performed all statistical analyses with SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC). We defined each season as July of one year through June of the next.
PCV7 coverage estimates.
We developed an age cohort model strategy similar to that of a recent CDC study (23) to convert detailed survey information into corrected historic vaccine uptake. We estimated vaccination coverage rates by 1-year infant age cohort for each calendar year and then summed these to compute PCV7 coverage for all children 0 to 59 months old, defining as covered all children who received either three or more doses, with the first occurring at <1 year of age, or one or more catch-up doses, with the first occurring at >1 year of age. We converted these annual coverage estimates to seasonal estimates for the 1999–2000 through 2005–2006 winter seasons by averaging coverage in each pair of years (e.g., we took the average of the 2002 and 2003 coverage to arrive at the coverage for the 2002–2003 season).
ICD9-coded outcome definitions and rate comparisons.
For each state and season, we extracted the numbers of hospitalizations for each outcome from among all of the listed diagnosis codes. We defined IPD as ICD9 code 320.1 or 038.2 or as codes 320.8, 790.7, or 038.9 and 041.2; pneumonia with diagnosed S. pneumoniae infection as ICD9 code 481; and all-cause pneumonia as ICD9 codes 480 to 486. Nonbacteremic pneumococcal pneumonia cases were defined as those with any mention of an ICD9 code 481 diagnosis but without mention of a diagnosis of IPD as defined above. We scanned across discharge diagnoses in each patient record for any mention of these disease codes. We used the disposition information at discharge to identify the subset of hospitalizations for each outcome that resulted in inpatient death. We constructed time series of each outcome for each state and for six age groups, <2 years old, 2 to 4 years old, 5 to 17 years old, 18 to 39 years old, 40 to 64 years old, and ≥65 years old. Baseline rates before introduction of PCV7 were defined as the average annualized rates during the 1996–1997 through 1998–1999 seasons; incidence RR estimates and 95% CIs were calculated using outcome-specific Poisson regression models.
Attributing pneumococcal and influenza-associated pneumonia hospitalizations.
Because influenza virus infection is rarely confirmed by laboratory testing and because a triggering influenza virus infection is often resolved by the time a patient presents with secondary complications such as bacterial pneumonia, it is not possible to directly assess the influenza disease burden. Modeling the pediatric burden of influenza is further complicated by the concurrent impact of RSV during winter months. Moreover, because most pneumonia hospitalizations are not linked to a specific pathogen, pneumonia due to infection with S. pneumoniae is frequently not recorded as such on hospital discharge forms.
To overcome these limitations, we applied a Poisson regression modeling strategy to monthly time series of outcome incidences per 100,000 in order to estimate the seasonal influenza-related and pneumococcal pneumonia-associated burdens in our six age groups. Our strategy was similar to that used by Thompson et al. (16) to assess influenza-related pneumonia. However, instead of weekly laboratory virus surveillance data for influenza and RSV epidemic patterns, we used ICD9-coded counts of influenza (ICD9 487, any mention) and RSV infection (ICD9 480.1, any mention) as explanatory variables, similar to Pitman et al. (22); we also included S. pneumoniae pneumonia (ICD9 481, any mention) as a third explanatory respiratory pathogen variable. A linear trend and a sinusoidal wave component accounted for seasonality and secular trends not captured by the “pathogen” explanatory variables. The best-fitting model was of the form
where AC pneumonia is all-cause pneumonia, defined as ICD codes 480 to 486 as the primary cause (removing records with first-listed ICD9 codes of 480.1 and 481); influenza (ICD code 487), RSV (ICD code 480.1), and pneumococcus (ICD code 481) are the monthly rates of hospitalizations specifically associated with each outcome in each state (all ages, any mention); and month is the running month variable. The cyclical terms track additional seasonality in the pneumonia data.
We computed fractions of all-cause pneumonia attributed to influenza virus, RSV, and S. pneumoniae for all available state/age group time series. For most state/age group combinations, all of the variables in the model were significant. However, we did not change the model form to accommodate states or age groups in cases where not all explanatory variables were significant at the P < 0.05 level. If any parameter value was less than zero, we set the number of attributed cases to zero. And finally, we summed the model attribution and the ICD9-coded attribution to generate the total attributed fraction of all-cause pneumonia to S. pneumoniae, RSV, and influenza virus, respectively. Figure S3 in the supplemental material shows a typical model fit, here for data from children 2 to 4 years of age in New Jersey.
Modeling reductions in pneumococcal disease burden associated with PCV7 use.
We first constructed Poisson regression models to assess the effect of PCV7 coverage on hospitalization and in-hospital mortality. We analyzed time series extracted from SID data from 10 states spanning the 1996–1997 through 2005–2006 seasons, with the exception of Utah, for which data were unavailable for the 1996–1997 season. We constructed time series for six age groups, younger than 2 years old, 2 through 4 years old, 5 through 17 years old, 18 through 39 years old, 40 through 64 years old, and 65 years old or older. RRs and accompanying 95% CIs were calculated to represent the association between 10 percentage point increments in PCV7 coverage and outcome rates.
For hospitalization rates, we ran age-specific Poisson regressions on monthly time series for each state and age group. The model form that best fit the data was
where Y is the number of hospitalizations during a particular month for a specific age group, N is the population offset, and t is the calendar month, β0 yielded the intercept while β1 and β2 accounted for seasonal changes in hospitalizations and β3 accounted for the effect of PCV7 coverage in children <5 years old. For in-hospital mortality rates, we applied the same model form to seasonal counts of cases in which a patient had both a diagnosis code associated with each outcome and a discharge status code indicating that the patient died in the hospital.
We used the results of these analyses to estimate cumulative national reductions in disease and mortality burdens associated with PCV7 use in each age group. For each outcome, we first used U.S. census data and the aggregate seasonal rates for the 1996–1997 through the 1998–1999 seasons from 10 states to estimate the national burden. Then, for each season and age group, we used national PCV7 coverage, the RR per percentage point increment in coverage, and U.S. census data to estimate the PCV7-associated change in burden for each season and summed these reductions for the 1999–2000 through the 2005–2006 seasons.
Single-season analysis of IPD and model-attributed influenza-related pneumonia.
Using SID data from 10 states for each age group, we determined the ratio of the rates of IPD and model-attributed influenza-related pneumonia in each post-PCV7 season compared to a baseline determined by the average of rates in the 1996–1997 through the 1998–1999 seasons. We then used Poisson regression to model the relative rate reduction associated with a 10 percentage point increase in PCV7 coverage and calculated two-sided P values to test the hypothesis of no effect of vaccination.
SUPPLEMENTAL MATERIAL
ACKNOWLEDGMENTS
We acknowledge financial support from Pfizer for this study.
The sponsors were not involved in the study design, execution, or interpretation and reporting of the results. K.P.K. has received consulting fees from Pfizer, Merck, GSK, Novartis, and Sanofi Aventis. L.S. has served on advisory panels for Merck and Novartis and has received consulting fees from BioCryst and SDI Health. R.J.T. has received consulting fees from SDI Health. Y.Y.X. has received consulting fees from Sanofi-Aventis, Sanofi-Pasteur, and Pfizer.
Footnotes
Citation Simonsen, L., R. J. Taylor, Y. Young-Xu, et al. 2010. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. mBio 2(1):e00309-10. doi:10.1128/mBio.00309-10.
REFERENCES
- 1. Black R. E., Cousens S., Johnson H. L., Lawn J. E., Rudan I., Bassani D. G., Jha P., Campbell H., Walker C. F., Cibulskis R., Eisele T., Liu L., Mathers C. 2010. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375:1969–1987 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization 2004. World health report. WHO, Geneva, Switzerland [Google Scholar]
- 3. Klugman K. P., Madhi S. A., Huebner R. E., Kohberger R., Mbelle N., Pierce N. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341–1348 [DOI] [PubMed] [Google Scholar]
- 4. Cutts F. T., Zaman S. M., Enwere G., Jaffar S., Levine O. S., Okoko J. B., Oluwalana C., Vaughan A., Obaro S. K., Leach A., McAdam K. P., Biney E., Saaka M., Onwuchekwa U., Yallop F., Pierce N. F., Greenwood B. M., Adegbola R. A. 2005. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in the Gambia: randomised, double-blind, placebo-controlled trial. Lancet 365:1139–1146 [DOI] [PubMed] [Google Scholar]
- 5. Black S., Shinefield H., Fireman B., Lewis E., Ray P., Hansen J. R., Elvin L., Ensor K. M., Hackell J., Siber G., Malinoski F., Madore D., Chang I., Kohberger R., Watson W., Austrian R., Edwards K. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente vaccine study center group. Pediatr. Infect. Dis. J. 19:187–195 [DOI] [PubMed] [Google Scholar]
- 6. Whitney C. G., Farley M. M., Hadler J., Harrison L. H., Bennett N. M., Lynfield R., Reingold A., Cieslak P. R., Pilishvili T., Jackson D., Facklam R. R., Jorgensen J. H., Schuchat A. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737–1746 [DOI] [PubMed] [Google Scholar]
- 7. Poehling K. A., Talbot T. R., Griffin M. R., Craig A. S., Whitney C. G., Zell E., Lexau C. A., Thomas A. R., Harrison L. H., Reingold A. L., Hadler J. L., Farley M. M., Anderson B. J., Schaffner W. 2006. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA 295:1668–1674 [DOI] [PubMed] [Google Scholar]
- 8. Kyaw M. H., Lynfield R., Schaffner W., Craig A. S., Hadler J., Reingold A., Thomas A. R., Harrison L. H., Bennett N. M., Farley M. M., Facklam R. R., Jorgensen J. H., Besser J., Zell E. R., Schuchat A., Whitney C. G. 2006. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 354:1455–1463 [DOI] [PubMed] [Google Scholar]
- 9. Pilishvili T., Lexau C., Farley M. M., Hadler J., Harrison L. H., Bennett N. M., Reingold A., Thomas A., Schaffner W., Craig A. S., Smith P. J., Beall B. W., Whitney C. G. , Moore M. R. 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201:32–41 [DOI] [PubMed] [Google Scholar]
- 10. Pelton S. I., Loughlin A. M., Marchant C. D. 2004. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr. Infect. Dis. J. 23:1015–1022 [DOI] [PubMed] [Google Scholar]
- 11. Grijalva C. G., Nuorti J. P., Arbogast P. G., Martin S. W., Edwards K. M., Griffin M. R. 2007. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 369:1179–1186 [DOI] [PubMed] [Google Scholar]
- 12. De Wals P., Robin E., Fortin E., Thibeault R., Ouakki M., Douville-Fradet M. 2008. Pneumonia after implementation of the pneumococcal conjugate vaccine program in the province of Quebec, Canada. Pediatr. Infect. Dis. J. 27:963–968 [DOI] [PubMed] [Google Scholar]
- 13. Jardine A., Menzies R. I., McIntyre P. B. 2010. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr. Infect. Dis. J. 29:607–612 [DOI] [PubMed] [Google Scholar]
- 14. Simonsen L., Fukuda K., Schonberger L. B., Cox N. J. 2000. The impact of influenza epidemics on hospitalizations. J. Infect. Dis. 181:831–837 [DOI] [PubMed] [Google Scholar]
- 15. Cate T. R. 1987. Clinical manifestations and consequences of influenza. Am. J. Med. 82:15–19 [DOI] [PubMed] [Google Scholar]
- 16. Thompson W. W., Shay D. K., Weintraub E., Brammer L., Bridges C. B., Cox N. J., Fukuda K. 2004. Influenza-associated hospitalizations in the United States. JAMA 292:1333–1340 [DOI] [PubMed] [Google Scholar]
- 17. Mullooly J. P., Bridges C. B., Thompson W. W., Chen J., Weintraub E., Jackson L. A., Black S., Shay D. K. 2007. Influenza- and RSV-associated hospitalizations among adults. Vaccine 25:846–855 [DOI] [PubMed] [Google Scholar]
- 18. Berendt R. F., Long G. G., Walker J. S. 1975. Influenza alone and in sequence with pneumonia due to Streptococcus pneumoniae in the squirrel monkey. J. Infect. Dis. 132:689–693 [DOI] [PubMed] [Google Scholar]
- 19. Peltola V. T., McCullers J. A. 2004. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr. Infect. Dis. J. 23(1 Suppl.):S87–S97 [DOI] [PubMed] [Google Scholar]
- 20. Seki M., Yanagihara K., Higashiyama Y., Fukuda Y., Kaneko Y., Ohno H., Miyazaki Y., Hirakata Y., Tomono K., Kadota J., Tashiro T., Kohno S. 2004. Immunokinetics in severe pneumonia due to influenza virus and bacteria coinfection in mice. Eur. Respir. J. 24:143–149 [DOI] [PubMed] [Google Scholar]
- 21. Madhi S. A., Klugman K. P. 2004. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat. Med. 10:811–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pitman R. J., Melegaro A., Gelb D., Siddiqui M. R., Gay N. J., Edmunds W. J. 2007. Assessing the burden of influenza and other respiratory infections in England and Wales. J. Infect. 54:530–538 [DOI] [PubMed] [Google Scholar]
- 23. Nuorti J. P., Martin S. W., Smith P. J., Moran J. S., Schwartz B. 2008. Uptake of pneumococcal conjugate vaccine among children in the 1998–2002 United States birth cohorts. Am. J. Prev. Med. 34:46–53 [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention, Atlanta, GA. 2010. Datasets and related documentation for the national immunization survey. [Google Scholar]
- 25. Centers for Disease Control and Prevention 2009. Pneumonia hospitalizations among young children before and after introduction of pneumococcal conjugate vaccine—United States, 1997–2006. MMWR Morb. Mortal. Wkly. Rep. 58:1–4 [PubMed] [Google Scholar]
- 26. Hausdorff W. P., Feikin D. R., Klugman K. P. 2005. Epidemiological differences among pneumococcal serotypes. Lancet Infect. Dis. 5:83–93 [DOI] [PubMed] [Google Scholar]
- 27. Pilishvili T., Zell E. R., Farley M. M., Schaffner W., Lynfield R., Nyquist A. C., Vazquez M., Bennett N. M., Reingold A., Thomas A., Jackson D., Schuchat A., Whitney C. G. 2010. Risk factors for invasive pneumococcal disease in children in the era of conjugate vaccine use. Pediatrics 126:e9–e17 [DOI] [PubMed] [Google Scholar]
- 28. Nelson J. C., Jackson M., Yu O., Whitney C. G., Bounds L., Bittner R., Zavitkovsky A., Jackson L. A. 2008. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine 26:4947–4954 [DOI] [PubMed] [Google Scholar]
- 29. Serfling R. E., Sherman I. L., Houseworth W. J. 1967. Excess pneumonia-influenza mortality by age and sex in three major influenza A2 epidemics, United States, 1957–58, 1960 and 1963. Am. J. Epidemiol. 86:433–441 [DOI] [PubMed] [Google Scholar]
- 30. Simonsen L., Reichert T. A., Viboud C., Blackwelder W. C., Taylor R. J., Miller M. A. 2005. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch. Intern. Med. 165:265–272 [DOI] [PubMed] [Google Scholar]
- 31. Thompson W. W., Weintraub E., Dhankhar P., Cheng P. Y., Brammer L., Meltzer M. I., Bresee J. S., Shay D. K. 2009. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respi. Viruses 3:37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention 2009. Influenza vaccination coverage among children aged 6 months–18 years—eight immunization information system sentinel sites, United States, 2008–09 influenza season. MMWR Morb. Mortal. Wkly. Rep. 58:1059–1062 [PubMed] [Google Scholar]
- 33. Jackson L. A., Neuzil K. M., Yu O., Benson P., Barlow W. E., Adams A. L., Hanson C. A., Mahoney L. D., Shay D. K., Thompson W. W. 2003. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N. Engl. J. Med. 348:1747–1755 [DOI] [PubMed] [Google Scholar]
- 34. Jackson M. L., Nelson J. C., Weiss N. S., Neuzil K. M., Barlow W., Jackson L. A. 2008. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet 372:398–405 [DOI] [PubMed] [Google Scholar]
- 35. Fireman B., Lee J., Lewis N., Bembom O., van der Laan M., Baxter R. 2009. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am. J. Epidemiol. 170:650–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simonsen L., Taylor R. J., Viboud C., Miller M. A., Jackson L. A. 2007. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect. Dis. 7:658–666 [DOI] [PubMed] [Google Scholar]
- 37. Simonsen L. 2010. Available evidence points to low effectiveness of influenza vaccines for older people. Evid. Based Med. 15:109–110 [DOI] [PubMed] [Google Scholar]
- 38. Madhi S. A., Adrian P., Kuwanda L., Cutland C., Albrich W. C., Klugman K. P. 2007. Long-term effect of pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae—and associated interactions with Staphylococcus aureus and Haemophilus influenzae colonization—in HIV-infected and HIV-uninfected children. J. Infect. Dis. 196:1662–1666 [DOI] [PubMed] [Google Scholar]
- 39. Steiner C., Elixhauser A., Schnaier J. 2002. The healthcare cost and utilization project: an overview. Eff. Clin. Pract. 5:143–151 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.