Abstract
Breast cancer is the most prevalent type of cancer in American women. Exercise appears to diminish many of the side effects resulting from breast cancer and its treatment. Very little research, however, has compared the outcomes of varying lengths of combined aerobic and resistance training exercise interventions on physiological and psychological parameters in breast cancer survivors. The purpose of this study was to compare the physiological and psychological outcomes following 3 and 6 months of exercise in breast cancer survivors. Breast cancer survivors (N = 114) participated in either 3 months of prescriptive, individualized exercise (3M; n = 29), 6 months of prescriptive, individualized exercise (6M; n = 68), or served as sedentary controls (C; n = 17). Cancer survivors completed a medical evaluation and assessment at baseline followed by a predetermined 3- or 6-month exercise intervention. Cancer survivors in the control group performed no exercise between the initial assessment and 6-month reassessment. Cardiovascular endurance, pulmonary function, muscular endurance, fatigue, and symptoms of depression were assessed at baseline and post intervention. Repeated measures ANCOVA revealed improvements (P < 0.05) in cardiovascular endurance, fatigue, and symptoms of depression in breast cancer survivors undergoing 3- and 6-month individualized exercise interventions. Breast cancer survivors exercising for 6 months showed additional improvements (P < 0.05) in pulmonary function and muscular endurance. Cancer survivors in the control group did not improve in cardiovascular endurance, pulmonary function, muscular endurance, or fatigue. Three months of individualized, prescriptive exercise leads to improved cardiovascular endurance, fatigue, and symptoms of depression in breast cancer survivors. Additional benefits are seen if exercise is continued for a total of 6 months.
Keywords: Breast cancer survivors, Cancer-related fatigue, Depression, Exercise, Muscular endurance, Pulmonary function
Introduction
Breast cancer is the most commonly diagnosed cancer in American women, with one in eight women expected to develop breast cancer during her lifetime [1]. Fortunately, earlier detection and more aggressive therapies have improved the survival rate by nearly 15% over the past two decades, to an estimated overall survival rate of 89% across all breast cancer stages [1]. Despite the prevalence of breast cancer, the majority of women are surviving and facing the task of combating the physiological and psychological problems resulting from cancer and its treatment.
Treatment for breast cancer often includes surgery followed by adjuvant treatment with chemotherapy and/or radiation [2]. Surgical treatment and radiation can result in significant upper body limitations [3-5] including impaired range of motion [6, 7], reduction in muscle activity [8], and the development of lymphedema [9] which can lead to a reduction in quality of life and psychological distress [10]. Chemotherapy is commonly used as an adjuvant treatment to reduce the likelihood of metastasis [2]. Antineoplastic drugs are a highly effective treatment against cancer cells, increasing the time between initial diagnosis and recurrence and improving relative survival rates [11]. However, nonneoplastic cells are also affected. The serious side effects of chemotherapy may be apparent immediately following treatments or not until years after treatment has ended [12]. The manifestations of chemotherapy-induced tissue damage can include cardiovascular toxicity [13-16], pulmonary toxicity [17, 18], and hematological toxicity [19-22], all of which may result in impaired functional capacity.
Breast cancer and the treatment combinations that are employed often lead to cancer-related fatigue, the most common and persistent side effect of cancer treatment, often lasting months to years after treatment completion [23-26]. Cancer-related fatigue in breast cancer survivors can be associated with depression, sleep disturbances, and pain [27]. Similarly, depression increases in the first year after breast cancer diagnosis [28], and increased depression following breast cancer diagnosis is positively correlated with mortality [29].
Exercise has previously been found to be a valuable rehabilitation tool for cancer survivors during and following treatment [30-37]. Exercise interventions implemented concurrent with breast cancer treatment can improve cardiovascular fitness [38, 39], muscular strength [40], and body composition [40, 41] while attenuating a reduction in bone mineral density [42] and reducing fatigue [38, 43, 44]. Exercise following the completion of breast cancer treatment can result in improved cardiovascular fitness [45, 46], pulmonary function [38], muscular strength and endurance [45-47], body composition [46], quality of life [46, 48], and reduced fatigue [38]. Exercise has also been found to increase survival rates in breast cancer survivors [49]. Exercise interventions combining aerobic and resistance training, lasting from 2 to 6 months, are beneficial for breast cancer survivors. Cardiorespiratory fitness [39, 46, 50], muscular strength and endurance [46, 47, 50], and quality of life [48, 50] improved in breast cancer survivors undergoing 2 months of exercise. Quality of life also improved in breast cancer survivors who undergo 3 months of exercise upon completion of treatment [45]. Breast cancer survivors who participated in supervised resistance or aerobic training lasting a median of 17 weeks showed improvements in aerobic fitness, muscular strength, and body composition despite undergoing chemotherapy concurrently [41]. A 21-week exercise intervention in breast cancer survivors receiving cancer treatment resulted in significant improvements in muscular strength and body composition [40], and 6-month exercise interventions produce improved cardiovascular function [38], pulmonary function [38], attenuated reduction in bone mineral density [42], and reduced fatigue [38] in breast cancer survivors.
Despite the range of exercise intervention durations previously investigated, little research has compared the outcomes of varying lengths of comparable exercise interventions on physiological and psychological parameters in breast cancer survivors. The purpose of this study was to investigate the physiological and psychological alterations that occur as a result of 3 versus 6 months of similar individualized exercise interventions in breast cancer survivors.
Methods
Setting and participants
The investigation was conducted at the Rocky Mountain Cancer Rehabilitation Institute (RMCRI) at the University of Northern Colorado (UNC). The Institutional Review Board granted approval for all study procedures, cancer survivors were provided detailed written and verbal information concerning the assessment and training protocols including the confidential and voluntary nature of the study, and all cancer survivors provided written informed consent prior to participation. A convenience sample was used to assign cancer survivors to the exercise and control groups.
Assessment
Cancer survivors underwent comprehensive screening, including medical examination, prior to inclusion into the study. Cardiovascular endurance, pulmonary function, muscular endurance, fatigue, and symptoms of depression were assessed initially and following the predetermined 3 or 6-month exercise intervention in cancer survivors in the exercise groups (3M and 6M) and initially and following 6 months of usual care in the control (C) group. The Bruce Protocol, a multi-stage, variable speed and elevation treadmill protocol, was used to evaluate cardiovascular endurance [51]. Cancer survivors continued to a predetermined heart rate or until reaching volitional fatigue.
Pulmonary function was assessed using a Flowmate™ spirometer. Forced vital capacity (FVC) and forced expiratory volume in one-second (FEV1) were measured, and the absolute results of each parameter were compared to the predicted normal value (FVC %pred and FEV1 %pred), based on age, gender, height, and weight for each cancer survivor. Muscular endurance was assessed using the leg press, bench press, lat pull-down and shoulder press machines, as well as crunches. The muscular test battery was developed specifically for cancer survivors, with the weight lifted dependent on the body weight and age of each cancer survivor. Repetitions were performed at a rate of 12.5 repetitions per minute until reaching volitional fatigue [52]. Core muscular endurance was assessed using the curlup crunch test [51, 52].
The Piper Fatigue Inventory was used to assess total cancer-related fatigue, as well as subscales of fatigue, including behavioral, affective, sensory, and cognitive/mood fatigue [53]. The impact of cancer-related fatigue on school or work, social interaction, and interference with enjoyable activities was assessed with the behavioral fatigue subscale. The affective fatigue subscale assessed the emotional meaning attributed to fatigue. Mental, physical, and emotional symptoms of fatigue were included in the sensory fatigue subscale, and the cognitive/mood subscale assessed the impact of fatigue on memory and concentration [53]. The Beck Depression Inventory was used to assess symptoms of depression among cancer survivors. The 21 question Beck Depression Inventory corresponds to 21 symptoms or attitudes commonly observed in depressed patients, including mood, pessimism, sense of failure, lack of satisfaction, guilt feelings, sense of punishment, self-dislike, self-accusation, suicidal wishes, crying, irritability, social withdrawal, indecisiveness, distortion of body image, work inhibition, sleep disturbance, fatigue, loss of appetite, weight loss, somatic preoccupation, and loss of libido, each of which are scored on a 0–4 scale. The total Beck Depression Inventory score is the sum of the scores from the 21 questions. [54]. Higher scores on the Piper Fatigue Inventory and Beck Depression Inventory represent greater levels of fatigue and symptoms of depression, respectively [53, 54].
Following the 3- or 6-month exercise intervention and six months after the initial assessment was given to the C group, a reassessment was performed. The same physiological and psychological parameters were assessed using protocols identical to those used initially.
Intervention
Individualized exercise prescriptions were developed by certified Cancer Exercise Specialists based on the results of the initial assessment and designed to meet the specific needs of each cancer survivor. Cancer survivors in the 3M and 6M groups attended supervised exercise sessions 2 or 3 days per week for the duration of the study. Cancer survivors in the C group did not attend exercise sessions. Exercise sessions were 60 min in duration and “whole body” in focus [52]. Although sessions were individualized to meet the needs and achieve the goals of each cancer survivor, sessions generally included a warm-up lasting 10 min, aerobic exercise and resistance training for 40 min, and stretching and cooling down for 10 min. The exercise intensity prescribed was based on the results of the initial treadmill assessment, and ranged from 30 to 55% of heart rate reserve (HRR). The Karvonen, or percent HRR method, was used to determine exercise heart rate [51, 52]. The aerobic exercise mode for each individual was selected based on the patient’s preference, in consultation with the trainer, to determine the mode offering the most benefit. Treadmill and outdoor walking, stationary cycling, recumbent stepping, and use of an AquaCiser® underwater treadmill were all options. Resistance training and flexibility exercises emphasized all of the major muscle groups as did the low intensity cool-down.
Statistical analysis
Data are presented as means ± SEM. The main effect of supervised exercise compared baseline to post exercise intervention using repeated measures analysis of variance (RM-ANOVA). Following main effects significance, Tukey HSD post hoc tests were used to determine where significance occurred. The primary analyses compared change from baseline to post exercise intervention and between treatment groups using univariate analysis of covariance (ANCOVA) procedures in which the post value was the dependent variable, the baseline value of the same variable was the covariate, and the treatment group was the grouping variable. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS Inc, Chicago, IL). Statistical significance was set at a probability of α ≤ 0.05.
Results
The flow of cancer survivors through the study is represented in Fig. 1. All cancer survivors began the study with an initial assessment. Three months after the initial assessment and individualized exercise intervention, 3M cancer survivors underwent a post assessment. Six months after the initial assessment and individualized exercise intervention, 6M cancer survivors underwent a post assessment. Six months after the initial assessment, C cancer survivors underwent a post assessment.
Fig. 1.
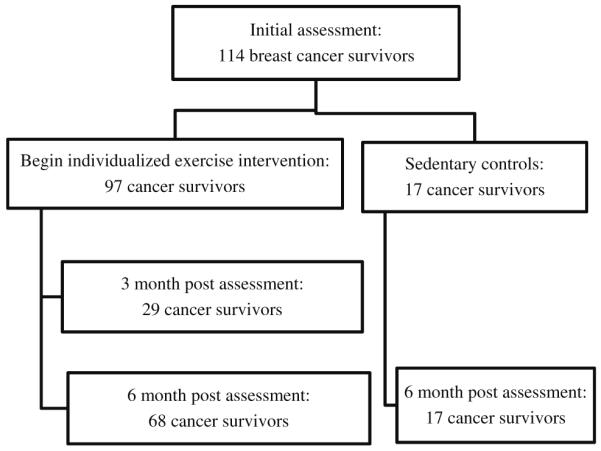
Flow of cancer survivors through the study
Table 1 presents cancer survivor characteristics at baseline. The groups were not significantly different in age, body weight, height, or percentage of cancer survivors in each group undergoing chemotherapy or radiation. The only significant difference at baseline was seen between the 3M and 6M in time since treatment, with 3M cancer survivors having been out of treatment for a shorter period of time [3M = 12.5 ± 1.2 mo., 6M = 29.0 ± 1.3 mo., P < 0.05].
Table 1.
Cancer survivor characteristics
| Variable | Sedentary n = 17 |
3M n = 29 |
6M n = 68 |
|---|---|---|---|
| Age (years ± SEM) | 61.2 (4.0) | 60.3 (2.4) | 57.6(1.2) |
| Body weight (lb ± SEM) | 160.9 (10.1) | 163.7 (6.9) | 170.2 (4.8) |
| Height (in ± SEM) | 63.9 (0.8) | 63.2 (0.5) | 64.5 (0.3) |
| Time since tx (M ± SEM) | 35.3 (4.8) | 12.5 (1.2) | 29.0 (1.3) |
| Chemotherapy | |||
| Yes | 11 (62%) | 16 (56%) | 41 (61%) |
| No | 6 (38%) | 13 (44%) | 27 (39%) |
| Radiation | |||
| Yes | 9 (51%) | 11 (38%) | 35 (51%) |
| No | 8 (49%) | 18 (62%) | 33 (49%) |
Physiological changes in outcomes over the 3 and 6-month interventions are presented in Table 2. Cardiovascular endurance as measured by time on treadmill significantly improved in both exercise groups [3M = 1.74 min improvement, 6M = 1.63 min improvement, P < 0.05], whereas no significant improvement was found in C. Pulmonary function was significantly greater following 6 months of individualized exercise. Forced vital capacity and FEV1, both presented as percentage of the predicted value, improved in 6 M [FVC = 4.6% improvement, FEV1 = 5.6% improvement, P < 0.05].
Table 2.
Changes in physiological outcomes over 3- and 6-month intervention
| Variable | C n = 17 |
3M n = 29 |
6M n = 68 |
|||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| TM time (min ± SEM) | 6.96 (0.5) | 7.20 (0.6) | 5.38 (0.5) | 7.12 (0.5)*,# | 5.22 (0.3) | 6.85 (0.3)*,# |
| Pulmonary function | ||||||
| FVC (%pred ± SEM) | 103.4 (5.3) | 98.5 (4.9) | 108.5 (3.2) | 109.6 (3.2) | 88.6 (1.8) | 92.8 (2.1)*,#,^ |
| FEV1 (%pred ± SEM) | 92.2 (6.2) | 92.8 (5.7) | 100.9 (4.4) | 104.2 (4.4) | 83.6 (2.1) | 89.2 (1.9)* |
| Muscular endurance | ||||||
| Leg Pr (reps ± SEM) | 20 (13) | 32 (2.7)* | 16 (1.3) | 22 (3.2) | 14 (1.0) | 25 (1.6)* |
| Bench Pr (reps ± SEM) | 12 (9) | 16 (1.9) | 12 (0.9) | 14 (1.1) | 9 (0.6) | 16 (0.8)*,^ |
| Lat PD (reps ± SEM) | 16 (8) | 23 (2.9)* | 15 (1.7) | 20 (2.2)* | 13 (.8) | 21 (1.7)* |
| Sh Pr (reps ± SEM) | 9 (5) | 12 (1.5) | 8 (0.7) | 13 (2.0) | 8 (0.5) | 11 (0.4) |
| Crunches (reps ± SEM) | 29 (17) | 25 (4.1) | 17 (1.1) | 26 (4.5)*,# | 20 (1.2) | 27 (1.9)*,# |
P < 0.05 pre versus post
P < 0.05 versus control group
P < 0.05 versus 3-month group
Muscular endurance was assessed with the leg press, bench press, lat pull-down and shoulder press machines, as well as the crunch test. Both C and 6M significantly improved in number of repetitions to volitional fatigue on the leg press [C = 12 repetition improvement, 6M = 11 repetition improvement, P < 0.05]. Number of repetitions to volitional fatigue was significantly greater in 6M only when performing the bench press [6M = 7 repetition improvement, P < 0.05). All three groups showed significant improvements in repetitions to fatigue when performing the lat pulldown exercise [C = 7 repetition improvement, 3M = 5 repetition improvement, 6M = 8 repetition improvement, P < 0.05]. No significant improvements were seen in the shoulder press exercise. Cancer survivors in 3M and 6M significantly improved in repetitions to failure when performing crunches [3M = 9 repetition improvement, 6M = 7 repetition improvement, P < 0.05].
Table 3 presents psychological changes in outcomes over the 3 and 6-month interventions. Total fatigue scores as well as subscales of fatigue scores were reduced in 3M and 6M. Behavioral fatigue was reduced in 3M and 6M [3M = 1.75 point reduction, 6M = 2.13 point reduction, P < 0.05], affective fatigue was reduced in 3M and 6M [3M = 1.26 point reduction, 6M = 1.88 point reduction, P < 0.05], sensory fatigue was reduced in 3M and 6M [3M = 1.19 point reduction, 6M = 1.97 point reduction, P < .05], cognitive fatigue was reduced in 6M [1.36 point reduction, P < 0.05], and total fatigue was reduced in 3M and 6M [3M = 1.15 point reduction, 6M = 1.88 point reduction, P < 0.05]. Depression symptom scores were significantly less following the intervention for C, 3M and 6M [C = 3.00 point reduction, 3M = 2.78 point reduction, 6M = 3.63 point reduction, P < 0.05].
Table 3.
Changes in psychological outcomes over 3- and 6-month intervention
| Variable | C n = 17 |
3M n = 29 |
6M n = 68 |
|||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Fatigue | ||||||
| Behavioral | 3.87 (0.5) | 2.62 (0.6) | 4.87 (0.5) | 3.12 (0.5)* | 4.65 (0.3) | 2.52 (0.3)* |
| Affective | 3.96 (0.5) | 3.21 (0.7) | 5.37 (0.4) | 4.11 (0.5)* | 5.66 (0.3) | 3.78 (0.3)*,^ |
| Sensory | 4.61 (0.4) | 4.05 (0.6) | 5.23 (0.4) | 4.04 (0.4)* | 5.67 (0.3) | 3.70 (0.5)* |
| Cognitive | 4.42 (0.4) | 3.97 (0.5) | 4.68 (0.4) | 4.14 (0.3) | 4.98 (0.3) | 3.62 (0.2)* |
| Total | 4.09 (0.4) | 3.40 (0.6) | 4.95 (0.4) | 3.80 (0.4)* | 5.16 (0.2) | 3.28 (0.2)* |
| Depression | 10.94 (1.7) | 7.94 (2.0)* | 11.22 (1.2) | 8.44 (1.0)* | 11.95 (0.9) | 8.32 (0.9)* |
Represented as score ± SEM.
P < .05 pre versus post
P < 0.05 versus control group
P < 0.05 versus 3-month group
Discussion
As anticipated, an individualized aerobic and resistance training “whole body” exercise intervention of 3 or 6 months in duration resulted in improvements in physiological and psychological parameters. Three months of individualized, prescriptive exercise led to improved cardiovascular endurance, fatigue, and symptoms of depression in breast cancer survivors. Additional improvements in pulmonary function and muscular endurance were found in cancer survivors who exercised for 6 months in duration.
Previous researchers [45, 46] have found an exercise intervention including aerobic and resistance training lasting as little as 8 weeks in duration can result in significant improvements in cardiovascular fitness. Therefore, improvements in cardiovascular endurance as evidenced by increased time on treadmill during the graded exercise test were expected and achieved in both 3M and 6M. Conversely, cancer survivors not engaged in individualized, prescriptive exercise did not significantly improve in cardiovascular endurance during the 6 month study. These results attest to the measurable benefits of a low to moderate intensity exercise intervention for cancer survivors.
Pulmonary function, as measured by FVC and FEV1, improved only in those cancer survivors who exercised for 6 months. Control group cancer survivors and 3M did not show significant improvements in either FVC or FEV1. These results are in accordance with previous findings in which a 6-month exercise intervention similar in nature to that prescribed in this study resulted in improvements in FVC and FEV1 in cancer survivors who had completed treatment before beginning exercise [38].
Improvements in muscular endurance were generally greater in 6M, although 3M did improve in repetitions to fatigue when performing lat pulldowns and crunches. Cancer survivors who exercised for 6 months improved in repetitions to volitional fatigue when performing the lat pulldowns and crunches as well as the leg press and bench press. Surprisingly, cancer survivors not undergoing individualized, prescriptive exercise interventions also significantly improved in repetitions to volitional fatigue in the leg press and lat pulldown exercises. The increase in ability to perform additional lat pulldown repetitions may have been due in part to the increased time since breast cancer treatment with greater recovery and less apprehension about overhead movements allowed by the additional time since breast cancer treatment.
Cancer-related fatigue and depression are serious consequences of the disease and its treatment processes. Cancer-related fatigue is the most commonly reported symptom of cancer survivors [55, 56]. Exercise interventions have been found to attenuate this debilitating side effect [31, 33, 57]. In agreement with previous research, we found both three- and six-month exercise interventions resulted in a reduction in total fatigue, as well as reductions in the behavioral, affective, and sensory fatigue subscales. In contrast, cancer survivors who did not participate in individualized, prescriptive exercise did not have a significant reduction in fatigue levels. Symptoms of depression were reduced in 3M and 6M as well as in C. We anticipated a reduction in depression symptom scores following the exercise interventions but did not expect a similar result in the sedentary, control group. However, it is likely that an additional 6 months between the baseline and post assessment in the sedentary control group may have given cancer survivors additional time to process the cancer diagnosis, treatment, and treatment effects and regain a normal way of life. Additional time since treatment without recurrence may have given cancer survivors in all three treatment groups greater optimism towards survival.
Participants were not randomized to the 3M, 6M and C groups; therefore, those who agreed to participate may have been more motivated and able to begin an exercise intervention. In addition, the study was not placebo-controlled or fully blinded. Participant expectancy and experimenter bias may have played a role in improvements. Despite these limitations the positive outcomes from this study provide evidence supporting individualized exercise for breast cancer survivors.
Three months of individualized, prescriptive exercise led to improved cardiovascular endurance, fatigue, and symptoms of depression in breast cancer survivors. Additional benefits, including increased muscular endurance of additional musculature and improved pulmonary function, are seen if exercise is continued for greater lengths of time. Exercise is a safe and effective way to provide rehabilitation for breast cancer survivors. The physiological and psychological benefits are seen as early as 3 months after beginning an intervention.
Contributor Information
Lisa K. Sprod, Rocky Mountain Cancer Rehabilitation Institute, University of Northern Colorado, Campus Box 6, Greeley, CO 80639, USA; University of Rochester, Rochester, NY, USA
City C. Hsieh, Yuanpei University, Hsinchu, Taiwan
Reid Hayward, Rocky Mountain Cancer Rehabilitation Institute, University of Northern Colorado, Campus Box 6, Greeley, CO 80639, USA.
Carole M. Schneider, Rocky Mountain Cancer Rehabilitation Institute, University of Northern Colorado, Campus Box 6, Greeley, CO 80639, USA
References
- 1.American Cancer Society . Cancer facts and figures. American Cancer Society; Atlanta: 2009. [Google Scholar]
- 2.DeVita VT, Hellman S, Rosenberg SA. Cancer, principles & practice of oncology. 7th edn. Lippincott Williams & Wilkins; Philadelphia: 2005. p. Ixxv.p. 2898. [Google Scholar]
- 3.Hayes S, Battistutta D, Newman B. Objective and subjective upper body function six months following diagnosis of breast cancer. Breast Cancer Res Treat. 2005;94(1):1–10. doi: 10.1007/s10549-005-5991-z. [DOI] [PubMed] [Google Scholar]
- 4.Westrup JL, et al. Risk of decline in upper-body function and symptoms among older breast cancer patients. J Gen Intern Med. 2006;21(4):327–333. doi: 10.1111/j.1525-1497.2006.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert US, et al. Early self-reported impairments in arm functioning of primary breast cancer patients predict late side effects of axillary lymph node dissection: results from a population-based cohort study. Breast Cancer Res Treat. 2006;100(3):285–292. doi: 10.1007/s10549-006-9247-3. [DOI] [PubMed] [Google Scholar]
- 6.Blomqvist L, et al. Evaluation of arm and shoulder mobility and strength after modified radical mastectomy and radiotherapy. Acta Oncol. 2004;43(3):280–283. doi: 10.1080/02841860410026170. [DOI] [PubMed] [Google Scholar]
- 7.Box RC, et al. Shoulder movement after breast cancer surgery: results of a randomised controlled study of postoperative physiotherapy. Breast Cancer Res Treat. 2002;75(1):35–50. doi: 10.1023/a:1016571204924. [DOI] [PubMed] [Google Scholar]
- 8.Shamley DR, et al. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res Treat. 2007;106(1):19–27. doi: 10.1007/s10549-006-9466-7. [DOI] [PubMed] [Google Scholar]
- 9.Leidenius M, et al. The consequences of long-time arm morbidity in node-negative breast cancer patients with sentinel node biopsy or axillary clearance. J Surg Oncol. 2005;92(1):23–31. doi: 10.1002/jso.20373. [DOI] [PubMed] [Google Scholar]
- 10.Pyszel A, et al. Disability, psychological distress and quality of life in breast cancer survivors with arm lymphedema. Lymphology. 2006;39(4):185–192. [PubMed] [Google Scholar]
- 11.Dow KH. Jones and Bartlett series in oncology. 2nd ed. Jones and Bartlett; Sudbury: 2004. Contemporary issues in breast cancer: a nursing perspective; p. xvi.p. 349. [Google Scholar]
- 12.Nygren P. What is cancer chemotherapy? Acta Oncol. 2001;40:166–174. doi: 10.1080/02841860151116204. [DOI] [PubMed] [Google Scholar]
- 13.Doyle JJ, et al. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol. 2005;23(34):8597–8605. doi: 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- 14.Meinardi MT, et al. Evaluation of long term cardiotoxicity after epirubicin containing adjuvant chemotherapy and locoregional radiotherapy for breast cancer using various detection techniques. Heart. 2002;88(1):81–82. doi: 10.1136/heart.88.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erselcan T, et al. Subclinical cardiotoxicity following adjuvant dose-escalated FEC, high-dose chemotherapy, or CMF in breast cancer. Br J Cancer. 2000;82(4):777–781. doi: 10.1054/bjoc.1999.0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Graaf H, et al. Cardiotoxicity from intensive chemotherapy combined with radiotherapy in breast cancer. Br J Cancer. 1997;76(7):943–945. doi: 10.1038/bjc.1997.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilczynski SW, et al. Delayed pulmonary toxicity syndrome following high-dose chemotherapy and bone marrow transplantation for breast cancer. Am J Respir Crit Care Med. 1998;157(2):565–573. doi: 10.1164/ajrccm.157.2.9705072. [DOI] [PubMed] [Google Scholar]
- 18.Chap L, et al. Pulmonary toxicity of high-dose chemotherapy for breast cancer: a non-invasive approach to diagnosis and treatment. Bone Marrow Transplant. 1997;20(12):1063–1067. doi: 10.1038/sj.bmt.1701028. [DOI] [PubMed] [Google Scholar]
- 19.Hurria A, et al. A prospective, longitudinal study of the functional status and quality of life of older patients with breast cancer receiving adjuvant chemotherapy. J Am Geriatr Soc. 2006;54(7):1119–1124. doi: 10.1111/j.1532-5415.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 20.Moon YW, et al. Neoadjuvant chemotherapy with infusional 5-fluorouracil, adriamycin and cyclophosphamide (iFAC) in locally advanced breast cancer: an early response predicts good prognosis. Ann Oncol. 2005;16(11):1778–1785. doi: 10.1093/annonc/mdi360. [DOI] [PubMed] [Google Scholar]
- 21.Erman M, et al. A phase II study on the safety and efficacy of 5-fluorouracil, epirubicin, cyclophosphamide (FEC) followed by paclitaxel in the adjuvant treatment of breast cancer. Cancer Investig. 2005;23(3):215–221. doi: 10.1081/cnv-200055956. [DOI] [PubMed] [Google Scholar]
- 22.Vassilomanolakis M, et al. First-line chemotherapy with docetaxel and cisplatin in metastatic breast cancer. Breast. 2005;14(2):136–141. doi: 10.1016/j.breast.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Hofman M, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 24.Meeske K, et al. Fatigue in breast cancer survivors two to five years post diagnosis: a HEAL Study report. Qual Life Res. 2007;16(6):947–960. doi: 10.1007/s11136-007-9215-3. [DOI] [PubMed] [Google Scholar]
- 25.Servaes P, et al. The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Psychooncology. 2007;16(9):787–795. doi: 10.1002/pon.1120. [DOI] [PubMed] [Google Scholar]
- 26.Bower JE, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4):751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 27.Bower JE, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 28.Burgess C, et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330(7493):702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hjerl K, et al. Depression as a prognostic factor for breast cancer mortality. Psychosomatics. 2003;44(1):24–30. doi: 10.1176/appi.psy.44.1.24. [DOI] [PubMed] [Google Scholar]
- 30.Schneider CM, et al. Cancer treatment-induced alterations in muscular fitness and quality of life: the role of exercise training. Ann Oncol. 2007;18(12):1957–1962. doi: 10.1093/annonc/mdm364. [DOI] [PubMed] [Google Scholar]
- 31.Schneider CM, et al. Exercise training manages cardiopulmonary function and fatigue during and following cancer treatment in male cancer survivors. Integr Cancer Ther. 2007;6(3):235–241. doi: 10.1177/1534735407305871. [DOI] [PubMed] [Google Scholar]
- 32.Valenti M, et al. Physical exercise and quality of life in breast cancer survivors. Int J Med Sci. 2008;5(1):24–28. doi: 10.7150/ijms.5.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlson LE, et al. Individualized exercise program for the treatment of severe fatigue in patients after allogeneic hematopoietic stem-cell transplant: a pilot study. Bone Marrow Transplant. 2006;37(10):945–954. doi: 10.1038/sj.bmt.1705343. [DOI] [PubMed] [Google Scholar]
- 34.Adamsen L, et al. The effect of a multidimensional exercise intervention on physical capacity, well-being and quality of life in cancer patients undergoing chemotherapy. Support Care Cancer. 2006;14(2):116–127. doi: 10.1007/s00520-005-0864-x. [DOI] [PubMed] [Google Scholar]
- 35.Windsor PM, Nicol KF, Potter J. A randomized controlled trial of aerobic exercise for treatment-related fatigue in men receiving radical external beam radiotherapy for localized prostate carcinoma. Cancer. 2004;101(3):550–557. doi: 10.1002/cncr.20378. [DOI] [PubMed] [Google Scholar]
- 36.Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Med Sci Sports Exerc. 2002;34(12):1863–1867. doi: 10.1097/00005768-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Mock V, et al. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract. 2001;9(3):119–127. doi: 10.1046/j.1523-5394.2001.009003119.x. [DOI] [PubMed] [Google Scholar]
- 38.Schneider CM, et al. Effects of supervised exercise training on cardiopulmonary function and fatigue in breast cancer survivors during and after treatment. Cancer. 2007;110(4):918–925. doi: 10.1002/cncr.22862. [DOI] [PubMed] [Google Scholar]
- 39.Kim CJ, et al. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nurs. 2006;29(2):156–165. doi: 10.1097/00002820-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Battaglini C, et al. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Med J. 2007;125(1):22–28. doi: 10.1590/S1516-31802007000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Courneya KS, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz AL, Winters-Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum. 2007;34(3):627–633. doi: 10.1188/07.ONF.627-633. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz AL, et al. Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Med Sci Sports Exerc. 2001;33(5):718–723. doi: 10.1097/00005768-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz AL. Daily fatigue patterns and effect of exercise in women with breast cancer. Cancer Pract. 2000;8(1):16–24. doi: 10.1046/j.1523-5394.2000.81003.x. [DOI] [PubMed] [Google Scholar]
- 45.Milne HM, et al. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;108(2):279–288. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- 46.Cheema BS, Gaul CA. Full-body exercise training improves fitness and quality of life in survivors of breast cancer. J Strength Cond Res. 2006;20(1):14–21. doi: 10.1519/R-17335.1. [DOI] [PubMed] [Google Scholar]
- 47.Sprod LK, et al. The effects of walking poles on shoulder function in breast cancer survivors. Integr Cancer Ther. 2005;4(4):287–293. doi: 10.1177/1534735405282212. [DOI] [PubMed] [Google Scholar]
- 48.Daley AJ, et al. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25(13):1713–1721. doi: 10.1200/JCO.2006.09.5083. [DOI] [PubMed] [Google Scholar]
- 49.Holmes MD, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 50.Herrero F, et al. Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. Int J Sports Med. 2006;27(7):573–580. doi: 10.1055/s-2005-865848. [DOI] [PubMed] [Google Scholar]
- 51.Dwyer GB, Davis SE, editors. ACSM’s health-related physical fitness assessment manual. Lippincott Williams & Wilkins; Philadelphia: 2005. p. 180. [Google Scholar]
- 52.Schneider CM, Dennehy CA, Carter SD. Human Kinetics. Champaign: 2003. Exercise and cancer recovery; p. xi.p. 219. [Google Scholar]
- 53.Piper BF, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25(4):677–684. [PubMed] [Google Scholar]
- 54.Beck AT, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 55.Wagner LI, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. Br J Cancer. 2004;91(5):822–828. doi: 10.1038/sj.bjc.6602012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young KE, White CA. The prevalence and moderators of fatigue in people who have been successfully treated for cancer. J Psychosom Res. 2006;60(1):29–38. doi: 10.1016/j.jpsychores.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Hewitt JA, et al. Exercise for breast cancer survival: the effect on cancer risk and cancer-related fatigue (CRF) Int J Fertil Womens Med. 2005;50(5 Pt 1):231–239. [PubMed] [Google Scholar]


