Abstract
Determining the in vivo significance of a specific enzyme, transporter or xenobiotic receptor in drug metabolism and pharmacokinetics may be hampered by gene multiplicity and complexity, levels of expression and interaction between various components involved. The development of knockout (loss-of-function) and transgenic (gain-of-function) mouse models opens the door to the improved understanding of gene function in a whole body system. There is also growing interest in the development of humanized mice to overcome species difference in drug metabolism and disposition. This review, therefore, aims to summarize and discuss some successful examples of drug-metabolizing enzyme, transporter, and nuclear receptor genetically modified mouse models. These genetically modified mouse models have proven as invaluable models for understanding in vivo function of drug-metabolizing enzymes, transporters and xenobiotic receptors in drug metabolism and transport, as well as predicting potential drug-drug interaction and toxicity in humans. Nevertheless, concerns remain about interpretation of data obtained from such genetically modified mouse models in which the expression of related genes is altered significantly.
Keywords: Transgenic, knockout, humanized, drug metabolism, pharmacokinetics, drug interaction, toxicity, cancer
Introduction
Understanding the metabolism and disposition of drugs is critical for efficient drug discovery and development. Drugs may be structurally altered by Phase 1 oxidative enzymes [e.g., cytochrome P450 (P450 or CYP) and flavin-containing monooxygenase (FMO)] and Phase 2 conjugative enzymes [e.g., uridine 5'-diphospho-glucuronosyltransferase (UGT), sulfotransferase (SULT) and glutathione S-transferase (GST)] in liver, small intestine or other tissues. Drug accessibility, through intestinal absorption, tissue distribution, cell uptake and efflux, and renal and biliary excretion could be ultimately limited or facilitated by efflux [e.g., adenosine triphosphate (ATP)-binding cassette (ABC) family] or influx [e.g., solute carrier (SLC) family] drug transporters. Furthermore, levels of expression of enzyme and transporter proteins are subject to complex regulation by xenobiotic receptors [e.g., aryl hydrocarbon receptor (AhR), pregnane X receptor (PXR), and constitutive androstane receptors (CAR)], as well as epigenetic factors recognized recently (Gonzalez and Yu, 2006; Ingelman-Sundberg et al., 2007; Yu, 2007; Gomez and Ingelman-Sundberg, 2009; Yu, 2009). Differences in the functions or the degree of regulation of enzymes and transporters inevitably affect pharmacokinetic profiles, which may result in considerable inter-individual variability in drug response or toxicity.
Animals are common models used to predict drug metabolism and disposition in humans because they share many similarities in anatomy, physiology and genetics. Mouse models are of particular interest. The small size features relative low husbandry and maintenance costs. The fairly short life span, large litter size and fast breeding rate allow for growth of a large number of animals in a relatively short time, and therefore many studies can be carried out in a desired period. Mice are also of great value for genetic modification through transgenesis and targeted gene disruption; a target gene may be disrupted and a new gene inserted into the mouse genome (Manis, 2007). The resultant gene knockout and transgenic mouse models have been widely used for improved understanding of the role of specific enzyme, transporter or xenobiotic receptor in drug metabolism and disposition at the systemic level (Gonzalez, 2003; Gonzalez and Kimura, 2003; Henderson et al., 2003b; Henderson and Wolf, 2003; Dai and Wan, 2005; Xia et al., 2007; Muruganandan and Sinal, 2008; Lagas et al., 2009; Ou et al., 2010). In addition, species difference is a major confounding factor in metabolism and disposition of many xenobiotics such as chemical carcinogens and medications. The overall metabolic stability of the same drug could be very different across species. There are also human-specific metabolites that cannot be produced or produced at similar levels in wild-type mouse models, where pharmacological and toxicological properties of a particular metabolite need to be understood. Since species differences in drug metabolism and disposition are generally caused by the difference in composition, expression and/or function of enzymes, transporters or xenobiotic receptors, one approach to overcome this issue is to use humanized transgenic mouse models that can be developed by breeding a knockout mouse with a transgenic mouse created separately, or directly replacing a target mouse gene with the corresponding human gene or cDNA (“knock-in”). Studies with these humanized mouse models may not only provide mechanistic understanding of species difference but also improve prediction of risks of xenobiotics in humans (Henderson and Wolf, 2003; Liggett, 2004; Gonzalez and Yu, 2006). The following provides a brief review on enzyme, transporter and xenobiotic receptor gene knockout, transgenic and humanized mouse models that have been developed and used for studies on drug metabolism and disposition of xenobiotics or endobiotics.
Drug-Metabolizing Enzyme Genetically Modified Mouse Models
CYP1A1/CYP1A2
A number of Cyp1a1(−/−) and Cyp1a2(−/−) mouse lines have been generated and used to study the function of human CYP1A orthologues in xenobiotic metabolism (Pineau et al., 1995; Liang et al., 1996; Dalton et al., 2000), given a high degree of interspecies conservation in CYP1A1/1A2 expression, function and gene regulation. The Cyp1a2(−/−) mouse (Pineau et al., 1995) was among the first reported P450-knockout mouse models (Table 1), which was created by disruption of the exon 2 of Cyp1a2, and exhibited neonatal lethality associated with severe respiratory distress symptom, that ultimately was bred out yielding a mouse that was phenotypically indistinguishable from a wild-type mouse. Another Cyp1a2(−/−) mouse model was created by targeted disruption of the exons 2–5 of Cyp1a2 (Liang et al., 1996) (Table 1). The human and rodent CYP1A enzymes do exhibit some difference in metabolic capacity and substrate selectivity (Bogaards et al., 2000), and thus extrapolation of rodent data to humans is prone to error. Therefore, CYP1A1_1A2 humanized mice were generated by crossing the Cyp1a1(−/−), Cyp1a2(−/−) or Cyp1a1/1a2(−/−) mice with the CYP1A1_1A2(+/+) mice, which the latter were developed by incorporating a bacterial artificial chromosomes (BAC) containing human CYP1A1 and CYP1A2 into mouse genome (Jiang et al., 2005; Dragin et al., 2007) (Table 1). These knockout and humanized mouse models have been successfully used for studying the role of CYP1A1/1A2 in the toxicology of a number of environmental carcinogens including polycyclic aromatic hydrocarbons and heterocyclic aromatic amines/amides (Uno et al., 2004; Cheung et al., 2005a; Uno et al., 2006), and in the pharmacokinetics of many drugs including caffeine and theophylline (Buters et al., 1996; Derkenne et al., 2005).
Table 1.
Drug-metabolizing enzyme genetically modified mouse models.
| Gene | Category | Example applications | Reference |
|---|---|---|---|
| Cyp1a1 | Knockout | Gene expression and function | (Dalton et al., 2000) |
| Cyp1a2 | Knockout | Potential physiological role of Cyp1a2 Metabolism of xenobiotics |
(Pineau et al., 1995) (Liang et al., 1996) |
|
Cyp1a1/1a2 CYP1A1/1A2 |
Knockout Humanized |
Assess the risk of environmental toxicant | (Dragin et al., 2007) |
| Cyp1b1 | Knockout | Evaluate the role of Cyp1b1 in DMBA carcinogenesis | (Buters et al., 1999) |
|
Cyp2a5 CYP2A6 |
Knockout Transgenic |
Role of Cyp2a5 in clearance of nicotine and cotinine Function of CYP2A6 in coumarin metabolism |
(Zhou et al., 2009) (Zhang et al., 2005) |
|
CYP2C18/ 2C19 |
Transgenic | Gene expression and function in drug metabolism | (Lofgren et al., 2008; Lofgren et al., 2009) |
| CYP2D6 | Transgenic/ humanized |
Debrisoquine metabolism and disposition Indolealkylamine metabolic pharmacogenetics |
(Corchero et al., 2001) (Yu et al., 2003c) |
|
Cyp2e1 CYP2E1 |
Knockout Humanized |
Acetaminophen toxicity | (Lee et al., 1996) (Cheung et al., 2005b) |
| CYP3A4 | Transgenic | Intestinal metabolism of midazolam, drug-drug metabolism, and physiological regulation | (Granvil et al., 2003) (van Herwaarden et al., 2005) |
|
Cyp3a CYP3A4 |
Knockout Humanized |
Docetaxel metabolism and pharmacokinetics | (van Herwaarden et al., 2007) |
| Cpr | Knockout | Drug metabolism and toxicity | (Shen et al., 2002; Otto et al., 2003) (Gu et al., 2003; Henderson et al., 2003a; Wu et al., 2003) |
| Gstp | Knockout | DMBA carcinogenesis Acetaminophen toxicity |
(Henderson et al., 1998) (Henderson et al., 2000) |
| Sult1e1 | Knockout | Physiological and pathological role of Sult1e1 | (Qian et al., 2001; Tong et al., 2005) |
|
Ugt1 UGT1 |
Knockout Transgenic Humanized |
Tissue distribution, induction and hormonal regulation Physiological role in bilirubin homeostasis |
(Nguyen et al., 2008) (Chen et al., 2005) (Cai et al., 2010; Fujiwara et al., 2010) |
CYP2A6/Cyp2a5
Human CYP2A6 and the mouse orthologue Cyp2a5 share many common substrates (e.g., coumarin, nicotine and cotinine). To assess the role of CYP2A6 in drug metabolism in vivo, a CYP2A6(+/+) mouse model with liver-specific expression of human CYP2A6 was generated using the CYP2A6 cDNA, driven by a mouse liver-specific transthyretin promoter/enhancer (Zhang et al., 2005) (Table 1). More recently, a Cyp2a5(−/−) mouse model, in which the exon 9 of Cyp2a5 was disrupted with a tk-neo-bpA cassette, was developed on the C57BL/6J background (Zhou et al., 2009) (Table 1). CYP2A6 transgenic and Cyp2a5 knockout mice, showing no apparent developmental defects with normal viability and fertility, were used to establish that CYP2A6/Cyp2a5 is involved in the clearance of various drugs including coumarin, nicotine and cotinine (Zhang et al., 2005; Zhou et al., 2009).
CYP2C19
CYP2C19 metabolizes many drugs including antidepressants and proton pump inhibitors and has many genetic variations in different populations. A CYP2C18/2C19 transgenic mouse model was created using a CYP2C18/2C19-containing BAC clone (Lofgren et al., 2008; Lofgren et al., 2009) (Table 1). Gender difference in CYP2C18/C19 expression was observed at the mRNA level in transgenic mice, while only CYP2C19 protein was detectable in heterozygous CYP2C18/19(+/−) mice. In addition, CYP2C18/19(+/−) mouse liver microsomes showed higher catalytic capacity in metabolism of R-omeprazole and S-mephenytoin. These findings suggest this mouse line as an appropriate model for assessing hormonal regulation of CYP2C in vivo, as well as CYP2C19-mediated drug metabolism (Lofgren et al., 2008; Lofgren et al., 2009).
CYP2D6
There is remarkable species difference in xenobiotic metabolism between mice and humans mediated by the CYP2D family of P450s. Thus a humanized CYP2D6(+/+) (Tg-CYP2D6) mouse model was generated with a λ phage genomic clone carrying wild-type CYP2D6 gene (Corchero et al., 2001) (Table 1). Functional CYP2D6 protein was expressed in CYP2D6(+/+) mouse liver, intestine and kidney, resulting in an enhanced debrisoquine metabolism and clearance. Furthermore, urinary debrisoquine metabolic ratio (UMR) profiles (debrisoquine/4-hydroxydebrisoquine) in CYP2D6(+/+) versus wild-type mice closely resembled those in human CYP2D6 extensive metabolizers versus poor metabolizers, suggesting that CYP2D6(+/+) mice could be good animal models for studying CYP2D6 pharmacogenetics (Yu et al., 2004). Indeed, the CYP2D6(+/+) mice have been used to successfully define the role of CYP2D6 in pinoline metabolism (Jiang et al., 2009), as well as the effects of CYP2D6 status on harmaline pharmacokinetics and pharmacodynamics (Figure 1) (Wu et al., 2009). In contrast, when desipramine (DMI), another well-known CYP2D6 substrate, was administered to Tg-CYP2D6 and wild-type control mice, the UMRs of DMI were similar in the two genotyped mice (Shen and Yu, 2009). The results indicate that mouse enzymes have notable contribution to DMI 2-hydroxylation, which is mainly catalyzed by CYP2D6 in humans. In addition, this mouse model was used to identify potential endogenous substrates for CYP2D6 (Yu et al., 2003a; Yu et al., 2003b; Yu et al., 2003c; Yu et al., 2004), indicating its application to the assessment of CYP2D6-mediated reactions in vivo.
Figure 1.
Studies with wild-type and Tg-CYP2D6 mice revealed a significant (P < 0.05; two-way ANOVA) impact of CYP2D6 status on (a) harmaline pharmacokinetics (5 and 15 mg/kg, i.p.; N = 3 at each time point), and (b) drug effects on mouse marble-burying behavior (0–10 mg/kg, i.p.; N = 14 in each group) (Wu et al., 2009).
CYP2E1
To evaluate CYP2E1-mediated biotransformation in vivo, a Cyp2e1(−/−) mouse model was generated (Lee et al., 1996) (Table 1). The Cyp2e1(−/−) mice were less sensitive to acetaminophen (APAP) than wild-type mice, thus establishing an important role for Cyp2e1 in the activation of APAP to a hepatotoxic metabolite. Since species differences in CYP2E1 function and regulation were documented between mice and humans (Bogaards et al., 2000), a CYP2E1-humanized mouse model was developed by introducing a CYP2E1-containing BAC clone into the Cyp2e1(−/−) mice (Cheung et al., 2005b) (Table 1). Comparative studies revealed that CYP2E1-humanized and wild-type mice did differ in APAP toxicity. The CYP2E1-humanized mice should be useful in assessing the pharmacological and toxicological effects of CYP2E1 substrates.
CYP3A4
CYP3A4 is the most abundant P450 enzyme in human liver and gut, and contributes to the metabolism of many different drugs including benzodiazepines, HIV antivirals, immunomodulators and steroids. To develop a CYP3A4 transgenic mouse model, a BAC clone containing complete CYP3A4 gene was used (Granvil et al., 2003) (Table 1). In contrast to constitutive CYP3A4 expression in small intestines, CYP3A4 expression in transgenic mouse livers was subject to developmental and hormonal regulation (Figure 2) (Yu et al., 2005; Cheung et al., 2006). Pharmacokinetic studies in adult male mice with CYP3A4 expression only in the gut supported a role for CYP3A4 in first-pass metabolism of midazolam (Granvil et al., 2003). Furthermore, the impact of ketoconazole on midazolam pharmacokinetics in transgenic mice seems to be closer to that in humans (Table 2). This CYP3A4-transgenic mouse model may offer better assessment of intestinal CYP3A4-mediated drug metabolism and drug-drug interaction. Nevertheless, the expression of some mouse P450 enzymes (e.g., Cyp3a and Cyp2c) was altered in CYP3A4-transgenic mouse livers (Figure 2), which may be due to the change of epigenetic regulation (Li et al., 2009; Pan et al., 2009). The change in murine P450s might complicate interpretation of data obtained from these transgenic mouse models (Yu et al., 2005; Felmlee et al., 2008).
Figure 2.
Sexual dimorphism and developmental expression of human CYP3A4 transgene, as well as murine P450s. (a) Expression of the CYP3A4 transgene and murine Cyp3a and Cyp2b is dependent on gender and age [2–16 week (W)] in mouse livers (Yu et al., 2005). (b) Hepatic CYP3A4 and murine P450 expression profiles are changed in mice following growth hormone (GH) infusion, compared to the SHAM control (Cheung et al., 2006). HLM, human liver microsomes.
Table 2.
Effects of ketoconazole on midazolam pharmacokinetics (AUC(ketoconazole)/AUC(control)) in humans and animal models.
| Intravenous administration | Oral administration | Reference | |
|---|---|---|---|
| Humans | 5 | 16 | (Tsunoda et al., 1999) |
| Rats | 2 | 5 | (Kotegawa et al., 2002) |
| Wild-type mice | n.a. | 3 | (Granvil et al., 2003) |
| Tg-CYP3A4 mice | n.a. | 8 | (Granvil et al., 2003) |
Another CYP3A4-transgenic mouse model was created with an ApoE promoter-HCR1 driven expression cassette containing CYP3A4 cDNA (van Herwaarden et al., 2005) (Table 1). CYP3A4 was selectively expressed in transgenic mouse livers, leading to a reduced systemic exposure to intravenously-administered midazolam and cyclosporin A. A villin-CYP3A4-transgenic mouse model was also developed with selective CYP3A4 expression in small intestines (van Herwaarden et al., 2007). Furthermore, Cyp3a(−/−) mice lacking all functional murine Cyp3a genes were generated and bred with the ApoE- and villin-CYP3A4-transgenic mice to produce liver (Cyp3a−/−A) and intestine (Cyp3a−/−V) CYP3A4-humanized mice, respectively (van Herwaarden et al., 2007) (Table 1). Studies in the CYP3A4-genetically modified mice showed that docetaxel pharmacokinetics and toxicity were largely affected by Cyp3a. In addition, intestinal CYP3A4 was shown to determine the systemic exposure to orally administered triazolam, as well as the drug-drug interaction between ketoconazole and triazolam (van Waterschoot et al., 2009). These genetically modified mouse lines are useful models for understanding Cyp3a-mediated drug metabolism and drug-drug interaction. Nevertheless, change in mouse Cyp2c expression was observed in Cyp3a(−/−) mice (van Waterschoot et al., 2008). Since Cyp2c enzymes contribute to the metabolism of many Cyp3a substrates, it is necessary to be cautious when extrapolating data obtained from the related mouse models.
Cytochrome P450 reductase (CPR)
Disruption of CPR is anticipated to cause a global inactivation of metabolism catalyzed by all microsomal P450s because CPR is the sole electron donor for microsomal P450s. Indeed, ubiquitous disruption of Cpr led to embryonic lethality (Shen et al., 2002; Otto et al., 2003). The Cre-loxP approach was thus utilized to generate conditional knockout mouse lines lacking Cpr expression in livers (Gu et al., 2003; Henderson et al., 2003a; Wu et al., 2003) (Table 1). Other tissue-specific Cpr(−/−) mouse models were also created for intestine (Finn et al., 2007; Zhang et al., 2009), lung (Weng et al., 2007) and cardiomyocytes (Fang et al., 2008b), besides the global Cpr-low expression mice (Wu et al., 2005; Gu et al., 2007). These Cpr genetically modified mouse models were used to investigate the physiological role of Cpr (Gu et al., 2003; Wu et al., 2005; Mutch et al., 2006), and the importance of Cpr in the metabolic clearance or detoxification of many xenobiotic drugs (Pass et al., 2005; Henderson et al., 2006; Weng et al., 2007; Fang et al., 2008a; Fang et al., 2008b; Zhang et al., 2009).
Glutathione S-transferase pi (GSTP)
Human GSTP is one of the most important GST enzymes catalyzing the conjugation of glutathione (GSH) with electrophilic compounds and being responsible for cellular defense against endobiotic and xenobiotic toxins. A GstP1/P2(−/−) mouse model was created to study the function of GSTP in vivo (Henderson et al., 1998) (Table 1). Mice lacking GstP did not show any obvious phenotype, although some GstP null mice had higher body weights than controls. Studies using the GstP knockout mouse model have demonstrated that GstP could be an important metabolic determinant of 7,12-dimethylbenz anthracene (DMBA)-induced carcinogenesis (Henderson et al., 1998), APAP-induced hepatotoxicity (Henderson et al., 2000), and tobacco-induced endothelial dysfunction (Conklin et al., 2009).
Sulfotransferase 1E1 (SULT1E1)
Estrogen sulfotransferase (SULT1E1) catalyzes the sulfation of estrogens at the 3-hydroxyl position and contributes to estrogen homeostasis. A Sult1e1(−/−) mouse model was developed to examine the physiological role of Sult1e1 (Qian et al., 2001) (Table 1). Male Sult1e1(−/−) mice showed age-dependent Leydig cell hypertrophy/hyperplasia and seminiferous tubule damage, which could be recapitulated by estradiol supplementation. Further studies in Sult1e1(−/−) female mice demonstrate that Sult1e1 is a critical estrogen modulator in placenta and affects estrogen levels and placental thrombosis (Tong et al., 2005). This unique mouse model has not yet been used to study xenobiotic metabolism.
Uridine 5'-diphospho-glucuronosyltransferase 1 (UGT1)
A UGT1-transgenic mouse model was created using a BAC clone containing the entire human UGT1 locus (Chen et al., 2005) (Table 1). UGT1A proteins were differentially expressed in transgenic mouse liver and gastrointestinal tract. UGT1 transgene was inducible by AhR, PXR and PPARα ligands (Chen et al., 2005; Bonzo et al., 2007; Senekeo-Effenberger et al., 2007). The disruption of murine Ugt1 gene cluster resulted in severe hyperbilirubinemia in newborn Ugt1(−/−) mice, which resembles Crigler-Najjar Type I disease in humans (Nguyen et al., 2008) (Table 1). Furthermore, humanized mice expressing the Gilbert's UGT1A1*28 allele or the wild-type UGT1A1*1 allele were developed on the Ugt1-null background to evaluate UGT1A-dependent drug clearance (Cai et al., 2010), as well as the role of UGT1 in developmental hyperbilirubinemia and CNS toxicity (Fujiwara et al., 2010) (Table 1). These UGT1 genetically modified mice would be useful models to investigate regulation and function of UGT1 enzymes.
Drug Transporter Genetically Modified Mouse Models
P-glycoprotein (P-gp/MDR1/ABCB1)
Human P-gp, expressed at high levels on the apical membrane of enterocytes, biliary surface of hepatocytes, and the luminal (apical) side of kidney proximal tubule cells, largely affects drug absorption, distribution and excretion. To investigate the role of P-gp in vivo, mice deficient in Mdr1a [Mdr1a(−/−)] (Schinkel et al., 1994) or both Mdr1a and Mdr1b [Mdr1a/b(−/−)] (Schinkel et al., 1997) were generated (Table 3). Comparative studies in wild-type versus knockout mice demonstrated the importance of Mdr1a or Mdr1a/1b in brain uptake, pharmacokinetics and toxicity of many drugs including ivermectin, vinblastine, dexamethasone, digoxin, cyclosporine A, ondansetron, loperamide and rhodamine (Schinkel et al., 1994; Schinkel et al., 1995; Schinkel et al., 1996; Schinkel et al., 1997). For instance, Mdr1a-knockout mice showed 100-fold higher sensitivity to the neurotoxic pesticide ivermectin (Figure 3) and 3-fold higher sensitivity to the carcinostatic drug vinblastine (Schinkel et al., 1994). The Mdr1 knockout mice are useful animal models to evaluate the pharmacological and toxicological role of P-gp. Nevertheless, the obvious increase in Mdr1b expression in Mdr1a(−/−) mice (Schinkel et al., 1994) reminds one to be cautious when translating data from transgenic mice into humans.
Table 3.
Drug transporter genetically modified mouse models.
| Gene | Category | Example applications | References |
|---|---|---|---|
| Mdr1a (Abcb1a) | Knockout | Drug absorption, distribution and elimination, including brain penetration | (Schinkel et al., 1994; Schinkel et al., 1995; Schinkel et al., 1996) |
| Mdr1a/1b (Abcb1a/1b) | Knockout | Drug distribution | (Schinkel et al., 1997) |
| Bsep (Abcb11) | Knockout | Bile acid distribution and excretion | (Wang et al., 2001; Wang et al., 2003) |
| Mrp1 (Abcc1) | Knockout | Disposition of xenobiotic drugs and endogenous leukotrienes | (Wijnholds et al., 1997) (Lorico et al., 1997) |
| Mrp2 (Abcc2) | Knockout | Glutathione excretion, bile flow and drug disposition | (Vlaming et al., 2006) (Chu et al., 2006) |
| Mrp3 (Abcc3) | Knockout | Acetaminophen disposition; biliary excretion | (Belinsky et al., 2005) (Zelcer et al., 2006) |
| Mrp4 (Abcc4) | Knockout | Drug resistance and brain penetration. | (Leggas et al., 2004) |
| Bcrp1 (Abcg2) | Knockout | Drug absorption, distribution and elimination, including the secretion of compounds into milk | (Zhou et al., 2002) (Jonker et al., 2002) |
| Ostα | Knockout | Bile acid disposition | (Rao et al., 2008) |
| Pept2 (Slc15a2) | Knockout | Role of Pept2 in drug distribution and excretion | (Rubio-Aliaga et al., 2003) (Shen et al., 2003; Shen et al., 2007) |
| Mate1 (Slc47a1) | Knockout | Renal secretion of metformin | (Tsuda et al., 2009) |
| Oct1 (Slc22a1) | Knockout | Hepatic uptake and intestinal disposition of organic cation drugs | (Jonker et al., 2001) |
|
Oct2 (Slc22a2) Oct1/2 (Slc22a1/2) |
Knockout | Renal excretion of drugs | (Jonker et al., 2003) |
| Oct3 (Slc22a3) | Knockout | Role of Oct3 in drug distribution | (Zwart et al., 2001) |
| Oat1 (Slc22a6) | Knockout | Excretion of organic anions and drugs | (Eraly et al., 2006) |
| Oat3 (Slc22a8) | Knockout | Uptake of organic anions and drugs | (Sweet et al., 2002) (Sykes et al., 2004) |
| OATP1B1 (SLCO1B1) | Transgenic | Hepatic uptake of mitoxantrone | (van de Steeg et al., 2009) |
| Oatp1b2 (Slco1b2) | Knockout | Drug disposition and hepatic uptake | (Zaher et al., 2008) (Lu et al., 2008) (Chen et al., 2008) |
Figure 3.
Sharp difference in ivermectin (p.o.) toxicity between Mdr1a-null and wild-type mice (Schinkel et al., 1994).
Bile salt export pump (BSEP/ABCB11)
A Bsep knockout mouse model (Table 3) was developed after disruption of the coding region of Walker A of the N-terminal ATP-binding domain of Bsep (Wang et al., 2001). The Bsep(−/−) mice were viable and fertile, but showed growth retardation. In addition, the Bsep(−/−) mice exhibited various phenotypic characteristics including intrahepatic cholestasis, reduced secretion of cholic acid and output of bile salt, liver necrosis, high mortality, and change of the expression of many liver genes, supporting the critical role of Bsep in the transport of bile acid and lipid homeostasis (Wang et al., 2001; Wang et al., 2003). However, no study on drug disposition in this Bsep(−/−) mouse model has been reported.
Multidrug resistance-associated proteins (MRP/ABCC)
MRP1/ABCC1 is ubiquitously expressed in humans and has an important role in disposition of endobiotic and xenobiotic agents. Two different Mrp1(−/−) mouse lines (Table 3) were generated by targeted disruption of either the exons encoding the first ATP-binding domain of mouse Mrp1 gene (Wijnholds et al., 1997) or part of the second putative ATP-binding domain (Lorico et al., 1997). Both mouse lines exhibited increased sensitivity to anticancer drugs such as etoposide and vincristine (Lorico et al., 1997; Wijnholds et al., 1997), indicating the critical role of Mrp1 in drug disposition. Two Mrp2(−/−) mouse lines were also developed (Chu et al., 2006; Vlaming et al., 2006) (Table 3). Absence of Mrp2 resulted in a decreased biliary excretion of Mrp2 substrates and an increased systemic exposure to several drugs and carcinogens after oral administration, illustrating the critical role of Mrp2 in disposition of these substances.
To examine the function of MRP3 in vivo, two Mrp3 knockout mouse models (Table 3) were developed after targeted disruption of exons 6–8 (Belinsky et al., 2005) or exons 2–8 (Zelcer et al., 2006). The resulting Mrp3(−/−) mice have been employed to assess the function of Mrp3 in disposition of endogenous bile acids and bilirubin glucuronide (Belinsky et al., 2005; Zelcer et al., 2006), as well as morphine, morphine-6-glucuronide and APAP (Manautou et al., 2005; Zelcer et al., 2006). A Mrp4 knockout mouse model (Table 3) was also generated by disruption of exon 27, and utilized to evaluate the role of Mrp4 in renal elimination and brain penetration of topotecan (Leggas et al., 2004). The Mrp4(−/−) mouse model was further employed to demonstrate the contribution of Mrp4 to renal elimination of antiviral drugs (e.g., adefovir and tenofovir) (Imaoka et al., 2007), and cytotoxicity and tissue distribution of 9-(2-(phosphonomethoxy)ethyl)-adenine (Takenaka et al., 2007).
Breast cancer resistance protein (BCRP/ABCG2)
BCRP/ABCG2 plays an important role in disposition of many xenobiotic drugs (e.g., mitoxantrone, doxorubicin and topotecan) and metabolites (e.g., estrone-3-sulfate and estradiol-17-β-D-glucuronide). Two Bcrp1(−/−) mouse models (Table 3) were created to investigate the physiological and pharmacological function of BCRP/ABCG2 (Jonker et al., 2002; Zhou et al., 2002). Studies using Bcrp1(−/−) mouse models have revealed a critical role for Bcrp1 in protection of hematopoietic stem cells from toxins (Zhou et al., 2002), and in prevention of diet-dependent photoxocity (Jonker et al., 2002). In addition, the Bcrp1(−/−) mice were used to demonstrate the effects of Bcrp1 expression on absorption, distribution, and elimination of dietary carcinogens (e.g., PhIP) and medications (e.g., nitrofurantoin) (van Herwaarden et al., 2003; Breedveld et al., 2005; Jonker et al., 2005; Merino et al., 2005; Merino et al., 2006). The Bcrp1(−/−) mice may serve as unique animal models for understanding the disposition of drugs transported by BCRP.
Organic solute transporter α (OSTα)
OSTα and OSTβ are coexpressed on the intestinal basolateral membrane, forming a functional heteromer to transport many organic compounds such as taurocholate, digoxin and prostaglandin E1. An Ostα knockout mouse model (Table 3) was developed to evaluate the function of the Ostα-Ostβ heteromeric transporter in intestinal bile acid reabsorption after deletion of the proximal promoter and exons 1 and 2 of murine Ostα gene (Rao et al., 2008). The transileal transport study using the everted gut sac prepared from Ostα(−/−) mice demonstrated that Ostα-Ostβ heteromer is critical for intestinal transport of bile acids.
Peptide transporter 2 (PEPT2/SLC15A2)
A constitutive Pept2-deficient mouse model and a conditional Pept2 knockout mouse model (Table 3) were developed to examine the physiological and pharmacological role of PEPT2 (Rubio-Aliaga et al., 2003; Shen et al., 2003). Comparative studies in knockout versus wild-type mice demonstrated a critical role for PEPT2 in uptake of dipeptides (Rubio-Aliaga et al., 2003; Shen et al., 2003). Further studies revealed that renal PEPT2 was fully responsible for reabsorption of cefadroxil in kidney, and choroid plexus PEPT2 limited brain penetration of cefadroxil (Shen et al., 2007), indicating that the Pept2 knockout mouse model could be useful in the development of peptidomimetic drugs.
Organic cation transporters (OCT/SLC22A)
A number of Oct-knockout mouse models (Table 3) were developed to examine the physiological and pharmacological functions of OCT transporters (Jonker et al., 2001; Zwart et al., 2001; Jonker et al., 2003). Studies in the Oct1(−/−) mice demonstrated an important role for Oct1/Slc22a1 in the distribution and excretion of tetraethylammonium, as well as hepatic accumulation of neurotoxin 1-methyl-4-phenylpyridium and anticancer drug metaiodobenzylguanidine (Jonker et al., 2001). Studies in Oct2 single knockout and Oct1/2 double knockout mice indicated that Oct1 and Oct2/Slc22a2 together were critical for renal secretion of organic cations (Jonker et al., 2003). The mouse homolog of OCT3 was also disrupted to generate an Orct3(−/−) mouse line, which was used to reveal a critical role for Oct3/Slc22a3 in the accumulation of 1-methyl-4-phenylpyridium in the heart (Zwart et al., 2001).
Organic anion transporters (OAT/SLC22A)
OAT/SLC22 transporters are located on the barrier epithelia of diverse tissues such as the proximal tubule of kidney and choroid plexus of brain, where they mediate the absorption and excretion of many organic anions (e.g., β-lactam antibiotics). Disruption of murine Oat1/Slc22a6 or Oat3/Slc22a8 did not alter the fertility of the resulting gene knockout mice (Sweet et al., 2002; Eraly et al., 2006) (Table 3). Studies with Oat1(−/−) mice demonstrated a critical role for Oat1 in the renal excretion of organic anions and drugs (Eraly et al., 2006). The Oat3(−/−) mice were utilized to investigate the contribution of Oat3 to renal and brain uptake of several drugs including para-aminohippurate and estrone sulfate taurocholate (Sweet et al., 2002; Sykes et al., 2004).
Organic anion transporting polypeptides (OATP/SLCO)
To investigate the role of Oatp1b2 in hepatic uptake of xenobiotic drugs, Oatp1b2(−/−) mouse models (Table 3) were developed by the targeted disruption of exons 10–12 (Zaher et al., 2008) or exon 3 of Oatp1b2/Slco1b2 (Lu et al., 2008). The Oatp1b2(−/−) mice were used to demonstrate an important role for Oatp1b2 in the hepatic uptake, systemic disposition and toxicity of many xenobiotic drugs including phalloidin, microcystin-LR, pravastatin, rifampicin and lovastatin (Chen et al., 2008; Lu et al., 2008; Zaher et al., 2008). Recently, a human OATP1B1-transgenic mouse model with liver-specific OATP1B1 expression (Table 3) was generated and found to have significantly higher hepatic accumulation of methotrexate than wild-type control mouse (van de Steeg et al., 2009). These genetically modified mouse models should be of great value for defining the role of OATP in drug disposition.
Multidrug and toxin extrusion 1 (MATE1/SLC47A1)
MATE1/SLC47A1 is a proton-dependent transporter mainly expressed on the luminal side of renal proximal tubules and bile canaliculi, and mediates the excretion of many exogenous or endogenous organic cations. Targeted disruption of murine Mate1/Slc47a1 did not alter fertility of the resultant knockout mice (Tsuda et al., 2009) (Table 3). However, lack of Mate1 expression caused a sharp decrease in renal clearance of metformin and a considerable increase in systemic exposure to metformin, indicating an important role for Mate1 in metformin disposition.
Xenobiotic Receptor Genetically Modified Mouse Models
Aryl hydrocarbon receptor (AHR)
A number of Ahr knockout mouse models (Table 4) were developed independently through disruption of exon 1 or exon 2 (Fernandez-Salguero et al., 1995; Schmidt et al., 1996; Mimura et al., 1997). These Ahr-null mouse strains showed some physiological changes including lower growth rate, decreased fertility, and/or liver deficiency. Studies in Ahr(−/−) versus wild-type mice demonstrated a crucial role for Ahr in induction of Cyp1a enzymes, dioxin-induced toxicity and carcinogenesis. In addition, an AHR-humanized mouse model (Table 4), in which murine Ahr was replaced by human AHR after homologous recombination, was generated to investigate species difference in dioxin toxicity (Moriguchi et al., 2003). Compared with wild-type Ahr(+/+) mice, the AHR-humanized mice indeed exhibited a different induction profile following the treatment of AHR ligands. These genetically modified mice are useful models for predicting the risk of xenobiotic drugs in humans.
Table 4.
Xenobiotic receptor genetically modified mouse models.
| Gene | Category | Example applications | References |
|---|---|---|---|
| Ahr | Knockout | Role of Ahr in gene regulation and liver function | (Fernandez-Salguero et al., 1995) (Schmidt et al., 1996) (Mimura et al., 1997) |
| AHR | Humanized | Species difference in AHR-mediated gene regulation | (Moriguchi et al., 2003) |
| Car (Nr1i3) | Knockout | Role of Car in gene regulation and liver toxicity | (Wei et al., 2000) |
| CAR (NR1I3) | Humanized Transgenic |
Species differences in CAR-mediated gene regulation Gene regulation and bile acid detoxification |
(Zhang et al., 2002; Huang et al., 2004) (Saini et al., 2004) |
| Pparα (Nr1c1) | Knockout | Gene regulation and peroxisome proliferation | (Lee et al., 1995) |
| PPARα (NR1C1) | Humanized | Species difference in PPARα-mediated gene regulation | (Cheung et al., 2004) |
| Pxr (Nr1i2) | Knockout | Role of Pxr in regulation of drug metabolism and disposition | (Xie et al., 2000) (Staudinger et al., 2001) |
| PXR (NR1I2) | Humanized | Species difference in PXR-mediated gene regulation | (Staudinger et al., 2001) (Ma et al., 2007) |
| Rxrα (Nr2b1) | Knockout | Physiological role of Rxrα (embryonic lethality) | (Kastner et al., 1994; Sucov et al., 1994) |
| Rxrα (hepatocyte) | Knockout | Regulation of endobiotics and xenobiotics metabolism | (Wan et al., 2000) |
Pregnane X receptor (PXR/NR1I2)
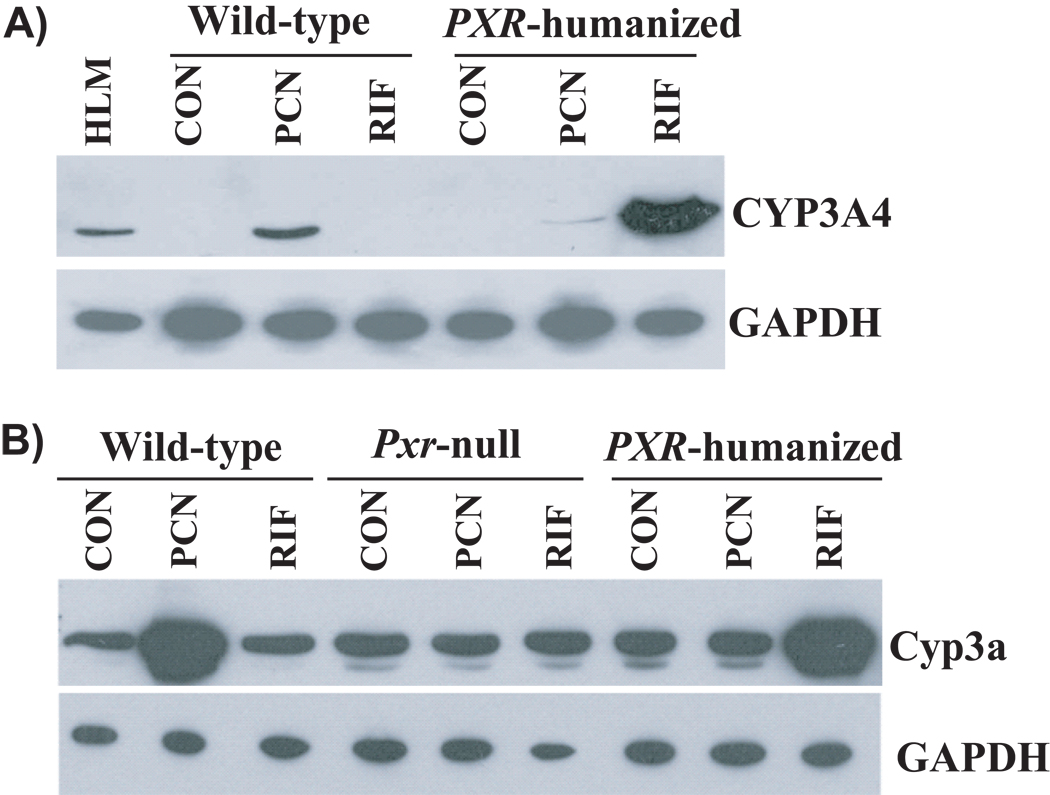
PXR regulates the expression of many enzymes (e.g., CYP3A4 and UGT1A) and transporters (e.g., ABCB1) important in drug metabolism and disposition. Some Pxr(−/−) mouse models (Table 4) were developed to study its physiological and pharmacological role after targeted disruption of exons 2–3 or exon 1 (Xie et al., 2000; Staudinger et al., 2001). Induction of various drug-metabolizing enzymes and transporters by PXR ligands was abolished in mice lacking Pxr expression (Xie et al., 2000; Staudinger et al., 2001). In addition, human PXR-transgenic mice were generated using PXR cDNA or a BAC clone, and then crossbred with Pxr-null mice to produce PXR-humanized mouse models (Xie et al., 2000; Ma et al., 2007) (Table 4). Differential regulation of drug metabolism by human PXR and mouse Pxr was demonstrated by studies in wild-type versus PXR-humanized mice where they exhibited differences in ligand specificity (Figure 4). These findings indicate that PXR-humanized and Pxr-knockout mice are unique animal models to study PXR ligands and PXR-mediated drug-drug interaction.
Figure 4.
Differential induction of CYP3A4/Cyp3a by pregnenolone-16α-carbonitrile (PCN) and rifampicin (RIF) in wild-type and PXR-humanized mice (Ma et al., 2007). Mice treated with drug vehicle were used as control (CON).
Constitutive androstane receptor (CAR/NR1I3)
CAR is an important xenobiotic receptor regulating the expression of various enzymes (e.g., CYP2B6, UGT1A1 and GSTA1) and transporters (e.g., ABCC2). A Car(−/−) mouse model was developed to study the function of CAR in vivo (Wei et al., 2000) (Table 4). The absence of Cyp2b10 induction by Car activators (e.g., phenobarbital) in Car(−/−) mice supports the essential role for CAR in the regulation of Cyp2b10 expression (Wei et al., 2000). The Car(−/−) mice were resistant to APAP hepatotoxicity, indicating CAR as a modulator in APAP-induced toxicity (Zhang et al., 2002). A transgenic mouse model [VP-CAR(+/+)] with human CAR expression was also developed (Saini et al., 2004) (Table 4), which showed higher hepatic sulfation activity toward lithocholic acid (LCA) and increased resistance to LCA-induced hepatotoxicity, compared to wild-type mice (Saini et al., 2004). Another CAR-transgenic mouse model was created using human CAR cDNA, and crossbred with Car(−/−) mice to produce a CAR-humanized mouse model (Huang et al., 2004) (Table 4). Interestingly, meclizine was revealed as an agonist for mouse Car and an inverse agonist for human CAR by studies in wild-type, Car(−/−) and CAR-humanized mice, illustrating the potential application of these mouse models to preclinical assessment of CAR-mediated regulation of drug metabolism.
Peroxisome proliferator-activated receptor α (PPARα/NR1C1)
PPARα regulates CYP4A expression and provokes peroxisome proliferation and hepatocarcinogenesis in rodents. A Pparα(−/−) mouse model was developed after targeted disruption of exon 8 of murine Pparα (Lee et al., 1995) (Table 4). The absence of peroxisome proliferation and Cyp4a induction in Pparα(−/−) mice treated with Pparα ligands (e.g., clofibrate and Wy-14,643) demonstrated a critical role for PPARα in peroxisome proliferation and regulation of target genes (Lee et al., 1995; Gonzalez et al., 1998). In addition, a PPARα-humanized mouse model (Table 4) was generated to investigate the species difference in PPARα functionality (Cheung et al., 2004). Despite peroxisome proliferator induced hypolipidemia and many enzymes in fatty acid oxidation, synthesis, transport in both wild-type and PPARα-humanized mice, the increase in peroxisome and induction of cell cycle genes, hepatocellular proliferation and hepatomegaly were not observed in PPARα-humanized mice but only in wild-type mice (Cheung et al., 2004; Morimura et al., 2006; Foreman et al., 2009) (Table 5). Thus the PPARα-humanized mice may be a better animal model to predict risk of peroxisome proliferators (e.g., lipid lowering fibrate drugs) in humans.
Table 5.
Comparison of the responses to peroxisome proliferators (e.g., Wy-14,643) in wild-type, Pparα-knockout, PPARα-humanized mice and humans.
| Response to peroxisome proliferators |
Wild-type mice |
Ppara-null mice |
PPARa-humanized mice |
Humans |
|---|---|---|---|---|
| PPARa expression | Murine Pparα | None | Human PPARα | Human PPARα |
| Increase in peroxisomes | Yes | No | No | No |
| Induction of enzymes in fatty acid oxidation, synthesis, transport | Yes | No | Yes | No |
| Induction of cell cycle genes | Yes | No | No | No |
| Cell proliferation | Yes | No | No | No |
| Hypolipidemia | Yes | No | Yes | Yes |
| Increased risk of cancer | Yes | No | No | No |
Retinoid X receptor α (RXRα/NR2B1)
The RXRα is a heterodimer partner for PXR, CAR and PPARα regulating liver functions. The conventional Rxrα(−/−) mice (Table 4) developed by targeted disruption of exon 3 or exon 4 of RXRα/NR2B1 showed embryonic lethality, which was probably due to cardiac or placental developmental defects (Kastner et al., 1994; Sucov et al., 1994). Therefore, a Rxrα(−/−)hep mouse model (Table 4), in which RXRα is conditionally and selectively disrupted in hepatocytes, was developed for investigation of the physiological functions of RXRα (Wan et al., 2000). While no gross liver abnormalities were found in the Rxrα(−/−)hep mice, many hepatic metabolic pathways mediated by heteromerization between RXRα and xenobiotic receptors (e.g., PPARα, CAR and PXR) were changed (Wan et al., 2000; Wu et al., 2004; Dai et al., 2005). The Rxrα(−/−)hep mouse line is an unparalleled animal model for studying the function of RXRα in the homeostasis of cholesterol, fatty acids, bile acids and steroids, as well as in drug metabolism and disposition.
Conclusions
Genetically modified mice with lost (knockout) or gained (transgenic) function are unique and valuable additions to in vitro and wild-type animal models for preclinical investigations. Studies with these mouse models help better understand the in vivo role of specific drug-metabolizing enzyme, transporter or xenobiotic receptor in drug metabolism and pharmacokinetics, and consequent effects on drug efficacy or toxicity. Development of humanized mouse models is of particular interest, which may ultimately overcome species difference that impairs accurate prediction of drug metabolism and disposition in humans. However, whether the level of transgene expression in mice could be translated into humans is undefined in many cases. In addition, the disruption of target genes or introduction of genes of interest can sometimes lead to changes in the expression of other related genes in the genetically modified mice. Mouse genetic backgrounds could also affect the results. One should not overlook the limitations when studying drug metabolism and pharmacokinetics with such genetically modified mouse models.
Acknowledgements
A-M. Yu wants to thank the support (award number R01DA021172) from the National Institute On Drug Abuse, National Institutes of Health (NIH). X-L. Jiang is supported by a Pfizer fellowship.
References
- Belinsky MG, Dawson PA, Shchaveleva I, Bain LJ, Wang R, Ling V, Chen ZS, Grinberg A, Westphal H, Klein-Szanto A, Lerro A, Kruh GD. Analysis of the in vivo functions of Mrp3. Mol Pharmacol. 2005;68:160–168. doi: 10.1124/mol.104.010587. [DOI] [PubMed] [Google Scholar]
- Bogaards JJ, Bertrand M, Jackson P, Oudshoorn MJ, Weaver RJ, van Bladeren PJ, Walther B. Determining the best animal model for human cytochrome P450 activities: a comparison of mouse, rat, rabbit, dog, micropig, monkey and man. Xenobiotica. 2000;30:1131–1152. doi: 10.1080/00498250010021684. [DOI] [PubMed] [Google Scholar]
- Bonzo JA, Belanger A, Tukey RH. The role of chrysin and the ah receptor in induction of the human UGT1A1 gene in vitro and in transgenic UGT1 mice. Hepatology. 2007;45:349–360. doi: 10.1002/hep.21481. [DOI] [PubMed] [Google Scholar]
- Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, Schellens JH. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65:2577–2582. doi: 10.1158/0008-5472.CAN-04-2416. [DOI] [PubMed] [Google Scholar]
- Buters JT, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, Doehmer J, Ward JM, Jefcoate CR, Gonzalez FJ. Cytochrome P450 CYP1B1 determines susceptibility to 7, 12-dimethylbenz[a]anthracene-induced lymphomas. Proc Natl Acad Sci U S A. 1999;96:1977–1982. doi: 10.1073/pnas.96.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buters JT, Tang BK, Pineau T, Gelboin HV, Kimura S, Gonzalez FJ. Role of CYP1A2 in caffeine pharmacokinetics and metabolism: studies using mice deficient in CYP1A2. Pharmacogenetics. 1996;6:291–296. doi: 10.1097/00008571-199608000-00002. [DOI] [PubMed] [Google Scholar]
- Cai H, Nguyen N, Peterkin V, Yang YS, Hotz K, La Placa DB, Chen S, Tukey RH, Stevens JC. A humanized UGT1 mouse model expressing the UGT1A1*28 allele for assessing drug clearance by UGT1A1-dependent glucuronidation. Drug Metab Dispos. 2010;38:879–886. doi: 10.1124/dmd.109.030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Stock JL, Liu X, Shi J, Van Deusen JW, DiMattia DA, Dullea RG, de Morais SM. Utility of a novel Oatp1b2 knockout mouse model for evaluating the role of Oatp1b2 in the hepatic uptake of model compounds. Drug Metab Dispos. 2008;36:1840–1845. doi: 10.1124/dmd.108.020594. [DOI] [PubMed] [Google Scholar]
- Chen S, Beaton D, Nguyen N, Senekeo-Effenberger K, Brace-Sinnokrak E, Argikar U, Remmel RP, Trottier J, Barbier O, Ritter JK, Tukey RH. Tissue-specific, inducible, and hormonal control of the human UDP-glucuronosyltransferase-1 (UGT1) locus. J Biol Chem. 2005;280:37547–37557. doi: 10.1074/jbc.M506683200. [DOI] [PubMed] [Google Scholar]
- Cheung C, Akiyama TE, Ward JM, Nicol CJ, Feigenbaum L, Vinson C, Gonzalez FJ. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor alpha. Cancer Res. 2004;64:3849–3854. doi: 10.1158/0008-5472.CAN-04-0322. [DOI] [PubMed] [Google Scholar]
- Cheung C, Ma X, Krausz KW, Kimura S, Feigenbaum L, Dalton TP, Nebert DW, Idle JR, Gonzalez FJ. Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in mice humanized for CYP1A1 and CYP1A2. Chem Res Toxicol. 2005a;18:1471–1478. doi: 10.1021/tx050136g. [DOI] [PubMed] [Google Scholar]
- Cheung C, Yu AM, Chen CS, Krausz KW, Byrd LG, Feigenbaum L, Edwards RJ, Waxman DJ, Gonzalez FJ. Growth hormone determines sexual dimorphism of hepatic cytochrome P450 3A4 expression in transgenic mice. J Pharmacol Exp Ther. 2006;316:1328–1334. doi: 10.1124/jpet.105.094367. [DOI] [PubMed] [Google Scholar]
- Cheung C, Yu AM, Ward JM, Krausz KW, Akiyama TE, Feigenbaum L, Gonzalez FJ. The cyp2e1-humanized transgenic mouse: role of cyp2e1 in acetaminophen hepatotoxicity. Drug Metab Dispos. 2005b;33:449–457. doi: 10.1124/dmd.104.002402. [DOI] [PubMed] [Google Scholar]
- Chu XY, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, Wang RW, Yabut J, Hartley DP, Evans DC, Evers R. Characterization of mice lacking the multidrug resistance protein MRP2 (ABCC2) J Pharmacol Exp Ther. 2006;317:579–589. doi: 10.1124/jpet.105.098665. [DOI] [PubMed] [Google Scholar]
- Conklin DJ, Haberzettl P, Prough RA, Bhatnagar A. Glutathione-S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke. Am J Physiol Heart Circ Physiol. 2009;296:H1586–H1597. doi: 10.1152/ajpheart.00867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corchero J, Granvil CP, Akiyama TE, Hayhurst GP, Pimprale S, Feigenbaum L, Idle JR, Gonzalez FJ. The CYP2D6 humanized mouse: effect of the human CYP2D6 transgene and HNF4alpha on the disposition of debrisoquine in the mouse. Mol Pharmacol. 2001;60:1260–1267. doi: 10.1124/mol.60.6.1260. [DOI] [PubMed] [Google Scholar]
- Dai G, Chou N, He L, Gyamfi MA, Mendy AJ, Slitt AL, Klaassen CD, Wan YJ. Retinoid X receptor alpha Regulates the expression of glutathione s-transferase genes and modulates acetaminophen-glutathione conjugation in mouse liver. Mol Pharmacol. 2005;68:1590–1596. doi: 10.1124/mol.105.013680. [DOI] [PubMed] [Google Scholar]
- Dai G, Wan YJ. Animal models of xenobiotic receptors. Curr Drug Metab. 2005;6:341–355. doi: 10.2174/1389200054633862. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Dieter MZ, Matlib RS, Childs NL, Shertzer HG, Genter MB, Nebert DW. Targeted knockout of Cyp1a1 gene does not alter hepatic constitutive expression of other genes in the mouse [Ah] battery. Biochem Biophys Res Commun. 2000;267:184–189. doi: 10.1006/bbrc.1999.1913. [DOI] [PubMed] [Google Scholar]
- Derkenne S, Curran CP, Shertzer HG, Dalton TP, Dragin N, Nebert DW. Theophylline pharmacokinetics: comparison of Cyp1a1(−/−) and Cyp1a2(−/−) knockout mice, humanized hCYP1A1_1A2 knock-in mice lacking either the mouse Cyp1a1 or Cyp1a2 gene, and Cyp1(+/+) wild-type mice. Pharmacogenet Genomics. 2005;15:503–511. doi: 10.1097/01.fpc.0000167326.00411.50. [DOI] [PubMed] [Google Scholar]
- Dragin N, Uno S, Wang B, Dalton TP, Nebert DW. Generation of 'humanized' hCYP1A1_1A2_Cyp1a1/1a2(−/−) mouse line. Biochem Biophys Res Commun. 2007;359:635–642. doi: 10.1016/j.bbrc.2007.05.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, Monte JC, Rieg T, Truong DM, Long JM, Barshop BA, Kaler G, Nigam SK. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem. 2006;281:5072–5083. doi: 10.1074/jbc.M508050200. [DOI] [PubMed] [Google Scholar]
- Fang C, Behr M, Xie F, Lu S, Doret M, Luo H, Yang W, Aldous K, Ding X, Gu J. Mechanism of chloroform-induced renal toxicity: non-involvement of hepatic cytochrome P450-dependent metabolism. Toxicol Appl Pharmacol. 2008a;227:48–55. doi: 10.1016/j.taap.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, Gu J, Xie F, Behr M, Yang W, Abel ED, Ding X. Deletion of the NADPH-cytochrome P450 reductase gene in cardiomyocytes does not protect mice against doxorubicin-mediated acute cardiac toxicity. Drug Metab Dispos. 2008b;36:1722–1728. doi: 10.1124/dmd.108.021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee MA, Lon HK, Gonzalez FJ, Yu AM. Cytochrome P450 expression and regulation in CYP3A4/CYP2D6 double transgenic humanized mice. Drug Metab Dispos. 2008;36:435–441. doi: 10.1124/dmd.107.018838. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- Finn RD, McLaren AW, Carrie D, Henderson CJ, Wolf CR. Conditional deletion of cytochrome P450 oxidoreductase in the liver and gastrointestinal tract: a new model for studying the functions of the P450 system. J Pharmacol Exp Ther. 2007;322:40–47. doi: 10.1124/jpet.107.121780. [DOI] [PubMed] [Google Scholar]
- Foreman JE, Chang SC, Ehresman DJ, Butenhoff JL, Anderson CR, Palkar PS, Kang BH, Gonzalez FJ, Peters JM. Differential hepatic effects of perfluorobutyrate mediated by mouse and human PPAR-alpha. Toxicol Sci. 2009;110:204–211. doi: 10.1093/toxsci/kfp077. [DOI] [PubMed] [Google Scholar]
- Fujiwara R, Nguyen N, Chen S, Tukey RH. Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus. Proc Natl Acad Sci U S A. 2010;107:5024–5029. doi: 10.1073/pnas.0913290107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A, Ingelman-Sundberg M. Pharmacoepigenetics: its role in interindividual differences in drug response. Clin Pharmacol Ther. 2009;85:426–430. doi: 10.1038/clpt.2009.2. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ. Role of gene knockout and transgenic mice in the study of xenobiotic metabolism. Drug Metab Rev. 2003;35:319–335. doi: 10.1081/dmr-120026496. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Kimura S. Study of P450 function using gene knockout and transgenic mice. Arch Biochem Biophys. 2003;409:153–158. doi: 10.1016/s0003-9861(02)00364-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Peters JM, Cattley RC. Mechanism of action of the nongenotoxic peroxisome proliferators: role of the peroxisome proliferator-activator receptor alpha. J Natl Cancer Inst. 1998;90:1702–1709. doi: 10.1093/jnci/90.22.1702. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Yu AM. Cytochrome P450 and xenobiotic receptor humanized mice. Annu Rev Pharmacol Toxicol. 2006;46:41–64. doi: 10.1146/annurev.pharmtox.45.120403.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granvil CP, Yu AM, Elizondo G, Akiyama TE, Cheung C, Feigenbaum L, Krausz KW, Gonzalez FJ. Expression of the human CYP3A4 gene in the small intestine of transgenic mice: in vitro metabolism and pharmacokinetics of midazolam. Drug Metab Dispos. 2003;31:548–558. doi: 10.1124/dmd.31.5.548. [DOI] [PubMed] [Google Scholar]
- Gu J, Chen CS, Wei Y, Fang C, Xie F, Kannan K, Yang W, Waxman DJ, Ding X. A mouse model with liver-specific deletion and global suppression of the NADPH-cytochrome P450 reductase gene: characterization and utility for in vivo studies of cyclophosphamide disposition. J Pharmacol Exp Ther. 2007;321:9–17. doi: 10.1124/jpet.106.118240. [DOI] [PubMed] [Google Scholar]
- Gu J, Weng Y, Zhang QY, Cui H, Behr M, Wu L, Yang W, Zhang L, Ding X. Liver-specific deletion of the NADPH-cytochrome P450 reductase gene: impact on plasma cholesterol homeostasis and the function and regulation of microsomal cytochrome P450 and heme oxygenase. J Biol Chem. 2003;278:25895–25901. doi: 10.1074/jbc.M303125200. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Otto DM, Carrie D, Magnuson MA, McLaren AW, Rosewell I, Wolf CR. Inactivation of the hepatic cytochrome P450 system by conditional deletion of hepatic cytochrome P450 reductase. J Biol Chem. 2003a;278:13480–13486. doi: 10.1074/jbc.M212087200. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Otto DM, McLaren AW, Carrie D, Wolf CR. Knockout mice in xenobiotic metabolism. Drug Metab Rev. 2003b;35:385–392. doi: 10.1081/dmr-120026869. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Pass GJ, Wolf CR. The hepatic cytochrome P450 reductase null mouse as a tool to identify a successful candidate entity. Toxicol Lett. 2006;162:111–117. doi: 10.1016/j.toxlet.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc Natl Acad Sci U S A. 1998;95:5275–5280. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CJ, Wolf CR. Transgenic analysis of human drug-metabolizing enzymes: preclinical drug development and toxicology. Mol Interv. 2003;3:331–343. doi: 10.1124/mi.3.6.331. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Wolf CR, Kitteringham N, Powell H, Otto D, Park BK. Increased resistance to acetaminophen hepatotoxicity in mice lacking glutathione S-transferase Pi. Proc Natl Acad Sci U S A. 2000;97:12741–12745. doi: 10.1073/pnas.220176997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhang J, Moore DD. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Invest. 2004;113:137–143. doi: 10.1172/JCI200418385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaoka T, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007;71:619–627. doi: 10.1124/mol.106.028233. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007;116:496–526. doi: 10.1016/j.pharmthera.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Jiang XL, Shen HW, Yu AM. Pinoline may be used as a probe for CYP2D6 activity. Drug Metab Dispos. 2009;37:443–446. doi: 10.1124/dmd.108.025056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Dalton TP, Jin L, Wang B, Tsuneoka Y, Shertzer HG, Deka R, Nebert DW. Toward the evaluation of function in genetic variability: characterizing human SNP frequencies and establishing BAC-transgenic mice carrying the human CYP1A1_CYP1A2 locus. Hum Mutat. 2005;25:196–206. doi: 10.1002/humu.20134. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, Beijnen JH, Schinkel AH. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, Mesman E, Dale TC, Schinkel AH. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med. 2005;11:127–129. doi: 10.1038/nm1186. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Mol CA, Buitelaar M, Koepsell H, Smit JW, Schinkel AH. Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Mol Cell Biol. 2001;21:5471–5477. doi: 10.1128/MCB.21.16.5471-5477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol. 2003;23:7902–7908. doi: 10.1128/MCB.23.21.7902-7908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, Vonesch JL, Dolle P, Chambon P. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Kotegawa T, Laurijssens BE, Von Moltke LL, Cotreau MM, Perloff MD, Venkatakrishnan K, Warrington JS, Granda BW, Harmatz JS, Greenblatt DJ. In vitro, pharmacokinetic, and pharmacodynamic interactions of ketoconazole and midazolam in the rat. J Pharmacol Exp Ther. 2002;302:1228–1237. doi: 10.1124/jpet.102.035972. [DOI] [PubMed] [Google Scholar]
- Lagas JS, Vlaming ML, Schinkel AH. Pharmacokinetic assessment of multiple ATP-binding cassette transporters: the power of combination knockout mice. Mol Interv. 2009;9:136–145. doi: 10.1124/mi.9.3.7. [DOI] [PubMed] [Google Scholar]
- Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, Mercer KE, Zhuang Y, Panetta JC, Johnston B, Scheper RJ, Stewart CF, Schuetz JD. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24:7612–7621. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cui Y, Hart SN, Klaassen CD, Zhong XB. Dynamic patterns of histone methylation are associated with ontogenic expression of the Cyp3a genes during mouse liver maturation. Mol Pharmacol. 2009;75:1171–1179. doi: 10.1124/mol.108.052993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang HC, Li H, McKinnon RA, Duffy JJ, Potter SS, Puga A, Nebert DW. Cyp1a2(−/−) null mutant mice develop normally but show deficient drug metabolism. Proc Natl Acad Sci U S A. 1996;93:1671–1676. doi: 10.1073/pnas.93.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett SB. Genetically modified mouse models for pharmacogenomic research. Nat Rev Genet. 2004;5:657–663. doi: 10.1038/nrg1429. [DOI] [PubMed] [Google Scholar]
- Lofgren S, Baldwin RM, Carleros M, Terelius Y, Fransson-Steen R, Mwinyi J, Waxman DJ, Ingelman-Sundberg M. Regulation of human CYP2C18 and CYP2C19 in transgenic mice: influence of castration, testosterone, and growth hormone. Drug Metab Dispos. 2009;37:1505–1512. doi: 10.1124/dmd.109.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgren S, Baldwin RM, Hiratsuka M, Lindqvist A, Carlberg A, Sim SC, Schulke M, Snait M, Edenro A, Fransson-Steen R, Terelius Y, Ingelman-Sundberg M. Generation of mice transgenic for human CYP2C18 and CYP2C19: characterization of the sexually dimorphic gene and enzyme expression. Drug Metab Dispos. 2008;36:955–962. doi: 10.1124/dmd.107.019349. [DOI] [PubMed] [Google Scholar]
- Lorico A, Rappa G, Finch RA, Yang D, Flavell RA, Sartorelli AC. Disruption of the murine MRP (multidrug resistance protein) gene leads to increased sensitivity to etoposide (VP-16) and increased levels of glutathione. Cancer Res. 1997;57:5238–5242. [PubMed] [Google Scholar]
- Lu H, Choudhuri S, Ogura K, Csanaky IL, Lei X, Cheng X, Song PZ, Klaassen CD. Characterization of organic anion transporting polypeptide 1b2-null mice: essential role in hepatic uptake/toxicity of phalloidin and microcystin-LR. Toxicol Sci. 2008;103:35–45. doi: 10.1093/toxsci/kfn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Shah Y, Cheung C, Guo GL, Feigenbaum L, Krausz KW, Idle JR, Gonzalez FJ. The PREgnane X receptor gene-humanized mouse: a model for investigating drug-drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos. 2007;35:194–200. doi: 10.1124/dmd.106.012831. [DOI] [PubMed] [Google Scholar]
- Manautou JE, de Waart DR, Kunne C, Zelcer N, Goedken M, Borst P, Elferink RO. Altered disposition of acetaminophen in mice with a disruption of the Mrp3 gene. Hepatology. 2005;42:1091–1098. doi: 10.1002/hep.20898. [DOI] [PubMed] [Google Scholar]
- Manis JP. Knock out, knock in, knock down--genetically manipulated mice and the Nobel Prize. N Engl J Med. 2007;357:2426–2429. doi: 10.1056/NEJMp0707712. [DOI] [PubMed] [Google Scholar]
- Merino G, Alvarez AI, Pulido MM, Molina AJ, Schinkel AH, Prieto JG. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab Dispos. 2006;34:690–695. doi: 10.1124/dmd.105.008219. [DOI] [PubMed] [Google Scholar]
- Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol. 2005;67:1758–1764. doi: 10.1124/mol.104.010439. [DOI] [PubMed] [Google Scholar]
- Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, Fujii-Kuriyama Y. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Motohashi H, Hosoya T, Nakajima O, Takahashi S, Ohsako S, Aoki Y, Nishimura N, Tohyama C, Fujii-Kuriyama Y, Yamamoto M. Distinct response to dioxin in an arylhydrocarbon receptor (AHR)-humanized mouse. Proc Natl Acad Sci U S A. 2003;100:5652–5657. doi: 10.1073/pnas.1037886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimura K, Cheung C, Ward JM, Reddy JK, Gonzalez FJ. Differential susceptibility of mice humanized for peroxisome proliferator-activated receptor alpha to Wy-14,643-induced liver tumorigenesis. Carcinogenesis. 2006;27:1074–1080. doi: 10.1093/carcin/bgi329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruganandan S, Sinal CJ. Mice as clinically relevant models for the study of cytochrome P450-dependent metabolism. Clin Pharmacol Ther. 2008;83:818–828. doi: 10.1038/clpt.2008.50. [DOI] [PubMed] [Google Scholar]
- Mutch DM, Crespy V, Clough J, Henderson CJ, Lariani S, Mansourian R, Moulin J, Wolf CR, Williamson G. Hepatic cytochrome P-450 reductase-null mice show reduced transcriptional response to quercetin and reveal physiological homeostasis between jejunum and liver. Am J Physiol Gastrointest Liver Physiol. 2006;291:G63–G72. doi: 10.1152/ajpgi.00565.2005. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Bonzo JA, Chen S, Chouinard S, Kelner MJ, Hardiman G, Belanger A, Tukey RH. Disruption of the ugt1 locus in mice resembles human Crigler-Najjar type I disease. J Biol Chem. 2008;283:7901–7911. doi: 10.1074/jbc.M709244200. [DOI] [PubMed] [Google Scholar]
- Otto DM, Henderson CJ, Carrie D, Davey M, Gundersen TE, Blomhoff R, Adams RH, Tickle C, Wolf CR. Identification of novel roles of the cytochrome p450 system in early embryogenesis: effects on vasculogenesis and retinoic Acid homeostasis. Mol Cell Biol. 2003;23:6103–6116. doi: 10.1128/MCB.23.17.6103-6116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Z, Huang M, Zhao L, Xie W. Use of transgenic mice in UDP-glucuronosyltransferase (UGT) studies. Drug Metab Rev. 2010;42:119–127. doi: 10.3109/03602530903208983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YZ, Gao W, Yu AM. MicroRNAs regulate CYP3A4 expression via direct and indirect targeting. Drug Metab Dispos. 2009;37:2112–2117. doi: 10.1124/dmd.109.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass GJ, Carrie D, Boylan M, Lorimore S, Wright E, Houston B, Henderson CJ, Wolf CR. Role of hepatic cytochrome p450s in the pharmacokinetics and toxicity of cyclophosphamide: studies with the hepatic cytochrome p450 reductase null mouse. Cancer Res. 2005;65:4211–4217. doi: 10.1158/0008-5472.CAN-04-4103. [DOI] [PubMed] [Google Scholar]
- Pineau T, Fernandez-Salguero P, Lee SS, McPhail T, Ward JM, Gonzalez FJ. Neonatal lethality associated with respiratory distress in mice lacking cytochrome P450 1A2. Proc Natl Acad Sci U S A. 1995;92:5134–5138. doi: 10.1073/pnas.92.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YM, Sun XJ, Tong MH, Li XP, Richa J, Song WC. Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology. 2001;142:5342–5350. doi: 10.1210/endo.142.12.8540. [DOI] [PubMed] [Google Scholar]
- Rao A, Haywood J, Craddock AL, Belinsky MG, Kruh GD, Dawson PA. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A. 2008;105:3891–3896. doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Frey I, Boll M, Groneberg DA, Eichinger HM, Balling R, Daniel H. Targeted disruption of the peptide transporter Pept2 gene in mice defines its physiological role in the kidney. Mol Cell Biol. 2003;23:3247–3252. doi: 10.1128/MCB.23.9.3247-3252.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini SP, Sonoda J, Xu L, Toma D, Uppal H, Mu Y, Ren S, Moore DD, Evans RM, Xie W. A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol Pharmacol. 2004;65:292–300. doi: 10.1124/mol.65.2.292. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, van der Valk MA, Voordouw AC, Spits H, van Tellingen O, Zijlmans JM, Fibbe WE, Borst P. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci U S A. 1997;94:4028–4033. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senekeo-Effenberger K, Chen S, Brace-Sinnokrak E, Bonzo JA, Yueh MF, Argikar U, Kaeding J, Trottier J, Remmel RP, Ritter JK, Barbier O, Tukey RH. Expression of the human UGT1 locus in transgenic mice by 4-chloro-6-(2,3-xylidino)-2-pyrimidinylthioacetic acid (WY-14643) and implications on drug metabolism through peroxisome proliferator-activated receptor alpha activation. Drug Metab Dispos. 2007;35:419–427. doi: 10.1124/dmd.106.013243. [DOI] [PubMed] [Google Scholar]
- Shen AL, O'Leary KA, Kasper CB. Association of multiple developmental defects and embryonic lethality with loss of microsomal NADPH-cytochrome P450 oxidoreductase. J Biol Chem. 2002;277:6536–6541. doi: 10.1074/jbc.M111408200. [DOI] [PubMed] [Google Scholar]
- Shen H, Ocheltree SM, Hu Y, Keep RF, Smith DE. Impact of genetic knockout of PEPT2 on cefadroxil pharmacokinetics, renal tubular reabsorption, and brain penetration in mice. Drug Metab Dispos. 2007;35:1209–1216. doi: 10.1124/dmd.107.015263. [DOI] [PubMed] [Google Scholar]
- Shen H, Smith DE, Keep RF, Xiang J, Brosius FC., 3rd Targeted disruption of the PEPT2 gene markedly reduces dipeptide uptake in choroid plexus. J Biol Chem. 2003;278:4786–4791. doi: 10.1074/jbc.M207397200. [DOI] [PubMed] [Google Scholar]
- Shen HW, Yu AM. Difference in desipramine metabolic profile between wild-type and CYP2D6-humanized mice. Drug Metab Lett. 2009;3:234–241. doi: 10.2174/187231209790218118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucov HM, Dyson E, Gumeringer CL, Price J, Chien KR, Evans RM. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994;8:1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem. 2002;277:26934–26943. doi: 10.1074/jbc.M203803200. [DOI] [PubMed] [Google Scholar]
- Sykes D, Sweet DH, Lowes S, Nigam SK, Pritchard JB, Miller DS. Organic anion transport in choroid plexus from wild-type and organic anion transporter 3 (Slc22a8)-null mice. Am J Physiol Renal Physiol. 2004;286:F972–F978. doi: 10.1152/ajprenal.00356.2003. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Morgan JA, Scheffer GL, Adachi M, Stewart CF, Sun D, Leggas M, Ejendal KF, Hrycyna CA, Schuetz JD. Substrate overlap between Mrp4 and Abcg2/Bcrp affects purine analogue drug cytotoxicity and tissue distribution. Cancer Res. 2007;67:6965–6972. doi: 10.1158/0008-5472.CAN-06-4720. [DOI] [PubMed] [Google Scholar]
- Tong MH, Jiang H, Liu P, Lawson JA, Brass LF, Song WC. Spontaneous fetal loss caused by placental thrombosis in estrogen sulfotransferase-deficient mice. Nat Med. 2005;11:153–159. doi: 10.1038/nm1184. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Terada T, Mizuno T, Katsura T, Shimakura J, Inui K. Targeted disruption of the multidrug and toxin extrusion 1 (mate1) gene in mice reduces renal secretion of metformin. Mol Pharmacol. 2009;75:1280–1286. doi: 10.1124/mol.109.056242. [DOI] [PubMed] [Google Scholar]
- Tsunoda SM, Velez RL, von Moltke LL, Greenblatt DJ. Differentiation of intestinal and hepatic cytochrome P450 3A activity with use of midazolam as an in vivo probe: effect of ketoconazole. Clin Pharmacol Ther. 1999;66:461–471. doi: 10.1016/S0009-9236(99)70009-3. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, Shertzer HG, Gonzalez FJ, Nebert DW. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol Pharmacol. 2006;69:1103–1114. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Sinclair PR, Gorman N, Wang B, Smith AG, Miller ML, Shertzer HG, Nebert DW. Cyp1a1(−/−) male mice: protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicol Appl Pharmacol. 2004;196:410–421. doi: 10.1016/j.taap.2004.01.014. [DOI] [PubMed] [Google Scholar]
- van de Steeg E, van der Kruijssen CM, Wagenaar E, Burggraaff JE, Mesman E, Kenworthy KE, Schinkel AH. Methotrexate pharmacokinetics in transgenic mice with liver-specific expression of human organic anion-transporting polypeptide 1B1 (SLCO1B1) Drug Metab Dispos. 2009;37:277–281. doi: 10.1124/dmd.108.024315. [DOI] [PubMed] [Google Scholar]
- van Herwaarden AE, Jonker JW, Wagenaar E, Brinkhuis RF, Schellens JH, Beijnen JH, Schinkel AH. The breast cancer resistance protein (Bcrp1/Abcg2) restricts exposure to the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res. 2003;63:6447–6452. [PubMed] [Google Scholar]
- van Herwaarden AE, Smit JW, Sparidans RW, Wagenaar E, van der Kruijssen CM, Schellens JH, Beijnen JH, Schinkel AH. Midazolam and cyclosporin a metabolism in transgenic mice with liver-specific expression of human CYP3A4. Drug Metab Dispos. 2005;33:892–895. doi: 10.1124/dmd.105.004721. [DOI] [PubMed] [Google Scholar]
- van Herwaarden AE, Wagenaar E, van der Kruijssen CM, van Waterschoot RA, Smit JW, Song JY, van der Valk MA, van Tellingen O, van der Hoorn JW, Rosing H, Beijnen JH, Schinkel AH. Knockout of cytochrome P450 3A yields new mouse models for understanding xenobiotic metabolism. J Clin Invest. 2007;117:3583–3592. doi: 10.1172/JCI33435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Waterschoot RA, Rooswinkel RW, Sparidans RW, van Herwaarden AE, Beijnen JH, Schinkel AH. Inhibition and stimulation of intestinal and hepatic CYP3A activity: studies in humanized CYP3A4 transgenic mice using triazolam. Drug Metab Dispos. 2009;37:2305–2313. doi: 10.1124/dmd.109.029397. [DOI] [PubMed] [Google Scholar]
- van Waterschoot RA, van Herwaarden AE, Lagas JS, Sparidans RW, Wagenaar E, van der Kruijssen CM, Goldstein JA, Zeldin DC, Beijnen JH, Schinkel AH. Midazolam metabolism in cytochrome P450 3A knockout mice can be attributed to up-regulated CYP2C enzymes. Mol Pharmacol. 2008;73:1029–1036. doi: 10.1124/mol.107.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaming ML, Mohrmann K, Wagenaar E, de Waart DR, Elferink RP, Lagas JS, van Tellingen O, Vainchtein LD, Rosing H, Beijnen JH, Schellens JH, Schinkel AH. Carcinogen and anticancer drug transport by Mrp2 in vivo: studies using Mrp2 (Abcc2) knockout mice. J Pharmacol Exp Ther. 2006;318:319–327. doi: 10.1124/jpet.106.101774. [DOI] [PubMed] [Google Scholar]
- Wan YJ, An D, Cai Y, Repa JJ, Hung-Po ChenT, Flores M, Postic C, Magnuson MA, Chen J, Chien KR, French S, Mangelsdorf DJ, Sucov HM. Hepatocyte-specific mutation establishes retinoid X receptor alpha as a heterodimeric integrator of multiple physiological processes in the liver. Mol Cell Biol. 2000;20:4436–4444. doi: 10.1128/mcb.20.12.4436-4444.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Lam P, Liu L, Forrest D, Yousef IM, Mignault D, Phillips MJ, Ling V. Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology. 2003;38:1489–1499. doi: 10.1016/j.hep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, Helgason CD, Ackerley C, Phillips MJ, Ling V. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A. 2001;98:2011–2016. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- Weng Y, Fang C, Turesky RJ, Behr M, Kaminsky LS, Ding X. Determination of the role of target tissue metabolism in lung carcinogenesis using conditional cytochrome P450 reductase-null mice. Cancer Res. 2007;67:7825–7832. doi: 10.1158/0008-5472.CAN-07-1006. [DOI] [PubMed] [Google Scholar]
- Wijnholds J, Evers R, van Leusden MR, Mol CA, Zaman GJ, Mayer U, Beijnen JH, van der Valk M, Krimpenfort P, Borst P. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat Med. 1997;3:1275–1279. doi: 10.1038/nm1197-1275. [DOI] [PubMed] [Google Scholar]
- Wu C, Jiang XL, Shen HW, Yu AM. Effects of CYP2D6 status on harmaline metabolism, pharmacokinetics and pharmacodynamics, and a pharmacogenetics-based pharmacokinetic model. Biochem Pharmacol. 2009;78:617–624. doi: 10.1016/j.bcp.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Gu J, Cui H, Zhang QY, Behr M, Fang C, Weng Y, Kluetzman K, Swiatek PJ, Yang W, Kaminsky L, Ding X. Transgenic mice with a hypomorphic NADPH-cytochrome P450 reductase gene: effects on development, reproduction, and microsomal cytochrome P450. J Pharmacol Exp Ther. 2005;312:35–43. doi: 10.1124/jpet.104.073353. [DOI] [PubMed] [Google Scholar]
- Wu L, Gu J, Weng Y, Kluetzman K, Swiatek P, Behr M, Zhang QY, Zhuo X, Xie Q, Ding X. Conditional knockout of the mouse NADPH-cytochrome p450 reductase gene. Genesis. 2003;36:177–181. doi: 10.1002/gene.10214. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang X, Bardag-Gorce F, Robel RC, Aguilo J, Chen L, Zeng Y, Hwang K, French SW, Lu SC, Wan YJ. Retinoid X receptor alpha regulates glutathione homeostasis and xenobiotic detoxification processes in mouse liver. Mol Pharmacol. 2004;65:550–557. doi: 10.1124/mol.65.3.550. [DOI] [PubMed] [Google Scholar]
- Xia CQ, Milton MN, Gan LS. Evaluation of drug-transporter interactions using in vitro and in vivo models. Curr Drug Metab. 2007;8:341–363. doi: 10.2174/138920007780655423. [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- Yu AM. Small interfering RNA in drug metabolism and transport. Curr Drug Metab. 2007;8:700–708. doi: 10.2174/138920007782109751. [DOI] [PubMed] [Google Scholar]
- Yu AM. Role of microRNAs in the regulation of drug metabolism and disposition. Expert Opin Drug Metab Toxicol. 2009;5:1513–1528. doi: 10.1517/17425250903307448. [DOI] [PubMed] [Google Scholar]
- Yu AM, Fukamachi K, Krausz KW, Cheung C, Gonzalez FJ. Potential role for human cytochrome P450 3A4 in estradiol homeostasis. Endocrinology. 2005;146:2911–2919. doi: 10.1210/en.2004-1248. [DOI] [PubMed] [Google Scholar]
- Yu AM, Granvil CP, Haining RL, Krausz KW, Corchero J, Kupfer A, Idle JR, Gonzalez FJ. The relative contribution of monoamine oxidase and cytochrome p450 isozymes to the metabolic deamination of the trace amine tryptamine. J Pharmacol Exp Ther. 2003a;304:539–546. doi: 10.1124/jpet.102.043786. [DOI] [PubMed] [Google Scholar]
- Yu AM, Idle JR, Byrd LG, Krausz KW, Kupfer A, Gonzalez FJ. Regeneration of serotonin from 5-methoxytryptamine by polymorphic human CYP2D6. Pharmacogenetics. 2003b;13:173–181. doi: 10.1097/01.fpc.0000054066.98065.7b. [DOI] [PubMed] [Google Scholar]
- Yu AM, Idle JR, Gonzalez FJ. Polymorphic cytochrome P450 2D6: humanized mouse model and endogenous substrates. Drug Metab Rev. 2004;36:243–277. doi: 10.1081/dmr-120034000. [DOI] [PubMed] [Google Scholar]
- Yu AM, Idle JR, Herraiz T, Kupfer A, Gonzalez FJ. Screening for endogenous substrates reveals that CYP2D6 is a 5-methoxyindolethylamine O-demethylase. Pharmacogenetics. 2003c;13:307–319. doi: 10.1097/01.fpc.0000054094.48725.b7. [DOI] [PubMed] [Google Scholar]
- Zaher H, zu Schwabedissen HE, Tirona RG, Cox ML, Obert LA, Agrawal N, Palandra J, Stock JL, Kim RB, Ware JA. Targeted disruption of murine organic anion-transporting polypeptide 1b2 (Oatp1b2/Slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol Pharmacol. 2008;74:320–329. doi: 10.1124/mol.108.046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, van de Wetering K, de Waart R, Scheffer GL, Marschall HU, Wielinga PR, Kuil A, Kunne C, Smith A, van der Valk M, Wijnholds J, Elferink RO, Borst P. Mice lacking Mrp3 (Abcc3) have normal bile salt transport, but altered hepatic transport of endogenous glucuronides. J Hepatol. 2006;44:768–775. doi: 10.1016/j.jhep.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang W, Chua SS, Wei P, Moore DD. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science. 2002;298:422–424. doi: 10.1126/science.1073502. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Fang C, Zhang J, Dunbar D, Kaminsky L, Ding X. An intestinal epithelium-specific cytochrome P450 (P450) reductase-knockout mouse model: direct evidence for a role of intestinal p450s in first-pass clearance of oral nifedipine. Drug Metab Dispos. 2009;37:651–657. doi: 10.1124/dmd.108.025429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QY, Gu J, Su T, Cui H, Zhang X, D'Agostino J, Zhuo X, Yang W, Swiatek PJ, Ding X. Generation and characterization of a transgenic mouse model with hepatic expression of human CYP2A6. Biochem Biophys Res Commun. 2005;338:318–324. doi: 10.1016/j.bbrc.2005.08.086. [DOI] [PubMed] [Google Scholar]
- Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 2002;99:12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhuo X, Xie F, Kluetzman K, Shu YZ, Humphreys WG, Ding X. Role of CYP2A5 in the clearance of nicotine and cotinine: insights from studies on a Cyp2a5-null mouse model. J Pharmacol Exp Ther. 2009;332:578–587. doi: 10.1124/jpet.109.162610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP. Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol Cell Biol. 2001;21:4188–4196. doi: 10.1128/MCB.21.13.4188-4196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]