Abstract
In order to understand the brain networks that mediate cognitive reserve, we explored the relationship between subjects’ network expression during the performance of a memory test and an index of cognitive reserve. Using H215O positron emission tomography, we imaged 17 healthy older subjects and 20 young adults while they performed a serial recognition memory task for nonsense shapes under two conditions: low demand, with a unique shape presented in each study trial; and titrated demand, with a study list size adjusted so that each subject recognized shapes at 75% accuracy. A factor score that summarized years of education, and scores on the NART and the WAIS-R Vocabulary subtest was used as an index of cognitive reserve. The scaled subprofile model was used to identify a set of functionally connected regions (or topography) that changed in expression across the two task conditions and was differentially expressed by the young and elderly subjects. The regions most active in this topography consisted of right hippocampus, posterior insula, thalamus, and right and left operculum; we found concomitant deactivation in right lingual gyrus, inferior parietal lobe and association cortex, left posterior cingulate, and right and left calcarine cortex. Young subjects with higher cognitive reserve showed increased expression of the topography across the two task conditions. Because this topography, which is responsive to increased task demands, was differentially expressed as a function of reserve level, it may represent a neural manifestation of innate or acquired reserve. In contrast, older subjects with higher cognitive reserve showed decreased expression of the topography across tasks. This suggests some functional reorganization of the network used by the young subjects. Thus, for the old subjects this topography may represent an altered, compensatory network that is used to maintain function in the face of age-related physiological changes.
Keywords: compensation, covariance analysis, education, H215O PET, IQ
Introduction
Older adults demonstrate deficits, relative to young adults, in episodic or explicit tests of memory, e.g. free recall, cued recall and recognition tests, that require the conscious retrieval of previously experienced events (for reviews see Light, 1991; Grady and Craik, 2000). Several studies have suggested that there is differential susceptibility to age-related memory changes and dementia that is related to variables such as education, literacy, IQ and engagement in leisure activities (Schaie, 1984; Stern et al., 1994; Gold et al., 1995; Hultsch et al., 1999; Wilson et al., 2000, 2002; Scarmeas et al., 2001; Manly et al., 2003). These studies provide epidemiological evidence for the presence of cognitive reserve (CR), where subjects with greater CR may show less severe effects of the aging process.
The concept of CR posits that individual differences in how tasks are processed might provide differential reserve against brain pathology or age-related changes. That is, brain networks that are more efficient or flexible may be less susceptible to disruption. However, our understanding of how CR might be implemented in the form of brain networks is in the very early stages. We have hypothesized that there may be two complementary facets to the neural implementation of CR (Stern, 2002): neural reserve and neural compensation.
Neural reserve may represent normally occurring individual differences in the capacity to perform tasks or cope with increases in task difficulty. These differences may result from innate differences (e.g. in intelligence) or they may be modulated through life events such as educational or occupational experience or leisure activities. Higher neural reserve might be implemented in the form of brain networks that are either more efficient or have greater capacity in the face of increased demand. Thus, the same or network can deal with various levels of difficulty of some specific task, and people with greater reserves can use this same region at higher levels of task difficulty than other people can. Individuals who have these more efficient or higher capacity brain networks at their disposal might be able to withstand a greater degree of age-related change while maintaining intact functioning.
There are two conceptual points to emphasize regarding neural reserve. First, since it is based on individual differences that pre-exist any brain pathology, differential expression of brain networks as a function of CR should be observed even in young subjects that are free of any pathology. In fact, using both voxel-wise and network analyses, we have demonstrated individual variability in task-related activation as a function of proxies for CR such as IQ and education (Habeck et al., 2003; Stern et al., 2003). Second, because neural reserve is based on differences in network utilization that pre-exist brain pathology or age-related changes, the networks that subserve neural reserve should be comparable in young and old subjects. Although there may be differences in the efficiency or capacity of a reserve network as a function of age (or pathology), the network itself should be the same. This observation leads to the prediction that, if older individuals express the same reserve network as young individuals, the directionality of the relationship between network recruitment and CR should be the same in both groups.
It is also possible to imagine a situation where a task is too difficult to be performed by the appropriate brain network. One possible outcome is that the same network continues to carry out the processing, but inefficiently, and so task performance suffers. An alternative is that a different network takes over or supplements the first network. Several reports have noted the recruitment of additional brain areas or networks with increased task difficulty, even in healthy younger subjects (Grady et al., 1996; Rypma and D'Esposito, 1999; Jansma et al., 2000; Jha and McCarthy, 2000; Glahn et al., 2002). To the extent that the recruitment of an alternate network occurs in healthy young adults, and not in response to brain damage, we would classify such recruitment as an alternate instantiation of neural reserve. Thus the term neural reserve would encompass the normally occurring responses to increased task difficulty, which include differential recruitment of the same network, and perhaps recruitment of alternate networks when the capacity of the original network is exceeded.
We contrast these implementations of neural reserve to a complementary facet of CR, which we call neural compensation. We propose to use this term in a situation where the physiological effects of aging or brain pathology cause the alteration of a brain network, resulting in a network that would not normally be used by unaffected individuals. In this altered network, the same brain areas might be used in a different way, or additional brain areas might be recruited. We call this neural compensation because we presume that the altered network is used to compensate for the inability to utilize the healthy brain's responses to increased task difficulty. The ability of the compromised brain to express or optimize compensatory networks may also vary as a function of CR.
Note that our definition of the neural compensation does not require evidence that the use of the alternate network results in improved performance. Compensatory networks may simply serve to maintain performance in the face of aging or pathology. We consider implications of the use of the compensatory network for performance to be a separate question.
Our proposed distinction between neural reserve and compensation has both theoretical and practical implications for the interpretation of the results of functional imaging studies that compare old and young individuals (or any ‘normal’ and brain-damaged group). It forces one to distinguish between normally occurring responses to increased task demand, and compensatory responses to brain damage. For example, it is quite possible for a study to find that young and elderly individuals recruit a different brain area or network without the alternate areas recruited by the elders meeting our criteria for compensation. This could easily occur when there is a mismatch in task difficulty across the two subject groups. If the activation task is more difficult for the elders than the young subjects, it could elicit an alternate area or network in the elders that might also be seen in the young subjects if task difficulty were increased further for them.
One possible way to distinguish between a compensatory and reserve network is to determine the relationship between network expression and CR. As explained above, the relation between CR and the expression of reserve networks should be the same in young and older subjects. However, if the directionality of the relationship between network expression and CR differed in old and young subjects, this would provide evidence of compensatory reorganization of the network in the face of age-related neural changes. This prediction is based on the idea that individuals with more CR use a network in a more optimal fashion. For example, if high CR young individuals show greater expression than low CR young individuals of specific areas in a network, while high CR elders show lower network expression than low CR elders, this would suggest that some alteration in the network has occurred that makes it more optimal for the elders to express these areas to a lesser degree.
To test this idea, we used H215O positron emission tomography (PET) to explore the relationship between indices of subject's CR, such as education and IQ, and their expression of specific brain networks during the performance of a non-verbal serial recognition test. There were two task conditions. The low demand condition was designed to require encoding and recognition of individual items. The titrated demand condition required the subject to encode and then recognize a longer list of items. Prior to scanning, the list length in the titrated demand condition was adjusted for each subject such that recognition accuracy was 75%. This procedure was intended to control for task difficulty, matching difficulty across each subject in both the young and old groups. Our intention was to explore how individual differences in measured CR are related to changes in brain network expression as subjects moved from the low to the titrated demand task.
Our desire to explore network activity prompted our selection of the scaled subprofile model as the mode of data analysis. In this model, each cognitive operation that involves one or more brain regions may be associated with a unique regional pattern of physiological activity, or topography, that is extractable from the imaging data (Moeller et al., 1987, 1998; Alexander and Moeller, 1994). These topographies consist of constituent brain areas whose task-related activation co-varies. Thus, it is likely that the covariance pattern results from the activity of one or more underlying neural networks. Our strategy was to identify a topography whose mean change in expression across the two task conditions differed maximally across the two groups. Such a covariance pattern would be a candidate for containing a neural network that is used differently by young and old subjects. We then examined the correlation between change in expression of the identified topography across the two task conditions, and CR as measured by a factor score summarizing IQ measures and education. A network that expressed such a correlation in young individuals would be candidate for a system that is especially related to the neural instantiation of reserve. A similar correlation between network expression and CR measures in elders would suggest that they express the same reserve network. However, if the relationship between topographic expression and the CR factor score differed in old and young subjects, this would provide evidence that the old subjects were using a network that differed from that used by the young subjects. This altered network would meet our criteria for neural compensation in the face of age-related changes.
Materials and Methods
Subjects
Seventeen healthy older subjects (8 men, 10 women), and 20 healthy young adults (8 men, 12 women) participated (Table 1). All subjects were carefully screened with medical, neurological, psychiatric and neuropsychological evaluations to ensure that they had no neurological or psychiatric disease or cognitive impairment. In particular, older subjects were carefully evaluated to ensure the absence of dementia. All subjects were right-handed. Informed consent was obtained after the nature and risks of the study were explained.
Table 1.
Demographic characteristics and activation task performance in the two subject groups.
| Elders (n = 17) |
Young (n = 20) |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 70.9 | 7.0 | 23.4a | 2.2 |
| Education | 15.0 | 0.7 | 15.7 | 1.8 |
| NART estimated IQ | 121.1 | 6.9 | 121.2 | 3.3 |
| Vocabulary (age-scaled score) | 13.8 | 2.5 | 13.5 | 1.5 |
| Reserve factor score | 135.9 | 1.9 | 137.1 | 1.9 |
| Digit Symbol (age-scaled score) | 12.7 | 2.4 | 12.5 | 3.3 |
| Selective reminding test: total recall | 46.9 | 7.7 | 58.8a | 6.9 |
| Study list size (titrated demand condition) | 7.5 | 1.3 | 13.9a | 1.2 |
| % Correct (low demand condition) | 97.5 | 4.3 | 94.7 | 4.8 |
| % Correct (titrated demand condition) | 72.3 | 7.0 | 76.9 | 8.9 |
Young and old subjects differ significantly.
Reserve Index and Neuropsychological Tests
Three variables were used as proxy measures for each subject's level of CR: years of education, and two indices of intelligence, the New Adult Reading Test (NART)-North American Version (Nelson, 1982), and age-scaled scores on the Vocabulary subtest of the WAIS-R (Wechsler, 1981). The two latter tests are considered to be good estimates of premorbid Verbal IQ. Using a factor analysis, we derived a CR factor score which summarized the three reserve variables. The CR factor accounted for 70% of the common variance in these three measures. This factor score was used to represent CR in the subsequent analyses.
Subjects also received a series of neuropsychological tests including the WAIS-R Digit Symbol subtest (Wechsler, 1981) and the Selective Reminding Test (Buschke and Fuld, 1974).
Activation Task
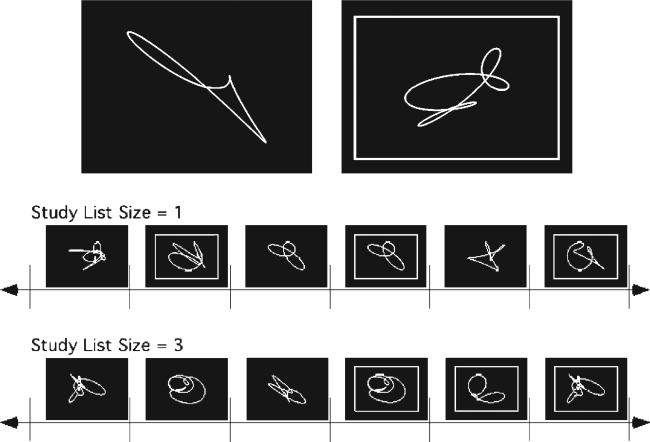
Two conditions of a continuous performance nonverbal recognition test were considered in the current analyses. The task is shown schematically in Figure 1 along with sample task stimuli. The basic task consisted of the serial presentation of one or more single unnamable shapes followed by a series of the same number of recognition probes. Probes were distinguished from study items by a circumscribed rectangular frame. Subjects made a ‘new’ or ‘old’ response for each probe item. In the low demand condition, the list length was one (i.e. one study shape followed by one recognition probe). The titrated demand condition used a list length that was predetermined to elicit a recognition accuracy of 75% for the subject being tested. For example, if the list length was 10, then 10 target items were presented followed by 10 test items, 5 of which were targets. One day prior to the PET scan, each subject completed a standardized training session and then two 2-20 min titration sessions. During these sessions, the particular regions of interest at which the subject achieved 75% overall accuracy (to both targets and lures) was determined using a staircase method of regions of interest adjustment. This list length was then used in the titrated demand condition on the day of the scan.
Figure 1.
Top: Samples of the shapes used in the nonverbal recognition task. A probe item is denoted by a surrounding rectangle. Bottom: Schematic of the nonverbal recognition task for study list sizes of 1 and 3.
Study shapes were displayed for 5 s for older subjects and 4 s for younger subjects as a partial control for differential task difficulty in the two groups. A 500 ms delay occurred at the transition to test trials, after which the recognition probes were displayed. The ‘new’ or ‘old’ response for each probe item was made by pressing one of two microswitches with the left or right thumb. A 6 s response time limit was imposed, with a premature response cutoff of 200 ms. Accuracy was emphasized over speed. A new test probe was displayed immediately following the button press. ‘New’ and ‘old’ test probes occurred with a frequency of 50%. Test probes were pseudorandomized so that no more than four consecutive trials required the same response.
Each shape was used only once for each subject. The set of shapes was designed to have similar characteristics that varied randomly within given sets of parameters. Looped shapes were used since their level of complexity made verbal encoding difficult (Fig. 1). The stimuli were prescreened to ensure that they could not be easily given a name, and a separate screening study established that elderly subjects could recognize each shape.
PET-scan Acquisition and Processing
The activation task was initiated 50 s prior to the start of the scan and continued throughout the scan period. Subjects viewed the shape stimuli on an overhead monochrome monitor while lying in a supine position.
Scans were separated by 10 min. After a scan at rest, the two conditions considered here were obtained in context of scanning session that included another shape condition and three conditions of a verbal activation task. The other four scans are not discussed here. The order of acquisition of the verbal and nonverbal scans was counter-balanced across subjects.
For each scan, a bolus of 30 mCi H215O was injected intravenously. Scan acquisition was triggered by the detection of a threshold level of true counts from the camera. Employing a Siemens HR+ PET camera, 230 s scan frames were acquired in two-dimensional mode. After measured attenuation correction (15 min transmission scan) and reconstruction by filtered back-projection, image resolution was 4.6 mm full width half maximum. Arterial blood sampling was not conducted.
Using modules from the Statistical Parametric Mapping program, each subject's scans were realigned to each other and normalized into a standardized Talairach space. MEDX software was then used to overlay a standardized three-dimensional region of interest template on the scan slices. This template was drawn on a standard magnetic resonance image that conformed to Tailarach space, and defined 22 regions of interest (ROIs) per hemisphere, comprising major cortical and sub-cortical regions (Stern et al., 2000). To prepare the data for scaled subprofile modeling analyses, the activity of each ROI was computed to be the average of the upper 20% of pixel blood flow values. In these calculations, radioactive counts per pixel were used to estimate the relative blood flow rates (Fox and Mintun, 1989).
Network Analysis
Covariance analysis techniques are considered appropriate methods to explore network activity (McIntosh et al., 1996; Poline et al., 1997). We used the scaled subprofile model (Moeller et al., 1987, 1998; Alexander and Moeller, 1994), a type of analysis methodologically similar to other current covariance analysis techniques. This method has been used previously in resting imaging studies of normal aging and a variety of diseases (Eidelberg et al., 1995; Moeller et al., 1996; Stern et al., 2000). In this model, each cognitive operation that involves one or more brain regions may be associated with a unique regional pattern of physiological activity, or topography, that is extractable from the imaging data. A change in the degree of expression of a topography across conditions is presumably related to the change in demands of the task on its associated cognitive operation.
Region of interest data from both task conditions for the young and old subjects were simultaneously included in a single scaled subprofile model analysis, which captured the major sources of between- and within-group variation. This analysis produced a series of principal components. The change in the expression of each principal component across the two task conditions is quantified for each subject by a subject scaling factor.
To identify a covariance pattern whose expression differed across the young and old groups, each subject's expression (subject scaling factor) of the first two identified principal components (capturing 22.4% of the variance) was entered into a linear regression model as the independent variable. Group membership (young versus old) was the dependent variable. This regression resulted in a linear combination of the two principal components that best discriminated the two groups. This linear combination of the first two principal components is described as the ‘age-related’ topography. A ‘common’ topography was then derived which consisted of a linear combination of the first two principal components that was orthogonal to the age-related topography. Together, the age-related and common topographies can be interpreted as a new set of basis vectors for the space spanned by the first two principal components.
A topography consists of a series of weights or loadings, which quantify the degree to which each ROI participates in that topography. The loadings may be either positive or negative, and express the covariance structure (i.e. the strength of the interaction) between the ROIs that participate in the principal component. Regions of interest with positive loadings can be conceived as exhibiting concomitant increased flow and those with negative loading can be conceived as exhibiting concomitant decreased flow. These loadings are fixed and the same for all subjects. For localization purposes, it is of interest to identify ROIs that participate in the topography with high confidence. The stability and robustness of ROI loadings was assessed using a bootstrap resampling technique (Efron and Tibshirani, 1998). This analysis produces inverse coefficient-of-variation values for each ROI loading by forming the ratio of the point estimate divided by the standard deviation computed through the bootstrap procedure. The inverse coefficient-of-variation values approximately obey a standard-normal distribution; large inverse coefficient-of-variation values indicate that bootstrap resampling produced small variability of the regional loadings around the original point estimate values. Thus, large absolute inverse coefficient-of-variation values indicate reliability of regional contributions to the activation pattern. Only ROIs whose inverse coefficient-of-variation values were > 2.33 (implying a one-tailed P-value of 0.01) are reported as participating reliably in a topography.
As mentioned above, change in subject expression of a topography across the two task conditions is quantified by a subject scaling factor. For each subject, a subject scaling factor was calculated which summarized the change in the expression of both the age-related and common topographies across the two task conditions. The more positive the subject scaling factor value, the greater the concomitant increase in activation across the two task conditions of the regions with positive loadings and the greater the concomitant decrease in activation of the regions with negative loadings. A more negative subject scaling factor value indicates the opposite — increased activation of areas with negative loadings and concomitant decreased activation of areas with positive loadings. It should be noted that subject scaling factor scores used in all subsequent analyses are difference measures which summarize the change in topographic expression across the two task conditions.
To explore the relationship between the change in the expression of the identified topographies and CR, subjects’ subject scaling factor scores for each network were correlated with the CR factor score. A significant correlation between expression of a network and the CR score would provide evidence that change in expression of a network across conditions varies with the level of CR. Thus the network may mediate an aspect of CR. By comparing this correlation in the young and old subject groups, we could determine whether the relation between CR and the expression of each topography remains stable in the face of normal aging.
Results
Behavioral Data
Demographics and performance data are summarized in Table 1. Mean age was 71.0 in the old group and 23.4 in the young group. Mean years of education were comparable in the two groups (t = 1.79, NS), as was performance on the NART (t = 0.03, NS), WAIS-R Vocabulary (t = 0.49, NS), the CR index (t = 0.44, NS) and WAIS-R Digit Symbol (t = –0.26, NS). Performance on the Selective Reminding Test was significantly lower in the old group (t = 4.92, P < 0.01), but was within normal limits for this age group.
In the low demand condition, accuracy was high in both groups. As expected, mean list length in the titrated demand condition was significantly higher in the young group (t = 3.52, P < 0.01). However, as intended, mean percent correct recognition in this condition was comparable in the two groups (t = 1.73, P = 0.09).
We examined the relationship between subjects’ CR index and the titrated list length that they achieved. This relationship did not reach significance in the old subjects (r = 0.21, P < 0.41). The correlation reached significance in the young subjects, but was driven by one outlying value (r = 0.56, P < 0.01; with the outlier removed r = 0.20, P = 0.36).
Physiological Results
The scaled subprofile model analysis identified the two topographies that changed in expression between low and titrated demand conditions: an age-related topography whose mean change in expression across the two conditions differed in the old and young groups; and an orthogonal, common topography, whose mean change in expression across the two conditions was comparable in the two groups.
Common Topography
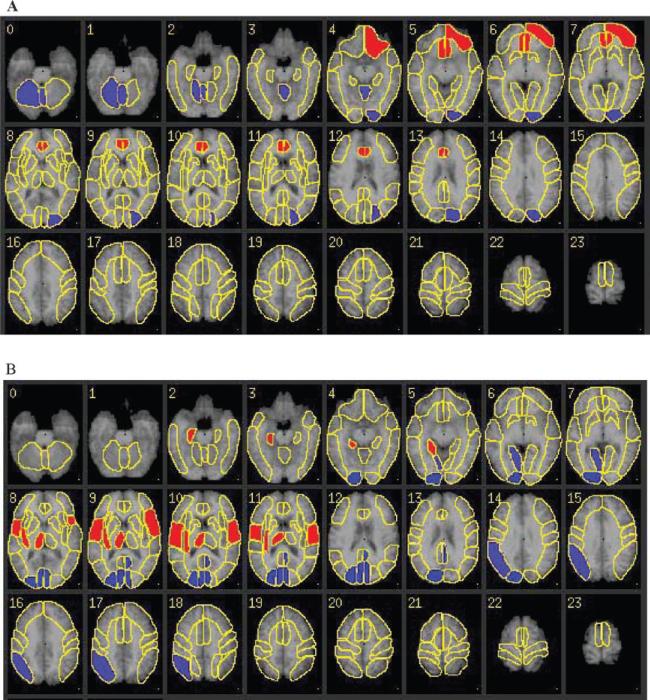
The strongest loadings for ROI participation in the common topography consisted of positive loadings in the right and left anterior cingulate and left orbital frontal cortex, with associated negative loadings in left association cortex and right cerebellum and vermis (Fig. 2A).
Figure 2.
The common (A) and age-related (B) covariance patterns (topographies) noted in the 20 young and 17 healthy elderly subjects. Weights for regions with most marked participation in the topography have been overlaid on standard Tailarach-transformed axial MRI sections, with positive weights indicated in red and negative weights indicated in blue. Radiological convention is used. (A) Common topography. This topography consisted of positive loadings in the right and left anterior cingulate and left orbital frontal cortex, with associated negative loadings in left association cortex and right cerebellum and vermis. (B) Age-related topography. This topography consisted of positive loadings in the right hippocampus, posterior insula, thalamus, and right and left operculum; negative loadings in right lingual gyrus, inferior parietal lobe and association cortex, left posterior cingulate, and right and left calcarine cortex.
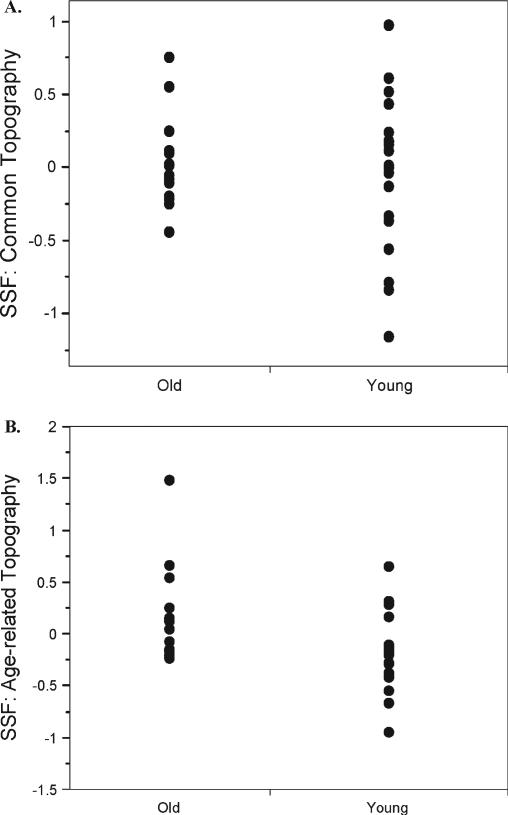
The mean change in expression of this topography, as measured by subjects’ subject scaling factors, was comparable in the two groups (young mean 0.019, old mean 0.018) (Fig. 3A). The range and variability of subject scaling factor values was significantly higher in the young group (young SD = 0.58; old SD = 0.29; Levene F = 5.12, P < 0.03).
Figure 3.
Subjects’ expression, as measured by the subject scaling factor of the common and age-related topographies in the young and old groups. (A) Common topography. Note that mean expression is comparable across the two groups, but that the range is greater in the younger group. (B) Age-related topography. Note that mean expression of this topography is lower in the young than the old subjects.
Expression of the topography did not correlate with the reserve factor or age in either group.
Age-related Topography
The strongest loadings for ROI participation in the age-related topography consisted of positive loadings in the right hippocampus, posterior insula, thalamus and right and left operculum; negative loadings in right lingual gyrus, inferior parietal lobe and association cortex, left posterior cingulate, and right and left calcarine cortex (Fig. 2B).
Mean expression of this topography differed significantly in the two groups, with expression more negative in the young group and more positive in the old group (mean young = –0.19; mean old = 0.17; t = –2.78, P < 0.01) (Fig. 3B). The variability in each group's topographic expression was comparable (Levene F = 0.41, P = 0.53).
The negative mean for the young subjects indicates that when moving from low to titrated demand task, the young subjects on average increased activation in areas with negative loadings — right lingual gyrus, inferior parietal lobe and association cortex, left posterior cingulate, and right and left calcarine cortex — with concomitant decreased activation in areas with positive loadings — right hippocampus, posterior insula, thalamus, and right and left operculum. The positive mean for the old group suggests that, on average, their pattern of recruitment was the opposite.
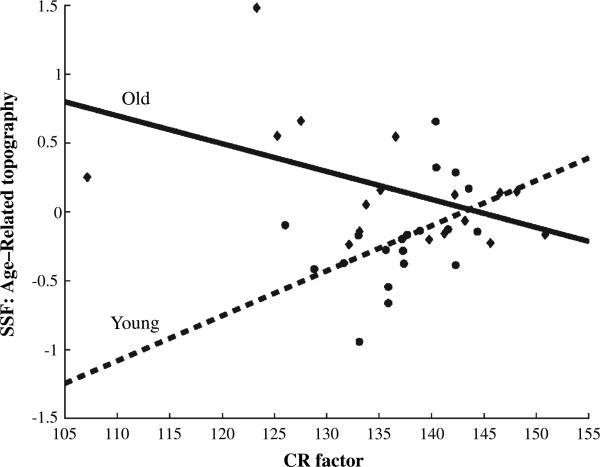
In the young subjects, there was a positive correlation between subject scaling factor values of the age-related topography and the CR factor score (Table 2; r = 0.45, P < 0.05). This indicates that, when moving from the low to the titrated demand condition, higher CR in the young subjects was associated with relatively greater increases in flow in positively weighted areas and concomitantly decreased flow in negatively weighted areas. In contrast, in the old subjects there was negative correlation between these CRs and subject scaling factor values of the age-related topography (r = –0.50, P < 0.05). Figure 4 summarizes the relationship between subject scaling factor values and the CR factor in both groups. Note that the age differences in expression of the topography may be driven primarily by the low CR subjects in both groups.
Table 2.
Correlations of key variables in the two subject groups.
| Elders |
Young |
|||||
|---|---|---|---|---|---|---|
| SSF: age-related Topography | SLS | Age | SSF: age-related topography | SLS | Age | |
| CR factor | –0.50* | 0.59** | –0.10 | 0.45* | 0.30 | 0.24 |
| Age | 0.52* | –0.09 | 0.20 | –0.05 | ||
| Study list size (SLS) | –0.21 | 0.56** | ||||
CR, cognitive reserve; SSF, subject scaling factor; measure of change in expression of age-related topography from the low to titrated demand condition.
P < 0.05
P < 0.01
NS with one outlier removed.
Figure 4.
Correlation between expression of the differential topography and the cognitive reserve factor in the young and old subjects. Note that this correlation is positive in the young group and negative in the old group.
In the old subjects, expression of age-related topography correlated positively with age (r = 0.52, P < 0.05), indicating increased expression of the positively weighted components of the topography with more advanced age. However, controlling for age did not alter the negative correlation between topographic expression and the reserve factor (partial r = –0.53, P < 0.05). This is because the CR score was uncorrelated with age (r = 0.10, P = 0.63).
Discussion
This study evaluated changes in network activity as a nonverbal recognition memory task increased in difficulty from a list length of one to a titrated list length that was intended to challenge each subject to the same degree. We identified an ‘age-related’ topography whose change in mean expression across these two conditions differed in the young and old subjects. The mean expression of this topography was more positive for the older group. Also, topographic expression correlated positively with age in the older group, such that the expression was more positive in the older members of that group. The analytic approach focused on identifying this age-related topography because such a topography would be most likely to shed light on differences in how the two groups respond to increases in task load.
Since young subjects operate without the burden of any age-related physiological changes, we begin by evaluating expression of the age-related topography in the young group. The mean of young subjects’ subject scaling factors for expressing this topography was lower than for the old subjects, but the correlation between topographic expression and reserve was positive. Thus, in the transition from low to titrated demand condition, the higher their level of CR, the more young subjects increased their activation in regions with positive loadings, with concomitantly decreased activation in regions with negative loadings. The differential utilization of this topography by young subjects as a function of CR is consistent with our prediction of the behavior of a neural reserve network.
In the older subjects, the correlation between the CR index and their expression of the age-related topography was negative. That is, the higher their level of CR, the more old subjects increased their activation in regions with negative loadings and decreased their activation in regions with positive loadings in the transition from low to titrated demand condition. Thus, as in the young subjects, individual differences in elder subjects’ network expression in response to increased task demand correlated with a measure of CR. However, the direction of this relationship was opposite that seen in the young subjects.
Since the young subjects have no age-related neural changes, we can speculate that the different relationship between CR and topographic expression in the two groups is due to some age-related physiological change in the older subjects. As a response to these changes, perhaps as a function of longer-term brain adaptation, the older subjects make use of an altered network, causing the activation of the regions captured in the covariance pattern to switch sign. This results in higher CR being associated with increased utilization of some brain areas with more positive network expression in one group, and more negative expression in the other. The age-related changes in network expression are thus most consistent with our definition of neural compensation.
It should be noted that the finding of a difference between young and old adults for expression of the age-related topography was obligated by how that topography was defined and derived. However, the direction of the correlation with CR within each group was not.
The age-related topography consisted of positive loadings in the right hippocampus, posterior insula, thalamus, and right and left operculum; negative loadings in right lingual gyrus, inferior parietal lobe and association cortex, left posterior cingulate, and right and left calcarine cortex. The increase in the activation of older subjects with increased task difficulty in medial temporal, temporal and frontal areas might be consistent with episodic encoding and retrieval. For example, one paper showed higher activation in elders for temporal regions BA 37 and insula (Grady et al., 1994). When going from low to titrated demand, the younger subjects relied more on the occipital and parietal areas implicated in early visual and spatial attentional processing. These differences may in part be due to poorer vision in the older subjects. Similar findings were noted in two studies using a face-matching task (Grady et al., 1994, 2000). These papers show higher activation in posterior areas BA 18, BA 40, BA 39 in young people, in accordance with our findings. The young subjects also showed increased involvement of bilateral calcarine cortex with increased task demand. It is not unusual to find less activation for older adults in early visual regions.
We also identified a common topography whose mean expression was comparable in the young and old groups. This represents a functional network whose expression increases with increased task difficulty in groups. It was possible a priori that expression of the common topography could also have correlated with CR as they are probably aspects of the implementation of reserve that do not differ across age. However, no correlation was observed with the CR index in either group. This topography consisted of positive loadings in the right and left anterior cingulate and left orbital frontal cortex, with associated negative loadings in left association cortex and right cerebellum and vermis. The areas with positive and negative loadings here correspond in many ways to areas that have shown increased and decreased activation with aging across many tasks (Cabeza, 2001). While the mean expression of this topography did not differ across the two age groups, this topography represents activation changes associated with increased task demand.
These analyses differ from traditional analyses of task-related activation data in several ways. These include the use of multivariate network analysis, and more importantly, an emphasis on individual variation in network expression. Another conceptually important feature of the design of this study was the fact that we adjusted list length for each subject in order to control for task difficulty on this episodic memory task. Matching task difficulty in each subject makes it more likely that activation differences between young and old subjects, or across subjects within each group, reflect true differences in the brain networks underlying task performance. Without controling for task difficulty, within- or between-group differences might simply reflect modulation of the same networks as a function of task difficulty.
One consequence of this adjustment is that list length actually differs across subjects. We could have chosen to evaluate how differential network expression across subjects related to list length. However, the intention of titrating list length was to hold task difficulty constant in order to isolate networks that might be differentially activated as a function of reserve. It might be contended that some of the differential network expression that we observed across the young and older subjects was due to lower list length in the older subjects invoking different cognitive processes than would be used when working with longer lists. However, there are several design features and study outcomes that indicate that this is not the case. First, the titration procedure ensured that each subject worked to maximum capacity given the fixed accuracy level, and pushed each subject to work with a list length that was above the capacity of their working memory. Second, while the mean list length in the older group was 7.5, individual subjects worked with list lengths up to 19, which is beyond the range of visual working memory capacity. These older subjects with longer lists expressed the topographies in the same parametric manner as elder subjects with shorter lists. Third, expression of both topographies was uncorrelated with list length, suggesting that group differences in expression are not directly linked to list length. We therefore conclude that task demands were similar in both groups.
The CR factor score that was used includes variables that have been associated with CR in previous studies. To the extent that the younger subjects were still in school, years of education could be truncated in that group. We still felt that education was an important variable to include in the older subjects and wanted to keep the CR index constant across the two groups. In should be noted, however, that the correlation between topographic expression and CR is still present in the young group using only NARTIQ as an index of CR.
We used nonverbal stimuli in our memory activation task. This was intended to preclude differential verbal mediation across subjects. The main intent of the task was simply to impose varying task loads on the subjects. We do not posit differential effects of CR for verbal and nonverbal tasks.
There have been many reports of age-related increased activation in areas not activated in young individuals (Grady et al., 1994; Madden et al., 1999b; McIntosh et al., 1999; Cabeza et al., 2002; Reuter-Lorenz et al., 2000; Logan et al., 2002; Reuter-Lorenz, 2002). This activation has been viewed as compensatory reallocation of brain function (Cabeza et al., 2002). However, the interpretation of either increased or decreased activation can be problematic. In some cases the additional activation seen in elders appears to be associated with poorer performance (Grady et al., 1996; Cabeza et al., 1997a; Madden et al., 1999b; Rypma and D'Esposito, 2001); while in other cases it appears to be beneficial to performance (Madden et al., 1999a; McIntosh et al., 1999; Reuter-Lorenz et al., 2000; Cabeza et al., 2002; Morcom et al., 2003).
While demonstrating that alternate patterns of activation in aging associated with maintained or improved function is consistent with the concept of compensation, our proposed definition of a compensatory network does not require such evidence. Rather, in the face of equal task difficulty/performance in the two groups, we simply require evidence that a brain network or brain area is not used by older subjects in the same manner as it is used young subjects. We assume that compensatory networks arise when normally used brain networks are altered as a result of age-related neural changes. Although it is important to evaluate whether a compensatory network assists in the maintenance of optimal function, we propose that the issue be separated from the initial task of differentiating reserve and compensatory networks. We did not find a relationship between network expression and list length attained in the titrated demand condition.
Typically, functional imaging studies evaluate the relation between activation and performance on the activation task. We evaluated the relationship between the expression of a compensatory network and the level of CR, as opposed to the level of task performance. We reasoned that CR is not synonymous with any specific cognitive ability or performance on any one task, but that some aspect of CR should be invoked in any challenging task. This is consistent with the implicit assumption that tasks are mediated differently by individuals with higher and lower education and IQ. Similarly, we did not relate activation to Digit Symbol performance because we were interested in more generic measures of CR. Very few studies have looked at the relation between measures that would be proxies for CR- and task-related activation (Gray et al., 2003; Habeck et al., 2003; Scarmeas et al., 2003; Stern et al., 2003).
Another unique aspect of this study was the covariance approach to data analysis. Most studies of compensation in normal aging have not considered functional connectivity across brain regions, but a few published studies have suggested age-related changes in connectivity (Grady et al., 1995; Cabeza et al., 1997b; McIntosh et al., 1999). Here we focused on identifying a single topography that was differentially utilized by the two groups. This approach differs from these other papers, where the focus was on identifying networks comprised of different regions in the two groups. We focused here on differential utilization of the same brain areas as opposed to seeking areas that were used by one group and not the other.
In summary, the present data provide information about the neural implementation of the two aspects of CR, neural reserve and compensation, in normal aging. Further studies are needed to determine if the differential utilization as a function of CR of these reserve and compensation networks assists in coping with later age- or disease-related brain changes.
Acknowledgments
Supported by Federal Grants AG14671 and RR00645.
References
- Alexander GE, Moeller JR. Application of scaled subprofile modeling to functional imaging in neuropsychiatric disorders: a principal component approach to modeling brain function in disease. Hum Brain Mapp. 1994;2:79–94. [Google Scholar]
- Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Functional neuroimaging of cognitive aging. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. MIT Press; Cambridge, MA: 2001. pp. 331–377. [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997a;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, McIntosh AR, Tulving E, Nyberg L, Grady CL. Age-related differences in effective neural connectivity during encoding and recall. Neuroreport. 1997b;8:3479–3483. doi: 10.1097/00001756-199711100-00013. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. CRC Press; Boca Raton, FL: 1994. [Google Scholar]
- Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Chaly T, Belakhlef A, Mandel F, Przedborski S, Fahn S. Early differential diagnosis of Parkinson's disease with 18F-fluorodeoxyglucose and positron emission tomography. Neurology. 1995;45:1995–2004. doi: 10.1212/wnl.45.11.1995. [DOI] [PubMed] [Google Scholar]
- Fox PT, Mintun MA. Noninvasive functional brain mapping by change-distribution analysis of averaged PET images of H2150 tissue activity. J Nucl Med. 1989;30:141–149. [PubMed] [Google Scholar]
- Glahn DC, Kim J, Cohen MS, Poutanen VP, Therman S, Bava S, Van Erp TGM, Manninen M, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Cannon TD. Maintenance and manipulation in spatial working memory: dissociations in the prefrontal cortex. Neuroimage. 2002;17:201–213. doi: 10.1006/nimg.2002.1161. [DOI] [PubMed] [Google Scholar]
- Gold DP, Andres D, Etezadi J, Arbuckle T, Schwartzman A, Chaikelson J. Structural equation model of intellectual change and continuity and predictors of intelligence in older men. Psychol Aging. 1995;10:294–303. doi: 10.1037//0882-7974.10.2.294. [DOI] [PubMed] [Google Scholar]
- Grady CL, Craik FI. Changes in memory processing with age1. Curr Opin Neurobiol. 2000;10:224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Grady CL, Horwitz B, Pietrini P, Mentis MJ, Ungerleider LG, Rapoport SI, Haxby JV. Effect of task difficulty on cerebral blood flow during perceptual matching for faces. Hum Brain Mapp. 1996;4:227–239. doi: 10.1002/(SICI)1097-0193(1996)4:4<227::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Rapoport SI. Age-related changes in the neural correlates of degraded and nondegraded face processing. Cogn Neuropsychol. 2000;17:165–186. doi: 10.1080/026432900380553. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Habeck C, Hilton HJ, Zarahn E, Flynn J, Moeller JR, Stern Y. Relation of cognitive reserve and task performance to expression of regional covariance networks in an event-related fMRI study of non-verbal memory. Neuroimage. 2003;20:1723–1733. doi: 10.1016/j.neuroimage.2003.07.032. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Small GW, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Coppola R, Kahn RS. Specific versus nonspecific brain activity in a parametric n-back task. Neuroimage. 2000;12:688–697. doi: 10.1006/nimg.2000.0645. [DOI] [PubMed] [Google Scholar]
- Jha AP, McCarthy G. The influence of memory load upon delay-interval activity in a working-memory task: an event-related functional MRI study. J Cogn Neurosci. 2000;12:90–105. doi: 10.1162/089892900564091. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and aging: four hypotheses in search of data. Annu Rev Psychol. 1991;42:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–40. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Gottlob LR, Denny LL, Turkington TG, Provenzale JM, Hawk TC, Coleman RE. Aging and recognition memory: changes in regional cerebral blood flow associated with components of reaction time distributions. J Cogn Neurosci. 1999a;11:511–20. doi: 10.1162/089892999563571. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, Coleman RE. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp. 1999b;7:115–35. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Touradji P, Tang M-X, Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol. 2003;5:680–690. doi: 10.1076/jcen.25.5.680.14579. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–57. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Sekuler AB, Penpeci C, Rajah MN, Grady CL, Sekuler R, Bennett PJ. Recruitment of unique neural systems to support visual memory in normal aging. Curr Biol. 1999;9:1275–8. doi: 10.1016/s0960-9822(99)80512-0. [DOI] [PubMed] [Google Scholar]
- Moeller JR, Strother SC, Sidtis JJ, Rottenberg DA. Scaled subprofile model: A statistical approach to the analysis of functional patterns in positron emission tomographic data. J Cereb Blood Flow Metab. 1987;7:649–658. doi: 10.1038/jcbfm.1987.118. [DOI] [PubMed] [Google Scholar]
- Moeller JR, Ishizaki T, Dhawan V, et al. The metabolic topography of normal aging. J Cerebral Blood Flow Metab. 1996;16:385–398. doi: 10.1097/00004647-199605000-00005. [DOI] [PubMed] [Google Scholar]
- Moeller JR, Ghez C, Antonini A, Ghilardi MF, Dahwan V, Kazumata K, Eidelberg D. Brain networks of motor behavior assessed by principal component analysis. In: Carson R, editor. Quantitative functional brain imaging with positron emission tomography. Academic Press; San Diego, CA: 1998. pp. 247–252. [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Nelson HE. The national adult reading test (NART): test manual. NFER-Nelson; Windsor: 1982. [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P. New visions of the aging mind and brain. Trends Cogn Sci. 2002;6:394. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–87. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Psychology. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Age-related changes in brain-behaviour relationships: evidence from event-related functional MRI studies. Eur J Cogn Psychol. 2001;13:235–256. [Google Scholar]
- Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, Hilton HJ, Flynn J, Van Heertum RL, Sackeim HA, Stern Y. Cognitive reserve modulates functional brain responses during memory tasks: a PET study in healthy young and elderly subjects. Neuroimage. 2003;19:1215–1227. doi: 10.1016/s1053-8119(03)00074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW. Midlife influences upon intellectual functioning in old age. Int J Behav Devel. 1984;7:463–478. [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. J Am Med Assoc. 1994;271:1004–1010. [PubMed] [Google Scholar]
- Stern Y, Moeller JR, Anderson KE, Luber B, Zubin N, Dimauro A, Park A, Campbell CE, Marder K, Van Heertum RL, Sackeim HA. Different brain networks mediate task performance in normal aging and AD: defining compensation. Neurology. 2000;55:1291–1297. doi: 10.1212/wnl.55.9.1291. [DOI] [PubMed] [Google Scholar]
- Stern Y, Zarahn E, Hilton HJ, Delapaz R, Flynn J, Rakitin B. Exploring the neural basis of cognitive reserve. J Clin Exp Neuropsychol. 2003;5:691–701. doi: 10.1076/jcen.25.5.691.14573. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale — revised. The Psychological Corporation; New York: 1981. [Google Scholar]
- Wilson RS, Bennett DA, Gilley DW, Beckett LA, Barnes LL, Evans DA. Premorbid reading activity and patterns of cognitive decline in Alzheimer disease. Archs Neurol. 2000;57:1718–1723. doi: 10.1001/archneur.57.12.1718. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. J Am Med Assoc. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]