Abstract
Introduction
Prior reports of dysferlinopathy suggest a clinically heterogeneous group of patients. We identified specific novel molecular and phenotypic features that help distinguish dysferlinopathies from other forms of limb-girdle muscular dystrophy (LGMD).
Methods
A detailed history, physical exam, protein and mutation analysis of genomic DNA was done in all subjects.
Results
Five of 21 confirmed DYSF gene mutations were not previously reported. A distinct “bulge” of the deltoid muscle in combination with other findings was a striking feature in all patients. Six subjects had atypical calf enlargement, and three of these exhibited a paradoxical pattern of dysferlin expression: severely reduced by direct immunfluorescence with overexpression by western blots. Six patients showed amyloid deposits in muscle that extended these findings to new domains of the dysferlin gene including the C2G domain. Correlative studies showed co-localization of amyloid with deposition of dysferlin.
Discussion
This data further serves to guide clinicians facing the expensive task of molecular characterization of patients with an LGMD phenotype.
Keywords: muscular dystrophy, LGMD2B, dysferlin, amyloid, calf myopathy
Introduction
Limb girdle muscular dystrophies (LGMD) include 7 autosomal dominant and 14 autosomal recessive disorders.1-3 Based on our own and studies of others,2 a specific diagnosis can be a laborious exercise and yields a confirmed molecular diagnosis in fewer than 60% of cases. Mutations in the gene that encodes dysferlin (DYSF) cause allelic autosomal recessive disorders that arise from mutations in the same genetic locus on chromosome 2p13.4,5 The classification of the dysferlinopathies suggests four distinct phenotypes that include LGMD2B with predominantly proximal weakness, Miyoshi myopathy (MM) with calf muscle weakness and atrophy, a distal anterior compartment myopathy (DACM) with tibialis muscle atrophy, and a less common subtype with rigid spine syndrome.6-10 Clinical experience, however, suggests that there is significant overlap in presentation of these presumably distinct entities.7,10,11 In addition, in some families, the same mutation can be associated with LGMD2B, MM, or DACM.12-15 As new treatment strategies evolve for muscular dystrophies, and enrollment in clinical trials is dependent on a specific diagnosis, the goal for most neuromuscular centers is to arrive at a specific diagnosis, proven by molecular analysis. In cases of suspected dysferlin deficiency, the large size of the dysferlin gene discourages molecular testing. In our dysferlin patient cohort of 21 subjects with confirmed DYSF gene mutations we have identified novel mutations and phenotypic features that provide insight for further testing.
Methods
Subjects
Twenty one patients were evaluated through an NIH supported LGMD characterization study (NIAMS U54 AR050733-05). Consistent information was obtained for each patient to establish ethnic and geographical origin, family history, consanguinity, age at onset, initial distribution of symptoms, pattern of muscle involvement, ambulatory status, disease progression, and serum creatine kinase (CK) levels.
Muscle Biopsy and Peripheral Blood Monocyte Analysis for Dysferlin
Skeletal muscle sections fixed in acetone (10-12 μm) were stained with NCL-Hamlet monoclonal antibody (Novocastra Laboratories Ltd.) to dysferlin diluted 1:10. Secondary antibody consisted of fluorecein-conjugated goat anti-mouse IgG diluted 1:200. Dysferlin was also analyzed by western blots using muscle homogenates prepared in SDS lysis buffer (125mM Tris-HCl pH 6.8, 4% SDS, 4M urea & protease inhibitor cocktail). For each subject, 15 μg of protein lysate were electrophoresed on 3-8% Tris-acetate NuPage gels (Invitrogen) and transferred to PVDF membrane (Amersham Biosciences). After blocking for 1hr in 5% nonfat dry milk in TBST (100 mM Tris-HCl, pH 8.0, 167 mM NaCl, 0.1% Tween), the western blot (WB) was incubated overnight with NCL-Hamlet (1:5000) dysferlin antibody followed by horseradish-peroxidase–labeled goat antimouse IgG (GE Healthcare). Immunoreactive bands were visualized with the use of the ECL Plus Western blotting detection system (GE Healthcare) and Hyperfilm ECL (Amersham Biosciences). Signal intensities were measured with ImageQuantTL software (GE Healthcare). For peripheral blood monocyte analysis, cells were isolated from whole blood using a Ficoll-Hypaque density gradient followed by protein extraction. Western blots for dysferlin were performed as described above (except 25 μg of protein lysate were electrophoresed). Similar methods have been described by Ho et al.16
Sequence Analysis of the Dysferlin (DYSF) Gene
Genomic DNA was isolated from each patient (PureGene, Qiagen) as previously described.17 Each of the 55 DYSF exons and at least 50 intronic bases were amplified using primers designed by Primer 3.0 (Whitehead Institute: http://www.wi.mit.edu/). The primers were tagged at the 5′ end with either the M13F (sense primers) or M13R (antisense primers) sequence. All exons were amplified with 35 cycles of 94° C for 30 seconds, 60° C for 30 sequences, and 72° C for 45 seconds. Bidirectional sequence analysis was performed using the M13F and M13R primers, as well as internal primers for larger exons, using the Applied Biosystems Big Dye Terminator 3.1 sequencing mix. All sequences were aligned to the reference sequence (NM_003494) using Sequencer (GeneCodes Corp) alignment software. For any nucleotide change suspected to be a pathogenic mutation, the exon was reamplified and resequenced in order to confirm that the mutation was not an amplification artifact.
Results
Genetic Analysis
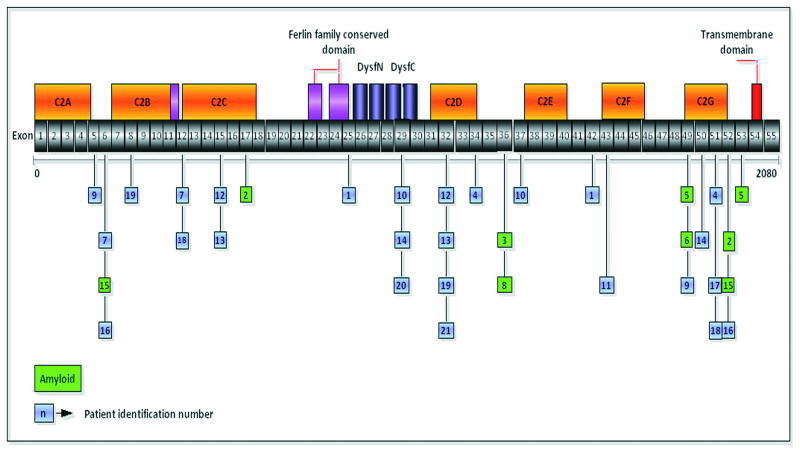
Twenty one patients were found to have DYSF gene mutations. One subject, the son of a consanguineous marriage, had an affected mother. Four subjects were Hispanic, one was Asian-Indian, one African-American, and the others were Caucasian non-Hispanic. We identified 27 different DYSF mutations (Fig. 1), five of which are novel (Table 1). No apparent mutational hot spots were observed. The mutations can be characterized as follows: eight missense, seven splice-site, five nonsense resulting in premature termination codons, and seven frameshift mutations. Twenty-three mutations affected C2 domains, and three affected the DysF domain (SMART nomenclature 18). In 15 of these cases, other proteins were affected (Table 2): calpain-3 (9 subjects) and caveolin-3 (1 subject) were reduced, and utrophin was upregulated (5 subjects). The secondary reductions of calpain-3 and caveolin-3 have been reported in patients with primary dysferlinopathy, but further information is needed to understand the functional implications and interactions between these proteins. 19-25 The cause for upregulation of utrophin is more elusive in dysferlin deficiency. There was no alteration of dystrophin to account for this finding in our cases.26-28
Figure 1.
Localization of the mutations in the dysferlin gene and the corresponding exons and protein domains. A green background color was used to distinguish the patients with amyloid deposits in their muscle biopsy. The number inside the box corresponds to the number of the patient in Table 2.
Table 1.
Dysferlin mutations in the 21 patients.
| Patient | Mutation |
|---|---|
| 1 | [c.2643+1G>A]+[c.4577A>C] |
| 2 | [c.1481-1G>A]+[c.5836_5839del] |
| 3 | [c.3892A>G] |
| 4 | [c.3843+2T>A]+[c.5698_5699delAG] |
| 5 | [c.5497G>T]+[c.5946+1G>A] |
| 6 | [c.5509G>A]+[c.5509G>A] |
| 7 | [c.610C>T]+[c.1120G>C] |
| 8 | [c.3892A>G] |
| 9 | [c.353delT]+[c.5444G>T] |
| 10 | [c.3065G>A; c.3992G>T] |
| 11 | [c.4685dupT]+[c.4685dupT] |
| 12* | [c.1392dupA]+[c.3516_3517delTT] |
| 13* | [c.1392dupA]+[c.3516_3517delTT] |
| 14 | [c.3041A>G]+[c.5526-1G>A] |
| 15* | [c.610C>T]+ [c.5884C>T] |
| 16* | [c.610C>T]+ [c.5884C>T] |
| 17 | [c.5668-7G>A] |
| 18 | [c.1120G>C]+[c.5713C>T] |
| 19 | [c.855+1delG]+[c.3505insC] |
| 20 | [c.3041A>G]+[c.3041A>G] |
| 21 | [c.3478C>T]+[c.3478C>T] |
12, 13 siblings; 15, 16 brothers.
In bold: novel mutations.
Table 2.
Histopathology features.
| Immunohistochemistry | Western Blot | |||||
|---|---|---|---|---|---|---|
| Patient | Dysferlin Membrane | Dysferlin Cytoplasm | Caveolin-3 | Utrophin | Dysferlin | Calpain-3 |
| 1 | Absent | Absent | Normal | Normal | ND | ND |
| 2Amyloid | Severely reduced | Absent | Normal | Normal | Absent | Reduced |
| 3Amyloid | Severely reduced | Present | Normal | Normal | Normal (2-5 fold increased) | Positive |
| 4 | Severely reduced | Absent | Normal | Normal | Absent | Reduced |
| 5Amyloid | Severely reduced | Absent | Normal | Upregulated | Absent | Positive |
| 6Amyloid | Absent | Absent | Reduced | Normal | Absent | Positive |
| 7 | Severely reduced | Absent | Normal | Normal | ND | ND |
| 8Amyloid | Absent | Absent | Normal | Normal | Normal (2-5 fold increased | Positive |
| 9 | Absent | Absent | Normal | Normal | Reduced | Positive |
| 10 | Severely reduced | Absent | Normal | Normal | Normal (2-5 fold increased | Reduced |
| 11 | Absent | Absent | Normal | Normal | Absent | Reduced |
| 12 | Absent | Absent | Normal | Normal | Absent | Positive |
| 13 | Absent | Absent | Normal | Normal | Absent | Positive |
| 14 | Absent | Absent | Normal | Normal | Absent | Reduced |
| 15Amyloid | Severely reduced | Absent | Normal | Upregulated | ND | ND |
| 16 | ND | ND | ND | ND | ND | ND |
| 17 | Severely reduced | Absent | Normal | Upregulated | Absent | Reduced |
| 18 | Severely reduced | Absent | Normal | Upregulated | Absent | Reduced |
| 19 | ND | ND | ND | ND | ND | ND |
| 20 | Absent | Absent | Normal | Normal | Absent | Reduced |
| 21 | Severely reduced | Absent | Normal | Upregulated | Absent | Reduced |
 Amyloid in muscle biopsy; ND= not done.
Amyloid in muscle biopsy; ND= not done.
Clinical Features
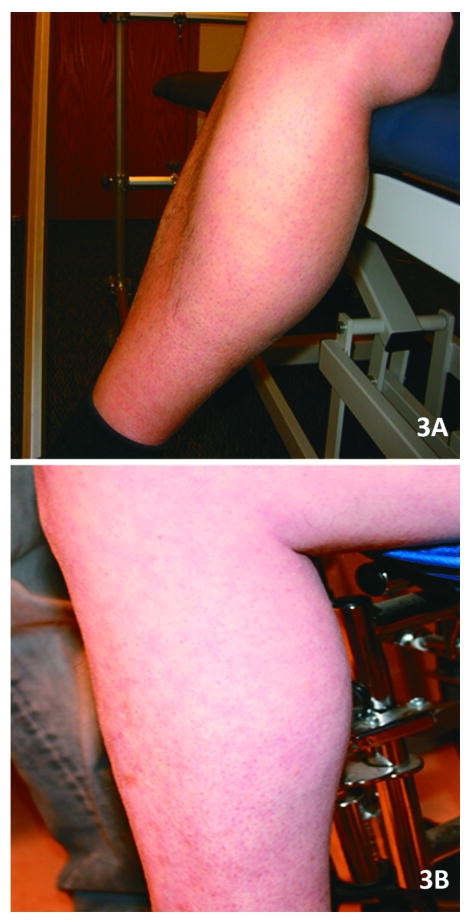
In this cohort of 21 subjects (Table 3), the mean age of onset was 19 years (range from 3 - 34 yrs). The experience of patients in this cohort was similar to that recently described by Klinge et al.29 Twenty-four percent of patients presented symptoms before age 15, and the majority described participating in physical activities without limitations prior to the onset of symptoms. One patient was a very successful wrestler up to the age of 21, when his muscle condition became apparent after a sports related injury. Another patient was also a renowned boxer until his late twenties when he became aware of his muscle disease after a direct knee injury in a car accident. Anecdotal reports of traumatic events coincident with the onset illness in individuals with pre-symptomatic fitness have been described in other patients with dysferlinopathies.29-31 Eight subjects became wheelchair-dependent at a mean age of 38 years old (range 24-54). Serum CK levels were strikingly divergent (6248 U/L ± 5091, normal 37-430 U/L), ranging from normal (280 U/L) to markedly elevated (17972 U/L). All 21 patients, ages 24 to 60 years (mean age 38 ± 9.7), displayed a pattern of both proximal and distal muscle upper and lower extremity weakness at the time of examination by our group (Table 3 shows distribution of weakness at onset). A consistent finding exhibited in all patients was preservation of the deltoid muscle. This resulted in a distinct “bulge” in contrast to the surrounding muscle groups of the shoulder girdle, scapular stabilizers, and upper arm muscles (trapezius, levator scapulae, teres major and minor, pectoralis major, biceps) (Fig. 2). The biceps atrophy and preserved deltoids present a striking contrast not previously commented on in previous reports of LGMD2B patients. The sarcoglycanopathies also have relative preservation of deltoids and markedly atrophied biceps but are distinguished from the dysferlin deficient patients when there is associated calf muscle atrophy. Rare FSHD patients with facial sparing may also have overlapping features, but they can usually be distinguished by asymmetric weakness, axillary folds, scapular winging with riding up of the scapula from the frontal view, and pectoralis major muscle atrophy.32,33 Phenotypic uncertainty arises, however, when DYSF gene mutations are associated with calf enlargement, as we have seen in six of the twenty one subjects including patients #3, #4, #8, #10, #18, and #20 (Table 3; Fig. 3). Patients who had calf muscle hypertrophy were previously misdiagnosed with Becker muscular dystrophy. A slowly progressive course of muscle weakness was common to all subjects.
Table 3.
Phenotypic features.
| Clinical Data | |||||
|---|---|---|---|---|---|
| Patient | Sex/Age | Age of Onset | Onset mode | Age = loss of ambulation | CK |
| 1 | M/48 | 30 | DUE+PLE | Ambulatory | 3481 |
| 2 | M/40 | 17 | DLE | 28 | 7938 |
| 3† | M/46 | 27 | PLE | 46 | 9468 |
| 4† | F/47 | 34 | PLE | Ambulatory | 4393 |
| 5 | M/35 | 18 | DLE | Ambulatory | ND |
| 6 | M/38 | 17 | DLE | Ambulatory | 17972 |
| 7 | M/34 | 22 | PLE | Ambulatory | 3156 |
| 8† | M/32 | 3 | PLE | Ambulatory | 280 |
| 9 | M/31 | 19 | PUE | Ambulatory | 14650 |
| 10† | M/46 | 6 | PLE | 43 | 428 |
| 11 | F/25 | 22 | DLE | Ambulatory | 2661 |
| 12* | M/41 | 21 | PLE | 35 | 695 |
| 13* | F/42 | 16 | DLE | 31 | 320 |
| 14 | M/26 | 21 | DLE | Ambulatory | 11040 |
| 15* | M/60 | 15 | DLE | 54 | 1758 |
| 16* | M/52 | 14 | DLE | 45 | ND |
| 17 | M/24 | 20 | DLE | Ambulatory | 8877 |
| 18† | F/31 | 19 | PLE | Ambulatory | 3046 |
| 19 | M/33 | 20 | DLE | Ambulatory | 8477 |
| 20† | M/42 | 24 | PLE | Ambulatory | 4000 |
| 21 | M/25 | 12 | PLE | 24 | 10147 |
12, 13 siblings; 15, 16 brothers.
Patients with calf hypertrophy; CK, creatine kinase; DLE, distal lower extremity; DUE, distal upper extremity; PLE, proximal lower extremity; PUE, proximal upper extremity; ND, not done.
Figure 2.
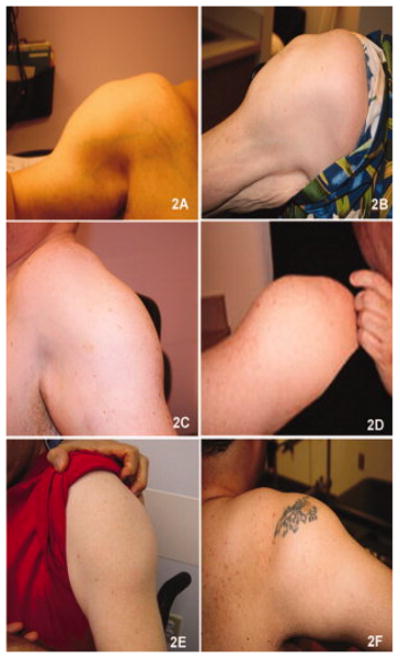
(A-F) Shows deltoid hypertrophy in 6 cases of dysferlin deficiency. The surrounding muscles are atrophic. This is well seen in (A) and (B), where the atrophy of the biceps is clearly demonstrated.
Figure 3.
(A,B) Calf muscle hypertrophy in patients with dysferlin gene mutations. These patients had been previously mislabeled with a diagnosis of Becker muscular dystrophy.
Histology and Immunohistochemical analysis
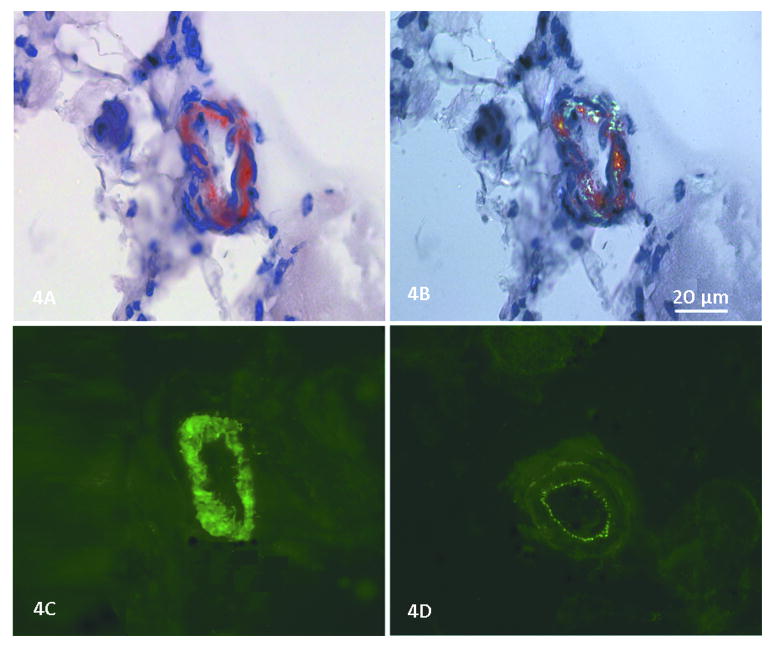
The muscle biopsies showed non-specific dystrophic features to varying degrees with fiber size variability, muscle fiber necrosis, and endomysial connective tissue proliferation. Inflammatory infiltrates, mainly perivascular, were observed in several cases as previously described.34-36 The most distinctive histological finding was the presence of amyloid deposits identified by Congo red (Fig. 4) in blood vessel walls and in the perimysial connective tissue in the muscle biopsies of six dysferlin deficient subjects (Table 2, Figure 1). This unique finding has previously been described in LGMD2B37,38 and is distinct to this form of dystrophy. It should not be confused with the intracellular deposits observed within the muscle fibers of sporadic inclusion body myositis subjects.39 To ensure amyloid specificity for dysferlinopathies we performed Congo red stains on muscle biopsies from the following cases with proven LGMD mutations (part of our LGMD characterization study): LGMD2A (calpain-3 deficiency, n = 5), LGMD2C (gamma-SG deficiency, n = 3), LGMD2D (alpha-SG deficiency, n = 6), LGMD2E (beta-SG deficiency, n = 5), LGMD2I (FKRP deficiency, n = 5) and LGMD1B (lamin A/B deficiency, n = 5). None were found to have amyloid deposits in the muscle. In the cohort we report here, amyloidogenic mutations included exons not previously described that extend over a gene distribution from exons 5-53 (patient 2 = exons 17 and 52; patient 3 = exon 36; patient 5 = exons 49 and 53; patient 6 = exons 49; patient 8 = exon 36; patient 15 = exons 5 and 52), (Figure 1). In prior reports the amyloidogenic dysferlin mutations preferentially clustered toward the N- terminal region between exons 7-16, corresponding to the C2B and C2C domains of DysF.37,38 In addition, we were able to unequivocally demonstrate dysferlin co-localized to the amyloid deposits in arterioles in the muscle biopsy specimen of patient 3 (exon 36 mutation), (Fig. 4). This removes any doubt that amyloid is derived from the mutant dysferlin. It most likely represents a fragment of the full length protein that has been degraded and then folded to form a β-pleated sheet with typical green birefringence under polarized light. Similar findings were demonstrated by Spuler et al.,37,38 in their cases.
Figure 4.
(A) Congo red stain shows amyloid deposited in the smooth muscle layer of a skeletal muscle arteriole in patient 3. (B) Using polarized light, the green birefringence of amyloid is seen. (C) In a consecutive serial section, dysferlin deposition is seen in this same blood vessel (NCL-Hamlet Novocastra Laboratories Ltd.). (D) In the same section a blood vessel wall lacks dysferlin deposition; only the autofluorescence of the internal elastic membrane (tunica intima) and adventitia (tunica externa) can be seen, but the smooth muscle of the tunica media is devoid of dysferlin.
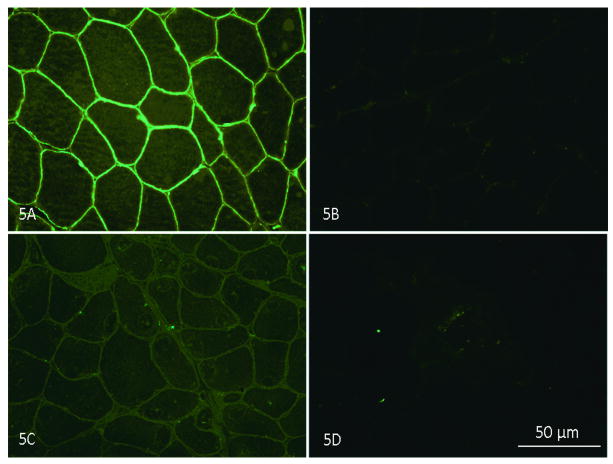
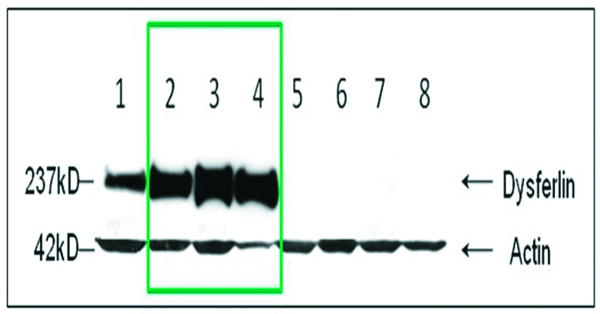
Another previously unreported, unique finding in our cohort was the paradoxical reduction or absence of dysferlin expression by immunofluorescence (IF) on muscle sections (Fig. 5), compared with overexpression (increased by 2-5 fold over normal) of this same protein by WB in three of the 21 cases (Table 2 patients 3, 8, 10), (Fig. 6). These three patients have DYSF gene mutations that have been reported in previous publications, 7, 15, 40,41 and listed in the Leiden database. Patients #3 and #8 have heterozygous mutations at one allele at exon 36 (c.3892A>G). A recently published algorithm that derives a pathogenicity score for missense mutations suggests that this amino acid substitution, isoleucine to valine, is in a non-pathologic range.42 Despite the fact that the mutation has been called into question, these patients fulfill criteria for dysferlinopathy based upon: 1) severely reduced (#3) and absent (#8) dysferlin membrane staining; 2) the presence of amyloid in their muscle that is highly specific for LGMD. The third (patient 10) has two dysferlin mutations, one of unequivocal pathogenicity [exon 37, c.3992G>T, sequence change p.(R133L)] clearly defining this patient with a dysferlinopathy. The second mutation is controversial (exon 29, c.3065G>A) with disagreement as to whether this nucleotide change is a polymorphism, overrepresented in normal population43,44 or should carry a designation of “probably pathogenic”.41 Of particular interest, patients 3 and 10 returned for analysis of peripheral blood monocyte dysferlin performed by WB. In both subjects, dysferlin was present in the monocyte WB (patient 3 done by a commercial laboratory; patients 3 and 10 done by our laboratory at Nationwide Children's Hospital). Control cases, patients 1, 5, 15, and 21 showed absent monocyte dysferlin and these same cases also demonstrated reduced or absent dysferlin by IF and WBs of skeletal muscle. It is important to emphasize that dysferlin may be abnormal in the cytoplasm as a non-specific finding, but the results of membrane staining were the most predictive of gene mutation in our series.
Figure 5.
(A) Dysferlin staining in the sarcolemma of normal muscle; (B) negative control (normal muscle without dysferlin antibody stained only with goat anti-mouse IgG); (C) reduced dysferlin membrane staining is seen in patient 2 (Table 2); (D) absent dysferlin membrane staining in patient 8 (Table 2). NCL-Hamlet antibody Novocastra Laboratories Ltd in A,C, D.
Figure 6.
Western blot in LGMD2B patients. Total protein extracted from the patient muscle samples was probed with dysferlin antibody (upper panel). Lane 1: a normal control; lanes 2-4: LGMD2B patients showing overexpression of dysferlin in patients 3, 8, 10, Table 2); band signal intensities were quantified by ImageQuant T2 software (GE Healthcare) and were to 2 to 5 fold increased over normal controls; lanes 5-8: LGMD2B patients showing no dysferlin expression. Protein loading is assessed by probing with muscle specific actin antibody (lower panel).
Discussion
Defining a specific LGMD diagnosis for patients is a challenging objective for neuromuscular clinicians. The data presented in this study further arm clinicians with additional information to help attain this goal. Potential experimental treatment strategies rely on a specific molecular diagnosis45-48 prior to entry into a clinical trial. Presently, only about 60% 2 of LGMD patients can be further classified into one of the 14 recessive or 7 dominant forms of the overall group of LGMDs. The dysferlinopathies represent a particular diagnostic challenge because of the size of the gene and the expense incurred in gene sequencing, since hot spots for mutations have not been identified. The observations provided in this cohort analysis will strengthen the clinical suspicion and provide a rationale for dysferlin gene sequencing. For example, the bulging deltoid with loss of muscle bulk in surrounding muscles combined with either typical lower leg posterior compartment (calf muscle) atrophy 49-51 or calf muscle hypertrophy should be considered to be a signature of the disease. Under such conditions the muscle biopsy evaluation must include dysferlin staining. Further novel features detected in this cohort can be summarized as follows: 1) First, the presence of amyloid deposits in the muscle is unique to LGMD2B, different from all other LGMDs. Although, this has previously been reported, 37,38 the findings in the patient group in this analysis confirms and extends the observation by recognizing that amyloidogenic domains extend over a wide distribution from N- to C-terminal. In this report, we added important confirmatory co-localization observations linking amyloid to dysferlin. Presumably these are misfolded dysferlin proteins that emphasize the importance of including Congo red staining as part of the muscle biopsy analysis in the LGMD work up. 2) Secondly, the discrepancy on muscle biopsy between the absence of dysferlin by direct IF stains on muscle sections and the finding of overexpressed amounts by WB potentially leads to misdiagnosis. The exact mechanism for this profile has not been established but may represent a mutant protein that fails to insert into the muscle membrane. This finding was observed in a patient 10 (Table 1) with an unequivocal DYSF mutation with a pathologic amino acid substitution [p.(R133L) reported in the Leiden database. The other two cases (patients 3 and 8) have the c.3892A>G mutation that is less certain to be disease causing. It should be considered, however, that in the Italian family where this mutation was first considered to be disease-causing, 7, 15 three siblings with the same nucleotide change each demonstrated the classic phenotypes of dysferlinopathy: Myoshi myopathy, LGMD, and DACM. This still does not preclude the obvious possibility that this is a familial non-pathogenic mutation. 3) In addition to the discrepancy between dysferlin IF (severely reduced) and WB (overexpressed) on muscle tissue, our study demonstrates a further difference with peripheral blood monocytes where dysferlin appeared normal (patient 10 with established pathologic mutation). Thus, if the index of suspicion is high, a muscle biopsy or DNA test for dysferlin is still warranted despite positive dysferlin in monocytes. 4) The final contribution of this report is the addition of five novel dysferlin mutations that will ease the potential burden of molecular diagnosis. An integrated summary of the clinical, biopsy and gene mutation findings can be found in the tables 1-3.
Acknowledgments
This work was supported by NIH NIAMS U54 AR050733-05, Jesse's Journey, and the Muscular Dystrophy Association
Abbreviations
- BMD
Becker muscular dystrophy
- CK
creatine kinase
- C2 domain
calcium sensitive domain of the dysferlin gene
- C2B
the second calcium sensitive domain of the dysferlin gene
- C2C
the third calcium sensitive domain of the dysferlin gene
- C2G
the seventh calcium sensitive domain of the dysferlin gene
- DACM
distal anterior compartment myopathy
- DMD
Duchene muscular dystrophy
- DNA
Deoxyribonucleic acid
- DYSF
dysferlin gene
- ECL
enhanced chemiluminescence
- FKRP
fukutin related protein
- FSHD
Facioscapulohumeral Muscular Dystrophy
- IF
Immunofluorescence
- IHC
immunohistochemistry
- LGMD
limb girdle muscular dystrophy
- LGMD1B
limb girdle muscular dystrophy type 1B
- LGMD2A
limb girdle muscular dystrophy type 2A
- LGMD2B
limb girdle muscular dystrophy type 2B
- LGMD2C
limb girdle muscular dystrophy type 2C
- LGMD2D
limb girdle muscular dystrophy type 2D
- LGMD2E
limb girdle muscular dystrophy type 2E
- LGMD2I
limb girdle muscular dystrophy type 2I
- MM
miyoshi myopathy
- M13F
sense primers
- M13R
antisense primers
- NIAMS
National Institute of Arthritis and Musculoskeletal and Skin Diseases
- NIH
National Institute of Health
- PBMC
Peripheral blood mononuclear cell
- PBS
phosphate-buffered saline
- PVDF
Polyvinylidene fluoride
- SDS
sodium dodecyl sulfate
- SG
sarcoglycan
- TBST
Tris buffered saline Tween
- WB
western blot
Footnotes
Material included in this manuscript was presented in the annual meeting at the American Academy of Neurology in Seattle, WA., on April 29, 2009.
References
- 1.Guglieri M, Straub V, Bushby K, Lochmuller H. Limb-girdle muscular dystrophies. Curr Opin Neurol. 2008;21:576–584. doi: 10.1097/WCO.0b013e32830efdc2. [DOI] [PubMed] [Google Scholar]
- 2.Guglieri M, Magri F, D'Angelo MG, Prelle A, Morandi L, Rodolico C, et al. Clinical, molecular, and protein correlations in a large sample of genetically diagnosed Italian limb girdle muscular dystrophy patients. Hum Mut. 2008;29:258–266. doi: 10.1002/humu.20642. [DOI] [PubMed] [Google Scholar]
- 3.Jarry J, Rioux MF, Bolduc V, Robitaille Y, Khoury V, Thiffault I, et al. A novel autosomal recessive limb-girdle muscular dystrophy with quadriceps atrophy maps to 11p13-p12. Brain. 2007;130:368–380. doi: 10.1093/brain/awl270. [DOI] [PubMed] [Google Scholar]
- 4.Bashir R, Keers S, Strachan T, Passos-Bueno R, Zatz M, Weissenbach J, et al. Genetic and physical mapping at the limb-girdle muscular dystrophy locus (LGMD2B) on chromosome 2p. Genomics. 1996;33:46–52. doi: 10.1006/geno.1996.0157. [DOI] [PubMed] [Google Scholar]
- 5.Bejaoui K, Hirabayashi K, Hentati F, Haines JL, Ben Hamida C, Belal S, et al. Linkage of Miyoshi myopathy (distal autosomal recessive muscular dystrophy) locus to chromosome 2p12-14. Neurology. 1995;45:768–772. doi: 10.1212/wnl.45.4.768. [DOI] [PubMed] [Google Scholar]
- 6.Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998;20:37–42. doi: 10.1038/1689. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20:31–36. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- 8.Illa I, Serrano-Munuera C, Gallardo E, Lasa A, Rojas-Garcia R, Palmer J, et al. Distal anterior compartment myopathy: a dysferlin mutation causing a new muscular dystrophy phenotype. Ann Neurol. 2001;49:130–134. [PubMed] [Google Scholar]
- 9.Seror P, Krahn M, Laforet P, Leturcq F, Maisonobe T. Complete fatty degeneration of lumbar erector spinae muscles caused by a primary dysferlinopathy. Muscle Nerve. 2008;37:410–414. doi: 10.1002/mus.20910. [DOI] [PubMed] [Google Scholar]
- 10.Nagashima T, Chuma T, Mano Y, Goto Y, Hayashi YK, Minami N, et al. Dysferlinopathy associated with rigid spine syndrome. Neuropathology. 2004;24:341–346. doi: 10.1111/j.1440-1789.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- 11.Ueyama H, Kumamoto T, Horinouchi H, Fujimoto S, Aono H, Tsuda T. Clinical heterogeneity in dysferlinopathy. Int Med. 2002;41:532–536. doi: 10.2169/internalmedicine.41.532. [DOI] [PubMed] [Google Scholar]
- 12.Weiler T, Bashir R, Anderson LV, Davison K, Moss JA, Britton S, et al. Identical mutation in patients with limb girdle muscular dystrophy type 2B or Miyoshi myopathy suggests a role for modifier gene(s) Hum Mol Genet. 1999;8:871–877. doi: 10.1093/hmg/8.5.871. [DOI] [PubMed] [Google Scholar]
- 13.Weiler T, Greenberg CR, Nylen E, Halliday W, Morgan K, Eggertson D, et al. Limb-girdle muscular dystrophy and Miyoshi myopathy in an aboriginal Canadian kindred map to LGMD2B and segregate with the same haplotype. Am J Hum Genet. 1996;59:872–878. [PMC free article] [PubMed] [Google Scholar]
- 14.Illarioshkin SN, Ivanova-Smolenskaya IA, Tanaka H, Poleshchuk VV, Markova ED, Tsuji S. Refined genetic location of the chromosome 2p-linked progressive muscular dystrophy gene. Genomics. 1997;42:345–348. doi: 10.1006/geno.1997.4725. [DOI] [PubMed] [Google Scholar]
- 15.Aoki M, Liu J, Richard I, Bashir R, Britton S, Keers SM, et al. Genomic organization of the dysferlin gene and novel mutations in Miyoshi myopathy. Neurology. 2001;57:271–278. doi: 10.1212/wnl.57.2.271. [DOI] [PubMed] [Google Scholar]
- 16.Ho M, Gallardo E, McKenna-Yasek D, De Luna N, Illa I, Brown RH., Jr A novel, blood-based diagnostic assay for limb girdle muscular dystrophy 2B and Miyoshi myopathy. Ann Neurol. 2002;51:129–133. doi: 10.1002/ana.10080. [DOI] [PubMed] [Google Scholar]
- 17.D'Souza G, Sugar E, Ruby W, Gravitt P, Gillison M. Analysis of the effect of DNA purification on detection of human papillomavirus in oral rinse samples by PCR. J Clin Microbiol. 2005;43:5526–5535. doi: 10.1128/JCM.43.11.5526-5535.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel P, Harris R, Geddes SM, et al. Solution structure of the inner DysF domain of myoferlin and implications for limb girdle muscular dystrophy type 2B. J Mol Biol. 2008;379:981–990. doi: 10.1016/j.jmb.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 19.Glover L, Brown RH., Jr Dysferlin in membrane trafficking and patch repair. Traffic. 2007;8:785–794. doi: 10.1111/j.1600-0854.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Verheesen P, Roussis A, Frankhuizen W, Ginjaar I, Haldane F, et al. Protein studies in dysferlinopathy patients using llama-derived antibody fragments selected by phage display. Eur J Hum Genet. 2005;13:721–730. doi: 10.1038/sj.ejhg.5201414. [DOI] [PubMed] [Google Scholar]
- 21.Anderson LV, Harrison RM, Pogue R, Vafiadaki E, Pollitt C, Davison K, et al. Secondary reduction in calpain 3 expression in patients with limb girdle muscular dystrophy type 2B and Miyoshi myopathy (primary dysferlinopathies) Neuromuscul Disord. 2000;10:553–559. doi: 10.1016/s0960-8966(00)00143-7. [DOI] [PubMed] [Google Scholar]
- 22.Walter MC, Braun C, Vorgerd M, Poppe M, Thirion C, Schmidt C, et al. Variable reduction of caveolin-3 in patients with LGMD2B/MM. J Neurol. 2003;250:1431–1438. doi: 10.1007/s00415-003-0234-x. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda C, Hayashi YK, Ogawa M, Aoki M, Murayama K, Nishino I, et al. The sarcolemmal proteins dysferlin and caveolin-3 interact in skeletal muscle. Hum Mol Genet. 2001;10:1761–1766. doi: 10.1093/hmg/10.17.1761. [DOI] [PubMed] [Google Scholar]
- 24.Chrobakova T, Hermanova M, Kroupova I, Vondracek P, Marikova T, Mazanec R, et al. Mutations in Czech LGMD2A patients revealed by analysis of calpain3 mRNA and their phenotypic outcome. Neuromuscul Disord. 2004;14:659–665. doi: 10.1016/j.nmd.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Deviez DJ, Martin S, Laval SH, Lo HP, Cooper ST, North KN, et al. Aberrant dysferlin trafficking in cells lacking caveolin or expressing dystrophy mutants of caveolin-3. Hum Mol Genet. 2006;15:129–142. doi: 10.1093/hmg/ddi434. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno Y, Nonaka I, Hirai S, Ozawa E. Reciprocal expression of dystrophin and utrophin in muscles of Duchenne muscular dystrophy patients, female DMD-carriers and control subjects. J Neurol Sci. 1993;119:43–52. doi: 10.1016/0022-510x(93)90190-a. [DOI] [PubMed] [Google Scholar]
- 27.Kawajiri M, Mitsui T, Kawai H, Kobunai T, Tsuchihashi T, Saito S. Dystrophin, utrophin and beta-dystroglycan expression in skeletal muscle from patients with Becker muscular dystrophy. J Neuropathy Exp Neurol. 1996;55:896–903. doi: 10.1097/00005072-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Kleopa KA, Drousiotou A, Mavrikiou E, Ormiston A, Kyriakides T. Naturally occurring utrophin correlates with disease severity in Duchenne muscular dystrophy. Hum Mol Genet. 2006;15:1623–1628. doi: 10.1093/hmg/ddl083. [DOI] [PubMed] [Google Scholar]
- 29.Klinge L, Aboumousa A, Eagle M, Hudson J, Sarkozy A, Vita G, et al. New aspects on patients affected by dysferlin deficient muscular dystrophy. J Neurol Neurosurg Psychiatry. 2009 June 14; doi: 10.1136/jnnp.2009.178038. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagaraju K, Rawat R, Veszelovszky E, Thapliyal R, Kesari A, Sparks S, et al. Dysferlin deficiency enhances monocyte phagocytosis: a model for the inflammatory onset of limb-girdle muscular dystrophy 2B. Am J Pathol. 2008;172:774–785. doi: 10.2353/ajpath.2008.070327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahjneh I, Marconi G, Bushby K, Anderson LV, Tolvanen-Mahjneh H, Somer H. Dysferlinopathy (LGMD2B): a 23-year follow-up study of 10 patients homozygous for the same frameshifting dysferlin mutations. Neuromuscul Disord. 2001;11:20–26. doi: 10.1016/s0960-8966(00)00157-7. [DOI] [PubMed] [Google Scholar]
- 32.Felice KJ, North WA, Moore SA, Mathews KD. FSH dystrophy 4q35 deletion in patients presenting with facial-sparing scapular myopathy. Neurology. 2000;54:1927–1931. doi: 10.1212/wnl.54.10.1927. [DOI] [PubMed] [Google Scholar]
- 33.Felice KJ, Moore SA. Unusual clinical presentations in patients harboring the facioscapulohumeral dystrophy 4q35 deletion. Muscle Nerve. 2001;24:352–356. doi: 10.1002/1097-4598(200103)24:3<352::aid-mus1005>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 34.Gallardo E, Rojas-Garcia R, de Luna N, Pou A, Brown RH, Jr, Illa I. Inflammation in dysferlin myopathy: immunohistochemical characterization of 13 patients. Neurology. 2001;57:2136–2138. doi: 10.1212/wnl.57.11.2136. [DOI] [PubMed] [Google Scholar]
- 35.Anderson LV, Davison K, Moss JA, Young C, Cullen MJ, Walsh J, et al. Dysferlin is a plasma membrane protein and is expressed early in human development. Hum Mol Genet. 1999;8:855–861. doi: 10.1093/hmg/8.5.855. [DOI] [PubMed] [Google Scholar]
- 36.McNally EM, Ly CT, Rosenmann H, Mitrani Rosenbaum S, Jiang W, Anderson LV, et al. Splicing mutation in dysferlin produces limb-girdle muscular dystrophy with inflammation. Am J Med Genet. 2000;91:305–312. doi: 10.1002/(sici)1096-8628(20000410)91:4<305::aid-ajmg12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 37.Carl M, Rocken C, Spuler S. Amyloidosis in muscular dystrophy. Der Pathologe. 2009;30:235–239. doi: 10.1007/s00292-009-1129-0. [DOI] [PubMed] [Google Scholar]
- 38.Spuler S, Carl M, Zabojszcza J, Straub V, Bushby K, Moore SA, et al. Dysferlin-deficient muscular dystrophy features amyloidosis. Ann Neurol. 2008;63:323–328. doi: 10.1002/ana.21309. [DOI] [PubMed] [Google Scholar]
- 39.Mendell JR, Sahenk Z, Gales T, Paul L. Amyloid filaments in inclusion body myosits. Novel findings provide insight into nature of filaments. Arch Neurol. 1991;48:1229–1234. doi: 10.1001/archneur.1991.00530240033013. [DOI] [PubMed] [Google Scholar]
- 40.Fanin M, Nascimbeni AC, Angelini C. Muscle protein analysis in the detection of heterozygotes for recessive limb girdle muscular dystrophy type 2B and 2E. Neuromuscul Disord. 2006;16:792–799. doi: 10.1016/j.nmd.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Krahn M, Beroud C, Labelle V, Nguyen K, Bernard R, Bassez G, et al. Analysis of the DYSF mutational spectrum in a large cohort of patients. Hum Mutat. 2009;30:E345–375. doi: 10.1002/humu.20910. [DOI] [PubMed] [Google Scholar]
- 42.Frédéric MY, Lalande M, Boileau C, et al. UMD-predictor, a new prediction tool for nucleotide substitution pathogenicity -- application to four genes: FBN1, FBN2, TGFBR1, and TGFBR2. Hum Mutat. 2009;30:952–959. doi: 10.1002/humu.20970. [DOI] [PubMed] [Google Scholar]
- 43.Cagliani R, Fortunato F, Giorda R, et al. Molecular analysis of LGMD-2B and MM patients: identification of novel DYSF mutations and possible founder effect in the Italian population. Neuromuscul Disord. 2003;13:788–795. doi: 10.1016/s0960-8966(03)00133-0. [DOI] [PubMed] [Google Scholar]
- 44.Kawabe K, Goto K, Nishino I, Angelini C, Hayashi YK. Dysferlin mutation analysis in a group of Italian patients with limb-girdle muscular dystrophy and Miyoshi myopathy. Eur J Neurol. 2004;11:657–661. doi: 10.1111/j.1468-1331.2004.00755.x. [DOI] [PubMed] [Google Scholar]
- 45.Hattori H, Nagata E, Oya Y, Takahashi T, Aoki M, Ito D, et al. A novel compound heterozygous dysferlin mutation in Miyoshi myopathy siblings responding to dantrolene. Eur J Neurol. 2007;14:1288–1291. doi: 10.1111/j.1468-1331.2007.01958.x. [DOI] [PubMed] [Google Scholar]
- 46.Leriche-Guerin K, Anderson LV, Wrogemann K, Roy B, Goulet M, Tremblay JP. Dysferlin expression after normal myoblast transplantation in SCID and in SJL mice. Neuromuscul Disord. 2002;12:167–173. doi: 10.1016/s0960-8966(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 47.Kong KY, Ren J, Kraus M, Finklestein SP, Brown RH., Jr Human umbilical cord blood cells differentiate into muscle in sjl muscular dystrophy mice. Stem cells. 2004;22:981–993. doi: 10.1634/stemcells.22-6-981. [DOI] [PubMed] [Google Scholar]
- 48.Vieira NM, Bueno CR, Jr, Brandalise V, Moraes LV, Zucconi E, Secco M, et al. SJL dystrophic mice express a significant amount of human muscle proteins following systemic delivery of human adipose-derived stromal cells without immunosuppression. Stem cells. 2008;26:2391–2398. doi: 10.1634/stemcells.2008-0043. [DOI] [PubMed] [Google Scholar]
- 49.Ueyama H, Kumamoto T, Nagao S, Masuda T, Horinouchi H, Fujimoto S, et al. A new dysferlin gene mutation in two Japanese families with limb-girdle muscular dystrophy 2B and Miyoshi myopathy. Neuromuscul Disord. 2001;11:139–145. doi: 10.1016/s0960-8966(00)00168-1. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki N, Aoki M, Takahashi T, Takano D, Asano M, Shiga Y, et al. Novel dysferlin mutations and characteristic muscle atrophy in late-onset Miyoshi myopathy. Muscle Nerve. 2004;29:721–723. doi: 10.1002/mus.20025. [DOI] [PubMed] [Google Scholar]
- 51.Katz JS, Rando TA, Barohn RJ, Saperstein DS, Jackson CE, Wicklund M, et al. Late-onset distal muscular dystrophy affecting the posterior calves. Muscle Nerve. 2003;28:443–448. doi: 10.1002/mus.10458. [DOI] [PubMed] [Google Scholar]