Summary
Cell polarity involves transport of specific membranes and macromolecules at the right time to the right place. In budding yeast, secretory vesicles are transported by the myosin-V Myo2p to sites of cell growth. We show that phosphatidylinositol 4-phosphate (PI4P) is present in late secretory compartments and is critical for their association with, and transport by, Myo2p. Further, the Trans-Golgi network (TGN) Rab Ypt31/32p and secretory vesicle Rab Sec4p each bind directly, but distinctly, to Myo2p, and these interactions are also required for secretory compartment transport. Enhancing Myo2p's interaction with PI4P bypasses the requirement for interaction with Ypt31/32p and Sec4p. Together with additional genetic data, the results indicate that Rab proteins and PI4P collaborate in the association of secretory compartments with Myo2p. Thus, we show that a coincidence detection mechanism coordinates inputs from PI4P and the appropriate Rab for secretory compartment transport.
Introduction
Cell polarity is achieved largely through the selective transport of cargoes by molecular motors moving along microtubules and/or microfilaments (Goode et al., 2000). By selectively localizing macromolecules and organelles to specific areas of the cell, processes such as migration, secretion, growth, and division can occur, which ultimately, are essential for the development of the organism. To achieve such transport, mechanisms must exist for motors to recognize specific organelles for their transport to the correct place at the appropriate time. A potential candidate to provide organelle selective transport includes Rab GTPases that associate with specific membrane compartments (Grosshans et al., 2006; Zerial and McBride, 2001). Indeed, Rab27a is linked through melanophilin to a specific splice form of myosin-Va for melanosome capture at the cell cortex (Wu et al., 2002). Similarly, Rab11 associates with myosin-Vb to facilitate exit from the recycling endosome (Hales et al., 2002; Lapierre et al., 2001). Another family of candidate molecules is the phosphoinositides that are enriched in specific membrane-bound compartments. For example, PI3P is enriched in endosomes where it binds effectors and, together with Rab5, plays a critical role in endocytic trafficking (Wurmser and Emr, 1998; Zoncu et al., 2009); PI4,5P2 is enriched at the plasma membrane and regulates a myriad of processes, from endocytosis to cytoskeletal organization (Audhya et al., 2004; Zoncu et al., 2007); and PI4P is enriched in the Golgi compartment where it is critical for exit of cargo from that organelle (D'Angelo et al., 2008; Szentpetery et al., 2010; Walch-Solimena and Novick, 1999). In these ways PIs can regulate and control membrane trafficking, often together with Rab GTPases (Di Paolo and De Camilli, 2006). In this study we extend this concept by demonstrating a collaborative role for both Rab GTPases and PI4P in the myosin-V based transport of secretory compartments in the budding yeast Saccharomyces cerevisiae.
The ability of this yeast to grow is dependent on the selective and polarized transport of secretory vesicles into the bud where cargo enzymes remodel the cell wall for bud expansion. Transport of secretory vesicles is dependent on the presence of polarized actin cables that arise from the bud cortex and the neck, and extend into the mother cell (Pruyne et al., 1998). The cables serve as tracks for the transport of secretory vesicles by yeast's essential myosin-V (Schott et al., 1999, 2002), whose heavy chain is encoded by MYO2 (Johnston et al., 1991). Myo2p also contributes to organelle segregation during the cell cycle by actively transporting vacuole fragments (Hill et al., 1996), peroxisomes (Hoepfner et al., 2001), mitochondria (Altmann et al., 2008), and the TGN (Arai et al., 2008; Rossanese et al., 2001) into the bud, and by binding the ends of cytoplasmic microtubules for nuclear orientation prior to mitosis (Yin et al., 2000). Cargo-specific receptors for Myo2p on almost all of these compartments have been identified, including the vacuole receptor Vac17p (Ishikawa et al., 2003), the peroxisome receptor Inp2p (Fagarasanu et al., 2006), and Kar9p that binds to Bim1p on microtubule ends (Yin et al., 2000). However, no receptor has been described for secretory vesicles, the only essential cargo of Myo2p.
Earlier work has shown that PI4P performs an essential role in the secretory pathway regulating exit of cargo from the TGN (Hama et al., 1999; Walch-Solimena and Novick, 1999). Cells depleted of Golgi PI4P fail to make secretory vesicles, and accumulate secretory cargo internally. Because of the defect in secretion, they stop growing at the restrictive temperature. As Myo2p transports secretory vesicles from the Golgi complex we set out to explore whether PI4P might also participate in the recognition mechanism by which Myo2p associates with secretory vesicles. We have described seven conditional mutations in the cargo-binding tail domain of Myo2p that very rapidly uncouple the motor from secretory vesicles at the restrictive temperature (Schott et al., 1999). These mutants are not defective in secretion per se, as the cells grow at the restrictive temperature in an isotropic manner, giving rise to large round cells that cannot divide. In this study we show that PI4P plays an additional specific role in the association of Myo2p with both the TGN and secretory vesicles. Additionally, we confirm that Myo2p binds Ypt31/32p, the yeast homologues of Rab11, and show that it also binds directly to Sec4p, the yeast homologue of Rab8, on secretory vesicles. We report that enhanced association of Myo2p with PI4P can bypass the need for an association with Ypt31/32p or Sec4p, suggesting a model where PI4P and a compartment-specific Rab protein contribute to Myo2p binding and polarized transport of membrane-bound compartments of the secretory pathway.
Results
Increasing Golgi-localized PI4P specifically rescues defects in Myo2p-dependent polarized secretion
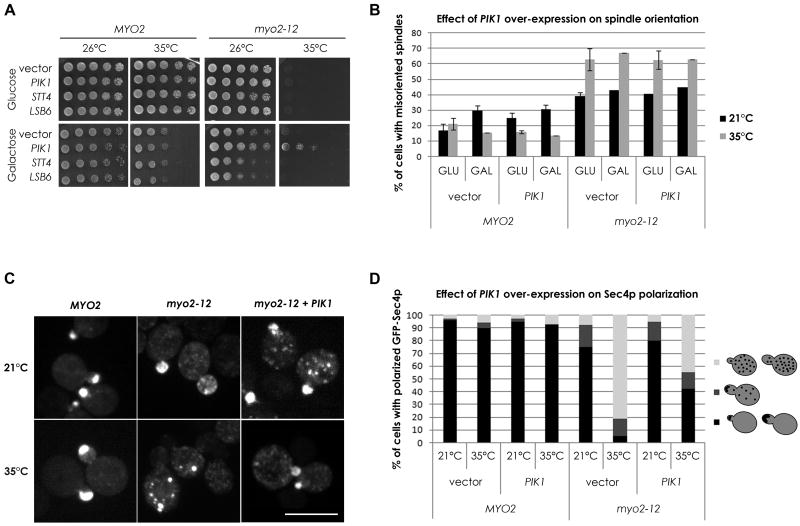
Yeast contains three PI 4-kinases, two of which are essential, the plasma membrane localized Stt4p (Audhya and Emr, 2002; Cutler et al., 1997) and the Golgi localized Pik1p (Flanagan et al., 1993), and the non-essential endosomal/plasma membrane Lsb6p (Han et al., 2002). If PI4P is important for the association of Myo2p with secretory vesicles, we reasoned that some of our mutants might be sensitive to alterations in PI4P levels. We therefore examined if over-expression of any of the PI 4-kinases might elevate PI4P and suppress the growth defects of any of our conditional Myo2p-tail mutants. Galactose regulated expression of PIK1, but not of STT4 or LSB6, partially suppressed the conditional growth defect of one allele, myo2-12, without affecting the growth of wild-type cells (Figure 1A and Table 1). Under these conditions, the total level of cellular PI4P rises about 1.5-fold as measured by thin layer chromatographic separation of radioactive PIs (Figure S1A). This suppression requires the kinase activity of PIK1 as kinase-dead mutants of PIK1 cannot suppress myo2-12, although they are all expressed at similar levels (Figure S1B, S1C). The suppression of myo2-12 is specific for its defect in secretion, as myo2-12 is also defective in spindle orientation (Yin et al., 2000) and this is not corrected by PIK1 over-expression (Figure 1B).
Figure 1. Modestly increasing Golgi PI4P can rescue a specific myo2 conditional tail mutant.
(A) Wild-type MYO2 and the myo2-12 cells expressing the indicated genes from the GAL1 promoter after growth on glucose (promoter off) or galactose (promoter on) at the indicated temperatures. See also Figure S1. (B) The percentage of cells with misoriented spindles (scored as in Yin et al., 2000) was determined before and after a 10-min shift to 35°C under conditions that repress (GLU) or over-express (GAL) PIK1. Error bars represent standard deviation of the sample. (C) GFP-Sec4p localization in the indicated strains at either room temperature or after 30-min incubation at 35°C. (D) Quantitation of GFP-Sec4p localization of cells in (C). >100 small/medium budded cells were examined as wild-type cells polarize GFP-Sec4p to the bud tip. Since there is punctate GFP-Sec4p staining in the mothers of myo2-12 cells even at the permissive temperature, a separate category was used to score these (dark gray). Bar is 5μm.
Table 1. Summary of genetic interactions with myo2 tail mutants.
| Allele | Restrictive temperature1 |
Over- expression of PIK12 |
Combined with pik1-139 |
Combined with sac1Δ |
Expression of myo2ΔCTD-PHWT3 |
Expression of myo2ΔCTD-PHEE3 |
Over- expression of SEC44 |
Over- expression of YPT314 |
|---|---|---|---|---|---|---|---|---|
| MYO2+ | >37°C | NE | 36°C | NE | NE | NE | NE | NE |
| myo2-12 | 33°C | 35°C | 30°C | 37°C | >37°C | 30°C | 36°C | 35°C |
| myo2-13 | 35°C | NE | NE | >37°C | 37°C | 33°C | NE | NE |
| myo2-14 | 35°C | NE | NE | NE | NE | 33°C | NE | NE |
| myo2-16 | 32°C | NE | 30°C | 36°C | >37°C | 30°C | 35°C | NE |
| myo2-17 | 37°C | NE | NE | NE | NE | NE | NE | NE |
| myo2-18 | 36°C | NE | NE | 37°C | 37°C | NE | NE | NE |
| myo2-20 | 37°C | NE | NE | NE | NE | NE | NE | NE |
The highest temperatures showing growth are reported for strains with either wild-type MYO2, or myo2 alleles with mutations in the C-terminal domain (Schott et al., 1999). NE, no effect
Restrictive temperature on synthetic complete medium.
PIK1 was expressed behind the GAL1 promoter.
The fusion constructs were expressed from the MYO2 promoter.
SEC4 and YPT31 were expressed on a low copy plasmid from the ADH1 promoter.
The polarized transport of secretory vesicles can be visualized by the localization of GFP-Sec4p (Schott et al., 2002), the Rab protein associated with secretory vesicles (Goud et al., 1988). In wild-type cells, GFP-Sec4p localizes to sites of cell growth, namely the bud tip in budding cells, and the bud neck in cells just before and after cytokinesis. In myo2-12 cells shifted to the restrictive temperature for just 5 minutes, the normal polarized distribution of GFP-Sec4p is lost (Figure 1C). However, in myo2-12 cells over-expressing PIK1, GFP-Sec4p remains at least partially polarized after shifting the cells to elevated temperatures, thereby accounting for the ability of these cells to grow (Figures 1C, 1D).
The conditional allele pik1-139 has reduced levels of PI4P even at the permissive temperature, but not sufficiently low to affect secretion (Sciorra et al., 2005). We examined which of the myo2 alleles might be affected by lower PI4P levels by combining each of them with the pik1-139 mutation. Just two of the alleles, myo2-12 and myo2-16, showed synthetic growth defects when combined with pik1-139 (Figure S1D and Table 1). Thus, raising Golgi PI4P is not a general mechanism by which all myo2 mutants can be suppressed, and the myo2-12, and to a lesser extent the myo2-16 allele, are especially sensitive to reductions in Pik1p's PI4P pool.
Our data, combined with earlier studies on Pik1p, suggest that PI4P plays two important roles in the secretory pathway. First, acute depletion of PI4P blocks secretion (Walch-Solimena and Novick, 1999). Second, a partial reduction of PI4P does not block secretion, but affects Myo2p-dependent membrane transport in an appropriately sensitized background.
Modulating PI4P turnover at the Golgi affects Myo2p-dependent polarized secretion
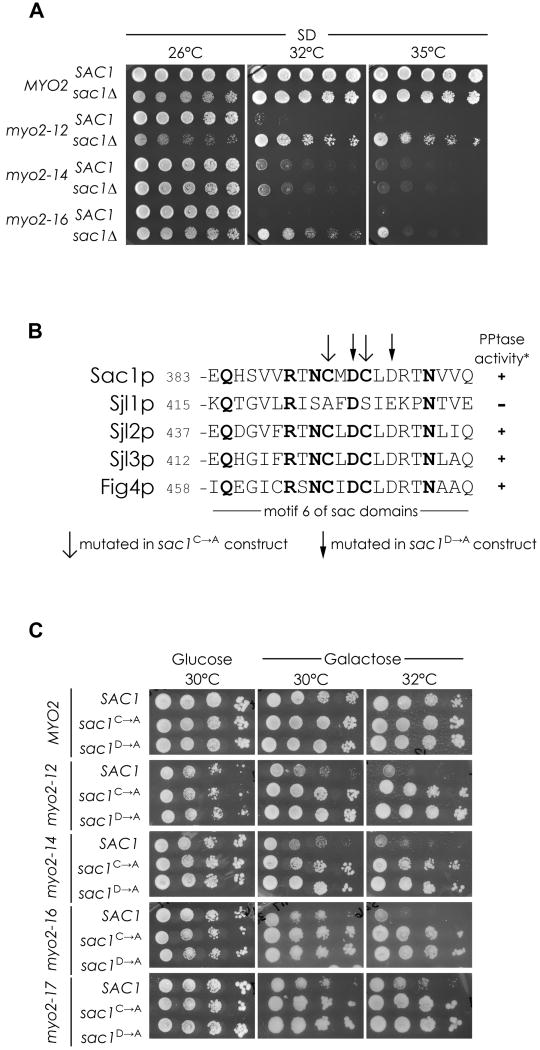
Yeast contains three lipid phosphatases that can dephosphorylate PI4P to PI. Two of these, Sjl2p and Sjl3p, function at the plasma membrane (Stefan et al., 2005), and the third, Sac1p, functions at the ER/Golgi (Foti et al., 2001; Tahirovic et al., 2005). However, Sac1p is the main phosphatase since the level of PI4P in sjl2Δsjl3Δ double mutants does not increase significantly (Stefan et al., 2002) whereas in sac1Δ cells, the level of PI4P rises more than eightfold (Foti et al., 2001; Hughes et al., 2000; Figure S1A). We therefore explored whether this method of acutely elevating PI4P levels would also suppress some of the conditional myo2-tail mutants. Although sac1Δ slows cell growth, the higher level of PI4P was able to suppress a broader spectrum of myo2 mutants, namely it suppressed myo2-12 and myo2-16 very well, and myo2-13 and myo2-18 to a lesser extent (Figure 2A, Table 1). Thus, altering PI4P modestly through PIK1 manipulation, or acutely by SAC1 deletion, reveals that the two alleles, myo2-12 and myo2-16, are especially sensitive to alterations in PI4P levels.
Figure 2. Acute modulation of PI4P turnover at the Golgi affects a broader array of myo2 tail mutants.
(A) The effect sac1Δ on growth of conditional myo2 tail alleles. (B) Alignment of the putative catalytic motif in yeast sac1 domain containing proteins, showing the two different mutations made in Sac1p to render it non-functional based on Sjl1p lack of PPtase activity (Guo et al. 1999). (C) SAC1+, or non-functional sac1 mutants, were over-expressed in the myo2ts strains and growth at different temperatures assessed, four alleles are shown. See also Figure S2.
As an additional way to examine if decreasing Golgi-localized PI4P affects polarized secretion, the effect of moderate over-expression of SAC1 on the myo2 mutants was assessed. Expression of SAC1 from a weakened GAL1 promoter (Mumberg et al., 1994) has little effect on wild-type yeast, but it lowers the restrictive temperature of all the myo2-tail mutants (Figure 2C). This effect is dependent on the phosphatase activity as expression of equivalent levels of Sac1p with mutations in the catalytic domain (Figure 2B, S2B) has no effect when over-expressed (Figure 2C) or when added back to the myo2ts sac1Δ strains (Figure S2C).
There are at least two pools of PI4P in cells, in the Golgi apparatus and at the plasma membrane (Roy and Levine, 2004). To assess the location where the Sac1p phosphatase activity affects the myo2 tail mutants, we made use of gene fusions that target the Sac1p phosphatase domain to different intracellular compartments to see which would compromise the ability of sac1Δ to suppress the myo2 conditional mutants. The Sac1p phosphatase domain was targeted by the PH domain of PLCδ to the PI-4,5P2 at the plasma membrane, or by the FYVE domain of EEA1 protein that binds PI-3P on endosomes, or by the PH domain of FAPP1 that binds specifically to the PI4P present at the Golgi (Parrish et al., 2004). Targeting the phosphatase to the Golgi reversed the ability of sac1Δ to rescue myo2-12, whereas targeting to the plasma membrane or endosome did not (Figure S2D). Again the suppression is dependent on PI4P, as expression of targeted mutant sac1 versions did not restore the temperature sensitivity of the myo2 alleles (Figure S2D). These data therefore support the hypothesis that PI4P at the Golgi plays an important role in Myo2p's essential function of transporting membranes for cell growth.
PI4P is important for coupling secretory membranes to Myo2p
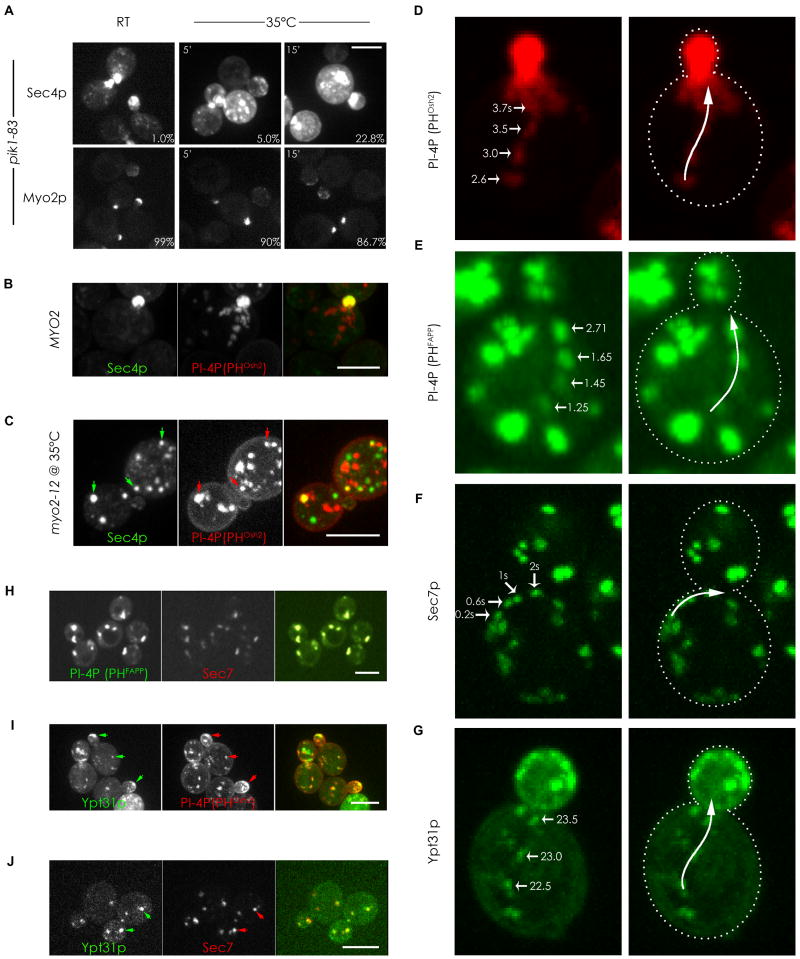
In wild-type cells, there is a strong colocalization between Myo2p and the secretory vesicle marker, Sec4p (Schott et al., 1999). To examine how this colocalization is affected by PI4P depletion, we localized Myo2p-GFP and GFP-Sec4p in the fast-acting pik1-83 conditional mutant after shifting to the restrictive temperature. At the permissive temperature, Myo2p and Sec4p polarize as well as they do in wild-type cells. Upon shifting to the restrictive temperature of 35°C for just 5 minutes, Myo2p is still polarized, but Sec4p has become somewhat depolarized, and an increased percentage of cells showed this uncoupling at longer timepoints (Figure 3A). Likewise, the polarized localization of GFP-Snc1p, the v-SNARE associated with secretory vesicles, also becomes affected after inactivating Pik1p (Figure S3A, S3B), indicating that the affect on GFP-Sec4p is not a consequence of it being lost from secretory membranes but of secretory membranes no longer being transported in a directed manner. Consistent with this, subcellular fractionation of pik1-83 cells showed no change in the amount of transport vesicle-associated Sec4p before and after shift to the restrictive temperature (P100 fraction, Figure S3C) in the same timeframe that results in a partial depolarization of GFP-Sec4p. Moreover, in the presence of a constitutive active Sec4p (Q79L), GFP-Snc1p is partially depolarized immediately after Pik1p inactivation (Figure S3A, S3B), indicating that the uncoupling of vesicles from Myo2p in the pik1-83 strain is not due to changes in Sec4p activation but due to a reduction of PI4P on secretory membranes. Thus, PI4P is a critical component necessary for the transport of secretory vesicles by Myo2p.
Figure 3. PI4P is present in compartments of the late secretory pathway and is required for their transport.
(A) pik1-83 cells expressing either GFP-Sec4p or Myo2p-GFP were fixed and examined at different times after shifting to 35°C and the percentage of small/medium budded cells with delocalized GFP-Sec4p and polarized Myo2p-3XGFP was determined (percentages in lower right corners). (B) Small budded wild type cell expressing GFP-Sec4p and mCherry-PHOsh2p. (C) myo2-12 cell expressing GFP-Sec4p and mCherry-PHOsh2p after 15-min shift to the restrictive temperature. Arrows indicate examples of colocalization. (D and E) Frames from Supplementary movie 1 (D) and 2 (E) showing vesicular-like structures labeled by the indicated PI4P reporters moving rapidly towards the bud. Times in the movies are indicated. (F) Frames from Supplementary movie 3 showing the directed movement of GFP-Sec7p towards the bud. (G) Frames from Supplementary movie 4 showing the directed movement of the GFP-Ypt31p towards the bud. (H, I, and J) Cells co-expressing a PI4P reporter and different secretory markers showing colocalization (arrows) of PI4P with Sec7p (H), with Ypt31p (I), or between Sec7p and Ypt31p (J). See also Figure S3. Bars represent 5μm.
If secretory vesicles require PI4P for their transport by Myo2p, a marker for PI4P should colocalize with secretory vesicles. The PH domain from yeast Osh2p recognizes all pools of PI4P, including the significant concentrations in the Golgi and at the plasma membrane (Roy and Levine, 2004). We therefore used mCherry-PHOsh2p to explore if PI4P colocalizes with GFP-Sec4p. mCherry-PHOsh2p localizes to many punctate structures, and live cell imaging revealed many of these showed directed transport to the bud (Figure 3B, 3D and movie S1). The PI4P-rich structures overlap with GFP-Sec4p enrichments at the bud tip and bud neck, which are sites of active growth (Figure 3B, 3D). However, very few instances of colocalization in the mother cells were seen in wild-type cells, possibly because most of the GFP-Sec4p puncta accumulate at growth sites. In myo2-12 cells shifted to the restrictive temperature, where the secretory vesicles are not transported and accumulate in the mother cell, most of the PI4P and Sec4p puncta were distinct, but a significant number now colocalized (Figure 3C). We conclude that at least some Sec4p-containing secretory vesicles contain PI4P. This may be an underestimate as it is limited by our ability to image low levels of PI4P.
Secretory membranes transported by Myo2p are rich in PI4P
Since PI4P is necessary for the transport of secretory vesicles by Myo2p, we explored whether other Golgi membranes enriched in PI4P are also transported by Myo2p. The PH domain from human FAPP1 binds only to a PI4P rich Golgi compartment as it also requires the presence of Arf1p (Levine and Munro, 2002). We therefore used GFP-PHFAPP1 to image the movements of this compartment in vivo. Directed movements towards the bud at 2.54μm/sec (±0.23) were observed in wild-type cells (Figure 3E, movie S2), a rate close to that found for secretory vesicles (Schott et al., 2002). To determine whether this movement is dependent on Myo2p, we observed it both in a myo2 mutant that transports organelles at a slower velocity due to a shortened lever-arm (Schott et al., 2002), and in the myo2 tail mutants at their restrictive temperature. The movement of the GFP-PHFAPP1 was slower in a cell with the shortened Myo2p lever arm, and transport of GFP-PHFAPP1 ceased in a myo2-12 conditional mutant shifted to the restrictive temperature. These data suggest that the PI4P rich compartment is recognized and transported by Myo2p in a manner similar to that used for secretory vesicles.
The compartment marked by GFP-PHFAPP1 precisely colocalizes with RFP-Sec7p (Figure 3H), a guanine nucleotide exchange factor for Arf1p, located in a late Golgi compartment (Franzusoff et al., 1992; Rossanese et al., 2001). Consistent with the GFP-PHFAPP1 results, the Sec7p compartment also exhibits rapid directed movements towards the bud (Figure 3F, movie S3). The related and redundant Rab proteins Ypt31/32p are enriched in the TGN (Jedd et al., 1997), and overlap extensively with RFP-Sec7p (Figure 3J). Imaging GFP-Ypt31p or Ypt32p in wild-type and myo2 lever-arm mutants showed that this compartment too is transported by Myo2p (Figure 3G, movie S4), consistent with the recent results of others (Casavola et al., 2008; Lipatova et al., 2008). Moreover, co-imaging of GFP-Ypt31p with the PI4P reporter shows that most of these compartments are also rich in PI4P (Figure 3I), as expected. No directed movement of these compartments was observed in pik1-83 cells at their restrictive temperature. Thus, all the post-Golgi membranes, the Sec7p late Golgi, the Ypt31p/32p TGN, and the Sec4p-containing secretory vesicles, are all enriched in PI4P and transported by Myo2p in a PI4P-dependent manner.
Linking Myo2p directly to PI4P can rescue specific myo2 alleles
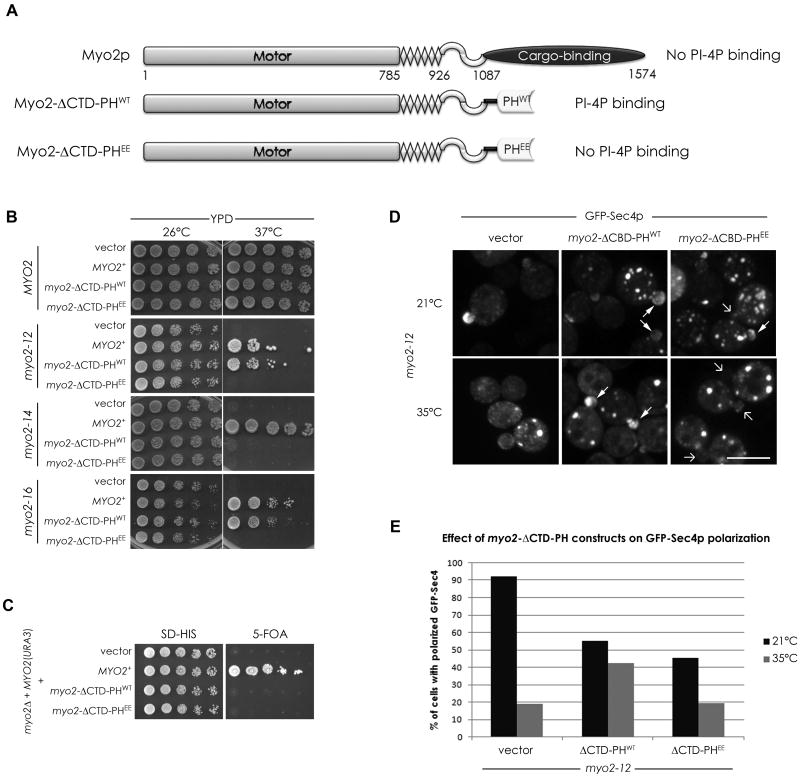
Our data suggest that PI4P is important for the association of secretory compartments with the Myo2p tail, and that some of our myo2 alleles are especially sensitive to changes in PI4P levels. To test if Myo2p's compromised association with secretory vesicles in these alleles is in part due to defective PI4P binding, we explored the possibility that the Myo2p tail itself binds PI4P. However, bacterially expressed tail constructs failed to show any PI specificity by lipid-protein blot overlays (not shown) or showed any preference for PI4P-containing vesicles by liposome floatation assays (Figure S4B). Another possibility is that Myo2p interacts with PI4P through an adaptor protein. We reasoned that linking Myo2p directly to the normal levels of Golgi PI4P might bypass the need for such an adaptor protein. We made a construct in which the C-terminal domain (CTD) of Myo2p was replaced by either the PHFAPP1 domain (Myo2-ΔCTD-PHWT) or an equivalent construct with mutations (Q16E, R18E) that compromise the ability of the PH domain to bind PI4P (Myo2-ΔCTD-PHEE; Figure 4A, S4A). Since Myo2p dimerizes, introduction of these constructs into strains with conditional myo2 mutations is expected to generate a subpopulation of heterodimers – having one chain with the PH domain in place of the CTD, and one with the mutated CTD. The Myo2-ΔCTD-PHWT completely suppressed the temperature sensitivity of myo2-12 and myo2-16, the two alleles that are especially sensitive to reduced levels of PI4P (Figure 4B and Table 1). Importantly, it also partially suppressed just those mutants that could also be suppressed by sac1Δ, but not those, like myo2-14, unaffected by elevation of PI4P (Figure 4B and Table 1), indicating that it is not a general non-specific mechanism to suppress conditional myo2 mutants. Thus, the ability of Myo2-ΔCTD-PHWT to rescue a specific class of myo2 mutants indicates that it cannot supply all the essential functions of the tail. Consistent with this, Myo2-ΔCTD-PHWT was not able to replace the function of the chromosomal MYO2 gene (Figure 4C). The Myo2-ΔCTD-PHEE construct defective in lipid binding was unable to rescue any alleles albeit being expressed at similar levels as the PHWT fusion (Figure 4B, S4C), indicating that the effect is due to an interaction with PI4P. Moreover, expression of this mutated version had a dominant-negative effect causing a decrease in the restrictive temperature of many myo2 alleles (Figure S4D and Table 1). Consistent with this dominant negative effect, expression of either Myo2-ΔCTD-PHWT or Myo2-ΔCTD-PHEE caused a partial depolarization of GFP-Sec4p in myo2-12 cells at the permissive temperature (Figure 4E). Nevertheless, at the restrictive temperature, Myo2-ΔCTD-PHWT allowed sufficient polarization of GFP-Sec4p for growth, whereas Myo2-ΔCTD-PHEE did not (Figure 4D and 4E). Thus, while the myo2-12 allele disrupts association with secretory vesicles at the restrictive temperature, enhancing the connection of Myo2-12p with PI4P now allows for an association with membranes that contain GFP-Sec4p.
Figure 4. Linking Myo2p directly to PI4P can contribute to its essential function.
(A) Schematic representation of the Myo2p constructs used. The C-terminal globular tail domain was replaced with either the PH domain from FAPP1 (PHWT) or a mutant version (PHEE) that does not recognize PI4P. (B) Growth of myo2 mutants as indicated expressing either an empty vector, wild-type Myo2p, Myo2-ΔCTD-PHWT, or Myo2-ΔCTD-PHEE. (C) A strain deleted for genomic MYO2, covered by a URA3-based plasmid, and transformed with the constructs in (A), was spotted on media with or without 5-FOA, to select against the URA3 plasmid. (D) Localization of GFP-Sec4p in myo2-12 cells expressing the Myo2-PH fusion proteins. Arrows point to small buds with (filled arrowheads) or without (arrows) GFP-Sec4p enrichments. Quantitation of GFP-Sec4p polarization in small/medium budded cells in these strains is shown in (E). See also Figure S4. Bar represent 5μm.
The complementation of the essential function of myo2-12 by the introduction of Myo2-ΔCTD-PHWT did not extend to other functions of Myo2p; indeed it acted as a dominant negative for both spindle orientation and vacuole inheritance (Figure S4E, S4F). Thus, it specifically rescues the transport of compartments of the secretory pathway.
The Rab proteins Ypt31/32p and Sec4p provide an additional linkage between Myo2p tail and secretory membranes
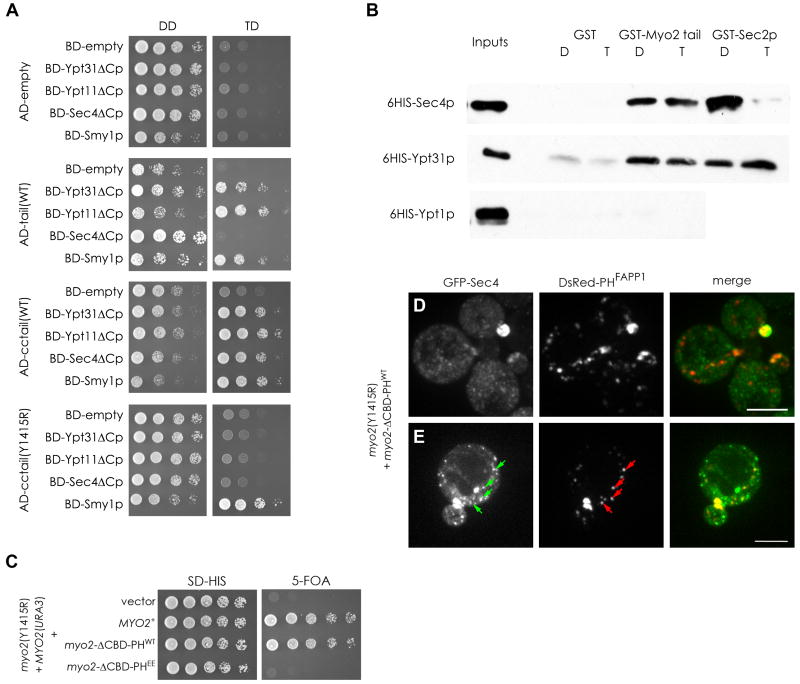
Two-hybrid studies have shown that Ypt31/32p binds the tail of Myo2p (Casavola et al., 2008; Lipatova et al., 2008), as does another Rab protein, Ypt11p (Itoh et al., 2002), and Smy1p (Beningo et al., 2000). Our two-hybrid studies confirm these interactions and additionally show that Sec4p also binds the Myo2p tail (Figure 5A). Interestingly, the interactions of the Rabs with the tail are not identical, as we get a comparable 2-hybrid signal with Sec4p only when the upstream coiled-coil region of Myo2p is present (Figure 5A). Additionally, by two-hybrid analysis, the interaction of Sec4p and Ypt31p with the Myo2p tail is GTP-dependent (Figure S5A). In vitro, Myo2p exhibited Rab specificity by binding directly to both 6HIS-Ypt31p and 6HIS-Sec4p, but not to 6HIS-Ypt1p, although we were unable to recapitulate the GTP-dependence seen by two-hybrid analysis (Figures 5B, S5B). Residues required for Ypt31/32p to bind the tail, including the Y1415R mutation, were recently reported (Casavola et al., 2008; Lipatova et al., 2008). This mutation also abolishes the interaction with Sec4p and Ypt11p, but not Smy1p (Figure 5A). When introduced into the chromosomal locus, this mutation renders Myo2p non-functional and is therefore lethal (Casavola et al., 2008; Lipatova et al., 2008; Figure 5C). Remarkably, when the Myo2-ΔCTD-PHWT construct, with the PI4P-binding module, is introduced into the myo2-Y1415R strain it can now grow, whereas with the lipid binding defective construct, Myo2-ΔCTD-PHEE, it cannot (Figure 5C). Thus, a lethal mutation that abolishes the interaction with the Rab proteins can be bypassed by providing a domain to enhance the association with normal levels of PI4P. In these cells, as expected, the PI4P rich compartment is polarized, but so is GFP-Sec4p (Figure 5D). Thus, although Myo2-Y1415R and Myo2-ΔCTD-PHWT cannot bind to Sec4p, Myo2-ΔCTD-PHWT can nevertheless associate with the PI4P and Sec4p-containing compartment to allow for both polarized membrane delivery and for Sec4p to perform its essential function in exocytosis. Even in the cells with no clear enrichment of GFP-Sec4p, the PI4P compartment was polarized which presumably provides sufficient Sec4p for bud growth (Figure 5D, lower cells). Moreover, in these cells colocalization and movement of GFP-Sec4p with PI4P in mother cells is clear (Figure 5E, S3D). This is similar to our result with myo2-12 cells, where Sec4p is uncoupled from Myo2p at the restrictive temperature, yet can be rescued by expression of Myo2-ΔCTD-PHWT with partial restoration of GFP-Sec4p polarization (Figure 4D). The results indicate that there are two important components for the association of the Myo2p tail with compartments of the secretory pathway. The first is a Rab protein – either Ypt31/32p or Sec4p – and the second is the lipid PI4P, and enhancing the interaction with the latter can compensate for a defective interaction with the former.
Figure 5. The essential interaction of Rab GTPases with the Myo2p tail can be by-passed by enhancing PI4P binding.
(A) Yeast 2-hybrid interactions with the Myo2p-tail (tail), or coiled-coil region and tail (cctail), or cctail with the Y1415R mutation, fused to the activating domain (AD) and the Rab GTPases and Smy1p fused to the DNA binding domain (BD). All Rab constructs were mutated in their C-terminal CXC motif (eg. Ypt31ΔCp) to eliminate prenylation. Growth on the control (DD) and test (TD) plates is shown. (B) GST, GST fused to Myo2p-cctail, or GST-Sec2p, was used to retain 6xHis-Rab GTPases in the presence of GDP (D) or GTP-γS (T). Binding assays were carried out as described in Experimental Procedures and visualized with antibodies to 6xHis. (C) A strain with the genomic myo2 Y1415R mutation, covered by a URA3-based plasmid, was transformed with the constructs as indicated and spotted on media with or without 5-FOA, to select against the URA3 plasmid. (D and E) Images of GFP-Sec4p and DsRed-PHFAPP1 localization in the Rab-binding deficient myo2-Y1415R mutant expressing the Myo2-ΔCTD-PHWT construct. Arrows indicates examples of colocalization in the mother cell. See also Figure S5. Bar represent 5μm.
PI4P and Rab GTPases collaborate in the association of Myo2p with secretory compartments
Our results suggest a coincidence detector model for the recruitment of Myo2p to secretory membranes. A prediction of this model is that over-expression of Sec4p might be able to suppress Myo2p tail mutants that are sensitive to low levels of PI4P. Indeed, mild over-expression of Sec4p, driven by the ADH1 promoter, is sufficient to suppress the myo2-12 and myo2-16 alleles (Figure 6A and Table 1), precisely those that are especially sensitive to reductions in PI4P levels. Likewise, over-expression of Ypt31p can rescue myo2-12 specifically, albeit only partially. These results show that Myo2p requires interactions with both PI4P and Rabs, especially Sec4p, and these are affected in the myo2-12 and myo2-16 alleles.
Figure 6. Both PI4P and Rab GTPases are limiting factors in the association of Myo2p with secretory vesicles.
(A) Effect of SEC4 and YPT31 over-expression (from the strong ADH1 promoter) on the growth of myo2 alleles, either those sensitive to PI4P levels (myo2-12 and myo2-16), or not sensitive (myo2-14). (B) Wild-type cells co-transformed to over-express on galactose media the GST-Myo2p-tail and different Rab proteins (Sec4p or Ypt31p), PI 4-kinases (Pik1p and Stt4p) or Smy1p. (C) Western blots of strains in (B) after induction with galactose for 3 hours. Same number of OD600 units were loaded in a 10% protein gel, resolved and blotted with anti-Myo2p-tail antibodies and anti-HA antibodies. Asterisk marks endogenous Myo2p. (D) Wild-type cells expressing the Myo2-PH fusions and transformed to over-express the GST-Myo2p-tail. The no tail control has two empty plasmids. (E) Working model for secretory compartment association with and transport by Myo2p along polarized actin cables. The membrane lipid PI4P (red) is required for association of all transported compartments with Myo2p through an unknown factor (X). A specific Rab protein, either Ypt11p (late Golgi), Ypt31/32 (TGN) or secretory vesicles (Sec4p), that associates with morphologically distinct compartments is also required. The association of Ypt11p with Myo2p at the late Golgi is based on the work of others (Arai et al., 2008). See text for discussion.
Additional support for this model comes from analysis of over-expression of the Myo2p tail. Earlier studies have shown that Myo2p tail over-expression is lethal because it depolarizes secretion, presumably by binding the secretory vesicle receptor and thereby uncoupling secretory compartments from the motor (Reck-Peterson et al., 1999; Schott et al., 1999). If there are two components to the association between secretory vesicles and Myo2p, it might be possible to overcome this lethality by enhancing one of the associations. We therefore examined whether PIK1 over-expression (to enhance the PI4P component), or Sec4p over-expression (to enhance the Rab component), can suppress the lethal phenotype conferred by over-expression of the Myo2p tail. Consistent with our model, over-expression of either PIK1 or SEC4 was able to partially suppress the lethal phenotype of the Myo2p tail, whereas STT4, YPT31 or SMY1 were not (Figure 6B). These growth differences were not due to decreased expression of the GST-Myo2p-tail construct as they remained equivalent in all the strains (Figure 6C). Moreover, in cells expressing the Myo2-ΔCTD-PHWT construct, the GST-Myo2p-tail is also unable to completely inhibit growth (Figure 6D). These results indicate that both PI4P and Sec4p are limiting factors for the association of Myo2p with secretory vesicles, and under conditions where one of the components can be bypassed, the GST-Myo2p-tail construct no longer completely interferes with endogenous Myo2p.
Discussion
Most of the receptor proteins that bridge Myo2p to its organelles have been identified with the exception of the secretory compartments, which are the only essential cargo. One strategy used for delivery of organelles involves degradation of the organelle-specific receptor at its destination, as has been seen for Vac17p mediated delivery of the vacuole (Tang et al., 2003). This is well suited for organelles that just require a one-way trip to be delivered at a specific point in the cell cycle. By contrast, compartments of the secretory pathway are transported to specific locations throughout the cell cycle, so the receptor is likely to persist and be recycled. Several studies have suggested Rab proteins as compartment-specific components involved in bridging to Myo2p (Casavola et al., 2008; Lipatova et al., 2008; Schott et al., 1999). In this study, we present evidence to support this idea, and importantly extend it to also include PI4P as a factor required to bridge secretory compartments to Myo2p.
In a simplistic model, organelle-specific lipids, such as phosphoinositides, could account for Myo2p's selectivity in cargo selection. We therefore focused on PI4P's role in Myo2p-dependent secretion for two reasons: first, PI4P is greatly enriched at the TGN, where secretory vesicles originate; second, genes encoding enzymes responsible for PI4P regulation show genetic interactions with myo2-66 and the Rab proteins sec4-8 (Walch-Solimena and Novick, 1999) and ypt31Δ (Sciorra et al., 2005). These observations point to a functional relationship between Myo2p, Pik1p, Ypt31p and Sec4p. In agreement, we found that two myo2 alleles that are especially sensitive to levels of PI4P can be rescued by increases in PI4P while being highly sensitive to reductions of the lipid. However, PI4P alone cannot account for the recruitment of Myo2p to secretory membranes because we failed to see an in vitro interaction between Myo2p tail and PI4P and, most importantly, PI4P is also present in the plasma membrane. To achieve specificity, a factor must also be present only on the membranes to be transported by Myo2p. Indeed, we found that the exocytic Rabs that identify specific secretory membranes moved by Myo2p bind the Myo2p tail directly, an interaction that is essential as demonstrated by the lethality of the myo2-Y1415R mutation. Therefore, our results indicate that PI4P and exocytic Rabs function as a coincidence detector for the polarized transport of secretory compartments by Myo2p.
Which compartments of the secretory pathway are transported by Myo2p in a PI4P-dependent manner? By using two different PI4P reporters, we have shown that all the secretory membranes actively transported by Myo2p, the Sec7p late Golgi, the TGN Ypt31/32p compartment, and the Sec4p-labeled secretory vesicles, are enriched in PI4P. The PI4P compartment labeled by PHFAPP1 coincides precisely with the Sec7p compartment. PHOsh2p illuminates additional smaller tubular and punctate structures that overlap with Ypt31p staining, and Sec4p overlaps strongly with PHOsh2p in small budded cells. Thus, all three compartments contain PI4P. A more stringent test would be the ability to co-visualize markers for both PI4P and each secretory compartment during Myo2-directed movement. This is technically challenging due to their small size and rapid rate of movement. When we look at PI4P using PHOsh2p, we see small vesicular as well as tubular structures moving in a Myo2p-dependent manner. Many of the tubular structures seem to fuse and divide while they move towards growth sites, and they are usually bigger than the GFP-Sec4p puncta. These vesiculotubular structures are reminiscent of the membranes labeled by Ypt31p. Secretory vesicles, however, with their small size may have amounts of PI4P that are generally below the level of detection for the 10 frames per second required to document their movement. To alleviate this problem, we localized PI4P and Sec4p in the myo2-12 conditional mutant at the restrictive temperature, where vesicles are uncoupled from Myo2p and do not exhibit rapid movements. Under these conditions, as well as in the myo2-Y1415R mutant covered by the Myo2-PH fusion, we can see an increased amount of colocalization of Sec4p with the PI4P reporter in the mother cell. However, not all Sec4p-vesicles have detectable PI4P even under these conditions, which could mean that the amount of PI4P diminishes as the compartment matures, to a point below detection. Supporting this hypothesis, the Novick lab recently suggested that the vesicle's PI4P levels have to decrease in order to recruit Sec15p to its surface (Mizuno-Yamasaki et al., 2010). It is not clear where, and by what phosphatase (or kinase), this happens, but vesicle PI4P hydrolysis (or conversion) could be one way membrane transport by Myo2p is integrated with exocytosis. Nevertheless, Sec7p, Ypt31p, and Sec4p directed movements are all lost in the pik1-83 mutant at the restrictive temperature, indicating that PI4P throughout the secretory pathway is needed for Myo2p-dependent movement.
Although secretory vesicles do not need to deliver PI4P to the plasma membrane, since the PI 4-kinase Stt4p synthesizes it there (Audhya and Emr, 2002), the strong accumulation of PI4P seen in small buds where secretory vesicles accumulate suggests that PI4P is present during transport of secretory vesicles to their destination. Further, under special conditions of growth, the PI 4-kinase Stt4p is not essential (Cutler et al., 1997), and, although the cells grow very slowly, arriving secretory vesicles can supply a sufficient trickle of PI4P for plasma membrane PI(4,5)P2 synthesis and cell viability, again supporting the concept that secretory vesicles must contain PI4P. This last statement is clearly shown in a recent paper where they assess the role of PI4P in trafficking (Szentpetery et al., 2010). Lastly, in mutants where Myo2p cannot associate directly with GFP-Sec4p-labeled membranes, the Myo2-ΔCTD-PHFAPP1 construct can still polarize these membranes, indicating that PI4P and Sec4p are present, to some extent, in the same membranes.
Our data therefore suggest the following model. PI4P is a general factor necessary for secretory compartments to associate with and be transported by Myo2p. Since a Myo2p-tail mutation that eliminates interactions with the exocytic Rab proteins is lethal, an additional requirement is for a Rab protein, potentially Ypt11p, Ypt31/32p and Sec4p. Ypt11p is known to contribute to the polarized transport of the Sec7p compartment (Arai et al., 2008), Ypt31/32p could contribute to the transport of the TGN, and Sec4p to the transport of secretory vesicles. The idea that different Rabs can use the same molecular motor is consistent with the fact that Myo2p recognition of Ypt31/32p and Sec4p is not entirely equal. Although they both need determinants in the globular tail, Sec4p binding also requires the coiled coil region of Myo2p. This is not unprecedented, as Myosin Vb shows similar structural requirements for Rab11a and Rab8a, the homologues of Ypt31p and Sec4p, respectively (Roland et al., 2007). The requirement for both a Rab protein as well as PI4P may serve as a coincidence detector to allow Myo2p to distinguish between the plasma membrane and secretory compartment pools of PI4P. At the same time, since the structural requirements for Myo2p binding of the Rabs are distinct, this might still allow for compartment-specific regulation.
How does Myo2p associate with PI4P? Since we have not found a direct interaction in vitro between the Myo2p tail and PI4P, we hypothesize that the binding to PI4P is indirect through an adaptor protein. We reasoned that if the myo2 alleles sensitive to low PI4P were partially defective in binding the PI4P adaptor protein, we could bypass it by tethering Myo2p directly to PI4P. Instead of fusing a PI4P-binding module to the tail domain, we replaced the whole tail domain with the lipid-binding module to make the Myo2-ΔCTD-PHFAPP1 construct. Expression of this construct can restore viability to just those myo2 alleles sensitive to low PI4P, indicating that PI4P is an important factor, but not the only one necessary for tethering Myo2p to secretory compartments. Importantly, the construct cannot suppress many of the other conditional myo2 alleles, and cannot replace the function of wild-type Myo2p, thereby demonstrating the requirement for additional components. One of these necessary components, as described above, is the compartment-specific Rab protein. This requirement can also be bypassed by the Myo2-ΔCTD-PHFAPP1 construct, indicating that Rabs are not the only other component of the receptor. Furthermore, this result implies a model whereby PI4P and Rabs collaborate as part of the receptor for secretory compartments. It would be very interesting to test if enhancing Rab binding could bypass the need for PI4P, the same way we show the opposite is true (Figure 4 and 5). Although this experiment would be extremely difficult since PI4P has other essential functions, we also showed that you can selectively rescue the PI4P sensitive myo2 alleles by over-expressing the Rabs YPT32 or SEC4. This result suggests that high levels of Rab proteins, by enhancing the Myo2p-Rab interaction, provide a remedial effect to strains sensitive to low levels of PI4P. This interpretation is also supported by our finding that the lethality associated with Myo2p tail over-expression can be suppressed by over-expression of either PIK1 or SEC4. However, in these experiments, the lethality of the GST-Myo2p-tail over-expression is only partially suppressed, suggesting that another component may be titrated out by the over-expressed fusion protein. Our model therefore proposes at least three components for the Myo2p secretory compartment receptor: PI4P, a compartment-specific Rab, and an unidentified third component that probably binds PI4P and/or the Rab.
How general might the requirement for phosphoinositides be in tethering cargo to a molecular motor? In plants, the delivery of secretory vesicles necessary for pollen tube and root tip growth depend on PI4P, synthesized and regulated by the Pik1p and Sac1p homologs PI4Kβ1 and SAC7 (Preuss et al., 2006; Thole et al., 2008). Moreover, PI4Kβ1 is an effector of RabA, the homologue of yeast Ypt31p, an interaction that is conserved in mammals between PI4Kβ and Rab11 (de Graaf et al., 2004). Microtubule based kinesins also function with phosphoinositides. The nematode kinesin Unc104 has a PH domain that binds PI-4,5P2 directly to transport synaptic vesicles (Klopfenstein et al., 2002), and special vesicles rich in PIP3 are delivered to the tips of neurites by the kinesin GAKIN linked through a PIP3-interacting protein (Horiguchi et al., 2006). As far as we are aware, Myo2p is the first myosin shown to require a phosphoinositide to bind its cargo.
We propose a model of the late secretory pathway (Figure 6E) in which PI4P is present from the donor compartment until the target membrane is reached, but the Rab protein changes along the way together with the morphology of the carrier. We speculate that PI4P may be an ancient conserved component of this pathway. As cells evolved and different compartmentalization was required, Rabs diversified to subdivide the already segregated PI4P marked membranes. Thus PI4P would be the identification marker for secretory compartments in general, whereas the Rab protein would identify the specific compartment. The whole process can then be terminated at the plasma membrane with the degradation or conversion of PI4P into PI or PI-4,5P2, respectively. The simple view where Myo2p tethers secretory vesicles through Rab GTPases can now be expanded to include another component, PI4P, and an additional unidentified adapter protein that may bind PI4P.
Experimental Procedures
Yeast Strains and molecular biology techniques
Strains used in this study are listed in Table S1. Standard media and techniques for growing and transform yeast were used (Sherman, 1991). For more details on strain and plasmid construction see Supplemental Experimental Procedures.
Microscopy
Micrographs were acquired with a spinning disc confocal microscopy system (3I Corp) using a DMI 6000B microscope (Leica) and a digital camera (QuantEM; Photometrics). Movie S2 and figure 3D were taken using an Axiovert conventional fluorescence microscope. Images were further analyzed and adjusted using Slidebook 5. For panel assembly, the images were cropped and further adjusted in Photoshop (Adobe) to give the clearest presentation of the results. Quantification of GFP markers was done as described in figure legends.
Analysis of PI4P-Myo2p tail interaction
Binding of recombinant Myo2-tail to PI4P was tested by phosphoinositide-protein dot blots (PIP strips) and by liposome floatation assays. See Supplemental Experimental Procedures for details.
In vitro binding assays
Purification of GST-Myo2p-tail and 6xHis-Rab GTPases recombinant proteins was done as described (Legesse-Miller et al., 2006; Du and Novick, 2001). GST-PHFAPP and GST-Sec2p were purified following the standard pGEX fusion system instructions, except that for GST-Sec2p the induction was done at 20°C with 0.4mM IPTG overnight. The Rab GTPases were preloaded with GDP or GTP-γS by resuspending 125nM of the protein in binding buffer (25mM Tris, pH 7.4, 80mM NaCl, 1mM MgCl2, 1mM DTT) containing 1mM EDTA and 1mM of the nucleotide and incubating at 30°C for 30-min. The reaction was terminated by the addition of MgCl2 to 5mM final. The GST-Myo2p-tail bound to the beads was added (∼1mM, usually 10-20μL of slurry) and the total volume adjusted to 600μL Reactions were incubated for 1hr at 4°C with rotation. Beads were washed four times with wash buffer (binding buffer except 5mM MgCl2, 10% glycerol, 0.1% triton X-100) and then resuspended in 2× protein sample buffer. Bound Rabs were separated by SDS-PAGE and analyzed by western blotting with anti-6xHIS antibodies (Sigma).
Yeast two-hybrid analyses
Yeast strain AH109 was co-transformed with both activation and DNA binding domain fusion proteins and the presence of both plasmids selected in media lacking leucine and tryptophan (DD) while an interaction was tested by growing in media additionally lacking histidine (TD). The Myo2p-tail constructs were done in pGADT7, the Rab proteins in pBridge, and the Smy1p construct in pAS2-1.
Supplementary Material
Acknowledgments
The authors are very grateful to Ruth Collins, Susan Henry, and Scott Emr for strains and plasmids. We thank Chris Stefan, Chris Fromme, and the members of the Bretscher lab for discussions and comments on the manuscript, and Katherine Wilhelmy for assistant with making constructs. Supported by NIH GM39066 to AB and NIH predoctoral fellowship F31GM077098 to F.H.S-T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann K, Frank M, Neumann D, Jakobs S, Westermann B. The class V myosin motor protein, Myo2, plays a major role in mitochondrial motility in Saccharomyces cerevisiae. J Cell Biol. 2008;181:119–130. doi: 10.1083/jcb.200709099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Noda Y, Kainuma S, Wada I, Yoda K. Ypt11 functions in bud-directed transport of the Golgi by linking Myo2 to the coatomer subunit Ret2. Curr Biol. 2008;18:987–991. doi: 10.1016/j.cub.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Audhya A, Emr SD. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev Cell. 2002;2:593–605. doi: 10.1016/s1534-5807(02)00168-5. [DOI] [PubMed] [Google Scholar]
- Audhya A, Loewith R, Parsons AB, Gao L, Tabuchi M, Zhou H, Boone C, Hall MN, Emr SD. Genome-wide lethality screen identifies new PI4,5P2 effectors that regulate the actin cytoskeleton. Embo J. 2004;23:3747–3757. doi: 10.1038/sj.emboj.7600384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Lillie SH, Brown SS. The yeast kinesin-related protein Smy1p exerts its effects on the class V myosin Myo2p via a physical interaction. Mol Biol Cell. 2000;11:691–702. doi: 10.1091/mbc.11.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casavola EC, Catucci A, Bielli P, Di Pentima A, Porcu G, Pennestri M, Cicero DO, Ragnini-Wilson A. Ypt32p and Mlc1p bind within the vesicle binding region of the class V myosin Myo2p globular tail domain. Mol Microbiol. 2008 doi: 10.1111/j.1365-2958.2008.06106.x. [DOI] [PubMed] [Google Scholar]
- Cutler NS, Heitman J, Cardenas ME. STT4 is an essential phosphatidylinositol 4-kinase that is a target of wortmannin in Saccharomyces cerevisiae. J Biol Chem. 1997;272:27671–27677. doi: 10.1074/jbc.272.44.27671. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Vicinanza M, Di Campli A, De Matteis MA. The multiple roles of PtdIns(4)P -- not just the precursor of PtdIns(4,5)P2. J Cell Sci. 2008;121:1955–1963. doi: 10.1242/jcs.023630. [DOI] [PubMed] [Google Scholar]
- de Graaf P, Zwart WT, van Dijken RA, Deneka M, Schulz TK, Geijsen N, Coffer PJ, Gadella BM, Verkleij AJ, van der Sluijs P, et al. Phosphatidylinositol 4-kinasebeta is critical for functional association of rab11 with the Golgi complex. Mol Biol Cell. 2004;15:2038–2047. doi: 10.1091/mbc.E03-12-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Du LL, Novick P. Purification and properties of a GTPase-activating protein for yeast Rab GTPases. Methods Enzymol. 2001;329:91–99. doi: 10.1016/s0076-6879(01)29070-3. [DOI] [PubMed] [Google Scholar]
- Fagarasanu A, Fagarasanu M, Eitzen GA, Aitchison JD, Rachubinski RA. The peroxisomal membrane protein Inp2p is the peroxisome-specific receptor for the myosin V motor Myo2p of Saccharomyces cerevisiae. Dev Cell. 2006;10:587–600. doi: 10.1016/j.devcel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Flanagan CA, Schnieders EA, Emerick AW, Kunisawa R, Admon A, Thorner J. Phosphatidylinositol 4-kinase: gene structure and requirement for yeast cell viability. Science. 1993;262:1444–1448. doi: 10.1126/science.8248783. [DOI] [PubMed] [Google Scholar]
- Foti M, Audhya A, Emr SD. Sac1 lipid phosphatase and Stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol 4-phosphate that functions in the control of the actin cytoskeleton and vacuole morphology. Mol Biol Cell. 2001;12:2396–2411. doi: 10.1091/mbc.12.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzusoff A, Lauze E, Howell KE. Immuno-isolation of Sec7p-coated transport vesicles from the yeast secretory pathway. Nature. 1992;355:173–175. doi: 10.1038/355173a0. [DOI] [PubMed] [Google Scholar]
- Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol. 2000;12:63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Goud B, Salminen A, Walworth NC, Novick PJ. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988;53:753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Stolz LE, Lemrow SM, York JD. SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. J Biol Chem. 1999;274:12990–5. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- Hales CM, Vaerman JP, Goldenring JR. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J Biol Chem. 2002;277:50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–34300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Han GS, Audhya A, Markley DJ, Emr SD, Carman GM. The Saccharomyces cerevisiae LSB6 gene encodes phosphatidylinositol 4-kinase activity. J Biol Chem. 2002;277:47709–18. doi: 10.1074/jbc.M207996200. [DOI] [PubMed] [Google Scholar]
- Hill KL, Catlett NL, Weisman LS. Actin and myosin function in directed vacuole movement during cell division in Saccharomyces cerevisiae. J Cell Biol. 1996;135:1535–1549. doi: 10.1083/jcb.135.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner D, van den Berg M, Philippsen P, Tabak HF, Hettema EH. A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J Cell Biol. 2001;155:979–990. doi: 10.1083/jcb.200107028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi K, Hanada T, Fukui Y, Chishti AH. Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity. J Cell Biol. 2006;174:425–436. doi: 10.1083/jcb.200604031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang E, Kusch J, Barral Y, Huffaker TC. Spindle orientation in Saccharomyces cerevisiae depends on the transport of microtubule ends along polarized actin cables. J Cell Biol. 2003;161:483–488. doi: 10.1083/jcb.200302030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Catlett NL, Novak JL, Tang F, Nau JJ, Weisman LS. Identification of an organelle-specific myosin V receptor. J Cell Biol. 2003;160:887–897. doi: 10.1083/jcb.200210139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Watabe A, Toh EA, Matsui Y. Complex formation with Ypt11p, a rab-type small GTPase, is essential to facilitate the function of Myo2p, a class V myosin, in mitochondrial distribution in Saccharomyces cerevisiae. Mol Cell Biol. 2002;22:7744–7757. doi: 10.1128/MCB.22.22.7744-7757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GC, Prendergast JA, Singer RA. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Tomishige M, Stuurman N, Vale RD. Role of phosphatidylinositol(4,5)bisphosphate organization in membrane transport by the Unc104 kinesin motor. Cell. 2002;109:347–358. doi: 10.1016/s0092-8674(02)00708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW, Jr, Mercer JA, Bahler M, Goldenring JR. Myosin Vb is associated with plasma membrane recycling systems. Mol Biol Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine TP, Munro S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr Biol. 2002;12:695–704. doi: 10.1016/s0960-9822(02)00779-0. [DOI] [PubMed] [Google Scholar]
- Lipatova Z, Tokarev AA, Jin Y, Mulholland J, Weisman LS, Segev N. Direct interaction between a myosin V motor and the Rab GTPases Ypt31/32 is required for polarized secretion. Mol Biol Cell. 2008;19:4177–4187. doi: 10.1091/mbc.E08-02-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P. Phosphatidylinositol 4-Phosphate Controls Both Membrane Recruitment and a Regulatory Switch of the Rab GEF Sec2p. Dev Cell. 18:828–840. doi: 10.1016/j.devcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D, Muller R, Funk M. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 1994;22:5767–5768. doi: 10.1093/nar/22.25.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish WR, Stefan CJ, Emr SD. Essential role for the myotubularin-related phosphatase Ymr1p and the synaptojanin-like phosphatases Sjl2p and Sjl3p in regulation of phosphatidylinositol 3-phosphate in yeast. Mol Biol Cell. 2004;15:3567–3579. doi: 10.1091/mbc.E04-03-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson SL, Novick PJ, Mooseker MS. The tail of a yeast class V myosin, Myo2p, functions as a localization domain. Mol Biol Cell. 1999;10:1001–1017. doi: 10.1091/mbc.10.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JT, Kenworthy AK, Peranen J, Caplan S, Goldenring JR. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol Biol Cell. 2007;18:2828–2837. doi: 10.1091/mbc.E07-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossanese OW, Reinke CA, Bevis BJ, Hammond AT, Sears IB, O'Connor J, Glick BS. A role for actin, Cdc1p, and Myo2p in the inheritance of late Golgi elements in Saccharomyces cerevisiae. J Cell Biol. 2001;153:47–62. doi: 10.1083/jcb.153.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Levine TP. Multiple pools of phosphatidylinositol 4-phosphate detected using the pleckstrin homology domain of Osh2p. J Biol Chem. 2004;279:44683–44689. doi: 10.1074/jbc.M401583200. [DOI] [PubMed] [Google Scholar]
- Schott D, Ho J, Pruyne D, Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J Cell Biol. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott DH, Collins RN, Bretscher A. Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J Cell Biol. 2002;156:35–39. doi: 10.1083/jcb.200110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorra VA, Audhya A, Parsons AB, Segev N, Boone C, Emr SD. Synthetic genetic array analysis of the PtdIns 4-kinase Pik1p identifies components in a Golgi-specific Ypt31/rab-GTPase signaling pathway. Mol Biol Cell. 2005;16:776–793. doi: 10.1091/mbc.E04-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan CJ, Audhya A, Emr SD. The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Mol Biol Cell. 2002;13:542–557. doi: 10.1091/mbc.01-10-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan CJ, Padilla SM, Audhya A, Emr SD. The phosphoinositide phosphatase Sjl2 is recruited to cortical actin patches in the control of vesicle formation and fission during endocytosis. Mol Cell Biol. 2005;25:2910–2923. doi: 10.1128/MCB.25.8.2910-2923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentpetery Z, Varnai P, Balla T. Acute manipulation of Golgi phosphoinositides to assess their importance in cellular trafficking and signaling. Proc Natl Acad Sci USA. 2010;107:8225–8230. doi: 10.1073/pnas.1000157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahirovic S, Schorr M, Mayinger P. Regulation of intracellular phosphatidylinositol-4-phosphate by the Sac1 lipid phosphatase. Traffic. 2005;6:116–130. doi: 10.1111/j.1600-0854.2004.00255.x. [DOI] [PubMed] [Google Scholar]
- Tang F, Kauffman EJ, Novak JL, Nau JJ, Catlett NL, Weisman LS. Regulated degradation of a class V myosin receptor directs movement of the yeast vacuole. Nature. 2003;422:87–92. doi: 10.1038/nature01453. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase Pik1 regulates secretion at the Golgi. Nat Cell Biol. 1999;1:523–525. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JA., 3rd Identification of an organelle receptor for myosin-Va. Nat Cell Biol. 2002;4:271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Emr SD. Phosphoinositide signaling and turnover: PtdIns(3)P, a regulator of membrane traffic, is transported to the vacuole and degraded by a process that requires lumenal vacuolar hydrolase activities. EMBO J. 1998;17:4930–4942. doi: 10.1093/emboj/17.17.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Pruyne D, Huffaker TC, Bretscher A. Myosin V orientates the mitotic spindle in yeast. Nature. 2000;406:1013–1015. doi: 10.1038/35023024. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De Camilli P. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli PV. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.