Abstract
Regulation of alternative splicing is controlled by pre-mRNA sequences (cis-elements) and trans-acting protein factors that bind them. The combinatorial interactions of multiple protein factors with the cis-elements surrounding a given alternative splicing event lead to an integrated splicing decision. The mechanism of multifactorial splicing regulation is poorly understood. Using a splicing-sensitive DNA microarray, we assayed 352 Caenorhabditis elegans alternative cassette exons for changes in embryonic splicing patterns between wild-type and 12 different strains carrying mutations in a splicing factor. We identified many alternative splicing events that are regulated by multiple splicing factors. Many splicing factors have the ability to behave as splicing repressors for some alternative cassette exons and as splicing activators for others. Unexpectedly, we found that the ability of a given alternative splicing factor to behave as an enhancer or repressor of a specific splicing event can change during development. Our observations that splicing factors can change their effects on a substrate during development support a model in which combinatorial effects of multiple factors, both constitutive and developmentally regulated ones, contribute to the overall splicing decision.
INTRODUCTION
Alternative splicing is a common mechanism for the generation of alternative isoforms of transcripts and proteins. This process can be regulated in tissue-specific or developmental stage-specific manners, and can be responsive to signaling cues. The regulation of alternative splicing is achieved through the interplay between sequence elements of the pre-mRNA, known as cis-elements, and trans-acting splicing factor proteins that bind to them. Multiple splicing factor proteins that act to repress or activate splicing have been identified. Many of these splicing factors can be grouped into families, and different families can have antagonistic effects on alternative splicing decisions. Two main families of splicing factors have been described with detail: the SR (serine/arginine) and the hnRNPs proteins (heterogeneous nuclear ribonucleoparticles) (1,2). SR proteins are generally described as splicing enhancers but they are also known to negatively regulate splicing in particular cases (3). Comparatively, proteins of the hnRNP family are generally described as negative regulators of splicing, but opposite effects have also been reported (4). Identification of alternative splicing factors and the sequences that they interact with has led to a model of a splicing code. A goal of research in the field is to solve this code so that knowledge of the regulatory elements on a pre-mRNA and their relative location, combined with knowledge of the array of splicing factors present in the nucleus, will allow for the ability to predict the outcome of alternative splicing (5–7).
Detailed biochemical studies of several alternatively spliced genes have shown that splicing regulation can occur through multiple distinct pre-mRNA splicing factors interacting with multiple distinct cis-elements. Many of these factors are ubiquitously expressed, but in combination with tissue-specific factors, splicing specificity can be achieved. The combinatorial control of alternative splicing by various cis-elements on specific events has been described earlier (6). A detailed analysis of the RNA features that control alternative splicing was recently published; this work takes into account many features in pre-mRNA sequences and is able to make successful predictions for how alternatively spliced regions are regulated in different tissues (8). However, the mechanisms by which cis-elements interacting with protein factors regulate the actual assembly of an active spliceosome are poorly understood.
Combinatorial regulation of a specific splicing event by the binding of multiple splicing factors to cis-elements has been demonstrated for many genes. For example PTB and hnRNP A1/A2 were recently described as common regulators of PKM alternative splicing (9,10). hnRNP H and hnRNP A1 can collaborate to regulate 5′-splice site selection (11). Detailed study of the neural-specific alternative cassette exon of the c-src gene has identified polypyrimidine tract binding protein (PTB), its neural specific homolog nPTB, hnRNP H, Fox2 and other factors as participating in this tissue-specific splicing regulation (12). In a careful study to identify alternative splicing events regulated by different members of the hnRNP family of factors, RNA interference was performed on 14 different hnRNPs. Analysis of splicing by reverse transcription–polymerase chain reaction (RT–PCR) on 56 different alternative splicing events indicated that all of the splicing factors had a regulatory role for at least one of the events. Some hnRNPs, like hnRNP K, act like global regulators having effects on many alternative splicing events (>40%), while others, such as hnRNP M, are highly specific regulators affecting <2% of the alternative splicing events studied (13). A recent study of the binding affinities of four genes of the hnRNP A/B family shows that they have a combinatorial network of interactions, where they might regulate similar populations of mRNAs (14). A recent report also shows that hnRNP L can act as a splicing enhancer and silencer (15). In many aspects, alternative splicing decisions are analogous to transcriptional initiation; multiple factors, both positive and negative, assemble onto a nucleic acid control region, and the combination of assembled factors leads to an integrated decision (which isoform to generate by splicing or whether to initiate transcription). In order to understand splicing regulation on a global level, it will be key to uncover the interactions of multiple splicing factors with any given pre-mRNA and to reveal how these interactions lead to splicing decisions.
Most of the studies of alternative splicing regulation that uncovered multiple interacting splicing factors were performed using ex vivo systems. The Caenorhabditis elegans model system provides an excellent platform from which to probe the combinatorial interactions of alternative splicing factors on target genes in animals. Regulated alternative splicing occurs in this species, and homologs of all the major vertebrate alternative splicing factors are present in the genome (16). The use of two-color alternative splicing reporter transgenes to study splicing regulation has indicated that multiple factors in the same or different families play a role in the regulation of alternative splicing in a tissue-specific and developmental manner in these worms (17–19). Genetic analysis of the regulation of the alternative splicing of the C. elegans unc-52 gene has uncovered four different splicing factor genes, mec-8, smu-1, smu-2 and sym-2, which play a role in this splicing regulation (20–22).
In our lab, we have developed a DNA microarray that can measure changes in alternative splicing in 352 alternative cassette exons. We have previously used this platform to measure changes in splicing during C. elegans development and speciation, and we have used it to uncover evidence of alternative splicing coupled to developmentally-regulated non-sense-mediated decay (23–25). In this report, we use the splicing-sensitive DNA microarrays to analyze the differences between mRNA isolated from wild-type worms and 13 different C. elegans strains carrying viable genetic defects in alternative splicing factors. Our analysis uncovers many examples of coordinated regulation of alternative splicing. Examples of splicing factors that are functionally redundant, as well as splicing factors that appear to work antagonistically, are also revealed. In addition, we identify examples in which one splicing factor functions as a suppressor of an alternative splicing event at one stage of development and as an enhancer of the same alternative splicing event at a separate stage, indicating the importance of the combinatorial effect of multiple factors on splicing.
MATERIALS AND METHODS
Strains and RNA samples
Splicing factors mutant strains: KH1125 [asd-1(yb978)] (17), VC176 [exc-7(ok370)], CB398 [mec-8(e398)] (26), VC463 [rsp-2(ok639)], RB1451 [rsp-5(ok324)], SP2230 [sym-2(mn617)] (20), CB5380 [fox-1(e2643)] (27), VC119 [ptb-1(gk113)], VC659 [hrp-1(ok963)], CB950 [unc-75(e950)] (28) and RW2306 [sup-12(st89)] (29) were obtained from the Caenorhabditis Genetics Center (CGC). Strains RB1451, VC119, VC176, VC659 and VC463 were generated by the C. elegans Reverse Genetics Core Facility at UBC, which is part of the International C. elegans Gene Knockout Consortium. Strains TM3406 [hrpf-1(tm3406)] and TM367 [rsp-6(tm367)], were generated and obtained from the National BioResource Project-C. elegans, Japan. The tm3406;mn617 double mutant strain was generated by conventional genetic crosses. F2 worms were screened using PCR analysis for those that were homozygous for both mutants. Worm samples: large quantities of mixed-stage worms were grown on egg–NGM plates with HB101 until plates were confluent; at that point worms were synchronized using 1% sodium hypochlorite and 0.5 M NaOH to isolate embryos. Embryo samples were taken after axenization of adults from mixed-stage cultures. Larval and adult stages were synchronized from embryos that we let hatch overnight in M9 buffer at room temperature. The next morning synchronized L1s were washed in fresh M9 and plated onto egg–NGM plates with HB101, and collected at the fourth larval stage. Total RNA samples were extracted with Trizol reagent (Invitrogen). mRNA was purified from total RNA using the PolyA Tract mRNA isolation system (Promega).
Splicing-sensitive microarrays and data analysis
We previously reported a DNA microarray capable of detecting changes in the isoform ratios (IR) for 352 alternative cassette exons in C. elegans (25). cDNA derived from 2 µg of purified messenger RNA per channel were labeled with Alexa Fluor dyes (555 and 647) using the SuperScript Indirect Labeling System (Invitrogen) for each of the strains used. Hybridizations were done in duplicate with dye swaps. Data were normalized, further processed and isoform ratios (IR) were calculated as described earlier (25). In brief, a positive IR ratio equals more inclusion of the cassette exon in the reference sample (N2) while a negative IR ratio means more inclusion in the experimental sample (splicing factor mutant).
Semiquantitative and quantitative RT–PCR
RT–PCR was performed using SuperScriptIII One-Step RT–PCR Kit (Invitrogen). An amount of 25 ng of mRNA from a sample representing a biological replica of the sample used for the microarray analysis were used in each reaction, and the number of PCR cycles was 27–30 depending on the specific mRNA targeted. Primer sequences are available upon request. PCR products were first analyzed using ethidium bromide-stained agarose gels and later quantified using an Agilent Bioanalyzer 2100 with the Agilent DNA 1000 kit (Agilent). AS ratios and inclusion proportions were calculated from the molar concentrations of each isoform as reported by the Bioanalyzer 2100 software (Agilent). For expression analysis of mec-8, sup-12, asd-1, rsp-2 and rsp-5 quantitative RT–PCR was performed for 45 cycles using Lightcycler DNA Master SYBRgreen I (Roche Applied Science, Pensberg, Germany) in 384-well plates using Lightcycler 480 (Roche), individual PCR amplifications were carried out in triplicates and two biological replicas were used to calculate average and standard deviations. The log2 ratios were calculated as described earlier (30), by using gpd-2 and rps-1 as reference housekeeping genes to normalize between samples. The log2 ratios represent the changes between embryos and adults, with negative values representing a downregulation of expression in adults compared to the embryo sample.
RESULTS
In order to analyze the global effects on alternative splicing for different alternative splicing factors, we employed splicing-sensitive microarrays that monitor the isoform ratios for 352 events of alternative splicing in C. elegans (25). Thirteen different strains carrying mutations in 12 alternative splicing factors and one double-mutant strain were used in this study (Table 1). These represent mutant alleles of homologs of a range of known mammalian splicing factors. These include members of the hnRNP F/H family (sym-2, hrpf-1), the SR protein family (rsp-2, rsp-5, rsp-6), hnRNP A1 (hrp-1), the Fox1/2 family (fox-1, asd-1), a muscle-specific factor (sup-12), a neural-specific ELAV homolog (exc-7), a regulator of splicing whose loss leads to mechanosensory defects (mec-8) and a homolog of PTB (ptb-1). Eight of these mutants correspond to genomic deletions that remove several exons of each splicing factor (it is assumed that many are null alleles but we have no additional data as to whether these are complete or partial loss-of-function); mec-8(e398) is an amber mutation; the asd-1, sup-12 and sym-2 alleles used here are missense mutations that were previously characterized as null alleles (17,22,31). Messenger RNA was isolated from synchronized embryos for each of the mutant strains and compared against wild-type Bristol N2 embryonic mRNA on the microarrays, with replicates used in dye-swap experiments. The number of alternative splicing events for each strain that show >2-fold changes in isoform ratios (IR) are indicated in Table 1 [See ‘Materials and Methods’ section and (23) for details in how IR was calculated].
Table 1.
Alternative splicing ratio changes in mutant strains
| Mutant | Genes with isoform | Human homolog | Mutation | References |
|---|---|---|---|---|
| Ratio changes >2-fold | ||||
| asd-1(yb978) | 3 | FOX1/2 | Missense mutation G140R | (17) |
| exc-7(ok370) | 0 | ELAV 4 | 1404-bp deletion | C. elegans Gene Knockout consortium |
| fox-1(e2643) | 30 | FOX1/2 | 1255-bp deletion | (27) |
| hrp-1(ok963) | 11 | hnRNP A1 | 843-bp deletion | C. elegans Gene Knockout consortium |
| hrpf-1(tm3406) | 1 | hnRNP F/H | 426-bp deletion | National BioResource Project, C. elegans |
| mec-8(e398) | 17 | RBPMS2 | Amber mutation Q177X | (39) |
| ptb-1(gk113) | 8 | PTB | 542-bp deletion | C. elegans Gene Knockout consortium |
| rsp-2(ok639) | 10 | SRSF4 (SRp75) | 984-bp deletion | C. elegans Gene Knockout consortium |
| rsp-5(ok324) | 13 | SRSF2 (SC35) | 1116-bp deletion | C. elegans Gene Knockout consortium |
| rsp-6(tm367) | 12 | SRSF3 (SRp20) | 395-bp deletion | National BioResource Project, C. elegans |
| sup-12(st89) | 20 | RBM24 | Missense mutation within RRM, G77E | (31) |
| sym-2(mn617) | 3 | hnRNP F/H | Missense mutation Y163N | (40) |
| unc-75(e950) | 0 | CUG-BP | 6900-bp deletion | (33) |
| hrpf-1; sym-2 | 17 | hnRNP F/H | 843-bp deletion and missense mutation | This work |
Microarray analysis of embryonic mRNA between indicated splicing factor mutant strains and N2. The number of genes on the microarray undergoing >2-fold changes in isoform ratios in the mutant strain are indicated. Human homologs of the genes are indicated, as well as the type of mutation in the C. elegans genome.
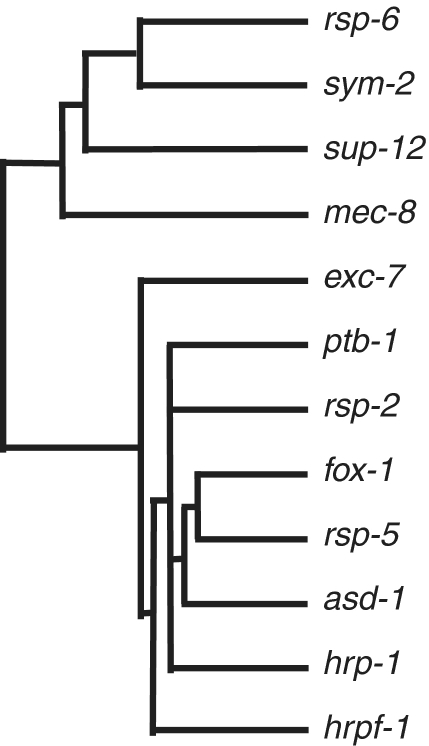
In total, we found that 134/352 (38%) events of alternative splicing measured by our microarray have at least a 2-fold change in isoform ratio in at least one mutant strain. The strain harboring a mutant allele of the splicing factor fox-1 shows the greatest number of splicing events with >2-fold changes, while a strain carrying a mutant allele of the neuro-specific exc-7 gene had no detectable alternative splicing changes at this threshold (Table 1). Supplementary Table S1 shows the isoform ratios of each gene tested on the microarray for each of the mutant strains. In order to ascertain whether there are splicing factors that have similar functions in this analysis, we performed a hierarchical clustering of the results for the 12 single splicing factor mutant strains for all the 352 genes on the microarray (Figure 1). One interesting result is that splicing factors belonging to different families cluster together (for example the SR protein rsp-5 with the Fox1/2 protein fox-1), suggesting that they are involved in the co-regulation of particular splicing events and that both are required for proper control. It is important to note that the mutation to hrp-1 is unique in this group in that homozygous mutants have very severe phenotypes and developmental delays (C. elegans Gene Knockout Consortium). As such, the strain used to grow the hrp-1 mutant contains a balancer, so that the embryos tested were a mixture of worms homozygous and heterozygous for the hrp-1 mutation. Therefore, the 11 alternative splicing events showing changes above the threshold in the hrp-1 mutant worms in Table 1 represent a minimum estimate of targets for hrp-1 alternative splicing regulation.
Figure 1.
Hierarchical clustering of splicing factors mutant strains. Isoform ratios for all 352 alternative splicing events included in the microarray were used to cluster the different mutant strains.
Several examples of redundancy in the regulation of splicing by members of the same family of factors have been reported earlier [for examples in C. elegans see (18,32)]. In our analysis in Figure 1 we noted that some members of the same family, such as fox-1 and asd-1, clustered fairly closely, indicating that they have cooperative or partially overlapping functions on similar substrates. In another interesting case we saw that pairs of proteins from the same family, the hnRNP F/H genes sym-2 and hrpf-1, were fairly distant in the clustering analysis. This could indicate that they function on a small number of distinct substrates, or that they have redundant function so that the majority of hnRNP F/H-dependent splicing events are unaffected by the loss of one family member. In order to test this, we generated an hrpf-1;sym-2 double mutant strain and observed that it was viable without any obvious phenotypic defects, similar to either allele on its own. hrpf-1 and sym-2 represent two of the three hnRNP F/H splicing factors genes present in the worm genome, the third family member is hrpf-2 for which no mutant allele is available. Our array analysis indicates that the double-mutant strain has many more changes in splicing isoform ratio at the >2-fold level when compared to either mutant alone (17 changes >2-fold for the double-mutant compared to three for the sym-2 mutant and one for the hrpf-1 mutant) (Table 1). This indicates that there are extensive alternative splicing substrates for which these two splicing factors have redundant function, and that a small number of the substrates are uniquely regulated by each. The use of the double-mutant strain for these two family members allows for the discovery of more hnRNP F/H-dependent alternative splicing events.
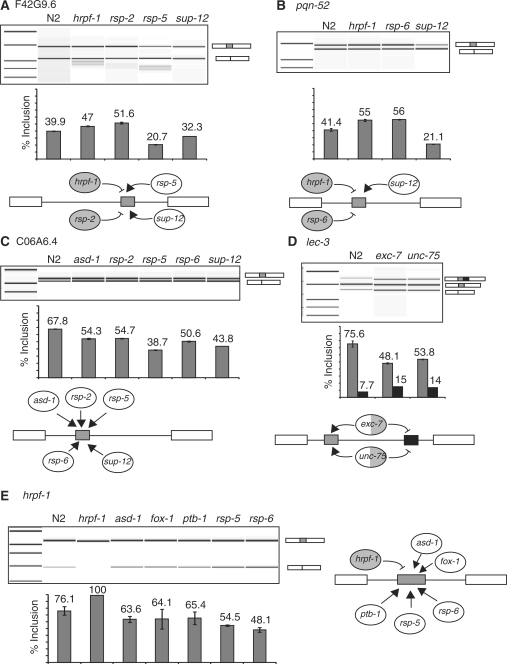
After examining the microarray data across the mutant strains, we were able to uncover examples of individual alternative splicing events that are regulated by multiple splicing factors. To aid in this analysis, we created a term called the co-regulation value; it is the sum for each gene of the positive value for all splicing isoform ratios relative to N2 from each of the mutant strains. The top 18 coordinately regulated genes that have at least one isoform ratio in one mutant change >1.5 log2 are shown in Table 2. In order to further analyze these results, we confirmed by RT–PCR the alternative splicing in wild-type and mutant strains for events with high co-regulation values for RNA samples representing biological replicates of the ones used for the microarray experiments. Figure 2 shows the RT–PCRs for F42G9.6, pqn-52, C06A6.4, lec-3 and hrpf-1. For these splicing events we performed RT–PCRs for all twelve splicing factors mutants and found a validation for the microarray predictions for many events, but found also other regulators that were not predicted by the microarray. We also found that several of these alternative splicing events which the array data suggested were regulated by multiple factors could not be validated. In these cases it was due to one of the alternative isoforms representing <5% of the final transcript; this led to a technical limitation in measuring reproducible changes of these low abundance minor isoforms by semi-quantitative RT–PCR.
Table 2.
Genes showing highest co-regulation in multiple mutant strains tested on the microarray
| Gene | Coregulation |
|---|---|
| value | |
| F42G9.6 | 10.75 |
| hrpf-1 | 7.95 |
| gcy-31 | 7.05 |
| K04H4.2 | 8.72 |
| lec-3 | 9.42 |
| egl-3 | 8.03 |
| rnp-6 | 6.03 |
| phy-2 | 6.11 |
| F11E6.1 | 6.31 |
| C06A6.4 | 6.37 |
| C06G8.3 | 6.53 |
| H14E04.2 | 6.07 |
| mbk-2 | 5.62 |
| ZC518.1 | 6.21 |
| rme-8 | 5.03 |
| pqn-52 | 4.82 |
| gsy-1 | 3.81 |
| tnt-3 | 3.59 |
The co-regulation value is the sum of the positive values of the isoform ratios (log2) for each gene over all mutant strains tested.
Figure 2.
RT–PCR validations of splicing changes for alternative splicing events with high co-regulation values; (A) F42G9.6, (B) pqn-52, (C) C06A6.4, (D) lec-3 and (E) hrpf-1. An Agilent Bioanalyzer 2100 was used to quantitate the experimental results, error bars are shown representing results from at least two different RT–PCR reactions, and an electropherogram for each experiment is shown.
In the analysis of the effects of mutations of different factors on substrates, certain themes emerge. For example, sup-12 and hrpf-1 have antagonistic effects on exon inclusion for both F42G9.6 and pqn-52 indicating that their activities counterbalance each other on these substrates (Figure 2A and B). rsp-2 and rsp-5 mutants have opposite effects on exon inclusion for F42G9.6, but similar effects on exon inclusion for C06A6.4 (Figure 2A and C). The lec-3 gene contains two alternative cassette exons, and mutation of the neural-specific factor exc-7 leads to a 2-fold increase in use of the downstream cassette exon and a 2-fold increase in the skipping of both cassette exons (Figure 2D). unc-75 is also a neural-specific RNA binding protein homologous to mammalian CELF/BRUNO alternative splicing factors (21). We analyzed an unc-75 mutant for changes in lec-3 splicing by RT–PCR and we found that, similar to mutations in exc-7, mutations in unc-75 lead to a 2-fold increase in inclusion of the downstream cassette exon. These results indicate that neural-specific factors control lec-3 alternative splicing in neurons, and that these changes are significant enough to be detected in total worm mRNA. The inclusion of the cassette exon of the splicing factor hrpf-1 is itself regulated by other splicing factors (Figure 2E). It is interesting to note that the lesion in the hrpf-1 mutant strain is a 426-bp deletion in a region of the gene upstream of the alternative cassette exon, which should lead to a non-functional transcript; this deletion also leads to constitutive inclusion of the alternative cassette exon in those transcripts. We previously demonstrated that the skipping isoform of hrpf-1 is a substrate for non-sense-mediated decay (25), so by lowering the inclusion of the cassette exons, the mutations in asd-1, fox-1, ptb-1, rsp-5 and rsp-6 lead to a decrease in the level of HRPF-1. With the exception of the effect of the deletion in hrpf-1 on its own splicing, it is important to note that we could not identify any particular alternative splicing isoform that is entirely dependent on the activity of a single alternative splicing factor. This implies that inputs from multiple factors contribute to alternative splicing decisions, but that no individual decision that we assayed for is completely dependent on a single specific factor.
Changes in splicing factor activities during development
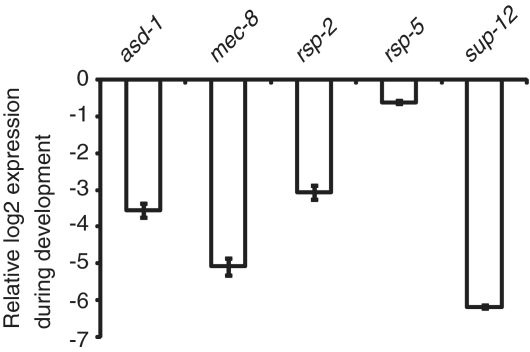
Several of the alternative splicing factors mutants that have been studied in C. elegans have been reported to show phenotypes specifically in adult worms (25,31,33,34). These splicing factors may also have a role in embryonic splicing that is without an obvious phenotype. To study their function in more detail we performed microarray experiments with adult mRNA for sup-12(st89) and mec-8(e398) mutant strains. MEC-8 is important for mechanosensory behavior in adult worms, and SUP-12 is a known muscle-specific splicing factor (26,31). We found examples of genes whose embryonic alternative splicing is different between the sup-12 mutant strain and wild-type but whose splicing is identical between these same strains in adults (i.e. F42G9.6 and pqn-52). Table 3 summarizes the results from the analysis of the effects of sup-12 and mec-8 both in embryo and adult samples (Supplementary Table S2 shows the isoform ratios of each gene tested on the microarray for each of the mutant strains). Surprisingly, there is no overlap between the top-scoring embryo and adult targets for mec-8 and sup-12. We performed quantitative RT–PCR to detect the changes in expression levels of mec-8 and sup-12 between embryos and adults (Figure 4). The changes in mec-8 (log2 = −5.1) and sup-12 (log2 = −6.2) expression during development detected by qRT–PCR together with previous reports (20,31), let us conclude that both mec-8 and sup-12 change from broad expression in embryonic cells to tissue-specific expression in adult worms. This result suggests that developmental regulation of the transcription of target pre-mRNAs for these splicing factors, or changes in the relative number of cells in which the splicing factors are expressed, may lead to changes in detection of splicing targets. Alternatively, other factors that work in combinatorial coordination with these factors to regulate splicing may undergo changes in development that affect the outcome.
Table 3.
Developmental changes in splicing regulation in mec-8(e398) and sup-12(st89) mutant strains
| Embryos | Adults | ||
|---|---|---|---|
| Gene | Exon | mec-8 | mec-8 |
| Genes with high embryo regulation in mec-8 | |||
| rnp-6 | 6 | 1.5 | 0.91 |
| ZK1127.9 | 2 | 1.8 | 0.64 |
| Y55F3AM.3 | 3 | 2.1 | 0.35 |
| lin-10 | 6 | 1.5 | 0.02 |
| lat-1 | 3 | 1.9 | −0.44 |
| dct-17 | 11 | 1.7 | 0.00 |
| unc-53 | 17 | −2.2 | 0.32 |
| sox-2 | 2 | −1.8 | 0.32 |
| F55C12.1 | 2 | −1.7 | −0.46 |
| nlp-18 | 2 | −1.6 | 0.25 |
| Genes with high embryo regulation in sup-12 | |||
| F42G9.6 | 7 | −2.3 | −0.04 |
| phy-2 | 10 | −1.6 | −0.03 |
| gsy-1 | 5 | −1.6 | −0.26 |
| Genes with high adult regulation in mec-8 | |||
| unc-43 | 10 | −0.3 | 2.52 |
| ccch-1 | 3 | 0.3 | 2.35 |
| pqn-70 | 3 | −0.3 | 2.16 |
| ret-1 | 7 | −0.1 | 2.04 |
| gsy-1 | 5 | 0.9 | 2.02 |
| clp-1 | 4 | −0.1 | 1.67 |
| Y97E10AR.2 | 3 | 0.2 | 1.56 |
| nhx-5 | 17 | −1.0 | −1.52 |
| unc-2 | 18 | 0.7 | −1.57 |
| ketn-1 | 16 | 0.1 | −1.62 |
| gip-1 | 6 | 0.1 | −1.76 |
| F28E10.1 | 8 | 0.7 | −1.98 |
| hrpf-1 | 5 | 0.4 | −2.51 |
| unc-89 | 19 | 0.1 | −2.76 |
Splicing events with an isoform ratio ≥ 1.5 (log2) in at least one of the two stages (embryo or adults) for either mec-8 or sup-12. Note that for the sup-12 mutant strain adult RNA we could detect no isoform ratios >1.5 compared to N2 adults.
Figure 4.
Developmental changes in expression levels for splicing factors. Detection by quantitative RT–PCR of expression changes during development for five splicing factors. Housekeeping genes gpd-2 and rps-1 levels were used to normalize RNA levels between embryos and adults, and the log2 ratio of the comparison for each factor is displayed.
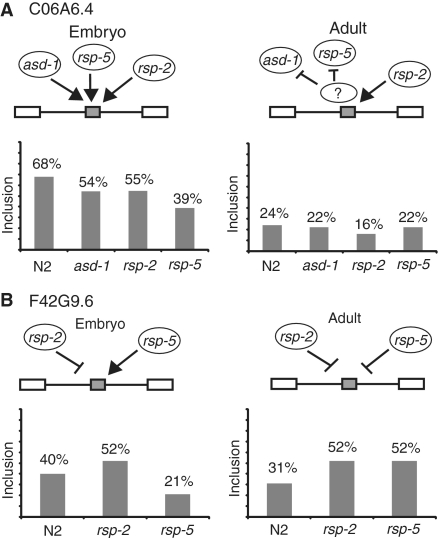
This change of targets for splicing regulation by specific factors during development prompted us to look at specific targets and ask whether there are changes in the factors that regulate their splicing during development. We measured the adult splicing regulation for F42G9.6 and C06A6.4, two genes with high co-regulation values in embryos as seen in Table 2. We performed RT–PCRs on mRNA samples from adult worms for these genes from wild-type N2 as well as asd-1, rsp-2 and rsp-5 mutant strains. The isoform proportions for these two genes in wild-type strains change during development (Figure 3). Several splicing factors that have an important role in the splicing regulation in the embryonic stage, lose this regulation in the adult stage. For example, rsp-5 mutants have a dramatic effect on C06A6.4 splicing in embryos but no effect on the splicing of this gene in adults (Figure 3A). Unexpectedly we identified a splicing factor that changes its role on a specific substrate from an enhancer in embryos to a repressor in adults. In the rsp-5 mutant strain there is a 2-fold decrease in F42G9.6 exon inclusion in embryos but this same strain shows a 1.7-fold increase in inclusion of this exon in adults. While there have been factors shown to act as a repressor or an enhancer of splicing on different substrates, to our knowledge this is the first example of an alternative splicing factor that acts as either a repressor or an enhancer of the same splicing event depending on the state of development. Expression levels of these splicing factors rule out that this change in splicing regulation could be due to the absence of rsp-5 in adult tissues (Figure 4). We detected by quantitative RT–PCR the levels of expression for asd-1, rsp-2 and rsp-5 and found that while asd-1 and rsp-2 have changes in expression between embryos and adults, log2 of −3.6 and −3.1 respectively, when compared to reference housekeeping genes, rsp-5 maintains similar levels of expression (log2 = −0.6). This together with previous reports that shows that rsp-5 is ubiquitously present in all nuclei of somatic cells of adult worms (35), shows that rsp-5 is a constitutively expressed splicing factor.
Figure 3.
Developmental changes in splicing regulation. RT–PCRs with embryo and adult samples were performed for C06A6.4 (A) and F42G9.6 (B) using mRNA samples from splicing factor mutant strains identified as regulators of embryonic splicing.
DISCUSSION
In this study, we report the splicing changes for 352 alternative cassette exons that occur in strains carrying 12 different viable mutations in alternative splicing factors. These viable mutants represent close to 10% of the 151 RNA Recognition Motif (RRM) containing genes in C. elegans (36). Close to 40% of the alternative cassette exons studied have splicing changes >2-fold in the presence of at least one of the viable mutations. One should keep in mind that we are measuring splicing changes in RNA extracted from whole worms, yet several of the alternative splicing factors whose mutants we studied are expressed in only a subset of tissues. Therefore, it is possible that many more of the events that were unchanged in our study are indeed regulated by the splicing factors targeted, but that the changes in splicing are happening in just a few cells so when we extract RNAs from whole worms these changes are masked by the transcripts present in other tissues. For example, exc-7 is only expressed in neurons, and in this study we were only able to identify one substrate, lec-3, whose splicing is regulated by exc-7. This number of exc-7 substrates would likely increase if we were to perform the microarray analysis with mRNA isolated specifically from neurons. Therefore, the number of splicing targets for any given factor in this study represents a minimum estimate. As data is not available for many of the mutant strains as to the number of times the alleles were outcrossed back to N2 wild-type, it is formally possible that some of the changes in alternative splicing we observe in the different strains may derive from mutations in the strains that are harbored outside of the splicing factor. However, the fox-1 mutant strain that showed the highest number of splicing changes in embryos (Table 1) has been outcrossed back to N2 less than five times. This is consistent with all those changes in splicing being due to the fox-1 mutation. In addition, we did not observe differentially-sized RT–PCR products in our validations, consistent with a lack of new cis-mutations in those targets. There is still a possibility that a small number of changes in Table 1 may derive from additional mutations in these strains outside of the documented splicing factor alleles, however our results indicate if these exist they would at best account for only a very small fraction of the changes we observe.
One advantage of this study is that it allowed us to identify and confirm several new examples of coordinated regulation of alternative splicing by multiple splicing factors (Table 2, Figures 2 and 3). Combinatorial regulation of alternative splicing has been identified in several systems using biochemical and genetic approaches (14,18,37,38). Previous work on the combinatorial regulation of hnRNP A/B proteins on splicing patterns concluded that each splicing factor studied has specific affinities for overlapping populations of transcripts (14). The assembly of multiple factors onto the introns and exons of an alternative cassette exon must allow for control of the eventual recruitment of the splicing machinery to that cassette exon. However, very little is understood about the interactions between the primary RNA-binding alternative splicing factors and the spliceosome. Identifying multiple examples of combinatorial alternative splicing regulation will help in establishing models for the interactions that lead to splicing decisions.
An unexpected result was the finding that the loss of a particular splicing factor has different effects on the splicing of the same substrate at different stages of development (Figure 3). We found evidence that some factors act to regulate splicing at one stage but not another. This might be due to the factor and the substrate pre-mRNA being expressed in the same cells at one stage of development but not at another. One unexpected result was the observation that mutation of the SR protein rsp-5, one of two C. elegans homologs of the mammalian splicing factor SC35, leads to opposite effects on splicing of the same substrate at different stages of development. It is an enhancer of F49G2.6 alternative cassette exon usage in embryos and a silencer of the splicing of the same exon in adults. This points to the possibility that rsp-5 alternative splicing activity is modulated by additional, still unidentified regulators that are present in one developmental stage but not the other. This possibility is further supported by the constant expression levels of rsp-5 during development as shown by the qRT–PCR experiments in Figure 4. That we can detect a constitutive splicing factor with important yet different effects on the splicing regulation of a specific substrate at different stages of development argues that the developmental regulation is modulated by stage-specific splicing factors that cooperate with rsp-5 to regulate splicing.
Functional redundancy on specific substrates by members of splicing factor families has been shown to occur both in vitro and in vivo, and in these cases it is important to target multiple family members in order to see a phenotype (18,32). We observed this phenomenon by targeting two different hnRNP F/H family members in a double-mutant strain (Table 1). This double-mutant strain remained viable, but this may be due to additional functional redundancy with a third hnRNP F/H family member in C. elegans, hrpf-2. The double mutant strain allowed us to uncover many more affected substrates than mutation of either family member alone. The logical next step for the current work is the study at a global level of the effects on particular alternative splicing events of the targeting of several splicing factors at the same time. As long as these strains are viable, this should aid in the discovery of new networks of regulation that are not detectable when just one factor at a time is mutated. Our results argue the importance of studying the combinatorial effect of multiple factors on specific splicing events in order to provide more information for the deconvolution of the splicing code.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Institutes of Health (R01-GM061646 to A.M.Z.); National Institutes of Health-funded UCSC Initiative for Maximizing Student Diversity (IMSD) Program (R25-GM058903 to J.E.); UCSC Minority Access to Research Careers Program (T34-GM007910 to P.M.). Funding for open access charge: National Institutes of Health (R01-GM061646 to A.M.Z.).
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors thank Lily Shiue and Manny Ares for establishing the UCSC microarray facility and for advice and assistance with its usage. The authors thank Annick Lamb for help growing worm strains. The authors also thank the Caenorhabditis Genetics Center, the International C. elegans Gene Knockout Consortium and the National BioResource Project-C. elegans for kindly providing strain
REFERENCES
- 1.Martinez-Contreras R, Cloutier P, Shkreta L, Fisette JF, Revil T, Chabot B. hnRNP proteins and splicing control. Adv. Exp. Med. Biol. 2007;623:123–147. doi: 10.1007/978-0-387-77374-2_8. [DOI] [PubMed] [Google Scholar]
- 2.Lin S, Fu XD. SR proteins and related factors in alternative splicing. Adv. Exp. Med. Biol. 2007;623:107–122. doi: 10.1007/978-0-387-77374-2_7. [DOI] [PubMed] [Google Scholar]
- 3.Kanopka A, Muhlemann O, Akusjarvi G. Inhibition by SR proteins of splicing of a regulated adenovirus pre-mRNA. Nature. 1996;381:535–538. doi: 10.1038/381535a0. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Contreras R, Fisette JF, Nasim FU, Madden R, Cordeau M, Chabot B. Intronic binding sites for hnRNP A/B and hnRNP F/H proteins stimulate pre-mRNA splicing. PLoS Biol. 2006;4:e21. doi: 10.1371/journal.pbio.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Dov C, Hartmann B, Lundgren J, Valcarcel J. Genome-wide analysis of alternative pre-mRNA splicing. J. Biol. Chem. 2008;283:1229–1233. doi: 10.1074/jbc.R700033200. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Burge CB. Splicing regulation: from a parts list of regulatory elements to an integrated splicing code. RNA. 2008;14:802–813. doi: 10.1261/rna.876308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463:457–463. doi: 10.1038/nature08909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barash Y, Calarco JA, Gao W, Pan Q, Wang X, Shai O, Blencowe BJ, Frey BJ. Deciphering the splicing code. Nature. 465:53–59. doi: 10.1038/nature09000. [DOI] [PubMed] [Google Scholar]
- 9.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–368. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clower CV, Chatterjee D, Wang Z, Cantley LC, Vander Heiden MG, Krainer AR. The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc. Natl Acad. Sci. USA. 2010;107:1894–1899. doi: 10.1073/pnas.0914845107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisette JF, Toutant J, Dugre-Brisson S, Desgroseillers L, Chabot B. hnRNP A1 and hnRNP H can collaborate to modulate 5' splice site selection. RNA. 16:228–238. doi: 10.1261/rna.1890310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Underwood JG, Boutz PL, Dougherty JD, Stoilov P, Black DL. Homologues of the Caenorhabditis elegans Fox-1 protein are neuronal splicing regulators in mammals. Mol. Cell. Biol. 2005;25:10005–10016. doi: 10.1128/MCB.25.22.10005-10016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venables JP, Koh CS, Froehlich U, Lapointe E, Couture S, Inkel L, Bramard A, Paquet ER, Watier V, Durand M, et al. Multiple and specific mRNA processing targets for the major human hnRNP proteins. Mol. Cell. Biol. 2008;28:6033–6043. doi: 10.1128/MCB.00726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanchette M, Green RE, MacArthur S, Brooks AN, Brenner SE, Eisen MB, Rio DC. Genome-wide analysis of alternative pre-mRNA splicing and RNA-binding specificities of the Drosophila hnRNP A/B family members. Mol. Cell. 2009;33:438–449. doi: 10.1016/j.molcel.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motta-Mena LB, Heyd F, Lynch KW. Context-dependent regulatory mechanism of the splicing factor hnRNP L. Mol. Cell. 37:223–234. doi: 10.1016/j.molcel.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahler AM. Alternative splicing in C. elegans. 2005 doi: 10.1895/wormbook.1.31.1. In The C. elegans Research Community (ed.), WormBook, doi/10.1895/wormbook.1.31.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroyanagi H, Kobayashi T, Mitani S, Hagiwara M. Transgenic alternative-splicing reporters reveal tissue-specific expression profiles and regulation mechanisms in vivo. Nat. Methods. 2006;3:909–915. doi: 10.1038/nmeth944. [DOI] [PubMed] [Google Scholar]
- 18.Kuroyanagi H, Ohno G, Mitani S, Hagiwara M. The Fox-1 family and SUP-12 coordinately regulate tissue-specific alternative splicing in vivo. Mol. Cell. Biol. 2007;27:8612–8621. doi: 10.1128/MCB.01508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohno G, Hagiwara M, Kuroyanagi H. STAR family RNA-binding protein ASD-2 regulates developmental switching of mutually exclusive alternative splicing in vivo. Genes Dev. 2008;22:360–374. doi: 10.1101/gad.1620608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spike CA, Davies AG, Shaw JE, Herman RK. MEC-8 regulates alternative splicing of unc-52 transcripts in C. elegans hypodermal cells. Development. 2002;129:4999–5008. doi: 10.1242/dev.129.21.4999. [DOI] [PubMed] [Google Scholar]
- 21.Spartz AK, Herman RK, Shaw JE. SMU-2 and SMU-1, Caenorhabditis elegans homologs of mammalian spliceosome-associated proteins RED and fSAP57, work together to affect splice site choice. Mol. Cell. Biol. 2004;24:6811–6823. doi: 10.1128/MCB.24.15.6811-6823.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies AG, Spike CA, Shaw JE, Herman RK. Functional overlap between the mec-8 gene and five sym genes in Caenorhabditis elegans. Genetics. 1999;153:117–134. doi: 10.1093/genetics/153.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barberan-Soler S, Lambert NJ, Zahler AM. Global analysis of alternative splicing uncovers developmental regulation of nonsense-mediated decay in C. elegans. RNA. 2009;15:1652–1660. doi: 10.1261/rna.1711109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barberan-Soler S, Zahler AM. Alternative splicing and the steady-state ratios of mRNA isoforms generated by it are under strong stabilizing selection in Caenorhabditis elegans. Mol. Biol. Evol. 2008;25:2431–2437. doi: 10.1093/molbev/msn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barberan-Soler S, Zahler AM. Alternative splicing regulation during C. elegans development: splicing factors as regulated targets. PLoS Genet. 2008;4:e1000001. doi: 10.1371/journal.pgen.1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalfie M, Sulston J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 1981;82:358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- 27.Skipper M, Milne CA, Hodgkin J. Genetic and molecular analysis of fox-1, a numerator element involved in Caenorhabditis elegans primary sex determination. Genetics. 1999;151:617–631. doi: 10.1093/genetics/151.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson P, Brenner S. A selection for myosin heavy chain mutants in the nematode Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 1984;81:4470–4474. doi: 10.1073/pnas.81.14.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francis GR, Waterston RH. Muscle organization in Caenorhabditis elegans: localization of proteins implicated in thin filament attachment and I-band organization. J. Cell. Biol. 1985;101:1532–1549. doi: 10.1083/jcb.101.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anyanful A, Ono K, Johnsen RC, Ly H, Jensen V, Baillie DL, Ono S. The RNA-binding protein SUP-12 controls muscle-specific splicing of the ADF/cofilin pre-mRNA in C. elegans. J. Cell. Biol. 2004;167:639–647. doi: 10.1083/jcb.200407085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longman D, Johnstone IL, Caceres JF. Functional characterization of SR and SR-related genes in Caenorhabditis elegans. EMBO J. 2000;19:1625–1637. doi: 10.1093/emboj/19.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loria PM, Duke A, Rand JB, Hobert O. Two neuronal, nuclear-localized RNA binding proteins involved in synaptic transmission. Curr. Biol. 2003;13:1317–1323. doi: 10.1016/s0960-9822(03)00532-3. [DOI] [PubMed] [Google Scholar]
- 34.Lundquist EA, Herman RK. The mec-8 gene of Caenorhabditis elegans affects muscle and sensory neuron function and interacts with three other genes: unc-52, smu-1 and smu-2. Genetics. 1994;138:83–101. doi: 10.1093/genetics/138.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawano T, Fujita M, Sakamoto H. Unique and redundant functions of SR proteins, a conserved family of splicing factors, in Caenorhabditis elegans development. Mech. Dev. 2000;95:67–76. doi: 10.1016/s0925-4773(00)00339-7. [DOI] [PubMed] [Google Scholar]
- 36.The Universal Protein Resource. (UniProt) in 2010. Nucleic Acids Res. 38:D142–D148. doi: 10.1093/nar/gkp846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian M, Maniatis T. Positive control of pre-mRNA splicing in vitro. Science. 1992;256:237–240. doi: 10.1126/science.1566072. [DOI] [PubMed] [Google Scholar]
- 38.Rooke N, Markovtsov V, Cagavi E, Black DL. Roles for SR proteins and hnRNP A1 in the regulation of c-src exon N1. Mol. Cell. Biol. 2003;23:1874–1884. doi: 10.1128/MCB.23.6.1874-1884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundquist EA, Herman RK, Rogalski TM, Mullen GP, Moerman DG, Shaw JE. The mec-8 gene of C. elegans encodes a protein with two RNA recognition motifs and regulates alternative splicing of unc-52 transcripts. Development. 1996;122:1601–1610. doi: 10.1242/dev.122.5.1601. [DOI] [PubMed] [Google Scholar]
- 40.Yochem J, Bell LR, Herman RK. The identities of sym-2, sym-3 and sym-4, three genes that are synthetically lethal with mec-8 in Caenorhabditis elegans. Genetics. 2004;168:1293–1306. doi: 10.1534/genetics.104.029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.