Abstract
Bacterial multiplication in the lung associated with murine Sendai virus pneumonia is caused by virus-induced defects in pulmonary bactericidal mechanisms. The nature of this effect has been studied in animals immunized against the challenge bacteria. Mice were immunized against Proteus mirabilis by intraperitoneal inoculation and by aerosol inhalation. After the development of immunity, mice were infected aerogenically with 104 TCID50 of Sendai virus. 7 days later, during the height of the bronchial inflammation and pulmonary consolidation, the mice were challenged with an aerosol of viable 35S-labeled Proteus mirabilis or 32P-labeled Staphylococcus aureus.
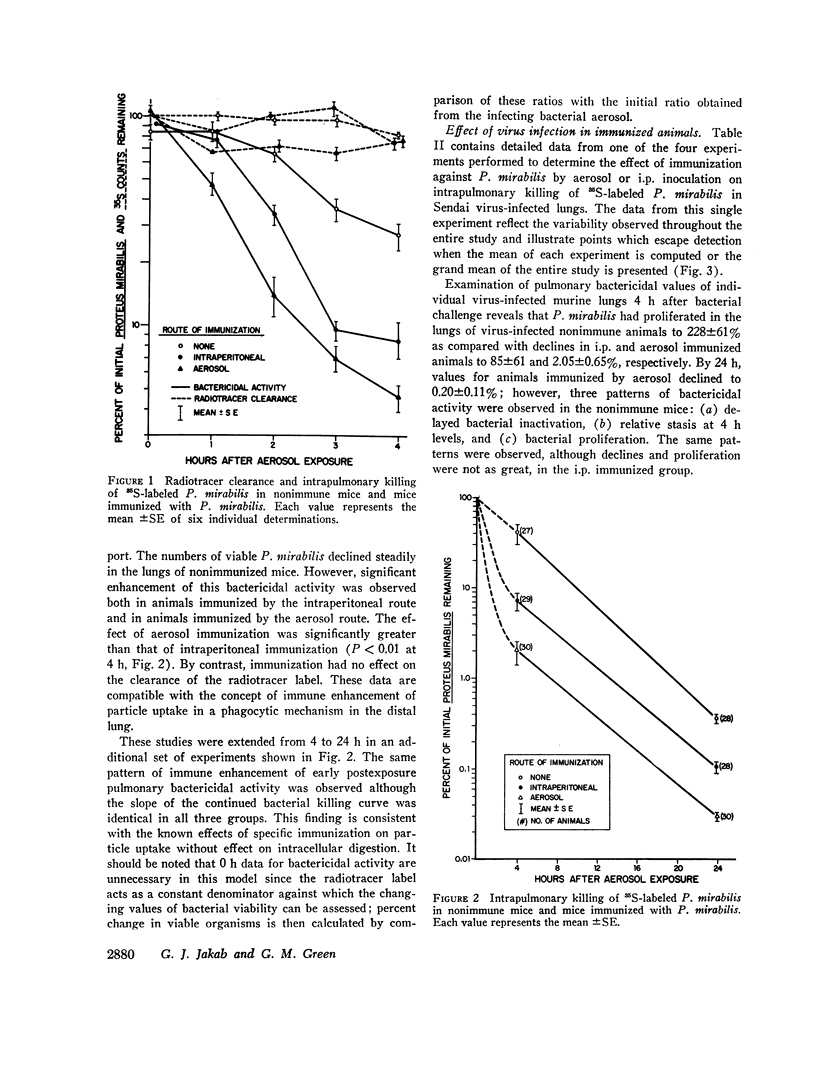
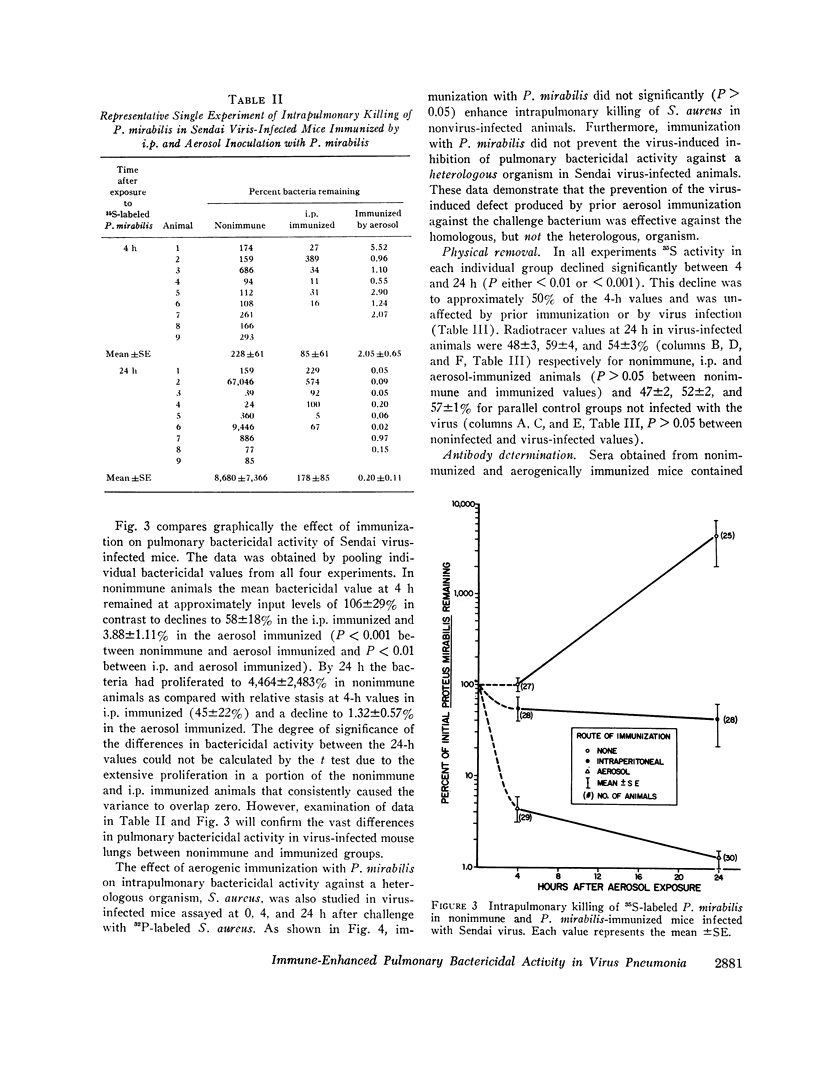
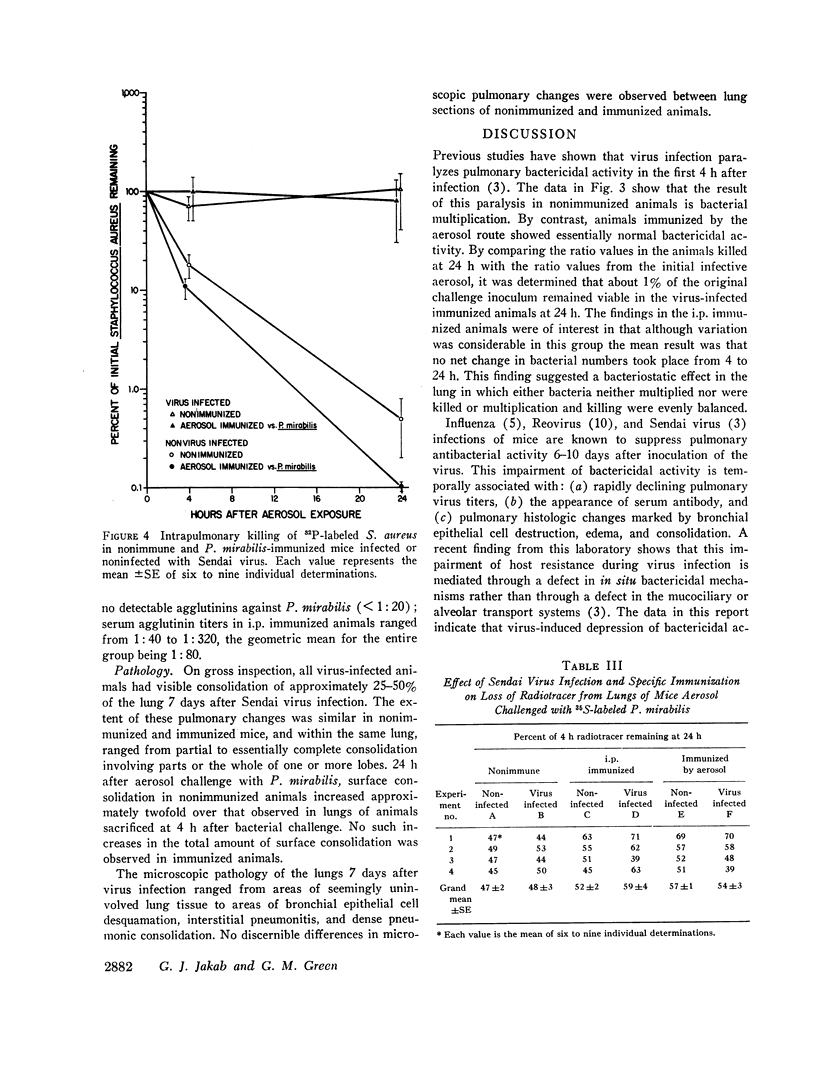
Nonimmunized virus-infected animals showed marked impairment of pulmonary bactericidal activity with subsequent multiplication of the bacterial strain in the case of Proteus mirabilis. Immunized nonvirus-infected animals showed enhancement of pulmonary bactericidal activity for the homologous and heterologous strains in comparison with nonimmunized animals. Virus-infected animals immunized by aerosol showed enhanced bactericidal activity against the homologous but not the heterologous bacterial strain. Neither virus infection nor immunization had a significant effect on the transport of particles in the lung. The data demonstrated that the bacterial multiplication associated with the virus pneumonia was prevented by preceding immunization against the homologous challenge organism. The data suggest a mechanism for controlling bacterial multiplication associated with virus pneumonias.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo B., Myrvik Q. N. Migratory response of granulomatous alveolar cells from BCG-sensitized rabbits. J Immunol. 1970 Jul;105(1):227–237. [PubMed] [Google Scholar]
- Green G. M., Goldstein E. A method for quantitating intrapulmonary bacterial inactivation in individual animals. J Lab Clin Med. 1966 Oct;68(4):669–677. [PubMed] [Google Scholar]
- Green L. H., Green G. M. Direct method for determining the viability of a freshly generated mixed bacterial aerosol. Appl Microbiol. 1968 Jan;16(1):78–81. doi: 10.1128/am.16.1.78-81.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Waldman R. H. Cell-mediated immunity shown by lymphocytes from the respiratory tract. Science. 1970 Aug 14;169(3946):696–697. doi: 10.1126/science.169.3946.696. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. The effect of Sendai virus infection on bactericidal and transport mechanisms of the murine lung. J Clin Invest. 1972 Aug;51(8):1989–1998. doi: 10.1172/JCI107005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. O., Green G. M., Tilles J. G., Kass E. H., Finland M. Effect of intranasal reovirus infection on antibacterial activity of mouse lung. J Infect Dis. 1969 Jan;119(1):43–50. doi: 10.1093/infdis/119.1.43. [DOI] [PubMed] [Google Scholar]
- Loosli C. G. Synergism between respiratory viruses and bacteria. Yale J Biol Med. 1968 Apr-Jun;40(5-6):522–540. [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. The phagocytosis and inactivation of staphylococci by macrophages of normal rabbits. J Exp Med. 1960 Jul 1;112:35–53. doi: 10.1084/jem.112.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The J. Burns Amberson LECTURE The induction and expression of cell-mediated hypersensitivity in the lung. Am Rev Respir Dis. 1971 Dec;104(6):813–828. doi: 10.1164/arrd.1971.104.6.813. [DOI] [PubMed] [Google Scholar]
- Maxwell K. W., Marcus S. Phagocytosis and intracellular fate of Mycobacterium tuberculosis: in vitro studies with guinea pig peritoneal and alveolar mononuclear phagocytes. J Immunol. 1968 Jul;101(1):176–182. [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. I. Analysis of protein and lipids in bronchial secretions and antibody responses after vaccination with pseudomonas aeruginosa. J Immunol. 1973 Aug;111(2):358–368. [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. II. Interaction of respiratory antibodies with Pseudomonas aeruginosa and alveolar macrophages. J Immunol. 1973 Aug;111(2):369–380. [PubMed] [Google Scholar]
- Smith C. B., Bellanti J. A., Chanock R. M. Immunoglobulins in serum and nasal secretions following infection with type 1 parainfluenza virus and injection of inactivated vaccines. J Immunol. 1967 Jul;99(1):133–141. [PubMed] [Google Scholar]
- Smith C. B., Purcell R. H., Bellanti J. A., Chanock R. M. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966 Nov 24;275(21):1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Henney C. S. Cell-mediated immunity and antibody responses in the respiratory tract after local and systemic immunization. J Exp Med. 1971 Aug 1;134(2):482–494. doi: 10.1084/jem.134.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Spencer C. S., Johnson J. E., 3rd Respiratory and systemic cellular and humoral immune responses to influenza virus vaccine administered parenterally or by nose drops. Cell Immunol. 1972 Feb;3(2):294–300. doi: 10.1016/0008-8749(72)90168-2. [DOI] [PubMed] [Google Scholar]
- Wigley F. M., Fruchtman M. H., Waldman R. H. Aerosol immunization of humans with inactivated parainfluenza type 2 vaccine. N Engl J Med. 1970 Dec 3;283(23):1250–1253. doi: 10.1056/NEJM197012032832303. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Anacker R. L., Ribi E. Macrophage Migration Inhibition Studies with Cells from Mice Vaccinated with Cell Walls of Mycobacterium bovis BCG: Relationship Between Inhibitory Activity of Lung Cells and Resistance to Airborne Challenge with Mycobacterium tuberculosis H37Rv. Infect Immun. 1970 Jun;1(6):595–599. doi: 10.1128/iai.1.6.595-599.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


