Abstract
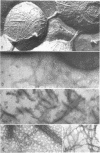
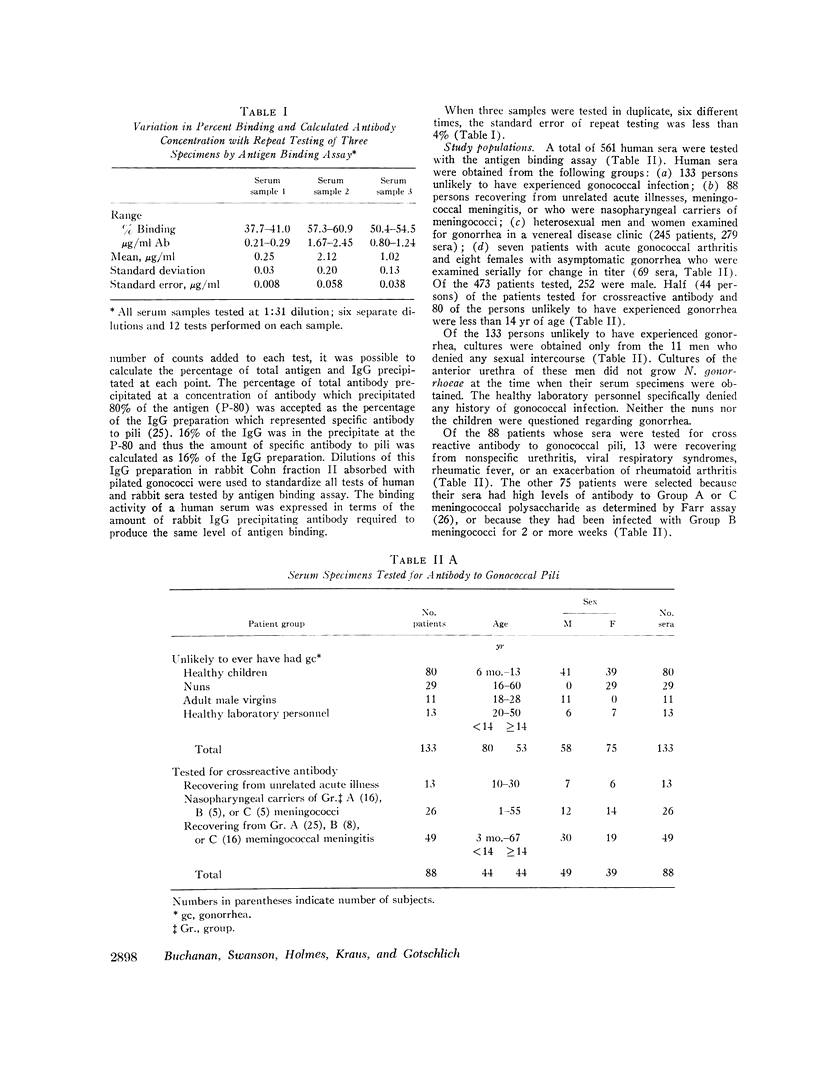
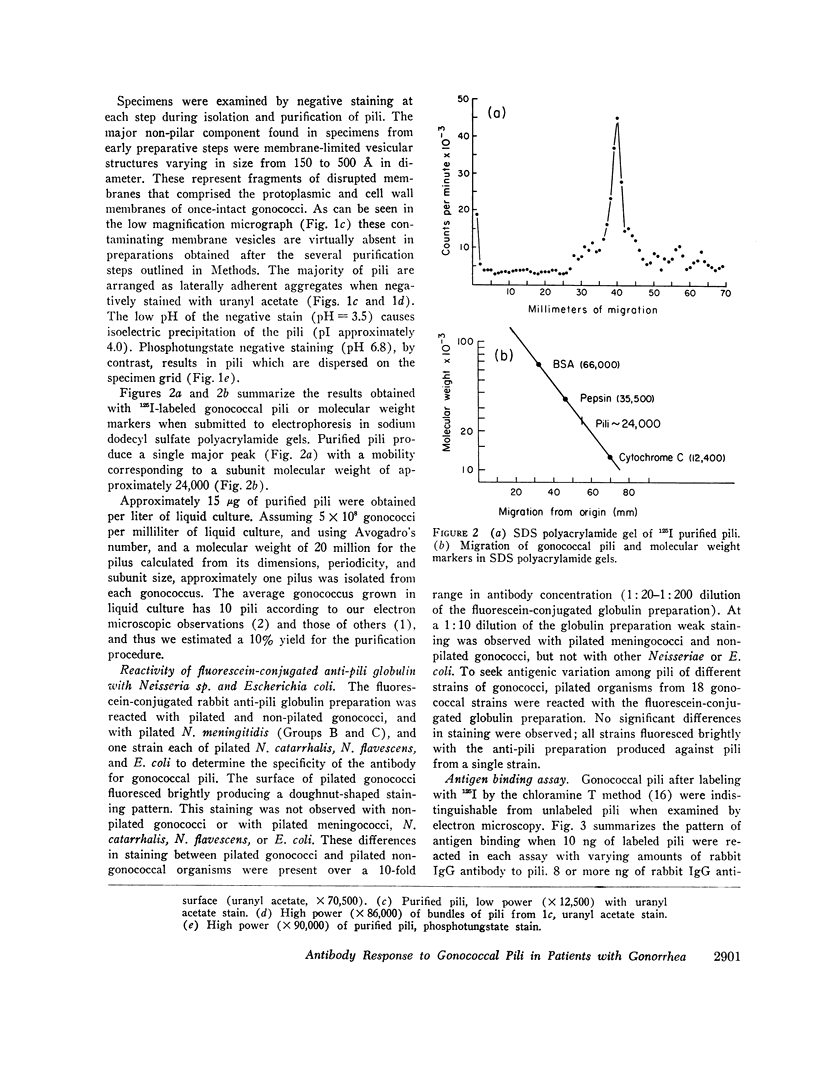
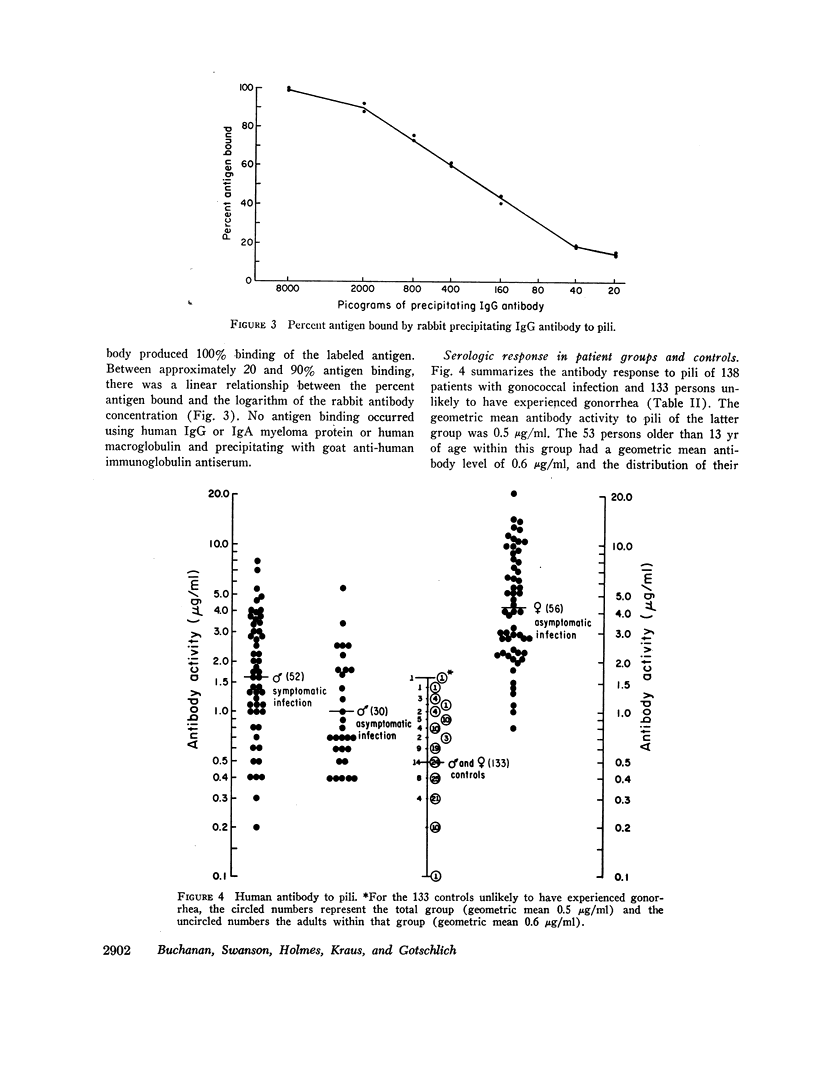
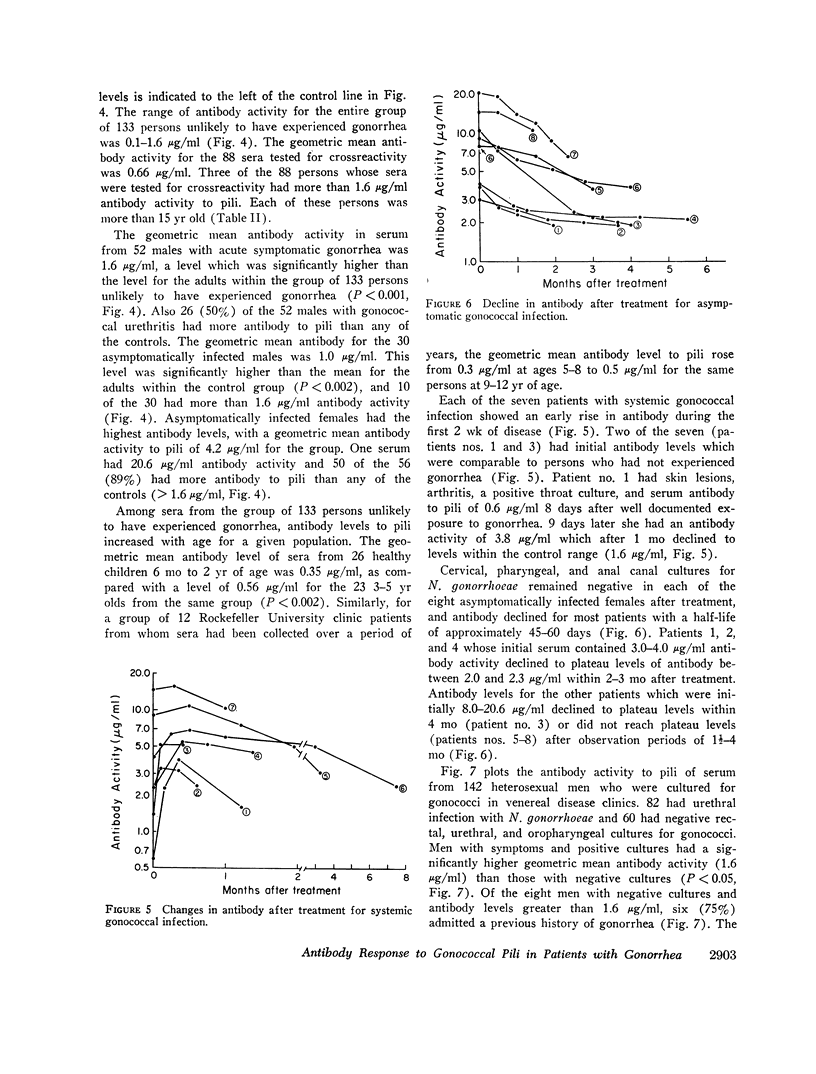
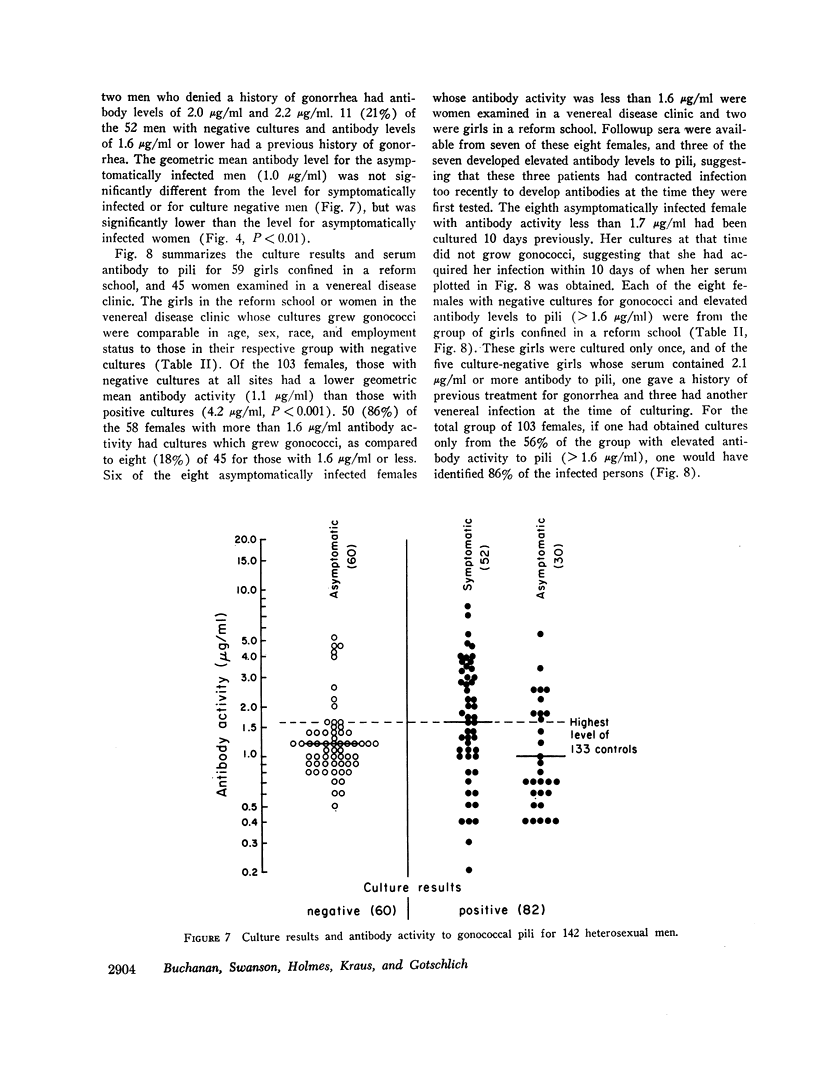
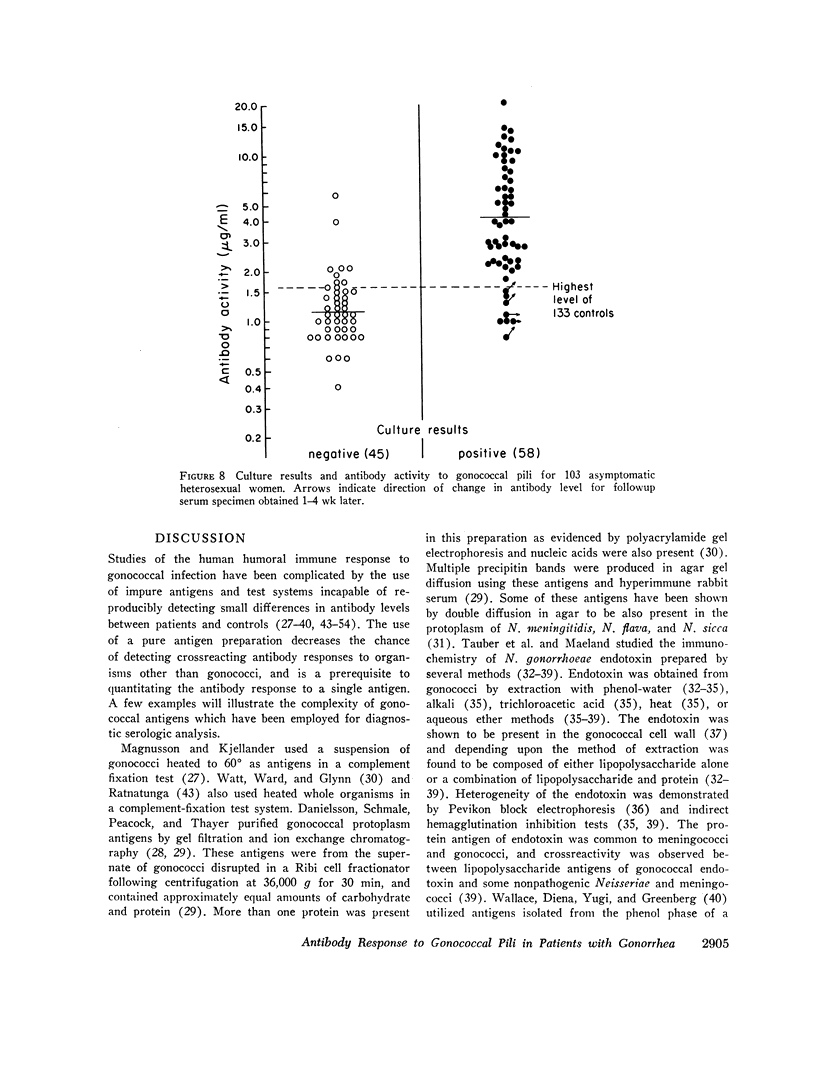
Gonococcal pili, pure by the criteria of electron microscopic examination and polyacrylamide gel electrophoresis in sodium dodecyl sulfate, have been prepared by repeated cycles of precipitation with 0.1 M MgCl2, followed by dissolution in 0.01 M Tris pH 8, 0.01 M NaN3. Using a fluorescein-conjugated antibody prepared to pili from a single strain, pilar antigen(s) was found to be present in each of 18 strains of gonococci tested, and absent from strains of pilated meningococci, nonpathogenic Neisseria sp., and Escherichia coli. Purified pili, labeled with 125I were used in an antigen binding assay to quantitatively measure antibody to pili in rabbit sera and in 561 human sera. The range of antibody activity for 133 persons unlikely to have experienced gonorrhea was 0.1-1.6 μg/ml with a geometric mean of 0.5 μg/ml. This geometric mean antibody activity was significantly lower than the geometric mean for asymptomatically infected males (1.0 μg/ml, P < 0.002), males with symptomatic gonococcal anterior urethritis (1.6 μg/ml, P < 0.001), or asymptomatically infected females (4.2 μg/ml, P < 0.001). Antibody appeared in elevated levels (> 1.6 μg/ml) 2-3 wk after infection and returned toward control levels 1 or more months after treatment. Antibody levels higher than 1.6 μg/ml were found in 26 (50%) of 52 males with gonococcal anterior urethritis, in 10 (33%) of 30 males with asymptomatic urethral infection and in 50 (89%) of 56 asymptomatically infected females. In a high-risk group of 103 females for whom culture results and antibody to pili were compared, 58 (57%) had elevated antibody levels to pili and 86% of the infected females were within this seropositive group. The antigen binding assay may provide a means to detect asymptomatic gonococcal infection in women.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainbender E., Weisinger R. B., Hevizy M., Hodes H. L. Difference in the immunoglobulin class of polioantibody in the serum of men and women. J Immunol. 1968 Jul;101(1):92–98. [PubMed] [Google Scholar]
- BROWN L., BROWN B. C., WALSH M. J., PIRKLE C. I. URETHRITIS IN MALES PRODUCED BY NEISSERIA GONORRHOEAE FROM ASYMPTOMATIC FEMALES. JAMA. 1963 Oct 12;186:153–155. doi: 10.1001/jama.1963.63710020016023b. [DOI] [PubMed] [Google Scholar]
- Barr J., Danielsson D. Septic gonococcal dermatitis. Br Med J. 1971 Feb 27;1(5747):482–485. doi: 10.1136/bmj.1.5747.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Gotschlich E. C. Studies on gonococcus infection. 3. Correlation of gonococcal colony morphology with infectivity for the chick embryo. J Exp Med. 1973 Jan 1;137(1):196–200. doi: 10.1084/jem.137.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko C. W., Nair G. M. Sero-diagnosis of gonorrhoea with a microprecipitin test using a lipopolysaccharide antigen from N. gonorrhoeae. Br J Vener Dis. 1969 Mar;45(1):33–39. doi: 10.1136/sti.45.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. R., Kellogg D. S., Jr, Norins L. C. Serum antibody response in experimental human gonorrhoea. Immunoglobulins G, A, and M. Br J Vener Dis. 1969 Dec;45(4):325–327. doi: 10.1136/sti.45.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. R. Natural and immune human antibodies reactive with antigens of virulent Neisseria gonorrhoeae: immunoglobulins G, M, And A. J Bacteriol. 1967 Jul;94(1):141–148. doi: 10.1128/jb.94.1.141-148.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIELSSON D. THE DEMONSTRATION OF N. GONORRHOEAE WITH THE AID OF FLUORESCENT ANTIBODIES. 3. STUDIES BY IMMUNOFLUORESCENCE AND DOUBLE DIFFUSION-IN-GEL TECHNIQUE ON THE ANTIGENIC RELATIONSHIP BETWEEN STRAINS OF N. GONORRHOEAE. Acta Pathol Microbiol Scand. 1965;64:243–266. doi: 10.1111/apm.1965.64.2.243. [DOI] [PubMed] [Google Scholar]
- DANIELSSON D. THE DEMONSTRATION OF N. GONORRHOEAE WITH THE AID OF FLUORESCENT ANTIBODIES. 4. STUDIES BY IMMUNOFLUORESCENCE AND DOUBLE DIFFUSION-IN-GEL TECHNIQUE ON THE ANTIGENIC RELATIONSHIP BETWEEN N. GONORRHOEAE AND OTHER NEISSERIA STRAINS. Acta Pathol Microbiol Scand. 1965;64:267–276. doi: 10.1111/apm.1965.64.2.267. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Danielsson D. G., Schmale J. D., Peacock W. L., Jr, Thayer J. D. Antigens of Neisseria gonorrhoeae: characterization by gel filtration, complement fixation, and agar-gel diffusion of antigens of gonococcal protoplasm. J Bacteriol. 1969 Mar;97(3):1012–1017. doi: 10.1128/jb.97.3.1012-1017.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Frantz I. D. Growth Requirements of the Meningococcus. J Bacteriol. 1942 Jun;43(6):757–761. doi: 10.1128/jb.43.6.757-761.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Ward M. E. Nature and Heterogeneity of the Antigens of Neisseria gonorrhoeae Involved in the Serum Bactericidal Reaction. Infect Immun. 1970 Aug;2(2):162–168. doi: 10.1128/iai.2.2.162-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C. A simplification of the radioactive antigen-binding test by a double label technique. J Immunol. 1971 Sep;107(3):910–911. [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Triau R., Sparks K. J. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J Clin Invest. 1972 Jan;51(1):89–96. doi: 10.1172/JCI106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESS E. V., HUNTER D. K., ZIFF M. GONOCOCCAL ANTIBODIES IN ACUTE ARTHRITIS. JAMA. 1965 Feb 15;191:531–534. doi: 10.1001/jama.1965.03080070015004. [DOI] [PubMed] [Google Scholar]
- Holmes K. K., Weisner P. J., Pedersen A. H. The gonococcal arthritis-dermatitis syndrome. Ann Intern Med. 1971 Sep;75(3):470–471. doi: 10.7326/0003-4819-75-3-470. [DOI] [PubMed] [Google Scholar]
- Jephcott A. E., Reyn A., Birch-Andersen A. Neisseria gonorrhoeae 3. Demonstration of presumed appendages to cells from different colony types. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):437–439. doi: 10.1111/j.1699-0463.1971.tb00086.x. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L., Schmale J. D. Identification of a gonococcal antigen important in the human immune response. Infect Immun. 1970 Feb;1(2):207–208. doi: 10.1128/iai.1.2.207-208.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan L. C., Cox P. M., Norins L. C. Reactivity of two gonococcal antigens in an automated microhemagglutination procedure. Appl Microbiol. 1970 Dec;20(6):907–909. doi: 10.1128/am.20.6.907-909.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. B. The national venereal disease problem. Med Clin North Am. 1972 Sep;56(5):1073–1086. doi: 10.1016/s0025-7125(16)32333-1. [DOI] [PubMed] [Google Scholar]
- MAGNUSSON B., KJELLANDER J. GONOCOCCAL COMPLEMENT-FIXATION TEST IN COMPLICATED AND UNCOMPLICATED GONORRHOEA. Br J Vener Dis. 1965 Jun;41:127–151. doi: 10.1136/sti.41.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C. R., LAZAROW A. Immunoassay of insulin using a two-antibody system. Proc Soc Exp Biol Med. 1962 May;110:29–32. doi: 10.3181/00379727-110-27411. [DOI] [PubMed] [Google Scholar]
- Maeland J. A. Antigenic determinants of aqueous ether extracted endotoxin from Neisseria gonorrhoeae. Acta Pathol Microbiol Scand. 1969;76(3):475–483. doi: 10.1111/j.1699-0463.1969.tb03277.x. [DOI] [PubMed] [Google Scholar]
- Maeland J. A. Antigenic properties of various preparations of Neisseria gonorrhoeae endotoxin. Acta Pathol Microbiol Scand. 1968;73(3):413–422. doi: 10.1111/j.1699-0463.1968.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Maeland J. A. Immunochemical characterization of aqueous ether extracted endotoxin from Neisseria gonorrhoeae. Acta Pathol Microbiol Scand. 1969;76(3):484–492. doi: 10.1111/j.1699-0463.1969.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Maeland J. A., Larsen B. Human serum antibodies reacting with endotoxin from Neisseria gonorrhoeae. Br J Vener Dis. 1971 Aug;47(4):269–272. doi: 10.1136/sti.47.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeland J. A. Serological cross-reactions of aqueous ether extracted endotoxin from Neisseria gonorrhoeae strains. Acta Pathol Microbiol Scand. 1969;77(3):505–517. doi: 10.1111/j.1699-0463.1969.tb04257.x. [DOI] [PubMed] [Google Scholar]
- Maeland J. A. Serological properties of aqueous ether extracted endotoxin from Neisseria gonorrhoeae. Acta Pathol Microbiol Scand. 1969;77(3):495–504. doi: 10.1111/j.1699-0463.1969.tb04256.x. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Michaels R. M., Rogers K. D. A sex difference in immunologic responsiveness. Pediatrics. 1971 Jan;47(1):120–123. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Pariser H. Asymptomatic gonorrhea. Med Clin North Am. 1972 Sep;56(5):1127–1132. doi: 10.1016/s0025-7125(16)32338-0. [DOI] [PubMed] [Google Scholar]
- Ratnatunga C. S. Evaluation of the gonococcal complement-fixation test. Br J Vener Dis. 1971 Aug;47(4):279–288. doi: 10.1136/sti.47.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reising G., Kellogg D. S., Jr Detection of gonococcal antibody. Proc Soc Exp Biol Med. 1965 Dec;120(3):660–663. doi: 10.3181/00379727-120-30617. [DOI] [PubMed] [Google Scholar]
- Reising G., Schmale J. D., Danielsson D. G., Thayer J. D. Reactivity of two selected antigens of Neisseria gonorrhoeae. Appl Microbiol. 1969 Sep;18(3):337–339. doi: 10.1128/am.18.3.337-339.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOM J. H., TALMAGE D. W. Nonprecipitating insulin antibodies. J Clin Invest. 1958 Jun;37(6):783–786. doi: 10.1172/JCI103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVEHAG S. E., MANDEL B. THE FORMATION AND PROPERTIES OF POLIOVIRUS-NEUTRALIZING ANTIBODY. I. 19S AND 7S ANTIBODY FORMATION: DIFFERENCES IN KINETICS AND ANTIGEN DOSE REQUIREMENT FOR INDUCTION. J Exp Med. 1964 Jan 1;119:1–19. doi: 10.1084/jem.119.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVEHAG S. E., MANDEL B. THE FORMATION AND PROPERTIES OF POLIOVIRUS-NEUTRALIZING ANTIBODY. II. 19S AND 7S ANTIBODY FORMATION: DIFFERENCES IN ANTIGEN DOSE REQUIREMENT FOR SUSTAINED SYNTHESIS, ANAMNESIS, AND SENSITIVITY TO X-IRRADIATION. J Exp Med. 1964 Jan 1;119:21–39. doi: 10.1084/jem.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmale J. D., Danielsson D. G., Smith J. F., Lee L., Peacock W. L., Jr Isolation of an antigen of Neisseria gonorrhoeae involved in the human immune response to gonococcal infection. J Bacteriol. 1969 Aug;99(2):469–471. doi: 10.1128/jb.99.2.469-471.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop J. W., Zegers B. J., Sander P. C., Ballieux R. E. Serum immunoglobulin levels in healthy children and adults. Clin Exp Immunol. 1969 Jan;4(1):101–112. [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. II. Freeze-fracture, freeze-etch studies on gonocci. J Exp Med. 1972 Nov 1;136(5):1258–1271. doi: 10.1084/jem.136.5.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALMAGE D. W., MAURER P. H. I131-Labelled antigen precipitation as a measure of quantity and quality of antibody. J Infect Dis. 1953 May-Jun;92(3):288–300. doi: 10.1093/infdis/92.3.288. [DOI] [PubMed] [Google Scholar]
- TAUBER H., GARSON W. Isolation of lipopolysaccharide endotoxin. J Biol Chem. 1959 Jun;234(6):1391–1393. [PubMed] [Google Scholar]
- TAUBER H., GARSON W. Preparation and some properties of Neisseria gonorrhoeae endotoxin. Proc Soc Exp Biol Med. 1957 Aug-Sep;95(4):669–672. doi: 10.3181/00379727-95-23323. [DOI] [PubMed] [Google Scholar]
- TAUBER H., RUSSELL H. Amino compounds in lipopolysaccharides. J Biol Chem. 1960 Apr;235:961–964. [PubMed] [Google Scholar]
- THAYER J. D., MARTIN J. E., Jr A SELECTIVE MEDIUM FOR THE CULTIVATION OF N. GONORRHOEAE AND N. MENINGITIDIS. Public Health Rep. 1964 Jan;79:49–57. [PMC free article] [PubMed] [Google Scholar]
- Wallace R., Diena B. B., Yugi H., Greenberg L. The bentonite flocculation test in assay of Neisseria antibody. Can J Microbiol. 1970 Aug;16(8):655–659. doi: 10.1139/m70-113. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Glynn A. A. Human antibody response to lipopolysaccharides from Neisseria gonorrhoeae. J Clin Pathol. 1972 Jan;25(1):56–59. doi: 10.1136/jcp.25.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt P. J., Ward M. E., Glynn A. A. A comparison of serological tests for the diagnosis of gonorrhoea. Br J Vener Dis. 1971 Dec;47(6):448–451. doi: 10.1136/sti.47.6.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wiktor T. J., Koprowski H., Dixon F. Radioimmunoassay procedure for rabies binding antibodies. J Immunol. 1972 Sep;109(3):464–470. [PubMed] [Google Scholar]
- Wistreich G. A., Baker R. F. The presence of fimbriae (pili) in three species of Neisseria. J Gen Microbiol. 1971 Feb;65(2):167–173. doi: 10.1099/00221287-65-2-167. [DOI] [PubMed] [Google Scholar]