Abstract
Laser ablation of a silver plate immersed in virgin coconut oil was carried out for fabrication of silver nanoparticles. A Nd:YAG laser at wavelengths of 1064 nm was used for ablation of the plate at different times. The virgin coconut oil allowed formation of nanoparticles with well-dispersed, uniform particle diameters that were stable for a reasonable length of time. The particle sizes and volume fraction of nanoparticles inside the solutions obtained at 15, 30, 45 min ablation times were 4.84, 5.18, 6.33 nm and 1.0 × 10−8, 1.6 × 10−8, 2.4 × 10−8, respectively. The presented method for preparation of silver nanoparticles in virgin coconut oil is environmentally friendly and may be considered a green method.
Keywords: silver nanoparticles, laser ablation, virgin coconut oil
Introduction
Applications of nanoparticles (NPs) depend on the particle size, charge, hydrophobicity, and surface functionlization, etc. These phenomena cause nanotechnology to play an important role in science and technology. Antibacterial applications and nanocomposite fabrications are some of the more important applications of silver NPs (Ag-NPs) among many.1–3 It was found that Ag-NPs show strong antibacterial efficacy in very small amounts.4
There are many methods for the preparation of NPs,5–9 and one of them is the laser ablation (LA) technique. This technique is based on the ablation of a solid target by pulsed laser. The target is located in a gas or liquid environment and NPs are collected in the form of colloidal solution or nanopowder. The method is fast, straightforward, and an easy method for the preparation of NPs compared to other methods, as it does not need multistep chemical synthetic procedures, long reaction times, and high temperatures. Different types of NPs from metallic to semiconducting and polymeric NPs, including semiconducting alloys or complex multielement metallic composites, can be produced by this technique. It does not require the use of hazardous, toxic chemical precursors for the synthesis of nanomaterials. Therefore this technique is safe for the laboratory, environmentally friendly, and can be considered a green method. LA in liquids has received much attention in comparison with LA in gas or vacuum. One of the interesting features of this technique is the influence of the surrounding solvent on particle size and stability which has been studied by many researchers.10,11 For example, it was found that the obtained NPs in polyvinyl pyrrolidone (PVP) solution were more stable than the preparation of NPs in pure water.12,13 PVP also prevents aggregation of ablated atoms, clusters, and droplets produced by LA in the solution and subsequently PVP controls the particle size.
Recently, synthesis of NPs using vegetable oils as stabilizing agents for preparation of NPs has been reported.14,15 These natural compounds contain triglycerides as amphiphilic molecules with polar carboxylic group which is able to coordinate to NPs and non polar long carbon chain that prevent NPs agglomeration through steric repulsion.
Among vegetable oils, the saturated fat in virgin coconut oil is a unique type comprised predominantly of medium chain triglycerides. This unique fat has a number of health benefits and is completely different from the saturated fat found in other vegetable oils and meats.16 The chain triglycerides in saturated fat of coconut oil are burned immediately and are not converted into cholesterol or body fat. Virgin coconut oil with this unique type of saturated fats can supply incredible health benefits such as protection against heart diseases and stroke. Therefore it is considered the healthiest of all dietary oils. In this article, we report the preparation of Ag-NPs in virg in coconut oil using the LA technique.
Experiment
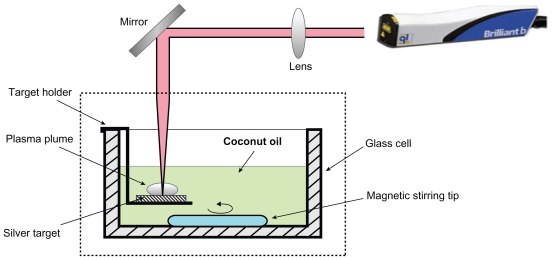
The schematic diagram of the LA experimental set up is illustrated in Figure 1. A pulsed Q-Switched Nd:YAG laser (Brilliant B; Quantel, Newbury, UK) with pulse duration of 5 ns and 10 Hz repetition rate at its original wavelength (1064 nm) was applied to fabricate Ag-NPs. A silver plate (99.99% purity; Sigma Aldrich, St. Louis, MO) was located in cubic glass cell containing 20 mL of virgin coconut oil. The plate firstly was washed using ultrasonic bath for 30 min and then immersed in the virgin coconut oil. The solution was stirred magnetically during the ablation process to disperse the produced NPs. The recorded laser output power by the optical power detector was 360 mJ/pulse with the power fluence of 162 J/cm2. The laser beam was focused at the silver target vertically by a 25-cm focal length lens and a flat mirror. The target plate was located about 4 mm below the oil surface. The ablation was carried out at room temperature with different duration times; 15, 30, and 45 minutes. We obtained the volume fraction of NPs inside the samples by the following equation:
Figure 1.
Schematic diagram of laser ablation experimental set up.
| (1) |
where VL is the oil volume and VS = m/ρ is the volume of the particles where ρ is the mass density of the silver and m is the particles mass dispersed in the oil. An atomic absorption spectrometer (S Series) has been used to measure m. The obtained volume fractions of samples are 1.0 × 10−8, 1.6 × 10−8, and 2.4 × 10−8 in order for 15, 30, 45 min ablation times. The prepared Ag-NPs were characterized using a UV-vis double beam photospectrometer (Shimadzu, Columbia, MD) with 1 cm optical path cell and transmission electron microscopy (TEM, Hitachi H-7100; Hitachi, Chula Vista, CA) at accelerating voltage of 120 kV. The samples were prepared for TEM experiments by depositing a drop of solution containing Ag-NPs onto copper grids and left for one day to dry completely at room temperature.
Results and discussion
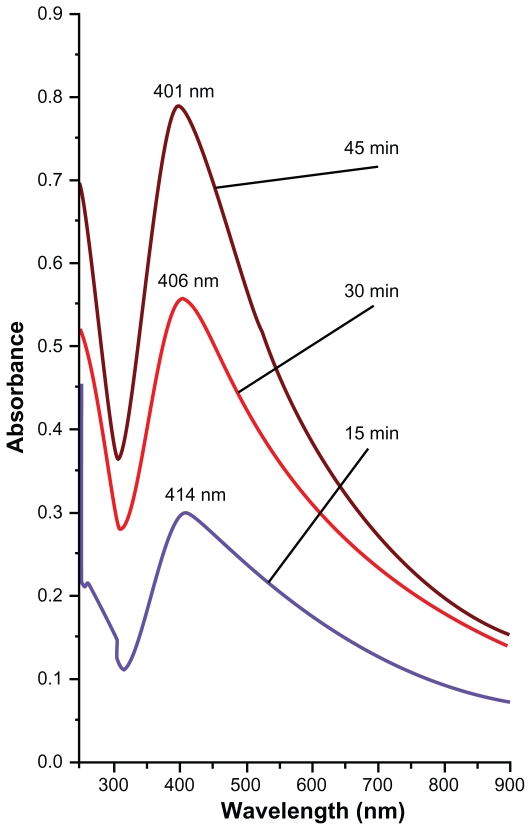
During the LA of silver plate the virgin coconut oil at first appeared colorless and transparent but after a few minutes it began to change to yellow-brown and finally to brown. The color for solutions with higher concentration of Ag-NPs is deeper. This was also confirmed by UV-vis absorption spectra and will be discussed later on. In Figure 2, the optical absorption spectra of the solutions containing Ag-NPs when the silver plate was irradiated by constant laser power under different ablation times is shown. The selected ablation times were 15, 30, and 45 minutes. From the figure we can relate that the peak intensity of the spectra depends on the volume fraction of NPs inside the samples. The peak intensity increased with an increase in particle volume fraction. The appearance of the 400 nm peak with broad tails that are extended toward the UV wavelength range confirmed formation of Ag-NPs inside the solutions.17 This peak arises from surface plasmon resonance of Ag-NPs while the tail part around 250 nm is originated from interband transition.18 At 400 nm the spectra peaks are prominent and single; this confirms that the presence of NPs in the solutions is in spherical shapes.19 It is clear from Figure 2 that the intensity of the absorption peak increases with the increase of ablation times, which means an increase in the formation efficiency of the NPs. When the NPs’ formation efficiency at longer times increases the intensity of the interband transition peak is also increased. On the other hand the shift toward the higher energy (lower wavelength) that appears in the spectrum indicates reduction in size of particles. This decrement in particle size with increasing ablation time can be explained by the interaction of generated particles from plate with laser light. As a result of this interaction, the large particles will fragment and become smaller. The efficiency of fragmentation increases by increasing the ablation time, therefore the obtained particles at longer ablation times are smaller.20
Figure 2.
UV-vis absorption spectra of samples containing Ag-NPs prepared for different ablation times in virgin coconut oil.
Abbreviations: Ag, silver; NPs, nanoparticles; UV, ultraviolet.
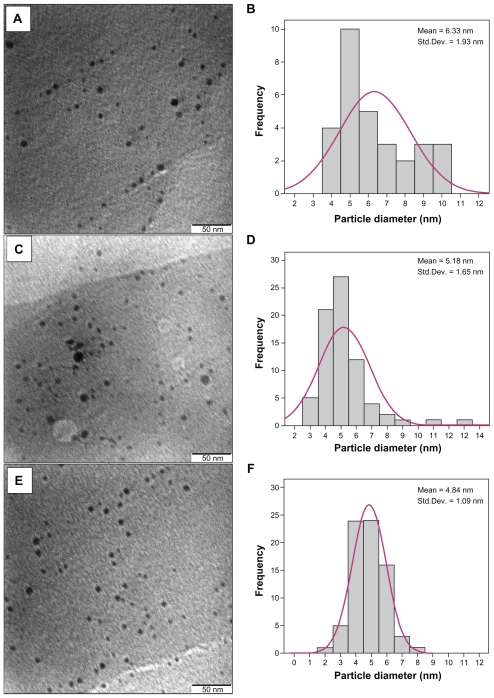
Figure 3 represents the electron micrograph and its corresponding size distribution of NPs prepared by this technique. TEM images showed nonagglomerated as well as scattered NPs with spherical shape. The spherical-shaped NPs can be very appropriate for drug loading and most biological applications, such as antibacterial properties.21
Figure 3.
TEM image and typical of statistical graph for Ag-NPs in virgin coconut oil under 15 min (A–B), 30 min (C–D), and 45 min (E–F) ablation times.
Abbreviations: Ag, silver; NPs, nanoparticles; SD, standard deviation; TEM, transmission electron microscopy.
The calculated average size diameters of Ag-NPs are about 6.33, 5.18, and 4.84 nm in order for 15, 30, and 45 minutes ablation time. This also confirms the observation of particle size decrement with respect to the ablation time (Table 1). The UV-visible spectrum for the sample prepared at 45 min ablation time didn’t show any specific change in intensity and spectral width around one month after preparation, which confirms the stability of the sample.
Table 1.
The particle size of silver nanoparticles and their corresponding volume fraction in virgin coconut oil
| Samples | Volume fraction | Particle size (nm) | Standard deviation for particle size (nm) |
|---|---|---|---|
| 15 min | 1.0 × 10−8 | 6.33 | 1.93 |
| 30 min | 1.6 × 10−8 | 5.18 | 1.65 |
| 45 min | 2.4 × 10−8 | 4.84 | 1.09 |
The mechanism of the role of the virgin coconut oil can be understood by the interaction between virgin coconut oil molecules and the particles produced during LA. NPs are formed during the process of LA through phase transition, nucleation, and crystal growth of emitted materials from the target plate. The ablated materials are silver atoms, clusters, and droplets.22 The NPs formed were adsorbed by the virgin coconut oil and this adsorbtion can prevent aggregation and growth of the particles.
The formation efficiency of NPs is related to the confinement of ablation products. The emitted materials from the plate are confined by the solvent, in this case virgin coconut oil. Because of the high density and viscosity of virgin coconut oil the confining effect of the solvent increased. The generated plasma which already confined near the plate surface is high pressured and therefore can etch further the surface to produce NPs.23,24 This process is called secondary ablation25 and can increase the formation efficiency of Ag-NPs. We compared the formation efficiency of Ag-NPs in virgin coconut oil with that in grape seed oil of lower density and viscosity. The preparations of NPs in both of the oils were completed with 10 min ablation time. The density of the Ag-NPs in virgin coconut oil was 0.055 mg/L whereas that for grape seed oil was 0.045 mg/L. This means the formation efficiency of NPs prepared in virgin coconut oil is higher.
Conclusion
In summary, we presented the synthesis of Ag-NPs in virgin coconut oil using a LA technique, which is a simple and green method. The obtained sizes for particle are 4.84, 5.18, and 6.33 nm for 45, 30, and 15 min ablation times, respectively. The virgin coconut oil controls the particles size and prevents agglomeration between the ablated NPs. Therefore the obtained NPs are stable for quite a long time. The particle size reduced with increasing ablation time, which is attributed to more particle fragmentation under longer times.
Acknowledgment
The authors are grateful to the Ministry of Higher Education of Malaysia for supporting this work under the Fundamental Research Grant Scheme No: 01-04-10-864 FR.
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Dowling DP, Betts AJ, Pope C, McConnell ML, Eloy R, Artnauld MN. Anti-bacterial silver coatings exhibiting enhanced activity through the addition of platinum. Surf Coat Technol. 2003;637:163–164. [Google Scholar]
- 2.Song C, Wang D, Lin Y, Hu Z, Gu G, Fu X. Formation of silver nano-shells on latex spheres. Nanotechnology. 2004;15:962–965. [Google Scholar]
- 3.Kokkoris M, Trapalis CC, Kossionides SK, et al. RBS and HIRBS studies of nanostructured AgSiO2 sol–gel thin coatings. Nucl Instrum Methods Phys Res B. 2005;188:67–72. [Google Scholar]
- 4.Li Y, Leung P, Yao L, Song QW, Newton E. Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect. 2006;62:58–63. doi: 10.1016/j.jhin.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Brust M, Walker M, Bethel D, Schiffrin D, Whyman DJR. Synthesis of thiol-derivatized gold nanoparticles in a two phase liquid–liquid system. J Chem Soc Chem Commun. 1994;15:801–802. [Google Scholar]
- 6.Pastoriza-Santos I, Lis-Marzan LM. Formation and stabilization of silver nanoparticles through reduction by N, N-Dimethylformamide. Langmuir. 1999;15:948–951. [Google Scholar]
- 7.Bonet F, Delmas V, Grugeon S, Herrera Urbina R, Silvert PY, Tekaia-Elhsissen K. Synthesis of monodisperse Au, Pt, Pd, Ru and Ir nanoparticles in ethylene glycol. Nanostruct Mater. 1999;11:1277–1284. [Google Scholar]
- 8.Pillai ZS, Kamat PV. What factors control the size and shape of silver nanoparticles in the citrate ion reduction method. J Phys Chem B. 2004;108:945–951. [Google Scholar]
- 9.Yamamoto M, Nakamato M. Novel preparation of mono-dispersed silver nanoparticles via amine adducts derived from insoluble silver-myristate in alkylamine. J Mater Chem. 2003;13:2064–2065. [Google Scholar]
- 10.Amendola V, Polizzi S, Meneghetti M. Free silver nanoparticles synthesized by laser ablation in organic solvents and their easy functionalization. Langmuir. 2007;23:6766–6770. doi: 10.1021/la0637061. [DOI] [PubMed] [Google Scholar]
- 11.Amendola V, Meneghetti M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys Chem Chem Phy. 2009;11:3805–3821. doi: 10.1039/b900654k. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji T, Thang DH, Okazaki Y, Nakanishi M, Tsuboi Y, Tsuji M. Preparation of silver nanoparticles by laser ablation in polyvinylpyrrolidone solutions. Appl Surf Sci. 2008;254:5224–5230. [Google Scholar]
- 13.Chandra M, Indi SS, Das PK. First hyperpolarizabilities of unprotected and polymer protected copper nanoparticles prepared by laser ablation. Chem Phys Lett. 2006;422:262–266. [Google Scholar]
- 14.Wu N, Fu L, Aslam M, Wong KC, Dravid VW. Interaction of fatty acid monolayers with cobalt nanoparticles. Nano Lett. 2004;4:383–386. [Google Scholar]
- 15.Song HT, Huh YM, Kim S, Jun WW, Suh JS, Cheon J. Surface modulation of magnetic nanocrystals in the development of highly efficient magnetic resonance probes for intracellular labeling. J Am Chem Soc. 2005;127:9992–9993. doi: 10.1021/ja051833y. [DOI] [PubMed] [Google Scholar]
- 16.Bruce FND. The Healing Miracle of Coconut Oil. Boise, ID: Healthwise; 2000. [Google Scholar]
- 17.Kerker M. The optics of colloidal silver: something old and something new. J Colloid Interf Sci. 1985;105:297–314. [Google Scholar]
- 18.Mafune F, Kohno J, Takeda Y, Kondow T, Sawabe H. Formation and size control of silver nanoparticles by laser ablation in aqueous solution. J Phys Chem B. 2000;104:9111–9117. [Google Scholar]
- 19.Tsuji T, Iryo K, Watanabe N, Tsuji M. Preparation of silver nanoparticles by laser ablation in solution: influence of laser wavelength on particle size. Appl Surf Sci. 2002;202:80–85. [Google Scholar]
- 20.Hajiesmaeilbaigi F, Mohammadalipour A, Sabbaghzadeh J, Hoseinkhani S, Fallah HR. Preparation of silver nanoparticles by laser ablation and fragmentation in pure water. Laser Phys Lett. 2006;5:252–256. [Google Scholar]
- 21.Khanna PK, Nair CKK. Synthesis of silver nanoparticles using cod liver oil (fish oil): green approach to nanotechnology. International Journal of Green Nanotechnology: Physics and Chemistry. 2009;1:3–9. [Google Scholar]
- 22.Yang GW. Laser ablation in liquids: Applications in the synthesis of nanocrystals. Prog Mater Sci. 2007;52(4):648–698. [Google Scholar]
- 23.Tomita Y, Robinson PB, Tong RP, Blake JR. Growth and collapse of cavitation bubbles near a curved rigid boundary. J Fluid Mech. 2002;466:259–283. [Google Scholar]
- 24.Kawaguchi Y, Ding X, Narazaki A, Sato T, Niino H. Transient pressure induced by laser ablation of toluene, a highly laser-absorbing liquid. Appl Phys A. 2005;80:275–281. [Google Scholar]
- 25.Kabashin AV, Meunier M. Synthesis of colloidal nanoparticles during femtosecond laser ablation of gold in water. J Appl Phys. 2003;94:7941–7944. [Google Scholar]