Abstract
The effect of impaired intestinal protein synthesis on chylomicron apoprotein composition was studied in mesenteric lymph fistula rats. Lymph was obtained from animals with impaired protein synthesis given intraperitoneal acetoxycycloheximide (ACH), a potent inhibitor of protein synthesis. Lymph chylomicrons were then isolated by ultracentrifugation and purified on agarose columns. Purified chylomicrons from control and ACH-treated animals were delipidated, and their apoprotein pattern was examined on sodium dodecyl sulfate (SDS) polyacrylamide gels.
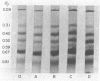
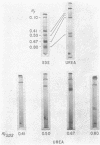
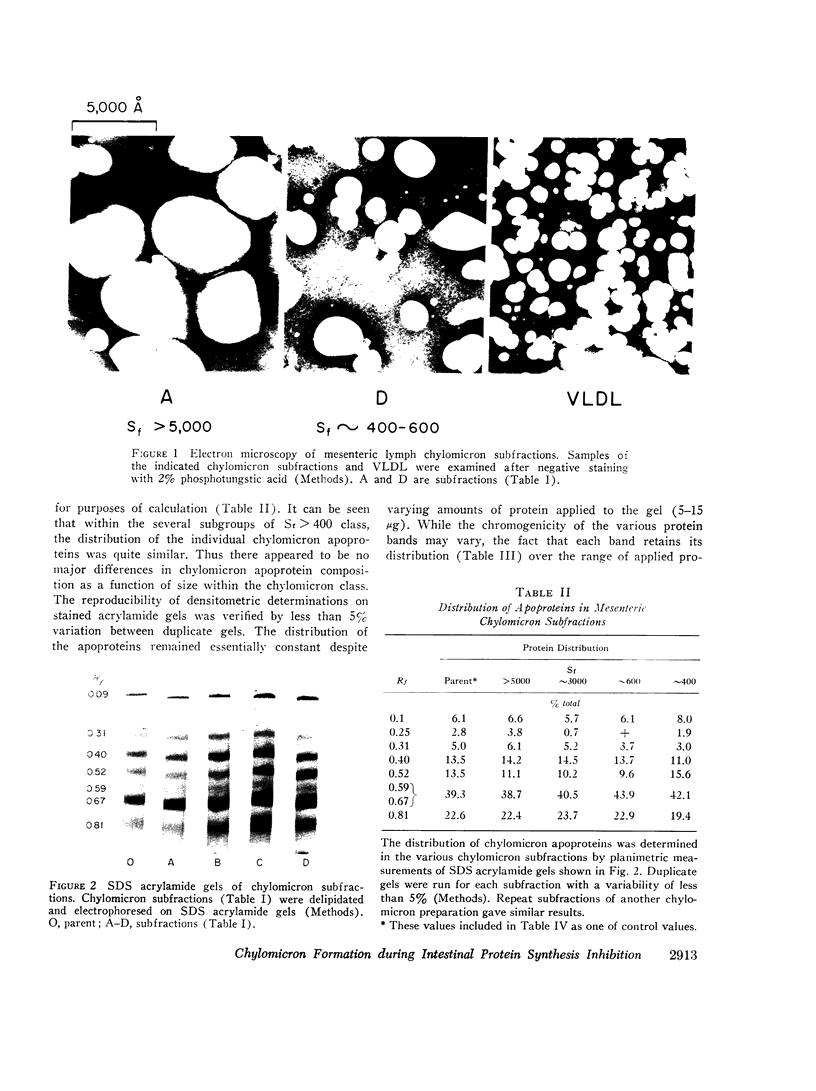
Because we had previously demonstrated a markedly increased lymph chylomicron size during the inhibition of protein synthesis, it was first necessary to determine whether chylomicron apoprotein composition normally varied with chylomicron size. Chylomicrons of varying sizes were prepared by differential ultracentrifugation, and their apoprotein composition was determined densitometrically on SDS polyacrylamide gels. No significant difference in apoprotein composition was found normally with varying chylomicron size.
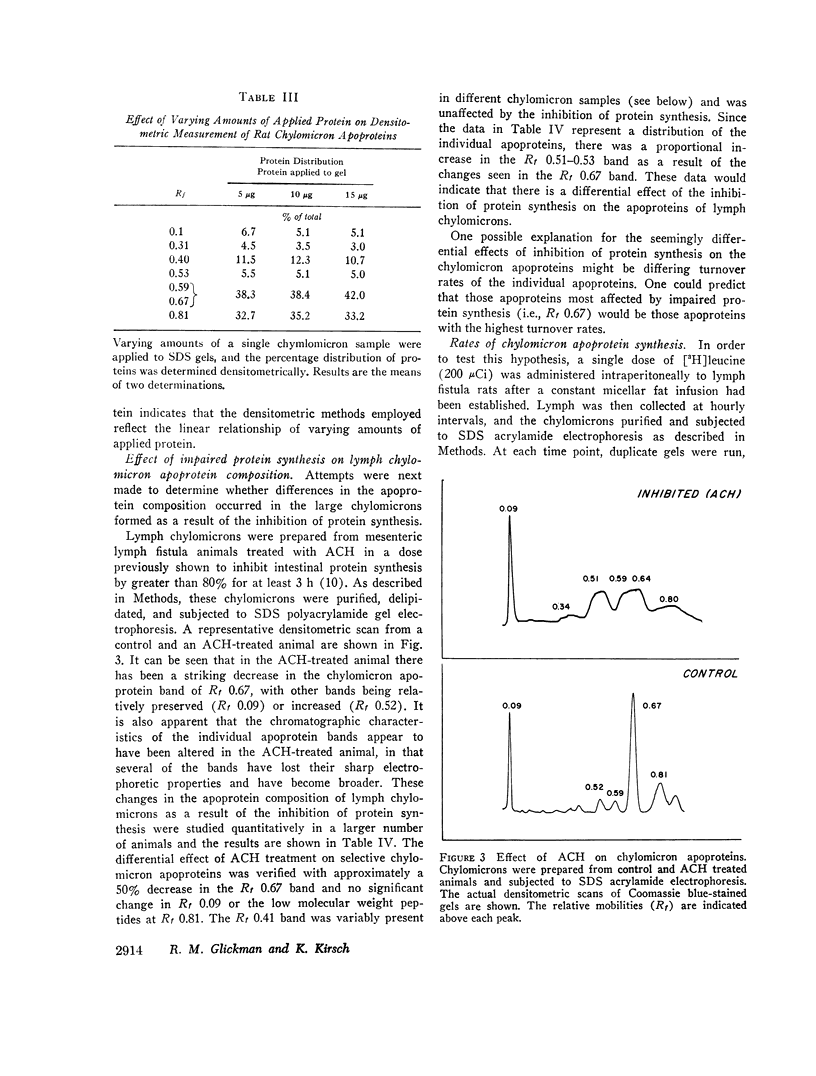
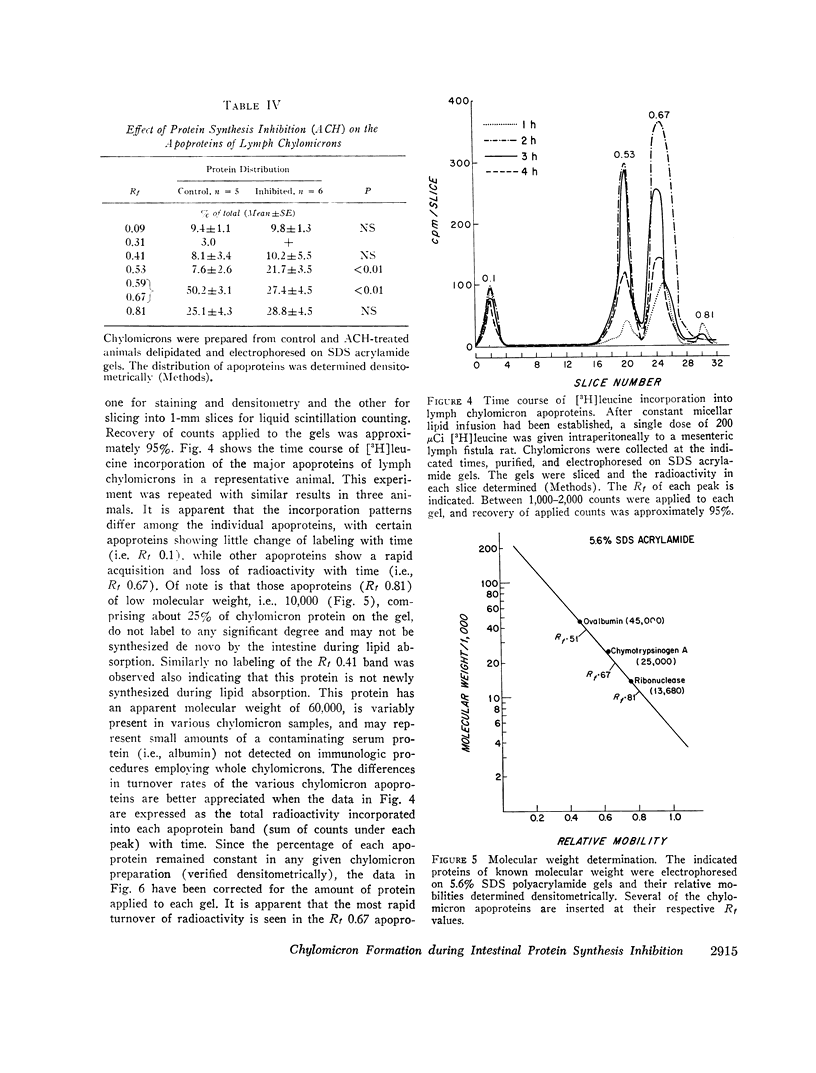
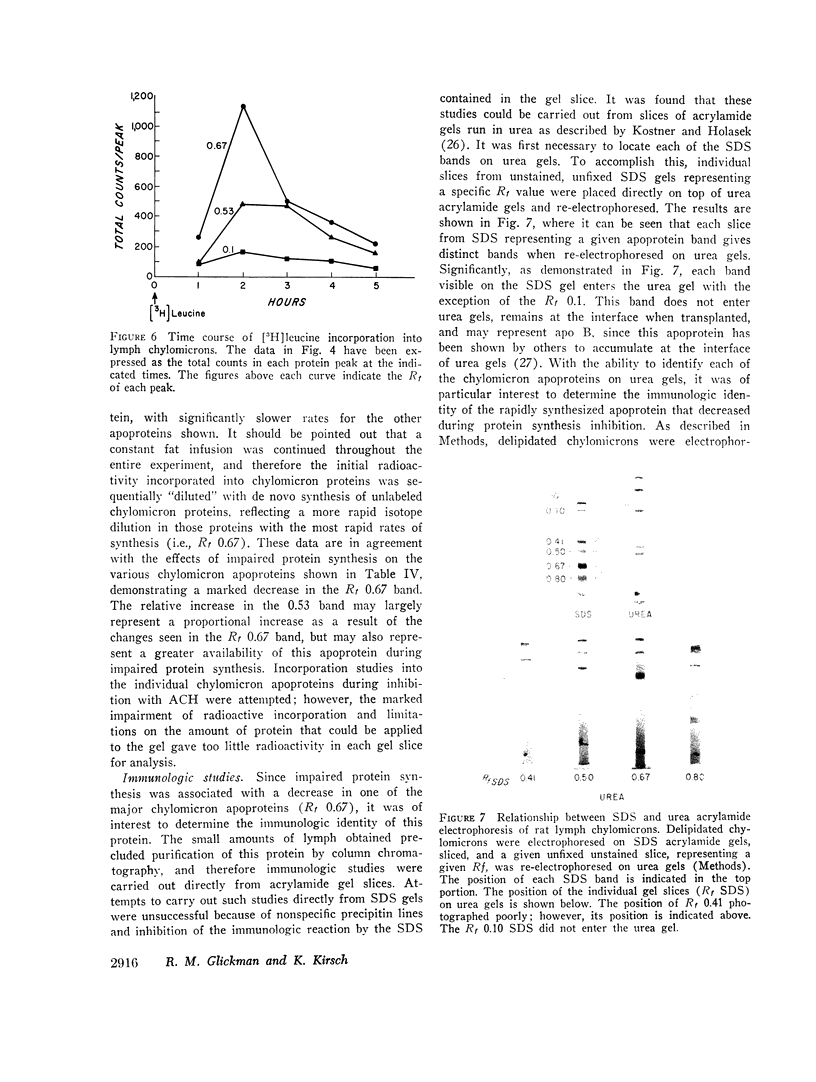
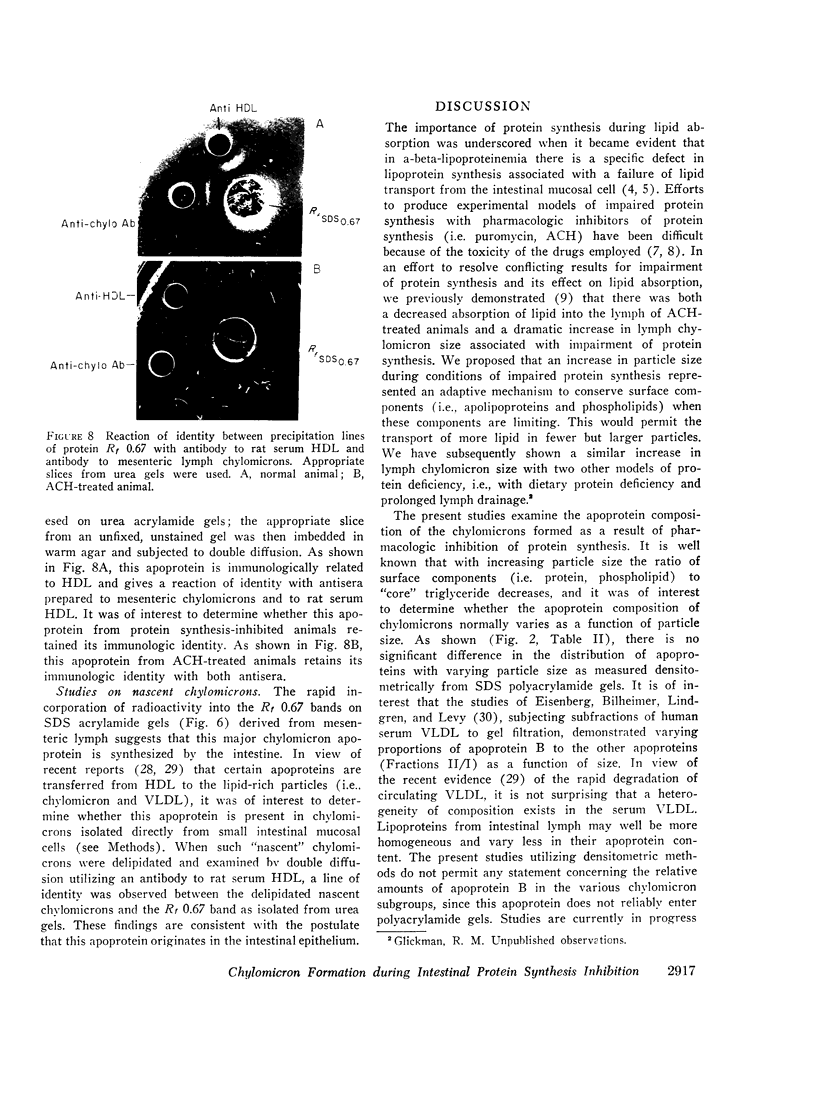
In contrast, however, chylomicrons from ACH-treated animals showed a 50% decrease in a major apoprotein band with R1 0.67. Other chylomicron apoproteins were not decreased as a result of impaired protein synthesis, suggesting differing rates of synthesis of the various chylomicron apoproteins. In vivo incorporation studies of [3H]leucine into the various apoproteins of lymph chylomicrons demonstrated that this apoprotein (R1 0.67) had the most rapid synthesis rate and suggested that it seemed most affected by impaired intestinal protein synthesis. Immunologic studies indicated that this apoprotein was immunologically related to high-density lipoproteins (HDL) and was present in chylomicrons isolated directly from small intestinal mucosa.
These studies demonstrate that impaired intestimal protein synthesis is associated with a deficiency in one of the major chylomicron apoproteins and may in part explain the impaired lipid absorption seen during states of impaired protein synthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bersot T. P., Brown W. V., Levy R. I., Windmueller H. G., Fredrickson D. S., LeQuire V. S. Further characterization of the apolipoproteins of rat plasma lipoproteins. Biochemistry. 1970 Aug 18;9(17):3427–3433. doi: 10.1021/bi00819a022. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown W. V., Levy R. I., Fredrickson D. S. Studies of the proteins in human plasma very low density lipoproteins. J Biol Chem. 1969 Oct 25;244(20):5687–5694. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., HAMLIN J. T., 3rd Particulate fat in lymph and blood. Physiol Rev. 1962 Oct;42:674–701. doi: 10.1152/physrev.1962.42.4.674. [DOI] [PubMed] [Google Scholar]
- Eggstein M., Kreutz F. H. Eine neue Bestimmung der Neutralfette im Blutserum und Gewebe. I. Prinzip, Durchführung und Besprechung der Methode. Klin Wochenschr. 1966 Mar 1;44(5):262–267. doi: 10.1007/BF01747716. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Bilheimer D., Lindgren F., Levy R. I. On the apoprotein composition of human plasma very low density lipoprotein subfractions. Biochim Biophys Acta. 1972 Feb 21;260(2):329–333. doi: 10.1016/0005-2760(72)90045-8. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Friedman H. I., Cardell R. R., Jr Effects of puromycin on the structure of rat intestinal epithelial cells during fat absorption. J Cell Biol. 1972 Jan;52(1):15–40. doi: 10.1083/jcb.52.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman R. M., Alpers D. H., Drummey G. D., Isselbacher K. J. Increased lymph alkaline phosphatase after fat feeding: effects of medium chain triglycerides and inhibition of protein synthesis. Biochim Biophys Acta. 1970 Feb 24;201(2):226–235. doi: 10.1016/0304-4165(70)90296-5. [DOI] [PubMed] [Google Scholar]
- Glickman R. M., Kirsch K., Isselbacher K. J. Fat absorption during inhibition of protein synthesis: studies of lymph chylomicrons. J Clin Invest. 1972 Feb;51(2):356–363. doi: 10.1172/JCI106821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotto A. M., Levy R. I., John K., Fredrickson D. S. On the protein defect in abetalipoproteinemia. N Engl J Med. 1971 Apr 15;284(15):813–818. doi: 10.1056/NEJM197104152841503. [DOI] [PubMed] [Google Scholar]
- Gustafson A., Alaupovic P., Furman R. H. Studies of the composition and structure of serum lipoproteins. Separation and characterization of phospholipid-protein residues obtained by partial delipidization of very low density lipoproteins of human serum. Biochemistry. 1966 Feb;5(2):632–640. doi: 10.1021/bi00866a033. [DOI] [PubMed] [Google Scholar]
- Kalab M., Martin W. G. Gel filtration of native and modified pig serum lipoproteins. J Chromatogr. 1968 Jun 4;35(2):230–233. doi: 10.1016/s0021-9673(01)82379-7. [DOI] [PubMed] [Google Scholar]
- Kessler J. I., Stein J., Dannacker D., Narcessian P. Biosynthesis of low density lipoprotein by cell-free preparations of rat intestinal mucosa. J Biol Chem. 1970 Oct 25;245(20):5281–5288. [PubMed] [Google Scholar]
- Koga S., Bolis L., Scanu A. M. Isolation and characterization of subunit polypeptides from apoproteins of rat serum lipoprotein. Biochim Biophys Acta. 1971 May 25;236(2):416–430. doi: 10.1016/0005-2795(71)90222-4. [DOI] [PubMed] [Google Scholar]
- Kostner G., Holasek A. Characterization and quantitation of the apolipoproteins from human chyle chylomicrons. Biochemistry. 1972 Mar 28;11(7):1217–1223. doi: 10.1021/bi00757a016. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahley R. W., Bennett B. D., Morré D. J., Gray M. E., Thistlethwaite W., LeQuire V. S. Lipoproteins associated with the Golgi apparatus isolated from epithelial cells of rat small intestine. Lab Invest. 1971 Nov;25(5):435–444. [PubMed] [Google Scholar]
- Ockner R. K., Bloch K. J., Isselbacher K. J. Very-low-density lipoprotein in intestinal lymph: evidence for presence of the A protein. Science. 1968 Dec 13;162(3859):1285–1286. doi: 10.1126/science.162.3859.1285. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Hughes F. B., Isselbacher K. J. Very low density lipoproteins in intestinal lymph: origin, composition, and role in lipid transport in the fasting state. J Clin Invest. 1969 Nov;48(11):2079–2088. doi: 10.1172/JCI106174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave T. G. Inhibition of protein synthesis and absorption of lipid into thoracic duct lymph of rats. Proc Soc Exp Biol Med. 1969 Mar;130(3):776–780. doi: 10.3181/00379727-130-33653. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G., Zilversmit D. B. Does puromycin block release of chylomicrons from the intestine? Am J Physiol. 1969 Aug;217(2):336–340. doi: 10.1152/ajplegacy.1969.217.2.336. [DOI] [PubMed] [Google Scholar]
- Rodgers J. B., Riley E. M., Drummey G. D., Isselbacher K. J. Lipid absorption in adrenalectomized rats: the role of altered enzyme activity in the intestinal mucosa. Gastroenterology. 1967 Oct;53(4):547–556. [PubMed] [Google Scholar]
- Rubenstein B., Rubinstein D. Interrelationship between rat serum very low density and high density lipoproteins. J Lipid Res. 1972 May;13(3):317–324. [PubMed] [Google Scholar]
- SABESIN S. M., ISSELBACHER K. J. PROTEIN SYNTHESIS INHIBITION: MECHANISM FOR THE PRODUCTION OF IMPAIRED FAT ABSORPTION. Science. 1965 Mar 5;147(3662):1149–1151. doi: 10.1126/science.147.3662.1149. [DOI] [PubMed] [Google Scholar]
- SALT H. B., WOLFF O. H., LLOYD J. K., FOSBROOKE A. S., CAMERON A. H., HUBBLE D. V. On having no beta-lipoprotein. A syndrome comprising a-beta-lipoproteinaemia, acanthocytosis, and steatorrhoea. Lancet. 1960 Aug 13;2(7146):325–329. doi: 10.1016/s0140-6736(60)91478-1. [DOI] [PubMed] [Google Scholar]
- Ward S., Wilson D. L., Gilliam J. J. Methods for fractionation and scintillation counting of radioisotope-labeled polyacrylamide gels. Anal Biochem. 1970 Nov;38(1):90–97. doi: 10.1016/0003-2697(70)90158-2. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Concanavalin A agglutination of intestinal cells from the human fetus. Science. 1972 Aug 11;177(4048):525–526. doi: 10.1126/science.177.4048.525. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Herbert P. N., Levy R. I. Biosynthesis of lymph and plasma lipoprotein apoproteins by isolated perfused rat liver and intestine. J Lipid Res. 1973 Mar;14(2):215–223. [PubMed] [Google Scholar]
- Windmueller H. G., Levy R. I. Production of beta-lipoprotein by intestine in the rat. J Biol Chem. 1968 Sep 25;243(18):4878–4884. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Fat transport and lymph and plasma lipoprotein biosynthesis by isolated intestine. J Lipid Res. 1972 Jan;13(1):92–105. [PubMed] [Google Scholar]
- Yousef I. M., Kuksis A. Release of chylomicrons by isolated cells of rat intestinal mucosa. Lipids. 1972 Jun;7(6):380–386. doi: 10.1007/BF02531507. [DOI] [PubMed] [Google Scholar]
- Zilversmit D. B. Formation and transport of chylomicrons. Fed Proc. 1967 Nov-Dec;26(6):1599–1605. [PubMed] [Google Scholar]