Abstract
Recent developments of automated methods for monitoring animal movement, e.g., global positioning systems (GPS) technology, yield high-resolution spatiotemporal data. To gain insights into the processes creating movement patterns, we present two new techniques for extracting information from these data on repeated visits to a particular site or patch (“recursions”). Identification of such patches and quantification of recursion pathways, when combined with patch-related ecological data, should contribute to our understanding of the habitat requirements of large herbivores, of factors governing their space-use patterns, and their interactions with the ecosystem.
We begin by presenting output from a simple spatial model that simulates movements of large-herbivore groups based on minimal parameters: resource availability and rates of resource recovery after a local depletion. We then present the details of our new techniques of analyses (recursion analysis and circle analysis) and apply them to data generated by our model, as well as two sets of empirical data on movements of African buffalo (Syncerus caffer): the first collected in Klaserie Private Nature Reserve and the second in Kruger National Park, South Africa.
Our recursion analyses of model outputs provide us with a basis for inferring aspects of the processes governing the production of buffalo recursion patterns, particularly the potential influence of resource recovery rate. Although the focus of our simulations was a comparison of movement patterns produced by different resource recovery rates, we conclude our paper with a comprehensive discussion of how recursion analyses can be used when appropriate ecological data are available to elucidate various factors influencing movement. Inter alia, these include the various limiting and preferred resources, parasites, and topographical and landscape factors.
Keywords: African buffalo, circular path, Fourier transform, GPS, herbivore foraging, Kruger National Park, South Africa, looping, net displacement, periodogram, resource recovery, Syncerus caffer
Introduction
Movement patterns of organisms have a direct effect on the dynamics and persistence of populations (Brown and Kodric-Brown 1977, Hanski and Gilpin 1997, Colbert et al. 2001, Kokko and Lopez-Sepulcre 2006). The increasing interest in the ecological aspects of movement (Sugden and Pennisi 2006, Getz and Saltz 2008, Nathan et al. 2008) has led to the development of automated telemetric methods for monitoring movement (White and Garrott 1990, Rodgers et al. 1996, Cooke et al. 2004), spatiotemporal statistical methods for analyzing movement (Turchin 1998, Preisler et al. 2004, Dalziel et al. 2008, Patterson et al. 2008, Wittemyer et al. 2008), and nonparametric kernel methods that are more stable than parametric kernel methods for constructing utilization distributions when location data sets become increasingly large (Getz and Wilmers 2004, Getz et al. 2007). Recent developments in global positioning systems (GPS) technology provide researchers with the opportunity to evaluate wildlife movements in detail (Dussault et al. 2005, Galanti et al. 2006, Bruggeman et al. 2007, Burdett et al. 2007, Schofield et al. 2007), based on high-resolution spatiotemporal data. However, because it is often difficult to identify specific patterns from large sets of GPS-location data (e.g., several years of sub-hourly data), there is a need for efficient methods for analyzing these data and identifying specific space-use patterns in a way that reveals the processes leading to their creation. Causal deconstruction of movement patterns may add to the theoretical understanding of behavioral processes influencing movement and of the factors governing these processes, e.g., need for food, water, and shelter, regulation of inter- and intraspecies interactions, avoidance of predation and contact with humans, and reduction of contact with contaminated and degraded landscapes (Patterson et al. 2008). Analyses can also serve as a basis for developing conservation strategies and management programs (Sinclair and Arcese 1995).
The spatial dynamics of large herbivores that constitute major animal biomass components of many natural terrestrial ecosystems have a critical impact on ecosystem function (Owen-Smith 1988). Space-use patterns of large herbivores may affect primary production (McNaughton 1985), the quantity and quality of forage (Polis et al. 1997), nutrient distribution and cycles (McNaughton et al. 1997), fire regimes (Hobbs et al. 1991), intra- and interspecific disease transmission (Cross et al. 2005), and the distribution and population dynamics of other herbivores (McNaughton 1985).
Among the factors that affect large herbivores' space use are resource distribution quality and abundance. There is considerable evidence that large herbivores exhibit nonrandom selective foraging and track areas of highest quality forage (Sinclair 1977, McNaughton 1985, Fryxell et al. 1988, 2005). Recent investigations demonstrate that herbivores may use spatial memory to locate preferred food patches and to return to high-quality foraging locations (Gillingham and Bunnell 1989, Edwards et al. 1996, Roguet et al. 1998, Hewitson et al. 2005). Returns to previously grazed areas may be a useful foraging strategy for large herbivores to consume regrowing vegetation in its high primary productivity stage (McNaughton 1985). Moreover, these returns may accelerate nutrient cycling in highly grazed sites (McNaughton et al. 1997). Identification and characterization of repeated visits to a particular site, hereafter referred to as “recursions,” should contribute to our theoretical understanding of the habitat requirements of large herbivores, the factors governing their space-use patterns, and interaction with the ecosystem. This understanding could lead to strategies for conservation and management of the populations and landscapes of interest.
In this paper we present two new techniques for analyzing recursions in animal movement patterns. We first apply them to the output of a simple spatial model that simulates movements of large herbivores in a herdlike structure based on minimal parameters: (1) resource availability; and (2) resource recovery, at a specific rate, after a local depletion. The model outputs show that under relatively simple rules, recursion patterns emerge. We then present and apply our new methods to empirical data on African buffalo (Syncerus caffer) herd movements collected in Klaserie Private Nature Reserve (KPNR) and in Kruger National Park (KNP), South Africa (see Plate 1). Unaided scrutiny of these data is not conducive to immediate interpretation. However, comparing output from analyses of simulated model and empirical KPNR and KNP data provides insights into possible rules governing the movement of African buffalo.
Plate 1.

African buffalo (Syncerus caffer) in Kruger National Park, South Africa. Photo credit: P. C. Cross.
The two techniques proceed from an analysis of finer scale features of the movement tracks of individuals to obtain general patterns: (1) recursion analysis, a new approach to movement data that identifies all closed paths, their length and locations, based on the observation that along a path that closes on itself, the sum of vector displacements is zero; and (2) circle analysis, a new approach to analyzing movement data that uses the complex Fourier transform to display the periodogram of clockwise and counterclockwise looping in the movement patterns.
Methods
Simulation model
We developed a simple, spatially explicit model, based on five rules, that simulates the spatial movement on a landscape over time of a herd of large herbivores. (1) The landscape is overlaid with a grid of grazing patches where the foraging value (equivalently habitat score) of each patch (pixel) is given by the elements of a landscape matrix (105 × 105 pixels in our case). (2) At each time step, the herd moves from the current patch to the patch with highest value among all patches at a particular distance. (3) This distance is specified by search radius. To keep the simulation simple, we assumed a constant stay time in a patch and a constant movement time (i.e., time step). (4) A resource depletion factor is incorporated into the model to account for the fact that after a group occupies a patch for a period of time, the available forage in that patch decreases (Funston et al. 1994). In our model we assumed that the habitat score declines from its current value to zero when occupied by a herd for one time step (but for a fixed level of reduction in each time step, see Getz and Saltz 2008). (5) Recovery to the maximum habitat score for that patch (Smax), with different patches having different maxima, occurs in a gradual process during a specified recovery period (tr) so that the habitat score, τ time steps after depletion at time t, is given by
When a group moves, the patch it occupies is recorded in a dynamic movement matrix, with dimensions equal to the landscape matrix, which presents the order of the spatial location of the group at each time step. Also, a dynamic landscape matrix, which represents the effect of the groups on the vegetation (i.e., on the landscape template), is generated and updated after each time step during model runs. To keep the simulation simple, we ignore other movement drivers such as water or predator avoidance.
Different recovery periods may reflect seasonal patterns and habitat parameters (e.g., as a result of landscape characteristics and variation in the amount of precipitation). To explore the influence of the tr periods on movement patterns, the tr value was changed in different model runs: tr = 9–90 time steps. A fast recovery rate (e.g., tr = 9 time steps) may reflect a wet-season rate, and a slow recovery rate (e.g., tr = 90 time steps) may reflect a dry-season rate (Hik and Jefferies 1990), although in some systems vegetation may grow only during a particular period of the year. We also explored the influence of the landscape structure on movement patterns by running the model for different random landscape matrices. Each landscape matrix, in the size of 105 × 105 pixels, was generated from a uniform distribution of [0,1]; the value in each pixel represented the habitat scores of each patch. We changed the search radius among model runs to explore its potential influence on model output. Model simulations were coded in MATLAB 6.1 (MathWorks 2001) and run for 250 time steps.
The simulations are kept simple because they are used purely to illustrate certain basic principles. Realistic models would need to take into account more factors than used in this model (Getz and Saltz 2008), such as different time spans within a patch and inter- and intraspecies interactions.
Empirical data
Spatial locations of an African buffalo herd in KPNR (Appendix A: Fig. A1) were recorded between 1997 and 1999 (see Appendix A: Fig. A2 for years 1997–1998, and Ryan et al. 2006). Direct observations of the KPNR herd were recorded every 1–3 hours onto surveyor's maps by hand and digitized, orthorectified, and georeferenced as per the methodology in Ryan et al. (2006).
Spatial locations of three individual African buffalo in KNP (Appendix A: Fig. A1) were recorded using GPS technology during 2005–2006 (Appendix A: Figs. A3 and A4). The GPS locations usually were provided every hour during the entire day; accuracy of the locations was within 10 m.
We defined the wet (December–February) and the dry (June–August) seasons based on monthly precipitation records (Macandza et al. 2004, Ryan et al. 2006). For the analyses with the two new techniques that we will describe, we used only cases in which we had a complete set of data for the same herd or individual through most of the study season: i.e., sequences of at least 40 (and up to 70) full days of observations during each season.
Data analysis techniques
Each time series of locations was analyzed using the two novel techniques: recursion and circle analyses. These techniques consider the displacements from a starting location to the location k time steps later as vectors in the complex plane, where X is the west–east and Y is the south–north axis, respectively. The advantage of this notation is that it retains directional information, which simplifies both analyses.
Recursion analysis
We developed an algorithm to identify closed paths in the movement patterns, defined as instances in which there is an exact (to the resolution of landscape discretization) recursion to a previous location at a later time, and to sort such paths according to the duration of the recursion in days. The algorithm is based on the observation that the sum of vector displacements along a closed path is zero and thus requires the identification of zero-valued partial summations of the X and Y coordinates of sequential locations.
Specifically, given a path, identified by the sequence of coordinates (X(k), Y(k)), k = 1,2,…, K, the algorithm proceeds as follows.
Write the locations coordinates as Z(k) = X(k) + iY(k) for k = 1, 2,…, K, and define vectors ν(k) = Z(k + 1) − Z(k).
-
Let t and s denote two arbitrary values of the index k, such that t > s. Define a time window (t, s), of duration D = t − s, as a segment of the path from Z(s) to Z(t) and denote by V(t, s) the sum of the vectors in the window:
A recursion of duration D is, then, a window for which V(t, s) = 0 and t – s = D.
-
Construct a lower triangular matrix V with elements (V)t,s = V(t, s) for t > s, using the recurrence:
For each column s find the first row t for which V(t, s) = 0 and call the respective pair (t, s) a “zero” event.
Count the number of zeros for each diagonal in the lower triangular matrix V. This count represents the sum of recursions that occurred for each specific duration D, because each duration value corresponds to one diagonal in the matrix.
Note that the scale of the recursion analysis is the resolution of the distance between two locations (patches). In our analysis of the buffalo data, we set the scale at one kilometer (i.e., locations X(k), Y(k) recorded at a resolution of meters were rounded to integer values using the transformation X(k)/1000 and Y(k)/1000). Hence, a recursion occurred when a new location was within (allowing for rounding errors) 0–1 km of a previous location, after a time interval of at least one day. Further, a 1-km scale is concordant with the fact that a buffalo herd typically ranges around 5 km per day (Mloszewski 1983), and a buffalo herd of about 300 individuals occupies 0.5–1 km2 while grazing (Ryan et al. 2006). In other applications, a finer or coarser scale may be appropriate.
The recursion analysis identifies all closed paths, their length, and locations. This is indeed true for the net displacement (Turchin 1998) as well, and the algorithm also could be formulated in terms of net displacement, but we found the complex notation to be preferable because it simplifies the recursion algorithm and furthermore it enables the circle analysis.
Circle analysis
While the previous technique identifies all recursions, it does not indicate whether the movement involved a circular path, clockwise or counterclockwise. However, the periodogram obtained from the complex Fourier transform (CFT),
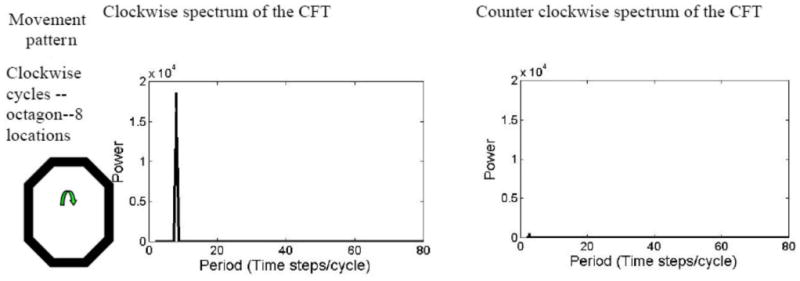
of the locations' coordinates Z(k)=X(k)+iY(k) for k=1, 2,…, K, provides an indication of the trend of circular motion, and can also be used to distinguish clockwise (CW) from counterclockwise (CCW) patterns. The CFT performed on Z(k) indicates the spectrum of CW circles in the movement patterns, whereas a CFT of the complex conjugate coordinates Z*(k)=X(k) − iY(K) indicates the spectrum of CCW circles. These spectra are functions of the frequency f, which is the reciprocal of duration, D (i.e., D = 1/f). The periodogram is defined as the magnitude of the CFT when viewed as a function of D.
To visualize the outcome of a CFT, consider the case of Z(k) representing movement CW around the circumference of a circle in N steps: Z(k)=ei2πfk for N=1/f and k = 1, 2,…, N. Then the CFT, at f = 1/N, equals K, indicating the high value attached to a CW circle. On the other hand, the CFT of a CCW circle, Z*(k) = e−i2πkt is zero (see Appendix B: Fig. B1, simulated for N = 8).
We conducted Monte Carlo tests to assess the confidence level of the circle analysis technique and to identify significant peaks in the periodograms. For the analysis of the model outputs, we simulated a “homogeneous landscape herd,” moving within the same matrix as the original modeled herd according to the same parameters for movement distances (search radius), but with no landscape preferences (i.e., no habitat score, habitat depletion, nor recovery). For the analysis of the empirical data: we simulated a “random directions herd,” moving within the same range as the real herd according to the actual parameters for movement distances, but with no information on past direction. These two kinds of simulated data were used to test the significance of the results when the effects of the additional information (landscape structure for the model outputs, past direction for the empirical data) are evaluated. In both types of simulations, we conducted 1000 repetitions and analyzed them using our circle analysis technique. We defined the 95% bounds obtained from the simulated populations (by taking the 95th percentile of the plots at each time point corresponding to simulated realizations). Data sets for which the actual periodogram exceeds the 95% bounds of the simulated reduced information cases are then considered to indicate that the additional information (landscape structure for the model outputs, past direction for the empirical data) plays a significant role in determining the structure of the movement paths in question.
Results
Movement patterns: model simulations
Recursions in movement patterns were obtained in the simulations as a function of the recovery period parameter tr. After a relative short recovery period (which may reflect the situation in the wet season), the recursion in movement patterns, i.e., return to previous visited locations, led to convergence into a repeated closed route (tr = 15; Fig. 1a), while as the recovery period value increased, the movement patterns broadened and the “herd” moved in a larger circuit/route that led to a decrease in the recursion events (Fig. 1b and Fig. 1c; tr = 45 and 60, respectively) and to “drifting across the landscape” in large recovery periods (e.g., Fig. 1d, tr =90). Long recovery period may reflect the situation in a drought (i.e., no recovery).
Fig. 1.
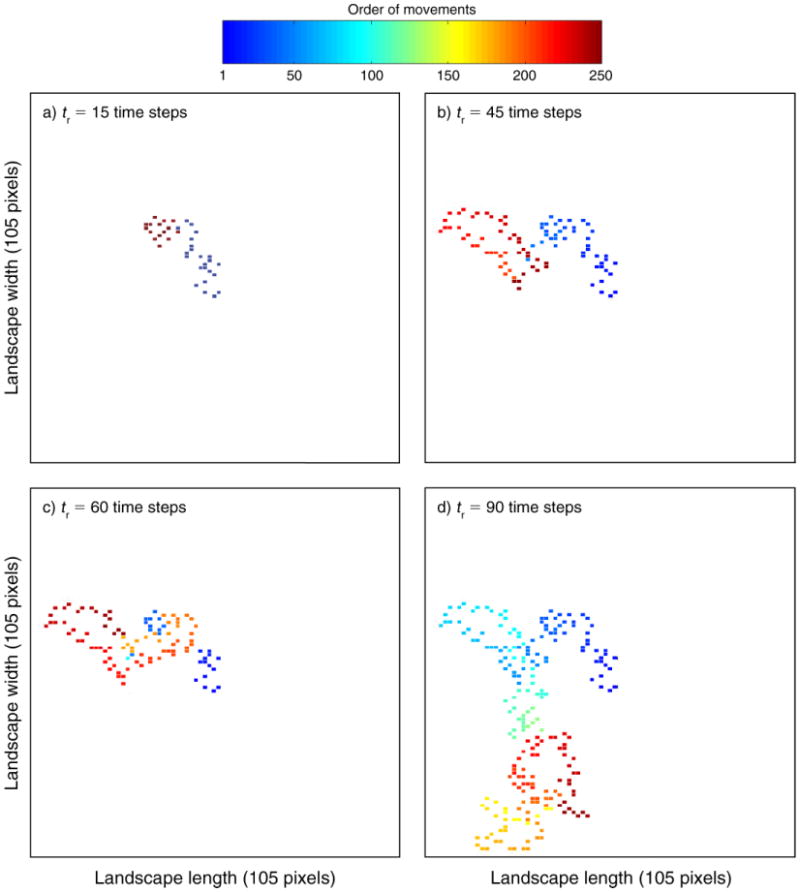
Simulation of space-use patterns of a herd of large herbivores as a function of different resource recovery periods, tr (time steps). All scenarios are on the same random landscape matrix, 105×105 pixels. We assume one movement between locations per time step and a radius of search of 2 cells (pixels). The color represents the order of the movement out of 250 movements (e.g., darkest blue is the first and darkest red the 250th move in the sequence).
The recursion analysis, which ensures that each and every closed path is accounted for, demonstrates that the number of recursions to previously visited sites is a function of recovery period; in a relatively short recovery period (i.e., tr =15), the number of recursions was highest after 16 time steps (∼180 recursions, Fig. 2b), while as the recovery period increased (on the same landscape), the number of recursions decreased and their duration increased (for tr = 45, ∼155 recursions of 48 time steps; for tr = 60, ∼70 recursions of 70 time steps; and for tr = 90, about 25 recursions of 98 time steps; Appendix C: Figs. C1b, C2b, and C3b, respectively).
Fig. 2.
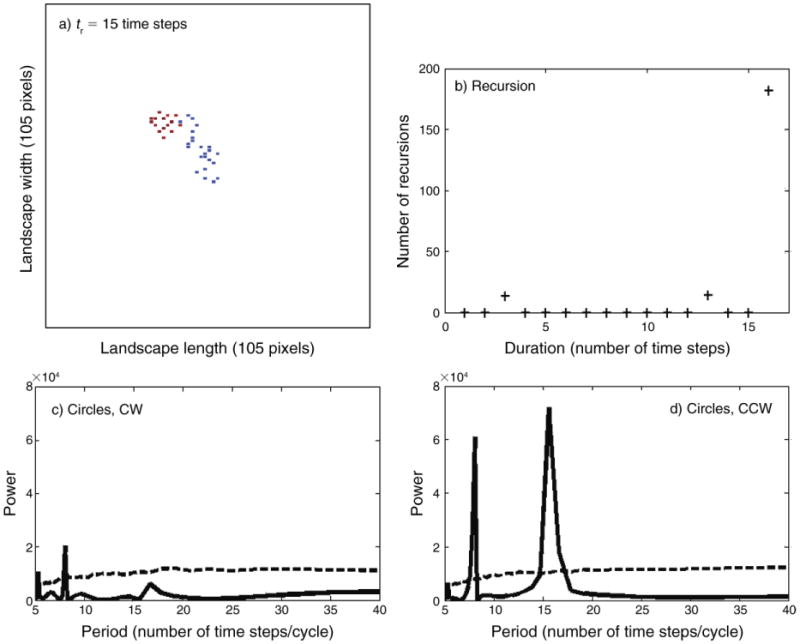
Model simulation outputs of space-use (movement) patterns analyzed by the different procedures. (a) Simulation of space-use patterns of a herd of large herbivores, when recovery period, tr, is 15 time steps in a landscape matrix 105×105 pixels (see Fig. 1). (b) Recursions analysis, the number of recursions (represented by + symbol), defined as the number of instances in which there is an exact (to the resolution of landscape discretization) return to a previous location at the specific duration (in time steps); i.e., where V(t, s) = 0. For details, see Methods: Data analysis techniques. Circle analysis is shown for (c) the spectrums of clockwise (CW) and (d) counterclockwise (CCW) circles in the movement patterns (based on the complex Fourier transform of the locations' coordinates in complex notation). CW and CCW spectrums of model outputs are solid lines, while the 95% bounds obtained from 1000 simulations of a “homogeneous landscape population” analyzed by the circle analysis (i.e., the 95th percentile of the plots at each time point) are dashed lines. At large values of the period (x-axis) the 95th percentile increases roughly with the square of the period. Thus, for clarity of presentation, instead of plotting power directly, we rather plotted power/(1 + [period/Pd]2) where the constant Pd was picked to appropriately reduce the rate of increase, thereby confining the range of the plots. In this figure, Pd = 8, while other values were selected for Figs. 3 and 4.
The periodograms of the circle analysis of the locations' coordinates indicate a tendency for circular patterns (looping) in movements: the main peak (i.e., the maximum power in the graph) in the CCW periodogram in Fig. 2d suggests the occurrence of circular routes of 16 time steps for the short-recovery-period scenario (i.e., tr = 15). The absence of a similar peak in the CW periodogram (Fig. 2c) indicates a circuit of a directional pattern according to the CCW direction. The 16-time-steps route was also identified as a recursion (Fig. 2b). This indicates that this closed route is of a circular pattern. Similarly, the recursion route of 48 time steps, of the scenario of a larger recovery period (i.e., tr = 45) (Appendix C: Fig. C1b) is also of a circular pattern, as indicated by the periodogram (Appendix C: Fig. C1d).
It should be noted that, in general, a zero event in the recursion algorithm indicates an exact recursion, but not necessarily a circular path, and certainly not its direction. On the other hand, a large peak in the clockwise or counterclockwise spectrum indicates circular movement in the appropriate direction, but does not ensure a recursion (i.e., a closed path). In this sense the two techniques are complementary. For example, as previously mentioned, the recursion analysis for tr = 15 indicated 16-time-steps recursion routes; this route was identified by the CCW periodogram as a circular route (Fig. 2d). The CCW periodogram also indicated a smaller peak of the 8-time-step durations (Fig. 2d). This 8-time-step route was with a circular tendency but was not a closed path, because it was not indicated by the recursion analysis as a recursion. Similarly, when tr = 60, the CCW periodogram indicated a large peak of 83-time-step/cycle duration and a small peak of the 42-time-step/cycle durations (Appendix C: Fig. C2d). Only the 83-time-step route is a close path, as it was identified by the recursion analysis (Appendix C: Fig. C2b). Hence, integration between the methods is required when the question is whether there are circular paths among the recursions. Otherwise, if the question is whether there are recursions and how long they take, then the recursion analysis stands by itself.
Although the model was found to be influenced by the landscape templates, which may reflect differences among habitats/areas (Appendix D) and the search radius parameter (Appendix E), the recursion movement pattern was eventually obtained in all of the simulations with short recovery period (tr = 15; Appendices D and E). This fact suggests that recovery period is a factor governing recursion movement dynamics.
Movement patterns: empirical data
Recursions of different durations occur during both the wet and the dry seasons (Figs. 3 and 4) in KPNR and KNP regions. We compared seasonal recursions for three cases in which we had a complete set of data for the same herd (KPNR herd in years 1997 and 1998) and individual #257 (KNP, year 2006) in the wet and dry seasons. Because the data have been collected over different time durations (total number of days), we also compared normalized results for: (1) the recursion proportion of specific duration (i.e., the ratio of the number of zeros for a specific duration to the total number of zero(s), and (2) the recursion proportion of all durations in terms of all paired locations (i.e., the ratio of the number of zeros to the total pairs of locations of duration D ≥ 1). We found no significant difference in the number of recursions or their distribution in wet vs. dry seasons (Appendix F, Table 1; data derived from Figs. 3 and 4). Most of the recursions occurred in a duration of up to ∼20 days (Fig. 3a–e, Fig. 4a–c); however, there were recursions that occurred after more than 40 days (e.g., Fig. 3b).
Fig. 3.
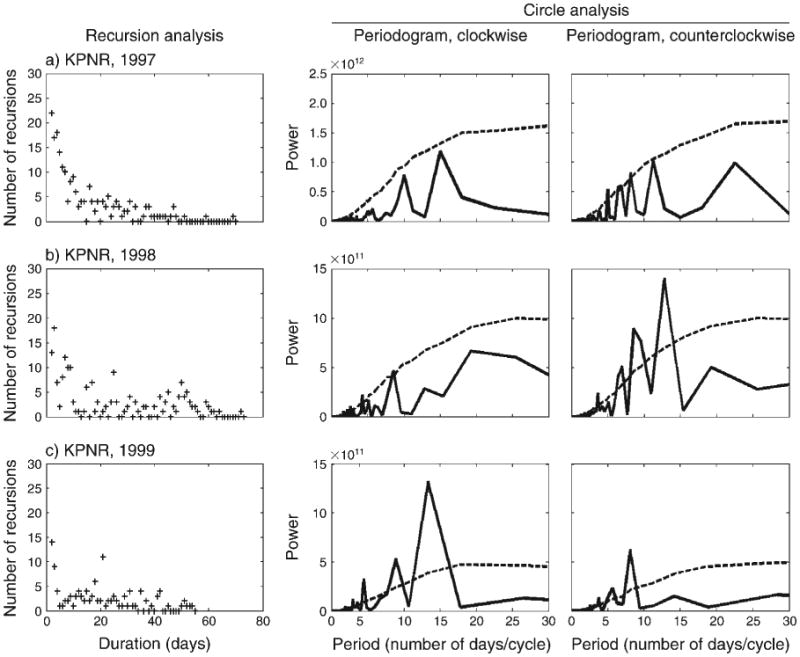
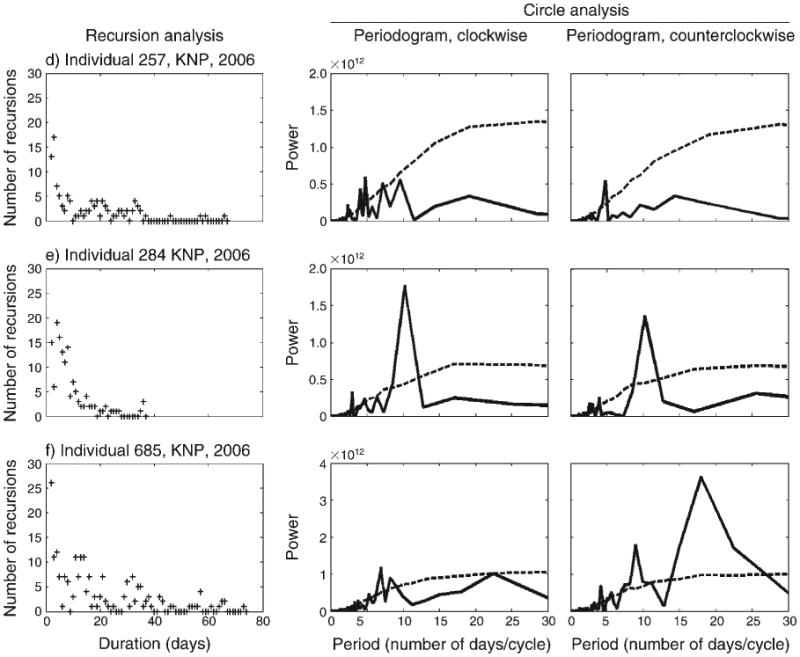
Buffalo movement patterns during the wet season. Movement patterns of (a–c) a Klaserie Private Nature Reserve (KPNR) herd during 1997–1999, and (d–f) individual buffalo (#257, #284, and #685) from the Kruger National Park (KNP) during 2006. The left-hand column shows recursion analysis; the middle and right-hand columns show circle analysis of the spectrums of clockwise (CW) and counterclockwise (CCW) circles, respectively. CW and CCW spectrums of the actual empirical data are solid lines; dashed lines show the 95% bounds obtained from 1000 simulations of a “random directions population” using circle analysis (i.e., the 95th percentile of the plots at each time point). For purpose of presentation, data in panels (c) and (d) were normalized with Pd = 200 (see details in Fig. 2).
Fig. 4.
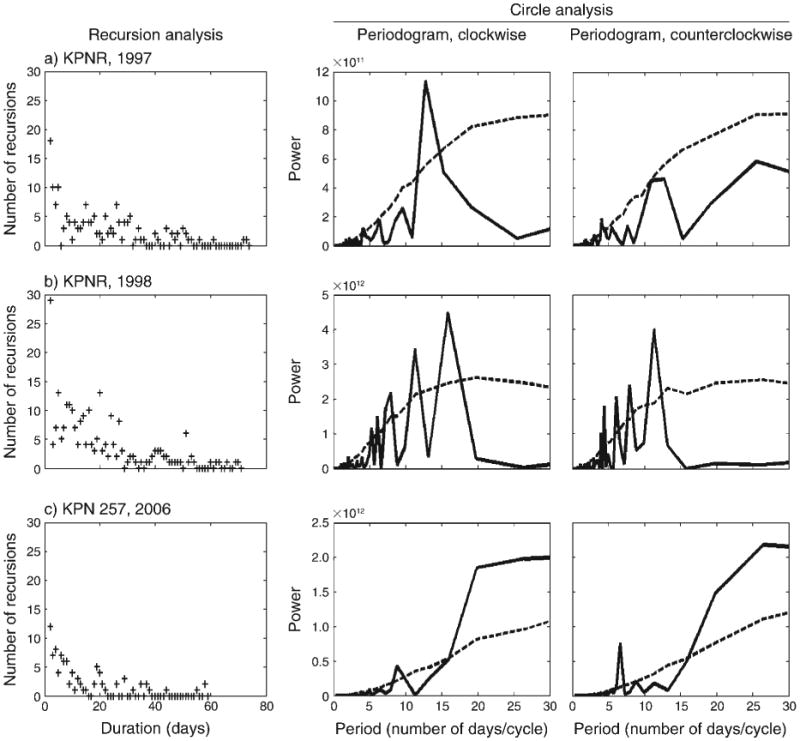
Buffalo movement patterns during the dry season. Movement patterns of (a, b) a Klaserie Private Nature Reserve (KPNR) herd during 1997–1998, and (c) of individual #257 from the Kruger National Park (KNP) during 2006. The left-hand column shows recursion analysis; the middle and right-hand columns show circle analysis of the spectrums of clockwise (CW) and counterclockwise (CCW) circles, respectively. CW and CCW spectrums of the actual empirical data are solid lines; dashed lines show the 95% bounds obtained from 1000 simulations of a “random directions population” using circle analysis (i.e., the 95th percentile of the plots at each time point). For purpose of presentations, data in the circle analysis were normalized with Pd = 200 (see details in Fig. 2).
The periodograms of the circle analysis indicate the tendency of circular patterns in movements in both CW (e.g., Fig. 3c, e; Fig. 4a, b) and CCW (e.g., Fig. 3b, c, e, f; Fig. 4b) directions. The peaks in the periodograms, integrated with the results of the recursion analysis (i.e., the duration of each peak was checked for a recursion), suggest the occurrence of circular closed routes among the recursion routes (e.g., Fig. 3b, c, e, f; Fig. 4a, b). These circular routes of different durations were taken by the buffalo in both the KPNR and the KNP regions. Recursions in circular routes of 10–16 days were relatively common and occurred in the wet (Fig. 3b, c, e) and in the dry seasons (Fig. 4a, b). There was no difference in the directionality of the circular routes, in both seasons and regions.
Discussion
Although considerable research and theory, such as optimal foraging theory (Pyke et al. 1977), focuses on patch choice, patch use, and optimal patch departure (Charnov 1976), little attention has been paid to returns to a previously occupied or depleted patch. Based on the analyses conducted in this study, recursions in movement patterns to pre-visited sites of African buffalo occur throughout the wet and dry seasons in both studied areas (KPNR and KNP).
Spatial dynamics of large herbivores is influenced by spatiotemporal variations in the quality and quantity of forage. Recursions to previously grazed areas may be a useful foraging strategy for large herbivores to consume regrowing vegetation in its stage of high primary productivity (McNaughton 1985, Gordon and Lindsay 1990). These recursions may accelerate nutrient cycling in highly grazed sites (Gordon and Lindsay 1990, McNaughton et al. 1997) and may maintain them as nutrient hotspots (Winnie et al. 2008). The recursion pattern may trigger and maintainapositive feedback loop (McNaughton et al. 1997) in which the large herbivores return to already grazed, high-quality sites, eating the regenerating vegetation that is in its high primary productivity stage. This positive feedback process thus appears to stimulate productivity and accelerate nutrient cycling, thereby leading to an increase in the foraging patch quality and availability on a cyclic basis.
Recursions to foraging sites may lead to cyclic movements among the sites: large herbivores may move in generally circular patterns among highly profitable feeding patches within the area of the home range. This may improve the rate of food intake by individual herbivores in a herd structure (Fryxell 1995). Because grass may be most attractive to herbivores at an intermediate stage of recovery (Fryxell 1995), herbivores would not be expected to wholly consume resources during a visit, and should return before resources have recovered fully. Effectively, they should revisit sites once these have regenerated abundant green regrowth (Gordon and Lindsay 1990). Circular patterns were documented for the African buffalo in Sabi Sand Game Reserve, Transvaal, South Africa, during the hot, wet summer (Funston et al. 1994). A larger spatiotemporal scale of circular passage was also documented in the Serengeti, in which herbivores moved through grasslands during the dry season, but returned to them when residual soil water stimulated new grass growth (McNaughton and Banyikwa 1995). Circular routes (“looping movement”) are considered to be an optimal mode of searching (Turchin 1998). Looping may occur in habitats of high nutritional value, in which individuals might spend more time (Dai et al. 2007); nonoverlapping looping movements may avoid foraging in the same place, and a circular trajectory is less likely to cross a previous pathway than a less directed meander. Therefore, this strategy may facilitate the recovery of utilized vegetation (Dai et al. 2007).
Recursion patterns emerged in our model outputs, under simple rules. Recursions in movement patterns appeared in the model as a function of the vegetation recovery period parameter: After a relatively short recovery period, the recursion pattern led to convergence into a repeated circular path, while, as the recovery period increased, the herd moved in a larger path, which led to a decrease in the recursion events. This pattern suggested that recovery period may be a factor, among other factors, governing recursion movement dynamics. The recovery period may represent the time it takes for the vegetation to reach the high primary productivity stage. The productivity stage of grassland was found to affect herbivore seasonal movement patterns (McNaughton 1985). In our model we assumed that herds had knowledge about the habitat quality of neighboring cells (within a search radius). The scale of movement relative to the scale of the habitat patches, and knowledge of the habitat quality of distant patches, are likely to affect movement dynamics. An important area of future research is to develop an understanding of how scale affects recursion patterns.
The vegetation recovery period may be a function of the season (Hik and Jefferies 1990) and habitat parameters, e.g., riverbeds and waterholes support greater plant growth throughout the year (Winnie et al. 2008); in nutrient hotspots there are considerably higher plant-available levels (McNaughton et al. 1997). Hence, under specific conditions such as near river beds, or in the nutrient hotspots, the recovery period may be shorter. This may lead to an increase in recursion patterns to profitable foraging sites and to convergence into circular patterns, as described for African buffalo in the wet season (Funston et al. 1994). It may also lead to a decrease in home range size in nutrient hotspots areas (Winnie et al. 2008). Future studies should estimate recovery periods in different areas and seasons (under different conditions such as the amount of precipitation and its distribution) and correlate them with recursion patterns.
We found that recursions in movement patterns of African buffalo in KPNR and KNP occur throughout the wet and dry seasons. Based on the simulation model, recursions were not expected to occur in the dry season (i.e., long recovery period), but this analysis did not account for the fact that patches are usually partially, rather than wholly, consumed during a visit, so a herd may return to high-quality, partially depleted patches in both seasons. Moreover, a high proportion of the recursions in circular routes occur within a time interval of ∼10–16 days since a previous visit in the area, in both seasons. The recovery period for vegetation in a depleted grazing patch may take longer than this time interval. However, African buffalo herds, which move through grassland areas, do not completely deplete the entire area that they pass through (Vesey-Fitzgerald 1960, Pienaar 1969, Leuthold 1972). They are selective feeders when they have the opportunity (Prins 1996, Macandza et al. 2004). Moreover, herds may have an advantage in leaving high-quality patches only partially depleted and returning to them some time later, as parasite control. While moving through grassland areas, African buffalo may contaminate these areas with their feces. Returning to a partially depleted patch too soon after leaving it may increase the risk of infection or disease transmission by fecal parasites (Ezenwa 2004). Thus, there may be a trade-off between “staying in” and “returning to” the high-quality, partially depleted patch and the risk of infection by fecal parasites. African buffalo might avoid areas of recent use for a specific time interval (e.g., ∼10–16 days) to decrease the risk of infection by recontacting their own feces, assuming that this is sufficient lag time for infectivity to dissipate. Further studies are needed to pursue this hypothesis.
With the potential advantage of recursions to foraging sites (increasing individual intake, accelerating nutrient cycles, and maintaining high-quality forage patches), one would expect to see a high frequency of such patterns among the movements of large herbivores. However, based on our analyses of African buffalo herds, these recursion patterns are not that frequent. Large herbivores should have the spatial memory to return to high-quality foraging sites, but they might “choose” another foraging strategy and continually resample the forage in other areas in order to ascertain its quality (Hewitson et al. 2005). Moreover, large herbivores not only respond to food resources but also are constrained by other environmental, abiotic (e.g., water and shelter), and biotic factors (e.g., inter- and intraspecies interactions), in their movement patterns (Roguet et al 1998). For example, among the high-quality sites are areas with a higher predation risk; herbivores might avoid these areas and choose lower quality patches (Winnie et al. 2008). Similarly, movement patterns of other herbivore herds (of conspecific or other species) might affect movement decisions. The interaction between the different factors that govern the movement dynamics of large herbivores can lead to a deviation from the expected circular patterns.
Recursions may be driven by other factors, e.g., by water sources (Redfern et al. 2003), salt licks (Ayotte et al. 2006, Mills and Milewski 2007), selected plants, favorable resting areas, and commonly used traveled routes. Future studies should characterize the recursion sites in terms of habitat structure, landscape characteristics, frequency, and time interval among recursions (i.e., the relative attractiveness of those sites) and should examine their potential importance for population dynamics. Characterizing these sites may help to identify the different factors involved in governing the recursion patterns in different seasons and may also serve as a basis for further modeling work.
Among the recursions identified by the recursion analysis, could be casual recursions as a result of roaming in a confined area, i.e., the herd while moving in its home range could casually cross its tracks (casual crossing site). This could be a function of the size of the home range and its topography. The time spent in a recursion site could be a measure for distinguishing between a “casual” and a “substantial” recursion site; if it is a casual crossing site, we expect that a herd will not stay there a long while. If it is a deliberately sought recursion site, we expect the herd to stay there longer. The time spent in a site could be measured by the number of GPS locations in a site out of sequential locations (it depends on the frequency of GPS measurements, the spatial resolution of a “site,” and the velocity of the herd when “heading” vs. when “local foraging”). This could be examined using the recursion analysis. Direct field observation on behavior patterns in identified recursion sites could help in classifying these sites as casual crossing sites or substantial recursion sites.
The methods developed in this study can help researchers to identify recursions and circular patterns in the maze of GPS location data of the studied organisms. These methods enable examination from coarser to finer scale patterns of movement. The exact advent of recursions (i.e., actual returns to previously occupied sites) is obtained using our recursion analysis, which identifies all closed paths, their length, and locations based on the observation that along a path that closes on itself, the sum of vector displacements is zero. The recursion analysis does not indicate the direction of the movement nor whether it involves a looping path. In these terms, the advantages of the circle analysis are in both providing indication of a trend of circular motion and in distinguishing between CW and CCW looping. On the other hand, the circle analysis by itself does not ensure a recursion; hence, a combination of the different techniques enables a comprehensive view of recursion patterns. If the question is whether there are recursions and how long they take, then the recursion analysis stands by itself. If the question is whether there is a circular tendency in the movement patterns, then use of the circle analysis could provide information. Finally, if the question is whether there are circular paths among the recursions, then integration between the recursion and circle analyses is required.
With an increase in the quantity and quality (i.e., resolution) of GPS data collected in wildlife studies, the methods that we present here should provide new insights into the form and function of recursion patterns of various organisms, thereby increasing our understanding of the factors governing their movement dynamics, with implications for disease transmission (e.g., recontacting feces) and herbivore–plant synergisms (e.g., nutrient cycling). This information could be further applied for conservation policy and management of large herbivores and their habitats; for example, in designing nature reserves so that they include the recursion sites and appropriate landscape connectivity, and in implementing disease control strategies at such sites. Furthermore, such information could also be used by managers in tourist areas and game resorts.
Acknowledgments
This research was funded by the U.S. National Science Foundation Ecology of Infectious Disease Grant and a James S. McDonnell 21st Century Science Initiative Award (WMG). S. J. Ryan was also supported by an EPA STAR grant and an NSF Bioinformatics Fellowship. We thank Justin Bowers, Craig Hay, Julie Wolhuter, Kutani Bulunga, Augusta Mabunda, Fernando Muhlovo, Markus Hofmeyr, Peter Buss, Lin-Mari de Klerk, Roy Bengis, Douw Grobler, N. Zambatis, C. Rowles, E. Leibnitz, M. Peel, KPNR rangers and camp-managers, KNP managers and scientists, and KNP Game Capture and State Veterinary technicians. C. U. Knechtel's fieldwork in KPNR was supported by the German Academic Exchange Service (DAAD), Daimler-Chrysler South Africa, and the State Chancellery Nordrhein-Westfalen/Germany. We thank Norman Owen-Smith and an anonymous reviewer for constructive comments on previous versions of this article. This is publication number 639 of the Mitrani Department of Desert Ecology.
APPENDIX A
Map of the study area and space-use patterns of African buffalo herds within the study area, South Africa (Ecological Archives E090-173-A1).
Fig. A1.
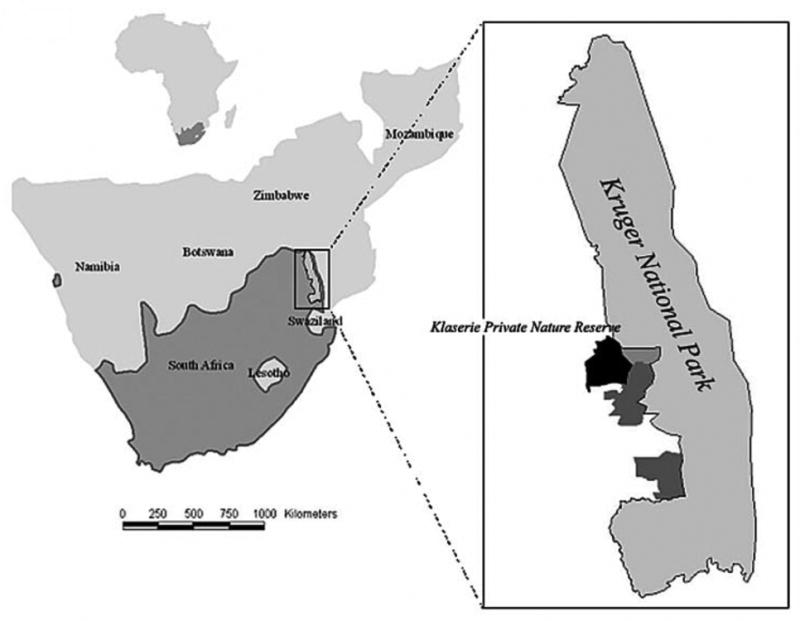
Map of the Klaserie Private Nature Reserve (KPNR), and Kruger National Park (KNP) South Africa.
Fig. A2.
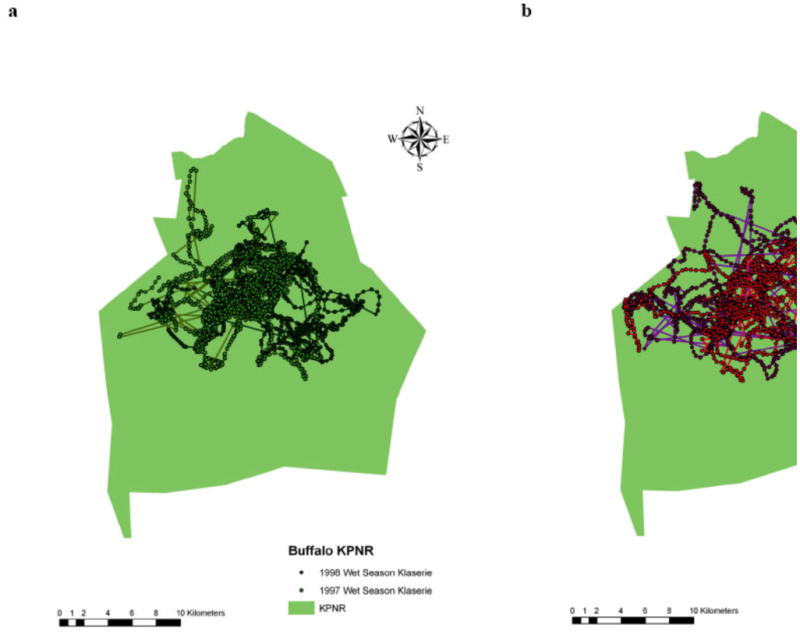
Space-use patterns of an African buffalo herd in Klaserie Private Nature Reserve (KPNR), South Africa during the (a) wet (December–February) and (b) dry (June–August) seasons of 1997 and 1998. Each dot represents a location of the herd (12-20 locations per day, for 21–month).
Fig. A3.
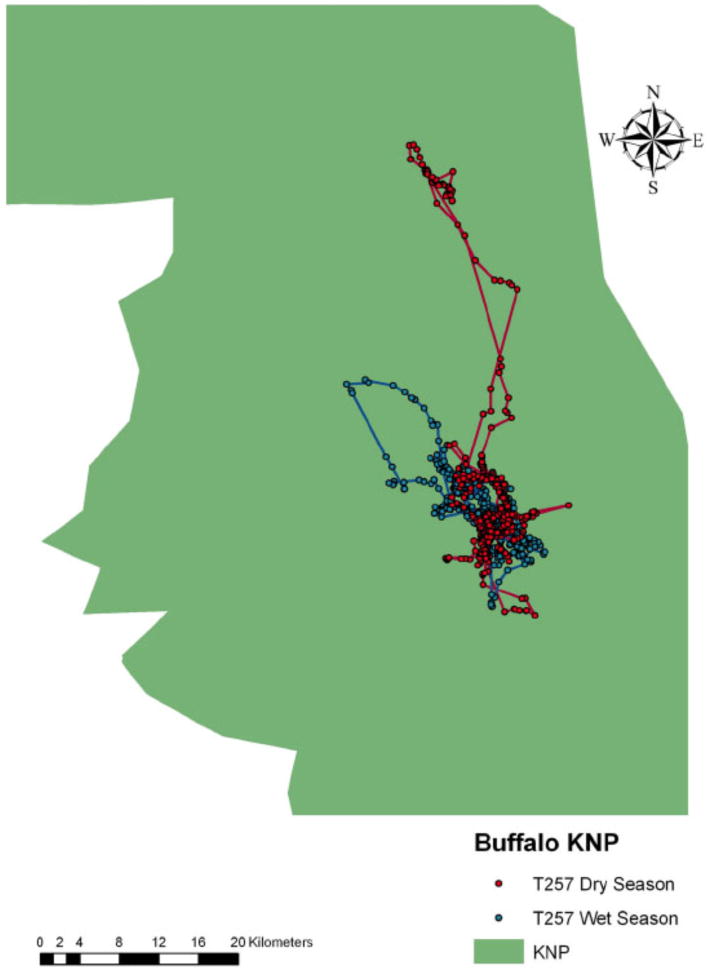
Space-use patterns of an African buffalo GPS-collared individual (#257), in Kruger National Park (KNP) South Africa during the wet (December–February) and dry (June–August) seasons of 2006. Each dot represents a location of the individual.
Fig. A4.
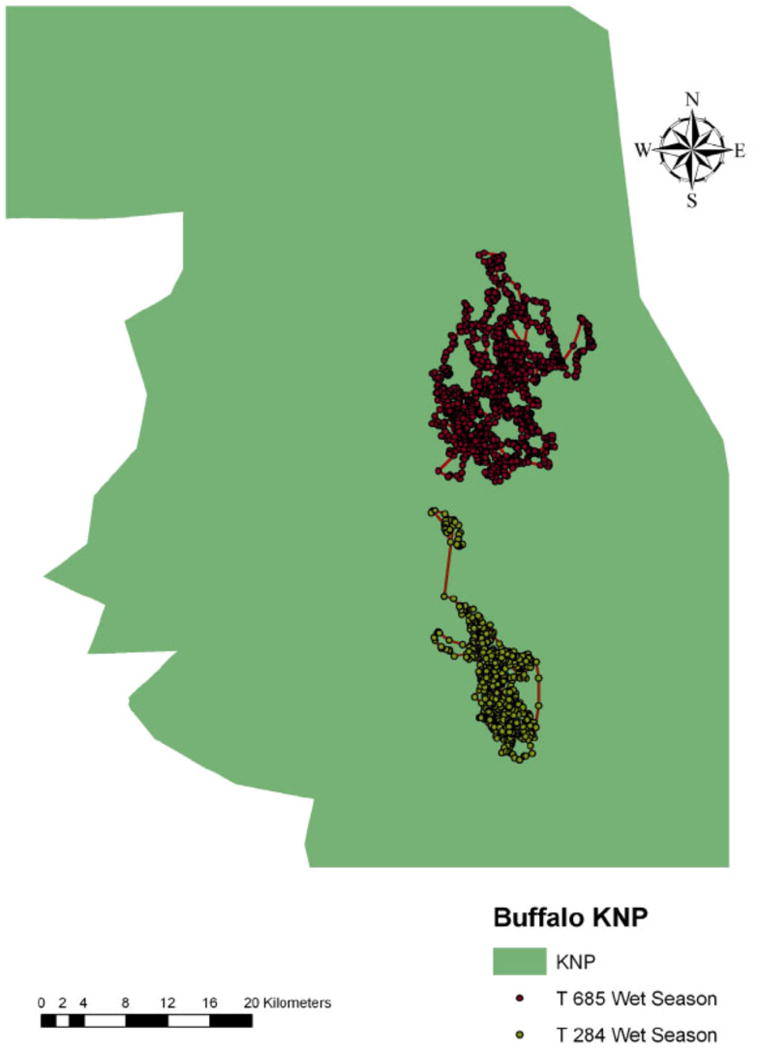
Space-use patterns of an African buffalo GPS-collared individuals (#284, #685), in Kruger National Park (KNP) South Africa during the wet season of 2006. Each dot in a specific color represents a location of one individual.
APPENDIX B
Circle analysis using complex Fourier transform (CFT) periodograms: an example (Ecological Archives E090-173-A2).
APPENDIX C
Model simulation outputs of space-use (movement) patterns with different recovery periods, analyzed by the different methods (Ecological Archives E090-173-A3).
Fig. C1.
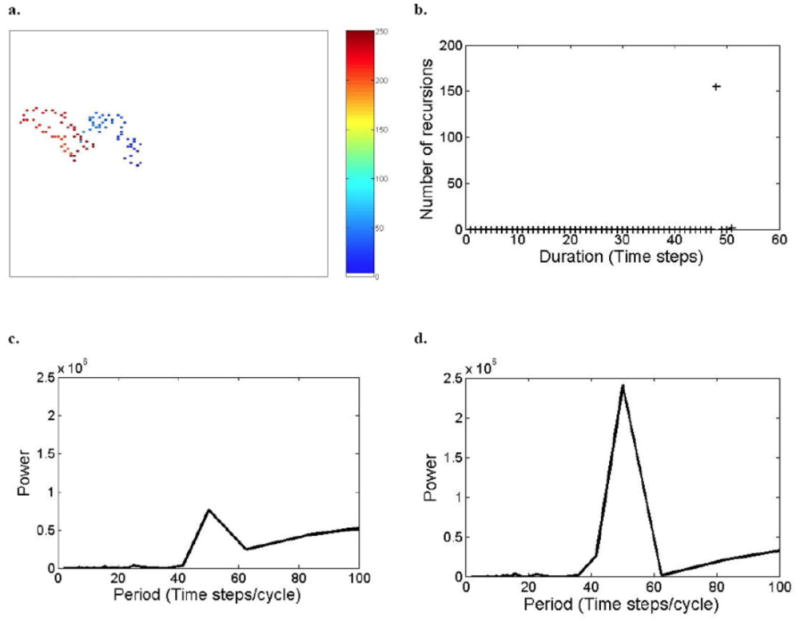
Model simulation outputs of space-use (movement) patterns analyzed by the different procedures. (a) Simulation of space use patterns of a herd of large herbivores on a random landscape matrix (105 by 105 pixels (“patches”), the same matrix as Figs. 2–3 in this appendix and Fig.1 in the main paper), when recovery period, tr = 45 time steps. We assume one movement between locations per time step and a “radius of search” of 2 pixels. The color represents the order of the movement out of 250 movements (e.g., lightest blue is the first and darkest red the 250th move in the sequence); (b) recursions analysis: the number of recursions (Y axis), presented by + symbol, is defined as the number of pairs of locations of which V(t,s) = 0 of a specific duration (X axis); (c, d) circle analysis: the spectrums of clockwise (c) and counter clockwise circles (d) in the movement patterns (based on the complex Fourier transform of the locations' coordinates in complex notation).
Fig. C2.
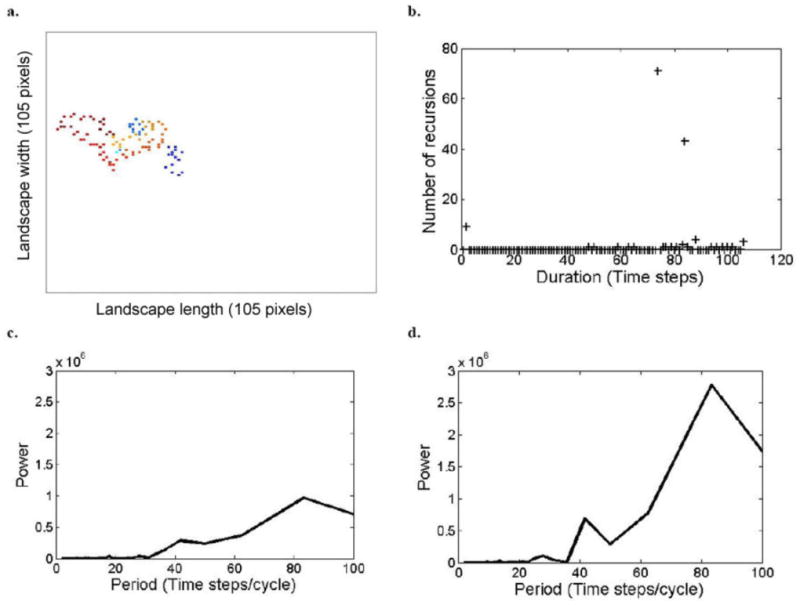
Model simulation outputs of space-use patterns of a herd of large herbivores, when Recovery Period, tr = 60 time steps, analyzed by the different methods. See caption of Fig. 1.
Fig. C3.
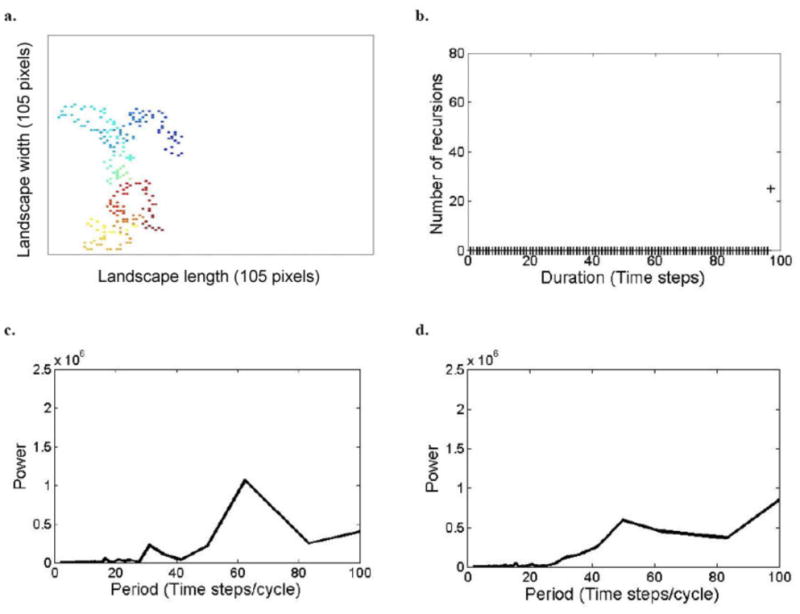
Model simulation outputs of space-use patterns of a herd of large herbivores, when recovery period, tr = 90 time steps, analyzed by the different methods. See caption of Fig. 1.
APPENDIX D
Model simulation outputs of space-use (movement) patterns over different random landscape matrices (Ecological Archives E090-173-A4).
Fig. D1.
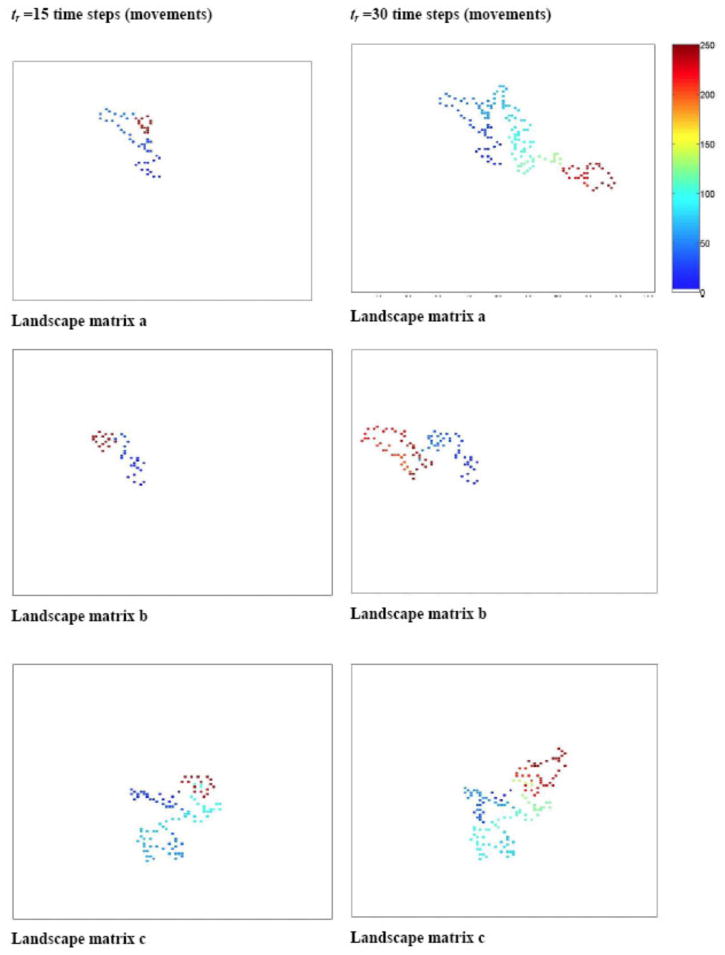
Simulation of space-use patterns of a herd of large herbivores over different random landscape matrices (a–c) as a function of resource recovery period (tr = 15 or tr = 30 time steps). We assume one movement between locations per time step and a radius of search of 2 pixels (patches). The color represents the order of the movement out of 250 movements (e.g., lightest blue is the first and darkest red the 250th move in the sequence).
APPENDIX E
Model simulation outputs of space-use (movement) patterns with different radius of search (Ecological Archives E090-173-A5).
Fig. E1.
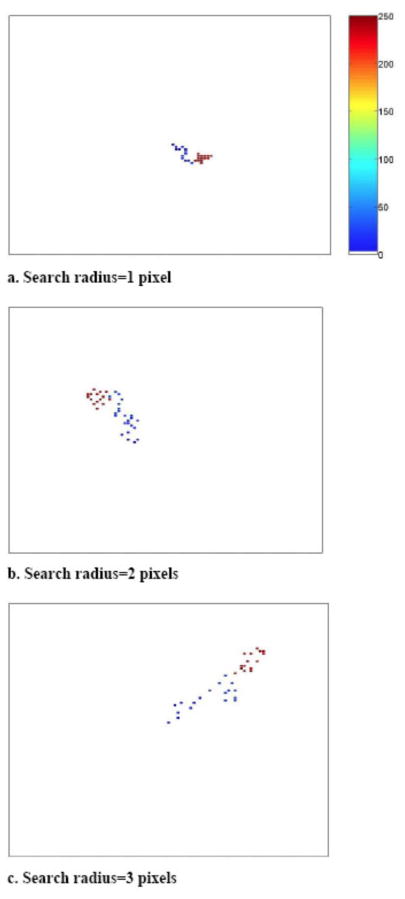
Simulated space-use patterns of a herd of large herbivores with different “radius of search” i.e., 1, 2, and 3 pixels (“patches”). All Scenarios are on the same random landscape matrix (105 × 105 pixels). We assume one movement between locations per unit time and resource recovery period, tr = 15 time steps. We assume one movement between locations per time step. The color represents the order of the movement out of 250 movements (e.g., lightest blue is the first and darkest red the 250th move in the sequence).
APPENDIX F
Recursion proportions within seasons (Ecological Archives E090-173-A6).
Table. F1.
(a) Recursion proportion of all durations, out of all paired locations — the ratio of the number of zeros (V(t,s) = 0) to the total pairs of locations (of duration D ≥ 1); (b) and (c) Recursion proportion of specific duration, out of all recursions — the ratio of the number of zeros for a specific duration (D ≥ 10 and D ≥ 20, respectively) to the total number of zeros.
| Name of individual/herd | Year | a) Recursion proportions out of all paired locations | b) Recursion proportions of D ≥ 10 days out of all recursions | c) Recursion proportions of D ≥ 20 days out of all recursions | |||
|---|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | ||
| Klaserie | 1997 | 0.0019 | 0.0012 | 0.1327 | 0.0706 | 0.086 | 0.0399 |
| Klaserie | 1998 | 0.0011 | 0.0015 | 0.1123 | 0.1001 | 0.0665 | 0.0794 |
| KNP 257 | 2006 | 0.003 | 0.0021 | 0.0891 | 0.072 | 0.0478 | 0.0458 |
| P value (paired t test, one-tailed) | 0.2133 | 0.097 | 0.2879 | ||||
Footnotes
Corresponding Editor: S. T. Buckland
Literature Cited
- Ayotte JB, Parker KL, Arocena JM, Gillingham MP. Chemical composition of lick soils: functions of soil ingestion by four ungulate species. Journal of Mammalogy. 2006;87:878–888. [Google Scholar]
- Brown JH, Kodric-Brown A. Turnover rates in insular biogeography: effect of immigration on extinction. Ecology. 1977;58:445–449. [Google Scholar]
- Bruggeman JE, Garrott RA, White PJ, Watson FGR, Wallen R. Covariates affecting spatial variability in bison travel behavior in Yellowstone National Park. Ecological Applications. 2007;17:1411–1423. doi: 10.1890/06-0196.1. [DOI] [PubMed] [Google Scholar]
- Burdett CL, Moen RA, Niemi GJ, Mech LD. Defining space use and movements of Canada lynx with global positioning system telemetry. Journal of Mammalogy. 2007;88:457–467. [Google Scholar]
- Charnov EL. Optimal foraging: marginal value theorem. Theoretical Population Biology. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-x. [DOI] [PubMed] [Google Scholar]
- Colbert J, Danchin E, Dhondt AA, Nichols JD. Dispersal. Oxford University Press; New York, New York, USA: 2001. [Google Scholar]
- Cooke SJ, Hinch SG, Wikelski M, Andrews RD, Kuchel LJ, Wolcott TG, Butler PJ. Biotelemetry: a mechanistic approach to ecology. Trends in Ecology and Evolution. 2004;19:334–343. doi: 10.1016/j.tree.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Cross PC, Lloyd-Smith JO, Johnson PLF, Getz WM. Duelling timescales of host movement and disease recovery determine invasion of disease in structured populations. Ecology Letters. 2005;8:587–595. [Google Scholar]
- Dai XH, Shannon G, Slotow R, Page B, Duffy KJ. Short-duration daytime movements of a cow herd of African elephants. Journal of Mammalogy. 2007;88:151–157. [Google Scholar]
- Dalziel BD, Morales JM, Fryxell JM. Fitting probability distributions to animal movement trajectories: dynamic models linking distance, resources, and memory. American Naturalist. 2008;172:248–258. doi: 10.1086/589448. [DOI] [PubMed] [Google Scholar]
- Dussault C, Ouellet JP, Courtois R, Huot J, Breton L, Jolicoeur H. Linking moose habitat selection to limiting factors. Ecography. 2005;28:619–628. [Google Scholar]
- Edwards GR, Newman JA, Parsons AJ, Krebs JR. The use of spatial memory by grazing animals to locate food patches in spatially heterogeneous environments: an example with sheep. Applied Animal Behaviour Science. 1996;50:147–160. [Google Scholar]
- Ezenwa VO. Selective defecation and selective foraging: antiparasite behavior in wild ungulates? Ethology. 2004;110:851–862. [Google Scholar]
- Fryxell JM. Aggregation and migration by grazing ungulates in relation to resources and predators. In: Sinclair ARE, Arcese P, editors. Serengeti II, dynamics, management, and conservation of an ecosystem. University of Chicago Press; Chicago, Illinois, USA: 1995. pp. 257–273. [Google Scholar]
- Fryxell JM, Greever J, Sinclair ARE. Why are migratory ungulates so abundant? American Naturalist. 1988;131:781–798. [Google Scholar]
- Fryxell JM, Wilmshurst JF, Sinclair ARE, Haydon DT, Holt RD, Abrams PA. Landscape scale, heterogeneity, and the viability of Serengeti grazers. Ecology Letters. 2005;8:328–335. [Google Scholar]
- Funston PJ, Skinner JD, Dott HM. Seasonal variation in movement patterns, home-range and habitat selection of buffalos in a semiarid habitat. African Journal of Ecology. 1994;32:100–114. [Google Scholar]
- Galanti V, Preatoni D, Martinoti A, Wauters LA, Tosi G. Space and habitat use of the African elephant in the Tarangire-Manyara ecosystem, Tanzania: Implications for conservation. Mammalian Biology. 2006;71:99–114. [Google Scholar]
- Getz WM, Fortmann-Roe S, Cross PC, Lyons AJ, Ryan SJ, Wilmers CC. LoCoH: nonparametric kernel methods for constructing home ranges and utilization distributions. PLoS ONE. 2007;2(2):e207. doi: 10.1371/journal.pone.0000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz WM, Saltz D. A framework for generating and analyzing movement paths on ecological landscapes. Proceedings of the National Academy of Sciences (USA) 2008;105:19066–19071. doi: 10.1073/pnas.0801732105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz WM, Wilmers CC. A local nearest-neighbor convex-hull construction of home ranges and utilization distributions. Ecography. 2004;27:489–505. [Google Scholar]
- Gillingham MP, Bunnell FL. Effects of learning on food selection and searching behavior of deer. Canadian Journal of Zoology. 1989;67:24–32. [Google Scholar]
- Gordon IJ, Lindsay WK. Could mammalian herbivores manage their resources? Oikos. 1990;59:270–280. [Google Scholar]
- Hanski IA, Gilpin ME. Metapopulation biology: ecology, genetics and evolution. Academic Press; San Diego, California, USA: 1997. [Google Scholar]
- Hewitson L, Dumont B, Gordon IJ. Response of foraging sheep to variability in the spatial distribution of resources. Animal Behaviour. 2005;69:1069–1076. [Google Scholar]
- Hik DS, Jefferies RL. Increases in the net aboveground primary production of a salt-marsh forage grass: a test of the predictions of the herbivore-optimization model. Journal of Ecology. 1990;78:180–195. [Google Scholar]
- Hobbs NT, Schimel DS, Owensby CE, Ojima DS. Fire and grazing in the tallgrass prairie: contingent effects on nitrogen budgets. Ecology. 1991;72:1374–1382. [Google Scholar]
- Kokko H, Lopez-Sepulcre A. From individual dispersal to species ranges: perspectives for a changing world. Science. 2006;313:789–791. doi: 10.1126/science.1128566. [DOI] [PubMed] [Google Scholar]
- Leuthold W. Home range, movements, and food of buffalo herd in Tsavo National Park. East Africa Wildlife Journal. 1972;10:237–243. [Google Scholar]
- Macandza VA, Owen-Smith N, Cross PC. Forage selection by African buffalo in the late dry season in two landscapes. South African Journal of Wildlife Research. 2004;34:113–121. [Google Scholar]
- MathWorks . MATLAB 6.1. MathWorks; Natick, Massachusetts, USA: 2001. [Google Scholar]
- McNaughton MM, Banyikwa FF. Plant community and herbivory. In: Sinclair ARE, Arcese P, editors. Serengeti II, dynamics, management, and conservation of an ecosystem. University of Chicago Press; Chicago, Illinois, USA: 1995. pp. 49–70. [Google Scholar]
- McNaughton SJ. Ecology of a grazing ecosystem: the Serengeti. Ecological Monographs. 1985;55:259–294. [Google Scholar]
- McNaughton SJ, Banyikwa FF, McNaughton MM. Promotion of the cycling of diet-enhancing nutrients by African grazers. Science. 1997;278:1798–1800. doi: 10.1126/science.278.5344.1798. [DOI] [PubMed] [Google Scholar]
- Mills A, Milewski A. Geophagy and nutrient supplementation in the Ngorongoro Conservation Area, Tanzania, with particular reference to selenium, cobalt and molybdenum. Journal of Zoology. 2007;271:110–118. [Google Scholar]
- Mloszewski MJ. The behavior and ecology of the African buffalo. Cambridge University Press; Cambridge, UK: 1983. [Google Scholar]
- Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. Moving forward with movement ecology. Proceedings of the National Academy of Sciences (USA) 2008;105:19052–19059. doi: 10.1073/pnas.0800375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Smith NR. Megaherbivores: The influence of very large body size on ecology. Cambridge University Press; Cambridge, UK: 1988. [Google Scholar]
- Patterson TA, Thomas L, Wilcox C, Ovaskainen O, Matthiopoulos J. State–space models of individual animal movement. Trends in Ecology and Evolution. 2008;23:87–94. doi: 10.1016/j.tree.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Pienaar UdV. Observations on developmental biology, growth and some aspects of the population ecology of African buffalo (Syncerus caffer caffer Sparrman) in the Kruger National Park. Koedoe. 1969;12:29–53. [Google Scholar]
- Polis GA, Anderson WB, Holt RD. Toward an integration of landscape and food web ecology: The dynamics of spatially subsidized food webs. Annual Review of Ecology and Systematics. 1997;28:289–316. [Google Scholar]
- Preisler HK, Ager AA, Johnson BK, Kie JG. Modeling animal movements using stochastic differential equations. Environmetrics. 2004;15:643–657. [Google Scholar]
- Prins HHT. Ecology and behaviour of the African buffalo. Social inequality and decision making. Chapman and Hall; London, UK: 1996. [Google Scholar]
- Pyke GH, Pulliam HR, Charnov EL. Optimal foraging: selective review of theory and tests. Quarterly Review of Biology. 1977;52:137–154. [Google Scholar]
- Redfern JV, Grant R, Biggs H, Getz WM. Surface-water constraints on herbivore foraging in the Kruger National Park, South Africa. Ecology. 2003;84:2092–2107. [Google Scholar]
- Rodgers AR, Rempel RS, Abraham KF. A GPS-based telemetry system. Wildlife Society Bulletin. 1996;24:559–566. [Google Scholar]
- Roguet C, Dumont B, Prache S. Selection and use of feeding sites and feeding stations by herbivores: a review. Annales de Zootechnie. 1998;47:225–244. [Google Scholar]
- Ryan SJ, Knechtel CU, Getz WM. Range and habitat selection of African buffalo in South Africa. Journal of Wildlife Management. 2006;70:764–776. [Google Scholar]
- Schofield G, Bishop CM, MacLean G, Brown P, Baker M, Katselidis KA, Dimopoulos P, Pantis JD, Hays GC. Novel GPS tracking of sea turtles as a tool for conservation management. Journal of Experimental Marine Biology and Ecology. 2007;347:58–68. [Google Scholar]
- Sinclair ARE. The African buffalo. University of Chicago Press; Chicago, Illinois, USA: 1977. [Google Scholar]
- Sinclair ARE, Arcese P. Serengeti II, dynamics, management, and conservation of an ecosystem. University of Chicago Press; Chicago, Illinois, USA: 1995. [Google Scholar]
- Sugden A, Pennisi E. When to go, where to stop: introduction. Science. 2006;313:775. [Google Scholar]
- Turchin P. Quantitative analysis of movement. Measuring and modeling population redistribution in animals and plants. Sinauer Associates; Sunderland, Massachusetts, USA: 1998. [Google Scholar]
- Vesey-Fitzgerald DF. Grazing succession among East African game animals. Journal of Mammalogy. 1960;41:161–172. [Google Scholar]
- White GC, Garrott RA. Analysis of wildlife radio-tracking data. Academic Press; New York, New York, USA: 1990. [Google Scholar]
- Winnie JA, Cross PC, Getz WM. Habitat quality and heterogeneity influences distribution, individual and herd behavior in African buffalo (Syncerus caffer) Ecology. 2008;89:1457–1468. doi: 10.1890/07-0772.1. [DOI] [PubMed] [Google Scholar]
- Wittemyer G, Polansky L, Douglas-Hamilton I, Getz WM. Nonstationary influences of season, location and sociality on properties of movement among African elephants (Loxodonta africana) Proceedings of the National Academy of Sciences (USA) 2008;105:19108–19113. doi: 10.1073/pnas.0801744105. [DOI] [PMC free article] [PubMed] [Google Scholar]


