Abstract
Background
Although inflammatory activity is known to play a role in depression, no work has examined whether experimentally induced systemic inflammation alters neural activity that is associated with anhedonia, a key diagnostic symptom of depression. To investigate this, we examined the effect of an experimental inflammatory challenge on the neural correlates of anhedonia—namely, reduced ventral striatum (VS) activity to reward cues. We also examined whether this altered neural activity related to inflammatory-induced increases in depressed mood.
Methods
Participants (n = 39) were randomly assigned to receive either placebo or low-dose endotoxin, which increases proinflammatory cytokine levels in a safe manner. Cytokine levels were repeatedly assessed through hourly blood draws; self-reported and observer-rated depressed mood were assessed regularly as well. Two hours after drug administration, neural activity was recorded as participants completed a task in which they anticipated monetary rewards.
Results
Results demonstrated that subjects exposed to endotoxin, compared with placebo, showed greater increases in self-reported and observer-rated depressed mood over time, as well as significant reductions in VS activity to monetary reward cues. Moreover, the relationship between exposure to inflammatory challenge and increases in observer-rated depressed mood was mediated by between-group differences in VS activity to anticipated reward.
Conclusions
The data reported here show, for the first time, that inflammation alters reward-related neural responding in humans and that these reward-related neural responses mediate the effects of inflammation on depressed mood. As such, these findings have implications for understanding risk of depression in persons with underlying inflammation.
Keywords: Anhedonia, depressed mood, immune, inflammation, proinflammatory cytokines, reward, ventral striatum
Substantial evidence has demonstrated that inflammation, characterized by increases in proinflammatory cytokine activity, plays a critical role in the onset and perpetuation of depression and depressive symptoms in those who are comorbid for inflammatory disorders (1). Consistent with this, experimental work has shown that exposure to inflammatory challenge increases depressed or negative mood in healthy samples (2–4). Based on these findings, there has been a growing interest in whether inflammatory processes contribute to depression in a causal manner and how such effects might occur.
Anhedonia—the lack of reactivity to pleasurable stimuli—is an important symptom of endogenous depression and, along with depressed mood, forms the key diagnostic criteria for depressive disorder (5). Whereas negative affect is a feature of most emotional disorders, anhedonia is a symptom that is unique to depression (6). However, even though anhedonia figures prominently in the specific etiology of depression, the effect of experimentally induced inflammation on anhedonia has not been examined in humans.
In animals, experimental immune system activation has been shown to produce anhedonic-like behavior (7). Experimental inflammatory challenge has been shown to decrease reward-related responding in rats as indexed by reductions in saccharin preference (8) and reductions in rewarding neural self-stimulation (9). This inflammatory-induced change in behavior is consistent with the notion that proinflammatory cytokines can signal the brain to initiate “sickness behavior,” a motivational response to infection or disease that is thought to promote recovery from illness (10,11). As such, anhedonia may be an adaptive response to sickness that promotes recuperative behavior and prevents individuals from engaging in activities that require excessive energy. Still, little is known about the effect of inflammation on anhedonia in humans or the neural mechanisms that underlie these changes.
Neuroimaging studies have successfully identified some of the neural regions involved in reward processing, which are hypothesized to contribute to the anhedonic symptoms of depression (12). Thus, the ventral striatum (VS) has been found to figure prominently in the anticipation of rewards (13,14). Dopamine projections to the VS fire selectively in response to the presentation of reward cues in monkeys (15), and the anticipation of monetary reward activates the VS in humans (16,17). Interestingly, comparative research suggests that dopamine release in the VS is greater during reward anticipation than reward consumption (13,18) and that the VS is particularly sensitive to the anticipation of reward (17,19).
Consistent with this, recent research has implicated reduced VS activity in the anhedonic symptoms of depression. For example, depressed patients (vs. healthy control subjects) showed reduced VS activity during a monetary reward task (20). Moreover, higher levels of anhedonia were associated with reduced VS responses to rewarding stimuli in both healthy (21) and depressed (22,23) subjects. In addition, even remitted depressed patients (vs. healthy control subjects) demonstrated evidence of reduced VS activity to rewarding stimuli (24).
In this study, we examined the effect of inflammatory challenge on the neural correlates of anhedonia—namely, reduced VS activity to reward cues. We also explored how these reward processes related to inflammatory-induced increases in depressed mood. Moreover, because previous studies in humans have focused primarily on self-reported depressed mood, we also included a measure of observer-rated depressed mood to obtain a more objective index of inflammatory-induced depressive symptoms. Thus, healthy participants were randomly assigned to receive either endotoxin, known to increase proinflammatory cytokines in a safe manner (25,26), or placebo and then completed a reward anticipation task. We hypothesized that endotoxin, compared with placebo, would lead to decreases in VS activity to reward anticipation as well, as to increases in depressed mood. We also investigated whether reductions in reward-related neural responding mediated the relationship between condition (endotoxin vs. placebo) and increases in depressed mood.
Methods and Materials
Participants
Thirty-nine participants (mean age: 21.8 ± 3.4 years; range: 18–36 years) were randomly assigned to receive either endotoxin (n = 23, 12 female participants; mean age: 21.57 ± 2.76 years; range: 18–28 years) or placebo (n =16, 8 female participants; mean age: 22.19 ± 4.26 years; range: 18–36 years). Sample size was based on previous studies of experimentally induced inflammatory challenge (3). Prospective participants with the following conditions were excluded through a structured telephone interview: claustrophobia or metal in their body (relevant for the neuroimaging component); chronic mental or physical illness; history of allergies, autoimmune, liver, or other chronic diseases; current use of prescription medications; and nightshift work or time zone shifts (>3 hours) within the previous 6 weeks. Participants also had to be right-handed.
Following the telephone interview, participants completed an additional screening interview in which they completed the Structured Clinical Interview for DSM-IV Axis I Disorders (27) and provided blood and urine samples for laboratory screening tests. Any participant who: 1) had a body mass index greater than 30, 2) reported physical health problems or medication use, 3) evidenced an Axis I psychiatric disorder based on the Structured Clinical Interview for DSM-IV Axis I Disorders assessment (Axis II disorders were not systematically evaluated), 4) showed evidence of drug use from a positive urine test, 5) had a positive pregnancy test, or 6) showed abnormalities on the screening laboratory tests were ineligible for the study. The final sample was 39% European American, 18% Asian, 18% Hispanic, 7% African American, and 18% other. The two experimental groups were not significantly different from each other in terms of racial composition [endotoxin: 35% European American, 26% Asian, 13% Hispanic, 9% African American, and 17% other; placebo: 44% European American, 6% Asian, 25% Hispanic, 6% African American, 19% other; χ2(4) = 3.10, p > .50]. After complete description of the study, written informed consent was obtained from each participant in accordance with the University of California Los Angeles Institutional Review Board.
Procedure
The study was conducted between January and November 2007 at the University of California Los Angeles General Clinical Research Center (GCRC) using a randomized, double-blind, placebo-controlled design. Upon arrival to the GCRC, a nurse, who was blind to condition, inserted a catheter with a heparin lock into the participant’s dominant forearm (right) for hourly blood draws and one into the nondominant forearm (left) for a continuous saline flush and for drug administration. Ninety minutes after arrival, participants were randomly assigned to receive either endotoxin (.8 ng/kg of body weight; Escherichia coli group O113, provided by the National Institutes of Health Clinical Center [26]) or placebo (same volume of .9% saline), which was administered by the nurse as an intravenous bolus.1 No significant differences in age, years of education, body weight, or body mass index were found between the two groups.
Throughout the study, vital signs (pulse, temperature) were assessed every half hour and blood draws (to assess interleukin-6 [IL-6], tumor necrosis factor-alpha [TNF-α]) were collected hourly for 6 hours postinjection. Participants completed hourly self-report measures of sickness symptoms and depressed mood. In addition, to obtain a more objective measure of depressed mood, an experimenter (the same one), who was blind to condition, made ratings of how depressed the subject seemed at baseline and at 2 hours postinjection (observer-rated depressed mood). Participants did not know that these ratings were being made.
Approximately 2 hours postinjection, participants were scanned as they completed the monetary reward task in the functional magnetic resonance imaging scanner (all scans took place between the hours of 12:00 PM and 2:00 PM). Following this, participants returned to the GCRC and completed the study procedures. For safety reasons, the study physician (M.R.I.) was aware of each participant’s group assignment and was on call during each experimental session but did not take part in the testing procedures. Participants were discharged from the GCRC approximately 6 hours after drug administration upon approval from the study’s physician; approval was granted if self-reported physical and psychological symptoms returned to baseline levels. There were no adverse events. At the end of the study, participants were thanked, debriefed, and paid for their participation ($200).
Behavioral Assessments
Self-Reported Sickness Symptoms
Sickness symptoms (muscle pain, nausea, breathing difficulties, fatigue) were assessed hourly. Participants rated the extent to which they felt the symptoms on a scale from 0 (no symptoms) to 4 (very severe symptoms).
Observer-Rated Depressed Mood
An experimenter who stayed with the participant at the GCRC and was blind to condition made ratings of how unhappy and gloomy (taken from the Profile of Mood States depression subscale [28] as ones that an observer could rate) the participant seemed on a scale from 0 (not at all) to 4 (extremely). These items were assessed at baseline and 2 hours postinjection and were averaged at each time point. The reliability of the scale (at peak response) was good (α = .60).
Self-Reported Depressed Mood
Self-reported depressed mood was assessed hourly, using an abbreviated version of the Profile of Mood States depression subscale (28). Participants rated the extent to which they felt: unhappy, blue, lonely, gloomy, and worthless on a scale from 0 (not at all) to 4 (extremely). Scores were calculated by averaging ratings at each time point. The reliability of the scale (at peak response) was good (α = .68).
Functional Magnetic Resonance Imaging Paradigm
To assess neural responses to reward anticipation, participants were scanned while completing the monetary incentive delay (MID) task in a manner similar to previous work (16,17). While still at the GCRC (immediately following endotoxin/placebo administration), participants were given instructions for the MID task. Participants were told that on each trial, they would see a cue indicating how much money they could win or lose and that following this cue, they would see a target. On trials in which the cue indicated a potential win, participants could win money if they pressed a button during the target presentation. Alternatively, on trials in which the cue indicated a potential loss, participants could avoid losing money if they pressed a button during the target presentation. During neutral trials, participants’ earnings would not change, but they were still instructed to press the button as quickly as possible following the cue. After receiving the instructions, participants completed a practice version of the task to produce an estimate of each participant’s reaction time for standardizing task difficulty in the scanner.
MID Task
During the MID task, participants saw 65 6-second trials. During each trial, participants saw one of five cue shapes (cue, 250 msec), then a crosshair as they waited a variable interval (delay, 2000–2500 msec), and then a white target square that appeared for a variable length of time (target, 166–333 msec). If participants pressed a button while the target was on the screen, they won money, avoided losing money, or stayed even (depending on the type of cue). Feedback information (feedback, 1753 msec) followed the target and notified participants about their earnings on the previous trial, as well as their cumulative earnings at that point (Figure 1). Task difficulty, based on reaction times collected during the practice session, was set such that participants would succeed on approximately 66% of their target responses. Cues signaled potential reward (circles), potential loss (squares), or no monetary outcome (triangles). The number of horizontal lines in the cues indicated the magnitude of the possible reward (+ $1.00: n = 13, one horizontal line; + $5.00: n = 13, three horizontal lines) or loss (−$1.00: n =13; one horizontal line; −$5.00: n =13; three horizontal lines). Trial types were pseudorandomly ordered within the scanning session.
Figure 1.
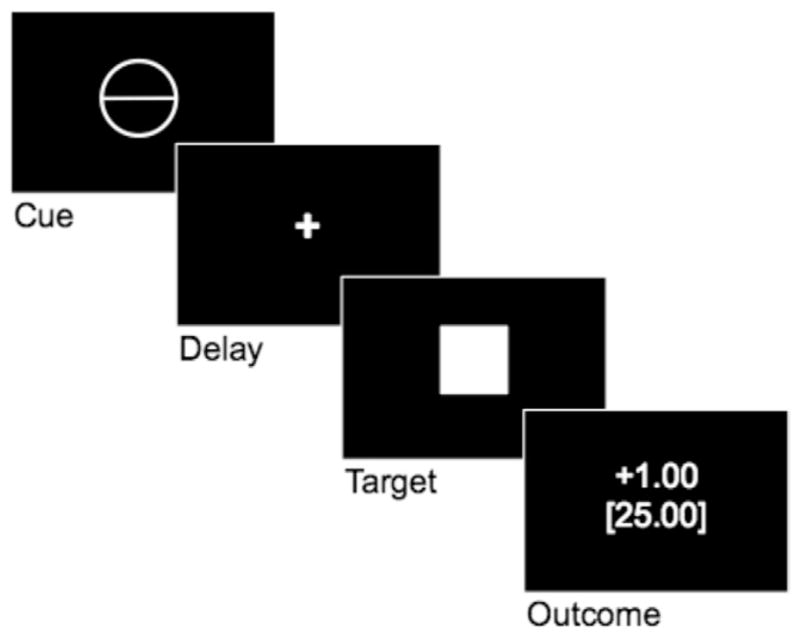
Sample trial from the monetary incentive delay task.
Functional Magnetic Resonance Imaging Data Acquisition and Data Analysis
Data were acquired on a Siemens Allegra 3T head-only scanner (Siemens Medical Systems). Head movements were restrained with foam padding and surgical tape placed across each participant’s forehead. For each participant, a high-resolution structural T2-weighted echo-planar imaging volume (spin-echo; repetition time = 5000 msec; echo time = 33 msec; matrix size 128 × 128; 36 axial slices; field of view = 20 cm; 3-mm thick, skip 1 mm) was acquired coplanar with the functional scans. One functional scan was acquired (echo-planar T2*-weighted gradient-echo, repetition time = 2000 msec, echo time = 25 msec, flip angle = 90°, matrix size 64 × 64; 36 axial slices, field of view = 20 cm; 3-mm thick, skip 1 mm), lasting approximately 9 minutes.
The imaging data were analyzed using SPM’5 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, United Kingdom). Images for each subject were realigned to correct for head motion, normalized into a standard stereotactic space, and smoothed with an 8 mm Gaussian kernel, full width at half maximum, to increase signal-to-noise ratio. For each participant, the presentation of the reward (+ $1.00, + $5.00) and neutral ($.00) cues were modeled as events. After the task was modeled for each participant, planned comparisons were computed as linear contrasts. Random effects analyses of the group were computed using the contrast images generated for each participant. Although loss trials were included, analyses focused on neural responses during the presentation of reward and neutral cues to examine reward processes (reward anticipation vs. neutral anticipation) that might relate to endotoxin-induced anhedonia. However, additional analyses revealed no significant between-group differences in reward-related neural activity during loss trials.
Plasma Levels of Cytokines
Plasma blood samples were collected in prechilled tubes containing sodium ethylenediaminetetraacetic acid and aprotinin. Tubes were immediately centrifuged at 4°C and plasma was harvested into multiple aliquots and then frozen in a −70°C freezer. Plasma levels of IL-6 and TNF-α were quantified by means of high-sensitivity enzyme-linked immunosorbent assays (Quantikine HS Human IL-6, Quantikine HS Human TNF-α, R&D Systems, Minneapolis, Minnesota). All assays were performed according to the manufacturer’s protocols, with reported intra-assay and interassay coefficients of variation less than 11%. For IL-6 assays, the lower limit of quantitation was .2 pg/mL; all samples were assayed in duplicate. For TNF-α assays, the lower limit of quantitation was .5 pg/mL; repeated measures on each individual were assayed in single wells.
Statistical Analyses
Behavioral Outcomes
To assess between-group differences (endotoxin vs. placebo) in behavioral outcomes, we conducted repeated-measures analyses of variance, testing time (baseline vs. each subsequent time point) by condition (endotoxin vs. placebo) interactions. Because observer-rated depressed mood was not normally distributed, we categorized this variable into two levels: 1) no evidence of depressed mood increases, and 2) evidence of depressed mood increases.
Neuroimaging Outcomes
To compare neural activity between the two groups in response to reward anticipation, we completed two kinds of analyses using SPM’5. First, based on a priori hypotheses regarding inflammatory-induced reductions in reward-related neural responding, we conducted region of interest (ROI) analyses focusing on activity within the left and right VS. Ventral striatum ROIs were structurally defined a priori using the Wake Forest University Pickatlas Tool (29) based on the Automated Anatomical Labeling atlas (30) and constrained in the following way: −12 <x <12, 4 <y <18, −12 <z <0. We then used the Marsbar toolbox (http://marsbar.sourceforge.net) to extract mean parameter estimates (that model the amplitude of the blood oxygenation level-dependent response during reward anticipation vs. neutral anticipation) averaged across all voxels in each ROI for each group (endotoxin, placebo). Standard statistical software was then used to examine between-group differences in these activation values (p < .05). To supplement these analyses, we conducted whole-brain analyses to further investigate between-group differences in the specific neural regions activated in response to reward anticipation (p < .001, 10 voxels) (31). Reward-related regions that demonstrated between-group differences in neural activity were further explored to see if they correlated with increases in depressed mood and mediated the relationship between condition and increases in depressed mood. Based on convention, all neuroimaging analyses were one-tailed. All coordinates are reported in Montreal Neurological Institute format.
Results
Physiological and Affective Responses
As reported previously (2,32), endotoxin led to a significant increase in IL-6 and TNF-α levels, body temperature, pulse, and sickness symptoms, and there were no sex differences in any of these effects. In addition, as reported previously, endotoxin (vs. placebo) led to a significant increase (from baseline to 2 hours postinjection) in self-reported depressed mood [F (1,35) = 8.13, p < .01; no sex differences], which was not altered by controlling for each type of sickness symptom, with the exception of fatigue [F (1,34) = .10, ns]. Interestingly, we also found significant between-group differences in observer-rated depressed mood increases (from baseline to 2 hours postinjection). Subjects in the endotoxin, compared with the placebo, group were rated by the experimenter (who was blind to condition) as more likely to show an increase in depressed mood [F (1,35) = 7.01, p <.05]. Thus, 43% of the subjects in the endotoxin group were rated as showing an increase in depressed mood; whereas, only 6% of the subjects in the placebo group were rated as showing an increase in depressed mood [χ2(1, n = 37) = 6.17, p < .05]. There was no sex difference in this effect (p > .18). Controlling for each type of sickness symptom did not alter this relationship.
Behavioral Responses to the MID Task
There were no significant between-group differences in hit rates (successful button presses during target presentation) to reward, loss, or neutral trials (ps > .25) or in reactions times to reward, loss, or neutral trials (ps > .59). In addition, there were no significant between-group differences in monetary earnings [t (32) =1.50, p = .14; endotoxin: M =$46.25, SD =$18.69; Placebo: M = $35.14, SD = $23.39). Thus, any between-group differences in neural responding during reward trials should reflect differences in neural sensitivity to reward and not differences in rewarding outcomes between the two groups.
Neural Responses to the MID Task
To examine whether subjects exposed to endotoxin showed evidence of anhedonia—characterized by reduced VS activity during reward anticipation—we examined whether there were between-groups differences in neural activity within the left and right VS ROIs during the anticipation of reward versus neutral trials. As predicted, subjects exposed to endotoxin versus placebo showed significantly less activity in the left VS (p < .05; Figure 2), and there was no sex difference in this effect (p > .38). There was no significant difference in activity in the right VS ROI. Follow-up analyses demonstrated that the placebo group showed significant bilateral VS activation (ts> 3.3, ps < .005), which is consistent with previous work (16,17). The endotoxin group, however, only showed significant activation in the right VS (t = 3.03, p < .005).
Figure 2.
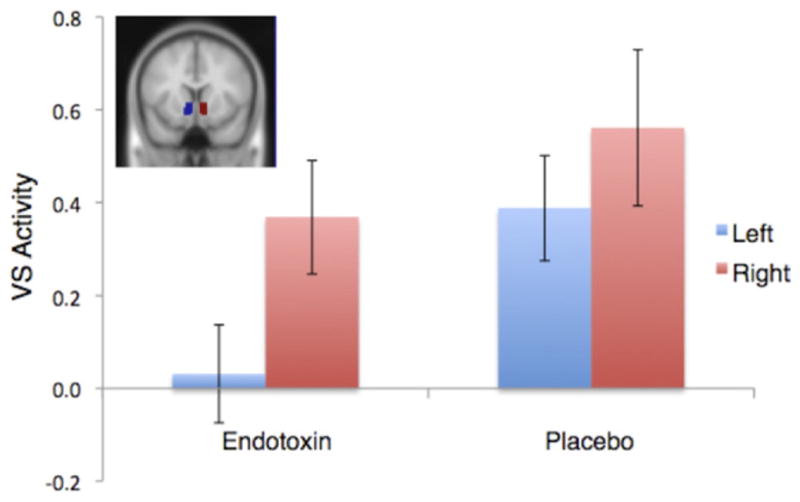
Neural activity (mean parameter estimates) for the left (blue) and right (red) ventral striatum regions of interest during reward anticipation versus neutral anticipation for participants in the endotoxin and placebo conditions. Shaded regions denote ventral striatum region of interest location (inset). VS, ventral striatum.
To further characterize the extent of the between-group differences in VS activity, we next ran whole-brain analyses. Consistent with the ROI analyses, whole-brain analyses also demonstrated reduced left VS activity in endotoxin subjects compared with placebo subjects (−6,18,−12, t =3.59, p <.001; Figure 3).2 There were no sex differences in this effect (p >.40). Follow-up analyses of this left VS cluster revealed that the placebo group showed significant activation in this region (t = 1.88, p < .05), whereas the endotoxin subjects showed significant deactivation in this region (t = −3.45, p < .005). In addition, there were no regions that were more active for endotoxin subjects than placebo subjects in response to reward anticipation (see Table 1 for a complete list of between-group differences in neural activation).
Figure 3.
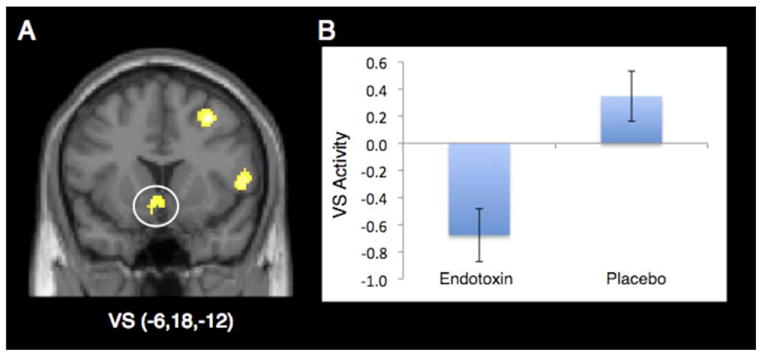
(A) Ventral striatum (VS) activity from the whole-brain analysis that was significantly more active for placebo, compared with endotoxin, subjects (displayed at p < .005, 10 voxels to show extent of activation; left = left). (B) Bar graph of ventral striatum activity from the whole-brain analysis showing significantly more activity in placebo, compared with endotoxin, subjects.
Table 1.
Between-Group Differences in Neural Activity to Reward Anticipation (vs. Neutral Anticipation)
| Anatomical Region | BA | x | y | z | t | k | p | |
|---|---|---|---|---|---|---|---|---|
| Placebo > Endotoxin | ||||||||
| Ventral Striatum | L | −6 | 18 | −12 | 3.59 | 19 | <.001 | |
| Premotor Cortex | 6 | R | 36 | 2 | 42 | 4.32 | 64 | <.0001 |
| 6/8 | R | 32 | 14 | 48 | 4.18 | 33 | <.0001 | |
| 6 | R | 52 | 10 | 6 | 4.14 | 37 | <.0001 | |
| Superior Parietal Cortex | 5/7 | R | 36 | −40 | 72 | 3.67 | 11 | <.0001 |
| Inferior Temporal Gyrus | 20 | R | 34 | 2 | −36 | 4.30 | 33 | <.0001 |
| Middle Temporal Gyrus | 21 | R | 50 | −42 | −6 | 4.15 | 33 | <.0001 |
| 21 | R | 48 | 0 | −22 | 3.66 | 12 | <.0001 | |
| Hippocampal Gyrus | 35/36 | L | −34 | −26 | −20 | 4.22 | 39 | <.0001 |
| Occipital Cortex | 19 | L | −28 | −98 | 16 | 4.09 | 30 | <.0001 |
Regions that showed greater activity in the placebo group compared with the endotoxin group during reward anticipation versus neutral anticipation trials (p < .001, 10 voxel extent threshold).
Column headings x, y, and z refer to Montreal Neurological Institute coordinates; t refers to the t score at those coordinates (local maxima); k refers to the number of contiguous activated voxels in a region; and p refers to the uncorrected p value for each region.
BA, Brodmann area; L, left hemisphere; R, right hemisphere.
Correlations between VS Activity and Depressed Mood
To further explore how the observed differences in VS activity related to increases in depressed mood, we examined correlations between left VS activity and increases in depressed mood from baseline to 2 hours postinjection. Left VS activity from the whole-brain analysis correlated negatively with increases in observer-rated depressed mood, such that subjects who showed reduced activity in the left VS during reward anticipation also showed a greater increase in observer-rated depressed mood (point-biserial correlation: r = −.54, p < .005). Left VS activity averaged across the entire ROI, however, did not correlate significantly with observer-rated depressed mood (r =−.15, ns). In addition, left VS activity (either from the ROI or whole-brain analysis) did not significantly correlate with increases in self-reported depressed mood (ps >.70).
Mediation Analyses
Finally, to examine whether endotoxin-induced changes in VS activity mediated the relationship between exposure to inflammatory challenge and increases in depressed mood, we conducted mediation analyses.3 These analyses revealed that the left VS cluster derived from the whole-brain analysis was a significant mediator of the relationship between condition and increases in observer-rated depressed mood (Sobel test =1.78, p <.05, one-tailed). In addition, to test the specificity of this meditational analysis, we investigated whether a control region also mediated this relationship. The hippocampal gyrus cluster (−34,−26,−20) was chosen as the control region, as it was significantly more activated for the placebo subjects compared with the endotoxin subjects but was not expected to uniquely mediate the relationship between condition and observer-rated depressed mood. Indeed, unlike the left VS, the hippocampal gyrus was not a significant mediator of this relationship (Sobel test = .04, ns).
Discussion
Although previous research has demonstrated that inflammatory activity contributes to depressive symptoms (1–4), no work in humans has examined the effect of experimentally induced inflammation on anhedonia—a key diagnostic feature of depression. The goal of the current study was to examine the effect of an experimental inflammatory challenge on the neural correlates of anhedonia—reduced VS activity to reward cues—and to explore whether this altered neural activity related to inflammatory-induced increases in depressed mood.
Results revealed that subjects exposed to endotoxin versus placebo showed greater increases in self-reported and observer-rated depressed mood and showed significant reductions in VS activity to reward cues. Moreover, the relationship between exposure to inflammatory challenge and increases in observer-rated depressed mood was mediated by between-group differences in VS activity to reward cues. Thus, inflammatory-induced decreases in reward-related VS activity were found to significantly contribute to the relationship between inflammatory activity and depressed mood.
Two points are worth noting regarding these results. First, although there were no between-group differences in behavioral responses to the monetary reward task, neuroimaging analyses revealed reliable differences in VS responsivity to reward cues that related to increases in observer-rated depressed mood. Thus, neuroimaging may be a useful tool for examining the mechanistic correlates of inflammatory-induced depressed mood that may not be revealed by behavioral observations alone. Second, while both self-reported and observed-rated depressed mood increased as a function of endotoxin, only observer-rated depressed mood correlated negatively with VS activity. This effect may have been due to sex differences in the tendency to report depressed mood, as male subjects showed a negative correlation between self-reported and observer-rated depressed mood (r =−.61, p =.08), whereas female subjects showed a positive correlation (r =.55, p =.08). Future studies would benefit from including both subjective as well as more objective assessments of depressed mood to further explore how altered reward-related responding contributes to depression. In addition, future studies would benefit from including measures of clinically assessed depressed mood as well, as the current measure of observer-rated depressed mood was not made by a clinician.
The findings reported here are consistent with animal research showing that inflammatory activity can increase anhedonic-like behavior (7), as well as human research demonstrating a role for altered reward-related neural responding in depressive states. Although it is not yet clear how cytokines alter reward-related neural responding, it is known that cytokines have central effects, either through leaky regions in the blood-brain barrier or through the transmission of cytokine signals through the vagus nerve (11). Furthermore, it has been shown that the administration of cytokine-producing agents can alter monoamine levels (dopamine, serotonin) in the brain more generally (1) and in the striatum specifically (32,33). To the extent that monoamines, such as dopamine and serotonin, play a role in both reward processing and depressive symptoms (12,34), it is possible that proinflammatory cytokines relate to reduced reward processing and increased depressive symptoms through altered activity in these neurotransmitter systems.
Together, the results reported here highlight the importance of examining altered reward-related processing in inflammatory-related depression. Previous work exploring the psychological and neural consequences of inflammatory activity has tended to focus on the negative affective processes that might relate to depression (2,35,36). However, to the extent that depressive symptoms relate to altered activity in neural systems associated with positive affective processes (e.g., reward), it will be important to continue to investigate the effects of inflammation on altered activity in reward-related processing.
In summary, although previous research in humans has demonstrated that proinflammatory cytokine activity can increase depressed mood (1–4), the neurocognitive mechanisms that mediate these effects remain unknown. Here, we demonstrate, for the first time, that alterations in reward-related processing are an important neural mediator of the effects of inflammation on depressed mood. Identifying the neural processes that are altered as a function of inflammation may allow for greater precision in treating and preventing inflammatory-associated depression. For example, future work could examine whether pharmacological agents that directly target reward-related neural regions (dopaminergic drugs) could attenuate the effects of inflammatory activity on depressed mood. As such, alterations in reward-related neural circuitry represent an important avenue for understanding inflammatory-associated depression.
Acknowledgments
This research was funded by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award, a Dana Foundation Grant, a University of California Los Angeles (UCLA) Faculty Senate Grant, a postdoctoral research fellowship (T32-MH19925), and a UCLA Integrative Study of Mood Disorders Award to NIE. In addition, the authors acknowledge the additional support provided by Grants HL-079955, AG-026364, CA-10014152, CA-116778, P30-AG028748, M01-RR00865, the UCLA Cousins Center at the Semel Institute for Neurosciences, the UCLA Claude D. Pepper Older Americans Independence Center Inflammatory Biology Core, and the General Clinical Research Centers Program (M01-RR00865).
We thank the staff and support of the UCLA General Clinical Research Center; Anthony Suffredini, M.D., and George Grimes, R.P., at the National Institutes of Health, Warren Grant Magnuson Clinical Center, for providing the standard reference endotoxin; Thanh Luu and Elizabeth Breen for performing the cytokine assays; and Brian Knutson for providing the Monetary Incentive Delay Task.
Footnotes
Random assignment was determined by a consultant who was not involved in running participants and was kept by the University of California Los Angeles Pharmacy to ensure proper drug preparation for each participant. The random allocation sequence was determined through the use of a random number generator with consideration for the inclusion of equal numbers of male and female subjects in each group.
Although, with neuroimaging analyses, each subject serves as his/her own control subject (e.g., neural activity during reward vs. neural activity during neutral) and thus the effects of sickness symptoms on neural activity should be subtracted out, we also conducted analyses in which we controlled for self-reported sickness symptoms. Controlling for sickness symptoms did not significantly change any of the VS findings in either the ROI or whole-brain analyses.
These mediation analyses are considered exploratory because: 1) measures of depressed mood were taken immediately before the scanning session rather than after it and thus the causal ordering of the mediating pathway is unclear, and 2) the test of mediation was not independent of the condition-VS relationship because the clusters of VS activity were determined based on between-group differences in condition (endotoxin vs. placebo).
The authors reported no biomedical financial interests or potential conflicts of interest.
ClinicalTrials.gov: Cytokine-Associated Depression and Social Pain; http://clinicaltrials.gov/ct2/show/NCT00949845; NCT00949845.
References
- 1.Miller AH, Maletic V, Raison CL. Inflammation and its disconnects: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. Neuroimage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 4.Wright CE, Strike PC, Brydon I, Steptoe A. Acute inflammation and negative mood: Mediation by cytokine activation. Brain Behav Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychological Association; 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- 6.Dryman A, Eaton WW. Affective symptoms associated either the onset of major depression in the community: Findings from the US National Institute of Mental Health Epidemiological Catchment Area Program. Acta Psychiatr Scand. 1991;84:1–5. doi: 10.1111/j.1600-0447.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 7.De La Garza R, Savitz KL. Endotoxin- or proinflammatory cytokine-induced sickness behavior as an animal model of depression: Focus on anhedonia. Neurosci Biobehav Rev. 2005;29:761–770. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 9.Borowski T, Kokkinidis L, Merali Z, Anisman H. Lipopolysaccharide sentral in vivo biogenic amine variations, and anhedonia. Neuroreport. 1998;9:3797–3802. doi: 10.1097/00001756-199812010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 11.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 14.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 15.Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectivity recruits nucleus accumbens. J Neurosci. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 18.Berridge KC, Robinsn TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 19.Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 20.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmediated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulated cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46:327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein J, Pan H, Kocis JH, Yang Y, Butler T, Chusid J, et al. Lack of ventral striatal response to positive stimuli in depressed versus non-depressed subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 23.Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 24.McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl) 2009;205:667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suffredini AF, O’Grady NP. Pathophysiological responses to endotoxin in humans. In: Morrison D, editor. Endotoxin in Health Diseases. New York: Marcel Dekker; 1999. pp. 817–830. [Google Scholar]
- 26.Suffredini AF, Hochstein HD, McMahon FG. Dose-related inflammatory effects of intravenous endotoxin in humans: Evaluation of a new clinical lot of Escherichia coli O: 113 endotoxin. J Infect Dis. 1999;179:1278–1282. doi: 10.1086/314717. [DOI] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, Version 2.0. New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- 28.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- 29.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI datasets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 30.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 31.Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: Re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamata M, Higuchi H, Yoshimoto M, Yoshida K, Shimizu T. Effect of single intracerebroventricular injection of α-interferon on monoamine concentrations in the rat brain. Eur Neuropsychopharmacol. 2000;10:129–132. doi: 10.1016/s0924-977x(99)00067-x. [DOI] [PubMed] [Google Scholar]
- 33.Maurino R, Machado A, Santiago M. Effect of in vivo striatal perfusion of lipopolysaccharide on dopamine metabolites. Neurosci Lett. 2010;475:121–123. doi: 10.1016/j.neulet.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 34.Ressler KJ, Nemeroff CB. The role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 35.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: An inflammatory challenge induces feelings of social disconnection. Brain Behav Immun. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


