Summary
In Duchenne muscular dystrophy (DMD), dystrophin mutation leads to progressive lethal skeletal muscle degeneration. For unknown reasons, dystrophin deficiency does not recapitulate DMD in mice (mdx), which have mild skeletal muscle defects and potent regenerative capacity. We postulated that human DMD progression is a consequence of loss of functional muscle stem cells (MuSC) and the mild mouse mdx phenotype results from greater MuSC reserve fueled by longer telomeres. We report that mdx mice lacking the RNA component of telomerase (mdx/mTR) have shortened telomeres in muscle cells and severe muscular dystrophy that progressively worsens with age. Muscle wasting severity parallels a decline in MuSC regenerative capacity, and is ameliorated histologically by transplantation of wild-type MuSC. These data show that DMD progression results in part from a cell-autonomous failure of MuSC to maintain the damage-repair cycle initiated by dystrophin deficiency. The essential role of MuSC function has therapeutic implications for DMD.
Keywords: Duchenne Muscular Dystrophy, mdx, muscle stem cells, telomere shortening, telomerase, dystrophin
Highlights.
Mdx mice lacking telomerase (mdx/mTRKO) exhibit severe muscular dystrophy
Telomere shortening results in progressive loss of muscle stem cell function
Continuous loss of functional stem cells is critical to DMD pathophysiology
Introduction
Duchenne Muscular Dystrophy (DMD) is a devastating muscle degenerative disease caused by a mutation in dystrophin (Hoffman et al., 1987), a cytoskeletal protein essential for the stability of the membrane of multinucleated myofibers in skeletal muscle (Durbeej and Campbell, 2002). Absence of dystrophin results in increased fragility of the sarcolemma, leading to injury in the presence of even mild stress (Petrof et al., 1993). DMD patients suffer from progressive loss of muscle function, leading to paralysis and death in the third decade of life (Emery, 2002). Under normal conditions, skeletal muscle is relatively quiescent when compared to high turnover tissues such as the blood and most epithelia. However, skeletal muscle harbors considerable regenerative capacity due to the presence of adult muscle stem cells (MuSC), also known as satellite cells, which play a major role in postnatal muscle growth and repair (Collins et al., 2005; Cornelison et al., 2004; Kuang et al., 2007; Montarras et al., 2005; Sacco et al., 2008). In DMD, unrelenting, recurrent myofiber damage elicits a constant need for regeneration. Eventually, muscle tissue is supplanted by fibrosis, calcium deposits and adipose accumulation that coincides with clinical manifestations. To date, the basis for the observed mild effects in mice and lethal effects in humans of the same genetic absence of dystrophin remain unknown. Furthermore, although clearly initiated by dystrophin deficiency, the pathophysiological cause of the failure of the repair process in muscles of DMD patients is not understood.
More than two decades ago, before the cloning of the DMD gene, we obtained data that suggested that muscle cells in DMD patients have diminished regenerative capacity as a consequence of replicative aging (Blau et al., 1983; Webster and Blau, 1990). Human DMD myoblasts exhibited a severe proliferation deficit in vitro that became more pronounced with patient age, resulting in a yield of myoblasts per gram muscle of 5% of normal, and the proliferative potential of the remaining myoblasts was severely impaired. However, this proliferative defect did not segregate with the X-chromosome in studies of myoblast clones from doubly heterozygous carriers for two X-linked loci, DMD and a Mediterranean histologically detectable heat-labile variant of G6PD, and was therefore dependent on additional factors (Webster et al., 1986).
Recent studies support and extend these early findings that myoblasts from DMD have impaired replicative potential and suggest that telomere shortening is a common feature of dystrophic human muscle cells with increasing age and correlates with their limited ability to regenerate DMD tissues upon transplant (Mouly et al., 2005). Indeed, a 14-fold greater shortening of telomeres in DMD patients relative to healthy individuals has been reported (Decary et al., 2000). Telomeres are DNA repeats that protect chromosome ends from illicit recombination, fusion, and degradation leading to genomic instability (Palm and de Lange, 2008). Telomere length is maintained by the enzyme telomerase, which adds telomere repeats to chromosome ends ensuring their proper replication (Greider and Blackburn, 1985). Cell proliferation in settings of insufficient telomerase results in progressive telomere shortening, ultimately leading to replicative senescence as chromosome end-protection is compromised at a subset of short telomeres (Rodier et al., 2005; Sherr and DePinho, 2000). Telomere shortening also accompanies aging of mitotically active human tissues with high turnover, including blood, liver, skin, testis, and kidneys (Aikata et al., 2000; Friedrich et al., 2000; Lindsey et al., 1991; Takubo et al., 2000; Vaziri et al., 1993). In contrast, analysis of telomeres in skeletal muscle during aging in whole tissue assays reveals only a mild shortening (Decary et al., 1997; Renault et al., 2002), presumably reflecting the low rate of proliferation of myogenic progenitors and muscle tissue turnover during normal aging. In agreement with these findings, studies of telomerase knockout mice revealed short dysfunctional telomeres that profoundly impaired progenitor cell function in actively renewing tissues leading to atrophy and reduced regenerative potential, whereas more quiescent low-turnover tissues such as muscle were unaffected (Allsopp et al., 2003; Lee et al., 1998; Rudolph et al., 1999).
A major challenge hindering the development of effective therapies for DMD has been the lack of an animal model that closely recapitulates the disease progression in humans. The most widely used animal model for DMD, the mdx mouse, exhibits only a mild dystrophic phenotype, although like DMD patients, it lacks functional dystrophin due to a point mutation in the dystrophin gene (Bulfield et al., 1984; Hoffman et al., 1987; Ryder-Cook et al., 1988). Muscles of mdx mice, like those in DMD patients, undergo repeated cycles of degeneration and regeneration, but for unknown reasons the mice exhibit only transient muscle weakness and never exhibit the profound loss of muscle strength and death observed in DMD patients (DiMario et al., 1991; Straub et al., 1997). Here we test the hypothesis that species-specific differences in telomere length account for the differential proliferative capacity of muscle cells derived from DMD patients and mdx mice, and consequent disparate disease progression between the two species. Humans have relatively short telomeres of ~5–15 kilobases in comparison to inbred strains of laboratory mice which have telomeres that are typically >40 kilobases (Kipling and Cooke, 1990). This greater telomere reserve could endow MuSC in mice with a prolonged regenerative capacity and mild muscle phenotype despite dystrophin deficiency. In support of this hypothesis, lack of a disease phenotype in mouse models of other human diseases, such as Werner and Ataxia-Telangiectasia syndromes, has been linked to species-specific differences in telomere length, as when these models were crossed with mice lacking telomerase activity, the disease became apparent (Chang et al., 2004; Wong et al., 2003).
Here we generate mdx mice lacking telomerase activity and show that dystrophin deficiency coupled with telomere dysfunction recapitulates the severe phenotypic characteristics of muscular dystrophy in humans, including profound loss of muscle force, poor performance on a treadmill, increased serum creatine kinase (CK) levels, accumulation of fibrosis and calcium deposits within skeletal muscle tissues, kyphosis, and shortened life-span. We show that the severity of the disease progressively worsens with age. Moreover, MuSC exhibit a severe proliferation deficit both in vitro and in vivo, inability to respond to tissue injury and markedly reduced engraftment and contribution to muscle repair. Transplantation of WT MuSC into mdx mice lacking telomerase activity ameliorates the dystrophic phenotype histologically. Together, theses results indicate that the combination of the structural defect of dystrophin deficiency that leads to muscle degeneration together with the progressive exhaustion of functional MuSC generates the dystrophic phenotype. These findings support the hypothesis that in humans, DMD is initiated by the genetic defect, but as suggested by our earlier findings (Blau et al., 1983; Webster et al., 1986) develops progressively into an exhaustion of cells with regenerative potential.
Results
We generated dystrophic mice lacking telomerase activity by crossing C57Bl6 mdx female mice homozygous for the dystrophin mutation (Im et al., 1996) with C57Bl6 mice heterozygous for the telomerase RNA component Terc (mTR)(Blasco et al., 1997)(Fig. 1A). The resulting mdx/mTRHet mice were further crossed to generate double mutant mdx mice totally lacking dystrophin and telomerase activity (designated herein as first generation mdx/mTRG1). The mdx/mTRG1 mice were in turn intercrossed to produce second-generation mdx/mTRG2 mice. This strategy yields same strain dystrophic mice that lack telomerase, which is critical, as telomere length differs among mouse strains (Hemann et al., 2001). In addition, the dystrophic phenotype is analyzed in mice with different telomere lengths, as shortening increases in mTR mice with successive generations.
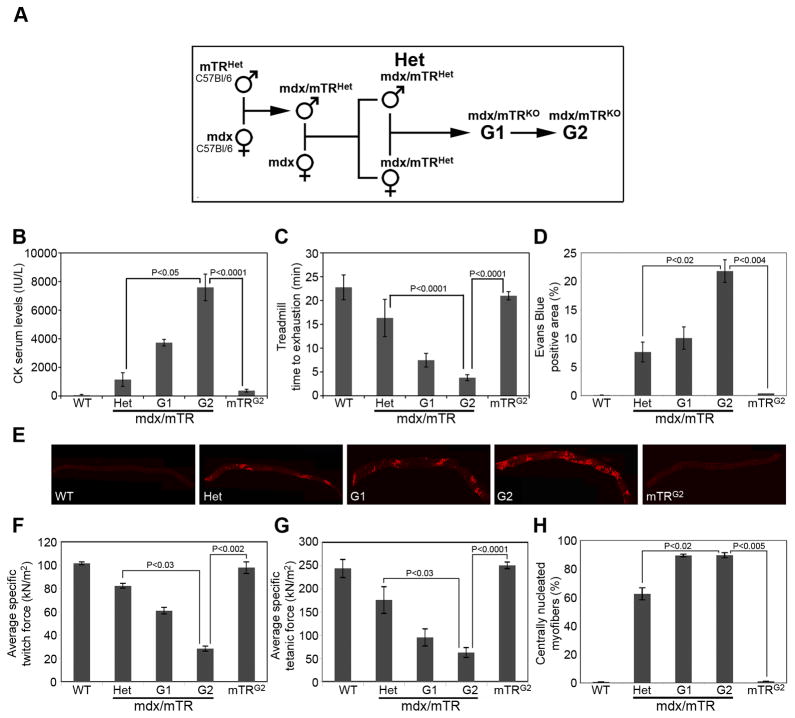
Figure 1. Evidence of severe muscular dystrophy in mdx/mTRG2 mice.
(A) Scheme of mouse breeding.
(B) Serum CK levels in 8 week old males. A marked increase in CK levels is seen in mdx/mTRG2 animals. Data are represented as average±s.e.m. (n≥10, P values are indicated in the graph). See also Fig. S1A.
(C) 8 week old males were subjected to the treadmill test and their performance was measured as time to exhaustion. mdx/mTRG2 animals were severely impaired in their muscle endurance, compared to WT or mdx/mTRHet. Data are represented as average±s.e.m. (n≥5, P values are indicated in the graph). See also Fig. S1B–C.
(D) Quantification of Evans Blue Dye uptake in diaphragm muscle of 8 week old non-exercised mice (% EBD+ area). Data are represented as average±s.e.m. (n=3, P values are indicated in the graph). See also Fig. S2.
(E) Representative images of Evans Blue Dye uptake in diaphragm muscles from the various genotypes.
(F–G) Muscle force was measured in the lateral gastrocnemius muscle in vivo in 8 week old anesthetized animals by electrical stimulation of the sciatic nerve and expressed relative to cross-sectional area (P0/CSA, force per square meter, kN/m2). Data are represented as average±s.e.m. (n≥4, P values are indicated in the graph). See also Fig. S1D.
(H) Quantification of centrally nucleated myofibers, in gastrocnemius muscles of 8 week old mice from the indicated genotypes. Data are represented as average±s.e.m. (n=3, P values are indicated in the graph).
Severe muscular dystrophy in mdx/mTRG2 mice
To assess the severity of muscular dystrophy, we carried out three essential controls. We compared mdx/mTRG1 and mdx/mTRG2 mice with mdx/mTRHet mice, as these mice lack dystrophin and one copy of the Terc gene, providing the most stringent same-strain control. As a second control we analyzed mTRG2 mice, which are generation-matched for telomerase deficiency, but have wild-type dystrophin, which was essential in order to rule out the possibility that a phenotype resulted from telomerase deficiency alone. Finally, our studies were restricted to male mice, as the disease is X-linked and differences in sex can confound results.
We first tested serum creatine kinase (CK) levels, an established indicator of skeletal muscle damage in mice that is a diagnostic indicator of DMD. 8 week old mdx/mTRG2 males already have remarkably elevated serum CK (7,594±928 IU/L), compared to mdx/mTRHet males (1,148±477 IU/L)(Fig. 1B), approximating human DMD levels (6,000–8,000 IU/L in 10 year old patients)(Zatz et al., 1991). However, by 60 weeks of age, CK levels decline in mdx/mTRG2 mice (Fig. S1A), consistent with CK levels in DMD patients which peak at 1–6 years of age when active muscle degeneration is occurring, and then decrease with age, as muscle tissue is progressively lost (Zatz et al., 1991). Heterozygous mdx/mTRHet mice with increased age show increased CK levels, presumably due to slower progression of the disease (Fig. S1A). Serum CK levels are indistinguishable for mTRG2 and healthy WT mice (Fig. 1B, P>0.05), consistent with previous studies showing that telomere deficiency preferentially affects highly proliferative organs, but not skeletal muscle (Allsopp et al., 2003; Artandi, 2006; Herrera et al., 1999; Lee et al., 1998). The progressive elevation of CK seen in mdx/mTRG1 and mdx/mTRG2 animals suggests that muscle damage deteriorated with successive generations, implicating telomere attrition as a cooperating factor with dystrophin deficiency in disease manifestation.
Skeletal muscle damage is also evident as myofiber membrane permeability, measured by Evans Blue Dye (EBD) uptake (Straub et al., 1997). Diaphragm and gastrocnemius muscles of 8 week old mdx/mTRG2 male mice exhibit a significant increase in the percentage of EBD positive areas, compared to mdx/mTRHet or mdx/mTRG1 mice (Figs. 1D-E and S2). Control mTRG2 mice are indistinguishable from WT mice, confirming that the absence of telomerase activity per se is not sufficient to trigger skeletal muscle damage. Membrane permeability significantly worsens with age, as 60 week old mdx/mTRG2 animals exhibit higher EBD incorporation (Fig. S2). Together, these data indicate that in the absence of telomerase, dystrophin deficiency causes progressive skeletal muscle damage in mice.
To assess muscle performance we first analyzed time to exhaustion on a treadmill. 8 week old mdx/mTRG2 mice run for a significantly shorter time than control WT (3.8±0.6 vs. 22.8±2.6min) or mdx/mTRHet mice (16.3±3.9min). Muscle performance further worsens with age, as 24 week old mdx/mTRG2 mice barely run at all (1.1±0.6min)(Figs. 1C and S1B). By contrast age-matched mTRG2 mice are indistinguishable from WT mice at both 8 and 24 weeks of age (Figs. 1C and S1B). A second well-established assay of muscle performance is the grid test, a measure of the length of time that mice can support their weight by holding onto a rod. The results of the grid test in 8 week old mice paralleled the treadmill experiment, as mdx/mTRG2 mice are able to hold onto the grid for a substantially shorter time than controls (Fig. S1C). Third, we measured specific muscle force on gastrocnemius muscles. To avoid potential artifacts due to muscle excision, we assayed force generation in vivo in intact muscles in 8 week old anesthetized animals (Blaauw et al., 2008). Lateral gastrocnemius muscle twitch force, tetanic force and tetanic tension are markedly decreased in mdx/mTRG1 and further decrease in mdx/mTRG2 mice, compared to control WT, mdx/mTRHet, and mTRG2 mice (Figs. 1F–G and S1D), providing direct evidence for progressively severe muscle weakness.
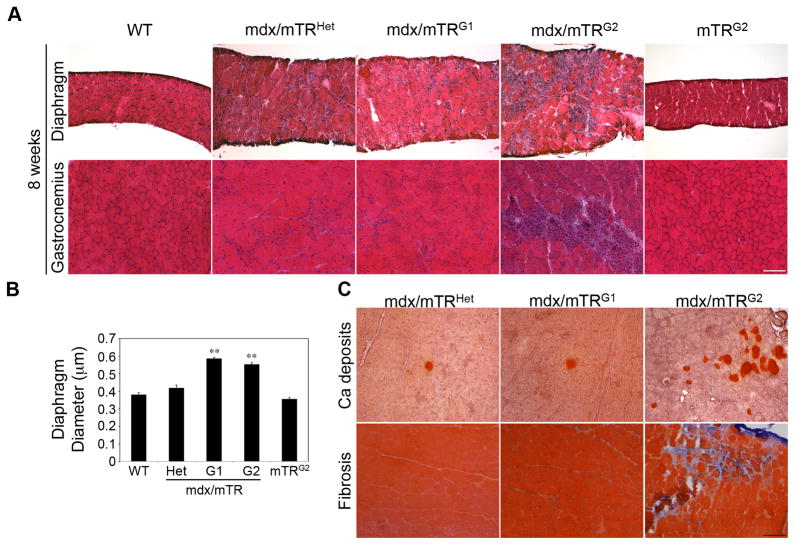
Histologically, the percentage of centrally nucleated myofibers, a hallmark of muscle regeneration, is increased in mdx/mTRHet mice (63±4%) relative to WT (0.7±0.1%), but is further increased in mdx/mTRG1 and mdx/mTRG2 muscles (90±1%)(Figs. 1H and 2A) providing evidence of a marked increase in tissue damage and regenerative activity in most fibers at 8 weeks of age. Further histological analyses of diaphragm and gastrocnemius muscles from 8 week old mdx/mTRG2 mice reveal a marked increase in myofiber diameter heterogeneity, mononuclear cellular infiltration, necrosis and overall altered tissue structure relative to age-matched controls (Fig. 2A). Importantly, muscle histology from mTRG2 control mice is indistinguishable from WT mice. Diaphragm diameter of mdx/mTRHet is increased compared to WT, consistent with ongoing tissue damage and repair (Fig. 2A–B), and further increases in mdx/mTRG1 and mdx/mTRG2 mice, due primarily to massive cellular infiltration (Fig. 2A–B). Aggregates of calcium deposition and fibrotic regions are substantially increased in mdx/mTRG2 muscle (Fig. 2C), by contrast with mdx/mTRHet and mdx/mTRG1 muscles. Thus, although the control mdx/mTRHet mice undergo substantial muscle regeneration as indicated by the relatively high frequency of centrally nucleated myofibers, the tissue histology is not compromised compared with mdx mice lacking telomerase activity (G1 and G2).
Figure 2. Histological evidence of severe muscular dystrophy in muscles from mdx/mTRG2 mice.
(A) Hematoxylin/eosin staining of transverse sections of diaphragm (top) and gastrocnemius (bottom) muscles from the indicated genotypes at 8 weeks of age. Scale bar, 120μm. See also Fig. S2.
(B) Average diaphragm diameter is significantly increased in 8 week old mdx/mTRKO mice. Data are represented as average±s.e.m. (n=3, P<0.05).
(C) Alizarin Red staining to visualize calcium deposits (top) and Gomori staining for collagen deposition (bottom) in muscle transverse sections from 8 week old mice. Scale bar, 120μm.
Muscular dystrophy in mdx/mTRG2 mice progresses with age
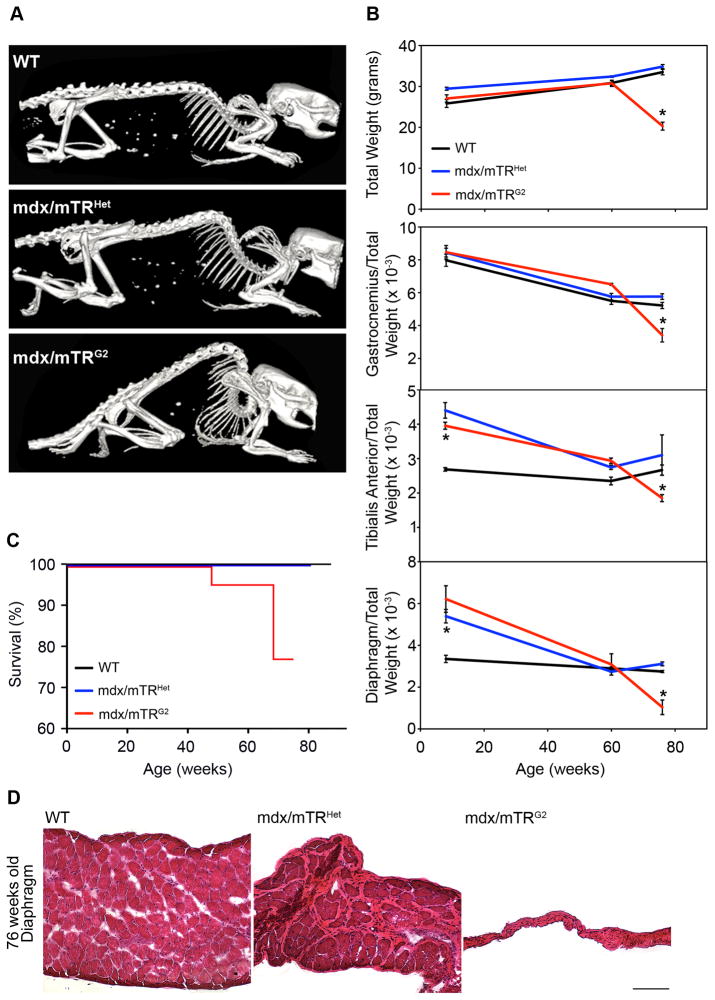
The severe progression of DMD associated with age is not recapitulated in mdx mice. Kyphosis of the spine is a classical clinical manifestation of DMD due to uneven weakening of trunk muscles and substitution with connective tissue and fat (Oda et al., 1993; Wilkins and Gibson, 1976). SPECT-CT imaging revealed that kyphosis in a cohort of aging mice is substantially more pronounced in mdx/mTRG2 mice than in age-matched mdx/mTRHet controls at 76 weeks of age (Fig. 3A).
Figure 3. Muscular dystrophy in mdx/mTRG2 mice progresses with age.
(A) Whole body SPECT/CT images. Pronounced skeletal deformity of the spine (kyphosis) is present in 76 week old mdx/mTRG2 mice, compared to age-matched controls.
(B) Animal weight significantly decreased in mdx/mTRG2 mice at 76 weeks of age (top, n≥3, P<0.05). Indicated muscles were harvested from mice at various ages and weighed. Data are represented as the weight of the tissue relative to total body weight, a standard control for telomere shortening (Lee et al., 1998). Results are shown as average±s.e.m (n≥3, P<0.05).
(C) Kaplan-Meyer survival curve. mdx/mTRG2 mice exhibited reduced life-span compared to mdx/mTRHet and WT controls (n≥12).
(D) Hematoxylin/eosin staining of diaphragm muscle from animals at 76 weeks of age showed dramatic atrophy of the tissue in mdx/mTRG2 animals compared to controls (Scale Bar=120μm). See also Fig. S3.
We showed the severity of muscle wasting by assessing a loss of both total body weight and specific muscle weight due to atrophy, which is typically exacerbated with increasing age in DMD patients. Total body weight is disproportionately reduced in mdx/mTRG2 mice compared to controls at 76 weeks of age (Fig. 3B). When controlled for total body weight, all genotypes exhibit a progressive decrease in weight with age, but the drop is accelerated in mdx/mTRG2 muscles after 60 weeks. In 8 week old mice, the diaphragm and tibialis anterior mdx/mTRG2 muscles exhibit an increase in weight relative to total body weight (Fig 3B), consistent with observations in DMD patients, whose body weight transiently increases at young age, but then decreases at later ages due to skeletal muscle atrophy (McDonald et al., 1995). The weight of control testis tissue, a high proliferative organ, does not change significantly among genotypes, indicating that the tissue atrophy is specific to muscles (Fig. S5). Kaplan-Meyer survival analyses show that mdx/mTRG2 mice have reduced life-span, starting to die at 48 weeks of age, while mdx/mTRHet do not show a difference compared to WT animals (Fig. 3C). Finally, histological analyses of limb muscles from 76 week old animals reveals pronounced tissue damage in mdx/mTRG2 mice, with extensive fibrosis and immune cell infiltration (Fig. S3). Most notably, diaphragm muscles from mdx/mTRG2 mice at 76 weeks of age have atrophied to a small remnant of their original size, suggesting that respiratory failure is the cause of premature death (Fig. 3D).
Impaired proliferation of mdx/mTRG2 MuSC in mice in vivo
We hypothesized that the severe muscle phenotype observed in mdx/mTRG2 animals is caused by defects in MuSC function. To test this possibility, we assessed MuSC proliferation in vivo by assaying undamaged or acutely injured Tibialis Anterior muscles (Harris and MacDonell, 1981) injected intraperitoneally with bromo-deoxyuridine (BrdU) 24hrs prior to harvesting. MuSC were then isolated by FACS to >90% purity for all genotypes (Fig. S4), as previously described (Sacco et al., 2008), and analyzed for BrdU incorporation. In WT mice in undamaged conditions, only 0.4±0.2% of MuSC were BrdU+ (Fig. 4A–B), consistent with previous reports that adult MuSC are largely quiescent in steady-state conditions (Cerletti et al., 2008). However, upon notexin injury, WT MuSC are activated, enter the cell cycle, and 8.6±2.9% are BrdU+ (Fig. 4A–B). In undamaged mdx/mTRHet mice, 2.5±1.4% of MuSC are BrdU+, which is higher than WT mice, consistent with ongoing tissue regeneration due to dystrophin deficiency. MuSC from mdx mice are able to respond appropriately to acute injury by entering the cell cycle (5.1±0.6%). In contrast, MuSC from mdx/mTRG1 (1.4±0.3%) and mdx/mTRG2 (1.3±0.2%) mice exhibit markedly reduced BrdU incorporation in the absence of notexin damage (Fig. 4A–B), which does not significantly increase upon injury in mdx/mTRG1 (1.6±0.1%) and mdx/mTRG2 mice (2.5±0.4%), indicating a severe defect in MuSC proliferation prior to and in response to tissue damage. Notably, proliferative MuSC behavior from control mTRG2 mice is not impaired and is indistinguishable from WT mice. These data highlight that MuSC become severely impaired when the absence of dystrophin is combined with the absence of telomerase activity.
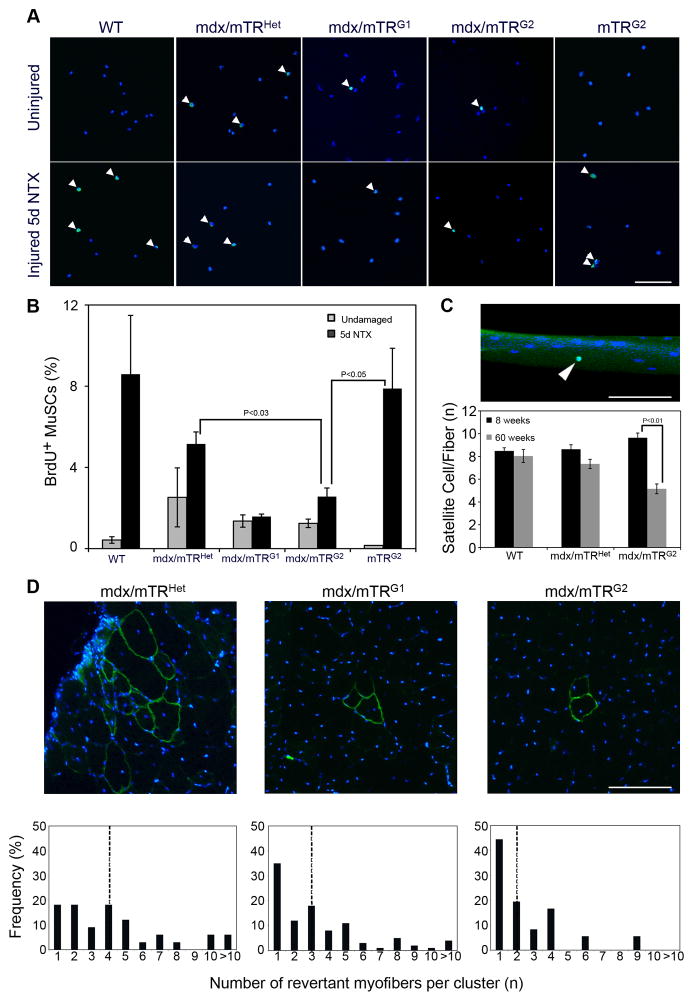
Figure 4. Muscle stem cells from mdx/mTRG2 mice exhibit impaired proliferation in vivo.
(A) 8 week old undamaged or NTX-damaged mice were pulsed with BrdU for 24hrs prior to tissue harvesting. MuSC were isolated by FACS, and immunofluorescence for BrdU (green) and Hoechst (blue) was performed. Representative images of MuSC from all genotypes are shown. Arrowheads indicate BrdU+ cells. Scale Bar, 80μm. See also Fig. S4.
(B) Percentage of BrdU+ MuSC represented as average±s.e.m. (n≥3, P values are indicated in the graph).
(C) Single fibers were isolated from tibialis anterior muscles at the indicated ages and stained for Pax7 (green) and nuclei (blue). Representative image showing a single fiber with a Pax7+ satellite cell (arrowhead). Scale bar, 50μm (top). Graph showing number of Pax7+ satellite cells/fiber. (n=3 animals/group and 50–100 myofibers/animal). Data are represented as average±s.e.m. (bottom).
(D) Representative images of transverse sections of gastrocnemius muscles from the indicated genotypes at 8 weeks of age immunostained for dystrophin (green) and nuclei (blue). Scale Bar, 100μm (top). Graphs showing distribution of size of revertant myofiber clusters (dotted lines in the graphs represent the median of cluster size)(bottom).
The number of Pax7+ satellite cells per myofiber (8.7±0.6 in WT, 7.7±0.1 in mdx/mTRHet, and 8.5±0.8 in mdx/mTRG2) is not statistically different among the different genotypes (P>0.05)(Fig. 4C), demonstrating that the observed decline in BrdU+ MuSC is not the result of reduced numbers of satellite cells, but rather a functional defect at this age. However, in older mice (60 weeks), the number of satellite cells is markedly reduced in aged mdx/mTRG2 mice (Fig. 4C), mirroring the severity of disease progression.
Another measure of MuSC proliferative potential in vivo is an analysis of clusters of revertant myofibers. Revertant myofibers express dystrophin protein, due to a compensatory mutation in somatic cells (Hoffman et al., 1990). If the mutation takes place in a MuSC, upon proliferation this MuSC will participate in the regeneration of myofibers in its close proximity, giving rise to a cluster of dystrophin+ myofibers. We observe a progressive reduction in revertant myofiber cluster size in successive mdx/mTR generations with the median cluster size in mdx/mTRHet, mdx/mTRG1 and mdx/mTRG2 declining progressively (Fig. 4D), consistent with a progressive reduction in MuSC proliferative potential.
Critically shortened telomeres in myoblasts from mdx/mTRG1 and mdx/mTRG2 mice
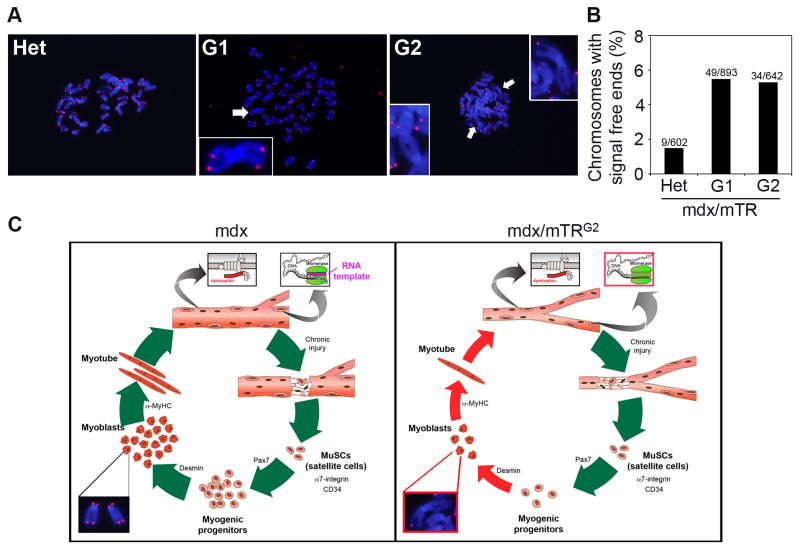
Telomeres were analyzed by hybridization of a telomere-specific probe to metaphase chromosomes by fluorescence in situ hybridization (telomere FISH). This assay requires large numbers of mitotic cells in metaphase. Since methods for cultivating MuSC in sufficient numbers do not yet exist, we analyzed the derivatives of MuSC, myoblasts, isolated as previously described (Rando and Blau, 1994). Chromosome ends lacking detectable telomere repeats (signal-free ends, SFEs), are detected at increased frequency 49/893 (5.5%) in mdx/mTRG1 and 34/642 (5.3%) in mdx/mTRG2 mice versus 9/602 (1.5%) in mdx/mTRHet mice (Fig. 5A–B). The 5% frequency of SFE seen in mdx/mTRG1 and mdx/mTRG2 mice is not detected even in highly proliferative organs until later generations in mice lacking Terc (Blasco et al., 1997), providing evidence that this increased frequency is not due to Terc absence alone. In addition, in metaphase spreads of myoblasts from mdx/mTRG1 and mdx/mTRG2 mice end-to-end chromosome fusions were detected (Fig. 5A, arrows), indicating that the telomere shortening in these cells leads to genomic instability. In marked contrast, in 602 chromosomes analyzed from mdx/mTRHet myoblast metaphase spreads no end-to-end fusions were detected. SFEs represent chromosome ends with the shortest telomeres and correlate with and increased incidence of chromosome fusions and tissue dysfunction in vivo in telomerase knockout mice (Chang et al., 2004). This result strongly supports our hypothesis that exhaustion of proliferative potential in dystrophic MuSC, via telomere dysfunction, is the direct cause of muscular dystrophy progression.
Figure 5. Telomere FISH analysis in primary myoblasts.
(A) Representative images of FISH on metaphase spreads from mdx/mTRHet, mdx/mTRG1 and mdx/mTRG2 (red, telomeres; blue, Hoechst). Arrows show end-to-end fusion of chromosomes, indicating genomic instability.
(B) Graph showing the quantification of percentage of chromosomes with signal free ends (total number of chromosomes analyzed are shown on top of each bar). P<0.03. See also Fig. S5.
(C) Scheme representing the series of events during muscle regeneration in the mdx mouse model (left) compared to the mdx/mTRG2 dystrophic model (right). Critical telomere shortening and chromosomal fusions in mdx/mTRG2 muscle cells results in impaired ability to sustain tissue regeneration.
Analyses of spleen and testis reveal that these two high proliferative organs are not affected in the generations analyzed (Fig. S5), which argues against the possibility that the effects observed are due to the absence of Terc alone and in favor of a muscle-specific etiology. A scheme depicts the effects of Terc loss in the etiology of DMD (Fig. 5C).
Impaired proliferation potential of MuSC from mdx/mTRG2 mice in culture
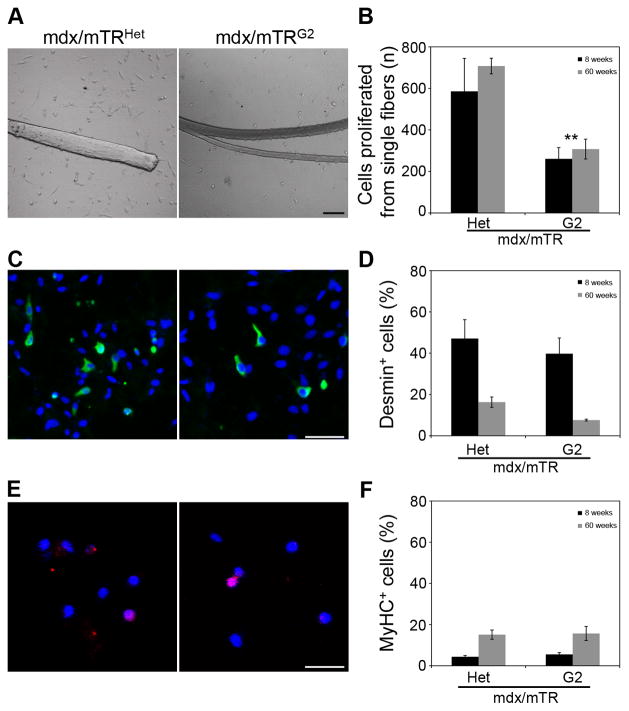
To further test whether the cell autonomous changes in muscle tissue were caused by reduced proliferative potential of MuSC in mdx/mTRG2 mice, we assessed their behavior in vitro. Single myofibers were isolated from Tibialis Anterior muscles from 8 week old animals and the proliferative potential of MuSC crawling off myofibers was analyzed after 4 days of culture. Satellite cells derived from mdx/mTRG2 myofibers exhibit reduced proliferative potential (261±54 cells/fiber) compared to mdx/mTRHet myofibers (586±157 cells/fiber)(Fig. 6A–B). As described above (Fig. 4C), the number of satellite cells per myofiber is not altered at this age, indicating that the observed differences in cell number result from a cell autonomous proliferative defect in MuSC from mdx/mTRG2 mice caused by dysfunctional telomeres. The reduction in satellite cell proliferation persists with age; at 60 weeks satellite cells from mdx/mTRG2 mice give rise only to 40% as many cells (307±47) as controls (707±37)(Fig. 6B).
Figure 6. Muscle stem cells from mdx/mTRG2 mice exhibit impaired proliferation in vitro.
(A) Single myofibers were isolated from 8 week and 60 week old animals and cultured for 96hrs. Representative images of cultures show a proliferation defect in mdx/mTRG2 muscles, compared to mdx/mTRHet muscles. Scale bar, 50μm. See also Fig. S6.
(B) Graph showing quantification of proliferation capacity as total number of cells derived per myofiber at 96hrs. Data are represented as average±s.e.m. (n=3, P<0.05).
(C) Representative images of immunofluorescence staining of fiber cultures for desmin (green) and nuclei by Hoechst 33258 (blue). Scale bar, 50μm.
(D) Graph showing quantification of percentage of desmin+ cells in cultures derived from single myofibers. Data are represented as average±s.e.m. (n=3, P>0.05).
(E) Representative images of immunofluorescence staining of fiber cultures for myosin heavy chain (red) and nuclei by Hoechst 33258 (blue). Scale bar, 50μm.
(F) Graph showing quantification of percentage of MyHC+ cells in cultures derived from single myofibers. Data are represented as average±s.e.m. (n=3, P>0.05).
To assess whether a reduction in the proliferation capacity is followed by a change in the differentiation efficiency, staining for desmin and myosin heavy chain, two established myogenic markers, was performed in vitro. The percentage of desmin+ cells is not significantly different between mdx/mTRHet (47±8%) and mdx/mTRG2 myofibers (40±21%), indicating that there is no impairment in mdx/mTRG2 satellite cells to transition to myoblasts (Fig. 6C–D). Notably, with age the myogenic potential (desmin expression) is substantially reduced in both genotypes (Fig. 6D), consistent with previous studies reporting a decreased myogenic potential in muscle cells from dystrophic and aged mice (Bockhold et al., 1998; Brack et al., 2007). Myosin heavy chain expression shows that spontaneous terminal differentiation does not differ significantly between mdx/mTRHet and mdx/mTRG2 cells in young or old mice (Fig. 6E–F). These data confirm that satellite cell differentiation properties do not differ, whereas the proliferative function does. With increased age the absolute number of MuSC declines (Fig. 4C), compounding the proliferative defect leading to an even more pronounced deficit in cells available for muscle repair.
mdx/mTRG2 MuSC are impaired in engrafting upon transplantation
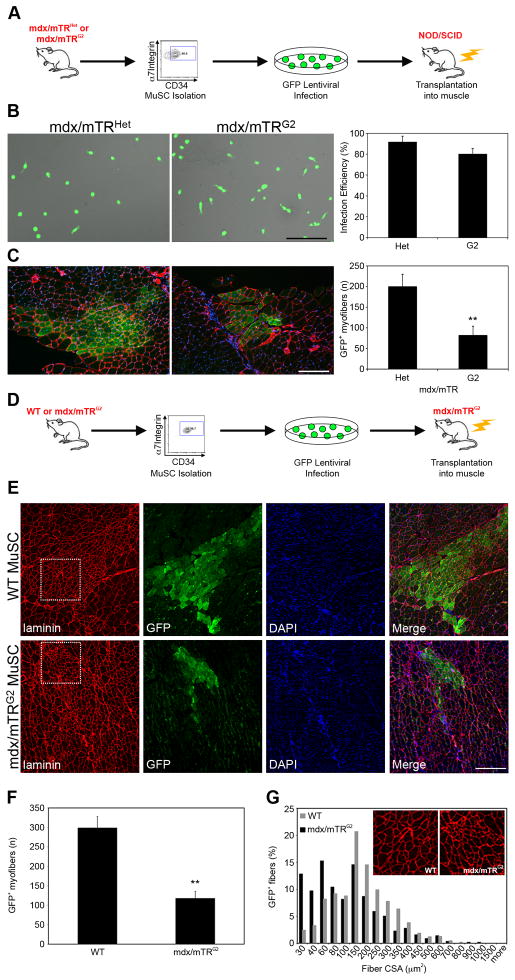
To test the combined effects of mdx mutation and telomere dysfunction on MuSC function in vivo, we assessed the ability of purified MuSC to engraft and proliferate upon transplantation into recipient muscles. Freshly isolated MuSC (Sacco et al., 2008) were infected with a lentivirus expressing GFP, and 3,000 cells were transplanted into irradiated Tibialis Anterior muscles of immunodeficient NOD/SCID mice (Fig. 7A). The infection efficiency approximated 90%, and was not statistically different among samples (Fig. 7B, P>0.05). Three weeks post transplantation, mdx/mTRHet MuSC contributed to an average of 200±30 GFP+ myofibers (Fig. 7C). Conversely, MuSC isolated from mdx/mTRG2 mice were significantly impaired in their engraftment capacity, contributing to substantially lower numbers of myofibers, 81±22 (Fig. 7C). These data corroborate our in vitro analyses and show that MuSC from mdx/mTRG2 mice have a cell autonomous defect in proliferation that compromises their ability to generate new muscle fibers in vivo.
Figure 7. Muscle stem cells from mdx/mTRG2 mice exhibit impaired engraftment following transplantation into mouse muscles.
(A) Scheme of transplantation into NOD/SCID mice.
(B) Representative images of lentivirus-infected MuSC. Scale bar, 80μm (left). Graph showing quantification of infection efficiency expressed as percentage of GFP+ cells. Data are represented as average±s.e.m. (right)(P>0.05).
(C) Three weeks after transplantation, muscles were harvested, sectioned and immunostained for GFP (green), Laminin (red) and nuclear Hoechst (blue). Representative images of transplanted muscles. Scale bar, 120μm (left). Graph showing total numbers of GFP+ myofibers scored per muscle. Data are represented as average±s.e.m. (n≥5, P<0.01)(right).
(D) Scheme of transplantation into mdx/mTRG2 mice.
(E) Three weeks after transplantation, muscles were harvested, sectioned and immunostained for GFP (green), Laminin (red) and nuclear DAPI (blue). Representative images of transplanted muscles. Scale bar, 100μm.
(F) Graph showing total numbers of GFP+ myofibers per muscle. Data are represented as average±s.e.m. (n=5, P<0.05).
(G) Analysis of GFP+ myofiber cross-sectional area after transplantation shows that WT MuSC give rise to larger myofibers compared to mdx/mTRG2 MuSC.
To investigate a potential effect of telomere erosion on myofiber degeneration, we induced acute injury by injection of notexin in 8 week old animals and performed histological analyses at day 3 after injury. The extent of muscle damage was comparable between mdx/mTRHet and mdx/mTRG2, indicating that mdx/mTRG2 mice are not more sensitive to this type of tissue injury (Fig. S6). In addition, the infiltrate of hematopoietic (CD45+ cells), macrophages (CD11b) and lymphocytes (B220) is equally represented in mdx/mTRHet and mdx/mTRG2 (Fig. S6). These results indicate that mdx/mTRG2 mice are competent to mount an inflammatory response to tissue injury and provide direct evidence that the hematopoietic compartment is not compromised. Finally, Sca1+ mesenchymal interstitial cells and CD31+ endothelial cells are also comparably present in mdx/mTRHet and mdx/mTRG2 muscle, suggesting that the severe phenotype is muscle-specific and is not the result of functional defects in other cell types.
Amelioration of the mdx/mTRG2 phenotype after transplantation of WT MuSC
To test if the dystrophic phenotype in mdx/mTRG2 mice results from diminished MuSC reserve, we performed MuSC transplantation experiments into mdx/mTRG2 dystrophic muscles. Freshly isolated MuSC, either WT or mdx/mTRG2, were infected with a lentivirus expressing GFP, and 5,000 cells were transplanted into irradiated Tibialis Anterior muscles of mdx/mTRG2 mice (Fig. 7D). The infection efficiency approximated 95% and was comparable among groups (P>0.05; data not shown). Three weeks post transplantation, WT MuSC efficiently engrafted into recipient muscles, contributed to an average of 299±29 GFP+ myofibers (Figs. 7E–F and S7F–J), while mdx/mTRG2 MuSC contributed to substantially lower numbers of GFP+ myofibers, 118±17 (Fig. 7E–F and S7A–E). Measurements of fiber cross-sectional area indicate that WT MuSC gave rise to larger myofibers compared to mdx/mTRG2 (Fig. 7G), indicating the ability to more robustly contribute to muscle upon transplantation. Further histological analysis revealed that the amount of inflammatory infiltration is substantially reduced in the muscle region into which the WT MuSC were injected, compared to mdx/mTRG2, as evidenced by the low numbers of CD45+ hematopoietic cells and overall improvement in tissue architecture apparent in H&E sections (Fig. S7K–L). The regenerative potential of transplanted MuSC from control mTRG2 mice into mdx/mTRG2 animals is similar to that observed with WT MuSC (data not shown). Together, these results provide strong evidence that the severe dystrophic phenotype observed in mdx/mTRG2 mice results from loss of regenerative potential of endogenous MuSC. Although we cannot completely rule out the possibility of defects in other cell types or a direct effect on the myofiber itself as a potential contributing factor, these results demonstrate that progressive loss of MuSC reserve plays a major role in determining the severity of the dystrophic phenotype.
Discussion
The absence of a mouse model for DMD that faithfully mimics key features of the human disease has limited our understanding of its pathophysiology and tests of potential therapies. A longstanding enigma has been why humans with a dystrophin mutation suffer from such severe muscle wasting, whereas mice with the same mutation do not. A major difference between humans and mice is the length of their telomeres, which are substantially shorter in humans. A reduction in telomere length in MuSC could severely limit the stem cell pool available for muscle repair over time. Here we show that a novel mouse model, mdx mice lacking telomerase activity (mdx/mTR), more closely recapitulates the DMD phenotype than mouse models described to date, as evidenced by characteristic severe progressive loss of muscle form and function.
We postulated that the severe muscle wasting phenotype observed in this mouse model is due to impaired function of the MuSC required for damage repair. We ruled out other possible stem cell etiologies such as premature MuSC differentiation or a reduction in MuSC numbers per myofiber at the time of disease onset. Instead, we determined both in vitro and in vivo that the MuSC from mdx/mTRG2 mice suffer from severe defects in proliferation. In vivo, MuSC exhibited reduced division (BrdU labeling), smaller revertant clone sizes, inability to respond to injury, and markedly reduced engraftment upon transplantation into mice. In vitro, the proliferation defect was manifested by a marked reduction in cells derived from single fibers. This proliferative defect correlated with an increase in signal free chromosome ends and evidence of chromosome fusions, normally not seen in low turnover tissues such as muscle. Over time, a decrease in the number of MuSC per muscle fiber was observed. Presumably the decrease in MuSC number in aged, dystrophic telomerase-null mice ultimately reflects a failure of stem cell self-renewal. This reduction in MuSC number exacerbates the already impaired regeneration resulting from the reduced proliferative capacity of the remaining MuSC.
We reasoned that the replicative capacity of MuSC plays a role in murine muscle tissue homeostasis, overcoming the defect observed in human muscle lacking dystrophin. We confirmed this hypothesis here by coupling the absence of telomerase together with a constant pressure for muscle cell proliferation and regeneration, caused by the mdx mutation. The result is a mouse model, mdx/mTRG2 that closely approximates the human disease. Muscles from DMD patients have been reported to have shorter telomeres than healthy individuals (Decary et al., 2000; Mouly et al., 2005). Similarly critical telomere shortening is evident here in cultured muscle progenitor cells isolated from mdx/mTRG2 mice with the muscular dystrophy phenotype. The generation of mouse models lacking telomerase activity, TERT and Terc knockout mice (Blasco et al., 1997; Liu et al., 2000) previously resulted in the demonstration that telomerase plays a crucial role in self-renewal of adult stem cells in high turnover tissues such as blood, liver, skin and testis (Allsopp et al., 2003; Lee et al., 1998). By contrast, skeletal muscle is a stable tissue, and a muscle phenotype in mice lacking TERT and Terc has never been reported. Even in the setting of continuous damage, the mdx model, only a mild muscle phenotype is apparent and there is no evidence for telomere shortening in myoblasts of these mice. By contrast, we show here that murine MuSC progeny from mice lacking both dystrophin and telomerase, exhibit a higher frequency of chromosomes with signal free ends (chromosomes with critically shortened telomeres), compared to control mdx/mTRHet, indicating that a persistent need for MuSC proliferation in vivo leads to telomere shortening, consequent senescence-like behavior, and tissue degeneration. These results provide definitive evidence that telomerase plays a crucial role in MuSC function in vivo. Although we cannot entirely rule out an effect of lack of telomerase on other cell types or mature myofibers, the findings highlight a cell autonomous or intrinsic limitation as causal in hindering MuSC function.
Our findings of stem cell autonomous defects are important to consider in light of recent studies that implicate a primary role of the environment in maintaining niche function and stem cell performance. Indeed, these studies show that stem cells from young and aged mice behave similarly when exposed to systemic factors from young mice via parabiosis (Brack et al., 2007; Conboy et al., 2005). Our results show that intrinsic characteristics such as telomere attrition are also critical to muscle regeneration and need to be taken into consideration when predicting the effects of modulating the environment on the function of human MuSC from aged or dystrophic muscles.
The etiology of the fibrosis observed in DMD remains unknown. Here we observe that when WT MuSC are transplanted into dystrophic mouse muscles, minimal fibrosis is observed, whereas fibrosis is extensive when proliferation impaired mdx/mTRG2 MuSC are transplanted. These results are similar to those observed in chronic liver cirrhosis in which fibrosis and telomere shortening are correlated (Kitada et al., 1995; Rudolph et al., 2000; Wiemann et al., 2002). These findings suggest the intriguing possibility that in chronically injured tissues fibrosis is secondary to cell loss, not a primary competing process that antagonizes repair.
In summary, we provide evidence that telomere shortening is the result of continuous proliferation in degenerating dystrophic muscle, and describe the generation of a novel DMD mouse model which more faithfully recapitulates the disease progression in humans. This novel model will prove useful for elucidating the pathophysiology of DMD and for testing potential therapeutic approaches for this and other muscle wasting diseases, which has been hindered by the mild phenotypes typical of currently available mouse models. Our results indicate that DMD, a muscle degenerative disease, is the result of a multifactorial process, due to both a structural defect of the tissue and to progressive exhaustion of its regenerative potential. Therapeutic interventions should consider both telomere length as well as myogenic potential of the regenerative cell source, in order to overcome previous limitations (Gussoni et al., 1999). Together, these data document the importance of telomerase in muscle homeostasis and provide the first direct experimental evidence that DMD progression, although initiated and driven by dystrophin deficiency, is ultimately a stem cell disease.
Experimental Procedures
Animals
All protocols were approved by the Stanford University Administrative Panel on Laboratory Animal Care. C57Bl6 mdx mice (purchased from Jackson Laboratories) and C57Bl6 mTRHet mice (a kind gift from Ron DePinho) were used to generate the double mutant animals. The NOD/SCID immunodeficient mice were purchased from the Jackson Laboratories.
Single fiber isolation and satellite cell staining
Single myofibers were isolated from Tibialis Anterior muscles for all genotypes as previously described (Collins et al., 2005). See Extended Experimental Procedures for a detailed description of the procedure.
Force Measurements
8 week old mice were anesthetized and force measurements of lateral gastrocnemius muscle were performed in vivo on anesthetized animals in accordance with Stanford guidelines. See Extended Experimental Procedures for a detailed description of the procedure.
Treadmill Test
The treadmill test was performed using the Exer3/6 (Columbus Instruments). Mice were acclimated to treadmill running for three times (every other day) before the test was performed. Mice ran on the treadmill at 20 degrees downhill, starting at a speed of 10meters/min. After three minutes, the speed was increased 1meter/min to a final speed of 20 meters/min. Exhaustion was defined as the inability of the animal to remain on the treadmill despite electrical prodding.
SPECT/CT Animal Imaging
Anesthetized (3% isofluorane) animals were placed into SPECT-CT scanner (Gamma-Medica-Ideas Pre-clinical Imaging) with high energy-resolution CZT detectors and multipin-hole collimators. See Extended Experimental Procedures for a detailed description of the procedure.
Irradiation, Cell Transplantation and Notexin Damage
NOD/SCID and mdx/mTRG2 mice were anesthetized and shielded in a lead-jig so that only the legs were exposed to the radiation source. A single dose of 18Gy was administered to the legs and cell transplantation was performed on the same day. Lentivirus-infected MuSC were resuspended in PBS and a 10μl of cell suspension was injected intramuscularly into the Tibialis Anterior (TA) muscles of recipient mice. For local tissue injury, mice were anesthetized with isofluorane and a single 10μl injection of notexin (10μg/ml, Latoxan, France) was injected into the TAs of recipient mice.
In vivo BrdU labeling
Mice were anesthetized with isofluorane, weighed and a single dose of BrdU in PBS was delivered intraperitoneally (100mg/Kg body weight). 24hrs later, mice were sacrificed and MuSC isolated, as previously described (Sacco et al., 2008). After FACS enrichment, MuSC were cytospun onto slides, fixed and stained using a BrdU labeling and detection kit (Roche).
Telomere fluorescence in situ hybridization (telomere FISH)
For telomere measurements, primary myoblasts were isolated as previously described (Rando and Blau, 1994) and maintained in culture in F10/DMEM+15%FBS+βFGF media. See Extended Experimental Procedures for a detailed description of the procedure.
Lentiviral Infection
The 5 plasmid system and infection protocol used here has been previously described (Westerman et al., 2007). See Extended Experimental Procedures for a detailed description of the procedure.
Cell Culture
Cells were isolated from muscle tissue by enzymatic dissociation as described above. Cells were plated on dishes coated with Laminin (Roche) in F10/DMEM (50/50)+15%FBS+2.5ng/ml βFGF (GM) for proliferation and in DMEM+2% horse serum (DM) for differentiation.
Image acquisition of immunofluorescence and histology
Images of muscle transverse sections were acquired using an epifluorescence microscope (Axioplan2, Carl Zeiss MicroImaging, Inc.), Fluar 20X/0.75 objective lens, and a digital camera (ORCA-ER C4742-95; Hamamatsu Photonics). The software used for acquisition was OpenLab 4.0.2 (Improvision). Images of cell cultures were acquired using a laser-scanning confocal microscope (LSM510, Carl Zeiss MicroImaging, Inc.) using Plan NeoFluar 10X/0.3 and 20X/0.75 objective lens and maximum optical sections with the LSM software. All images were composed and edited in Photoshop 7.0 (Adobe). Background was reduced using brightness and contrast adjustments, and color balance was performed to enhance colors. All the modifications were applied to the whole image using Photoshop 7.0 (Adobe).
Statistical Analysis
Data are presented as mean±s.e.m. Comparisons between groups used the Student’s t test assuming two-tailed distributions, with an alpha level of 0.01–0.05.
Supplementary Material
Acknowledgments
We thank Kassie Koleckar for excellent technical support and Fabio Rossi for providing the α7-integrin-PE conjugated antibody, Jackie Kustan for help with the scheme and Frezghi Habte at the Stanford Small Imaging Facility for help with the CT-Scan. This work was supported by American Heart Association Scientist Development Grant 10SDG3510024 to FM, NIH grants AG009521, AG020961, Muscular Dystrophy Association grant 4320 and the Baxter Foundation to HMB.
Footnotes
Authors Contributions A.S., J.P. and H.M.B. designed the research, A.S. and F.M. performed most of the experiments, P.K. maintained the mouse colony and performed treadmill experiments, M.L. performed force measurement experiments and S.D. analyzed the data, R.T. performed lentiviral infection and single fiber isolation, M.S. and J.C. performed Q-FISH assay and S.A. analyzed the data. A.S. analyzed the data. A.S., F.M., J.P., S.A. and H.M.B. discussed the results and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aikata H, Takaishi H, Kawakami Y, Takahashi S, Kitamoto M, Nakanishi T, Nakamura Y, Shimamoto F, Kajiyama G, Ide T. Telomere reduction in human liver tissues with age and chronic inflammation. Exp Cell Res. 2000;256:578–582. doi: 10.1006/excr.2000.4862. [DOI] [PubMed] [Google Scholar]
- Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102:517–520. doi: 10.1182/blood-2002-07-2334. [DOI] [PubMed] [Google Scholar]
- Artandi SE. Telomeres, telomerase, and human disease. The New England journal of medicine. 2006;355:1195–1197. doi: 10.1056/NEJMp068187. [DOI] [PubMed] [Google Scholar]
- Blaauw B, Mammucari C, Toniolo L, Agatea L, Abraham R, Sandri M, Reggiani C, Schiaffino S. Akt activation prevents the force drop induced by eccentric contractions in dystrophin-deficient skeletal muscle. Hum Mol Genet. 2008;17:3686–3696. doi: 10.1093/hmg/ddn264. [DOI] [PubMed] [Google Scholar]
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- Blau HM, Webster C, Pavlath GK. Defective myoblasts identified in Duchenne muscular dystrophy. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:4856–4860. doi: 10.1073/pnas.80.15.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockhold KJ, Rosenblatt JD, Partridge TA. Aging normal and dystrophic mouse muscle: analysis of myogenicity in cultures of living single fibers. Muscle & nerve. 1998;21:173–183. doi: 10.1002/(sici)1097-4598(199802)21:2<173::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science (New York, NY) 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Multani AS, Cabrera NG, Naylor ML, Laud P, Lombard D, Pathak S, Guarente L, DePinho RA. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat Genet. 2004;36:877–882. doi: 10.1038/ng1389. [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wilcox-Adelman SA, Goetinck PF, Rauvala H, Rapraeger AC, Olwin BB. Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes & development. 2004;18:2231–2236. doi: 10.1101/gad.1214204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decary S, Hamida CB, Mouly V, Barbet JP, Hentati F, Butler-Browne GS. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord. 2000;10:113–120. doi: 10.1016/s0960-8966(99)00093-0. [DOI] [PubMed] [Google Scholar]
- Decary S, Mouly V, Hamida CB, Sautet A, Barbet JP, Butler-Browne GS. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther. 1997;8:1429–1438. doi: 10.1089/hum.1997.8.12-1429. [DOI] [PubMed] [Google Scholar]
- DiMario JX, Uzman A, Strohman RC. Fiber regeneration is not persistent in dystrophic (MDX) mouse skeletal muscle. Developmental biology. 1991;148:314–321. doi: 10.1016/0012-1606(91)90340-9. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Current opinion in genetics & development. 2002;12:349–361. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Emery AE. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Friedrich U, Griese E, Schwab M, Fritz P, Thon K, Klotz U. Telomere length in different tissues of elderly patients. Mech Ageing Dev. 2000;119:89–99. doi: 10.1016/s0047-6374(00)00173-1. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Harris JB, MacDonell CA. Phospholipase A2 activity of notexin and its role in muscle damage. Toxicon. 1981;19:419–430. doi: 10.1016/0041-0101(81)90046-5. [DOI] [PubMed] [Google Scholar]
- Hemann MT, Rudolph KL, Strong MA, DePinho RA, Chin L, Greider CW. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Molecular biology of the cell. 2001;12:2023–2030. doi: 10.1091/mbc.12.7.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, Samper E, Blasco MA. Telomere shortening in mTR −/− embryos is associated with failure to close the neural tube. The EMBO journal. 1999;18:1172–1181. doi: 10.1093/emboj/18.5.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Morgan JE, Watkins SC, Partridge TA. Somatic reversion/suppression of the mouse mdx phenotype in vivo. J Neurol Sci. 1990;99:9–25. doi: 10.1016/0022-510x(90)90195-s. [DOI] [PubMed] [Google Scholar]
- Im WB, Phelps SF, Copen EH, Adams EG, Slightom JL, Chamberlain JS. Differential expression of dystrophin isoforms in strains of mdx mice with different mutations. Hum Mol Genet. 1996;5:1149–1153. doi: 10.1093/hmg/5.8.1149. [DOI] [PubMed] [Google Scholar]
- Kipling D, Cooke HJ. Hypervariable ultra-long telomeres in mice. Nature. 1990;347:400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- Kitada T, Seki S, Kawakita N, Kuroki T, Monna T. Telomere shortening in chronic liver diseases. Biochemical and biophysical research communications. 1995;211:33–39. doi: 10.1006/bbrc.1995.1774. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ. In vivo loss of telomeric repeats with age in humans. Mutat Res. 1991;256:45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Snow BE, Hande MP, Yeung D, Erdmann NJ, Wakeham A, Itie A, Siderovski DP, Lansdorp PM, Robinson MO, et al. The telomerase reverse transcriptase is limiting and necessary for telomerase function in vivo. Curr Biol. 2000;10:1459–1462. doi: 10.1016/s0960-9822(00)00805-8. [DOI] [PubMed] [Google Scholar]
- McDonald CM, Abresch RT, Carter GT, Fowler WM, Jr, Johnson ER, Kilmer DD, Sigford BJ. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74:S70–92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science (New York, NY) 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Mouly V, Aamiri A, Bigot A, Cooper RN, Di Donna S, Furling D, Gidaro T, Jacquemin V, Mamchaoui K, Negroni E, et al. The mitotic clock in skeletal muscle regeneration, disease and cell mediated gene therapy. Acta physiologica Scandinavica. 2005;184:3–15. doi: 10.1111/j.1365-201X.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- Oda T, Shimizu N, Yonenobu K, Ono K, Nabeshima T, Kyoh S. Longitudinal study of spinal deformity in Duchenne muscular dystrophy. J Pediatr Orthop. 1993;13:478–488. doi: 10.1097/01241398-199307000-00012. [DOI] [PubMed] [Google Scholar]
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. The Journal of cell biology. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault V, Rolland E, Thornell LE, Mouly V, Butler-Browne G. Distribution of satellite cells in the human vastus lateralis muscle during aging. Exp Gerontol. 2002;37:1513–1514. doi: 10.1016/s0531-5565(02)00095-5. [DOI] [PubMed] [Google Scholar]
- Rodier F, Kim SH, Nijjar T, Yaswen P, Campisi J. Cancer and aging: the importance of telomeres in genome maintenance. Int J Biochem Cell Biol. 2005;37:977–990. doi: 10.1016/j.biocel.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science (New York, NY) 2000;287:1253–1258. doi: 10.1126/science.287.5456.1253. [DOI] [PubMed] [Google Scholar]
- Ryder-Cook AS, Sicinski P, Thomas K, Davies KE, Worton RG, Barnard EA, Darlison MG, Barnard PJ. Localization of the mdx mutation within the mouse dystrophin gene. The EMBO journal. 1988;7:3017–3021. doi: 10.1002/j.1460-2075.1988.tb03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. The Journal of cell biology. 1997;139:375–385. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takubo K, Nakamura K, Izumiyama N, Furugori E, Sawabe M, Arai T, Esaki Y, Mafune K, Kammori M, Fujiwara M, et al. Telomere shortening with aging in human liver. J Gerontol A Biol Sci Med Sci. 2000;55:B533–536. doi: 10.1093/gerona/55.11.b533. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Schachter F, Uchida I, Wei L, Zhu X, Effros R, Cohen D, Harley CB. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993;52:661–667. [PMC free article] [PubMed] [Google Scholar]
- Webster C, Blau HM. Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet. 1990;16:557–565. doi: 10.1007/BF01233096. [DOI] [PubMed] [Google Scholar]
- Webster C, Filippi G, Rinaldi A, Mastropaolo C, Tondi M, Siniscalco M, Blau HM. The myoblast defect identified in Duchenne muscular dystrophy is not a primary expression of the DMD mutation. Clonal analysis of myoblasts from five double heterozygotes for two X-linked loci: DMD and G6PD. Hum Genet. 1986;74:74–80. doi: 10.1007/BF00278789. [DOI] [PubMed] [Google Scholar]
- Westerman KA, Ao Z, Cohen EA, Leboulch P. Design of a trans protease lentiviral packaging system that produces high titer virus. Retrovirology. 2007;4:96. doi: 10.1186/1742-4690-4-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, Flemming P, Franco S, Blasco MA, Manns MP, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. Faseb J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- Wilkins KE, Gibson DA. The patterns of spinal deformity in Duchenne muscular dystrophy. J Bone Joint Surg Am. 1976;58:24–32. [PubMed] [Google Scholar]
- Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu Y, Alt FW, DePinho RA. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature. 2003;421:643–648. doi: 10.1038/nature01385. [DOI] [PubMed] [Google Scholar]
- Zatz M, Rapaport D, Vainzof M, Passos-Bueno MR, Bortolini ER, Pavanello Rde C, Peres CA. Serum creatine-kinase (CK) and pyruvate-kinase (PK) activities in Duchenne (DMD) as compared with Becker (BMD) muscular dystrophy. J Neurol Sci. 1991;102:190–196. doi: 10.1016/0022-510x(91)90068-i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.