Abstract
Rationale
Low-dose acetylsalicylic acid (aspirin) is widely used in the treatment and prevention of vascular atherothrombosis. Cardiovascular doses of aspirin also reduce systemic blood pressure and improve endothelium-dependent vasorelaxation in patients with atherosclerosis or risk factors for atherosclerosis. Aspirin can acetylate proteins, other than its pharmacological target cyclooxygenase (COX), at lysine residues. The role of lysine acetylation in mediating the effects of low-dose aspirin on the endothelium is not known.
Objective
To determine the role of lysine acetylation of eNOS in the regulation of endothelial NO production by low-dose aspirin, and to examine whether the lysine deacetylase Histone Deacetylase-3 (HDAC3) antagonizes the effect of low-dose aspirin on endothelial NO production by reversing acetylation of functionally critical eNOS lysine residues.
Methods and results
Low concentrations of aspirin induce lysine acetylation of eNOS, stimulating eNOS enzymatic activity and endothelial NO production in a cyclooxygenase-1 (COX-1)-independent fashion. Low-dose aspirin in vivo also increases bioavailable vascular NO in an eNOS-dependent and COX-1-independent manner. Low-dose aspirin promotes the binding of eNOS to calmodulin. Lysine 609 in the calmodulin autoinhibitory domain of bovine eNOS mediates aspirin-stimulated binding of eNOS to calmodulin and eNOS-derived NO production. Overexpression of HDAC3 inhibits aspirin-stimulated lysine acetylation of eNOS, increase in eNOS enzymatic activity, eNOS-derived NO, and binding of eNOS to calmodulin. Similarly, downregulation of HDAC3 promotes lysine acetylation of eNOS, and endothelial NO generation.
Conclusions
Lysine acetylation of eNOS is a post-translational protein modification supporting low-dose aspirin-induced vasoprotection. HDAC3, by deacetylating aspirin-acetylated eNOS, antagonizes aspirin-stimulated endothelial production of NO.
Keywords: Aspirin, endothelial NOS, HDAC3, lysine acetylation, calmodulin
Introduction
Low-dose aspirin (81–325 mg/day) is a very useful tool in the armamentarium for the treatment of acute coronary syndromes, as well as for the secondary prevention of myocardial infarctions and stroke in high-risk patients1. The anti-thrombotic effects of low-dose aspirin are principally attributed to acetylation of a serine residue in platelet COX-1, irreversibly inhibiting COX-1 in platelets, and limiting platelet aggregation due to prostanoids produced by COX-12. In addition to preventing thrombosis, low-dose aspirin also improves vasomotor function mediated by the endothelium in humans3, 4, and in animal models of endothelial dysfunction5. Although low-dose aspirin is not commonly used in the treatment of hypertension, it is efficacious, when given at bedtime, for reduction of blood pressure in individuals with mild hypertension6, 7, or pre-hypertension8. Suppression of vasoconstricting prostanoids produced by COX-1 could be one mechanism by which low-dose aspirin improves endothelial function and reduces mildly and borderline elevated blood pressure. Notably, acetylation of alternate vasoregulatory targets by aspirin, other than COX-1, by aspirin could provide alternative explanations for its vasoprotective effects.
Low-dose aspirin increases NO produced by blood vessels9, but the mechanism responsible for this effect is not fully understood. Cardiovascular doses of aspirin increase nitric oxide synthase (NOS) enzymatic activity in endothelial cell homogenates9, and in platelets10, and aspirin at high concentrations acetylates eNOS serine residues10. Aspirin also acetylates lysine residues in other target proteins including hemoglobin11, 12, and ubiquitin13. However, unlike serine acetylation, lysine acetylation of biologically relevant cardiovascular targets by low-dose aspirin, and the physiological significance of such acetylation, has not been reported. Therefore, we asked whether cardiovascular doses of aspirin acetylate the epsilon amino groups of lysine residues in eNOS and investigated the physiological relevance of this chemical modification on eNOS-derived NO production. Moreover, reasoning that lysine acetylation in many proteins is reversible by endogenous lysine deacetylases, we explored the role of such deacetylases in antagonizing the effects of low-dose aspirin on lysine acetylation of eNOS, and endothelial NO production.
Methods
An expanded Methods section detailing the materials, methods, and statistics implemented in this study is available in the Online Data Supplement at http://circres.ahajournals.org.
Endothelial NOS −/− mice in a C57BL/6J background were purchased from Jackson Laboratories. The C57BL/6J strain was used as a wild-type control strain. Mice were administered aspirin or salicylic acid (3 mg/kg) intravenously or by gastric gavage and sacrificed after 45–60 minutes. The COX-1 inhibitor sc-560 (Cayman Chemicals) or vehicle control was injected intraperitoneally at 20 mg/kg 30 minutes before administration of aspirin or salicylic acid. All animals were treated in accordance with IACUC approved protocols.
Results and Discussion
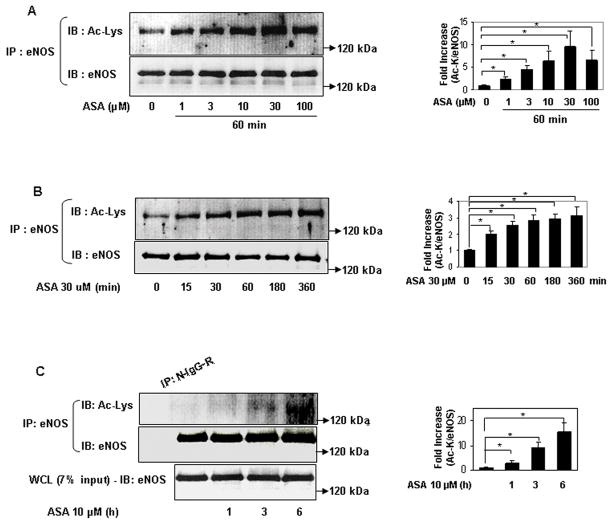
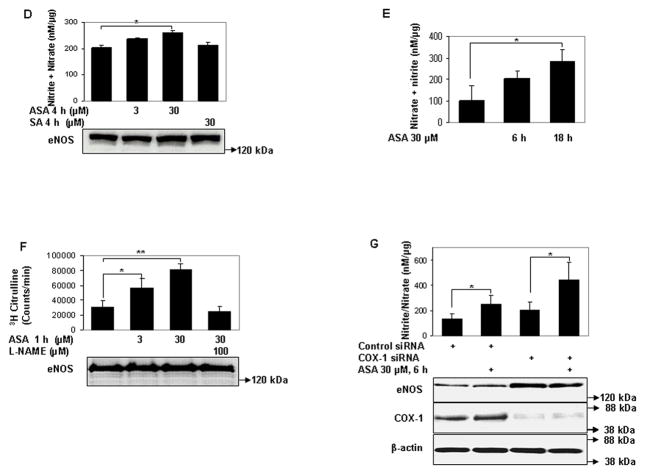
We first determined if eNOS is a target of lysine acetylation by low-dose aspirin. Within a concentration range that is acutely achieved in human plasma with oral administration of a 100 mg cardiovascular dose 14, aspirin rapidly and concentration-dependently acetylated eNOS expressed in HEK 293 cells (Figs 1a, 1b). A similar increase in lysine acetylation of endogenous eNOS in human umbilical vein endothelial cells (HUVEC) was observed with low concentrations of aspirin (Fig 1c). In parallel with the increase in lysine acetylation of eNOS, aspirin at low concentrations stimulated metabolites derived from NO produced by eNOS expressed in HEK 293 cells (Fig 1d), and by HUVEC (Fig 1e). In addition, low concentration of aspirin stimulated enzymatic activity of eNOS expressed in HEK 293 cells (Fig 1f). Because COX-1, a known target of low-dose aspirin is constitutively expressed in many cells including endothelial cells15, we then asked whether aspirin-stimulated NO in endothelial cells is dependent on COX-1. Knockdown of constitutive COX-1 expression with siRNA resulted in an increase in eNOS expression, but did not inhibit low-dose aspirin-stimulated NO production (Fig 1g). Similarly, treatment of HUVEC with sc-560, a COX-1 selective inhibitor16, did not abolish aspirin-stimulated NO (supplemental fig II). This reveals that clinically-relevant concentrations of aspirin acetylate lysine residues of endogenous and ectopically expressed eNOS in cultured cells, and suggests that acetylation mediates increased eNOS catalytic activity and NO production. Further, this implicates a COX-1-independent mechanism for aspirin modulation of endothelial NO production.
Figure 1.
Low concentrations of aspirin rapidly acetylate eNOS on lysine residues and stimulate eNOS enzymatic activity and NO production. A to C, Micromolar concentrations of aspirin rapidly stimulate lysine acetylation of eNOS. Representative immunoblots for acetylated lysine (Ac-Lys, Ac-K) in eNOS immunoprecipitated (from A to B) HEK 293 cells expressing ectopic eNOS and (C) HUVEC showing dose- and time-response to aspirin (ASA). Accompanying graphs show analysis by densitometry of Ac-K/total eNOS relative to no aspirin from independent blots. D to E, Low concentrations of aspirin increase NO metabolites (nitrite + nitrate) in conditioned media of (D) HEK 293 cells expressing ectopic eNOS and (E) HUVEC. F, Low concentrations of aspirin increase enzymatic activity (conversion of arginine to citrulline) of eNOS ectopically expressed in HEK 293 cells. (G) Knockdown of COX-1 does not suppress aspirin-stimulated NO (nitrite + nitrate) in conditioned media of HUVEC. *P<0.05 and **P< 0.01 (n=3 to 4).
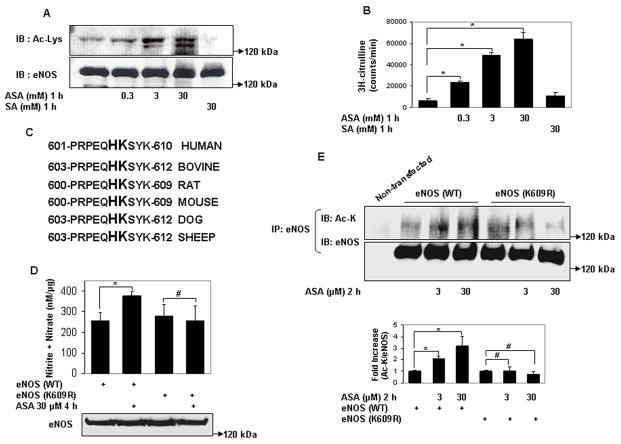
We then asked if eNOS is a direct target of lysine acetylation by aspirin. Aspirin acetylated recombinant eNOS on lysine residues in vitro (Fig 2a). Moreover, aspirin promoted the enzymatic activity of recombinant eNOS (Fig 2b). Increase in lysine acetylation and activation of recombinant eNOS was first evident at high micromolar concentrations, approximating the reported IC50 of aspirin for recombinant COX-117. However, to achieve a maximal effect on eNOS activity and lysine acetylation, millimolar concentrations of aspirin were required. The higher concentrations of aspirin required to achieve maximal lysine acetylation and activation of recombinant eNOS, when compared to eNOS expressed in cells, indicates that aspirin is a better acetylator of epsilon amino groups in vivo than in vitro. Such concentrations of aspirin are also required for in vitro lysine acetylation of other known target proteins, including hemoglobin11 and ubiquitin13. The relative insensitivity of recombinant eNOS to aspirin may also be reflective of the fact that mechanisms that stimulate and are required for optimal eNOS activity, such as phosphorylation by Akt kinase18 and binding to hsp9019, are present in intact cells but not in vitro. These findings show that eNOS is directly acetylated on lysine residues by aspirin, and this acetylation stimulates its enzymatic activity.
Figure 2.
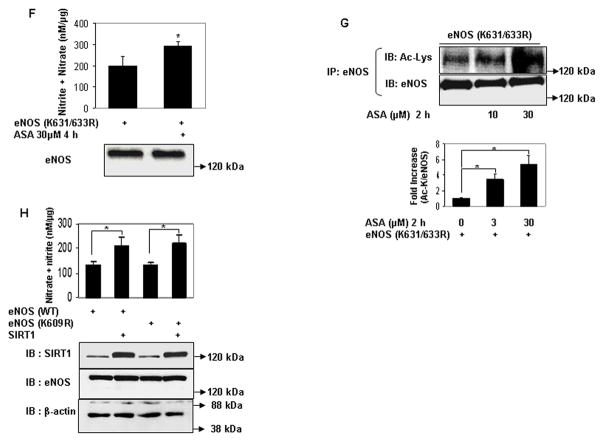
Aspirin directly acetylates eNOS on lysine residues, and acetylation of lysine 609 in eNOS mediates aspirin stimulated eNOS NO production. A and B, Lysine acetylation and activation of eNOS by aspirin in vitro. In vitro concentration dependent (A) lysine acetylation (Ac-Lys, Ac-K), and (B) enzymatic activity of recombinant eNOS by aspirin. C, Conservation of the tandem histidine-lysine pair consisting of lysine 609 (in bovine eNOS) in the calmodulin auto-inhibitory domains of eNOS of different species. D through G, Aspirin promotes eNOS-derived NO by specifically targeting lysine 609 in bovine eNOS. D and F, NO (nitrite + nitrate) measured in conditioned media, and E and G, lysine acetylation (Ac-Lys, Ac-K) of eNOS in lysates of HEK 293 cells expressing ectopic wildtype eNOS (eNOS WT), eNOS with lysine 609 mutated to nonacetylatable arginine (eNOS K609R or eNOS) nonacetylatable on lysines 631 and 633 (eNOS K631/633R) and treated with a low concentration of ASA. H, SIRT1 does not increase NO through deacetylation of lysine 609. NO (nitrite + nitrate) in conditioned media of HEK 293 cells expressing ectopic eNOS (WT) and eNOS (K609R), with and without SIRT1 overexpression. Representative immunoblots are shown with accompanying analysis by densitometry of Ac-K/total eNOS relative to no aspirin from independent blots. *P<0.05 and #P=NS (n=3 to 5).
Next, we examined the role of specific lysine residues in modulating NO production by low-dose aspirin. Lysine acetylation of ubiquitin by aspirin occurs by a transacetylation process in which a histidine residue, in proximity to the target lysine, is first acetylated by aspirin, and this acetylated histidine intermediate then transfers the acetyl group to the lysine residue13. The occurrence of histidine in the catalytic microenvironment has been similarly proposed to play an important role in formation of lysine glycation adducts of alcohol dehydrogenase20. Hypothesizing a similar catalytic role for histidine in facilitating the reactivity of lysine residues in eNOS to aspirin, we targeted lysines in proximity to histidine residues. Within bovine eNOS exists a single tandem histidine-lysine pair at position 608–609 (corresponding to positions 606–607 in human eNOS). This histidine-lysine pair is in the calmodulin autoinhibitory domain of eNOS, and is conserved across species (Fig 2c). We mutated lysine 609 to non-acetylatable arginine (eNOS (K609R)), and compared the stimulatory effect of low concentrations of aspirin on wild-type eNOS and eNOS (K609R). NO produced by eNOS (K609R) under resting conditions was similar to that of wild-type protein (Fig 2d). However, in contrast to its effect on wild-type eNOS aspirin did not stimulate NO produced by eNOS (K609R) (Fig 2d). Moreover, unlike wild-type eNOS, lysine acetylation of eNOS (K609R) was not stimulated by aspirin (Fig 2e). In addition to lysine 609, we also targeted other conserved lysine residues in the calmodulin autoinhibitory domain of eNOS. Because lysines 631 and 633 are in proximity to serine 635, phosphorylation of which stimulates eNOS activity21, we asked whether acetylation of these lysine residues, by mimicking phosphorylation of serine 635, could mediate aspirin-stimulated NO production. However, similar to its effect on NO produced by wild-type eNOS, aspirin stimulated NO production by the acetylation-resistant eNOS (K631/633R) (Fig 2f). In addition, unlike the lack of effect of aspirin on lysine acetylation of eNOS (K609R), low concentrations of aspirin increased lysine acetylation of eNOS (K631/633R) (Fig 2g). Thus, aspirin stimulates eNOS-derived NO production by targeting specific lysine residues in the calmodulin autoinhibitory domain of eNOS for acetylation.
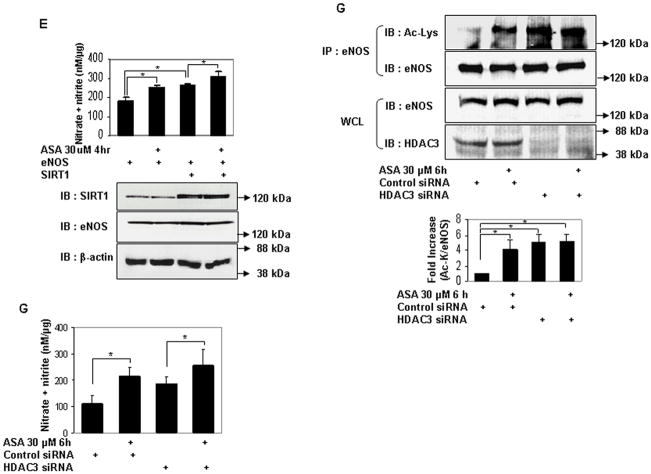
Sirtuin1 (SIRT1) is a class III histone deacetylase. It targets many non-histone proteins including eNOS, stimulating eNOS activity by deacetylating lysines in its calmodulin-binding domain22. Although K609 is not in the calmodulin-binding domain, we nevertheless questioned if SIRT1 plays any part in the regulation of eNOS–derived NO stimulated by low-dose aspirin. SIRT1 overexpression increased NO derived from both WT and K609R eNOS to the same extent (Fig 2h), indicating that lysine 609, which mediates aspirin-stimulated NO, is not a target of SIRT1.
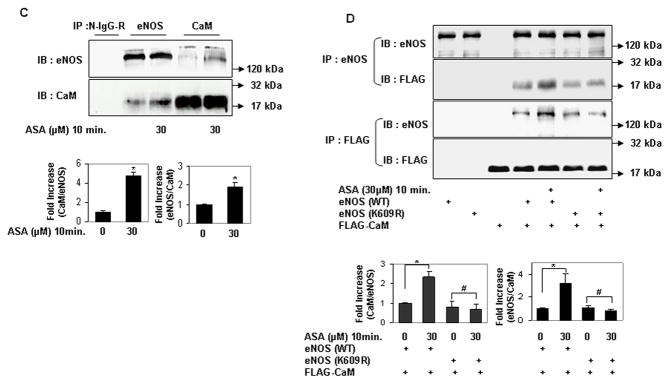
The autoinhibitory domain of eNOS inhibits its activation by interfering with its binding to calmodulin23. Because lysine 609 is in the autoinhibitory domain of eNOS, we hypothesized that its acetylation mediates aspirin-stimulated eNOS activation by promoting binding of eNOS to calmodulin. To investigate this possibility, we first examined the effect of low concentrations of aspirin on binding of calmodulin to eNOS. Aspirin rapidly stimulated the binding of eNOS and calmodulin expressed in HEK 293 cells (Fig 3a and 3b). A similar increase in the binding of endogenous eNOS to endogenous calmodulin was observed in endothelial cells exposed to aspirin (Fig 3c). In contrast, aspirin had no effect on binding of calmodulin to eNOS that is non-acetylatable on lysine 609 (K609R) (Fig 3d). These findings indicate that cardiovascular concentrations of aspirin stimulate eNOS activity by promoting its binding to calmodulin, and demonstrate the importance of lysine 609 in mediating aspirin-stimulated binding of eNOS to calmodulin.
Figure 3.
Lysine 609 mediates aspirin-stimulated binding of eNOS to calmodulin. A through C, Aspirin rapidly stimulates binding of eNOS to calmodulin. Coimmunoprecipitation of (A) calmodulin (CaM) in eNOS immunoprecipitates and (B) eNOS in CaM immunoprecipitates from HEK 293 cells expressing ectopic eNOS and FLAG-CaM treated with a low concentration of ASA for the indicated times. C, Coimmunoprecipitation of endogenous CaM in eNOS immunoprecipitates and endogenous eNOS in CaM immunoprecipitates from HUVEC, with and without treatment with ASA. D, Lysine 609 in bovine eNOS mediates its aspirin-stimulated binding to CaM. Coimmunoprecipitation of FLAG-CaM with eNOS (WT) and eNOS (K609R) ectopically expressed in HEK 293 cells treated with ASA. Representative immunoblots are shown with accompanying analysis by densitometry of immunoprecipitated CaM/eNOS and eNOS/CaM relative to no aspirin from independent blots. *P<0.05, **P<0.01, and #P=NS (n=3 to 4).
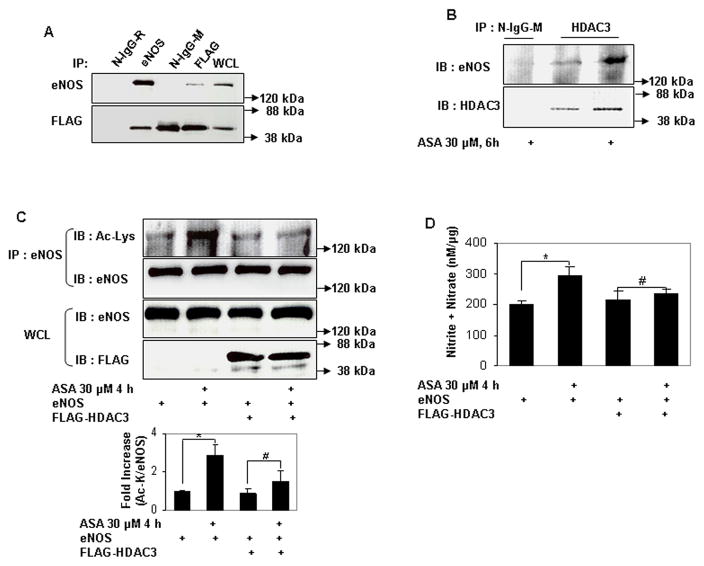
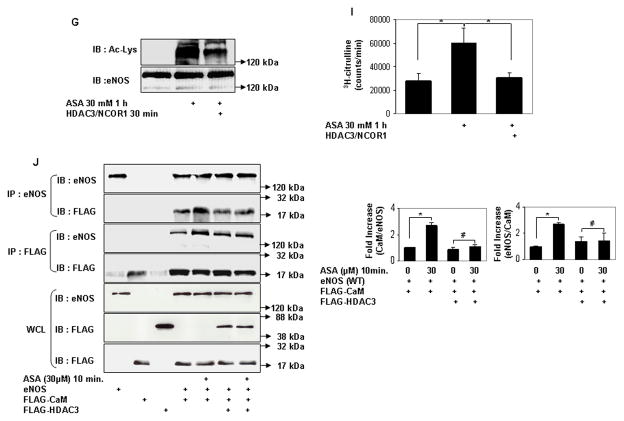
Serine acetylation of platelet COX-1 by cardiovascular doses of aspirin is non-reversible and may reflect the limited complement of cellular proteins found in non-nucleated platelets. In nucleated cells, acetylation of lysines is a reversible post-translational modification. Histone deacetylases (HDAC) remove acetyl residues from epsilon amino-acetylated lysines in histones and non-histone proteins, thereby modifying protein structure and function24. We therefore asked if lysine acetylation of eNOS is reversible by HDAC. Because eNOS is a cytosolic protein, we limited our search to HDAC that are expressed in the cytoplasm. HDAC3 belongs to class I HDAC and is expressed both in the nucleus and cytoplasm25, 26. In keeping with its presence in the cytoplasm, several nonhistone substrates of HDAC3 have been identified27–29. In endothelial cells, HDAC3 mediates a pro-thrombotic and pro-inflammatory phenotype30, 31. We therefore focused our attention on HDAC3 as a potential endogenous antagonist to aspirin-stimulated lysine acetylation of eNOS. We first asked if HDAC3 and eNOS associate with each other. HDAC3 and eNOS expressed in HEK 293 cells co-immunoprecipitated with each other (Fig 4a). Endogenous HDAC3 and eNOS also co-immunoprecipitated in endothelial cells, and this association was stimulated by aspirin (Fig 4b). We next determined if HDAC3 antagonizes aspirin-induced lysine acetylation of eNOS. Overexpression of HDAC3 in HEK293 cells abrogated the increase in lysine acetylation of eNOS (Fig 4c), and eNOS-derived NO (Fig 4d) stimulated by low concentrations of aspirin. Unlike HDAC3, the SIRT1 lysine deacetylase, which also binds to and deacetylates eNOS22, did not inhibit aspirin-stimulated NO (Fig 4e). Conversely, knockdown of endogenous HDAC3 in endothelial cells increased lysine acetylation of endogenous eNOS, without affecting eNOS expression (Fig 4f). The increase in lysine acetylation of eNOS corresponded with an increase in NO produced by endothelial cells in which HDAC3 was knocked down (Fig 4g). Importantly, recombinant active HDAC3 suppressed lysine acetylation of recombinant eNOS by aspirin in vitro (Fig 4g), and decreased aspirin-stimulated eNOS enzymatic activity (Fig 4i). Because aspirin led to an increase in binding of eNOS to calmodulin (Figs 3a–c), we then asked if HDAC3 modulates this binding. Aspirin-stimulated binding of eNOS to calmodulin was negated by overexpression of HDAC3 (Fig 3j). These findings show that HDAC3 targets lysine-acetylated eNOS for de-acetylation, thereby antagonizing aspirin-stimulated binding of eNOS to calmodulin, and inhibiting aspirin-triggered increase in eNOS activity and NO production. The increase in resting lysine acetylation of eNOS and NO in endothelial cells with knockdown of HDAC3, independent of aspirin, also hints at the presence of an endogenous lysine acetyltransferase that targets eNOS for acetylation.
Figure 4.
HDAC3 binds to eNOS and antagonizes aspirin-stimulated lysine acetylation of eNOS, eNOS activity, and endothelial NO generation. A and B, HDAC3 binds to eNOS, and aspirin stimulates this binding. Coimmunoprecipitation of (A) FLAG-HDAC3 and eNOS expressed in HEK 293 cells and (B) endogenous HDAC3 and eNOS in HUVEC. The molecular weight of FLAG-HDAC3 overlaps with that of mouse immunoglobulin heavy chain. N-IgG-M, mouse nonimmune immunoglobulin; N-IgG-R, rabbit nonimmune immunoglobulin; WCL, whole cell lysate. C through J, HDAC3, but not SIRT1, antagonizes the effects of ASA on eNOS. C and D, Overexpression of HDAC3 inhibits aspirin-stimulated (C) lysine acetylation (Ac-Lys, Ac-K) in lysates and (D) NO (nitrite + nitrate) in conditioned media of HEK 293 cells expressing ectopic eNOS. E, Overexpression of SIRT1 promotes basal and does not inhibit aspirin-stimulated NO (nitrite + nitrate) in conditioned media of HEK 293 cells expressing ectopic eNOS. F and G, Knockdown of HDAC3 promotes (F) lysine acetylation of endogenous eNOS in lysates and (G) NO (nitrite + nitrate) in conditioned media of HUVEC. H and I, HDAC3 directly targets lysine acetylated eNOS for deacetylation. HDAC3 reverses aspirin-stimulated (H) lysine acetylation (Ac-Lys) and (I) enzymatic activity of recombinant eNOS in vitro. J, HDAC3 inhibits aspirin-stimulated binding of eNOS to calmodulin. Coimmunoprecipitation of calmodulin (CaM) in eNOS immunoprecipitates and eNOS in CaM immunoprecipitates from HEK 293 cells expressing ectopic eNOS or nonacetylatable eNOS (K609R) and FLAG-CaM treated with ASA. Representative immunoblots are shown with accompanying analysis by densitometry of Ac-K/total eNOS, or immunoprecipitated CaM/eNOS and eNOS/CaM, relative to no aspirin, from independent blots. *P<0.05, **P<0.01, and #P=NS (n=3 to 4).
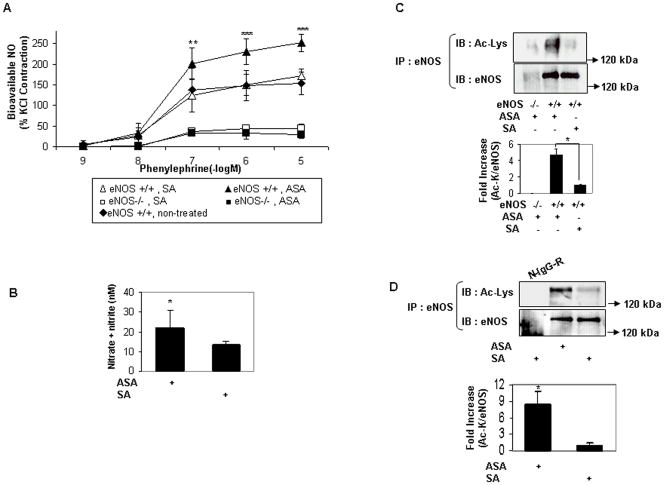
We next ascertained the role of eNOS in mediating the physiologic effects of low-dose aspirin on the vasculature. Mice were administered a cardiovascular dose of aspirin intravenously or by gastric gavage, followed by measurements of eNOS acetylation, serum NO, and vascular bioavailable NO. A 3mg/kg dose (corresponding to 300 mg given to an adult human) of aspirin given IV or by gastric gavage increased aortic bioavailable NO (Fig 4a), and serum NO (Fig 4b), respectively. The increase in serum and aortic bioavailable NO was accompanied by an increase in lysine acetylation of eNOS in hepatic tissue (Fig 4c, 4d). Importantly, aspirin did not increase aortic bioavailable NO in mice genetically deficient for eNOS (eNOS −/−) (Fig 4a). Because low-dose aspirin inhibits platelet COX-1 in vivo, we also determined the role of aspirin-induced COX-1 inhibition in mediating the increase in bioavailable vascular NO. Sc-560, the selective pharmacologic inhibitor of COX-1, was delivered in vivo at a dose that inhibits COX-1-derived prostanoids32. When compared to salicylic acid, low-dose aspirin led to an increase in bioavailable NO in aortas of mice pretreated with sc-560 just as it did in mice not treated with sc-560 (Fig 4e). The lack of effect of COX-1 inhibition on aspirin-stimulated increase in vascular NO bioavailability was evident at all concentrations of phenylephrine (Fig 4f). These findings indicate that a cardiovascular dose of aspirin administered in vivo, via oral or parenteral route, promotes lysine acetylation of eNOS, and acutely increases vascular NO levels in a COX-1-indpendent, eNOS-dependent fashion.
Anti-inflammatory concentrations of aspirin acetylate eNOS on serines 765 and 771 (767 and 773 in bovine eNOS)10. The functional significance of serine acetylation in regulating eNOS has not been reported. Therefore, we examined the importance of these serine residues by measuring aspirin-stimulated NO produced by eNOS that is non-acetylatable on these residues (eNOS S767/773R). Low-dose aspirin increased NO derived from eNOS (S767/773R), although the magnitude of increase was smaller when compared to WT eNOS (Supplemental Fig VI). Thus, acetylation of these serine residues by low-dose aspirin may play an important part in mediating the effects of aspirin on the endothelium. However, acetylation of these serine residues cannot fully account for aspirin’s stimulatory effect on eNOS.
Our work did not explore the effect of aspirin on endothelial NO in the context of vascular inflammation. It is known, however, that inducible NOS (iNOS) and eNOS mediate the anti-inflammatory effects of high-dose aspirin, and this has been attributed to aspirin-triggered epi-lipoxin generation in inflamed tissues33. In the inflammatory milieu, aspirin acetylates the active site of cycloxygenase-2 (COX-2) in endothelial or epithelial cells resulting in conversion of arachidonic acid to the intermediary (15R) hydroxyeicosotetraenoic acid which is rapidly metabolized in a transcellular fashion by lipoxygenases in leukocytes to epi-lipoxins34. Moreover, aspirin modulates COX-2 activity to generate omega-3 fatty acid derivatives such as docosohexanoic acid35, which increases eNOS activity36, 37. COX-2 can be induced in endothelial cells15, and is upregulated in blood vessels of individuals with cardiovascular risk factors38. Thus, the effect of aspirin on endothelial NO production during vascular inflammation may also be dependent on COX-2 and be mediated by aspirin-triggered epi-lipoxins and keto-fatty acid derivatives.
Aspirin is not a potent vasodilator but does reduce systemic blood pressure in mild hypertension and pre-hypertension6, 8. This effect is consistent with observations that low-dose aspirin stimulates production of endothelial NO to a much lesser degree than vasodilators such as acetylcholine and bradykinin9, 39, 40. In line with these observations, low-dose aspirin, when used alone, does not lead to vasodilation of pre-constricted vascular rings9. One limitation of our work is that we did not examine the effect of aspirin on vascular tone in vivo in a model of hypertension. However, we expect low-dose aspirin to have a small impact, at best, on blood pressure in vivo. Despite this, low-dose aspirin is efficacious in improving vascular relaxation and endothelial function in the setting of endothelial dysfunction, such as seen with aging and hypercholesterolemia4, 5. In such settings where endothelial NO production is decreased, the increase in vascular NO stimulated by aspirin, albeit modest, may be sufficient to elicit an effect on vascular tone.
Lysine acetylation-deacetylation has recently been recognized as a novel post-translational mechanism for the regulation of eNOS activity. The class III HDAC SIRT1 deacetylates and activates eNOS, and this activation is mediated by lysine residues in the calmodulin-binding domain of eNOS22. The role of class I HDAC (to which HDAC3 belongs) and class II HDAC in targeting eNOS for deacetylation has hitherto not been reported. The stimulatory effect of SIRT1-mediated deacetylation of lysine residues in the calmodulin-binding domain, compared to the inhibitory role of HDAC3-mediated deacetylation of a lysine residue in the calmodulin autoinhibitory domain, speaks to a finely tuned acetylation-deacetylation mechanism for the regulation of eNOS enzymatic activity.
We did not observe a significant change in eNOS expression with knockdown of HDAC3 in endothelial cells. However, non-specific inhibitors of class I and II HDAC lead to a change in eNOS expression. In non-endothelial cells in which eNOS is normally not expressed, inhibition of class I and II HDAC with Trichostatin A (TSA), combined with inhibition of DNA methyltransferase activity with 5-aza-2′-deoxycitidine resulted in induction of eNOS transcript41. In contrast, TSA and butyric acid, another non-specific HDAC inhibitor, suppressed eNOS protein levels in endothelial cells, and prevented NO-dependent relaxation of coronary arteries42. These somewhat contradictory findings hint at a complex role of class I and II HDAC in the regulation of eNOS expression, and may reflect the use of non-specific HDAC inhibitors. The added role of HDAC3 in post-translational modification of eNOS injects another layer of regulation of eNOS by HDAC, and indicates that specific HDAC may have distinct and perhaps opposing effects on endothelium-dependent vascular functions.
Endothelium-derived nitric oxide is not only important in the regulation of vascular tone, but also functions as an inhibitor of platelet adhesion to the endothelium43, suppression of platelet aggregation43, and smooth muscle cell migration into the intima44, all of which contribute to the pathological processes of atherosclerosis and thrombosis. Thus the stimulatory effect of low-dose aspirin on endothelial NO production through lysine acetylation of eNOS, and its antagonism by HDAC3, is likely to have relevance not only to endothelium-dependent regulation of vascular tone, but also to atherothrombosis which results in tissue ischemia and infarction. Therefore, selective inhibition of HDACs may form the basis of adjunctive therapies aimed to improve the efficacy of aspirin in the prevention and treatment of cardiovascular disease and stroke. In addition, epidemiologic data indicate that the use of low-dose aspirin also reduces the risk of proliferative disorders, including colorectal and prostate neoplasia45, 46. Because HDAC3 plays a part in the molecular pathogenesis of these disorders47, 48, aspirin and HDAC3 may also act in an antagonistic fashion to regulate the lysine acetylation status of targets that play a role in neoplastic development.
Although low-dose aspirin is widely used in the prevention and treatment of cardiovascular disease, a significant proportion of patients on aspirin experience atherothrombotic events. The pathophysiological and molecular mechanisms underlying this phenomenon, termed “aspirin resistance”, have not been clearly identified. There are likely multiple reasons for this phenomenon. Some that have been proposed include aspirin-insensitive thromboxane A2(TXA2) synthesis in platelets, increased collagen sensitivity of platelets, and mutations/polymorphisms in genes involved in the mechanisms of action of aspirin49. Suppression of aspirin-induced lysine acetylation and activation of eNOS, and consequent decreased endothelial NO production, could also be an endogenous mechanism responsible for the phenomenon of aspirin resistance. Importantly, HDAC inhibitors are presently under clinical trials for the treatment of solid and hematologic malignancies50, 51, raising the possibility that, if HDAC3 is an endogenous antagonist to the salutary effects of aspirin on the endothelium, specific HDAC3 inhibitors might offer therapeutic benefit when used in combination with aspirin for the treatment and prevention of cardiovascular diseases.
Novelty and Significance.
What is known?
Low-dose acetylsalicylic acid (aspirin) is widely used to treat and prevent acute coronary syndromes (ACS) and strokes.
Aspirin modifies cycloxygenase-1 in platelets, via acetylation of a serine residue, thereby inhibiting platelet aggregation.
Aspirin is also known to have beneficial effects outside of platelets, including the vasculature, where it stimulates nitric oxide (NO) production, thereby improving endothelium-dependent vascular function.
What New Information Does This Article Contribute?
Low-dose aspirin stimulates endothelial nitric oxide synthase (eNOS) by inducing lysine acetylation of eNOS, thus providing a new mechanism for the salutary effect of aspirin on the endothelium.
Lysine acetylation of eNOS by aspirin promotes binding of eNOS to its co-factor calmodulin.
The lysine deacetylase histone deacetylase-3 (HDAC3), targets aspirin-acetylated eNOS for deacetylation, thereby antagonizing the effect of aspirin on endothelial NO production.
Summary.
Aspirin improves endothelial function in individuals with atherosclerotic risk factors. The mechanisms underlying the beneficial effects of aspirin on the endothelium are not fully understood. We demonstrate that low-dose aspirin stimulates endothelial NO by direct lysine acetylation of eNOS. In addition, HDAC3 inhibits aspirin-induced endothelial NO by deacetylating aspirin-acetylated eNOS. These findings identify eNOS as a biologically relevant target of lysine acetylation by low-dose aspirin, and deacetylation by HDAC3. Endothelial NOS-derived NO also inhibits platelet activation and aggregation. Because many patients on low-dose aspirin therapy continue to have thrombotic events, inhibiting HDAC3 may augment the therapeutic benefit of aspirin in such “aspirin resistant” patients.
Supplementary Material
Figure 5.
Cardiovascular dose of aspirin in vivo increases vascular bioavailable NO in an eNOS-dependent and COX-1-independent fashion. A and B, Parenteral or oral low-dose aspirin increases aortic bioavailable and serum NO in an eNOS-dependent manner. A, Bioavailable NO in aortas of wildtype (eNOS +/+) and eNOS-deficient (eNOS −/−) mice 45 to 60 minutes after 3 mg/kg IV aspirin (ASA) or salicylic acid (SA) and nontreated animals. *P<0.05, **P<0.01, ***P<0.001 (n=3 to 4) compared with nontreated animals and animals treated with SA. B, Serum NO (nitrite + nitrate) 45 to 60 minutes after gastric gavage of wild-type (eNOS +/+) mice with ASA or SA (3 mg/kg). *P<0.05 (n=3). C and D, Parenteral or oral low-dose aspirin increases lysine acetylation of eNOS in tissue. Lysine acetylation of eNOS immunoprecipitated from liver of wild-type (eNOS +/+) and eNOS−/− mice 45 to 60 minutes after administration of 3 mg/kg ASA or SA (C) IV and (D) by gastric gavage. Wildtype mice were used in D. N-IgG-R, nonimmune rabbit immunoglobulin. Representative immunoblots are shown with accompanying analysis by densitometry of Ac-K/total eNOS relative to no aspirin from independent blots.
*P<0.05 (n=3 to 4). E and F, Aspirin-induced vascular bioavailable NO is independent of COX-1. E, Aortic bioavailable NO in wild-type mice 45–60 minutes after 3 mg/kg IV ASA or SA, pretreated with the COX-1 inhibitor sc-560. F, Increase in aortic bioavailable NO (difference between ASA and SA) in wild-type mice with and without pretreatment with sc-560. *P<0.05, **P<0.01, ***P<0.001, and #P=NS (n=3 to 4).
Acknowledgments
We thank M.K. Jain, T. Michel, S. Schreiber, E. Seto, and Y. Xia for providing the eNOS, HDAC3, and calmodulin cDNAs. We appreciate the advice and critique of Bruce A Freeman. This work is posthumously dedicated to Kenneth L Baughman, MD-a selfless leader who was instrumental in the career development of this author (KI), and many other investigators like him.
Sources of funding
This work was supported by Korea Research Foundation grant KRF-2006-214-E00003to S.-B. J., and NIH grants R01 HL070929, P01 HL065608, R01 HL094959, and R21 HL098892 to K.I.
Non-standard Abbreviations and Acronyms
- Aspirin
acetylsalicylic acid
- ASA
acetylsalicylic acid
- SA
salicylic acid
- COX
cyclooxygenase
- HDAC
Histone Deacetylase
- eNOS
endothelial nitric oxide synthase
- NO
nitric oxide
- HUVEC
human umbilical vein endothelial cells
- TSA
Trichostatin A
- SIRT1
sirtuin1
- Akt kinase
serine/threonine protein kinase
- hsp90
heat shock protein 90
- TXA2
thromboxane A2
Footnotes
Disclosures
None
References
- 1.Awtry EH, Loscalzo J. Aspirin. Circulation. 2000 Mar 14;101:1206–1218. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 2.Shimokawa T, Smith WL. Prostaglandin endoperoxide synthase. The aspirin acetylation region. J Biol Chem. 1992;267:12387–12392. [PubMed] [Google Scholar]
- 3.Husain S, Andrews NP, Mulcahy D, Panza JA, Quyyumi AA. Aspirin improves endothelial dysfunction in atherosclerosis. Circulation. 1998;97:716–720. doi: 10.1161/01.cir.97.8.716. [DOI] [PubMed] [Google Scholar]
- 4.Magen E, Viskoper JR, Mishal J, Priluk R, London D, Yosefy C. Effects of low-dose aspirin on blood pressure and endothelial function of treated hypertensive hypercholesterolaemic subjects. J Hum Hypertens. 2005;19:667–673. doi: 10.1038/sj.jhh.1001910. [DOI] [PubMed] [Google Scholar]
- 5.Bulckaen H, Prevost G, Boulanger E, Robitaille G, Roquet V, Gaxatte C, Garcon G, Corman B, Gosset P, Shirali P, Creusy C, Puisieux F. Low-dose aspirin prevents age-related endothelial dysfunction in a mouse model of physiological aging. Am J Physiol Heart Circ Physiol. 2008;294:H1562–1570. doi: 10.1152/ajpheart.00241.2007. [DOI] [PubMed] [Google Scholar]
- 6.Hermida RC, Ayala DE, Calvo C, Lopez JE. Aspirin administered at bedtime, but not on awakening, has an effect on ambulatory blood pressure in hypertensive patients. J Am Coll Cardiol. 2005;46:975–983. doi: 10.1016/j.jacc.2004.08.071. [DOI] [PubMed] [Google Scholar]
- 7.Hermida RC, Ayala DE, Calvo C, Lopez JE, Fernandez JR, Mojon A, Dominguez MJ, Covelo M. Administration time-dependent effects of aspirin on blood pressure in untreated hypertensive patients. Hypertension. 2003;41:1259–1267. doi: 10.1161/01.HYP.0000072335.73748.0D. [DOI] [PubMed] [Google Scholar]
- 8.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Ambulatory Blood Pressure Control With Bedtime Aspirin Administration in Subjects With Prehypertension. Am J Hypertens. 2009;22:896–903. doi: 10.1038/ajh.2009.83. [DOI] [PubMed] [Google Scholar]
- 9.Taubert D, Berkels R, Grosser N, Schroder H, Grundemann D, Schomig E. Aspirin induces nitric oxide release from vascular endothelium: a novel mechanism of action. Br J Pharmacol. 2004;143:159–165. doi: 10.1038/sj.bjp.0705907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Kane P, Xie L, Liu Z, Queen L, Jackson G, Ji Y, Ferro A. Aspirin acetylates nitric oxide synthase type 3 in platelets thereby increasing its activity. Cardiovasc Res. 2009;83:123–30. doi: 10.1093/cvr/cvp120. [DOI] [PubMed] [Google Scholar]
- 11.Bridges KR, Schmidt GJ, Jensen M, Cerami A, Bunn HF. The acetylation of hemoglobin by aspirin. In vitro and in vivo. J Clin Invest. 1975 Jul;56:201–207. doi: 10.1172/JCI108068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu AS, Macdonald JM, Labotka RJ, London RE. NMR study of the sites of human hemoglobin acetylated by aspirin. Biochim Biophys Acta. 1999;1432:333–349. doi: 10.1016/s0167-4838(99)00094-1. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald JM, Haas AL, London RE. Novel mechanism of surface catalysis of protein adduct formation. NMR studies of the acetylation of ubiquitin. J Biol Chem. 2000;275:31908–31913. doi: 10.1074/jbc.M000684200. [DOI] [PubMed] [Google Scholar]
- 14.Rosenkranz B, Frolich JC. Plasma concentrations and anti-platelet effects after low dose acetylsalicylic acid. Prostaglandins Leukot Med. 1985;19:289–300. doi: 10.1016/0262-1746(85)90142-8. [DOI] [PubMed] [Google Scholar]
- 15.Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, Talley JJ, Masferrer JL, Seibert K, Isakson PC. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci U S A. 1998;95:13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loll PJ, Picot D, Garavito RM. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat Struct Biol. 1995;2:637–643. doi: 10.1038/nsb0895-637. [DOI] [PubMed] [Google Scholar]
- 18.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 20.Shilton BH, Walton DJ. Sites of glycation of human and horse liver alcohol dehydrogenase in vivo. J Biol Chem. 1991;266:5587–5592. [PubMed] [Google Scholar]
- 21.Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, Huang W, Venema RC, Kemp BE. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem. 2002;277:42344–42351. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- 22.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salerno JC, Harris DE, Irizarry K, Patel B, Morales AJ, Smith SM, Martasek P, Roman LJ, Masters BS, Jones CL, Weissman BA, Lane P, Liu Q, Gross SS. An autoinhibitory control element defines calcium-regulated isoforms of nitric oxide synthase. J Biol Chem. 1997;272:29769–29777. doi: 10.1074/jbc.272.47.29769. [DOI] [PubMed] [Google Scholar]
- 24.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longworth MS, Laimins LA. Histone deacetylase 3 localizes to the plasma membrane and is a substrate of Src. Oncogene. 2006;25:4495–4500. doi: 10.1038/sj.onc.1209473. [DOI] [PubMed] [Google Scholar]
- 26.Takami Y, Nakayama T. N-terminal region, C-terminal region, nuclear export signal, and deacetylation activity of histone deacetylase-3 are essential for the viability of the DT40 chicken B cell line. J Biol Chem. 2000;275:16191–16201. doi: 10.1074/jbc.M908066199. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 28.Gregoire S, Xiao L, Nie J, Zhang X, Xu M, Li J, Wong J, Seto E, Yang XJ. Histone deacetylase 3 interacts with and deacetylates myocyte enhancer factor 2. Mol Cell Biol. 2007;27:1280–1295. doi: 10.1128/MCB.00882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thevenet L, Mejean C, Moniot B, Bonneaud N, Galeotti N, Aldrian-Herrada G, Poulat F, Berta P, Benkirane M, Boizet-Bonhoure B. Regulation of human SRY subcellular distribution by its acetylation/deacetylation. Embo J. 2004;23:3336–3345. doi: 10.1038/sj.emboj.7600352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue K, Kobayashi M, Yano K, Miura M, Izumi A, Mataki C, Doi T, Hamakubo T, Reid PC, Hume DA, Yoshida M, Aird WC, Kodama T, Minami T. Histone deacetylase inhibitor reduces monocyte adhesion to endothelium through the suppression of vascular cell adhesion molecule-1 expression. Arterioscler Thromb Vasc Biol. 2006;26:2652–2659. doi: 10.1161/01.ATV.0000247247.89787.e7. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Mahmud SA, Bitterman PB, Huo Y, Slungaard A. Histone deacetylase inhibitors suppress TF-kappaB-dependent agonist-driven tissue factor expression in endothelial cells and monocytes. J Biol Chem. 2007;282:28408–28418. doi: 10.1074/jbc.M703586200. [DOI] [PubMed] [Google Scholar]
- 32.Skill NJ, Theodorakis NG, Wang YN, Wu JM, Redmond EM, Sitzmann JV. Role of cyclooxygenase isoforms in prostacyclin biosynthesis and murine prehepatic portal hypertension. Am J Physiol Gastrointest Liver Physiol. 2008;295:G953–964. doi: 10.1152/ajpgi.00013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li P, Kim SW, Li X, Datta S, Pond WG, Wu G. Dietary supplementation with cholesterol and docosahexaenoic acid increases the activity of the arginine-nitric oxide pathway in tissues of young pigs. Nitric Oxide. 2008;19:259–265. doi: 10.1016/j.niox.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stebbins CL, Stice JP, Hart CM, Mbai FN, Knowlton AA. Effects of dietary decosahexaenoic acid (DHA) on eNOS in human coronary artery endothelial cells. J Cardiovasc Pharmacol Ther. 2008;13:261–268. doi: 10.1177/1074248408322470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szerafin T, Erdei N, Fulop T, Pasztor ET, Edes I, Koller A, Bagi Z. Increased cyclooxygenase-2 expression and prostaglandin-mediated dilation in coronary arterioles of patients with diabetes mellitus. Circ Res. 2006;99:e12–17. doi: 10.1161/01.RES.0000241051.83067.62. [DOI] [PubMed] [Google Scholar]
- 39.Kalinowski L, Dobrucki IT, Malinski T. Cicletanine stimulates nitric oxide release and scavenges superoxide in endothelial cells. J Cardiovasc Pharmacol. 2001;37:713–724. doi: 10.1097/00005344-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Malinski T, Taha Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature. 1992;358:676–678. doi: 10.1038/358676a0. [DOI] [PubMed] [Google Scholar]
- 41.Gan Y, Shen YH, Wang J, Wang X, Utama B, Wang J, Wang XL. Role of histone deacetylation in cell-specific expression of endothelial nitric-oxide synthase. J Biol Chem. 2005;280:16467–16475. doi: 10.1074/jbc.M412960200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossig L, Li H, Fisslthaler B, Urbich C, Fleming I, Forstermann U, Zeiher AM, Dimmeler S. Inhibitors of histone deacetylation downregulate the expression of endothelial nitric oxide synthase and compromise endothelial cell function in vasorelaxation and angiogenesis. Circ Res. 2002;91:837–844. doi: 10.1161/01.res.0000037983.07158.b1. [DOI] [PubMed] [Google Scholar]
- 43.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 44.Luscher TF. Endothelium in the control of vascular tone and growth: role of local mediators and mechanical forces. Blood Press Suppl. 1994;1:18–22. [PubMed] [Google Scholar]
- 45.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. 2007;99:608–615. doi: 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]
- 46.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, Steinbach G, Schilsky R. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 47.Liu P, Li S, Gan L, Kao TP, Huang H. A transcription-independent function of FOXO1 in inhibition of androgen-independent activation of the androgen receptor in prostate cancer cells. Cancer Res. 2008;68:10290–10299. doi: 10.1158/0008-5472.CAN-08-2038. [DOI] [PubMed] [Google Scholar]
- 48.Wilson AJ, Byun DS, Popova N, Murray LB, L’Italien K, Sowa Y, Arango D, Velcich A, Augenlicht LH, Mariadason JM. Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J Biol Chem. 2006;281:13548–13558. doi: 10.1074/jbc.M510023200. [DOI] [PubMed] [Google Scholar]
- 49.Mason PJ, Jacobs AK, Freedman JE. Aspirin resistance and atherothrombotic disease. J Am Coll Cardiol. 2005;46:986–993. doi: 10.1016/j.jacc.2004.08.070. [DOI] [PubMed] [Google Scholar]
- 50.Blanchard F, Chipoy C. Histone deacetylase inhibitors: new drugs for the treatment of inflammatory diseases? Drug Discov Today. 2005;10:197–204. doi: 10.1016/S1359-6446(04)03309-4. [DOI] [PubMed] [Google Scholar]
- 51.Kelly WK, Richon VM, O’Connor O, Curley T, MacGregor-Curtelli B, Tong W, Klang M, Schwartz L, Richardson S, Rosa E, Drobnjak M, Cordon-Cordo C, Chiao JH, Rifkind R, Marks PA, Scher H. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–3588. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.