Abstract
Psychiatrists are increasingly called upon to care for individuals with cognitive, emotional, and behavioral disturbances after TBI, especially in settings serving military service personnel and Veterans. In both the early and late post-injury periods, cognitive impairments contribute to disability among persons with TBI and are potentially substantial sources of suffering for persons with TBI and their families. In this article, the differential diagnosis, evaluation, and management of posttraumatic cognitive complaints is reviewed. The importance of pre-treatment evaluation as well as consideration of non-cognitive contributors to cognitive problems and functional limitations is emphasized first. The course of recovery after TBI, framed as a progression through posttraumatic encephalopathy, is reviewed next and used to anchor the evaluation and treatment of posttraumatic cognitive impairments in relation to injury severity as well as time post-injury. Finally, pharmacologic and rehabilitative interventions that may facilitate cognitive and functional recovery at each stage of posttraumatic encephalopathy are presented.
Cognitive impairments are common consequences of traumatic brain injury (TBI). In both the early and late post-injury periods, impairments of arousal, speed of information processing, attention, working memory, episodic memory, language and social communication, and executive functioning are common (1, 2). Cognitive impairments contribute to disability among persons with TBI (3-5) and are potentially substantial sources of suffering for persons with TBI and their families (6).
Psychiatrists are increasingly called upon to care for individuals with cognitive, emotional, and behavioral disturbances after TBI, especially in settings serving military service personnel and Veterans. Cognitive complaints are often prominent features of the clinical presentation of patients referred for psychiatric care after TBI (7). As such, familiarity with the evaluation, differential diagnosis, and management of posttraumatic cognitive complaints is needed to serve well these patients.
Toward that end, this article reviews the evaluation and treatment of posttraumatic cognitive impairments. We discuss first, in brief and in general terms, the importance of pre-treatment evaluation as well as consideration of non-cognitive contributors to cognitive problems and functional limitations. An approach to understanding posttraumatic cognitive impairments in relation to injury severity as well as time post-injury is presented. The overarching course of recovery after TBI, framed as a progression through the stages of posttraumatic encephalopathy, is reviewed next. Thereafter, pharmacologic and rehabilitative interventions that may facilitate cognitive and functional recovery at each stage of posttraumatic encephalopathy are discussed.
Pre-Treatment Evaluation
Comprehensive evaluation is a prerequisite to the treatment of any posttraumatic neuropsychiatric problem, including cognitive impairment. The content and organization of this type of clinical evaluation are described in detail elsewhere (7-9); readers may find these references useful guides to the development of a comprehensive assessment of a patient with neuropsychiatric problems after TBI.
Of utmost importance is determining whether one’s patient experienced a TBI and whether that patient’s cognitive difficulties are attributable to TBI. The American Congress of Rehabilitation Medicine (ARCM) definition of mild (or greater) TBI (10) (Table 1) and the clinical case definition used by the Centers for Disease Control and Prevention (11) (Table 2) are useful guides to the evaluation for TBI. In the inpatient setting, serial (daily) administration of either the Orientation Log (12) or the Galveston Orientation and Amnesia Test (GOAT) (13) is recommended to estimate duration of posttraumatic amnesia (PTA); this information is used to define injury severity, predict short- and long-term outcomes, and guide cognitive rehabilitation planning (3, 14-16). When prospective PTA data are not collected (as is often the case with mild TBI), retrospective estimation of PTA duration using an interview anchored to the GOAT is useful (17).
Table 1.
Definition of mild TBI, based on the definition developed by the Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine (1993)
| Application of a physical force to the brain, including: |
|
| The physical force induces a physiological disruption of brain function, as evidenced by one or more of the following: |
|
| To remain in the mild category of injury: |
|
| Injuries producing more than 30 minutes of lost consciousness, initial (e.g., 30-minute post-injury) Glasgow Coma Scale scores ≤ 12, or posttraumatic amnesia > 24 hours are considered more than mild (i.e., moderate or severe) |
|
|
Table 2.
Clinical case definition of TBI adapted from the Center for Disease Control (CDC) Guidelines for the Surveillance of Central Nervous System Injury (2002). This case definition requires occurrence of a injury to the head (brain) due to blunt or penetrating trauma or from acceleration-deceleration forces that results in one or more of the following: observed or self-reported alteration of consciousness; amnesia; objective neurological or neuropsychological abnormality; or diagnosed intracranial lesion. The CDC guidelines also permit skull fracture alone to constitute evidence of TBI. However, skull fracture alone is not a sufficiently reliable predictor of TBI to use as the sole clinical indicator of this condition and therefore is not included in the clinical case definition presented here
| Injury-Induced Clinical Phenomena | Description |
|---|---|
| Alteration of consciousness | There is observed or self-reported: |
| |
| Amnesia | Loss or impairment of episodic memory (posttraumatic amnesia): |
| |
| Objective neuropsychological abnormality |
Neuropsychological examination demonstrates: |
| |
| Objective neurological abnormality | On elementary neurological examination, there are: |
| |
| Diagnosed intracranial lesion | On computed tomography (CT) or magnetic resonance imaging (MRI), there is evidence of one or more of the following: |
|
Importantly, Glasgow Coma Scale (GCS) (18) scores are useful for gauging TBI severity but neither confirm nor refute a TBI diagnosis. Additionally, integrating neuroimaging findings in the assessment of TBI severity is encouraged; mild TBI with abnormal neuroimaging (of the types listed in Table 2) is referred to as “complicated mild TBI,” and outcomes from this injury type are more like those of persons with GCS-defined moderate TBI than otherwise “uncomplicated” mild TBI (19-21).
The Differential Diagnosis of Posttraumatic Cognitive Impairments
After determining that a patient experienced a TBI, the next step in the evaluation is to determine whether the patient’s cognitive problems (i.e., complaints, impairments, or both) are reasonably attributable to that injury. Although the development of cognitive impairments in the immediate post-injury period may appear, at first glance, to be easily attributable to TBI, the differential diagnosis for such problems in both the early and late post-injury periods is quite broad. Pre-morbid intellectual functioning; co-occurring neurological, medical, or psychiatric problems; other injury-related physical problems (e.g., headache, other pain, seizure, etc.); use of cognitively impairing substances or medications; substance or medication withdrawal states; trauma-induced dissociative states; and symptom elaboration (or malingering), among other problems, require consideration as contributors to or explanations for posttraumatic cognitive impairments.
Comparison of the course of the patient’s cognitive complaints and/or impairments with those expected for the patient’s type and severity of injury is also necessary. In general, these impairments and related functional limitations are worst in the immediate post-injury period and gradually (although sometimes incompletely) improve thereafter. Patterns or severities of cognitive impairment that deviate from this course or that produce patterns of functional disability that are not well explained by TBI alone should prompt consideration of alternate or concurrent explanations for those impairments.
Several of these alternate or concurrent etiologies for early posttraumatic cognitive impairments merit special attention. Among persons with moderate to severe TBI, seizures may occur in over 20% of patients and nearly 60% of these are nonconvulsive (22). During this period seizures must be entertained as a possible explanation for alterations in consciousness and/or behavior – especially for presentations that might otherwise be characterized as a delirium.
Seizure prophylaxis with anticonvulsants during the first week after moderate-to-severe TBI is the standard of care (23-25). Anticonvulsant prophylaxis should be discontinued after the first week post-injury (23-25). When early or late posttraumatic seizures (including non-convulsive types) occur, valproate or levetiracetam are reasonable first-line therapies but require careful monitoring for their effects on cognition and neurobehavioral status.
Additionally, other commonly prescribed medications may interfere with cognition after TBI (see Arciniegas and Silver (2006) for a detailed review of these issues). Medication of particular concern with respect to their effects on cognition in this population include: opiate analgesics; sedative-hypnotic agents, including benzodiazepines; dopamine type-2 (D2) receptor antagonists and agents that diminish cerebral norepinephrine activity, including typical antipsychotics; and agents with anticholinergic effects, including those used to treat dizziness (meclizine, scopolamine), tricyclic antidepressants, and paroxetine, among others. Because individual clinical circumstances may necessitate the use of one or more of these agents, a proscriptive approach to the pharmacotherapy in this population is not reasonable. Nonetheless, remaining mindful of possible cognitive complications of their use is essential. When possible, avoiding, eliminating, or using the minimum-necessary dose of such medications and discontinuing them as soon as is feasible is recommended, especially before drawing conclusions about the severity, prognosis, and also the need for treatment of posttraumatic cognitive impairments.
The pre-treatment evaluation is also usefully informed by structural neuroimaging (23). In clinical practice, we routinely perform magnetic resonance imaging (MRI) prior to developing a neuropsychiatric treatment plan. Neuroimaging demonstrating severe damage (including traumatic ablation) of areas required for specific cognitive functions suggests a relative lack of anatomic target for restorative pharmacotherapy. Such findings instead guide interventions toward environmental/behavioral, supportive/adaptive, and family education/counseling approaches. On the other hand, relatively “normal” neuroimaging suggests that pharmacologic interventions that augment cerebral function are more likely to be effective. When MRI is performed, including T1, fluid-attenuated inversion recovery (FLAIR), T2* gradient echo, susceptibility-weighted, and diffusion-weighted sequences is recommended.
Evaluation of Posttraumatic Cognitive Impairments
The relationship between posttraumatic cognitive impairments and daily function (or disability) is complex. At a minimum, it varies with the type (penetrating vs. nonpenetrating) and severity of TBI, time since injury, the types and severities of cognitive impairments, and the relevance of those impairments to the activities that affected individuals perform (2, 21). With this in mind, we will limit our discussion to non-penetrating TBI and organize our discussion of the psychiatric management of posttraumatic cognitive impairments according to time post-injury.
Early Posttraumatic Cognitive Impairments
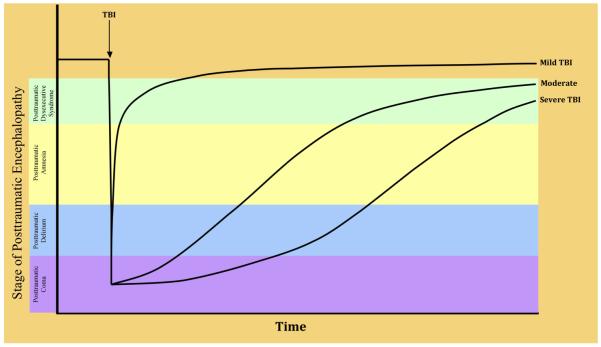
In the early post-injury period, neurobehavioral disturbances and recovery follow a reasonably consistent, although not invariate, course (26). There is a lack of consensus on the most useful semantic for describing the neurobehavioral features of the early period after moderate-to-severe TBI, and the framework presented here is one among several others in the TBI literature (9, 27-29). We suggest that the entirety of this course may be subsumed usefully under the heading of posttraumatic encephalopathy (PTE), which comprises five stages: posttraumatic coma, posttraumatic delirium, posttraumatic amnesia (PTA), posttraumatic dysexecutive syndrome, and recovery (Figure 1). Although all the stages of PTE entail multiple cognitive, emotional, and behavioral disturbances, each stage may be described according to its most salient neurobehavioral feature(s) (Table 3).
Figure 1.
Typical courses of progression through the stages of posttraumatic encephalopathy following mild, moderate, and severe TBI. The lines illustrating these courses for each level of injury severity are idealized; there is substantial variability in outcome at all levels of initial injury severity which, for the purpose of diagrammatic simplicity, is not illustrated here.
Table 3.
An overview of posttraumatic encephalopathy, its stages, and their features
| PTE Stage | Key Neurobehavioral Feature |
Description |
|---|---|---|
| Posttraumatic Coma | Impaired arousal | A complete impairment of arousal (wakefulness) in which there is no response to sensory input and no spontaneous behavior (purposeful or non-purposeful). |
| Posttraumatic Delirium | Impaired attention | A state in which there is reduced clarity of awareness of the environment, as evidenced by a reduced ability to focus, sustain, or shift attention. |
Additional features may include:
| ||
| Posttraumatic Amnesia | Impaired episodic memory | A state characterized by impaired new learning of declarative information, including orientation to time, place, and situation as well as autobiographical information for the peri- and immediate post- injury period; these impairments are not attributable to disturbances of wakefulness (coma) or awareness (delirium). |
| In this state, selective and sustained attention are relatively normal; impairments of higher-level (alternating, divided) attention, working memory, and executive function (including insight) are present; emotional and behavioral disturbances may persist (i.e., emotional lability, irritability, depression, anxiety, psychosis, apathy, aggression); based on the patient’s cognitive status, these problems are not attributable to posttraumatic delirium, but instead represent the neuropsychiatric sequelae of focal injuries (i.e., orbitofrontal syndrome) or damage to neurobehaviorally salient networks. | ||
| Posttraumatic Dysexecutive Syndrome |
Executive dysfunction, including executive control of ‘basic’ cognitive abilities |
A state characterized by impaired intrinsic executive function (e.g., conceptualization, judgment, insight) and impaired executive control of other cognitive functions, including attention (i.e., alternating, divided), working memory, language (impaired word retrieval, not confrontation naming), motor planning, and declarative memory (impaired retrieval, not new learning). |
| In this state, emotional and behavioral disturbances may persist (i.e., emotional lability, irritability, depression, anxiety, psychosis, apathy, aggression); based on the patient’s cognitive status, these problems are not attributable to posttraumatic delirium, but instead represent the neuropsychiatric sequelae of focal injuries (i.e., orbitofrontal syndrome) or damage to neurobehaviorally salient networks. |
The stages of PTE occur on a continuum, and patients may vacillate between PTE stages during recovery from TBI. Use of standardized assessments appropriate to the phase of PTE facilitates accurate diagnosis, guides prognostic formulations, and permits structured evaluation of recovery/treatment response. Using these measures also improve the reliability of examinations performed by members of multidisciplinary care teams, which are commonly employed in the settings in which patients receive care in the immediate post-injury period.
Posttraumatic Encephalopathy after Moderate-to-Severe TBI
The Coma Recovery Scale-Revised (30) is an easily administered and useful assessment of posttraumatic coma. The assessment of posttraumatic delirium may be facilitated by use of the Delirium Rating Scale-Revised-98 (31) or its TBI-specific derivative, the Delirium Diagnostic Tool-Provisional (28). During this period, it is also appropriate to begin serial (daily) assessment using the O-Log (12) or the GOAT (13). After patients emerge from posttraumatic delirium, daily assessment using the O-Log or the GOAT continues until the patient meets criteria for emergence from PTA (for two consecutive days, O-Log ≥ 25 or GOAT ≥ 76). During this stage of PTE, further evaluation of cognition using the Mini-Mental State Examination (MMSE) (32) and the Frontal Assessment Battery (FAB) (33) and interpreting performance using norms for these measures (34, 35) is encouraged. Data derived from these measures informs on cognitive function during and after PTA as well as inpatient rehabilitation outcomes (36). When neuropsychological testing resources are available (logistically and economically), a brief battery of neuropsychological tests developed specifically for use among persons with TBI receiving inpatient acute rehabilitation has been developed and may also provide useful information (4).
During the latter stages of PTE, neuropsychiatric disturbances also are common and require identification and treatment before cognition-specific interventions are undertaken. In light of the types of cognitive deficits experienced by patients in PTA (i.e., impaired episodic memory and executive deficits, including impaired insight), interviewing patients about their neuropsychiatric symptoms often is minimally productive. Instead, structured interviews of staff and family members regarding the patient’s neuropsychiatric symptoms are more useful. The Neuropsychiatric Inventory-Nursing Home version (37), in our experience, is well suited to the evaluation of neuropsychiatric disturbances among inpatients in the latter stages of PTE (36).
Posttraumatic Encephalopathy after Mild TBI
In the relatively early period after mild TBI (see Figure 1), many patients develop functionally significant impairments in higher-level attention, processing speed, working memory, memory retrieval (more than new learning), functional communication, and executive function. These impairments are frequently accompanied by other disturbances in emotional regulation and/or behavior. These problems individually and collectively reflect disturbances of frontally-mediated cognition (i.e., intrinsic executive function) as well as executive control of more basic cognitive functions, emotion, and social/interpersonal conduct. Accordingly, we use the term ‘posttraumatic dysexecutive syndrome’ here to refer to this constellation of problems.
Notwithstanding unusual circumstances in which a neuropsychiatrist is involved in the immediate post-injury care of persons with TBI, patients referred to psychiatrists for evaluation and management of cognitive problems after mild TBI are in the posttraumatic dysexecutive stage of PTE, if in PTE at all. The Neurobehavioral Rating Scale-Revised (38, 39) is a structured interview-based assessment of posttraumatic cognitive and neuropsychiatric symptoms. This measure is well suited to the evaluation of inpatients or outpatients with recent TBI who are able to participate fully in an assessment of such problems. If formal neuropsychological assessment is performed at all in the very early post-injury period, a brief battery like that described above (4) may be considered. It also is useful to assess cognitive and other neurobehavioral symptoms using a structured self-rating metric like the Neurobehavioral Functioning Inventory (40). Findings from these or similar assessments may help both clinician and patient identify symptoms that are most troubling and/or interfering with everyday function, and establish a baseline against which to gauge the effects of any treatments undertaken.
Late Posttraumatic Cognitive Impairments
Posttraumatic encephalopathy resolves gradually, albeit sometimes incompletely, in the weeks and months after TBI. As suggested by Figure 1, there is a dose-response relationship between injury severity and cognitive outcome, with greater numbers, severities, and persistence of cognitive impairments among persons with more severe injuries.
Moderate-to-Severe TBI
When cognitive impairments become persistent problems for persons with moderate-to-severe TBI, disturbances of episodic memory (although not generally simple orientation), slowed information processing, executive function, and attention are the most common problems (2). The character of these impairments is frequently captured within the construct of posttraumatic dysexecutive syndrome described earlier in this article, although patients with more severe injuries may also have substantial impairments in ‘basic’ cognitive functions as well. There is variability in cognitive outcome even at the more severe end of the injury spectrum despite this relationship, however, and substantial cognitive recovery after moderate-to-severe TBI is not uncommon. Additionally, the relationship between cognitive impairments and functional limitations is not entirely straightforward (41). A relatively recent study (14) observed 1-year post-injury returns to productive employment in: 70% of subjects with PTA of 1 day – 2 weeks; approximately 40% of subjects with PTA of 2-4 weeks; and approximately 20% (or fewer) with PTA > 4 weeks.
In light of these considerations, we encourage clinicians to use a neurorehabilitative approach to the evaluation and treatment of individuals with persistent cognitive impairments after moderate-to-severe TBI: one in which functional limitations are regarded as the primary clinical concern and cognitive (and other neurobehavioral and psychosocial) problems (42) are evaluated as factors that may be contributing to those limitations. Clinical interview of the patient and, when possible and relevant, his or her family, caregivers, and employers is performed to identify the functional problems requiring intervention. In many cases, formal assessment of the patient’s function in his or her usual home/work environment by a rehabilitation therapist will be necessarily and useful to define the highest priority clinical problems. The factors contributing to those problems – including cognitive, emotional, behavioral, physical, and psychosocial factors – are then evaluated in the manner described earlier in this article. In some cases, clinical observation, mental status examination, and ‘bedside’ cognitive assessment using the MMSE, FAB, or similar measures (43) may provide enough information to guide pharmacologic and rehabilitative interventions. When logistically and economically feasible to obtain, formal neuropsychological testing provides a more detailed assessment of a patient’s cognitive strengths and weaknesses. This information may inform on the relationship between cognitive impairments and function limitations, identify possible cognitive targets for pharmacotherapy, and facilitate the development of rehabilitative interventions that capitalize on a patient’s remaining cognitive strengths.
Mild TBI
As reviewed in detail elsewhere (8), the majority of individuals experience substantial recovery over the first year after mild TBI: 80-100% experience postconcussive symptoms (e.g., cognitive slowing, attention and/or memory problems, headache, etc.) immediately following TBI; about 50% remain symptomatic three months after TBI; and a minority (1-20%) experience persistence of such symptoms into the late (6-12 months or longer) post-injury period. In light of the generally favorable prognosis associated with mild TBI, the nature of persistent posttraumatic cognitive problems following mild TBI is a subject of considerable debate (2, 44).
Comparisons of late cognitive outcomes among groups of subjects with mild TBI and appropriately matched comparison subjects do not consistently demonstrate differences (45). This finding, however, is not particularly surprising: the natural course of recovery after mild TBI would be expected to leave only a minority of persons with cognitive impairments in prospectively followed groups of this type and therefore would tend to minimize the effects of any level of impairment experienced by this minority on the neuropsychological test scores of the group as a whole. Studies that carefully excluded subjects with pre- and post-TBI neurological, psychiatric, substance, and psychosocial confounds (46, 47) identify not only posttraumatic cognitive impairments but also their possible neurotrauma-induced biological correlates. However, such studies are often criticized for their lack of generalizability: their exclusion criteria create study groups with far fewer neuropsychiatric comorbidities (e.g., depression, anxiety, irritability, posttraumatic stress disorder; head or body pain (including headache); sleep disturbances and fatigue; substance use conditions) and psychosocial stressors that are generally encountered in this population (7, 48). These exclusory conditions are more the rule than not and they may cause, contribute to, and/or confound evaluation of posttraumatic cognitive complaints and impairments.
The need for comprehensive neuropsychiatric evaluation therefore is imperative prior to initiating treatment for chronic posttraumatic cognitive complaints and/or impairments after mild TBI. As outlined in Silver et al. (2009) (7), examining the relationship between cognitive, emotional, behavioral, and physical postconcussive symptoms, the patient’s pre-injury characteristics, and post-injury psychosocial stressors and supports is needed. This examination will determine whether that patient is best served initially by treating non-cognitive problems, cognitive problems, or both concurrently. For example, Fann et al. (2001) (49) observed that treatment of depression after TBI produced improvements not only in depression but also in neuropsychological performance and the severity of self-perceived cognitive difficulties. With this in mind, it is prudent in cases in which depression is comorbid with cognitive complaints to treat depression (perhaps using an agent with potential cognitive benefits as well) prior to initiating cognitive-specific interventions. Similarly, posttraumatic sleep disturbances exacerbate (and, in some cases, are responsible for) posttraumatic attention impairments (50); improving sleep may therefore alter (or obviate) treatment of such impairments.
When persistent posttraumatic cognitive impairments are the focus of treatment, baseline evaluation using neuropsychological testing (including symptom validity metrics) and measures like the Neurobehavioral Functioning Inventory (40) are recommended. Findings from these assessments can be used to guide medication selection and, through periodic reassessment, to monitor the effects of such treatments. Additionally, identifying specific everyday tasks that the patient believes are made more difficult by specific cognitive complaints or impairments is needed to guide rehabilitation interventions and compensatory strategy development. Formal assessment by a rehabilitation specialist of the patient’s performance in the contexts in which they are trying to perform these tasks may also be useful toward that end.
Treatment of Posttraumatic Cognitive Impairments
The selection of treatments of posttraumatic neurobehavioral disturbances is best guided by the published literature specific to TBI. The suggestions offered here therefore represent a combination of the published literature and our own clinical experience. Readers are referred to Arciniegas and Silver (2006) (1), Warden et al. (2006) (51), and Chew and Zafonte (2009) (52) for comprehensive reviews of pharmacotherapies of posttraumatic cognitive impairments. When medications are used for any purpose in this population, cautious dosing, frequent reassessment of effects and side effects, and monitoring for drug-drug interactions are strongly encouraged. Once a treatment is selected, dose titration to either beneficial effect or medication intolerance should be completed before discontinuing that treatment or adding additional agents (i.e., start-low, go-slow, but go). When a single medication does not provide adequate relief of symptoms or cannot be tolerated at therapeutic doses, augmentation using a second low-dose agent with a different mechanism of action may be useful. Readers are also encouraged to remain mindful that there are no FDA approved treatments for posttraumatic cognitive impairments and all those discussed here must be regarded as “off-label” uses. We encourage clinicians to consider the application of these agents to the treatment of any individual patient a matter of empiric trial.
The evidence-based reviews of cognitive rehabilitation performed by the Brain Injury Interdisciplinary Special Interest Group of American Congress of Rehabilitation Medicine (53, 54), the Task Force on Cognitive Rehabilitation of the European Federation of Neurological Societies (55, 56), and related meta-analyses (57) are also recommended readings for clinicians working with this population. In the interest of brevity, we will refer in this article to these reviews, rather than the primary sources they cite, except where noting key citations or identifying articles not included in these reviews is necessary.
Posttraumatic Coma
Reducing or eliminating cognitively-impairing medications, where possible, and addressing neurological problems (e.g., seizures) or medical comorbidities (i.e., hypotension, hypoglycemia, etc.) that may be contributing to impaired wakefulness (coma) are the most important elements of the neurobehavioral management of posttraumatic coma. Additionally, facilitating the patient’s engagement with staff, family (when available), and the environment is recommended; this includes entraining sleep-wake cycles with lighting cues; silencing alarms during rest periods; establishing appetitive/feeding rhythms with bolus rather than continuous feeding (where possible); avoiding elective procedures (i.e., blood draws, catheterization, line changes, etc.) at odd hours; and optimizing pain management while avoiding over-sedation, where possible. Formal sensory stimulation (“coma stimulation”) protocols are available, but the evidence of benefits from their use is limited (58). If offered to patients in posttraumatic coma (and, by extension, posttraumatic vegetative and minimally conscious states), it is important that staff and family maintain realistic expectations regarding the potential benefits of such interventions and weigh carefully the cost:benefits of their provision.
The benefits of pharmacotherapies targeting impaired arousal are only modestly more encouraging (59, 60). Although many agents, including bromocriptine, carbidopa/levodopa, methylphendiate, zolpidem, lamotrigine, and protriptyline, among others, are sometimes used for this purpose, the evidence favors amantadine as the first agent to use for the treatment of impaired arousal (i.e. coma and other disorders of consciousness) after TBI. Treatment generally begins with amantadine 50 mg twice daily, and may be increased weekly by 100 mg/day to achieve symptomatic improvement, medication intolerance, or a maximum dose of 200 mg twice daily. Monitoring for side effects, particularly evidence of potentiation of anticholinergic agents and seizures, is required. If amantadine is ineffective or is not tolerated, serial trials of other agents (e.g., bromocriptine, carbidopa/levodopa, methylphenidate, etc.) may be attempted (see Chew and Zafonte (2009) (52) for a detailed review of this topic).
Posttraumatic Delirium
Environmental and behavioral interventions as well as family and staff education are the cornerstones of posttraumatic delirium management. This includes the same steps to facilitate adaptive engagement with staff, family (when available), and the environment described for the management of posttraumatic coma. Balancing these interventions with the need to limit sensory overstimulation and resulting agitation is necessary, even if sometimes difficult to achieve in inpatient (including critical care) settings. Providing frequent reorientation cues regarding current circumstances and surroundings may help reduce agitation resulting from patients’ confusion on these points. Additionally, using one-to-one staffing (‘sitters’) in lieu of physical restraints is encouraged; physical restraint predictably increases agitation in confused patients, leading to their continued use and anti-agitation medication administration, which lead to further confusion and agitation and delayed emergence from posttraumatic delirium.
The general practice of using haloperidol (or other high-potency typical antipsychotics) and/or benzodiazepines to treat the perceptual and behavioral disturbances that occur during delirium requires reconsideration in this context, as these treatments may delay neurobehavioral recovery as well as neuroplasticity and repair after TBI (see Arciniegas and Silver (1) for review). Atypical antipsychotics appear to be as effective as haloperidol for the treatment of delirium in critically ill (including mechanically-ventilated) patients, interfere less with cerebral dopaminergic function, may facilitate normalization of cerebral cholinergic function, and produce fewer adverse motor effects than haloperidol. Collectively, these features make them attractive for the treatment of posttraumatic delirium (9). When enteral administration is possible, we generally use quetiapine 25-50 mg every 4-6 hours, with rapid dose escalation to achieve treatment response or until treatment intolerance develops. In our experience, quetiapine 600 mg (total daily dose) is generally needed to decrease agitated, aggressive, and other disinhibited behaviors during posttraumatic delirium. When enteral administration is not possible, we use olanzapine 2.5 mg IM every 12 hours, and may be advanced to olanzapine 5 mg every 6 hours as tolerated (but not exceeding a total daily dose of olanzapine IM 20 mg). If these agents are not fully effective in the management of agitation/aggression due to posttraumatic delirium, adjunctive or alternative treatment with valproate (starting at 250-500 mg three times) daily orally or intravenously may be helpful. If all of these interventions fail, then treatment with haloperidol 0.5 – 1 mg twice daily (by mouth, IM, or intravenously, total daily dose not exceeding 10 mg daily) may be considered. At haloperidol 10 mg daily, the apparent ‘effectiveness’ of high-dose haloperidol likely reflects its additional dose-dependent effects on alpha-1 adrenergic and serotonin type-2A receptors. If such effects are required to treat posttraumatic delirium, then using a lower dose of an atypical antipsychotic is likely to be as effective neurobehaviorally and less problematic with respect to extrapyramidal symptoms. The use of benzodiazpines is appropriate for the treatment of alcohol withdrawal or delirium tremens in the patient with TBI; their use in the acute post-injury period after TBI is otherwise discouraged strongly.
Posttraumatic Amnesia
Non-pharmacologic interventions are essential during PTA. The form and content of these interventions are organized around supporting cognition and providing environmental compensation for memory and executive disturbances. For example, errorless learning is an approach in which caregivers proactively provide patients with correct orientation and other necessary information in order to facilitate learning of that information, avoid encoding errors of comission (which interfere with learning correct information), and to minimize patient frustration/agitation associated with memory failures. Additionally, providing cueing and direction for daily tasks (given concurrent executive dysfunction), and providing patient/family/staff training and support are encouraged. Drill-and-practice tasks intended to ‘remediate’ cognitive deficits, including attention training exercises, visual imagery exercises, and the use of ‘memory notebooks,’ are sometimes offered to patients in PTA. These may be useful strategies for the treatment of patients with postacute and mild impairments in attention and memory; however, the benefits they confer are no better than those associated with spontaneous recovery or more general supportive cognitive interventions (53, 54) and therefore are not recommended for persons in PTA.
Cholinesterase inhibitors may hasten recovery from PTA; however, their effects on overall outcome after TBI remain uncertain (61, 62). When enteral administration is feasible, donepezil (5 mg daily for 2 weeks followed by 10 mg daily thereafter) is generally used as the first-line treatment. If enteral administration is not possible, intravenous physostigmine (3-12 mg daily, usually in 3-4 divided doses) or transdermal rivastigmine (4.6-9.5 mg/24 hour patch) may be considered. Of note, there are no published studies of transdermal rivastigmine among persons with TBI, but its safety profile and ease of administration make it a more appealing agent than physostigmine in this population. If used, the rivastigmine patch is initiated at 4.6 mg/24 hours, and should not be increased to 9.5 mg/24 hours prior to the end of 4 weeks of treatment at 4.6 mg/24 hours. Given the lack of population-specific safety data, this treatment should be used cautiously and with assiduous clinical monitoring, if it is used at all.
Impairments in speed of processing, higher-level (sustained, divided, alternating) attention, working memory, and executive function are also generally present during PTA. When patients respond to cholinesterase inhibitor therapy, these other cognitive impairments may improve as well. If specific treatment of these problems is needed, methylphenidate (5 mg twice daily, titrated in 5 mg twice daily increments every 2-3 days to a target dose of 0.3 mg/kg twice daily) may be useful. Alternatively, amantadine (50 mg twice daily, increased weekly by 100 mg/day to a maximum dose of 200 mg twice daily) may be considered.
Treatment of concurrent neuropsychiatric problems may be required during PTA. When depression, anxiety, or pathological laughing and crying develop during the period of PTA, treatment with selective serotonin reuptake inhibitors with relatively short half-lives, limited drug-drug interactions, and no antimuscarinic effects is preferred. These agents may also be useful for disinhibited, agitated, and/or aggressive behaviors. When they are ineffective or poorly tolerated, treatment with valproate (250 mg three times daily, titrated every 4-5 days in increments of 750 mg daily (divided doses) to effect or treatment intolerance may be useful. Alternatively, treatment with an atypical antipsychotic (as described above) may be considered. When any of these or other neurobehavioral problems develops in the early post-injury period, consultation with a neuropsychiatrist or behavioral neurologist experienced in their management is encouraged.
Posttraumatic Dysexecutive Syndrome
Whether it is developed as the last stage of a prolonged PTE after moderate or severe TBI, an early consequence of mild TBI, or a chronic form of posttraumatic cognitive impairments, posttraumatic dysexecutive syndrome is a clinical presentation of patients that are likely to present for psychiatric consultation and treatment. Our approach in this article has been to organize evaluation and treatment according to time post-injury and/or severity of initial TBI; however, the clinical features of this stage of PTE are quite similar across severities of TBI and throughout the post-injury period. We therefore will address first our overall approach to treating posttraumatic dysexecutive syndrome and, thereafter, comment on nuances of treatment that are informed by initial injury severity and time since injury.
As noted earlier, identifying and assiduously characterizing the patients cognitive problems (i.e., complaints, impairments, or both), the everyday tasks with which they are struggling, and the possible relationships between cognitive problems and functional status is essential. Interviewing family, staff, and other knowledgeable informants about these issues also is strongly encouraged. Information from all of these sources is needed to develop contextually relevant, patient-centered cognitive rehabilitation goals and interventions. It also is used to uncover discrepancies between presenting complaints and functional limitations that require additional – complementary or alternative – explanations for the patient’s clinical presentation and the need for further evaluation for such. Also as noted earlier, treatment of any such conditions is encouraged as an antecedent to the provision of cognition-specific rehabilitative and pharmacologic interventions.
Before considering referral for formal cognitive rehabilitation, psychiatrists and other mental health clinicians may assist patients during this stage of TBI recovery with learning and adopting several basic cognitive rehabilitation strategies. First among these strategies is helping the patient identify and, where possible, modify environmental antecedents to their cognitive failures. Understanding the contexts in which cognitive problems present functional challenges, as well as recognizing the relationship between perceived cognitive failures and affective/behavioral problems, facilitates the development of proactive approaches, rather than purely reactive responses, to these issues. This approach, in turn, facilitates improvements in the functional use of cognition, mitigates cognitive-impairment related disability, and alleviates patient and caregiver distress.
Outlining daily events/tasks and scheduling them to coincide with times during the day when the patient is well rested and/or refreshed is often very useful. This type of event/task scheduling reduces the likelihood (or extent to which) physical and/or cognitive fatigue affect function adversely. Everyday task performance as well as interpersonal function also may be improved by adjusting patient and family expectations about the number of tasks to be performed and the pace at which they will be completed. Waiting longer for verbal responses, teaching others not to respond or perform immediately for the individual, allowing longer intervals to accomplish tasks, and similar simple expectation-resetting interventions can be very useful functionally and psychologically for all involved.
Encouraging the use of ‘cognitive prosthetics’ such as memory notebooks, timers with alarms and messages, task lists, and verbal and/or non-verbal cues from others (or by signage posted in the patient’s environment), also may help compensate for impairments in higher-level attention, working memory, episodic memory retrieval difficulties, and intrinsic executive dysfunction. These sorts of cognitive prosthetics may also reduce performance failure- or frustration-related affective responses (e.g., anxiety, crying, anger/agitation, etc.). Similarly, assistive technologies such as communication devices, ‘smart phones,’ global positioning devices, and so forth also may help to compensate for impairments in language, topographical orientation, and executive function.
If, after assisting the patient and his or her family/caregiver/employer implement these strategies, it is clear that more specific cognitive rehabilitation interventions are needed, then formal consultation with rehabilitation therapist (usually a speech-language pathologist or an occupational therapist) experienced in TBI-related cognitive rehabilitation may be useful. As noted earlier, evidence-based reviews support the use of cognitive rehabilitation interventions for several types of posttraumatic cognitive problems (see Table 4). Split therapy between the psychiatrist or other clinician guiding the patient’s overall treatment plan and the rehabilitation therapist providing cognitive rehabilitation interventions may be highly productive. Toward that end, effective communication and sharing of information about the patient’s treatment goals and response is essential, particularly when medications also are used to facilitate cognitive recovery.
Table 4.
Rehabilitation interventions for posttraumatic cognitive impairments. Recommendation levels (Standard > Guideline > Option) are based on the evidence-based review performed by the Brain Injury Interdisciplinary Special Interest Group American Congress of Rehabilitation Medicine (Cicerone et al. 2000, 2005)
| Intervention | Evidence-Based Recommendation |
Comment |
|---|---|---|
| Memory strategy training | Practice standard | Recommended for persons with mild memory impairments; may include internalized strategies (e.g., visual imagery) or external memory compensations (e.g., notebooks). |
| Strategy training for attention deficits | Practice standard | Recommended during postacute rehabilitation, not during the acute phase of recovery. |
| Treatment of specific language impairments | Practice guideline | Recommended for specific language impairments only (e.g., reading comprehension and language formulation). |
| Training in compensatory strategies | Practice guideline | Recommended for persons with severe memory impairment when applied to functional activities; likely to be most useful when offered after emergence from PTA (include the late post-injury period). |
| Training in formal problem-solving strategies | Practice guideline | Recommended during the postacute rehabilitation phase and when targeted to everyday situations and functional activities that the person with injury is required to perform. |
| Comprehensive-holistic neuropsychologic rehabilitation |
Practice guideline | Recommended during post-acute rehabilitation to reduce cognitive and functional disability for persons with moderate to severe TBI. |
| Self-regulation and self-monitoring instruction |
Practice option | May be helpful for persons with impairments of executive function, attention (including neglect states), memory, and/or emotional regulation. |
| Computer-based interventions intended to produce extension of damaged visual fields |
Practice option | May be considered when posttraumatic visual field defects are present. |
| Rote practice on computer-based tasks without therapist involvement |
Not recommended |
There are two general approaches to the pharmacotherapy of cognitive impairments of the sort experienced during this stage of recovery after TBI: catecholaminergic augmentation and cholinergic augmentation. Methylphenidate augments cerebral dopaminergic and noradrenergic function and is generally regarded as a first-line treatment for posttraumatic impairments of processing speed; this agent also may improve arousal and, to a lesser extent, attention and memory disturbances (1, 51, 52). The dose initiation and titration procedure for this agent is similar whether it is offered to an inpatient or outpatient: begin with methylphenidate 5 mg twice daily, increase by 5 mg twice daily increments at intervals of no less than 2-3 days (and, in outpatients, it is often simpler to increase at weekly intervals) until a target dose of 0.3 mg/kg twice daily is achieved or treatment intolerance develops. If the latter occurs, reducing to the best previously tolerated dose is recommended. Onset of action is administration-related, with maximal benefit over 1-4 hours (more or less) after each dose taken. Timing administration of methylphenidate to match daily task demands is encouraged (i.e., for most patients, taking methylphenidate with breakfast and with lunch) as is avoiding administration within 4 hours of an anticipated sleep period. Methylphenidate may produce mild increases in heart rate and/or blood pressure, but these effects are infrequent and rarely require treatment discontinuation (63). Additionally, treatment with methylphenidate does not appear to increase seizure frequency in this population (63), even among those with previously-established seizures (64).
Cholinesterase inhibitors are most useful for the treatment of posttraumatic memory impairments (1, 51, 52), including impairments in retrieval of declarative information (i.e., executive control of verbal memory). Among patients with such impairments who respond to cholinesterase inhibitors, treatment-related improvements in attention and executive functioning also are common. For simplicity of daily administration and dose titration, donepezil (5 mg daily for 2 weeks followed by 10 mg daily thereafter) is commonly the first-line treatment. Oral rivastigmine (1.5 mg twice daily for 4 weeks, with increases in increments of 1.5 mg twice daily every four weeks) up to 12 mg/day is an alternative to this approach (65-68); the ability to incrementally adjust cholinesterase inhibitor dosing may be an advantage in some cases, although the adverse event profile of this agent in common clinical practice is generally less favorable than that associated with donepezil. When oral administration is not possible, transdermal rivastigmine (4.6-9.5 mg/24 hour patch) may be considered – however, the lack of population-specific safety data necessitate cautious use and assiduous clinical monitoring, if transdermal rivastigmine is used at all in this population. If used, the rivastigmine patch is initiated at 4.6 mg/24 hours, and it should not be increased to 9.5 mg/24 hours prior to the end of 4 weeks of treatment at 4.6 mg/24 hours.
Some patients respond robustly to catecholaminergic agents, others to cholinesterase inhibitors, some require treatment with some combination of these agents, and others respond poorly to all presently available medications. The use of these or any other such agents (see references (1, 51, 52) for the cognitive impairments during the posttraumatic dysexecutive syndrome stage of recovery after TBI must be approached as empiric trials with each patient.
Regardless of the pharmacotherapy or rehabilitation intervention used, periodic trials of treatment discontinuation are essential in order to determine whether it remains necessary. As noted earlier, spontaneous, even if sometimes incomplete, recovery is anticipated over first several months or longer after TBI. That recovery will obviate the need for continued pharmacologic and rehabilitative treatments in many patients. Accordingly, setting a schedule for tapering and/or discontinuing treatment and using a method of systematic and structured reassessment (e.g., using the same metrics employed at baseline) is recommended. In general, we perform these treatment tapers and discontinuations no later than 3-6 months after they are started and again at the end of the first year post-injury. The need for and types of treatments offered thereafter, if any, are predicated on the results of these treatment taper-related comprehensive clinical reassessments.
Acknowledgements
This work was supported in part by National Institutes of Health (grants NICHD R01 HD047242-04 and NICHD 5R01 HD047242-04S1) and the Veterans Health Administration VISN-19 Mental Illness Research, Education, and Clinical Center (MIRECC).
References
- 1.Arciniegas DB, Silver JM. Pharmacotherapy of posttraumatic cognitive impairments. Behav Neurol. 2006;17(1):25–42. doi: 10.1155/2006/460592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dikmen SS, Corrigan JD, Levin HS, Machamer J, Stiers W, Weisskopf MG. Cognitive outcome following traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):430–8. doi: 10.1097/HTR.0b013e3181c133e9. [DOI] [PubMed] [Google Scholar]
- 3.Sigurdardottir S, Andelic N, Roe C, Schanke AK. Cognitive recovery and predictors of functional outcome 1 year after traumatic brain injury. J Int Neuropsychol Soc. 2009;15(5):740–50. doi: 10.1017/S1355617709990452. [DOI] [PubMed] [Google Scholar]
- 4.Hanks RA, Millis SR, Ricker JH, Giacino JT, Nakese-Richardson R, Frol AB, et al. The predictive validity of a brief inpatient neuropsychologic battery for persons with traumatic brain injury. Arch Phys Med Rehabil. 2008;89(5):950–7. doi: 10.1016/j.apmr.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Hanks RA, Rapport LJ, Millis SR, Deshpande SA. Measures of executive functioning as predictors of functional ability and social integration in a rehabilitation sample. Arch Phys Med Rehabil. 1999;80(9):1030–7. doi: 10.1016/s0003-9993(99)90056-4. [DOI] [PubMed] [Google Scholar]
- 6.Ponsford J, Olver J, Ponsford M, Nelms R. Long-term adjustment of families following traumatic brain injury where comprehensive rehabilitation has been provided. Brain Inj. 2003;17(6):453–68. doi: 10.1080/0269905031000070143. [DOI] [PubMed] [Google Scholar]
- 7.Silver JM, McAllister TW, Arciniegas DB. Depression and cognitive complaints following mild traumatic brain injury. Am J Psychiatry. 2009;166(6):653–61. doi: 10.1176/appi.ajp.2009.08111676. [DOI] [PubMed] [Google Scholar]
- 8.Arciniegas DB, Anderson CA, Topkoff J, McAllister TW. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat. 2005;1(4):311–27. [PMC free article] [PubMed] [Google Scholar]
- 9.Arciniegas DB, McAllister TW. Neurobehavioral management of traumatic brain injury in the critical care setting. Crit Care Clin. 2008;24(4):737–65. viii. doi: 10.1016/j.ccc.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Kay T, Harrington DE, Adams RE, Anderson TW, Berrol S, Cicerone K, et al. Definition of mild traumatic brain injury: Report from the Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. J Head Trauma Rehabil. 1993;8(3):86–7. [Google Scholar]
- 11.Marr A, Coronado VG. Central Nervous System Injury Surveillance Data Submission Standards - 2002. National Center for Injury Control and Prevention, Centers for Disease Control and Prevention; Atlanta: 2002. [Google Scholar]
- 12.Jackson WT, Novack TA, Dowler RN. Effective serial measurement of cognitive orientation in rehabilitation: the Orientation Log. Arch Phys Med Rehabil. 1998;79(6):718–20. doi: 10.1016/s0003-9993(98)90051-x. [DOI] [PubMed] [Google Scholar]
- 13.Levin HS, O’Donnell VM, Grossman RG. The Galveston Orientation and Amnesia Test. A practical scale to assess cognition after head injury. J Nerv Ment Dis. 1979;167(11):675–84. doi: 10.1097/00005053-197911000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Nakase-Richardson R, Sepehri A, Sherer M, Yablon SA, Evans C, Mani T. Classification schema of posttraumatic amnesia duration-based injury severity relative to 1-year outcome: analysis of individuals with moderate and severe traumatic brain injury. Arch Phys Med Rehabil. 2009;90(1):17–9. doi: 10.1016/j.apmr.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Zafonte RD, Mann NR, Millis SR, Black KL, Wood DL, Hammond F. Posttraumatic amnesia: its relation to functional outcome. Arch Phys Med Rehabil. 1997;78(10):1103–6. doi: 10.1016/s0003-9993(97)90135-0. [DOI] [PubMed] [Google Scholar]
- 16.Frey KL, Rojas DC, Anderson CA, Arciniegas DB. Comparison of the O-Log and GOAT as measures of posttraumatic amnesia. Brain Inj. 2007;21(5):513–20. doi: 10.1080/02699050701311026. [DOI] [PubMed] [Google Scholar]
- 17.McMillan TM, Jongen EL, Greenwood RJ. Assessment of post-traumatic amnesia after severe closed head injury: retrospective or prospective? J Neurol Neurosurg Psychiatry. 1996;60(4):422–7. doi: 10.1136/jnnp.60.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 19.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27(3):422–8. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- 20.van der Naalt J, Hew JM, van Zomeren AH, Sluiter WJ, Minderhoud JM. Computed tomography and magnetic resonance imaging in mild to moderate head injury: early and late imaging related to outcome. Ann Neurol. 1999;46(1):70–8. doi: 10.1002/1531-8249(199907)46:1<70::aid-ana11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Kashluba S, Hanks RA, Casey JE, Millis SR. Neuropsychologic and functional outcome after complicated mild traumatic brain injury. Arch Phys Med Rehabil. 2008;89(5):904–11. doi: 10.1016/j.apmr.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Vespa P. Continuous EEG monitoring for the detection of seizures in traumatic brain injury, infarction, and intracerebral hemorrhage: “to detect and protect”. J Clin Neurophysiol. 2005;22(2):99–106. doi: 10.1097/01.wnp.0000154919.54202.e0. [DOI] [PubMed] [Google Scholar]
- 23.Marion DW. Evidenced-based guidelines for traumatic brain injuries. Prog Neurol Surg. 2006;19:171–96. doi: 10.1159/000095191. [DOI] [PubMed] [Google Scholar]
- 24.Chang BS, Lowenstein DH. Practice parameter: antiepileptic drug prophylaxis in severe traumatic brain injury: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;60(1):10–6. doi: 10.1212/01.wnl.0000031432.05543.14. [DOI] [PubMed] [Google Scholar]
- 25.Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. XIII. Antiseizure prophylaxis. J Neurotrauma. 2007;24(Suppl 1):S83–6. doi: 10.1089/neu.2007.9983. [DOI] [PubMed] [Google Scholar]
- 26.Sherer M, Yablon SA, Nakase-Richardson R. Patterns of recovery of posttraumatic confusional state in neurorehabilitation admissions after traumatic brain injury. Arch Phys Med Rehabil. 2009;90(10):1749–54. doi: 10.1016/j.apmr.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Stuss DT, Binns MA, Carruth FG, Levine B, Brandys CE, Moulton RJ, et al. The acute period of recovery from traumatic brain injury: posttraumatic amnesia or posttraumatic confusional state? J Neurosurg. 1999;90(4):635–43. doi: 10.3171/jns.1999.90.4.0635. [DOI] [PubMed] [Google Scholar]
- 28.Kean J, Trzepacz PT, Murray LL, Abell M, Trexler L. Initial validation of a brief provisional diagnostic scale for delirium. Brain Inj. 2010;24(10):1222–30. doi: 10.3109/02699052.2010.498008. [DOI] [PubMed] [Google Scholar]
- 29.Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil. 2005;20(1):76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85(12):2020–9. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 31.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–42. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 32.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55(11):1621–6. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 34.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–91. [PubMed] [Google Scholar]
- 35.Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa ML, et al. The Frontal Assessment Battery (FAB): normative values in an Italian population sample. Neurol Sci. 2005;26(2):108–16. doi: 10.1007/s10072-005-0443-4. [DOI] [PubMed] [Google Scholar]
- 36.Arciniegas DB. Neuropsychiatric Assessment of Traumatic Brain Injury during Acute Neurorehabilitation. In: Miyoshi K, Morimura Y, Maeda K, editors. Neuropsychiatric Disorders. Springer; Tokyo: 2010. pp. 125–46. [Google Scholar]
- 37.Wood S, Cummings JL, Hsu MA, Barclay T, Wheatley MV, Yarema KT, et al. The use of the neuropsychiatric inventory in nursing home residents. Characterization and measurement. Am J Geriatr Psychiatry. 2000;8(1):75–83. doi: 10.1097/00019442-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Vanier M, Mazaux JM, Lambert J, Dassa C, Levin HS. Assessment of neuropsychologic impairments after head injury: interrater reliability and factorial and criterion validity of the Neurobehavioral Rating Scale-Revised. Arch Phys Med Rehabil. 2000;81(6):796–806. doi: 10.1016/s0003-9993(00)90114-x. [DOI] [PubMed] [Google Scholar]
- 39.McCauley SR, Levin HS, Vanier M, Mazaux JM, Boake C, Goldfader PR, et al. The neurobehavioural rating scale-revised: sensitivity and validity in closed head injury assessment. J Neurol Neurosurg Psychiatry. 2001;71(5):643–51. doi: 10.1136/jnnp.71.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreutzer JS, Marwitz JH, Seel R, Serio CD. Validation of a neurobehavioral functioning inventory for adults with traumatic brain injury. Arch Phys Med Rehabil. 1996;77(2):116–24. doi: 10.1016/s0003-9993(96)90155-0. [DOI] [PubMed] [Google Scholar]
- 41.Royall DR, Lauterbach EC, Kaufer D, Malloy P, Coburn KL, Black KJ. The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2007;19(3):249–65. doi: 10.1176/jnp.2007.19.3.249. [DOI] [PubMed] [Google Scholar]
- 42.Cicerone KD, Mott T, Azulay J, Sharlow-Galella MA, Ellmo WJ, Paradise S, et al. A randomized controlled trial of holistic neuropsychologic rehabilitation after traumatic brain injury. Arch Phys Med Rehabil. 2008;89(12):2239–49. doi: 10.1016/j.apmr.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 43.Larson EB, Leahy B, Duff KM, Wilde MC. Assessing executive functions in traumatic brain injury: an exploratory study of the Executive Interview. Percept Mot Skills. 2008;106(3):725–36. doi: 10.2466/pms.106.3.725-736. [DOI] [PubMed] [Google Scholar]
- 44.Hoge CW, Goldberg HM, Castro CA. Care of war veterans with mild traumatic brain injury--flawed perspectives. N Engl J Med. 2009;360(16):1588–91. doi: 10.1056/NEJMp0810606. [DOI] [PubMed] [Google Scholar]
- 45.Anderson GD, Temkin NR, Dikmen SS, Diaz-Arrastia R, Machamer JE, Farhrenbruch C, et al. Haptoglobin phenotype and apolipoprotein E polymorphism: relationship to posttraumatic seizures and neuropsychological functioning after traumatic brain injury. Epilepsy Behav. 2009;16(3):501–6. doi: 10.1016/j.yebeh.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arciniegas DB, Topkoff JL. Applications of the P50 evoked response to the evaluation of cognitive impairments after traumatic brain injury. Phys Med Rehabil Clin N Am. 2004;15(1):177–203. viii. doi: 10.1016/s1047-9651(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 47.McAllister TW, Flashman LA, McDonald BC, Saykin AJ. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J Neurotrauma. 2006;23(10):1450–67. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- 48.Kim E, Lauterbach EC, Reeve A, Arciniegas DB, Coburn KL, Mendez MF, et al. Neuropsychiatric complications of traumatic brain injury: a critical review of the literature (a report by the ANPA Committee on Research) J Neuropsychiatry Clin Neurosci. 2007;19(2):106–27. doi: 10.1176/jnp.2007.19.2.106. [DOI] [PubMed] [Google Scholar]
- 49.Fann JR, Uomoto JM, Katon WJ. Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics. 2001;42(1):48–54. doi: 10.1176/appi.psy.42.1.48. [DOI] [PubMed] [Google Scholar]
- 50.Bloomfield IL, Espie CA, Evans JJ. Do sleep difficulties exacerbate deficits in sustained attention following traumatic brain injury? J Int Neuropsychol Soc. 2010;16(1):17–25. doi: 10.1017/S1355617709990798. [DOI] [PubMed] [Google Scholar]
- 51.Warden DL, Gordon B, McAllister TW, Silver JM, Barth JT, Bruns J, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468–501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- 52.Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury--a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851–79. doi: 10.1682/jrrd.2008.09.0120. [DOI] [PubMed] [Google Scholar]
- 53.Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, et al. Evidence-based cognitive rehabilitation: recommendations for clinical practice. Arch Phys Med Rehabil. 2000;81(12):1596–615. doi: 10.1053/apmr.2000.19240. [DOI] [PubMed] [Google Scholar]
- 54.Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil. 2005;86(8):1681–92. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 55.Cappa SF, Benke T, Clarke S, Rossi B, Stemmer B, van Heugten CM. EFNS guidelines on cognitive rehabilitation: report of an EFNS task force. Eur J Neurol. 2003;10(1):11–23. doi: 10.1046/j.1468-1331.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- 56.Cappa SF, Benke T, Clarke S, Rossi B, Stemmer B, van Heugten CM. EFNS guidelines on cognitive rehabilitation: report of an EFNS task force. Eur J Neurol. 2005;12(9):665–80. doi: 10.1111/j.1468-1331.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- 57.Rohling ML, Faust ME, Beverly B, Demakis G. Effectiveness of cognitive rehabilitation following acquired brain injury: a meta-analytic re-examination of Cicerone et al.’s (2000, 2005) systematic reviews. Neuropsychology. 2009;23(1):20–39. doi: 10.1037/a0013659. [DOI] [PubMed] [Google Scholar]
- 58.Meyer MJ, Megyesi J, Meythaler J, Murie-Fernandez M, Aubut JA, Foley N, et al. Acute management of acquired brain injury Part III: an evidence-based review of interventions used to promote arousal from coma. Brain Inj. 2010;24(5):722–9. doi: 10.3109/02699051003692134. [DOI] [PubMed] [Google Scholar]
- 59.Whyte J, Katz D, Long D, DiPasquale MC, Polansky M, Kalmar K, et al. Predictors of outcome in prolonged posttraumatic disorders of consciousness and assessment of medication effects: A multicenter study. Arch Phys Med Rehabil. 2005;86(3):453–62. doi: 10.1016/j.apmr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Wheaton P, Mathias JL, Vink R. Impact of early pharmacological treatment on cognitive and behavioral outcome after traumatic brain injury in adults: a meta-analysis. J Clin Psychopharmacol. 2009;29(5):468–77. doi: 10.1097/JCP.0b013e3181b66f04. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Plotkin RC, Wang G, Sandel ME, Lee S. Cholinergic augmentation with donepezil enhances recovery in short-term memory and sustained attention after traumatic brain injury. Arch Phys Med Rehabil. 2004;85(7):1050–5. doi: 10.1016/j.apmr.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 62.Kim YW, Kim DY, Shin JC, Park CI, Lee JD. The changes of cortical metabolism associated with the clinical response to donepezil therapy in traumatic brain injury. Clin Neuropharmacol. 2009;32(2):63–8. doi: 10.1097/WNF.0B013E31816F1BC1. [DOI] [PubMed] [Google Scholar]
- 63.Willmott C, Ponsford J, Olver J, Ponsford M. Safety of methylphenidate following traumatic brain injury: impact on vital signs and side-effects during inpatient rehabilitation. J Rehabil Med. 2009;41(7):585–7. doi: 10.2340/16501977-0369. [DOI] [PubMed] [Google Scholar]
- 64.Wroblewski BA, Leary JM, Phelan AM, Whyte J, Manning K. Methylphenidate and seizure frequency in brain injured patients with seizure disorders. J Clin Psychiatry. 1992;53(3):86–9. [PubMed] [Google Scholar]
- 65.Silver JM, Koumaras B, Chen M, Mirski D, Potkin SG, Reyes P, et al. Effects of rivastigmine on cognitive function in patients with traumatic brain injury. Neurology. 2006;67(5):748–55. doi: 10.1212/01.wnl.0000234062.98062.e9. [DOI] [PubMed] [Google Scholar]
- 66.Silver JM, Koumaras B, Meng X, Potkin SG, Reyes PF, Harvey PD, et al. Long-term effects of rivastigmine capsules in patients with traumatic brain injury. Brain Inj. 2009;23(2):123–32. doi: 10.1080/02699050802649696. [DOI] [PubMed] [Google Scholar]
- 67.Tenovuo O. Central acetylcholinesterase inhibitors in the treatment of chronic traumatic brain injury-clinical experience in 111 patients. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(1):61–7. doi: 10.1016/j.pnpbp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Tenovuo O, Alin J, Helenius H. A randomized controlled trial of rivastigmine for chronic sequels of traumatic brain injury-what it showed and taught? Brain Inj. 2009;23(6):548–58. doi: 10.1080/02699050902926275. [DOI] [PubMed] [Google Scholar]