Abstract
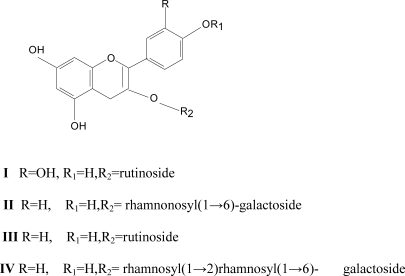
The bioactive N-butanol fraction of the ethanol extract of the leaves of Cissus ibuensis was fractionated over silica Gel column to give Quercetin 3-O-rutinoside (I) and mixtures of Flavonoids (A2). A2 was fractionated using reverse phase HPLC to give Kaempferol 3-O-α-rhamnopyranosyl (1→6)-β;-D-galactopyranoside (II), Kaempferol 3-O-rutinoside(III)and Kaempferol3-O-α-rhamnopyranosyl (1→6)-α-rhamnopyranosyl(1→2)-β;-D-galactopyranoside (IV). The structures were elucidated by NMR spectroscopy and compared with literature.
Keywords: Flavonoids, Kaempferol, Quercitin, anti-bacterial acitvity
Introduction
Cissus ibuensis Hook (F) a climber belongs to the family Vitaceae and is distributed in the tropical regions particularly in Nigeria, Niger, Togo, Benin and Ghana. Traditionally the leaves of the plant are used in Northern Nigeria to treat gastrointestinal disturbance, as remedy for rheumatism and arthritis (Irvine, 1961)). The fruits and leaves decoction are applied as liniment for rheumatism and arthritis (Dalziel, 1958). While this species has not been investigated before. Investigation of various species from the genus Cissus have been described. The leaves of C. rhifolia contain quinolizidine alkaloids, flavonoids and terpenoids (Siafah et al., 1983). The stem wood of C. pallida showed the presence of Stilbenes, triterpenoids and steroids (Khan et al., 1986), while stilbenoids have been isolated from C. quandrangularis (Singh et al., 2005).
As part of our research focusing on the genus Cissus here we report the isolation of four Flavonoids from the N-butanol soluble part of the ethanol extract, we also report here the anti-bacterial activity of the Acetone,Ethanol and the partitioned fractions: Ethylacetate and N-butanol.
Materials and methods
General experimental procedure
Column chromatography was performed on silica gel G (60 – 120µm) BDH,TLC was performed on precoated Kieselgel 60 F254 plates(Merck,Darmstadt,Germany);compounds were detected by spraying with Cerium sulphate in Sulphuric acid(Sigma-Aldrich). 1H-NMR and 13C-NMR were recorded on Bruker DRX spectrophotometer (300MHz) and 75MHz respectively in CD3OD with TMS as internal standard; Reversed phase (RP) HPLC separations were conducted on a waters 515 pumping system equipped with a Waters R401 refractive index detector, using a C18 bondapak column (30cm × 7.8mm) and a mobile phase consisting of Methanol:Water(40:60) at flow rate of 2ml/min.
Plant material: The leaves of the plant Cissus ibuensis was collected from Samaru — Zaria in the month of June,2004 and was identified by Mall.U. Gallah of the herbarium section, Department of Biological Sciences, Ahmadu Bello University, Zaria Nigeria where a voucher specimen No. 2708 was deposited.
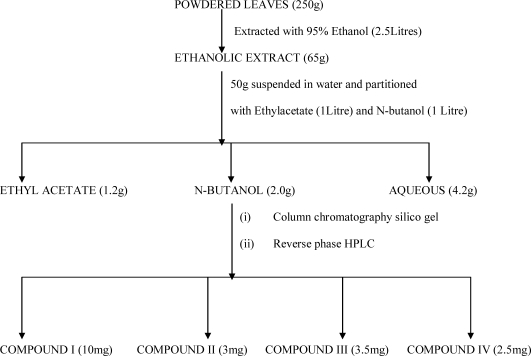
Extraction and isolation: The air dried powdered leaves (250g) was extracted with 95% ethanol (1X2.5L to exhaustion by cold process. The combined ethanolic extract was concentrated at reduced pressure to afford a greenish mass (65g). 50g of this was suspended in water and filtered. The water soluble part was extracted with ethylacetate, and N-butanol. The n-butanol soluble part (2.0g) was packed with silica gel G (100g) in a column (50cm × 1.2cm) and eluted gradiently with chloroform and chloroform : methanol mixtures. Progress of elution was monitored by silica TLC plates using n-butanol : Acetic acid : water (60 : 15 : 25)ethylacetate : methanol : water (100 : 16.5 : 13.5) and ethylacetate : formic acid : water (10 : 2 : 3).Elution with chloroform: methanol (8: 2) afforded a pale yellow solid which was crystallized in methanol to give compound I (10mg). Further elution with chloroform : methanol (8 : 2) gave A2(0.9g) which from TLC was shown to be mixtures of flavonoid. This was subjected to reverse-phase HPLC on a C18 µ-bondapak eluted with methanol : water (40 : 60) at 2ml/min to give compounds II(3mg), compound III(3.5mg) and compound IV(2.5mg) Compound IV was identified by 1H-NMR, and 2D13C-NMR (HSQC, HMBC, TOCSY), while compounds I ,II and III were identified by comparing their 1H-NMR spectra with that reported in literature.
Antibacterial studies: The test organisms: Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa are clinical isolates obtained from the Department of Pharmaceutical Microbiology and Biotechnology, Niger-Delta University, Wilberforce island Bayelsa state,Nigeria. The extracts at concentrations of 5 and 10mg/ml were screened for antibacterial activity by agar diffusion techniques as described by (Mendoza et al., 1997).
Results
Discussion
Antibacterial studies of the extracts showed that the Ethanol extracts is more active than the acetone at concentration 10mg/ml against the test pathogens(Table 3) and the partitioned extracts, N-butanol showed slightly higher activity than the ethyl acetate fractions and this fraction gave the isolated flavonoids. Activity of the extracts against S.aureus is comparable to the standard antibiotic Ciproflxacin while Gentmicin did not show any activity against S.aureus. Thus the extracts can be said to show more activity on S.aureus than the standard antibiotics used, however ciprofloxacin showed the best activity against the gram negative organisms used.
Table 3.
Antibacterial activity of Cissus ibuensis extracts#
| Tested material | S. aureus | B. subtilis | E. coli | P. aeruginosa |
| Acetone a | 15.0 | 17.0 | − | − |
| Ethanol a | 15.0 | 18.0 | 15.0 | 15.0 |
| Ethyl acetatea | 18.0 | 15.0 | 13.0 | 15.0 |
| N-Butanol a | 18.0 | 15.0 | 15.0 | 16.0 |
| Ciprofloxacinb | 12.0 | 28.0 | 32.0 | 32.0 |
| Gentamycinc | − | 10.0 | 16.0 | 12.0 |
-: No inhibition
Stock solution 10mg/ml
5µg/ml
=10µg/ml
Values are zone of inhibition diameter (mm) and mean of three replicates. The concentration of the standard antibiotic is 5 and 10 microgram microgram per disc.
The 1H-NMR spectrum of compound IV(Table 2) indicated a 5,7-dihydroxylated pattern for a ring A (two metacoupled protons at δ=6.13 and 6.29ppm and an A2B2 substitution pattern for ring B signals at δ=6.91 d,2H,J=8Hz and 8.01 d,J=8.0Hz allowing the aglycon to be recognized as Kaempferol(Young Lin et al,2001, Mabry at al.,1970). The 1H-NMR spectrum of compound IV also showed signals ascribable to sugar moieties (Table 1), three anomeric protons arising from sugar moieties at δ=4.55(s),5.25(s), and 5.5 d,J=(7.0Hz) which correlated respectively with signals at δ=102ppm,103ppm and 101ppm in HSQC spectrum. All the 1H and 13C signals of compound IV (Table 2) were assigned using HSQC and HMBC experiments. Complete assignments of protons and carbon chemical shifts of the sugar portions were accomplished by 1D-TOCSYexperiments and allowed the identification of the two terminal rhamnose units (d=1.04,d,3H,(J=6Hz)and d=1.13 d,3H(J=6Hz) and confirm by their corresponding carbon signals at d=17.2 and 18.1ppm respectively(Agrawal and Bansal,1989).
Table 2.
1H- and 13C-NMR data of IV(CD3OD) : 300/75MHz, J in Hz δ;in PPM)a
| C | δH | δC |
| Aglycone moeity | ||
| 2 | 156.7 | |
| 3 | 133.1 | |
| 4 | 177.5 | |
| 5 | 162.1 | |
| 6 | 6.13(s) | 101.5 |
| 7 | 164.3 | |
| 8 | 6.19 (s) | 96.0 |
| 9 | 156.7 | |
| 10 | 104.0 | |
| 1' | 120.8 | |
| 2' | 8.06 d, J=8.2Hz | 132.1 |
| 3' | 6.90 d, J=8.2Hz | 117.2 |
| 4' | 160.1 | |
| 5' | 6.90 d, J=8.2Hz | 117.2 |
| 6' | 8.06 d, J=.2Hz | 132.1 |
| β;-galactosyl | ||
| 1 | 5.58 d,(J=7.8Hz) | 100.6 |
| 2 | 3.96 | 77.5 |
| 3 | 3.72 | 75.5 |
| 4 | 3.79 | 70.6 |
| 5 | 3.64 | 75.2 |
| 6 | 3.48 | 66.8 |
| rhamnosyl in (2) | ||
| 1 | 5.25(s) | 102.3 |
| 2 | 4.03 | 72.2 |
| 3 | 3.84 | 72.1 |
| 4 | 3.37 | 73.9 |
| 5 | 4.11 | 69.8 |
| 6 | 1.02 d,(J=6.0Hz) | 17.5 |
| rhamnosyl in(6) | ||
| 1 | 4.55(s) | 101.6 |
| 2 | 3.53 | 72.1 |
| 3 | 3.60 | 71.9 |
| 4 | 3.30 | 73.9 |
| 5 | 3.56 | 69.1 |
| 6 | 1.2 d,(J=6.0Hz) | 17.6 |
(assignments are based on HSQC,HMBC and TOCSY)experiments.
Table 1.
1H-NMR of Compounds I-III (CD3OD) : 300MHz: J in Hz δ;in PPM
| C | δH | δH | δH |
| Aglycone moiety | I | II | III |
| 2 | |||
| 3 | |||
| 4 | |||
| 5 | |||
| 6 | 6.15(d) | 6.18(d),J=2.0 | 6.18(d),J=2.0 |
| 7 | 8 6.33 (d) | 6.37(d),J=2.0 | 6.37(d),J=2.0 |
| 9 | |||
| 10 | |||
| 1' | |||
| 2' | 7.65( d), J=8.2 | 8.05(d),J=8.8 | 8.05(d),J=8.5 |
| 3' | 6.86(d),J=8.8 | 6.89(d),J=8.5 | |
| 4' | |||
| 5' | 6.87 (d) J=8.4 | 6.86(d),J= 8.8 | 6.89(d),J=8.5 |
| 6' | 7.58 (d,d) J=2.0 | 8.07(d,),J= 8.8 | 8.05(d),J=8.5 |
| β;-glucose/galactose | |||
| 1 | 5.58 (d),J=7.8 | 5.11(d),J=8.5 | 5.11(d),J=8.5 |
| 2 | 3.96 | ||
| 3 | 3.72 | ||
| 4 | 3.79 | ||
| 5 | 3.64 | ||
| 6 | 3.48 | ||
| rhamnosyl | |||
| 1 | 4.51(s) | 4.51(s) | 4.51(s) |
| 2 | 4.03 | ||
| 3 | 3.84 | ||
| 4 | 3.37 | ||
| 5 | 4.11 | ||
| 6 | 1.02 (d), J=6.0 | 1.11(d),J=6.1 | 1.13(d),J=6.1 |
The rhamnogalactosyl linkage was evident from the down field shift of the C-2 and C-6 of galactose sugar from 71.3ppm to 77.49ppm and 60.8ppm to 66.8ppm and up field shift of the C-1 and C-5 from 102.0 to 100.4ppm and 75.8 to 75.16ppm provided evidence that the linkage of the two rhamnose sugar is at C-2 and C-6 of the galactose (Markham et al., 1978), complete sugar assignment was aided by direct C-H correlation: HSQC, and TOCSY experiments. Thus compound IV was identified as Kaempferol-3-O-α-rhamnosyl-(1.6)-α-rhamnopyranosyl-(1→2)-β;-galactopyranoside. The 1H-NMR spectra of compounds, I II and III (Table 1) were identified as Quercitin 3-O-rutinoside (rutin), Kaempferol 3-O-α-rhamnopyranosy(1→6)β-D-galcatopyranoside and Kaempferol-3-O-rutinoside by comparism with that reported in literature (Yun-Lian et al., 2000; Ahmed and Nordin, 1998).
The presence of compounds I, II and III (Table1) have been reported in leaf plants derived from Leguminasae and Moraceae (Luisa et al., 2000; Yun et al.,1991), but their presence in Cissus ibuensis is described for the first time. The isolated flavonoids might be responsible for the observed antibacterial activity as these plant constituents have been reported to have antibacterial actions (Harbone and Williams, 2000; Van Puyvelde et al., 1989). Work is ongoing to re-isolate these flavonoids and test their antibacterial activity.
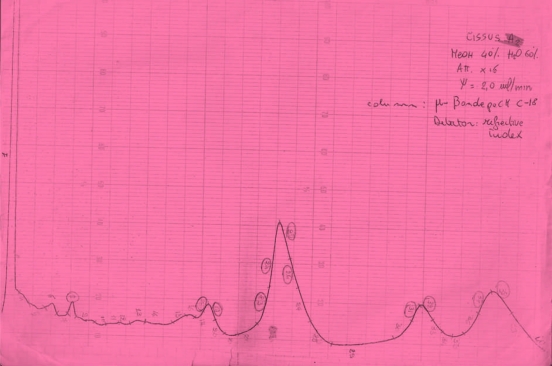
Figure 1.
HPLC Chomatogram of Fraction A2
Figure 2.
Isolation scheme of the N-Butanol extract
Figure 3.
Structures of isolated Flavonoids
References
- 1.Ahmed SH, Nordin HL. Chemical Constituents of Hedyotis herbacea. Asean Review of Biodiversity and Environmental conservation Article. II. 1998:1–6. [Google Scholar]
- 2.Agrawal PK, Bansal MC. 13C-NMR of Flavonoids. Amsterdam: 1989. pp. 283–294. [Google Scholar]
- 3.Dalziel JM. Flora of West Tropical Africa. A Crown Agent for Oversea Publication. 1958:280–281. [Google Scholar]
- 4.Harbone JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 5.Irvine FR. Woody plants of Ghana Oxford University Press. 1961:300–301. [Google Scholar]
- 6.Khan MA, Nabi SG, Prakash S, Zaman A. Pallidol, a resveratrol dimer from Cissus pallida. Phytochemistry. 1986;25:1945–1948. [Google Scholar]
- 7.Luisa P, Elisabetta EC, Morelli I. Flavonoids from Ficus Pumila Biochemical Systematics Ecology. 2000;28:287–289. [Google Scholar]
- 8.Mabry TJ, Markham KR, Thomas MB. The Systematic Identification of flavonoids. New York: Springer - Verlag Publication; 1970. p. 292. [Google Scholar]
- 9.Markham KR, Ternai B, Stanley R; Geiger H, Mabry TJ. 13C-NMR Studies of Flavonoids II, Tetrahedron. 1978;34:1391–1394. [Google Scholar]
- 10.Mendoza L, Wilkens M, Urzua A. Antimicrobial study of the Diterpenods and flavonoids from some Chilean Pseudographalium(Asteralae) J Ethnopharmacol. 1997;58(2):246–252. doi: 10.1016/s0378-8741(97)00084-6. [DOI] [PubMed] [Google Scholar]
- 11.Saifah E, Kelly C, Leary JD. Constituents of Cissus rheifolia. J Natural Products. 1983;46:353–358. [Google Scholar]
- 12.Singh G, Rawat P, Maurya R. Constituents of Cissus Quandrangularis Natural Products research. 2007;21(6):522–528. doi: 10.1080/14786410601130471. [DOI] [PubMed] [Google Scholar]
- 13.Van Puyvelde L, De Kimpe N, Costa J, Munyjabo V, Nyirankuliza A, Hakizamungu E, Schamp N. Isolation of Flavonoids and Chalcone from Helichrysun odoratissimum and synthesis of helichrysetin. J Nat Prod. 1989;52:629–633. doi: 10.1021/np50063a025. [DOI] [PubMed] [Google Scholar]
- 14.Young Leem K, Park EJ, Ken J, Kim VB, Kim SO, Kim YC. Neuroprotective Constituents of Hedyotis diffusa. J Nat Products. 2001;64:75–78. doi: 10.1021/np000327d. [DOI] [PubMed] [Google Scholar]
- 15.Yu R, Li X, Harigaya Y, Konda Y, Onda M. 2D NMR Spectroscopic Studies of Flavonoid from Oxytropis glabra Bopuxue Zazhi. 1991;8(1):99–110. [Google Scholar]
- 16.Yun -Lian L, Yueh HK, Mingshi S, Chen-Chih C, Jun-Chih O. Flavonoid glycosides from Terminalia Catappa. J Chinese Chem Soc. 2000;47:253–256. [Google Scholar]