Abstract
The authors characterized human immunodeficiency virus (HIV) and hepatitis C virus (HCV) incidence and prospective changes in self-reported risk behavior over 2 years among 1,158 injection drug users (IDUs) recruited in Chennai, India, in 2005–2006. At baseline, HIV prevalence was 25.3%, and HCV prevalence was 54.5%. Seropositive persons with prevalent HIV infection were used to estimate baseline HIV incidence by means of the Calypte HIV-1 BED Incidence EIA (Calypte Biomedical Corporation, Portland, Oregon). Longitudinal HIV and HCV incidence were measured among 865 HIV-negative IDUs and 519 HCV antibody-negative IDUs followed semiannually for 2 years. Participants received pre- and posttest risk reduction counseling at each visit. Estimated HIV incidence at baseline was 2.95 per 100 person-years (95% confidence interval (CI): 1.21, 4.69) by BED assay; observed HIV incidence over 1,262 person-years was 0.48 per 100 person-years (95% CI: 0.17, 1.03). HCV incidence over 645 person-years was 1.71 per 100 person-years (95% CI: 0.85, 3.03). Self-reported risk behaviors declined significantly over time, from 100% of participants reporting drug injection at baseline to 11% at 24 months. In this cohort with high HIV and HCV prevalence at enrollment, the authors observed low incidence and declining self-reported risk behavior over time. While no formal intervention was administered, these findings highlight the potential impact of voluntary counseling and testing in a high-risk cohort.
Keywords: cohort studies; hepacivirus; HIV; India; risk-taking; substance abuse, intravenous
India has approximately 2.4 million persons infected with human immunodeficiency virus (HIV), the virus that causes acquired immunodeficiency syndrome (AIDS) (1). Because 85% of HIV infections in India are acquired heterosexually (2), prevention programs have targeted heterosexual populations, resulting in stabilization of HIV incidence and prevalence in this group (3, 4). By contrast, national surveillance data suggest continued expansion of HIV among injection drug users (IDUs) (1). It is estimated that anywhere from 168,000 to 1.1 million IDUs reside in India (5, 6). Historically, HIV associated with injection drug use has been restricted to northeastern India (7). However, recent data suggest emergence of HIV epidemics among IDUs in other regions (1). IDUs represent a marginalized population with limited access to HIV prevention and treatment programs. Understanding the trajectory of the epidemic among IDUs will be critical to designing future interventions for this group, yet there are limited longitudinal data on HIV, other bloodborne infections such as hepatitis C virus (HCV) infection, and associated risk behaviors among IDUs in India.
Chennai is the capital of the southern state of Tamil Nadu. Together, the states of Tamil Nadu, Andhra Pradesh, Karnataka, and Maharashtra account for 60% of HIV infections in India (1). According to the 2007 surveillance report of the National AIDS Control Organisation, Tamil Nadu has a high prevalence of HIV infection among IDUs: 27.2% (1). We characterized HIV and HCV incidence and changes in risk behaviors determined semiannually over 24 months of follow-up in a cohort of IDUs in Chennai.
MATERIALS AND METHODS
Study population
The Madras Injection Drug User and AIDS Cohort Study is a community-based prospective cohort study designed to characterize HIV incidence among IDUs in Chennai. Between April 2005 and May 2006, a sample of volunteers was recruited through community outreach in venues frequented by IDUs (e.g., “shooting galleries” and burial grounds where IDUs congregate to purchase/use drugs). Participants were aged ≥18 years and reported having injected drugs at least once during the prior 6 months, as previously described (8, 9). This study was approved by the institutional review boards of the Y. R. Gaitonde Centre for AIDS Research and Education (Chennai, India) and the Johns Hopkins Bloomberg School of Public Health (Baltimore, Maryland).
Of 1,158 participants recruited, 293 (25.3%) were HIV-positive by double enzyme-linked immunosorbent assay (ELISA) (Murex HIV-1.2.O, Abbott Murex, Dartford, United Kingdom; and Vironostika HIV Uni-Form II Ag/Ab, bioMérieux Deutschland GmbH, Nürtingen, the Netherlands) and 631 (54.5%) demonstrated antibodies to HCV (Murex HIV-1.2.O, Abbott Murex, Johannesburg, South Africa; sensitivity = 100%, specificity = 99.98%) at baseline. Participants who tested HIV-positive at baseline or follow-up were referred to an on-site HIV clinic (10). Medical charts were maintained for all HIV-positive participants who made at least 1 visit to the clinic. The 865 HIV-negative participants were scheduled for semiannual follow-up; 706 (82%) returned for at least 1 visit and were included in the longitudinal analysis (Table 1). Of the 159 persons who did not return for any follow-up, 18 died within the first year (11). Of the remaining participants, only 10 were not traceable. Primary reasons for not returning for follow-up included relocation far from Chennai (n = 46), work responsibilities (n = 24), drug rehabilitation (n = 9), incarceration (n = 2), and hospitalization (n = 1) (11).
Table 1.
Incidence of Human Immunodeficiency Virus and Hepatitis C Virus Seroconversion Among Injection Drug Users Enrolled in the Madras Injection Drug Users and AIDS Cohort Study (n = 865), Chennai, India, 2005–2008
| No. Eligible for Visita | No. of Personsb | Person-Years of Follow-up | No. of Seroconversions | Incidence Rate/100 Person-Years | 95% Confidence Interval | |
| HIV incidence | ||||||
| Year 1 | 847 | 686 | 665 | 4 | 0.60 | 0.16, 1.53 |
| Year 2 | 820 | 616 | 597 | 2 | 0.34 | 0.04, 1.20 |
| Overall | 865 | 706 | 1,262 | 6 | 0.48 | 0.17, 1.03 |
| HCV incidence | ||||||
| Year 1 | 491 | 357 | 383 | 8 | 2.09 | 0.91, 4.07 |
| Year 2 | 464 | 277 | 262 | 3 | 1.15 | 0.23, 3.31 |
| Overall | 519 | 357 | 645 | 11 | 1.71 | 0.85, 3.03 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
A total of 865 persons were HIV-antibody-negative at baseline, and 519 were also negative for HCV antibodies at baseline. Eighteen persons who died in year 1 and 27 who died in year 2 were censored.
Numbers include persons who had either a 6-month or a 12-month visit in year 1 of follow-up and either an 18-month or a 24-month visit in year 2 of follow-up; 20 persons who did not make a follow-up visit in year 1 subsequently made a visit in year 2.
Study procedures
Plasma was pooled from the 159 HIV-negative IDUs who did not return for any follow-up visits (initial pool size = 50) for HIV RNA quantification using the ultrasensitive assay (limit of detection = 50 copies/mL) of the Roche AMPLICOR HIV-1 MONITOR Test, version 1.5 (Roche Diagnostics Corporation, Indianapolis, Indiana) to rule out the possibility that they were in the window period of HIV seroconversion at baseline.
Samples obtained from HIV-infected participants at baseline were tested using the Calypte HIV-1 BED Incidence EIA (Calypte Biomedical Corporation, Portland, Oregon) to estimate HIV incidence. This kit has been validated for HIV subtype C (12), the predominant HIV subtype in India. Medical records of the 12 HIV-infected participants identified as recent seroconverters by BED assay were available and reviewed to rule out false-positive findings (i.e., low CD4 receptor-positive T lymphocyte (CD4+ cell) counts, AIDS-defining illnesses, and use of antiretroviral therapy) (13).
HIV-negative participants completed a structured questionnaire and underwent blood drawing at each visit. The questionnaire was administered by trained interviewers and included questions on 1) frequency, types (e.g., heroin, buprenorphine), and route (injection, noninjection) of drugs used in the prior month and the prior 6 months; 2) injection practices, including frequency and types of needle-/paraphernalia-sharing, shooting gallery attendance, and needle-cleaning; 3) frequency, quantity, and types of alcohol consumed; and 4) numbers and types of sexual practices, condom use, and exchange sex. HIV antibodies were assessed at each visit using double ELISA (similar to baseline). HIV testing was accompanied by pre- and posttest counseling, which included a risk reduction component; counseling sessions followed questionnaire administration and lasted approximately 30 minutes. Participants were educated on the harms of injection drug use, needle-sharing, and inconsistent condom use in a one-on-one session with a trained counselor. The counselors tailored the sessions to the participants' responses to questions (e.g., appropriate cleaning/disposal methods if the participant reported inappropriate needle-cleaning). Following this, participants were asked whether they had any questions.
HCV antibody testing was performed at 12 and 24 months among participants who were HIV- and HCV-negative at baseline (519 eligible persons) using the same method as was used at baseline. A total of 357 participants (70%) had information from at least 1 of these visits; if a sample tested positive for HCV antibodies at 24 months, the 12-month sample was tested to estimate the seroconversion date.
Statistical analysis
HIV-negative IDUs with 1 or more follow-up visits were compared with IDUs who had no visits beyond baseline using the chi-squared test and the Mann-Whitney U test. An absorbance cutoff value of 0.8, corresponding to a seroconversion window of 155 days, was used to calculate HIV incidence (and 95% confidence intervals) by means of the BED assay, utilizing the manufacturer's formulae (12, 14, 15). Incidence was calculated as
where W = window period (155 days), Ni = number of recent infections, and Nu = number uninfected. Samples were excluded from the calculation if they had evidence of a false-positive test (e.g., CD4+ cell count < 200 cells/μL, AIDS-defining illness).
Longitudinal HIV incidence was calculated over the first 4 follow-up visits (24 months) by dividing the number of new infections by the total person-years of follow-up. Seroconversion date was defined as the midpoint between the last date on which a participant tested HIV-negative and the first date on which that person tested HIV-positive. Sensitivity analyses were performed to determine the impact of loss to follow-up on HIV incidence. Similar methods were used for HCV incidence. Poisson regression was used to determine whether there were statistically significant declines in HIV and HCV incidence over the course of follow-up and to ascertain whether these declines were associated with changes in behavior. In this analysis, all HIV-negative participants at baseline and those who tested positive by BED assay were considered to be at risk at baseline, and those who tested positive by BED were considered to have incident infection at baseline. Both of these groups were assigned 155 days of follow-up at baseline to correspond to the seroconversion window estimated by BED. For visits for which an HIV test result was not available (e.g., no blood could be drawn), HIV status was imputed as negative if both surrounding visits were negative.
Multivariate analysis was not performed because of the small number of events. However, we further explored changes in risk by examining visit-to-visit within-person changes in risk behaviors through matched-pair odds ratios calculated using conditional logistic regression. All statistical analyses were performed using Intercooled STATA, version 10.0 (Stata Corporation, College Station, Texas). Statistical significance was set at P < 0.05.
RESULTS
Demographic characteristics and risk behavior at baseline
The median follow-up time among the 706 participants with at least 1 follow-up visit was 1.92 years (interquartile range, 1.87–1.98). All 706 were male; the median age was 35 years (interquartile range, 29–40). The majority of participants followed up were married (67.7%); 26.6% had no formal education (Table 2). Sixty-two percent reported having injected drugs for nonmedical purposes for 5 or more years. Seventy-four percent reported injecting drugs in the prior month; 82.4% reported alcohol use. The median number of alcoholic drinks consumed per drinking episode was 4 (interquartile range, 4–4). Forty-nine percent reported having engaged in sexual intercourse during the prior month. No demographic or risk behaviors significantly differed between participants with at least 1 follow-up visit and the 159 participants who were lost to follow-up (P > 0.15 for all; Table 2). None of the HIV-antibody-negative IDUs who were lost to follow-up tested positive for HIV RNA by polymerase chain reaction (i.e., all persons lost to follow-up were truly HIV-negative at baseline).
Table 2.
Baseline Demographic Characteristicsa and Risk Behaviors of Injection Drug Users Enrolled in the Madras Injection Drug Users and AIDS Cohort Study, by Follow-up Status, Chennai, India, 2005–2008b
| Variable | IDUs With Follow-up (n = 706) |
IDUs Lost to Follow-up (n = 159) |
||
| No. | % | No. | % | |
| Marital status | ||||
| Married or live-in partner | 478 | 67.7 | 106 | 66.7 |
| Single | 203 | 28.8 | 48 | 30.2 |
| Separated | 16 | 2.3 | 3 | 1.9 |
| Divorced/widowed | 9 | 1.3 | 2 | 1.3 |
| Highest level of education | ||||
| None | 188 | 26.6 | 48 | 30.2 |
| Primary | 237 | 33.6 | 55 | 34.6 |
| Secondary | 185 | 26.2 | 36 | 22.6 |
| High school/university/professional | 96 | 13.6 | 20 | 12.6 |
| Frequency of alcohol consumption, times/week | ||||
| Never drinker | 124 | 17.6 | 27 | 17.0 |
| ≤2 | 419 | 59.4 | 99 | 62.3 |
| >2 | 163 | 23.1 | 33 | 20.8 |
| Duration of injection drug use, years | ||||
| <1 | 52 | 7.4 | 19 | 12.0 |
| 1–4.9 | 214 | 30.3 | 59 | 37.1 |
| 5–10 | 189 | 26.8 | 31 | 19.5 |
| >10 | 251 | 35.6 | 50 | 31.5 |
| No. of times drugs were injected in prior month | ||||
| 0 | 186 | 26.4 | 38 | 23.9 |
| 1–30 | 430 | 60.9 | 95 | 59.8 |
| >30 | 90 | 12.8 | 26 | 16.4 |
| Type(s) of drugs injected in prior month | ||||
| None | 185 | 26.2 | 38 | 23.9 |
| Heroin only | 333 | 47.2 | 77 | 48.4 |
| Buprenorphine only | 132 | 18.7 | 27 | 17.0 |
| Heroin and buprenorphine | 56 | 7.9 | 17 | 10.7 |
| Sharing needles in prior month | ||||
| No | 473 | 67.0 | 104 | 65.4 |
| Yes | 233 | 33.0 | 55 | 34.6 |
| Cleaning needles in prior month | ||||
| No | 347 | 49.2 | 78 | 49.1 |
| Yes | 359 | 50.9 | 81 | 50.9 |
| Injecting at a dealer's house in prior month | ||||
| No | 644 | 91.2 | 145 | 91.2 |
| Yes | 62 | 8.8 | 14 | 8.8 |
| Sexual intercourse in prior month | ||||
| No | 357 | 50.6 | 75 | 47.2 |
| Yes | 349 | 49.4 | 84 | 52.8 |
| Incarcerated in prior 6 months | ||||
| No | 617 | 87.4 | 145 | 91.2 |
| Yes | 89 | 12.6 | 14 | 8.8 |
Abbreviation: AIDS, acquired immunodeficiency syndrome; IDUs, injection drug users.
All IDUs were human immunodeficiency virus-negative at baseline. The median age was 35 years (interquartile range, 29–40) among IDUs with follow-up and 33 years (interquartile range, 28–41) among IDUs lost to follow-up.
The 2 groups were compared using the chi-squared test for categorical variables and the Mann-Whitney U test for continuous variables; all P's > 0.15.
Estimated incidence based on the BED assay
Of the 293 seropositive participants with prevalent HIV infection, 12 were identified as having incident infections by BED. One person's sample was excluded because the CD4+ cell count was 144 cells/μL. The median CD4+ cell count of the remaining 11 samples was 711 cells/μL (range, 356–1,171 cells/μL); none of these persons had ever used antiretroviral therapy, and none had an AIDS-defining illness. Assuming a follow-up period of 155 days, incidence was estimated (using the 11 infections identified as incident by BED and the 865 HIV-negative samples) to be 2.95 per 100 person-years (95% confidence interval (CI): 1.21, 4.69).
HIV incidence based on longitudinal follow-up
There were 6 HIV seroconversions in 1,262 person-years of follow-up (incidence rate per 100 person-years = 0.48, 95% CI: 0.17, 1.03) (Table 1). Five participants reported having injected drugs during the 6 months prior to their first seropositive visit; all of the 5 reported needle-sharing. Assuming an average of 24 months of follow-up for the 159 persons with no follow-up, we calculated incidence over a range of different assumptions, from an incidence comparable to that of persons who had follow-up (0.48 per 100 person-years) to more than 4 times' that incidence (1.92 per 100 person-years). Even with the highest value, estimated incidence for the entire cohort would have been only 0.76 per 100 person-years (95% CI: 0.39, 1.32).
There was a statistically significant decline in HIV incidence over the course of follow-up (incidence rate ratio (IRR) per visit = 0.51, 95% CI: 0.33, 0.79). The linear trend was significantly attenuated after we accounted for injection drug use in the prior 6 months (IRR = 0.79, 95% CI: 0.47, 1.34). No other risk behaviors affected the association between follow-up time and HIV incidence (data not shown).
HCV incidence
There were 11 HCV seroconversions in 645 person-years of follow-up (incidence rate per 100 person-years = 1.71, 95% CI: 0.85, 3.03) (Table 1). None of the HCV seroconversions occurred in HIV seroconverters. Six persons reported injecting drugs during the 6-month interval prior to seroconversion; 2 of them reported needle-sharing. Notably, in the 5 persons who did not report drug injection, the seroconversion window was greater than 6 months. HCV incidence also declined over the course of follow-up, but this trend was not statistically significant (IRR per year = 0.70, 95% CI: 0.35, 1.36).
Changes in self-reported risk behaviors
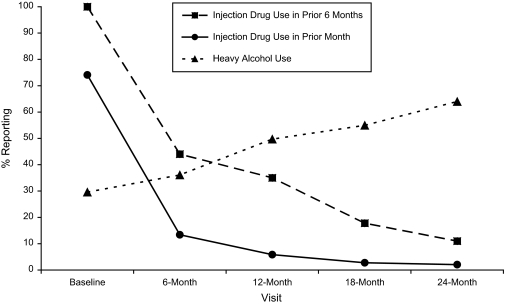
The prevalence of reported injection drug use in the prior 6 months by IDUs declined from 100% at baseline to 35% at 12 months and 11% at 24 months (Figure 1). The odds ratio for injecting any drug in the prior month at 6 months versus baseline was 0.02 (95% CI: 0.01, 0.04; Table 3), representing a 50-fold reduction in risk. Participants were also significantly less likely to report noninjection drug use at 6 months (odds ratio (OR) = 0.31, 95% CI: 0.22, 0.45). Declines in drug use continued through 24 months, with statistically significant declines being seen even from 12 months to 24 months. By 12 months, we also observed a significant reduction in needle-sharing (OR = 0.11, 95% CI: 0.003, 0.80).
Figure 1.
Changes in risk behavior among injection drug users enrolled in the Madras Injection Drug Users and AIDS Cohort Study, Chennai, India, 2005–2008. Heavy alcohol use was defined as consumption of 4 or more alcoholic drinks per day on 3 or more days per week.
Table 3.
Changes in Risk Behavior Over Time Among Injection Drug Users Enrolled in the Madras Injection Drug Users and AIDS Cohort Study, Chennai, India, 2005–2009
| Behaviora | 6 Months vs. 0 Months (n = 628) |
12 Months vs. 0 Months (n = 546) |
24 Months vs. 12 Months (n = 500) |
|||
| ORb | 95% CI | ORb | 95% CI | ORb | 95% CI | |
| Injecting any drug | 0.02 | 0.01, 0.04 | 0.01 | 0.004, 0.03 | 0.28 | 0.10, 0.67 |
| Any noninjection drug use | 0.31 | 0.22, 0.45 | 0.28 | 0.19, 0.40 | 0.97 | 0.69, 1.37 |
| Sharing needlesc | 0.71 | 0.28, 1.73 | 0.11 | 0.003, 0.80 | NCd | |
| Injecting at a dealer's housec | 0.38 | 0.22, 0.66 | NC | NC | ||
| Cleaning needlesc | 1.02 | 0.84, 1.23 | 0.94 | 0.60, 1.50 | NC | |
| Any sexual intercourse | 1.05 | 0.78, 1.42 | 0.91 | 0.68, 1.22 | 1.22 | 0.85, 1.76 |
| Condom use with a regular partnere | 1.00 | 0.27, 3.74 | 0.50 | 0.08, 2.34 | 0.67 | 0.06, 5.82 |
| Sex with a nonregular partner | 0.68 | 0.46, 0.99 | 0.56 | 0.37, 0.84 | 1.11 | 0.69, 1.79 |
| Condom use with a nonregular partnerf | 2.17 | 0.77, 6.95 | 3.33 | 0.86, 18.85 | 2.00 | 0.29, 22.11 |
| Exchanging money/drugs for sex | 0.95 | 0.61, 1.50 | 0.73 | 0.45, 1.18 | 1.15 | 0.66, 2.00 |
| Alcohol use >2 times/week | 1.28 | 0.99, 1.67 | 2.40 | 1.83, 3.16 | 3.14 | 2.05, 4.95 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; CI, confidence interval; NC, not calculated; OR, odds ratio.
All behaviors were self-reported and represented behaviors engaged in during the month prior to the interview.
Matched-pair odds ratio calculated using conditional logistic regression.
Data were restricted to participants who reported injecting drugs during the prior month at both time points.
Not calculated because too few people reported these behaviors at the 12-/24-month visit.
Data were restricted to participants who reported having had sex with a regular partner.
Data were restricted to participants who reported having had sex with a nonregular partner.
Compared with baseline, the odds ratio for having sex with a nonregular partner was 0.68 (95% CI: 0.46, 0.99) at 6 months (Table 3). This trend continued through 12 months, where we observed increased condom use among persons reporting sexual intercourse with nonregular partners, although the finding was not statistically significant (OR = 3.33, 95% CI: 0.86, 18.8). Trends in most sexual behaviors remained stable between 12 months and 24 months.
The only risk behavior that increased significantly over time was alcohol use. Compared with baseline, the proportion of participants who reported drinking alcohol more than 2 times per week increased consistently over time (Figure 1). In addition, we observed increases between 12 months and 24 months (OR = 3.14, 95% CI: 2.05, 4.95). The median number of reported drinks per occasion remained constant at 4.
DISCUSSION
To our knowledge, our estimates represent the first longitudinal assessment of HIV and HCV incidence among IDUs in India. Observed HIV and HCV incidence were lower than expected, particularly given the high baseline prevalence of both infections and the BED-estimated incidence. Based on HIV prevalence estimates, lack of access to antiretroviral therapy in this population, and a median survival of 92 months for HIV-infected persons in Chennai (16), an HIV incidence of 2–4 per 100 person-years was expected; expected HCV incidence would be even higher, as has been shown in other settings (17).
This finding was unexpected and in contrast to incidence estimates from IDU cohorts in Eastern Europe and Asia. Kozlov et al. (18) reported an incidence of 4.5 per 100 person-years among 520 IDUs in St. Petersburg, Russia, with a baseline prevalence of 30%. Zhang et al. (19) reported an incidence of 8.8 per 100 person-years among 508 IDUs in China with a baseline prevalence of 29%. Vanichseni et al. (20) observed an incidence of 5.8 per 100 person-years among IDUs in Bangkok, Thailand, with a baseline HIV prevalence of 30%. More recently, Ruan et al. (17) reported an incidence of 2.3 per 100 person-years among 333 IDUs in Xichang City, China, with a baseline prevalence of 11%. HCV incidence estimates from these regions are limited but when available are several orders of magnitude higher than what was observed in Chennai (e.g., 33 per 100 person-years in China) (17).
The longitudinal measured HIV incidence (0.48 per 100 person-years) was nearly 5 times lower than the BED-estimated incidence at baseline (2.95%). Indeed, the BED estimate was more consistent with the reports described above. While the BED assay has some acknowledged limitations (13, 21), which may lead to overestimation, we addressed these limitations by using double ELISA testing for confirmation, CD4+ cell counts, and review of medical charts to rule out AIDS. Further, our BED-estimated incidence is consistent with the recent suggestion that the expected annual HIV incidence in India is 10% of the baseline prevalence (22). Applying the method used for this estimate to the median survival time among HIV-infected persons in Chennai, we would expect an incidence that is 13.1% of the baseline prevalence, which is comparable to our estimate of 2.95 per 100 person-years (11.7% of baseline prevalence).
The discrepancies between our observed incidence and the BED results, as well as findings from other cohorts in the region, raise the question of whether our observation of low measured longitudinal incidence is valid or may reflect underlying unmeasured biases. Arguing against bias is the fact that the low HIV incidence rates were accompanied by dramatic declines in self-reported risk behaviors associated with HIV incidence. There were major reductions in self-reported injection as well as other risk behaviors (e.g., needle-sharing and anonymous sex) between baseline and 6 months, which continued through 24 months. On the other hand, several other factors should be considered before attributing the low incidence to declining risk behaviors.
Of concern is emigrative selection bias due to deaths and losses to follow-up. High mortality has previously been reported in this cohort; among HIV-negative persons, mortality was associated with daily drug injection (11). Thus, persons who died may also have been more likely to acquire HIV and HCV. However, those who were lost for other reasons were no different than those who remained with respect to HIV risk behaviors, and none had unrecognized incident infections. Further, the majority reported missing visits because of work or migration for work, which would be expected to result in greater stability, lower risk, and lower HIV incidence. Patterns of loss to follow-up in this cohort were also similar to those in other large IDU cohorts (23, 24). Finally, even under the assumption that HIV incidence was 4 times greater in those lost, incidence would have only reached 0.76 per 100 person-years.
When behaviors are primarily self-reported, information bias is a concern. It is possible that some persons may have falsely claimed a history of injection drug use at baseline in order to benefit from compensation. However, a number of preventive measures were in place. Participants were not informed of inclusion criteria before or after screening. Most participants were recruited from locations where IDUs congregate by field staff who themselves were former IDUs. Moreover, the HIV and HCV prevalence estimates were consistent with those observed in cross-sectional studies among IDUs in Chennai (25, 26). Mortality rates were also comparable with, if not higher than, those in other cohort studies of IDUs, and the leading cause of death among HIV-negative persons was overdose (11). Finally, we independently recruited 400 wives/sexual partners of these participants, and all confirmed their husbands' injection behavior (27).
Once someone is enrolled in a study, self-reported data on personal behaviors can be compromised by social desirability. As participants establish rapport with interviewers and undergo repeated HIV risk reduction counseling, they may underreport their risk behavior. While prior studies have confirmed the validity of self-reports among IDUs (28), these studies were not conducted in India, where cultural differences may exist. However, the questionnaires were based on surveys used in the United States, Thailand, and Vietnam (24), and participants would have also had a disincentive to report other high-risk behaviors (e.g., alcohol use) that increased over time. Finally, the low incidences of HIV and HCV further support declining rates of injection drug use.
Because our analysis focused on individual-level data, we did not have data on social networks or environmental factors. It is possible that the IDU community in Chennai comprises many small and dense social networks with minimal interaction between networks, implying that HIV and HCV are transmitted quickly within networks but more slowly between networks. We did not have data on environmental factors such as drug availability and pricing, which can fluctuate. Expanded harm reduction programs, including syringe exchange programs, drug treatment, and voluntary counseling and testing (VCT), have been associated with reduced HIV incidence in the West (29–31), so a period effect should be considered, particularly when comparing results with those of studies conducted earlier. However, an impact of such trends is unlikely, given that: 1) the Tamil Nadu government is just beginning to incorporate IDUs into interventions; 2) opiate substitution, particularly methadone maintenance, is not widely available in India, and the primary treatment available is detoxification (32); 3) fewer than 1% of participants reported procuring needles from syringe exchange programs; and 4) anecdotal evidence from focus groups suggests that the majority of IDUs in this cohort were exposed to VCT for the first time through this study.
Finally, it is well recognized that self-reported risk behaviors often decrease after people enroll in a study (the “Hawthorne effect”), and the sheer fact that people are being observed prompts them to change their behavior. While this bias would have played a role in the other cohort studies described above, it is possible that the impact was even greater in our study. Importantly, our data were not derived from a randomized clinical trial. No formal intervention was administered, so such a dramatic behavior change was not expected. All participants did receive the prevention standard of care at each visit, which included VCT. VCT has previously been associated with declines in risk behavior in other populations and regions, especially following first exposure (33). Although we did not specifically ask about prior VCT, anecdotal evidence from focus groups and clinic interactions suggests that this group had limited prior exposure to VCT. Thus, it is important to acknowledge the possibility that the “enhanced” VCT (VCT plus individually tailored risk reduction counseling) administered as part of our standard study procedures had some impact on behavior change.
We have previously observed similar effects of VCT in other populations in Chennai. In a study of 500 high-risk men and women from sexually transmitted infection clinics (2003–2004), a low HIV incidence of 0.44 per 100 person-years was observed after risk reduction counseling (34). In several HIV prevention trials where VCT was not actually the intervention being tested, low HIV incidence was observed in both intervention and control groups, suggesting that even the standard-of-care VCT received by the control group may have had some impact. For example, among 254 female sex workers in Chennai followed as part of a vaginal microbicide study, no seroconversions were observed (35). Among 3,521 wine-shop patrons and female sex workers followed for 24 months for assessment of a peer-based motivational intervention, only 6 HIV seroconversions were observed in more than 6,500 person-years of follow-up (David Celentano, Johns Hopkins Bloomberg School of Public Health, unpublished manuscript). These consistent reports support the hypothesis that VCT may influence behavior change to a large enough extent to affect disease incidence, at least in Chennai.
As we described in detail above, we were limited in this study by not being able to rule out other explanations. The question of whether the low incidence of bloodborne infections can be causally attributed to behavior change and potentially VCT could only be answered through a randomized trial, with the intervention arm being exposed to VCT and the control arm being unexposed. However, such a trial would not be ethical, as it would deny some participants the standard of care (VCT). Another valuable comparison might be with another cohort study in India where the risk reduction component of VCT may have been less intensive; however, to date, we know of no other longitudinal studies among IDUs in India. Additional factors may also affect generalizability. Compared with other Asian cohorts, there were fewer daily heroin injectors in the Madras Injection Drug Users and AIDS Cohort Study. For example, Kozlov et al. (18) and Zhang et al. (19) required for study inclusion that IDUs had to have injected drugs or shared injecting equipment at least 3 times per week during the prior month. The patterns of drug use observed in the Madras Injection Drug Users and AIDS Cohort Study may reflect lower levels of physiologic dependence in comparison with other cohorts; for example, Vanichseni et al. (20) recruited their cohort of IDUs from drug treatment centers, suggesting higher levels of dependence. Anecdotal reports have suggested that heroin in Chennai is of low purity, which can also affect dependence. Finally, we observed that declines in injection drug use were compensated for by simultaneous increases in alcohol use. In focus groups, participants reported “substituting” alcohol for drugs in order to maintain feelings of intoxication (unpublished data), which further supports the potential for psychological addiction to the practice of drug use rather than physiologic dependence on opioids. This finding is of particular concern given that 65% of the IDUs in this cohort were positive for HCV antibodies (8) and that alcohol accelerates the progression of liver disease (36).
These limitations notwithstanding, we observed low incidences of HIV and HCV infection coupled with substantial reductions in self-reported risk behaviors in this cohort of IDUs. Although we cannot definitively conclude that this was due to VCT, our data support increased access to VCT in this population. VCT targeted at IDUs has not been a key component of HIV prevention programs in India, especially outside of the northeast, but it represents a valuable first step and a cost-effective option that could be implemented without difficulty. Given the already high prevalence of HIV and HCV infection in this population, VCT may also serve as an entry point to medical care. Finally, it is important to factor in the impact of VCT on risk reduction and HIV/HCV incidence when estimating sample sizes for prevention trials in high-risk populations.
Acknowledgments
Author affiliations: Y. R. Gaitonde Centre for AIDS Research and Education, Chennai, India (Sunil Suhas Solomon, Aylur K. Srikrishnan, Canjeevaram K. Vasudevan, Kailapuri G. Murugavel, Syed H. Iqbal, Santhanam Anand, Muniratnam S. Kumar, Suniti Solomon); and Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Sunil Suhas Solomon, David D. Celentano, Carl Latkin, Shruti H. Mehta).
This study was partly supported by the US National Institutes of Health (grant R01-DA-12568) and the Indian Council of Medical Research (grant HIV/Indo-US/17/2007-ECD-II) as part of the US-India Bilateral Collaborative Research Partnership on the Prevention of HIV/AIDS. The study was also supported by the Fogarty International Center, US National Institutes of Health (grant 2D-43-TW000010-20-AITRP).
The authors thank the study and field staff at the Y. R. Gaitonde Centre for AIDS Research and Education and the Y. R. Gaitonde Centre for Substance Abuse-Related Research.
Conflict of interest: none declared.
Glossary
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- CI
confidence interval
- ELISA
enzyme-linked immunosorbent assay
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IDU
injection drug user
- IRR
incidence rate ratio
- OR
odds ratio
- VCT
voluntary counseling and testing
References
- 1.National AIDS Control Organisation, Ministry of Health and Family Welfare. HIV Sentinel Surveillance and HIV Estimation in India 2007—A Technical Brief. New Delhi, India: Ministry of Health and Family Welfare; 2008. ( http://www.nacoonline.org/upload/Publication/M&E%20Surveillance,%20Research/HIV%20Sentinel%20Surveillance%20and%20HIV%20Estimation%202007_A%20Technical%20Brief.pdf). (Accessed November 10, 2009) [Google Scholar]
- 2.National AIDS Control Organisation, Ministry of Health and Family Welfare. HIV Sentinel Surveillance Country Report 2006. New Delhi, India: Ministry of Health and Family Welfare; 2007. ( http://nacoonline.org/upload/NACO%20PDF/HIV%20Sentinel%20Surveillance%202006_India%20Country%20Report.pdf). (Accessed September 17, 2010) [Google Scholar]
- 3.Arora P, Kumar R, Bhattacharya M, et al. Trends in HIV incidence in India from 2000 to 2007. Lancet. 2008;372(9635):289–290. doi: 10.1016/S0140-6736(08)61105-8. [DOI] [PubMed] [Google Scholar]
- 4.Kumar R, Jha P, Arora P, et al. Trends in HIV-1 in young adults in south India from 2000 to 2004: a prevalence study. Lancet. 2006;367(9517):1164–1172. doi: 10.1016/S0140-6736(06)68435-3. [DOI] [PubMed] [Google Scholar]
- 5.Aceijas C, Friedman SR, Cooper HL, et al. Estimates of injecting drug users at the national and local level in developing and transitional countries, and gender and age distribution. Sex Transm Infect. 2006;82(suppl 3):iii10–iii17. doi: 10.1136/sti.2005.019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar S, Das N, Panda S, et al. Rapid spread of HIV among injecting drug users in north-eastern states of India. Bull Narc. 1993;45(1):91–105. [PubMed] [Google Scholar]
- 8.Solomon SS, Srikrishnan AK, Mehta SH, et al. High prevalence of HIV, HIV/hepatitis C virus coinfection, and risk behaviors among injection drug users in Chennai, India: a cause for concern. J Acquir Immune Defic Syndr. 2008;49(3):327–332. doi: 10.1097/QAI.0b013e3181831e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon SS, Desai M, Srikrishnan AK, et al. The profile of injection drug users in Chennai, India: identification of risk behaviours and implications for interventions. Subst Use Misuse. 2010;45(3):354–367. doi: 10.3109/10826080903452447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon SS, Hawcroft CS, Narasimhan P, et al. Comorbidities among HIV-infected injection drug users in Chennai, India. Indian J Med Res. 2008;127(5):447–452. [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon SS, Celentano DD, Srikrishnan AK, et al. Mortality among injection drug users in Chennai, India (2005-2008) AIDS. 2009;23(8):997–1004. doi: 10.1097/QAD.0b013e32832a594e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parekh BS, Kennedy MS, Dobbs T, et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res Hum Retroviruses. 2002;18(4):295–307. doi: 10.1089/088922202753472874. [DOI] [PubMed] [Google Scholar]
- 13.Karita E, Price M, Hunter E, et al. Investigating the utility of the HIV-1 BED capture enzyme immunoassay using cross-sectional and longitudinal seroconverter specimens from Africa. AIDS. 2007;21(4):403–408. doi: 10.1097/QAD.0b013e32801481b7. [DOI] [PubMed] [Google Scholar]
- 14.Janssen RS, Satten GA, Stramer SL, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280(1):42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- 15.Dobbs T, Kennedy S, Pau CP, et al. Performance characteristics of the immunoglobulin G-capture BED-enzyme immunoassay, an assay to detect recent human immunodeficiency virus type 1 seroconversion. J Clin Microbiol. 2004;42(6):2623–2628. doi: 10.1128/JCM.42.6.2623-2628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumarasamy N, Solomon S, Flanigan TP, et al. Natural history of human immunodeficiency virus disease in southern India. Clin Infect Dis. 2003;36(1):79–85. doi: 10.1086/344756. [DOI] [PubMed] [Google Scholar]
- 17.Ruan Y, Qin G, Yin L, et al. Incidence of HIV, hepatitis C and hepatitis B viruses among injection drug users in southwestern China: a 3-year follow-up study. AIDS. 2007;21(suppl 8):S39–S46. doi: 10.1097/01.aids.0000304695.54884.4f. [DOI] [PubMed] [Google Scholar]
- 18.Kozlov AP, Shaboltas AV, Toussova OV, et al. HIV incidence and factors associated with HIV acquisition among injection drug users in St Petersburg, Russia. AIDS. 2006;20(6):901–906. doi: 10.1097/01.aids.0000218555.36661.9c. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Shan H, Trizzino J, et al. HIV incidence, retention rate, and baseline predictors of HIV incidence and retention in a prospective cohort study of injection drug users in Xinjiang, China. Int J Infect Dis. 2007;11(4):318–323. doi: 10.1016/j.ijid.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Vanichseni S, Kitayaporn D, Mastro TD, et al. Continued high HIV-1 incidence in a vaccine trial preparatory cohort of injection drug users in Bangkok, Thailand. AIDS. 2001;15(3):397–405. doi: 10.1097/00002030-200102160-00013. [DOI] [PubMed] [Google Scholar]
- 21.McDougal JS, Parekh BS, Peterson ML, et al. Comparison of HIV type 1 incidence observed during longitudinal follow-up with incidence estimated by cross-sectional analysis using the BED capture enzyme immunoassay. AIDS Res Hum Retroviruses. 2006;22(10):945–952. doi: 10.1089/aid.2006.22.945. [DOI] [PubMed] [Google Scholar]
- 22.Lakshmi V, Sudha T, Dandona R, et al. Application of human immunodeficiency virus type 1 BED enzyme immunoassay on dried blood spots in India. J Med Microbiol. 2009;58(3):312–317. doi: 10.1099/jmm.0.005249-0. [DOI] [PubMed] [Google Scholar]
- 23.Nelson KE, Vlahov D, Solomon L, et al. Temporal trends of incident human immunodeficiency virus infection in a cohort of injecting drug users in Baltimore, MD. Arch Intern Med. 1995;155(12):1305–1311. [PubMed] [Google Scholar]
- 24.Vlahov D, Anthony JC, Munoz A, et al. The ALIVE Study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 25.Panda S, Kumar MS, Lokabiraman S, et al. Risk factors for HIV infection in injection drug users and evidence for onward transmission of HIV to their sexual partners in Chennai, India. J Acquir Immune Defic Syndr. 2005;39(1):9–15. doi: 10.1097/01.qai.0000160713.94203.9b. [DOI] [PubMed] [Google Scholar]
- 26.Kumar MS, Mudaliar S, Thyagarajan SP, et al. Rapid assessment and response to injecting drug use in Madras, south India. Int J Drug Policy. 2000;11(1-2):83–98. doi: 10.1016/s0955-3959(99)00057-2. [DOI] [PubMed] [Google Scholar]
- 27.Mehta SH, Srikrishnan AK, Celentano DD. The intersection between sex and drugs: HIV prevalence among sexual partners of IDUs in Chennai, India [abstract]. (Abstract W-157). Presented at the 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, California, February 16–19, 2010. [Google Scholar]
- 28.Samuels JF, Vlahov D, Anthony JC, et al. Measurement of HIV risk behaviors among intravenous drug users. Br J Addict. 1992;87(3):417–428. doi: 10.1111/j.1360-0443.1992.tb01942.x. [DOI] [PubMed] [Google Scholar]
- 29.Mehta SH, Galai N, Astemborski J, et al. HIV incidence among injection drug users in Baltimore, Maryland (1988–2004) J Acquir Immune Defic Syndr. 2006;43(3):368–372. doi: 10.1097/01.qai.0000243050.27580.1a. [DOI] [PubMed] [Google Scholar]
- 30.Des Jarlais DC, Perlis T, Arasteh K, et al. HIV incidence among injection drug users in New York City, 1990 to 2002: use of serologic test algorithm to assess expansion of HIV prevention services. Am J Public health. 2005;95(8):1439–1444. doi: 10.2105/AJPH.2003.036517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kral AH, Lorvick J, Gee L, et al. Trends in human immunodeficiency virus seroincidence among street-recruited injection drug users in San Francisco, 1987–1998. Am J Epidemiol. 2003;157(10):915–922. doi: 10.1093/aje/kwg070. [DOI] [PubMed] [Google Scholar]
- 32.Steinbrook R. HIV in India—a complex epidemic. N Engl J Med. 2007;356(11):1089–1093. doi: 10.1056/NEJMp078009. [DOI] [PubMed] [Google Scholar]
- 33.Voluntary HIV-1 Counseling and Testing Efficacy Study Group. Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. Lancet. 2000;356(9224):103–112. [PubMed] [Google Scholar]
- 34.Solomon SS, Solomon S, Masse BR, et al. Risk reduction counseling is associated with decreased HIV transmission-associated behaviors in high-risk Indian heterosexuals. J Acquir Immune Defic Syndr. 2006;42(4):478–483. doi: 10.1097/01.qai.0000221684.83057.2f. [DOI] [PubMed] [Google Scholar]
- 35.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359(5):463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 36.Poynard T, Ratziu V, Charlotte F, et al. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol. 2001;34(5):730–739. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]